Abstract
Eye-blink conditioning (EBC) is a form of associative learning that depends on the cerebellum. Previous reports suggested that sensory cortex is necessary for trace EBC but not for delay EBC. The trace and delay EBC procedures used in these studies differed by the presence or absence of a temporal gap between the end of the conditioned stimulus and the onset of the unconditioned stimulus (trace interval) and in the interval between the onset of the CS and the US (inter-stimulus interval, ISI). The current study examined the role of the visual cortex in delay, long-delay, and trace EBC, matching CS duration and inter-stimulus interval between groups. In Experiment 1, extensive removal of the visual cortex impaired acquisition of long-delay and trace EBC but had no effect on delay EBC. In Experiment 2, bilateral inactivation of the visual cortex impaired acquisition and retention of long-delay and trace EBC, but had no effect on delay EBC. In Experiment 3, unilateral inactivation of the visual cortex impaired long-delay EBC but had no effect on trace EBC. The results indicate that the visual cortex facilitates EBC with relatively long ISIs, regardless of whether there is a trace interval or not. Moreover, the ipsilateral projections from the visual cortex to the pontine nuclei are sufficient for modulating long-delay EBC, whereas trace EBC involves bilateral visual cortical interactions with forebrain systems including the hippocampus and prefrontal cortex.
Keywords: associative learning, eyeblink conditioning, visual cortex, cerebellum
1. Introduction
Eye-blink conditioning (EBC) has been used extensively to examine the mechanisms of cerebellar learning (Freeman & Steinmetz, 2011). Associative learning is established by pairing a conditioned stimulus (CS; e.g. tone or light) with an unconditioned stimulus (US; e.g. periorbital stimulation) that elicits eyelid closure. After repeated pairings of the CS and US an adaptive eyelid closure conditioned response (CR) emerges prior to US onset (Schneiderman et al., 1962). This type of associative learning depends upon synaptic plasticity mechanisms within the cerebellum (Freeman & Steinmetz, 2011).
In delay EBC, the offset of the CS overlaps with or is immediately followed by the US. Sensory pathways projecting the CS inputs to the cerebellum during delay EBC are subcortical but sensory cortex may modulate these subcortical pathways (Halverson & Freeman, 2006; 2010a,b; Halverson et al., 2008; 2009; 2010). Single unit recordings in rabbits indicate that auditory cortical activity undergoes learning-related modifications (Kraus & Disterhoft, 1982) and human auditory cortex shows large increases in activity (Molchan et al., 1994) during delay EBC. Delay EBC is acquired rapidly when stimulation of primary auditory or visual cortex is used as a CS (Knowlton & Thompson, 1992; Halverson et al., 2009) and inactivation of the auditory cortex partially impairs retention of delay EBC (Case et al., 2002), suggesting that sensory cortex may modulate subcortical CS pathways to facilitate expression of delay EBC.
Trace EBC is the same as delay EBC except that there is a stimulus-free “trace” interval between the CS and US. Learning-related changes in BOLD response have been observed during trace EBC in the rabbit primary visual cortex using fMRI (Miller et al., 2008). Cortical barrels have been shown to expand when the corresponding whiskers are stimulated as the CS in trace EBC and lesions of the cortical barrels disrupted acquisition and retention of trace EBC, but have no effect on delay EBC (Galvez et al., 2006; 2007). It is not known, however, whether the deficit in trace EBC with barrel cortex lesions is due to the longer inter-stimulus interval (ISI) relative to delay EBC or to the temporal gap (500 ms) in trace EBC. Taken together, these findings indicate that primary sensory cortex plays an essential role in acquisition of trace EBC, at least with a tactile CS.
An important implication of these findings is that sensory cortex may influence EBC to different degrees depending on the whether delay or trace conditioning procedures are used. An alternative hypothesis is that sensory cortex plays a role in EBC when longer ISIs are used, regardless of whether there is a temporal gap between the CS and US. The purpose of the current study was to test these hypotheses by examining the role of visual cortex in delay, long-delay, and trace EBC, matching CS duration and ISI across training conditions. In Experiment 1, aspiration lesions of the visual cortex were made prior to acquisition of delay, long-delay, or trace EBC. Inactivation of primary visual cortex was made either bilaterally (Experiment 2) or unilaterally (Experiment 3) during acquisition, retention, and extinction of delay, long-delay, or trace EBC.
2. Materials and Methods
2.1 Subjects
Subjects were 141 male Long-Evans rats, 300-350 g at the beginning of the experiment. The rats were housed in Spence Laboratories of Psychology at the University of Iowa with a 12 hr light-dark cycle and were given ad libitum access to food and water.
2.2 Surgery
One week prior to training, rats were removed from their home cage and anesthetized with isoflurane. After the onset of anesthesia they received aspirations of the visual cortex (Experiment 1) or were implanted with cannulae in the visual cortex (Experiments 2 and 3). For aspiration lesions, bilateral craniotomies were made from 1.5 to 4.6 mm in the medial-lateral dimension and from 6.0 to 9.2 mm in the anterior-posterior dimension. Aspiration lesions of the visual cortex were then made with a 19 G blunt needle. Gelfoam, soaked in cold sterile saline (0.9%), was packed into the wounds, and covered with low viscosity silicon. Control lesion surgery consisted of bilateral craniotomies but no cortex was removed. For cannula implantation, a 23 gauge guide cannula was implanted bilaterally (Experiment 2) or unilaterally to the contralateral to the trained eye (Experiment 3). A 30 gauge stylet was inserted into the guide cannula and extended 0.5 mm from the end of the guide cannula. The stereotaxic coordinates for visual cortex cannula, taken from bregma, were 6.7 mm posterior, 3.5 mm lateral, and 0.7 mm ventral to the skull surface. For all experiments, rats were fitted with differential electromyograph (EMG) electrodes (stainless steel) implanted into the upper left orbicularis oculi muscle. The reference electrode was a silver wire attached to a stainless steel skull screw. The EMG electrode leads terminated in gold pins in a plastic connector. A bipolar stimulating electrode (Plastics One, Roanoke, VA) for delivering the stimulation US was implanted subdermally, caudal to the left eye.
