Abstract
Background and Purpose
Much recent interest surrounds the use of action observation – observing another individual performing a motor task – in stroke rehabilitation, to promote motor recovery by engaging similar brain regions to action execution. This may be especially useful in individuals with limited mobility. Here we assess how cortical motor activity during action observation is affected by stroke and by stroke-related motor deficits.
Methods
We used fMRI to compare brain activity during right and left hand action observation in right-handed non-disabled participants and participants who were right-handed prior to left hemisphere stroke. All participants performed the same actions after their fMRI.
Results
Non-disabled participants show greater bilateral cortical motor activity when observing actions made using the left hand, whereas participants with stroke show greater ipsilesional cortical motor activity when observing actions made using the right (paretic) hand (p<.05, corrected). For both groups, action processing is modulated by motor capability: cortical motor activity is greater when observing the hand with lower motor scores (p<.05, corrected). Further, for stroke, the extent of ipsilesional activity correlates with lesion volume (p=.049), in a pattern that suggests adaptive plasticity.
Conclusions
We found that action observation activates specific motor plans in damaged motor circuits after stroke, and this activity is related to motor capability to perform the same actions. Cortical motor activity during action observation may be relevant to motor learning, and to motor relearning in stroke rehabilitation.
Keywords: stroke, rehabilitation, action observation, mirror neuron system, plasticity
Stroke is the leading cause of disability among adults, and motor deficits of the arm and hand are a major contributor to functional disability following stroke1. Stroke rehabilitation usually involves intensive motor practice aimed to promote adaptive plasticity in the damaged motor system toward recovery, to minimize motor deficits and develop new strategies in motor learning2. In patients with poor voluntary motor ability however, it is a challenge to provide relevant input to the sensorimotor system to promote optimal experience-dependent plasticity.
Much interest surrounds the potential to use action observation –observing another individual performing a motor task –to promote plasticity in stroke rehabilitation, especially in individuals with limited motor ability3, 4. Action observation is hypothesized to rebuild motor function despite impairments by engaging similar brain regions to action execution. The neurophysiological basis for this hypothesis is the putative human mirror neuron system, cortical motor regions that are active when we perform an action and when we observe similar actions being performed by others5. Recent work has described an “action observation network” (AON) of parietal, premotor, and occipitotemporal regions6. By engaging shared motor circuits with action execution, action observation may prime the motor system for subsequent motor practice, and enhance performance7. In rehabilitation, observing actions made by another individual, among other related approaches, can be used to promote activation of damaged motor circuits. While several small-scale clinical studies have found benefits from action observation after stroke8, success has been mixed9. Our proof-of-concept study was designed to address the lack of basic studies on the neural response to action observation after stroke. Here we provide neuroscientific support for the use of action observation in rehabilitation by demonstrating that action observation after stroke promotes activation in ipsilesional cortical motor regions considered to be relevant to neuroplasticity.
Prior work suggests that cortical motor regions respond most strongly to observation of actions that fall within the motor repertoire of the observer10, and for which the observer has motor expertise11, 12. Thus we were also interested in how activity in cortical motor regions is modulated when observing actions that fall outside the motor repertoire of the observer, as it is constrained by motor capability. In this case, how does an individual who cannot perform an action due to stroke process similar actions being performed by others? Such information may be used to inform clinical protocols of action observation in rehabilitation. We used fMRI to measure cortical motor activity during right and left hand action observation in right-handed non-disabled individuals and individuals with stroke, for whom the right hand was also the paretic hand. All participants performed the same actions after fMRI.
Methods
Subjects
Twelve individuals with stroke and twelve matched non-disabled individuals (Table 1) gave informed consent for the study in accordance with institutional guidelines. In order to test how individuals who cannot perform an action process arm actions made by others, we recruited right-handed individuals with dominant left hemisphere stroke and moderate-to-severe right hand hemiparesis. Because prior studies showing an effect of action observation in rehabilitation included individuals with chronic stroke, our inclusion criteria was also chronic, middle cerebral artery stroke. Based on these criteria, we incidentally recruited six individuals with stroke involving the internal capsule, and six with stroke involving the cortex and internal capsule. These subgroups enabled us to test how lesions involving cortical regions of the AON, or outside of the AON, affect action processing. All participants were right handed and had normal or corrected vision.
