Abstract
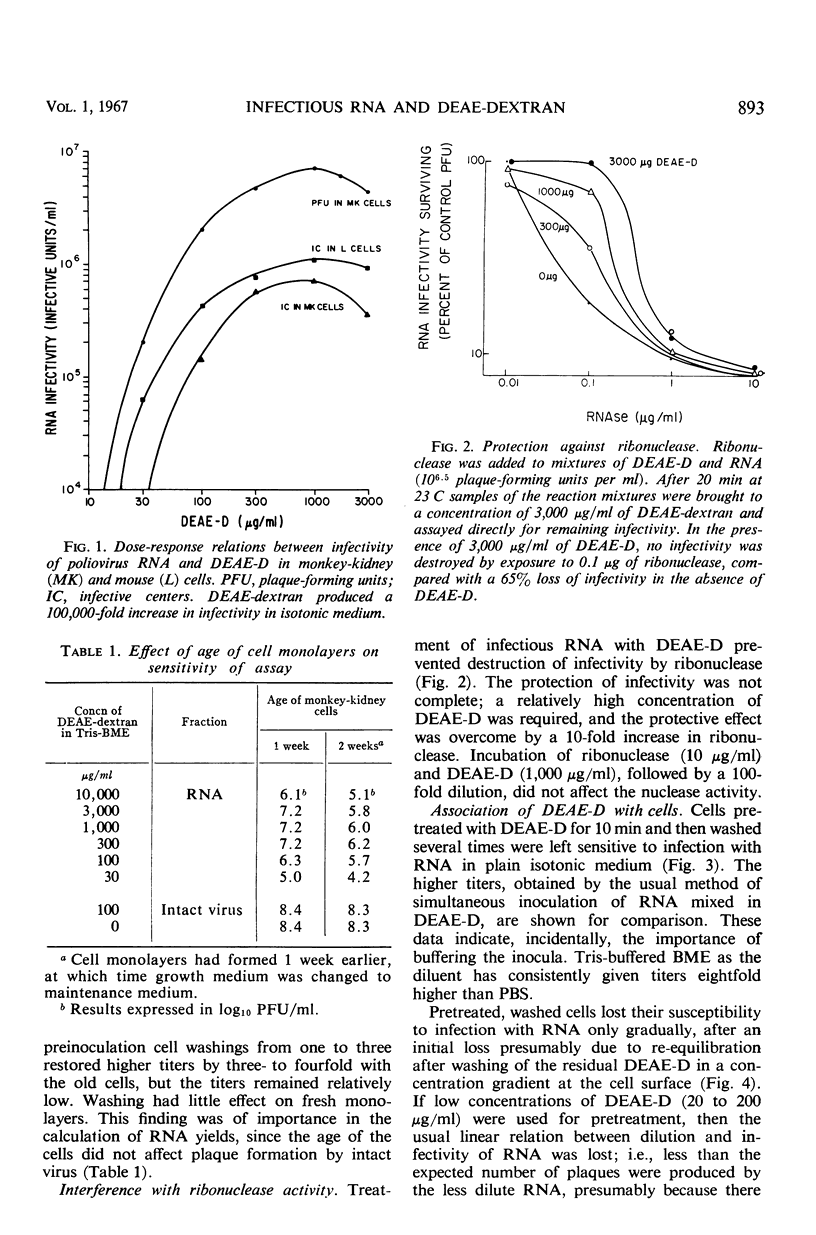
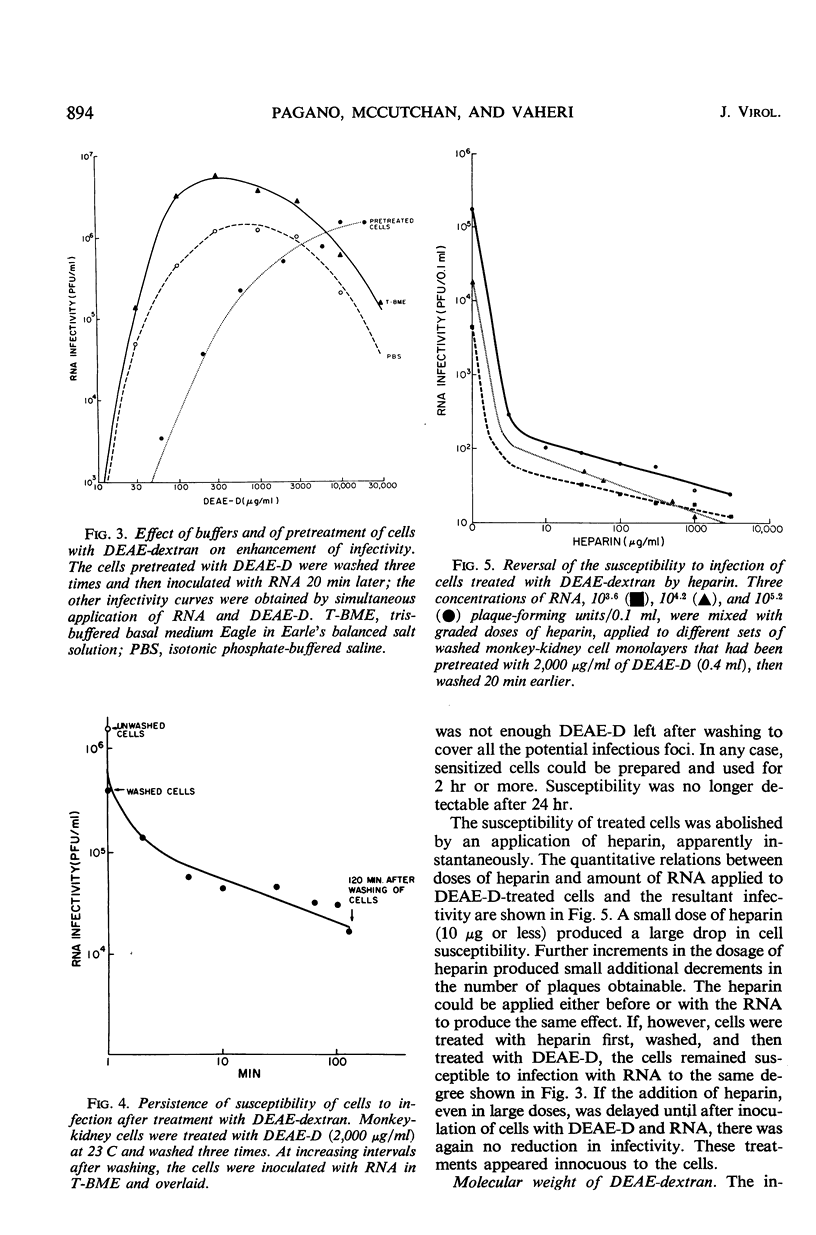
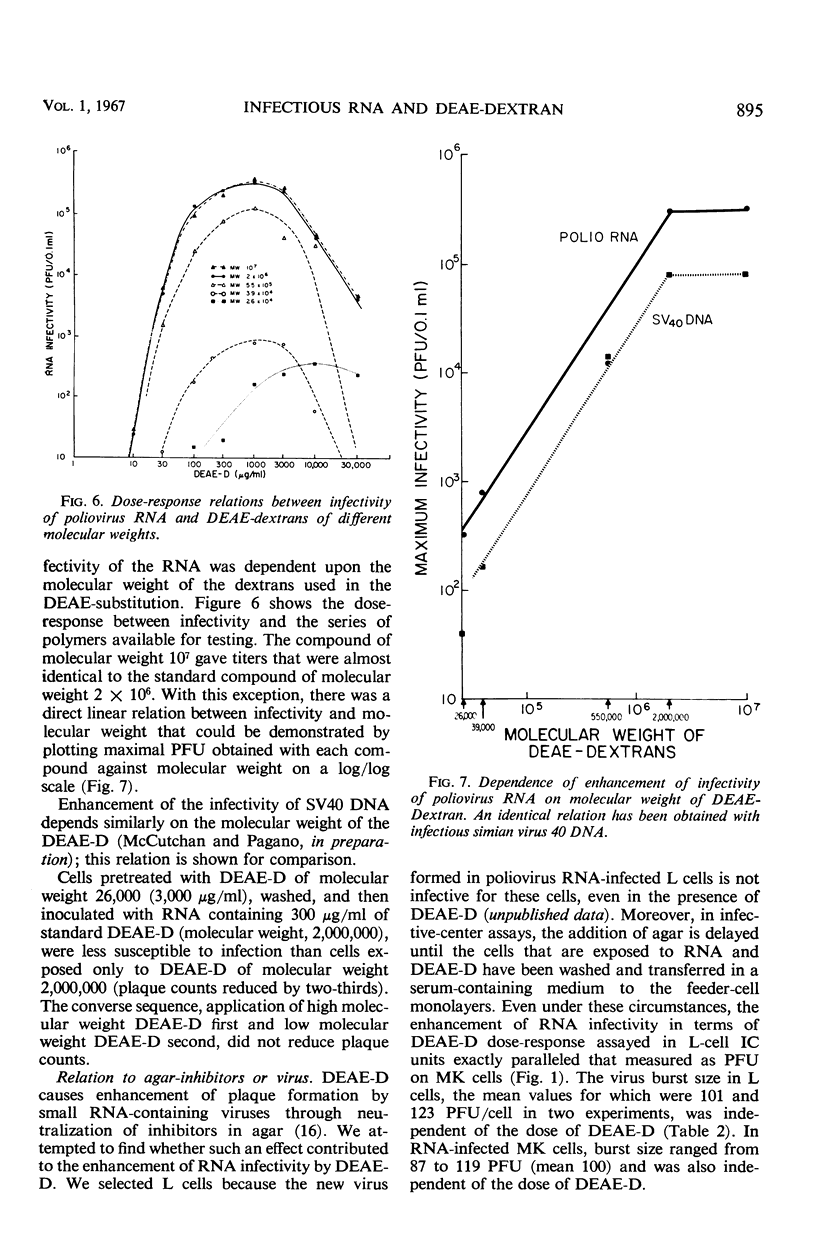
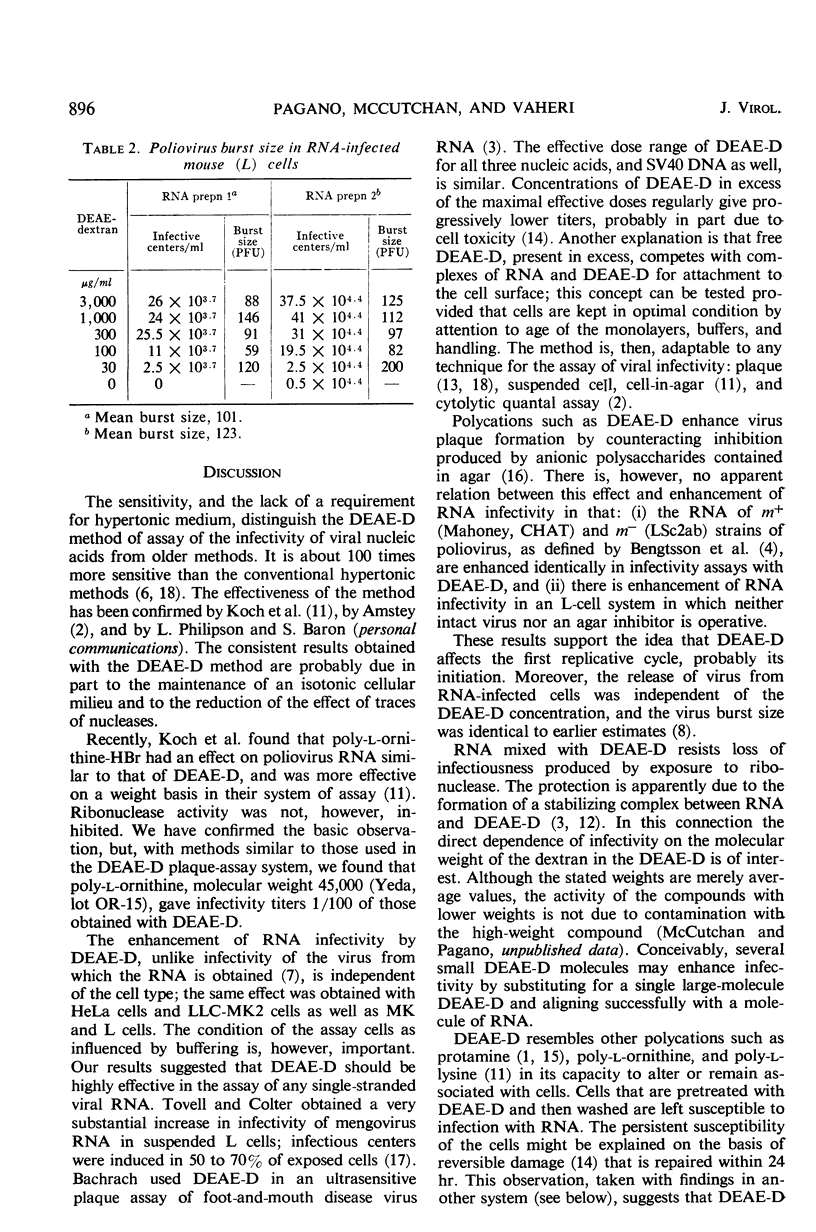
The enhancement by diethylaminoethyl-dextran (DEAE-D) of the infectivity of poliovirus ribonucleic acid (RNA) for cell cultures was demonstrated by infective-center as well as by plaque assays, both in nonprimate (L) and primate cell systems (MK, HeLa, LLC-MK2). The sensitivity of plaque assays was greatly improved by using a tris (hydroxymethyl)aminomethane-buffered synthetic medium (basal medium Eagle) and freshly confluent cell monolayers. Enhancement of nucleic acid infectivity was directly dependent on the molecular weight of the DEAE-D. Two observations bearing on the action of DEAE-D appeared important: ribonuclease activity was reduced by DEAE-D, and cells pretreated with DEAE-D remained susceptible to infection with RNA in isotonic medium. Appreciable susceptibility of the treated cells persisted for at least 2 hr; the susceptible state could be reversed at will by an application of heparin. Enhancement of nucleic acid infectivity was independent of an effect of DEAE-D on intact virus and agar inhibitors.