2.3 Apparatus
The conditioning apparatus consisted of four sound attenuation chambers (BRS/LVE, Laurel, MD). Within each sound attenuation chamber was a conditioning chamber (BRS/LVE, Laurel, MD). One wall of the operant chamber was fitted with a stimulus light (100 lux) for presenting the CS. Computer software controlled the delivery of stimuli and the recording of eyelid EMG activity (JSA Designs, Raleigh, NC). The stimulation US (2.5-3.5 mA, DC) was delivered through a stimulus isolator (Model number 365A, World Precision Instruments, Sarasota FL). EMG activity was recorded differentially, filtered (500–5000 Hz) and integrated by equipment (JSA Designs, Raleigh, NC) as described in other reports (Nicholson & Freeman 2002).
2.4 Conditioning procedures
Rats recovered from surgery for 1 week prior to the initiation of training. They were trained with one of three procedures: delay, long-delay, or trace EBC. Delay EBC consisted of a 250 ms CS that terminated with a 25 ms US; long-delay EBC consisted of a 750 ms CS that terminated with a 25 ms US; and trace EBC consisted of a 250 ms CS followed by a 500 ms trace interval, ending in a 25 ms US (Figure 1). Rats received both paired and extinction training sessions. Paired training sessions consisted of 10 blocks of 9 paired CS-US presentations and 1 CS-alone probe; whereas extinction training consisted of 100 CS-alone presentations. The US intensity was adjusted in each rat to elicit a blink and slight head movement (range = 2.5 – 3.5 mA). CRs were defined as EMG activity that exceeded a threshold of 0.4 units (amplified and integrated units) above the baseline mean during the CS period. EMG responses that exceeded the threshold during the first 80 ms of the CS period were defined as startle responses. On CS-alone probe trials, the duration for scoring CRs was extended beyond the CS to the end of the trial period. URs were defined as responses that crossed the threshold after the onset of the US.
Figure 1.
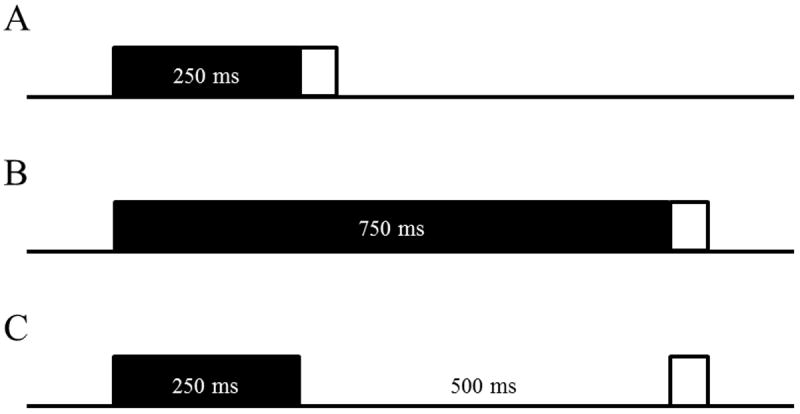
Schematic representation of trial types in Experiments 1, 2, and 3. Rats received either Delay EBC (A; 250 ms CS), Long-Delay EBC (B; 750 ms CS), or Trace EBC (C; 250 ms CS, 500 ms trace).
2.5 Infusions
Prior to infusions, the 30-gauge stylet was removed and replaced with a 30-gauge infusion needle that extended 1.0 mm past the tip of the guide cannula. A 10 μL syringe (Hamilton, Reno, NV) was connected to the infusion needle by a polyethylene tube (PE 10, 110-120 cm) and the syringe was secured to an infusion pump (Harvard Apparatus, Hilliston, MA). Rats were infused with .3 μL of either muscimol (2.0 mM) or PBS at a rate of 6.0 μL/hr 30 min in each site prior to the beginning of training.
2.6 Histology
Following the last training session, rats were euthanized with a lethal injection of sodium pentobarbital (150 mg/kg) and perfused transcardially with 0.9% saline followed by 10% buffered formalin. Brains were extracted and then post-fixed and cryo-protected in a 30% sucrose formalin solution. Brains with aspiration lesions were embedded in gelatin and sectioned at 50 μm with a sliding microtome. Sections were mounted on gel-coated slides and stained with thionin. The extent of lesions and the placements of cannulae were then verified using a stereotaxic brain atlas (Paxinos and Watson 2007).
3. Results
3.1 Experiment 1: Visual cortex lesions
Rats were given lesions of the visual cortex or control surgery 1 week prior to training on either delay (Control n = 7; Lesion n = 8), long-delay (Control n = 10; Lesion n = 10), or trace (Control n = 8; Lesion n = 11) EBC. There were an additional 9 rats that were not included in the analysis. Five of these rats died during or following surgery and 3 lost their head stage during acquisition training.
An example lesion is shown in Figure 2B, along with reconstructions of the largest and smallest lesions (Figure 2C). The extent of visual cortex lesions was examined using nissl-stained sections. The volume of tissue loss in the primary visual cortex (V1), primary visual cortex monocular area (V1M), primary visual cortex binocular area (V1B), secondary visual cortex (V2), secondary visual cortex lateral area (V2L), secondary visual cortex mediolateral area (V2ML), and secondary visual cortex mediomedial area (V2MM) was calculated as a percentage for each rat relative to the respective volumes of the visual areas in a subset of randomly chosen control rats (n=8). There were no significant differences between the three training groups in the extent of visual cortex loss (Figure 2A).