| I. Demographics. | ||||||||
|---|---|---|---|---|---|---|---|---|
| Stroke | ||||||||
| # | Gender | Age, Years |
Education, Years |
Duration, Years |
FMA-UE | Location | Volume, mm3 |
% IFGop |
| 2 | F | 64 | 20 | 5 | 48 | IC | 6161 | 0 |
| 6 | M | 55 | 16 | 4 | 46 | IC | 4897 | 0 |
| 7 | M | 74 | 20 | 17 | 18 | IC | 7845 | 0 |
| 10 | M | 39 | 20 | 2 | 40 | IC | 2360 | 0 |
| 12 | M | 73 | 14 | 4 | 13 | IC | 2912 | 0 |
| 13 | F | 85 | 14 | 8 | 31 | IC | 3831 | 0 |
| 4 | F | 64 | 20 | 15 | 13 | C+IC | 18753 | 0 |
| 14 | F | 51 | 16 | 6 | 14 | C+IC | 50196 | 56.5 |
| 15 | F | 74 | 20 | 9 | 47 | C+IC | 41825 | 0 |
| 16 | F | 68 | 16 | 6 | 15 | C+IC | 124318 | 11.4 |
| 18 | M | 71 | 20 | 8 | 37 | C+IC | 33711 | 3.9 |
| 21 | M | 71 | 20 | 4 | 35 | C+IC | 38044 | .2 |
| Mean | 6 F | 65.8 | 18 | 7.3 | 29.8 | 6 IC | 27904 | 6 |
| SEM | 3.6 | .74 | 1.3 | 4.1 | 10101 | 4.7 | ||
|
| ||||||||
| Non-disabled |
||||||||
| # | Gender | Age, Years |
Education, Years |
|||||
|
|
||||||||
| 8 | F | 71 | 20 | |||||
| 9 | M | 81 | 20 | |||||
| 17 | M | 75 | 18 | |||||
| 25 | M | 40 | 16 | |||||
| 27 | F | 64 | 20 | |||||
| 28 | M | 52 | 20 | |||||
| 19 | F | 68 | 14 | |||||
| 20 | M | 50 | 16 | |||||
| 22 | M | 71 | 20 | |||||
| 26 | F | 65 | 14 | |||||
| 29 | M | 71 | 16 | |||||
| 32 | F | 75 | 20 | |||||
| Mean | 5 F | 65.3 | 17.8 | |||||
| SEM | 3.5 | .72 | ||||||
|
|
||||||||
FMA-UE, Fugl-Meyer Motor assessment of the upper extremity; % IFGop, percent lesion overlap with inferior frontal gyrus pars opercularis; IC, internal capsule; C+IC, cortex plus internal capsule
fMRI Data Acquisition
fMRI included three 12s blocks: right or left hand action observation, and rest (fixation); each repeated 15 times, randomized across three 6min runs. During action observation, participants watched four 3s videos of a mean-age-matched non-disabled actor reach to grasp objects, from the first-person perspective. Actions were adapted from the Wolf Motor Function Test (WMFT)13: lift pencil, lift paperclip, stack checker (a round, flat game piece), flip card; and were included because they are difficult or impossible to perform using the paretic limb14, but are easy for non-disabled participants to perform as well using either hand. From this, we could make a within-subjects comparison between the paretic and non-paretic hands after stroke, and a between-subjects comparison with non-disabled participants. Participants were instructed to remain still, pay attention to the actions and to which hand the actor used, as they would be asked to imitate the actions after scanning. They first practiced the procedure in a mock scanner, and were monitored for movement during actual scanning. To ensure attention, after each run they were asked “In the most recent video…which hand was the actor using?” or “…what object did the actor pick up?” All participants answered correctly on all occasions.
We used a 3T Siemens Trio MRI, with a T2*-weighted gradient echo functional sequence (TR/TE = 2000/30ms, 37 slices, voxel size 3.5×3.5×3.5mm, flip angle 90°), and T1-weighted anatomical MPRAGE (stroke: TR/TE = 2350/3.09ms, 208 1mm slices, 256×256mm, flip angle 10°; non-disabled: TR/TE = 1950/2.26ms, 176 1mm slices, 256×256mm, flip angle = 9°).
