Abstract
Background & Aims
Mucosal healing, based on histologic analysis, is an endpoint of maintenance therapy for patients with ulcerative colitis (UC). There are few data on how histologic signs of inflammation correlate with endoscopic and peripheral blood measures of inflammation in these patients. We investigated patterns of histologic features of inflammation in patients with UC in clinical remission, and correlated these with endoscopic and biochemical measures of inflammation.
Methods
We performed a prospective observational study of 103 patients with UC in clinical remission undergoing surveillance colonoscopy while receiving maintenance therapy with mesalamine or thiopurines; 2674 biopsies were collected from 708 colonic segments. Each colonic segment was evaluated based on the Mayo endoscopic sub-score and the Geboes histology score (0–5.4). Biomarkers were measured in peripheral blood samples.
Results
Histologic features of inflammation were found in 54% of patients receiving maintenance therapy; 37% had at least moderate inflammation, based on histology scores. Of the 52 patients with endoscopic evidence only of left-sided colitis, 34% had histologic features of inflammation in their proximal colon. Histology scores correlated with endoscopic scores for per-segment inflammation (Spearman’s ρ=0.65; P<.001). Patients with histology scores >3.1 had a significantly higher mean level of C-reactive protein (CRP) than those with scores <3.1. There were no differences among treatment groups in percentages of patients with histologic scores >3.1.
Conclusions
Patients in clinical remission from UC still frequently have histologic features of inflammation, which correlate with endoscopic appearance. Patients with at least moderate levels of inflammation, based on histologic grading (score >3.1), have higher serum levels of CRP, which could be used as a surrogate marker of histologic inflammation.
Keywords: 5-ASA, response to therapy, therapeutic efficacy, mucosal healing, IBD
INTRODUCTION
The goal of therapy in patients with ulcerative colitis (UC) has shifted from symptom control alone to clinical remission in conjunction with mucosal healing (MH) 1. Clinical trials of drugs in patients with UC now routinely include a mucosal healing end-point, and expert consensus recommends healing as an end-point for optimal management in practice 2. The advantages of achieving resolution of mucosal inflammation can be seen in the reported lower rates of disease relapse, hospitalization, need for immunosuppressive therapy, and colon cancer in patients who obtain mucosal healing 3-5.
Although most studies on MH focus on endoscopic scores, such as the Mayo sub-score, some experts have suggested histological inflammation may be a valuable goal of therapy 6,7.The presence of histological inflammation is a better predictor of future clinical relapse than endoscopic appearance alone 8. A higher risk of relapse was noted in studies of patients with persistent active microscopic inflammation, when compared to patients with normal histology 9-11. Histological remission was also associated with a lower rate of hospitalization during a median 29 month follow up in a small cohort 12. A recent abstract from Rubin et al. reported that an increased level of histological inflammation could predict both colectomy and hospitalization in patients with UC 11.
In this context, validated scoring systems for evaluation of histological severity in clinical trials are desirable. The Riley index, Geboes Index and Chicago index have been developed for this purpose, but none universally utilized or independently validated 10,11,13. Recent expert guidelines have recommended a histological score be applied consistently as a secondary end-point in clinical trials 6. The Geboes score was first reported in 2000; it showed good reproducibility and modest agreement with the endoscopic grading system in 28 patients. Its independent validation has not been reported since then.
Evaluation of the severity of histological inflammation as an end-point for drug therapy has not been part of standard clinical practice, although persistent endoscopic and histological inflammation in the absence of clinical symptoms is common 14,15. Patients with quiescent UC with histological inflammation are difficult to identify as endoscopic measures of inflammation have variable correlation with symptoms. A small prospective study, presented only in abstract form, reported only modest agreement between clinical, endoscopic and histological measures of remission with complete agreement in just 58% of 91 patients (kappa 0.44) and 89% agreement between endoscopy and histology 16. Given the potential importance of histological healing in long-term outcomes with UC, and the limitations of using symptoms alone to screen for underlying macroscopic or microscopic inflammation, identification of markers of histological inflammation are needed in patients in remission.