Figure 2.
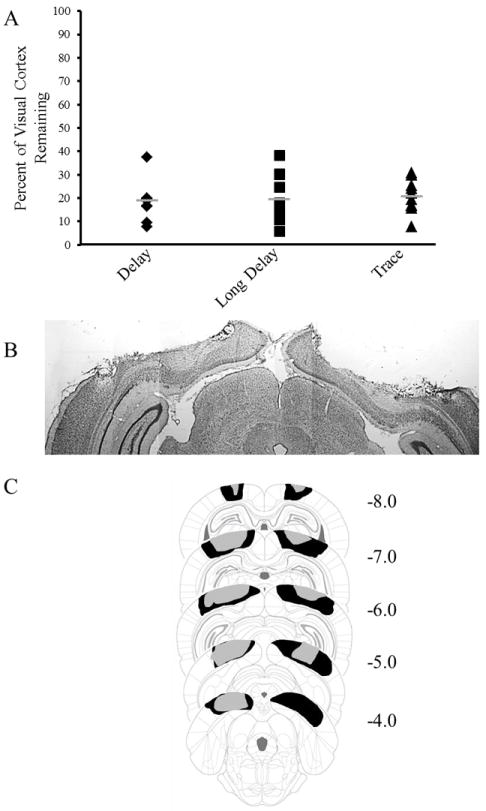
A. Graph depicting the percent of visual cortex remaining in rats given lesions for Delay EBC (black diamonds), Long-Delay EBC (black squares), and Trace EBC (black triangles) groups. Grey bars represent the mean. B. Example of lesion of visual cortex. B. Reconstruction of the largest lesion (black) and smallest lesion (gray). Values represent distance from bregma in mm.
Rats received either aspiration of the visual cortex or control surgery and were trained on either delay (250 ms CS), long-delay (750 ms CS), or trace EBC (250 ms CS, 500 ms trace) until they had two consecutive sessions with at least 80% CRs. Rats with lesions showed impaired rates of acquisition during long-delay and trace EBC (Figure 3). The number of sessions to reach criterion was calculated for each rat and a 2 Condition (Control or Lesion) × 3 Paradigm (delay, long-delay, or trace) ANOVA revealed a significant interaction [F(2,47)= 5.278; p= 0.009]. Post-hoc tests (Tukey-Kramer) indicated that both lesion groups given long-delay and trace EBC took more sessions to reach criterion than their respective control groups. Rats that received lesions and were trained with delay EBC did not significantly differ from their control group. No significant correlations were found for the amount of tissue loss in the visual cortex (V1, V1M, V1B, V2, V2L, V2ML, and V2MM), in either hemisphere or combined, and days to reach criterion.
Figure 3.
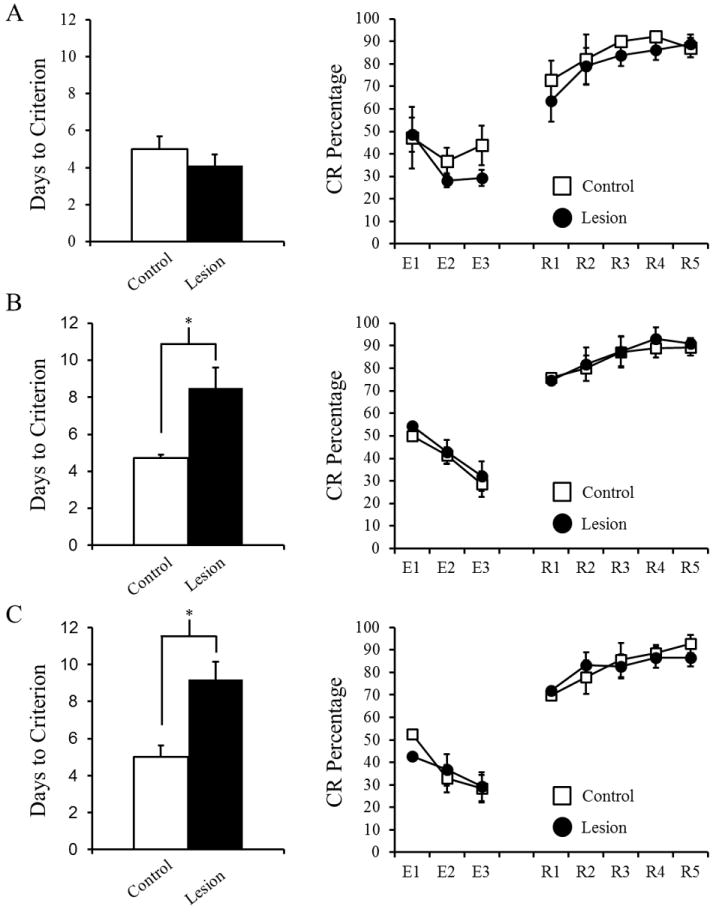
Mean number of days to reach criterion (two consecutive sessions with at least 80% conditioned responses) and for Delay EBC (A), Long-Delay EBC (B), and Trace EBC (C). After reaching criterion rats received extinction (E1-3) and reacquisition training (RA1-5). Significant effects are indicated (*).