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMOS H., KEARNS K. E. INFLUENCE OF BACTERIAL RIBONUCLEIC ACID ON ANIMAL CELLS IN CULTURE. II. PROTAMINE ENHANCEMENT OF RNA UPTAKE. Exp Cell Res. 1963 Oct;32:14–25. doi: 10.1016/0014-4827(63)90064-8. [DOI] [PubMed] [Google Scholar]
- BENGTSSON S., PHILIPSON L., PERSSON H., LAURENT T. C. THE BASIS FOR THE INTERACTION BETWEEN ATTENUATED POLIOVIRUS AND POLYIONS. Virology. 1964 Dec;24:617–625. doi: 10.1016/0042-6822(64)90216-8. [DOI] [PubMed] [Google Scholar]
- Bachrach H. L. Ribonucleic acid of foot-and-mouth disease virus: an ultrasensitive plaque assay. Proc Soc Exp Biol Med. 1966 Dec;123(3):939–945. doi: 10.3181/00379727-123-31644. [DOI] [PubMed] [Google Scholar]
- HOLLAND J. J. ENTEROVIRUS ENTRANCE INTO SPECIFIC HOST CELLS, AND SUBSEQUENT ALTERATIONS OF CELL PROTEIN AND NUCLEIC ACID SYNTHESIS. Bacteriol Rev. 1964 Mar;28:2–13. doi: 10.1128/br.28.1.2-13.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLAND J. J., HOYER B. H., McLAREN L. C., SYVERTON J. T. Enteroviral ribonucleic acid. I. Recovery from virus and assimilation by cells. J Exp Med. 1960 Nov 1;112:821–839. doi: 10.1084/jem.112.