Abstract
Objectives
Often peptides used in synthesis of radiopharmaceutical PET tracers are lipophilic and adhere to the walls of container closure systems (CCS) such that costly peptide and product are not fully recoverable after synthesis occurs. This investigation compares a standard United States Pharmacopeia (USP) Type I borosilicate glass CCS to a cyclic polyolefin copolymer Crystal Zenith® (CZ) CCS, for 68Ga-chloride and 68Ga-DOTATOC ([68Ga] Ga-DOTA-D-Phe1-Tyr3-octreotide) retention in the reaction vial after labeling.
Methods
68Gallium labeling of DOTATOC was conducted by adding 68Ga-chloride, 2M HEPES (4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid) or 0.75M sodium acetate, and 1µg to 30µg of DOTATOC into the CZ or glass CCS. The reaction mixture was heated for 15 minutes and cooled to room temperature. The crude reaction mixture was then withdrawn via syringe, for final processing. The CCS was then assayed using a dose calibrator to determine the amount of retained 68Ga-DOTATOC. Statistical significance was assessed using an unpaired Student's t-test.
Results
In all experiments (n=72) with various amounts of peptide and different buffering systems, the CZ CCS retained less activity than the glass CCS. Using 2M HEPES and 15µg or 30µg of DOTATOC, the CZ CCS retained approximately 10% less of the labeled DOTATOC compared to the glass CCS (p<0.05). Utilizing either a sodium acetate or a HEPES buffering system with 15µg or 30µg of DOTATOC, the CZ CCS retained approximately 2.5% less of the total reaction activity compared to the glass CCS (p<0.05). Product yield was equivalent in glass and CZ CCS under the same reaction conditions. Both the CZ and glass vials showed no retention of 68Ga-chloride.
Conclusion
For applications involving the labeling of peptides such as 68Ga-DOTATOC, the CZ CCS compared to the glass CCS, results in an improved recovery of product.
Keywords: 68Ga-DOTATOC, Crystal Resin Closure System, Peptide adsorption, Recovery
Introduction
Peptide-based radiopharmaceuticals are increasingly employed for diagnostic and therapeutic applications. Radioisotopes such as 68Ga, 18F, 86Y, and 64Cu, are positron emitters used in medical Positron Emission Tomography (PET) imaging. 68Ga is of recent interest as the technology for 68Ge/68Ga generators has greatly improved and allows PET imaging with a wide variety of small peptides and proteins at facilities without access to a cyclotron (Fani et al., 2008). The generator can be kept onsite and usually up to several GBq of activity can be acquired during each acid elution depending on the 68Ge/68Ga generator (Blois et al., 2010). 68Ga can be labeled to a chelator-conjugated peptide through a single step reaction (Leon-Rodriguez and Kovacs, 2008). With a half-life of 68 minutes and 89.1% of its decay through positron emission (Decristoforo et al., 2007) 68Ga is ideally suited as a PET imaging isotope.
Many peptides are lipophilic and can easily be adsorbed by the walls of container closure systems (CCS), such that costly peptide and product are not fully recoverable after the reaction has occurred. Methods which have been shown to mitigate the surface adhesion include the addition of a surfactant such as bovine serum albumin (BSA) or Tween 20 (Midwoud et al., 2006; Qadry et al., 2003), coating the CCS with polyethylene glycol (PEG) or siliconizing agents, and the addition of organic modifiers to improve the solubility of peptides (Goebel-Stengel et al., 2011). Surfactants and modifiers, such as ethanol and acetic acid, are known solvents that reduce the amount of adsorption to a CCS; however, the addition of these modifiers is often undesirable for the synthesis of radiopharmaceuticals. All of the previously discussed methods can require additional steps and may result in impurities that have the potential to render the radiopharmaceutical unfit for human use. Alternatively, a CCS with inherently reduced surface adhesion could improve the product recovery without the need for modifying the CCS surface properties by adding other chemicals to reaction mix. This investigation compares a USP Type I borosilicate glass CCS to a resin-based Crystal Zenith® (CZ) CCS to assess the difference of retained 68Ga-chloride and 68Ga-DOTATOC (68Gallium-DOTA-D-Phe1-Tyr3-octreotide) in the reaction vial after radiolabeling.