Behavioral Assessment
Participants performed the same actions (lift pencil, lift paperclip, stack checker, flip card) after scanning, according to the procedure of the WMFT, using each hand, as quickly as possible, to the best of their ability. They were videotaped for offline assessment of movement time (s), and movement functional ability scale (FAS) score, ranging from 0 = does not attempt movement, to 5 = movement appears to be normal. The WMFT was scored by a trained, blinded, research assistant (A.J.) with no other involvement in the study. Log mean movement time (given potential skewness) and mean FAS score were computed. For results, see http://stroke.ahajournals.org.
fMRI Analysis
SPM8 (www.fil.ion.ucl.ac.uk/spm) was used for batch preprocessing and analysis. Structural images were manually centered and reoriented with functional images to the anterior/posterior-commissure axis. Functional images were realigned for motion correction and parameters were used as regressors of no interest in the model. The structural image was coregistered to the mean functional image and segmented, and all images were normalized and smoothed using a 6mm full width at half maximum Gaussian kernel. BOLD signal was modeled using a separate regressor for each condition and a boxcar function convolved with a canonical hemodynamic response function, and fit using SPM8’s general linear model.
First level maps were generated for the main effect of right and left hand action observation. Second level maps were generated for stroke and non-disabled groups, using a whole-brain threshold of p<.05 False Discovery Rate (FDR) corrected for multiple comparisons. Anatomy was labeled using maximum probability cytoarchitectonic maps in SPM Anatomy (www.fz-juelich.de/inm/inm-1/DE/Forschung/_docs/SPMAnatomyToolbox). For a priori regions of interest (ROI), we used a threshold of p<.05 Family Wise Error (FWE), small volume corrected (SVC), for the inferior frontal gyrus pars opercularis (BA 44) and pars triangularis (BA 45), precentral gyrus (BA 6), and supramarginal gyrus (area PF). ROIs comprise the putative human mirror neuron system5 and are commonly reported in studies of action observation and imitation6 as part of the AON. ROIs were derived from SPM Anatomy, and analyzed using MarsBar (http://marsbar.sourceforge.net).
To compare between groups, we used a three-way mixed ANOVA for each ROI, testing hand observed (right/left) and hemisphere (right/left) within-subjects, and group (stroke/non-disabled) between-subjects; with a Bonferroni correction for the four ROIs.
Laterality index (LI) was calculated based on extent of activation: left – right / left + right, for above-threshold voxel counts in each hemisphere. We used LI-toolbox (www.medizin.uni-tuebingen.de/kinder/en/research/neuroimaging/software) bootstrap method to calculate weighted mean LI at a range of thresholds. LI ranges from +1 (all left hemisphere) to –1 (all right hemisphere) and is considered bilateral (|LI|<.1), hemisphere-dominant (.1<|LI|<.2), or lateralized (|LI|>.2)15.
Brain Behavior Analysis
To test whether brain activity during action observation is related to motor capability to perform the same actions, we tested for correlations between ROI activity during action observation and mean movement time (Pearson’s r) or FAS score (Spearman’s rho), for the same hand.
Lesion Analysis
Lesions were manually traced on participant’s individual anatomy using brainvox (www.nitrc.org/projects/brainvox). To test whether brain activity during action observation is related to stroke lesion, we ran a regression model for the main effect of action observation using lesion volume as a regressor. We used Automated Lesion Identification16 toolbox to visualize lesion overlap.
Results
Main Effect of Action Observation
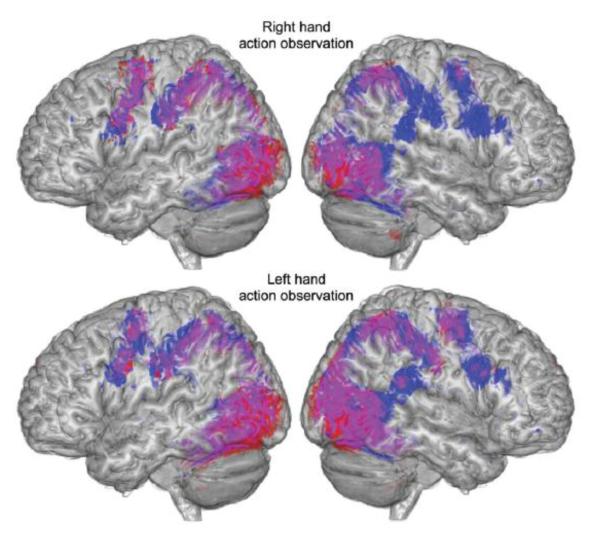
For non-disabled participants, relatively bilateral, symmetric cortical motor activity was found during right or left hand action observation, in brain regions commonly associated with action observation (the AON), including our ROIs (Figure 1). During right hand action observation, activations were found in the right inferior frontal gyrus pars opercularis, left inferior frontal gyrus pars triangularis, bilateral precentral gyrus and supramarginal gyrus, among others. During left (non-dominant) hand action observation, activations were found in the bilateral inferior frontal gyrus pars opercularis and supramarginal gyrus, and right inferior frontal gyrus pars triangularis, among others. For participants with stroke, similar bilateral, symmetric activity was found during left hand action observation (Figure 1), including the left inferior frontal gyrus pars opercularis, right inferior frontal gyrus pars triangularis and supramarginal gyrus, and bilateral precentral gyrus, among others. Yet during right (paretic) hand action observation, cortical motor activity is asymmetric, lateralized toward intact cortical motor regions of the left lesioned hemisphere, including the left inferior frontal gyrus pars opercularis and supramarginal gyrus, and bilateral precentral gyrus, among others. (All at p<.05 corrected; for a complete list of activations, see http://stroke.ahajournals.org).