The goal of this study was to enroll a cohort of patients with UC in clinical remission, to determine the prevalence of histological colitis in these patients using the Geboes grading system, and to compare the correlation between the Geboes score, and endoscopic and biochemical markers of disease activity in this setting.
MATERIALS AND METHODS
This was a prospective observational study performed at a single tertiary referral center. The study was approved for enrollment of human subjects by the local Institutional Review Board (protocol # 2009-P-000314). All patients with a confirmed history of ulcerative colitis who attended the endoscopy unit for a clinically-indicated surveillance colonoscopy were screened. All patients received the same bowel preparation (magnesium citrate). Clinical disease activity was determined using the Simple Clinical Colitis Activity Index (SCCAI), a validated score of colitis activity that has been shown to correlate well with endoscopic indices 17,18. In order to be considered “in remission” for enrollment in this study, participants had to have an SCCAI score <2.5 at the screening visit, and have had no changes in their UC medications or any steroid use in the prior month 17.
Each enrolled patient had baseline demographic and ulcerative colitis disease history recorded. This included disease location, duration, prior and current medication use, family history, extraintestinal disease, smoking status and NSAID use. During the index colonoscopy, the colon was divided into eight segments (cecum, ascending colon, hepatic flexure, transverse colon, splenic flexure, descending colon, sigmoid colon and rectum). Endoscopic activity in each colonic segment was classified by the endoscopist using the sigmoidoscopy subscore of the Mayo activity index (Suppl Table 1)19. A sub-set of 15 patients had their endoscopy images re-scored by a second blinded endoscopist to determine the interobserver agreement for the endoscopic score. The kappa statistic was 0.7, suggesting substantial agreement between endoscopists.
The protocol for the 4 gastroenterologists who performed the colonoscopies included the recommended four-quadrant biopsies every 10cm. Histological activity in all segments was classified using the Geboes scale, by a GI pathologist (JDG) blinded to the patient’s disease status and endoscopic scores 13. A baseline blood sample was drawn for measurement of white blood count (WBC), hematocrit (Hct), erythrocyte sedimentation rate (ESR in mm/hr) and C-reactive protein (CRP in mg/L) in all patients.
Histological scoring
The Geboes grading system is an instrument with 6 domains: structural (architectural change), chronic inflammatory infiltrate, lamina propia neutrophils and eosinophils, neutrophils in epithelium, crypt destruction, and erosions or ulcerations 13. Scores can range from 0 to 5.4, with higher scores indicating more severe histological inflammation (Suppl Figure 1). A total Geboes score was assigned to biopsies from each colonic segment and the highest score (most inflamed segment by histology) was used as the total histology score for each patient.
Statistical analysis
Dichotomous variables were analyzed for outcomes using ×2 test or Fisher’s exact test where appropriate, and continuous variables analyzed using t-test if normally distributed, or Wilcoxon test for non-normal data. Correlation between ordinal numeric scores was analyzed by Spearman’s rank correlation coefficient (ρ). A kappa agreement statistic was generated for assessment of dichotomous characterization of normal / not normal by endoscopic and histological scores. Data was analyzed with JMP 8.0 (SAS Institute Inc., North Carolina). Post-hoc power calculations were performed using the PS Power and sample size calculator (http://biostat.mc.vanderbilt.edu/PowerSampleSize).
RESULTS
One hundred and forty seven patients scheduled for surveillance were screened, and 103 enrolled in the study. Only 10% of the eligible population screen-failed due to clinically-active disease at the time of colonoscopy. The baseline characteristics of these 103 patients are summarized in Table 1A, and similar to our typical surveillance population. The biopsy sampling protocol during surveillance was extensive; the mean number of biopsies per colonoscopy was 26, and 2674 biopsies in total were taken during the 103 colonoscopies.