CR amplitude, onset latency, and peak latency were examined during the criterion sessions (at or above 80%) in order to examine potential effects of lesions on performance of the CR when there were relatively high numbers of CRs. A 2 Group (Lesion or Control) × 3 Paradigm (delay, long-delay, or trace) × 2 Session ANOVA was conducted for each parameter. For CR amplitude, CR onset latency, and CR peak latency no significant interactions were found, but a significant main effect of Paradigm was found: CR amplitude [F(2,47)= 18.654; p< 0.001], CR onset latency [F(2,47)= 47.292; p< 0.001], and CR peak latency [F(2,47)= 58.869; p< 0.001]. Post-hoc tests indicated that the delay EBC groups had larger CR amplitudes and earlier CR onset and peak latencies than either long-delay or trace EBC groups. To further examine the role of lesions during acquisition CR amplitude, CR onset latency, and CR peak latency were calculated for the 1st and 2nd halves of acquisition for each rat. A 2 Half × 2 Group (Lesion or Control) × 3 Paradigm (delay, long-delay, or trace) repeated measures ANOVA was conducted and found a significant Half × Paradigm interaction for CR amplitude [F(2,47)=12.853, p<.001]. Post-hoc tests revealed that the delay EBC group had larger CR amplitudes than both the long-delay EBC and trace EBC groups. A similar Half × Lesion × Paradigm repeated measures ANOVA was conducted for both CR onset and peak latencies. A main effect of Lesion was found for CR onset latency [F(1,47)=5.507, p=.023], while a significant Half × Lesion interaction was found for CR peak latency [F(1,47)=7.308, p=.010]. Post-hoc tests revealed that lesions caused significantly later timed CRs throughout acquisition (CR onset latency and during the 2nd half of learning (CR peak latency). These measures collectively indicate that lesions resulted in significantly later timed responses but no differences in the amplitudes.
After reaching criterion rats were given 3 sessions of CS-alone extinction training followed by reacquisition, which consisted of 5 sessions of paired CS-US training. Prior to extinction (the criterion session) there were no significant differences in CR percentage between the groups (Figure 3). A repeated measures ANOVA revealed a significant main effect of Session for extinction [F(2,47)= 7.276; p= 0.002] and reacquisition [F(2,47)= 10.994; p< 0.001], but no significant differences between paradigms or lesion and control groups. These main effects indicate that rats extinguished the CR and also reacquired the CR following training. Additionally, CR onset latency, peak latency, and amplitude were not significantly different between lesion and control groups during extinction or reacquisition.
In summary, lesions of visual cortex produced marked decrements in acquisition for both long-delay and trace EBC but had no effect on delay EBC. Additional CR measurements, including amplitude and timing were not significantly altered by the lesions. Lesioned rats did not exhibit deficits in extinction or reacquisition. These results, taken together, indicate a role for the visual cortex in acquisition of long-delay and trace EBC.
3.2 Experiment 2 Bilateral visual cortex inactivation
Rats were implanted with bilateral cannulae into the primary visual cortex (V1; Figure 4) and then trained with delay (250 ms CS), long-delay (750 ms CS), or trace (250 ms CS; 500 ms trace) EBC. Rats received muscimol or saline infusions prior to each of 5 CS-US acquisition sessions (Figure 5, P1-5). A 2 Drug (muscimol or saline) × 3 Paradigm (delay, long-delay, or trace), × 5 Session repeated measures ANOVA was conducted to examine the effects of muscimol on acquisition. Significant Paradigm × Session [F(8,88)=7.436 p<.001] and Drug × Paradigm [F(2,46)=5.461, p=.007] interactions were found. Post-hoc tests indicated that rats that received muscimol during long-delay and trace EBC exhibited significantly fewer CRs, whereas the delay EBC groups were not significantly different. A 2 Drug (muscimol or saline) × 3 Paradigm (delay, long-delay, or trace) × 5 Session repeated measures ANOVA was also conducted for CR amplitude, CR onset and peak latencies. A significant Paradigm × Session interaction was found for CR amplitude [F(2,47)=2.616, p=.015], CR onset latency [F(2,47)=2.551, p=.019], and CR peak latency [F(2,47)=7.390, p<.001]. Post-hoc tests indicated that the delay EBC groups had significantly larger CR amplitudes and earlier onset and peak latencies than both the long-delay and trace EBC groups.
Figure 4.
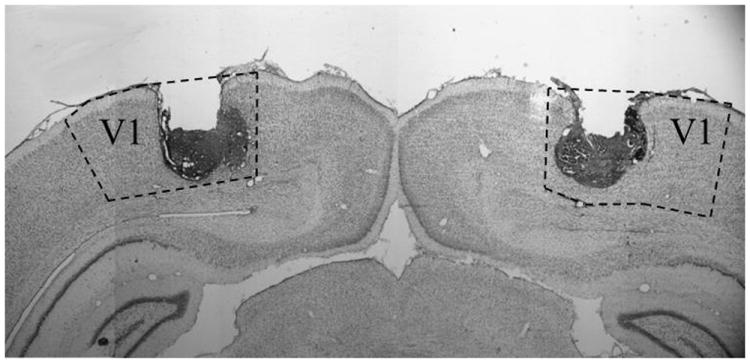
Example of bilateral cannula placements into the primary visual cortex (V1).
Figure 5.
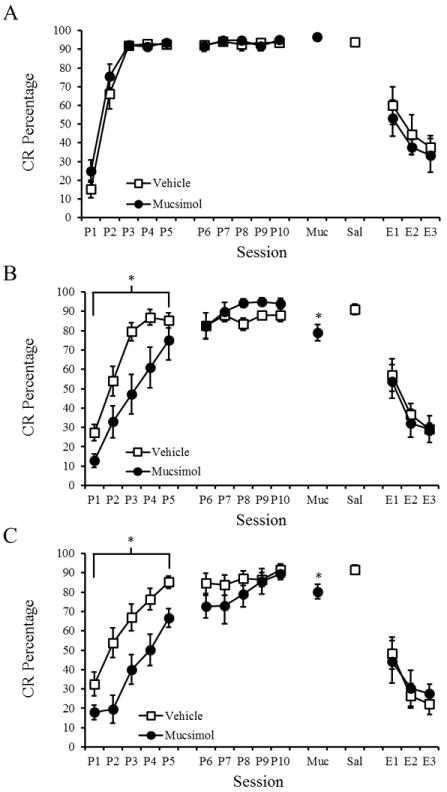
Mean (± SEM) conditioned response (CR) percentage for rats given bilateral muscimol or saline infusions into visual cortex during Delay (A), Long-Delay (B), and Trace (C) eyeblink conditioning. Significant effects are indicated (*).