Methods
2.1 68Gallium labeling procedures
The 68Gallium labeling of DOTATOC was carried out by first eluting the 68Ge/68Ga generator (iTHEMBA, South Africa) with a total of 6mL of 0.6M hydrochloric acid (HCl). Using a three step fractionation method (Blois et al., 2010) only the second 2mL of the eluant, which contained the highest 68Ga concentration, was directed into a cyclic polyolefin copolymer CZ (Daikyo/West), or a standard United States Pharmacopeia Type I borosilicate glass CCS. The CZ and glass CCS chosen for this study meet the United States Pharmacopeia (USP), European Pharmacopeia (EP) and Japanese Pharmacopeia (JP) requirements (Eu et al., 2011). Prior to the addition of the 68Ga-chloride, the CCS was pre-filled with either a HEPES (4-(2-Hydroxyethyl) piperazine-1-ethanesulfonic acid, Sigma Aldrich), or sodium acetate (Sigma Aldrich) buffer formulation.
The HEPES buffer formulation consisted of 2M HEPES and the sodium acetate buffer formulation consisted of 0.75M sodium acetate. With both buffer systems we used 4M hydrochloric acid (Sigma Aldrich) to adjust the pH of the reaction solution between 3.5 and 4.5 for ideal 68Ga-labeling conditions. The reaction volume was equivalent in each buffer system so the concentration of 68Ga remained approximately the same throughout all experiments, and a direct comparison could be made. Each CCS had 1µg, 15µg or 30µg of DOTATOC (Anaspec).
After the addition of 68Ga-chloride to the pre-loaded CCS, the vial was heated for 15 minutes at 100°C. The CCS was allowed to cool in a cold-water bath before the crude reaction mixture was extracted via syringe and passed through a C18 solid phase extraction column (Strata-x, Phenomenex). Dehydrated ethanol (Pharmco-AAPER) was used to elute 68Ga-DOTATOC off the column, which was collected in a separate vial. The CCS, which served as the reactor during the heating of the crude reaction mixture, and the final 68Ga-DOTATOC product were assayed in a dose calibrator (CRC-25 PET, CAPINTEC).
To verify that the radioactive compound adhering to the CCS was indeed the radiolabeled pharmaceutical, in a control experiment, 68Ga-chloride was added to both CCS types containing only buffer solution but no DOTATOC. The CCSs were then heated at 100°C for 15 minutes, as was done in the DOTATOC labeling procedure. The fluid inside each CCS was removed via syringe, and the empty CCS was assayed. Prior to these experiments, we confirmed that the amount of fluid retained in the CCS was 0.7±0.1% of the total reaction volume.
2.2 Validation
Thin layer chromatography (TLC), using alumina-backed silica gel, was used to validate the radiochemical purity of the final product. Two mobile phases, 0.1M sodium citrate (Sigma Aldrich) and 1M ammonium acetate/methanol (Sigma Aldrich) (1:1 v/v), were used to separate 68Ga3+, 68Ga-colloid and Ga-DOTATOC (Zhernosekov et al., 2007). With the 1 M ammonium acetate/methanol (1:1 v/v) mobile phase 68Ga3+ and 68Ga-colloid (hydrolyzed 68Gallium) remain at the origin (Rf=0.1) while 68Ga-DOTATOC moves towards the solvent front (Rf=0.4). Conversely, the 0.1M sodium citrate mobile phase retains 68Ga-DOTATOC and 68Ga-colloid at the origin (Rf=0), and 68Ga3+ ions move to the solvent front (Rf =0.9). The radiochemical purity was the determined as the area of the peak with Rf of 68Ga-DOTATOC divided by the area sum of all peaks. Plates were analyzed using a radio-TLC plate reader (AR-2000, Bioscan).
2.3 Data Analysis
Each condition was tested a minimum of three times. The unpaired Student's t-test was used to compare glass and crystal resin CCS data. Significance level was set at p<0.05 which is indicated by “*” on the histograms. Error bars in each figure represent standard error of the mean. All measurements of radioactivity were corrected for decay.