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWES D. W. The growth cycle of poliovirus in cultured cells. II. Maturation and release of virus in suspended cell populations. Virology. 1959 Sep;9:96–109. doi: 10.1016/0042-6822(59)90104-7. [DOI] [PubMed] [Google Scholar]
- HULL R. N., CHERRY W. R., TRITCH O. J. Growth characteristics of monkey kidney cell strains LLC-MK1, LLC-MK2, and LLC-MK2(NCTC-3196) and their utility in virus research. J Exp Med. 1962 May 1;115:903–918. doi: 10.1084/jem.115.5.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATCHALSKY A., DANON D., NEVO A. Interactions of basic polyelectrolytes with the red blood cell. II. Agglutination of red blood cells by polymeric bases. Biochim Biophys Acta. 1959 May;33(1):120–138. doi: 10.1016/0006-3002(59)90505-0. [DOI] [PubMed] [Google Scholar]
- Koch G., Quintrell N., Bishop J. M. An agar cell-suspension plaque assay for isolated viral RNA. Biochem Biophys Res Commun. 1966 Aug 12;24(3):304–309. doi: 10.1016/0006-291x(66)90155-0. [DOI] [PubMed] [Google Scholar]
- Pagano J. S., Vaheri A. Enhancement of infectivity of poliovirus RNA with diethylaminoethyl-dextran (DEAE-D). Arch Gesamte Virusforsch. 1965;17(3):456–464. doi: 10.1007/BF01241201. [DOI] [PubMed] [Google Scholar]
- Ryser H. J. Studies on protein uptake by isolated tumor cells. 3. Apparent stimulations due to pH, hypertonicity, polycations, or dehydration and their relation to the enhanced penetration of infectious nucleic acids. J Cell Biol. 1967 Mar;32(3):737–750. doi: 10.1083/jcb.32.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMULL C. E., LUDWIG E. H. Enhancement of the plaque-forming capacity of poliovirus ribonucleic acid with basic proteins. J Bacteriol. 1962 Nov;84:1035–1040. doi: 10.1128/jb.84.5.1035-1040.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto K. K. Plaque mutants of animal viruses. Prog Med Virol. 1966;8:314–348. [PubMed] [Google Scholar]
- Tovell D. R., Colter J. S. Observations on the assay of infectious viral ribonucleic acid: effects of DMSO and DEAE-dextran. Virology. 1967 May;32(1):84–92. doi: 10.1016/0042-6822(67)90255-3. [DOI] [PubMed] [Google Scholar]
- Vaheri A., Pagano J. S. Infectious poliovirus RNA: a sensitive method of assay. Virology. 1965 Nov;27(3):434–436. doi: 10.1016/0042-6822(65)90126-1. [DOI] [PubMed] [Google Scholar]