Rats were then trained for 5 additional CS-US sessions to reach asymptotic levels of learning prior to extinction training. After reaching asymptotic conditioning rats received CS-US retention test sessions with infusions of muscimol or saline. In order to examine the role of visual cortex in retention the session prior to muscimol, the muscimol session, and the saline session were examined using a 2 Drug (muscimol or saline) × 3 Paradigm (delay, long-delay, or trace) × 3 Session repeated measures ANOVA was conducted. A significant Drug × Paradigm × Session interaction [F(2,48)=4.543; p=.004] was found. Post-hoc tests indicated a significant muscimol-induced decrease in CR percentage for the long-delay and trace EBC groups but not for the short delay EBC group. A 2 Group (muscimol or saline) × 3 Paradigm (delay, long-delay, or trace) × 3 Session repeated measures ANOVA was conducted for CR amplitude, onset latency and peak latency. A Paradigm × Session interaction was found for CR amplitude [F(2,47)=6.149, p=.004], CR onset latency [F(2,47)=4.482, p=.002], and CR peak latency [F(2,47)=5.176, p=.001]. Post-hoc tests revealed that the delay EBC groups had larger CR amplitude and earlier CR onset and peak latencies than the long-delay and trace EBC groups.
Following retention, rats completed 3 sessions of CS-alone extinction training. Rats received either muscimol or saline 30 mins prior to each 100-trial session. A 2 Drug (muscimol or saline) × 3 Paradigm (delay, long-delay, or trace) × 3 Session repeated measures ANOVA was conducted and a main effect of session was found [F(2,35)=19.035, p<.001]. There were no interactions or main effects related to the muscimol administration. Additionally, there were no significant effects of muscimol on CR amplitude, onset latency, or peak latency during extinction.
In summary, bilateral administration of muscimol into the visual cortex impaired the rate of acquisition for long-delay and trace EBC but not delay EBC. Muscimol administration, however, did not impair CR amplitude, onset latency, or peak latency. Muscimol administration following learning resulted in a small, but significant decrease in CR percentage during retention of long-delay and trace EBC but not delay EBC. Visual cortex inactivation did not significantly alter the rate of extinction.
3.3 Experiment 3: Unilateral visual cortex inactivation
Bilateral inactivation of visual cortex resulted in decreases in the rate of acquisition for long-delay and trace EBC. To determine if these impairments were the result of unilateral or bilateral visual cortical contributions, rats were fitted with a cannula into the contralateral (to the trained eye) visual cortex. Long-delay and trace EBC paradigms were only used since delay EBC did not exhibit decrements in acquisition with bilateral lesions or inactivation. Rats received either muscimol or saline for 5 sessions during acquisition (Figure 6). A 2 Drug (muscimol or saline) × 2 Paradigm (long-delay or trace) × 5 Session repeated measures ANOVA was conducted to examine the potential effects of muscimol on acquisition. A significant Drug × Session interaction [F(1,28)=4.346, p=.046] was found. Post hoc tests (Tukey-Kramer) indicated that rats that received muscimol were impaired during long-delay EBC, but not during trace EBC. Repeated measures ANOVAs were also conducted for CR amplitude, onset latency, and peak latency. There was a main effect of session for CR amplitude [F(2,26)=4.432, p=.008], onset latency [F(2,26)=7.606, p<.001], and peak latency [F(2,26)=5.767, p=.002], indicating changes in these variables during acquisition.
Figure 6.
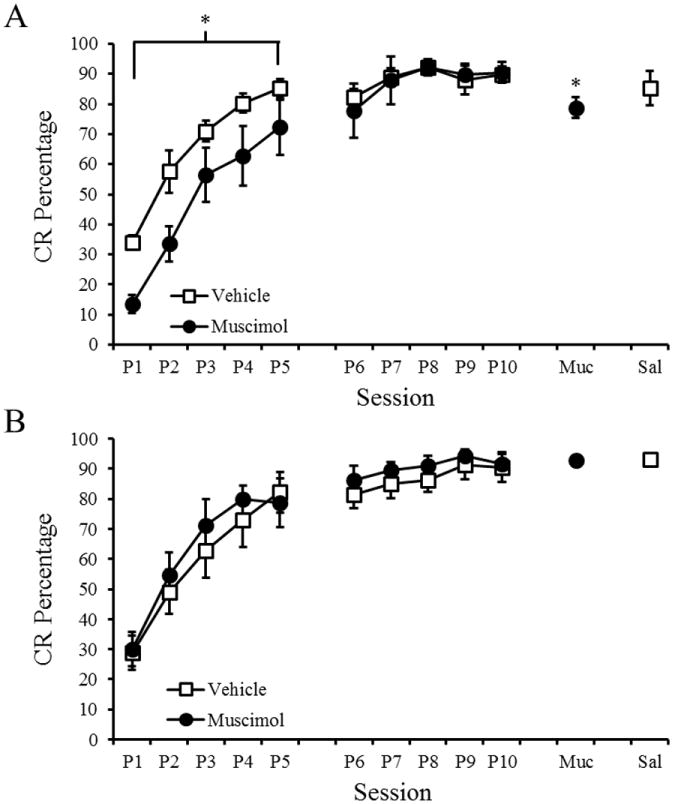
Mean (+ SEM) conditioned response (CR) percentage for rats given unilateral muscimol or saline infusions into visual cortex during Delay (A), Long-Delay (B), and Trace (C) eyeblink conditioning. Significant effects are indicated (*).