Results
3.1 Retention of 68Ga-DOTATOC using a sodium acetate buffer system
In all experiments using the sodium acetate buffering system and various amounts of DOTATOC, the CZ CCS retained less of the total activity than the glass CCS (Fig. 1a). With 30µg of DOTATOC, the glass and CZ retained 5.0±0.5% and 2.7±0.6% (n=3 each) of the total reaction activity, respectively (p<0.02). Using 15µg of peptide, 5.0±0.5% and 3.1±0.5% (n=3 each), of the total reaction activity adhered to the glass and CZ CCS, respectively (p<0.03). The overall decay corrected product yields for 15µg and 30µg of peptide were 54%±3.7% and 70%±2.4%, respectively. However, when using 1µg of peptide, the product yield was drastically reduced and fluctuated between 7% and 46%.
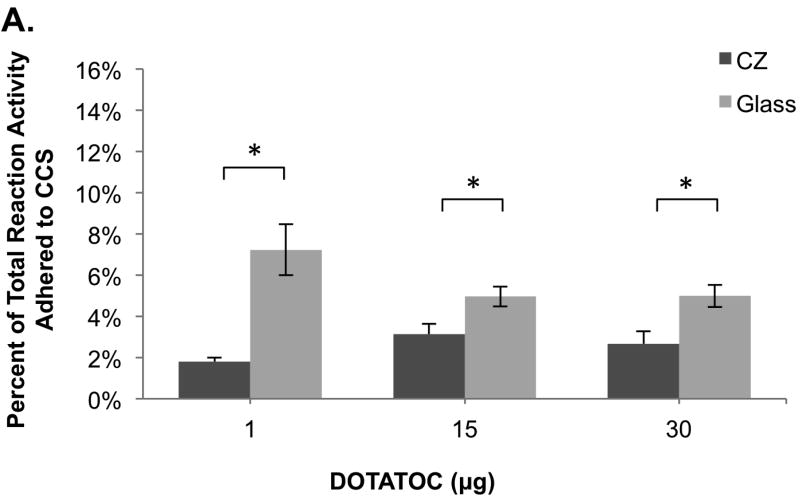
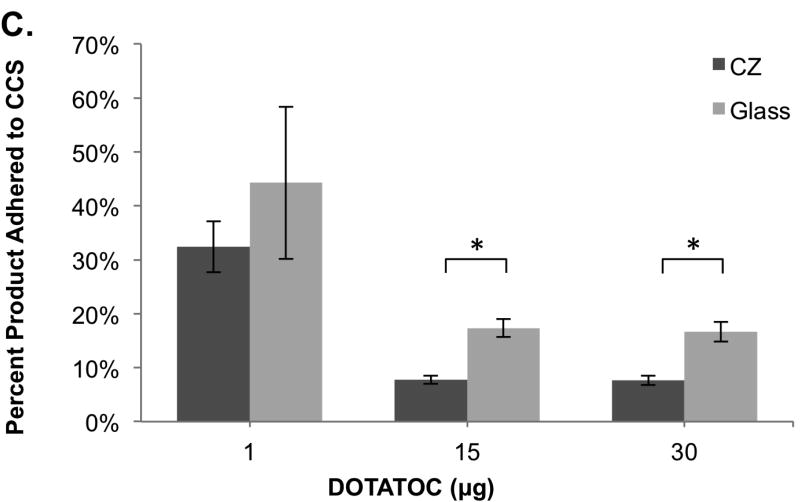
Figure 1.