Table 1.
A. Baseline characteristics of enrolled cohort (N=103)
| Mean Age in yrs (SD) | 50 (14) |
| Male (%) | 48% |
| Mean Disease Duration in yrs (SD) | 22 (26) |
| Clinical Remission < 6 months (%) | 79 |
| Median SCCAI score (IQR) | 1 (0-2) |
| Steroid use in last 12 months (%) | 17 |
| Disease geography | |
| Left-sided colitis (%) | 51 |
| Extensive colitis (%) | 49 |
| Current medications | |
| Mesalamine (%) | 78 |
| Azathioprine / mercaptopurine (%) | 18 |
| Infliximab (%) | 6 |
| Laboratory Markers | Mean (SD) |
| WCC (K/uL) | 6.3 (2) |
| Hematocrit (%) | 41 (5.6) |
| ESR (mm/hr) | 10 (9) |
| CRP (mg/L) | 3.7 (4.8) |
Of the 103 colonoscopies, 54% of patients had at least one biopsy with evidence of any histological inflammation, and 37% had biopsies that met the Geboes criteria for abnormal histological inflammation (score >3.1) 20. Eleven patients (11%) had a colonoscopy that showed histological inflammation in the right colon (score > 0) in the absence of endoscopic inflammation, and 6 of these had scores > 3.1. Of the 52 patients with endoscopic evidence only of left-sided colitis, 34% had histological inflammation in their right colon.
Amongst all colonic segments (n=708), 20% had histological evidence of inflammation, and 21% had an abnormal endoscopic appearance. In colonic segments with endoscopic inflammation (Mayo score > 1), 57% of biopsies from these segments had a Geboes score < 3.1, and 43% had a score > 3.1. In colonic segments with a Mayo score of 0 (normal endoscopic appearance), only 6% had underlying histological evidence of inflammation on biopsy.
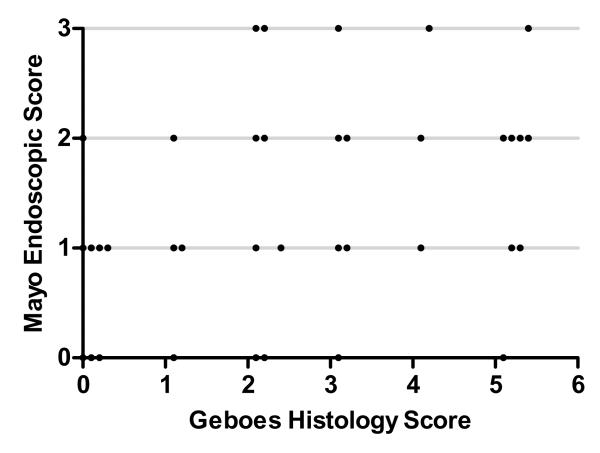
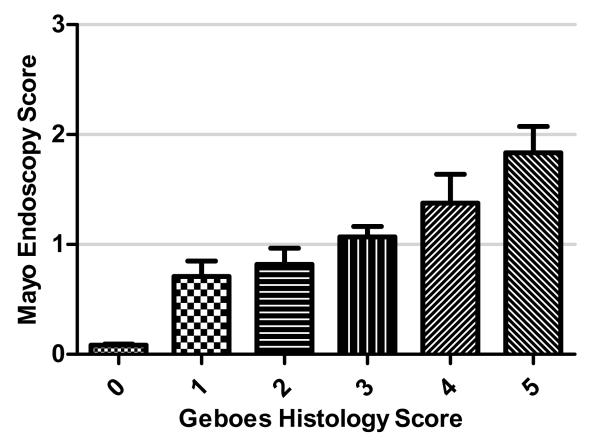
To determine the correlation between the Geboes histological score and Mayo endoscopic score, the score for each colonic segment was plotted on an x-y plot. There was a good correlation between histological and endoscopic scores, Spearman’s ρ=0.65, p<0.001 (Figure 1). When mean endoscopy scores for each segment were grouped according to Geboes grade (0-5), only segments with histological inflammation scores >3 had an endoscopic score >1 (Figure 2). If endoscopic and histological scores were dichotomized (0, >0), the agreement statistic (kappa) was 0.62 for the presence of normal histology in patients with normal endoscopic appearance.