As in Experiment 2, the rats were then trained for 5 sessions with no infusions to reach asymptotic levels of conditioning. The effect of inactivation on retention was analyzed by comparing CR percentage during session 10 (no infusion), session 11 (muscimol infusion), and session 12 (saline infusion). A 2 Paradigm (long-delay or trace) × 3 Session repeated measures ANOVA was conducted and a Paradigm × Session interaction was found [F(2,26)=6.067, p =.007]. Post-hoc tests (Tukey-Kramer) indicated a significant decrease in CR percentage during the muscimol session for the long-delay EBC group, but not for the trace EBC group. There were no significant differences during the muscimol session for CR amplitude, onset latency, or peak latency.
4. Discussion
Visual cortex removal impaired the rate of acquisition of long-delay and trace EBC but did not impair extinction or reacquisition. Bilateral reversible inactivation of the visual cortex produced acquisition and retention deficits for long-delay and trace EBC. Unilateral inactivation of the visual cortex contralateral to the trained eye impaired long-delay EBC but not trace EBC. Reversible inactivation of the visual cortex had no effect on extinction of EBC. Neither lesions nor inactivation of the visual cortex affected delay EBC.
Previous studies provided evidence that sensory cortex is not necessary for delay EBC. Decortication or decerebration does not prevent acquisition or retention of delay EBC (Oakley & Russell, 1972; 1977; Lovick & Zebrozyna, 1975; Norman et al., 1977; Mauk & Thompson, 1987; Hesslow, 1994). Moreover, removal of the visual cortex does not impair visual delay EBC (Marquis & Hilgard, 1936; 1937). A more recent study, however, found that inactivation of the auditory cortex with lidocaine impaired retention of delay EBC (Case et al., 2002), suggesting that lesions of sensory cortex could result in partial compensation or recovery, which would not be seen with reversible inactivation. The current results argue against that hypothesis by indicating that lesions or inactivation of the visual cortex impair long-delay EBC. Instead, the current findings suggest that visual cortex plays a role in delay EBC when the ISI is relatively long. The deficits in long-delay and trace EBC with visual cortex lesions or inactivation also demonstrate that the contributions of sensory cortex to trace EBC are not related to the presence of a temporal gap between the CS and US. Rather, the visual cortex plays a role in EBC when the ISI is relatively long, regardless of whether there is a trace interval during the ISI. The reason primary sensory cortex is necessary for EBC with longer ISIs is unclear. It is possible that the CS processing in the subcortical CS pathway provides only phasic input to the cerebellum, whereas the sensory cortex provides sustained input to the cerebellum throughout the ISI. The cerebellum needs CS input that is relatively close in time to the US, requiring a sustained input from the forebrain during trace EBC (Kalmbach et al., 2010). Visual cortex may play a role in providing sustained CS input to the cerebellum either directly through its ipsilateral projection to the pontine nuclei or indirectly through interactions with the ventral lateral geniculate (LGNv), hippocampus and/or medial prefrontal cortex.
The deficits in trace EBC following lesions or inactivation of the visual cortex are generally consistent with current models of the neural mechanisms underlying trace EBC (Weiss & Disterhoft, 2011). The hippocampus is thought to receive CS information through projections from sensory cortex and therefore blocking this pathway should completely block acquisition, which was reported for whisker-stimulation trace EBC following barrel cortex lesions (Galvez et al., 2007). The current findings contrast with Galvez et al. in that inactivation or lesions of the visual cortex slowed acquisition of trace EBC but did not completely block it, even in rats with 95% loss of visual cortex. Differences in the degree of dependence of visual and somatosensory trace EBC on primary sensory cortex might be related to the extent to which subcortical CS inputs to the cerebellum can partially compensate for the absence of sensory cortex. Direct projections from the LGNv to the pontine nuclei might be able to partially support trace conditioning whereas the absence of ventral posteromedial thalamic input to the pontine nuclei would leave whisker-stimulation trace EBC completely dependent upon the barrel cortex. It is also possible that there is more subcortical visual input to the medial prefrontal cortex and hippocampus (e.g., from the superior colliculus) than from the somatosensory system, resulting in partial compensation through subcortical activation of the forebrain systems that support trace EBC.
Unilateral inactivation of the visual cortex contralateral to the trained eye impaired long-delay EBC but had no effect on trace EBC. Moreover, the magnitude of the impairment in long-delay EBC was similar for unilateral and bilateral inactivation. Visual cortex has an exclusively ipsilateral projection to the lateral pontine nucleus (Glickstein et al., 1972; 1980; 1985; Wiesendanger & Wiesendanger, 1982; Legg et al., 1989; Wells et al., 1989). The pontine nuclei have bilateral projections into the cerebellum but only the contralateral projection is necessary for delay and trace EBC (Halverson et al., 2010; Halverson & Freeman, 2010a,b; Kalmbach et al., 2010). The results of Experiment 3 suggest that long-delay EBC is modulated by the ipsilateral projection of the visual cortex to the lateral pontine nucleus, whereas trace EBC is modulated by bilateral interactions with the hippocampus and prefrontal cortex.
Visual cortex inactivation resulted in a minor deficit in retention and no deficit in extinction of long-delay and trace EBC. Retrieval of the memory underlying long-delay and trace EBC may be partially mediated through subcortical visual systems and their projections, direct and indirect, to the prelimbic cortex (Takehara et al., 2003; Takehara-Nishiuchi & McNaughton, 2008). Extinction must also be mediated by subcortical projections to the prefrontal cortex or more directly to the cerebellum via the pontine nuclei (Medina et al., 2003).