Comparison of 68Ga-DOTATOC adherence to the glass and crystal resin CCS using HEPES and sodium acetate buffer formulations for 68Ga labeling. (A) Sodium acetate buffer system showing the percent of 68Ga-DOTATOC that adhered to the CCS compared to the total reaction activity, (n=3-5). (B) HEPES buffer system showing the percent of 68Ga-DOTATOC that adhered to the CCS compared to the total reaction activity, (n=3). (C) HEPES buffer formulation showing the percent of 68Ga-DOTATOC that adhered to the CCS compared to the final product activity, (n=3-5). *: p<0.05
3.2 Retention of 68Ga-DOTATOC using a HEPES buffer system
Over a range of different peptide amounts in the HEPES buffering system, less radioactivity remained in the CZ than the glass CCS with respect to both the percentage of total activity, (Fig. 1b) and the percentage of 68Ga-DOTATOC (Fig. 1c). With 15µg of peptide, the percentages of the total reaction activity that attached to the glass and CZ CCS were 9.0±0.8% and 6.3±0.6%, respectively (n=5 each). Utilizing the HEPES buffer and 30µg of peptide, 9.7±0.5% and 6.2±0.02% of the total reaction activity remained on the glass and CZ CCS, respectively, (n=5 each). The percentage of total activity that adhered to the glass and CZ CCS at both peptide quantities was statistically different (p<0.05). At a peptide amount of 1µg, the percentage of total activity adhered to the glass and CZ CCS was 12±1.2% and 12.±1.6%. With 1µg DOTATOC in the reaction mixture the retention levels of radioactivity in the two different CCS were not statistically different from one another; however, with the peptide amounts of 15µg and 30µg the percentages of total adhered activity were significantly different (p<0.05.) The overall decay corrected yields were similar to the acetate buffer system such that peptide amounts of 15 and 30µg gave yields above 50% and 1µg gave highly variable yields of 5% to 45%.
The percentage of 68Ga-DOTATOC product that adhered to the glass and CZ CCS using 15µg of DOTATOC, was 17±1.7% and 7.8±0.8%, respectively (n=5 each, p<0.03). Using 30µg of peptide, the percentage of the total product that adhered to the glass and CZ CCS was 16±1.9% and 7.7±0.9%, respectively, (n=5 each, p<0.01). Using both 15 and 30µg of peptide there was a statistically greater percentage of product adhering to the glass CCS, with about 10% less adsorption to the CZ CCS. Experiments with 1µg of DOTATOC showed a larger range of product adsorption, likely accounted for by the much lower and variable product yields that are obtained when a small amount of peptide is used.
3.3 68Ga-chloride
The generator eluant, 68Ga-chloride, did not significantly adhere to either the glass or CZ CCS. Less than 0.1% remained in both the glass and CZ CCS and values of the residual activity were not statistically different from one another.
3.4 Validation
Radiochemical purity was measured for synthesis runs using TLC (Fig. 2). Based on the area under the curves of the peaks as explained above we confirmed a radiochemical purity of >95% in our synthesis runs.
Figure 2.
Thin Layer Chromatography (TLC) of product, 68Ga-DOTATOC after C-18 purification. A) Mobile phase: 0.1M sodium citrate, Rf=Origin. B) Mobile phase: 1M ammonium acetate: methanol Rf=0.4.
Discussion
In this study, we demonstrated that the CZ CCS resulted in less product adhering to the vial compared to the commonly used USP Type I borosilicate single-use glass vials. Using radioisotope 68Ga to label the peptide, DOTATOC, we were able to quantify the adsorption to the glass and CZ CCS by assaying the radioactivity remaining on the CCS surface. This adhesion of radioactivity is primarily from the retention of the Ga-DOTATOC and not 68Ga, as 68Ga-chloride does not adsorb to either the glass or CZ CCS. For the intermediate and higher amounts of DOTATOC peptide (15µg and 30µg), the glass CCS retained approximately 10% more product, and 2.5% more total activity than the CZ CCS. Having a greater percentage of the product adhere, in comparison to the percentage of total activity, was expected and is congruent with the findings that 68Ga-chloride does not adhere to the CCS. Adsorption of 10% of the final radiopharmaceutical to a CCS may be important, not only for applications that require yield optimization but also for methods utilizing expensive peptides. An advantage of the CZ CCS is that one can achieve higher recoveries without the addition of other surfactants that may require extra purification steps as well as have negative effects on the reaction chemistry.
Overall product yields at peptide amounts of 15µg or 30µg in a HEPES or sodium acetate buffer system were comparable to literature values (Decristoforo et al., 2007). The adsorption of 68Ga-DOTATOC to CCS was slightly lower with sodium acetate buffer than the HEPES buffer. We hypothesize the varying amount of adsorption is due to difference in pKa of the buffer system. In addition, CZ and glass CCS had product yields within 10% of each other. Due to the unaffected product yields and TLC chromatograms (Fig. 2a, and 2b), we were able to conclude that the integrity of the radiopharmaceutical was not affected from being heated in a glass or CZ CCS. The TLC scans show a representative chromatogram of the final 68Ga-DOTATOC product to confirm that the radioactive compound adhered to the CCS is the radiopharmaceutical.