Figure 1.
Correlation between Geboes histological score and Mayo endoscopic score for each colonic segment
Figure 2.
Individual Geboes histological scores (0-5) plotted against Mayo endoscopic scores
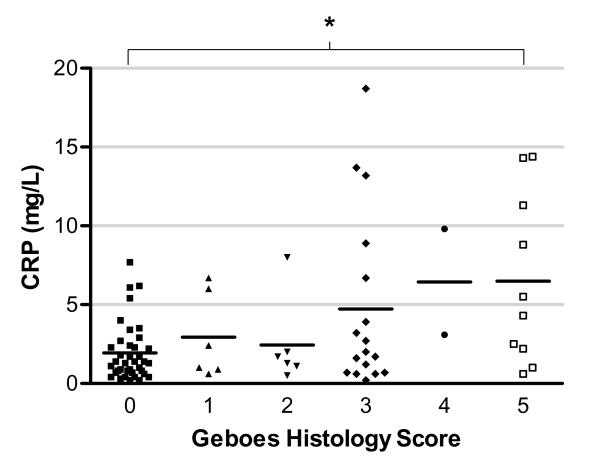
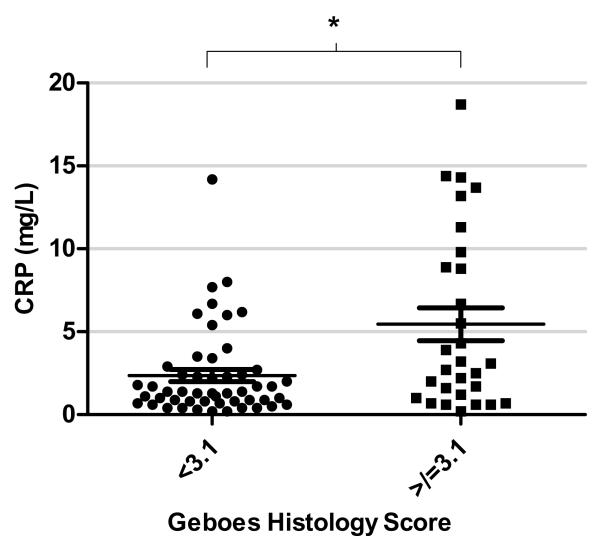
Since peripheral blood biomarkers have been associated with intestinal inflammation, we next sought to assess the blood levels of CRP and ESR in patients with histological inflammation. As can be seen in Figure 3, mean CRP levels were predominantly in the normal range (<5mg/L) for patients whose highest histological score was <3.1, but a wider distribution of levels was apparent in those with greater histological inflammation (p=0.008 by ANOVA). When Geboes scores were dichotomized (<3.1 or > 3.1), the mean CRP was higher in patients with a histological score > 3.1, than those with normal histological appearance (mean 5.4mg/L versus 2.3mg/L, p=0.001 by t-test) (Figure 4). The performance of CRP as a test for histological inflammation > 3.1 in patients in clinical remission was modest; area under the receiver-operator characteristic (ROC) curve was 0.67. A CRP cut-off of 2mg/L had the highest accuracy (66% sensitivity, 63% specificity) in this cohort. For correctly identifying patients with histological scores > 3.1, a CRP level above 10mg/L had an 86% positive predictive value, whereas levels below 0.5mg/L had an 88% negative predictive value. ESR was not significantly different between the two groups.
Figure 3.
Correlation between individual Geboes histological scores (0-5) and serum CRP levels
Figure 4.