Visual cortex differs from other sources of visual CS information in its projections to the PN. While primary visual cortex sends most projections to the lateral pontine nucleus (PN) and sparsely projects to the medial pontine nucleus (Wiesendanger & Wiesendanger, 1982), the LGNv sends projections primarily to the medial pontine nucleus (Halverson & Freeman, 2010b; Fig. 8). Furthermore, muscimol inactivation of the medial pontine nucleus prevents delay EBC with a light or LGNv stimulation CS (Halverson & Freeman, 2010). Also, lesions of the lateral pontine nucleus do not impair delay EBC with a light CS (Steinmetz et al., 1987). When combined, these findings indicate that EBC with a visual CS may rely on the medial PN for short delay EBC and lateral PN for long-delay and trace EBC.
Previous studies examining the role of sensory cortex in EBC have suggested that it is necessary for trace EBC but not for delay EBC. The current findings demonstrate that the visual cortex plays a role in delay EBC, as well as trace EBC, when a relatively long ISI is used. An implication of this finding is that visual cortex should not play a role in trace EBC when the ISI is relatively short. The differential effects of unilateral visual cortical inactivation on long-delay and trace EBC suggest that the visual cortex influences delay EBC through its ipsilateral projection to the pons, whereas bilateral interactions with forebrain systems mediate the visual cortical contributions to trace EBC. A surprising finding of the current study is that visual cortex lesions or inactivation had no effect on extinction of delay or trace EBC, suggesting that subcortical visual areas interact with forebrain system such as the prefrontal cortex or the cerebellum to mediate extinction. The current results indicate that visual cortex modulates associative cerebellar learning through interactions with forebrain and hindbrain neural circuitry; the specific nature of these interactions appears to differ between long-delay and trace EBC.
Highlights.
Visual cortex lesions impair long-delay and trace eyeblink conditioning
Bilateral visual cortex inactivation impairs long-delay and trace conditioning
Unilateral visual cortex inactivation impairs long-delay but not trace conditioning
Visual cortex facilitates eyeblink conditioning with relatively long ISIs
Visual cortical contributions to long-delay and trace conditioning differ
Acknowledgments
This work was supported by NIMH grant MH080005 to J.H.F.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Case GR, Lavond DG, Thompson RF. Cortical spreading depression and involvement of the motor cortex, auditory cortex, and cerebellum in eyeblink classical conditioning of the rabbit. Neurobiology of Learning and Memory. 2002;78(2):234–245. doi: 10.1006/nlme.2002.4061. [DOI] [PubMed] [Google Scholar]
- Freeman JH, Steinmetz AB. Neural circuitry and plasticity mechanisms underlying delay eyeblink conditioning. Learning & Memory. 2011;18(10):666–677. doi: 10.1101/lm.2023011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez R, Weible AP, Disterhoft JF. Cortical barrel lesions impair whisker-CS trace eyeblink conditioning. Learning & Memory. 2007;14(1-2):94–100. doi: 10.1101/lm.418407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez R, Weiss C, Weible AP, Disterhoft JF. Vibrissa-signaled eyeblink conditioning induces somatosensory cortical plasticity. The Journal of neuroscience. 2006;26(22):6062–6068. doi: 10.1523/JNEUROSCI.5582-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickstein M, Cohen JL, Dixon B, Gibson A, Hollins M, Labossiere E, Robinson F. Corticopontine visual projections in macaque monkeys. Journal of Comparative Neurology. 1980;190(2):209–229. doi: 10.1002/cne.901900202. [DOI] [PubMed] [Google Scholar]
- Glickstein M, Stein J, King RA. Visual input to the pontine nuclei. Science. 1972;178(4065):1110–1111. doi: 10.1126/science.178.4065.1110. [DOI] [PubMed] [Google Scholar]
- Glickstein M, May JG, Mercier BE. Corticopontine projection in the macaque: the distribution of labelled cortical cells after large injections of horseradish peroxidase in the pontine nuclei. Journal of Comparative Neurology. 1985;235(3):343–359. doi: 10.1002/cne.902350306. [DOI] [PubMed] [Google Scholar]
- Halverson HE, Freeman JH. Medial auditory thalamic input to the lateral pontine nuclei is necessary for auditory eyeblink conditioning. Neurobiology of learning and memory. 2010a;93(1):92–98. doi: 10.1016/j.nlm.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halverson HE, Freeman JH. Ventral lateral geniculate input to the medial pons is necessary for visual eyeblink conditioning in rats. Learning & Memory. 2010b;17(2):80–85. doi: 10.1101/lm.1572710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halverson HE, Hubbard EM, Freeman JH. Stimulation of the lateral geniculate, superior colliculus, or visual cortex is sufficient for eyeblink conditioning in rats. Learning & Memory. 2009;16(5):300–307. doi: 10.1101/lm.1340909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halverson HE, Lee I, Freeman JH. Associative plasticity in the medial auditory thalamus and cerebellar interpositus nucleus during eyeblink conditioning. The Journal of Neuroscience. 2010;30(26):8787–8796. doi: 10.1523/JNEUROSCI.0208-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halverson HE, Poremba A, Freeman JH. Medial auditory thalamus inactivation prevents acquisition and retention of eyeblink conditioning. Learning & Memory. 2008;15(7):532–538. doi: 10.1101/lm.1002508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halverson HE, Freeman JH. Medial auditory thalamic nuclei are necessary for eyeblink conditioning. Behavioral neuroscience. 