Figure 1.
Main effect of action observation. Brain regions activated during right hand (top) and left hand (bottom) action observation in participants with stroke (red), non-disabled participants (blue), and overlap between groups (violet). Shown at p<.01 uncorrected.
Laterality Index
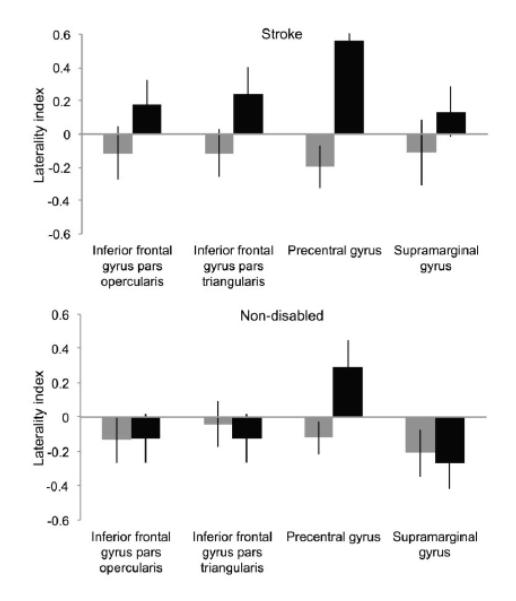
Based on lateralization found at the whole brain level, we used a laterality index (LI) to establish the laterality of ROI activity. We found that during right (paretic) hand action observation, participants with stroke show a clear pattern of left hemisphere-dominant activity across ROIs (Figure 2): inferior frontal gyrus pars opercularis (LI=.18, SEM=.15) and pars triangularis (LI=.24, SEM=.16), precentral gyrus (LI=.56, SEM=.08), and supramarginal gyrus (LI=.14, SEM=.15). In contrast, non-disabled participants show right hemisphere-dominant activity during right hand action observation in three ROIs: inferior frontal gyrus pars opercularis (LI=−.12, SEM=.14) and pars triangularis (LI=−.12, SEM=.14), supramarginal gyrus (LI=−.27, SEM=.15); and left-lateralized activity in the precentral gyrus (LI=.29, SEM=.16).
Figure 2.
Laterality index of brain activity during action observation. For participants with stroke (top), and non-disabled participants (bottom); during left hand (gray bars) and right hand (black bars) action observation, in regions of interest. Positive values indicate left hemisphere dominance, negative values indicate right hemisphere dominance.
For both groups, activity is right hemisphere-dominant during left hand action observation. For participants with stroke: inferior frontal gyrus pars opercularis (LI=−.11, SEM=.16) and pars triangularis (LI=−.12, SEM=.14), precentral gyrus (LI=−.2, SEM=.13), and supramarginal gyrus (LI=−.11, SEM=.2). For non-disabled participants: inferior frontal gyrus pars opercularis (LI=−.13, SEM=.13) and pars triangularis (LI=−.04, SEM=.13), precentral gyrus (LI=−.12, SEM=.09), and supramarginal gyrus (LI=−.21, SEM=.14).
For both groups, activity in the precentral gyrus is contralateral to the observed hand.
Right versus Left Hand Action Observation
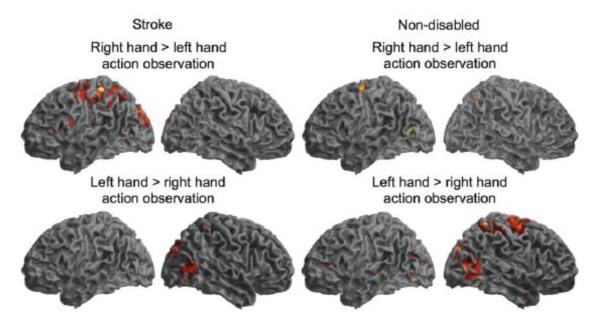
For participants with stroke, right (paretic) hand greater than left hand action observation activates the left inferior frontal gyrus pars opercularis, supramarginal gyrus, and postcentral gyrus (Figure 3). In contrast, for non-disabled participants, left (non-dominant) hand action observation more strongly activates right hemisphere cortical motor regions commonly associated with action observation (the AON), although not our ROIs. (All significant at p<.05 corrected; see http://stroke.ahajournals.org).