Correlation between Geboes histological scores (<3.1 and =/> 3.1) and serum CRP levels
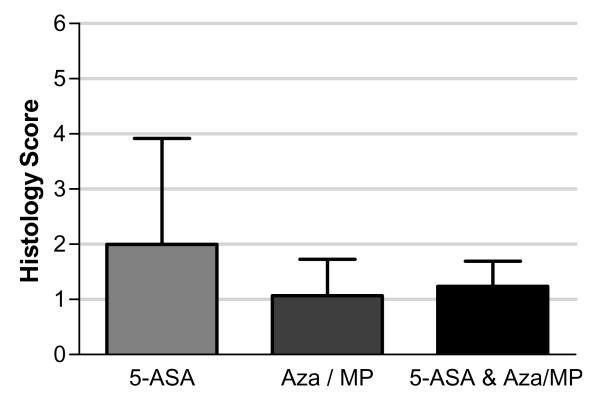
Finally, we examined whether there were difference in histological appearance according to maintenance therapy. Of the 18 patients taking thiopurines, all had been prescribed for > 3 months, and 52% were prescribed the recommended weight-based dose at time of enrollment (6MP 1.5mg/kg, azathioprine 2.5mg/kg). Of the 78 patients taking mesalamine, 80% were taking >2.4g/day. As can be seen in Figure 5, the patients’ overall mean histological level of inflammation was similar across treatment groups (mean scores 1.9, 1.1 and 1.2 for 5-ASA, Aza/MP, and 5-ASA & Aza/MP respectively). The proportion of patients taking 5-ASA or Aza/MP who had a histological score >3.1 was also similar (33% and 42% respectively). Although we did not measure adherence to these medications directly, prior studies from our patient population have reported over 70% adherence rates 21.
Figure 5.
Geboes histological scores in patients on maintenance therapy for quiescent ulcerative colitis
DISCUSSION
The goal of treating patients with IBD has evolved to focus on achieving mucosal healing, which has been associated with a reduced risk of long term complications 22. Histological assessment of underlying healing may be important in predicting future relapse and complications 11. The main limitations to the use of this end-point are the incomplete validation of the available histological scoring systems, and a lack of data on the patterns of histological healing when patients achieve clinical remission.
This study provides a number of novel pieces of information for the gastroenterology community. Firstly, more than one half of patients in clinical remission undergoing surveillance colonoscopy had histological evidence of inflammation, and a third had at least moderate histological inflammation. This was independent of which type of maintenance therapy they were receiving, but associated with higher mean serum CRP levels in our study. Baars et al. reported that 63% of their cohort of 98 UC patients in remission had histological inflammation, and 20% had at least moderate inflammation, using a non-validated scale 14. They did not correlate histological scores with endoscopic scores, or CRP. Cumulatively, this data provides some useful natural history information of histological healing in the era of wide-spread use of mesalamine and thiopurines as maintenance agents. Whether persistent histological inflammation is independently associated with a worse prognosis over time is unconfirmed, but raises interesting questions about how achievable histological healing is as a goal for maintenance therapy in practice 11,14.
Secondly, a small proportion of patients with left-sided endoscopic disease have right-sided histological inflammation, which may have implications for their colon cancer risk 23. Patients with distal colitis can have isolated areas of inflammation in more proximal regions, usually periappendiceal patches in the cecum 24. Its clinical significance in terms of disease activity is unknown, although a small study reported a similar overall disease severity when compared to those without right-sided inflammation 25. These findings support the role of complete colonic endoscopic surveillance with biopsies, even in patients with a history of only left-sided disease. Of interest, the proportion of patients with histological inflammation was similar, regardless of which maintenance agent patients were taking (Figure 5).
We have independently confirmed that the Geboes histological scoring scale correlates with endoscopic appearance, demonstrating good agreement between endoscopic and histological scales of inflammation, and determination of a “healed” colon in patients with UC in remission. Osada et al, in their study with 54 UC patients with both active and inactive disease, reported a similar degree of correlation between the Mayo endoscopic score and histological scores, although they did not use the Geboes scale, and only 20 patients were in clinical remission 26. The reported threshold for moderate histological inflammation of 3.1 on the Geboes scale is supported in our study by higher endoscopic scores, and higher CRP levels, in these patients 20.