In conclusion, the decrease in peptide adhesion to the CZ CSS in comparison to the glass CSS is a benefit of using CZ CCS in the labeling of radiopharmaceuticals such as 68Ga-DOTATOC.
Highlights.
We examined the adhesion of 68Ga-DOTATOC to glass and CZ CCS.
The adhesion of the 68Ga-DOTATOC was 10% less in CZ CCS compared to glass CCS
Overall recovery of 68Ga-DOTATOC reaction solution is higher in CZ CCS than glass CCS
Adhesion to the CCS is due to 68Ga-DOTATOC, not 68Ga-chloride.
Acknowledgments
This work was supported through a grant provided by West Pharmaceutical Services, Inc
References
- Blois ED, Chan HS, Naidoo C, Prince D, Krenning E, Breeman WAP. Characeristics of SnO2-based 68Ge/68Ga generator and aspects of radiolabelling DOTA-peptides. Applied Radiation and Isotopes. 2010;69:308–315. doi: 10.1016/j.apradiso.2010.11.015. [DOI] [PubMed] [Google Scholar]
- Decristoforo C, Knopp R, Guggenberg Ev, Rupprich M, Dreger T, Hess A, Virgolini I, Haubner R. A fully automated synthesis for the preparation of 68Ga-labelled peptides. Nuclear Medicine Communications. 2007;28:870–875. doi: 10.1097/MNM.0b013e3282f1753d. [DOI] [PubMed] [Google Scholar]
- Eu B, Cairns A, Ding G, Cao X, Wen ZQ. Direct visualization of protein adsorption to primary containers by gold nanoparticles. Journal of Pharmaceutical Sciences. 2011;100:1663–1670. doi: 10.1002/jps.22410. [DOI] [PubMed] [Google Scholar]
- Fani M, Andre JOP, Maecke HR. 68Ga-PET: a powerful generator-based alternative to cyclotron-based PET radiopharmaceuticals. Contrast Media and Molecular Imaging. 2008;3:67–77. doi: 10.1002/cmmi.232. [DOI] [PubMed] [Google Scholar]
- Goebel-Stengel M, Stengel A, Tache Y, R JR., Jr The importance of using the optimal plasticware and glassware in studies involving peptides. Analytical Biochemistry. 2011;414:38–46. doi: 10.1016/j.ab.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon-Rodriguez LMD, Kovacs Z. The Synthesis and Chelation Chemistry of DOTA-Peptides. Bioconjugate Chemistry. 2008;19:391–402. doi: 10.1021/bc700328s. [DOI] [PubMed] [Google Scholar]
- Midwoud PMv, Rieux L, Bischoff R, Verpoorte E, Niederlander HAG. Improvement of Recovery and Repeatability in Liquid Chromatography-Mass Spectrometry Analysis of Peptides. Journal of Proteome Research. 2006;6:781–791. doi: 10.1021/pr0604099. [DOI] [PubMed] [Google Scholar]
- Qadry SS, Roshdy TH, Char H, Terzo SD, Tarantino R, Moschera J. Evaluation of CZ-resin vials for packaging protein-based parenteral formulations. International Journal of Pharmaceutics. 2003;252:207–212. doi: 10.1016/s0378-5173(02)00641-5. [DOI] [PubMed] [Google Scholar]
- Woods EJ, Bagchi A, Goebel WS, Nase R, Vilivalam VD. Container system for enabling commerical production of cryopreserved cell therapy products. Future Medicine. 2010;5:659–667. doi: 10.2217/rme.10.41. [DOI] [PubMed] [Google Scholar]
- Zhernosekov KP, Filosofov DV, Baum RP, Aschoff P, Bihl H, Razbash AA, Jahn M, Jennewein M, Rosch F. Processing of Generator-Produced 68Ga for Medical Application. Journal of Nuclear Medicine. 2007;48:1741–1748. doi: 10.2967/jnumed.107.040378. [DOI] [PubMed] [Google Scholar]