Figure 3.
Right versus left hand action observation. Brain regions in participants with stroke and non-disabled participants showing greater activity during right than left hand action observation (top), or left than right hand action observation (bottom). Shown at p<.01 uncorrected.
Task by Hemisphere by Group Interactions
We tested ROIs for task by group interactions, rather than testing for a main effect between groups, to ensure that group differences are not due to changes in neurovasculature from stroke, which should influence all tasks similarly17. Three-way interactions were found between hand observed (right/left), hemisphere (right/left), and group (stroke/non-disabled), in the inferior frontal gyrus pars opercularis (F(1,22)=8.03, p=.01, p=.04 corrected) and pars triangularis (F(1,22)=6.49, p=.02, p=.07 corrected), and supramarginal gyrus (F(1,22)=4.06, p=.06, p=.22 corrected). For participants with stroke, activity was greater during right (paretic) than left hand action observation, in the left hemisphere; whereas for non-disabled participants, activity was greater during left (non-dominant) than right hand action observation, in both hemispheres. A two-way interaction was found between hand observed and hemisphere in the precentral gyrus (F(1,22)=42.14, p=.001, p=.001 corrected), where activity was greatest contralateral to the observed hand in both groups.
Brain Activity Related to Motor Scores
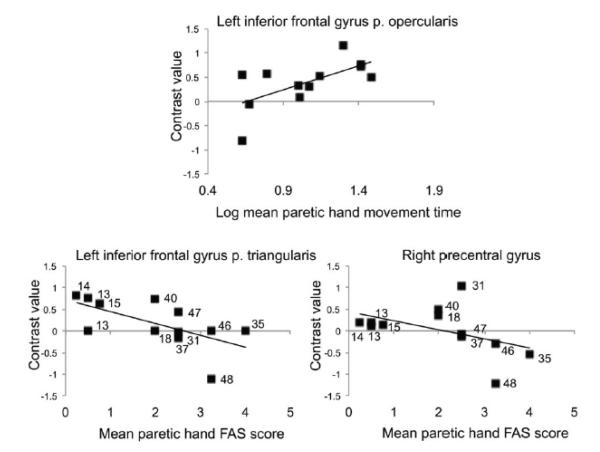
Mean movement time for the paretic right hand positively correlated with ROI activity during right hand action observation in the left inferior frontal gyrus pars opercularis (r(10)=.58, p=.05). Mean FAS score for the paretic right hand negatively correlated with ROI activity during right hand action observation in the left inferior frontal gyrus pars triangularis (rho(10)=−.79, p=.002) and right precentral gyrus (rho(10)=−.6, p=.04). Activity in these brain regions was greater for actions that are more difficult to perform (Figure 4).
Figure 4.
Brain activity during action observation correlated to motor scores. Positive correlation between mean paretic right hand movement time and activity during right hand action observation in the left inferior frontal gyrus pars opercularis (r(10)=.58, p=.05). Negative correlations between mean paretic right hand FAS score and activity during right hand action observation in the left inferior frontal gyrus pars triangularis (rho(10)=−.79, p=.002) and right precentral gyrus (rho(10)=−.6, p=.04). Point label: Fugl-Meyer Motor Assessment of the Upper Extremity score.
Brain Activity Related to Lesion
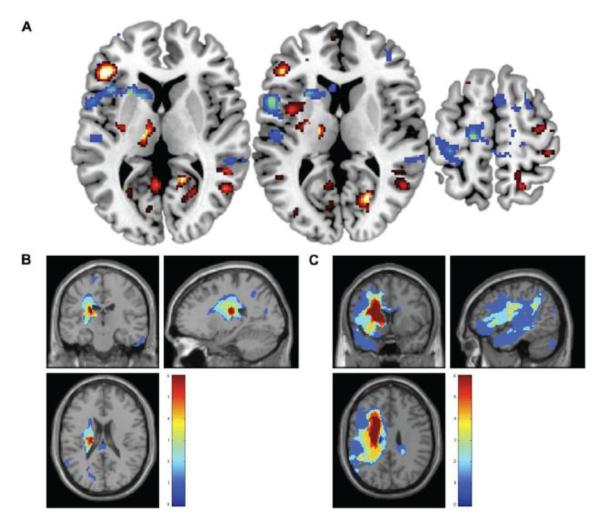
A whole brain correlation of lesion volume and brain activity during action observation revealed a marginally significant positive correlation in the left inferior frontal gyrus pars triangularis (p=.049 FWE SVC), marginally significant negative correlation in the left precentral gyrus (p=.054 FWE SVC), and trend toward negative correlation in the left inferior frontal gyrus pars opercularis (p=.069 FWE SVC; Figure 5).