Finally, our findings that mean CRP was higher in patients with a Geboes histological score > 3.1, than those with minimal histological changes suggests that CRP could be used to screen patients in the clinic for unresolved histological inflammation. As can be seen in Figure 3, the majority of patients with histologically normal or mildly inflamed colons had CRP levels less than 5mg/L. In contrast to its use as a marker in Crohn’s disease, CRP has been described as a less reliable correlate of disease activity in patients with ulcerative colitis, except perhaps for severe, extensive colitis 27. A small study of 34 patients with UC did not find an association between a CRP >8mg/L, and histological inflammation, although most of the patients had only mild histological inflammation 28. Our finding of an association between CRP levels and histological inflammation warrants further validation, as many factors independent of colitis can lead to false positive or negative CRP levels.
Limitations of this study include the small proportion of patients with moderate to severe histological inflammation, out of a total of 103 patients enrolled; variables that occur at small frequencies but differ between groups may have been subject to a type II error. A post-hoc power calculation predicted one would need to enroll a cohort of ~700 surveillance patients to detect difference between variables that occur at frequencies of less than 20%. However the study is strengthened by the prospective enrolment and standardized scoring of all patients with comprehensive clinical phenotypes. It is the largest study to prospectively describe histological patterns, and correlate the Geboes histological scale with endoscopic and biochemical markers in patients in remission.
In conclusion, this study highlights that many patients with UC in clinical remission have underlying histological inflammation, and this correlates with endoscopic appearances, and serum CRP levels. In practice, these findings should prompt clinicians to consider using CRP to screen patients with UC in clinical remission for underlying on-going histological inflammation. Future work will determine the significance of this microscopic inflammation in medium and long-term outcomes in patients, and may provide insights into the benefits of aiming for histological healing in this patient population.
Supplementary Material
Acknowledgments
Grant Support - ACM is supported by NIH grant K23DK084338 and the generosity of Doris Toby Axelrod & Lawrence J. Marks. The study design, implementation and analysis were independent of the funding sources.
Abbreviations
- UC
ulcerative colitis
- MH
mucosal healing
- SCCAI
Simple Colitis Clinical Activity Index
- CRP
C-reactive protein
- ESR
erythrocyte sedimentation rate
Footnotes
Supplementary Figure Legend 1. 100X image of H&E stain of colonic biopsy with Geboes score 1.2 (A), and biopsy with Geboes score 5.4 (B).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures - ACM has served on Advisory Boards for Janssen, Abbott, and UCB, and received research funding from Shire and Salix. ASC has served on Advisory Boards for Janssen, Abbott, and UCB.
Writing Assistance – none
Author contributions - LR, JG; acquisition of data; data analysis. AG, TZ, GL, KF, JW, AC; acquisition of data, critical review of manuscript. KSN; analysis and interpretation of data, drafting of the manuscript. ACM; study concept and design, critical revision of the manuscript for important intellectual content, statistical analysis, study supervision.