2006;120(4):880. doi: 10.1037/0735-7044.120.4.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesslow G. Inhibition of classically conditioned eyeblink responses by stimulation of the cerebellar cortex in the decerebrate cat. The Journal of physiology. 1994;476(2):245–256. doi: 10.1113/jphysiol.1994.sp020127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmbach BE, Ohyama T, Mauk MD. Temporal patterns of inputs to cerebellum necessary and sufficient for trace eyelid conditioning. Journal of neurophysiology. 2010;104(2):627–640. doi: 10.1152/jn.00169.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton BJ, Thompson RF. Conditioning using a cerebral cortical conditioned stimulus is dependent on the cerebellum and brain stem circuitry. Behavioral neuroscience. 1992;106(3):509. doi: 10.1037//0735-7044.106.3.509. [DOI] [PubMed] [Google Scholar]
- Kraus N, Disterhoft JF. Response plasticity of single neurons in rabbit auditory association cortex during tone-signalled learning. Brain research. 1982;246(2):205–215. doi: 10.1016/0006-8993(82)91168-4. [DOI] [PubMed] [Google Scholar]
- Legg CR, Mercier B, Glickstein M. Corticopontine projection in the rat: the distribution of labelled cortical cells after large injections of horseradish peroxidase in the pontine nuclei. Journal of Comparative Neurology. 1989;286(4):427–441. doi: 10.1002/cne.902860403. [DOI] [PubMed] [Google Scholar]
- Lovick TA, Zbrozyna AW. Classical conditioning of the corneal reflex in the chronic decerebrate rat. Brain research. 1975 doi: 10.1016/0006-8993(75)90724-6. [DOI] [PubMed] [Google Scholar]
- Marquis DG, Hilgard ER. Conditioned lid responses to light in dogs after removal of the visual cortex. Journal of Comparative Psychology. 1936;22(1):157. [Google Scholar]
- Marquis DG, Hilgard ER. Conditioned responses to light in monkeys after removal of the occipital lobes. Brain: A Journal of Neurology 1937 [Google Scholar]
- Mauk MD, Thompson RF. Retention of classically conditioned eyelid responses following acute decerebration. Brain research. 1987;403(1):89–95. doi: 10.1016/0006-8993(87)90126-0. [DOI] [PubMed] [Google Scholar]
- Medina JF, Nores WL, Mauk MD. Inhibition of climbing fibres is a signal for the extinction of conditioned eyelid responses. Nature. 2002;416(6878):330–333. doi: 10.1038/416330a. [DOI] [PubMed] [Google Scholar]
- Miller MJ, Weiss C, Song X, Iordanescu G, Disterhoft JF, Wyrwicz AM. Functional magnetic resonance imaging of delay and trace eyeblink conditioning in the primary visual cortex of the rabbit. The Journal of Neuroscience. 2008;28(19):4974–4981. doi: 10.1523/JNEUROSCI.5622-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molchan SE, Sunderland T, McIntosh AR, Herscovitch P, Schreurs BG. A functional anatomical study of associative learning in humans. Proceedings of the National Academy of Sciences. 1994;91(17):8122–8126. doi: 10.1073/pnas.91.17.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson DA, Freeman JH., Jr Neuronal correlates of conditioned inhibition of the eyeblink response in the anterior interpositus nucleus. Behavioral Neuroscience. 2002;116(1):22. [PubMed] [Google Scholar]
- Norman RJ, Villablanca JR, Brown KA, Schwafel JA, Buchwald JS. Classical eyeblink conditioning in the bilaterally hemispherectomized cat. Experimental Neurology. 1974;44(3):363–380. doi: 10.1016/0014-4886(74)90202-7. [DOI] [PubMed] [Google Scholar]
- Oakley DA, Russell IS. Neocortical lesions and Pavlovian conditioning. Physiology & Behavior. 1972;8(5):915–926. doi: 10.1016/0031-9384(72)90305-8. [DOI] [PubMed] [Google Scholar]
- Oakley DA, Steele Russell I. Subcortical storage of Pavlovian conditioning in the rabbit. Physiology & Behavior. 1977;18(5):931–937. doi: 10.1016/0031-9384(77)90203-7. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates: hard cover edition. Academic press; 2007. [Google Scholar]
- Schneiderman N, Fuentes I, Gormezano I. Acquisition and extinction of the classically conditioned eyelid response in the albino rabbit. Science. 1962;136:650–652. doi: 10.1126/science.136.3516.650. [DOI] [PubMed] [Google Scholar]
- Steinmetz JE, Logan CG, Rosen DJ, Thompson JK, Lavond DG, Thompson RF. Initial localization of the acoustic conditioned stimulus projection system to the cerebellum essential for classical eyelid conditioning. Proceedings of the National Academy of Sciences. 1987;84(10):3531–3535. doi: 10.1073/pnas.84.10.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehara K, Kawahara S, Kirino Y. Time-dependent reorganization of the brain components underlying memory retention in trace eyeblink conditioning. The Journal of Neuroscience. 2003;23(30):9897–9905. doi: 10.1523/JNEUROSCI.23-30-09897.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehara-Nishiuchi K, McNaughton BL. Spontaneous changes of neocortical code for associative memory during consolidation. Science. 2008;322(5903):960–963. doi: 10.1126/science.1161299. [DOI] [PubMed] [Google Scholar]
- Weiss C, Disterhoft JF. Exploring prefrontal cortical memory mechanisms with eyeblink conditioning. Behavioral Neuroscience. 2011;125(3):318. doi: 10.1037/a0023520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells GR, Hardiman MJ, Yeo CH. Visual projections to the pontine nuclei in the rabbit: Orthograde and retrograde tracing studies with WGA‐HRP. Journal of Comparative Neurology. 1989;279(4):629–652. doi: 10.1002/cne.902790410. [DOI] [PubMed] [Google Scholar]
- Wiesendanger R, Wiesendanger M. The corticopontine system in the rat. I. Mapping of corticopontine neurons. Journal of Comparative Neurology. 1982;208(3):215–226. doi: 10.1002/cne.902080302. [DOI] [PubMed] [Google Scholar]