Figure 5.
Brain activity during action observation correlated to lesion volume. (a) Positive whole brain correlation (hot) between lesion volume and activity during action observation in the left inferior frontal gyrus pars triangularis (−48, 32, 6); and negative correlations (cool) in the left inferior frontal gyrus pars opercularis (−54, 10, 10) and left dorsal precentral gyrus (−16, −20, 60). Shown at p<.05-.001 uncorrected. Lesion overlap map for participants with stroke involving (b) the internal capsule, and (c) the cortex and internal capsule. Color bar indicates N of 6 in each subgroup.
Discussion
This set of novel findings shows that in the healthy brain, cortical motor activity during action observation is greater for the non-dominant left hand; whereas after stroke, activity is greater for the right (paretic) hand. In both groups, activity is greater for the hand with lower motor scores. For stroke, right (paretic) hand action observation activates intact ipsilesional cortical motor regions, and extent of activation correlates with lesion volume.
The task specificity of cortical motor activity during action observation may be related to perceived effort. First, the observed actions are difficult or impossible to perform using the paretic limb14. Each requires control and coordination of multiple joints; multistep actions of reaching, grasping, and lifting; and high precision/accuracy. Second, inclusion criteria was no greater than FAS=3 for the paretic hand (attempts to use more-involved upper extremity, but movements are influenced to some degree by synergy or are performed slowly or with effort). Third, participants were instructed to observe in order to imitate the actions after scanning; observe to imitate leads to greater cortical motor activity than passive observation6, 18. Fourth, self-efficacy for expected task performance is lower for the non-dominant hand in non-disabled individuals, and for the paretic hand after stroke19.
Our findings may be related to motor learning. Motor recovery can be considered motor learning in a disrupted network20, especially in chronic stroke when the physiological attributes of recovery have attenuated. The AON is activated during motor learning21, more so by novel than practiced actions22 and unfamiliar than familiar actions23. This activity may reflect selection and combination of elementary motor acts, which decreases with learning as action representations become predictive; and may provide the basis for imitation24 and motor learning21, including motor learning by observing10, 25, and motor relearning after stroke.
For participants with stroke, we found a clear pattern of left hemisphere-dominant ROI activity during right (paretic) hand action observation. LI is commonly used to quantify hemispheric contributions to motor tasks after stroke; greater contralesional motor activity is associated with stroke, and with poorer motor recovery from stroke26. Better motor recovery is related to reorganization back toward the lesioned hemisphere27. We found that action observation provides a pattern of cortical motor activity lateralized toward the lesioned hemisphere that may be useful in rehabilitation to drive plasticity.
Further, extent of ipsilesional activity during action observation, particularly in the inferior frontal gyrus, correlated with lesion volume. In this study, lesion volume and location are not dissociable: larger lesions involved the left inferior frontal gyrus pars opercularis and precentral gyrus (Figure 5C), and are associated with less activity in these regions and greater activity in the adjacent inferior frontal gyrus pars triangularis. Thus for individuals with stroke involving brain regions typically activated by action observation in the healthy brain, activation during action observation is taken up by adjacent intact tissue; this suggests adaptive plasticity to support action observation and imitation after stroke.
A major approach for rehabilitation is to manipulate neuroplasticity to enhance recovery28. Toward this aim, it is promising that after stroke, rather than representing the available motor repertoire in favor of the non-paretic limb, action representations of the paretic limb are preserved, and may be activated by action observation. Prior work shows that action observation drives motor memory formation, whereby observed actions are encoded as specific motor representations similar to those from motor practice, a process that is facilitated by concurrent action observation and motor practice, and potentiated with respect to practice alone29. This process is preserved after chronic stroke30. Our findings support the use of action observation to prime the motor system for subsequent practice7, by showing that specific motor plans in damaged motor circuits are activated by action observation after stroke. Future studies will test whether repeated activation of motor plans by action observation drives plasticity after stroke.