Conflict of Interest - none
References
- 1.Reinisch W, Van Assche G, Befrits R, Connell W, D’Haens G, Ghosh S, Michetti P, Ochsenkuhn T, Panaccione R, Schreiber S, Silverberg MS, Sorrentino D, van der Woude CJ, Vermeire S, Panes J. Recommendations for the treatment of ulcerative colitis with infliximab: a gastroenterology expert group consensus. J Crohns.Colitis. 2012;6:248–258. doi: 10.1016/j.crohns.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Peyrin-Biroulet L, Ferrante M, Magro F, Campbell S, Franchimont D, Fidder H, Strid H, Ardizzone S, Veereman-Wauters G, Chevaux JB, Allez M, Danese S, Sturm A. Results from the 2nd Scientific Workshop of the ECCO. I: Impact of mucosal healing on the course of inflammatory bowel disease. J Crohns.Colitis. 2011;5:477–483. doi: 10.1016/j.crohns.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 3.Ardizzone S, Cassinotti A, Duca P, Mazzali C, Penati C, Manes G, Marmo R, Massari A, Molteni P, Maconi G, Porro GB. Mucosal healing predicts late outcomes after the first course of corticosteroids for newly diagnosed ulcerative colitis. Clin.Gastroenterol.Hepatol. 2011;9:483–489. doi: 10.1016/j.cgh.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 4.Froslie KF, Jahnsen J, Moum BA, Vatn MH. Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology. 2007;133:412–422. doi: 10.1053/j.gastro.2007.05.051. [DOI] [PubMed] [Google Scholar]
- 5.Gupta RB, Harpaz N, Itzkowitz S, Hossain S, Matula S, Kornbluth A, Bodian C, Ullman T. Histologic inflammation is a risk factor for progression to colorectal neoplasia in ulcerative colitis: a cohort study. Gastroenterology. 2007;133:1099–1105. doi: 10.1053/j.gastro.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Haens G, Sandborn WJ, Feagan BG, Geboes K, Hanauer SB, Irvine EJ, Lemann M, Marteau P, Rutgeerts P, Scholmerich J, Sutherland LR. A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology. 2007;132:763–786. doi: 10.1053/j.gastro.2006.12.038. [DOI] [PubMed] [Google Scholar]
- 7.Hanauer SB, Kirsner JB. Treat the patient or treat the disease? Dig.Dis. 2012;30:400–403. doi: 10.1159/000338139. [DOI] [PubMed] [Google Scholar]
- 8.Wright R, Truelove SR. Serial rectal biopsy in ulcerative colitis during the course of a controlled therapeutic trial of various diets. Am.J.Dig.Dis. 1966;11:847–857. doi: 10.1007/BF02233941. [DOI] [PubMed] [Google Scholar]
- 9.Bitton A, Peppercorn MA, Antonioli DA, Niles JL, Shah S, Bousvaros A, Ransil B, Wild G, Cohen A, Edwardes MD, Stevens AC. Clinical, biological, and histologic parameters as predictors of relapse in ulcerative colitis. Gastroenterology. 2001;120:13–20. doi: 10.1053/gast.2001.20912. [DOI] [PubMed] [Google Scholar]
- 10.Riley SA, Mani V, Goodman MJ, Dutt S, Herd ME. Microscopic activity in ulcerative colitis: what does it mean? Gut. 1991;32:174–178. doi: 10.1136/gut.32.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rubin D, Huo D, Hetzel J, Bunnag A, Sedrak M, Hart J. Increased degree of histological inflammation predicts colectomy and hospitalization in patients with ulcerative colitis. Gastroenterology. 2012;132(S1):A19. [Google Scholar]
- 12.Burger D, Thomas S, Walsh A, Von Herbay A, Buchell O, Brain O, Keshav S, Travis S. Depth of Remission may not Predict Outcome of UC over 2 Years. Gut. 2011;60(S1):A133. [Google Scholar]
- 13.Geboes K, Riddell R, Ost A, Jensfelt B, Persson T, Lofberg R. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut. 2000;47:404–409. doi: 10.1136/gut.47.3.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baars JE, Nuij VJ, Oldenburg B, Kuipers EJ, van der Woude CJ. Majority of patients with inflammatory bowel disease in clinical remission have mucosal inflammation. Inflamm.Bowel.Dis. 2012;18:1634–1640. doi: 10.1002/ibd.21925. [DOI] [PubMed] [Google Scholar]