This study highlights the need for a neuroscientific framework for clinical trials of action observation; our findings contribute to such a framework. Despite the small sample size, we provide proof-of-concept for the use of action observation in rehabilitation, although future studies must test other lesion groups, e.g., non-dominant stroke, and other patient populations, e.g., earlier after stroke. Despite patient heterogeneity, we find reliable group differences in cortical motor activity during action observation including a relation to motor capability, indicating the relevance to motor learning and possibly motor relearning after stroke.
Supplementary Material
Acknowledgements
We thank our participants, and Hanna Damasio, Mara Mather, Matthew Konersman, Alicia Johnson, and Julie Werner, for their contributions.
Funding American Heart Association (10SDG3510062); NIH, Eunice Kennedy Shriver National Institute of Child Health and Human Development (R03HD067475); National Institute of Biomedical Imaging and Bioengineering (EB00438). The content of this study is the responsibility of the authors and does not reflect the views of the funding bodies.
Footnotes
Disclosures None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.NINDS Final Report of the Stroke Progress Review Group [Accessed September 12, 2011];National Institutes of Neurological Disorders and Stroke web site. http://www.ninds.nih.gov/find_people/groups/stroke_prg/01-2012-stroke-prg-report.htm.
- 2.Winstein CJ, Wolf SL. Task-oriented training to promote upper extremity recovery. In: Stein J, Harvey R, Macko R, Winstein CJ, Zorowitz R, editors. Stroke Recovery and Rehabilitation. Demos Medical Publishing; 2008. pp. 267–290. [Google Scholar]
- 3.Garrison KA, Winstein CJ, Aziz-Zadeh L. The mirror neuron system: a neural substrate for methods in stroke rehabilitation. Neurorehabil Neural Repair. 2010;24:404–412. doi: 10.1177/1545968309354536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liew S, Garrison K, Werner J, Aziz-Zadeh L. The mirror neuron system: innovations and implications for occuptational therapy. OTJR: Occupation, participation and health. 2012;32:79–86. [Google Scholar]
- 5.Rizzolatti G, Craighero L. The mirror-neuron system. Annual Review of Neuroscience. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- 6.Caspers S, Zilles K, Laird AR, Eickhoff SB. ALE meta-analysis of action observation and imitation in the human brain. Neuroimage. 2010;50:1148–1167. doi: 10.1016/j.neuroimage.2009.12.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pomeroy V, Aglioti SM, Mark VW, McFarland D, Stinear C, Wolf SL, et al. Neurological principles and rehabilitation of action disorders: rehabilitation interventions. Neurorehabil Neural Repair. 2011;25:33S–43S. doi: 10.1177/1545968311410942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ertelt D, Small S, Solodkin A, Dettmers C, McNamara A, Binkofski F, et al. Action observation has a positive impact on rehabilitation of motor deficits after stroke. NeuroImage. 2007;36:T164–T173. doi: 10.1016/j.neuroimage.2007.03.043. [DOI] [PubMed] [Google Scholar]
- 9.Cowles T, Clark A, Mares K, Peryer G, Stuck R, Pomeroy V. Observation-to-imitate plus practice could add little to physical therapy benefits within 31 days of stroke: translational randomized controlled trial. Neurorehabil Neural Repair. 2013;27:173–182. doi: 10.1177/1545968312452470. [DOI] [PubMed] [Google Scholar]
- 10.Cross ES, Kraemer DJ, Hamilton AF, Kelley WM, Grafton ST. Sensitivity of the action observation network to physical and observational learning. Cereb Cortex. 2009;19:315–326. doi: 10.1093/cercor/bhn083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calvo-Merino B, Glaser DE, Grezes J, Passingham RE, Haggard P. Action observation and acquired motor skills: an fMRI study with expert dancers. Cereb. Cortex. 2005;15:1243–1249. doi: 10.1093/cercor/bhi007. [DOI] [PubMed] [Google Scholar]
- 12.Cross ES, Hamilton AF, Grafton ST. Building a motor simulation de novo: observation of dance by dancers. Neuroimage. 2006;31:1257–1267. doi: 10.1016/j.neuroimage.2006.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolf SL, Catlin PA, Ellis M, Archer AL, Morgan B, Piacentino A. Assessing Wolf Motor Function Test as outcome measure for research in patients after stroke. Stroke. 2001;32:1635–1639. doi: 10.1161/01.str.32.7.1635. [DOI] [PubMed] [Google Scholar]
- 14.Woodbury M, Velozo CA, Thompson PA, Light K, Uswatte G, Taub E, et al. Measurement structure of the Wolf Motor Function Test: implications for motor control theory. Neurorehabil Neural Repair. 2010;24:791–801. doi: 10.1177/1545968310370749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jansen A, Menke R, Sommer J, Forster AF, Bruchmann S, Hempleman J, et al. The assessment of hemispheric lateralization in functional MRI--robustness and reproducibility. Neuroimage. 2006;33:204–217. doi: 10.1016/j.neuroimage.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 16.Seghier ML, Ramlackhansingh A, Crinion J, Leff AP, Price CJ. Lesion identification using unified segmentation-normalisation models and fuzzy clustering. Neuroimage. 2008;41:1253–1266. doi: 10.1016/j.neuroimage.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D’Esposito M, Deouell LY, Gazzaley A. Alterations in the BOLD fMRI signal with ageing and disease: a challenge for neuroimaging. Nat Rev Neurosci. 2003;4:863–872. doi: 10.1038/nrn1246. [DOI] [PubMed] [Google Scholar]
- 18.Pomeroy VM, Clark CA, Miller JSG, Baron J-C, Markus HS, Tallis RC. The potential for utilizing the “mirror neurone system” to enhance recovery of the severely affected upper limb early after stroke: a review and hypothesis. Neurorehabil Neural Repair. 2005;19:4–13. doi: 10.1177/1545968304274351. [DOI] [PubMed] [Google Scholar]
- 19.Chen S, Lewthwaite R, Schweighofer N, Winstein CJ. Discriminant validity of a new measure of self-efficacy for reaching movements after stroke-induced hemiparesis. Journal of hand therapy : official journal of the American Society of Hand Therapists. 2013;26:116–123. doi: 10.1016/j.jht.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Fregni F, Pascual-Leone A. Hand motor recovery after stroke: tuning the orchestra to improve hand motor function. Cogn Behav Neurol. 2006;19:21–33. doi: 10.1097/00146965-200603000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Buccino G, Vogt S, Ritzl A, Fink GR, Zilles K, Freund H-J, et al. Neural circuits underlying imitation learning of hand actions: an event-related fMRI study. Neuron. 2004;42:323–334. doi: 10.1016/s0896-6273(04)00181-3. [DOI] [PubMed] [Google Scholar]
- 22.Vogt S, Buccino G, Wohlschlager AM, Canessa N, Shah NJ, Zilles K, et al. Prefrontal involvement in imitation learning of hand actions: Effects of practice and expertise. NeuroImage. 2007;37:1371–1383. doi: 10.1016/j.neuroimage.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Liew SL, Han S, Aziz-Zadeh L. Familiarity modulates mirror neuron and mentalizing regions during intention understanding. Hum Brain Mapp. 2011;32:1986–1997. doi: 10.1002/hbm.21164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, Rizzolatti G. Cortical mechanisms of human imitation. Science. 1999;286:2526–2528. doi: 10.1126/science.286.5449.2526. [DOI] [PubMed] [Google Scholar]
- 25.Mattar AA, Gribble PL. Motor learning by observing. Neuron. 2005;46:153–160. doi: 10.1016/j.neuron.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 26.Rossini PM, Calautti C, Pauri F, Baron JC. Post-stroke plastic reorganisation in the adult brain. Lancet Neurol. 2003;2:493–502. doi: 10.1016/s1474-4422(03)00485-x. [DOI] [PubMed] [Google Scholar]
- 27.Taub E, Uswatte G, Elbert T. New treatments in neurorehabilitation founded on basic research. Nat Rev Neurosci. 2002;3:228–236. doi: 10.1038/nrn754. [DOI] [PubMed] [Google Scholar]
- 28.Dimyan MA, Cohen LG. Neuroplasticity in the context of motor rehabilitation after stroke. Nat Rev Neurol. 2011;7:76–85. doi: 10.1038/nrneurol.2010.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stefan K, Classen J, Celnik P, Cohen LG. Concurrent action observation modulates practice-induced motor memory formation. Eur J Neurosci. 2008;27:730–738. doi: 10.1111/j.1460-9568.2008.06035.x. [DOI] [PubMed] [Google Scholar]
- 30.Celnik P, Webster B, Glasser DM, Cohen LG. Effects of action observation on physical training after stroke. Stroke. 2008;39:1814–1820. doi: 10.1161/STROKEAHA.107.508184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.