- 15.Rosenberg L, Lawlor G, Zenlea T, Goldsmith J, Gifford A, Falchuk K, Wolf J, Cheifetz A, Robson S, Moss A. Predictors of Endoscopic Inflammation in Patients with Ulcerative Colitis in Clinical Remission. Inflamm.Bowel.Dis. 2012 doi: 10.1097/MIB.0b013e3182802b0e. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas S, Von Herbay A, Walsh A. How much agreement is there between histological, endoscopic and clinical assessments of remission in ulcerative colitis? Gut. 2009;58(S1):A101. [Google Scholar]
- 17.Higgins PD, Schwartz M, Mapili J, Krokos I, Leung J, Zimmermann EM. Patient defined dichotomous end points for remission and clinical improvement in ulcerative colitis. Gut. 2005;54:782–788. doi: 10.1136/gut.2004.056358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walmsley RS, Ayres RC, Pounder RE, Allan RN. A simple clinical colitis activity index. Gut. 1998;43:29–32. doi: 10.1136/gut.43.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N.Engl.J Med. 1987;317:1625–1629. doi: 10.1056/NEJM198712243172603. [DOI] [PubMed] [Google Scholar]
- 20.Bessissow T, Lemmens B, Ferrante M, Bischops R, Geboes K, Van Assche G, Vermeire S, Rutgeerts P, De Hertogh G. Prognostic value of Serologic and Histologic Markers on Long-Term Outcomes in Ulcerative Colitis Patients with Mucosal Healing. Gastroenterology. 2012;142(5 (S1)):S653. doi: 10.1038/ajg.2012.301. [DOI] [PubMed] [Google Scholar]
- 21.Moss AC, Chaudhary N, Tukey M, Junior J, Cury D, Falchuk KR, Cheifetz AS. Impact of a patient-support program on mesalamine adherence in patients with ulcerative colitis--a prospective study. J Crohns.Colitis. 2010;4:171–175. doi: 10.1016/j.crohns.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Lichtenstein GR, Rutgeerts P. Importance of mucosal healing in ulcerative colitis. Inflamm.Bowel.Dis. 2010;16:338–346. doi: 10.1002/ibd.20997. [DOI] [PubMed] [Google Scholar]
- 23.Baumgart DC. Endoscopic surveillance in Crohn’s disease and ulcerative colitis: who needs what and when? Dig.Dis. 2011;29(Suppl 1):32–35. doi: 10.1159/000331683. [DOI] [PubMed] [Google Scholar]
- 24.D’Haens G, Geboes K, Peeters M, Baert F, Ectors N, Rutgeerts P. Patchy cecal inflammation associated with distal ulcerative colitis: a prospective endoscopic study. Am.J Gastroenterol. 1997;92:1275–1279. [PubMed] [Google Scholar]
- 25.Mutinga ML, Odze RD, Wang HH, Hornick JL, Farraye FA. The clinical significance of right-sided colonic inflammation in patients with left-sided chronic ulcerative colitis. Inflamm.Bowel.Dis. 2004;10:215–219. doi: 10.1097/00054725-200405000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Osada T, Ohkusa T, Okayasu I, Yoshida T, Hirai S, Beppu K, Shibuya T, Sakamoto N, Kobayashi O, Nagahara A, Terai T, Watanabe S. Correlations among total colonoscopic findings, clinical symptoms, and laboratory markers in ulcerative colitis. J Gastroenterol.Hepatol. 2008;23(Suppl 2):S262–S267. doi: 10.1111/j.1440-1746.2008.05413.x. [DOI] [PubMed] [Google Scholar]
- 27.Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys? Gut. 2006;55:426–431. doi: 10.1136/gut.2005.069476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solem CA, Loftus EV, Jr., Tremaine WJ, Harmsen WS, Zinsmeister AR, Sandborn WJ. Correlation of C-reactive protein with clinical, endoscopic, histologic, and radiographic activity in inflammatory bowel disease. Inflamm.Bowel.Dis. 2005;11:707–712. doi: 10.1097/01.mib.0000173271.18319.53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.