Abstract
Elevated triglycerides (TG) and low high-density lipoprotein cholesterol (HDL-C) are key metabolic abnormalities in insulin resistance (IR) states, including diabetes mellitus. The TG/HDL-C ratio has been advocated as a simple clinical indicator of IR, but studies have yielded inconsistent results. The total cholesterol/HDL-C ratio is widely used to assess lipid atherogenesis but its utility for assessing IR or its associated coronary heart disease (CHD) risk is unknown. We related the TG/HDL-C and total cholesterol/HDL-C ratios to IR (top quartile of the homeostasis model assessment of insulin resistance) in 3014 individuals (mean age 54 years; 55% women). Logistic regression was used to construct receiver-operating-characteristic curves for predicting IR, with lipid ratios as predictors. Multivariable Cox regression was used to evaluate if adjusting for lipid ratios attenuated the association of IR with CHD. Cross-sectionally, the age- and sex-adjusted correlations of IR were: 0.46 with TG/HDL-C, and 0.38 with total cholesterol/HDL-C. IR Prevalence increased across tertiles of lipid ratios (p<0.0001). The area under the receiver-operating-characteristic curves for predicting IR with TG/HDL-C ratio was 0.745, which was slightly higher than that for total cholesterol/HDL-C ratio (0.707; p<0.001 for comparison). On follow-up (mean 6.4 years), 112 individuals experienced initial CHD events. IR was associated with CHD risk (multivariable-adjusted hazards ratio 2.71, 95% CI 1.79–4.11), which remained significant even after adjustment for the lipid ratios. In conclusion, our observations suggest that the TG/HDL-C ratio is an imperfect surrogate for IR and its associated CHD risk, and it is only slightly better than the total cholesterol/HDL-C ratio for this purpose.
Keywords: insulin resistance, epidemiology, lipids, coronary risk
Introduction
We hypothesized that the TG/HDL-C ratio would predict IR cross-sectionally, and would attenuate any potential association of IR with coronary heart disease (CHD) risk (if the ratio reflected IR). We tested these hypotheses by evaluating the performance of several lipid measures (including the TG/HDL-C ratio and total cholesterol/HDL-C ratio) for predicting IR cross-sectionally, and evaluated the extent to which adjustment for proposed lipid measures of IR diminished the prognostic importance of IR longitudinally.
Methods
The Framingham Offspring Study was initiated in 1971, and the design and selection criteria have been described previously.11 Participants who attended the fifth examination cycle (1991 to 1995) were eligible for the present study (n=3799). At each quadrennial examination, Offspring cohort participants underwent a routine medical history, a physical examination that included blood pressure measurement and anthropometry, and blood sampling (after an overnight fast). We excluded 785 participants for the following reasons: age below 20 or above 74 (n=53), prevalent cardiovascular disease at baseline (n=359), lack of follow-up data (n=121), using insulin (n=28), or missing data on any lipid variable or insulin values (n=224). After these exclusions, 3014 individuals (mean age, 54 years; 55% women) were eligible, and constituted the study sample. The study protocol was approved by the Boston University Medical Center Institutional Review Board, and all participants provided written informed consent.
At the index examination, blood samples were obtained from attendees after an overnight fast (~10–12 hours). Phlebotomy was performed typically between 7:30 A.M. and 9:00 A.M. The fasting total cholesterol and triglyceride levels were measured enzymatically, and HDL-C determined after precipitation of low-density and VLDL with dextran sulfate-magnesium.12,13 Plasma glucose levels were measured using a hexokinase reagent kit.
Fasting insulin levels were measured in plasma as total immunoreactive insulin (Coat-A-Count Insulin, Diagnostic Products, Los Angeles, CA) and were standardized to serum levels for reporting purposes. The lower limit of sensitivity was 8.0 pmol/l (1.1 μU/mL) and the intra- and interassay coefficients of variation ranged from 5.0 to 10.0 %. Insulin resistance was assessed from fasting insulin and glucose levels, using the previously validated homeostasis model assessment.14 The homeostasis model assessment-IR = fasting glucose (mmol/L) × fasting insulin (μU/ml)/22.5. Insulin resistance (IR) was defined as a value of homeostasis model assessment -IR in the fourth quartile among individuals without diabetes in our sample.
The follow-up period for the present investigation was defined as from the baseline examination up to December 31, 2005. All Heart Study participants are under longitudinal surveillance for CHD occurrence, through periodic examinations at the Framingham Heart Study and through biennial health history updates between examinations. An endpoint adjudication committee consisting of three experienced investigators reviewed hospitalization and physician office visit records for all suspected CHD events. Incident CHD included recognized or unrecognized myocardial infarction, stable and unstable angina pectoris, or coronary heart disease death. Diagnosis criteria for these events have been described elsewhere.15
Two types of analyses were conducted: a cross-sectional evaluation relating different lipid measures to IR, and longitudinal analysis assessing if adjustment for lipid variables attenuated the CHD risk associated with IR. The following lipid variables or ratios were considered for analyses: TG, HDL-C, total cholesterol, TG/HDL-C and total cholesterol/HDL-C. We did not evaluate LDL-C because it was calculated (rather than measured) at the index examination and has not been reported to be a strong correlate of IR in the literature; nor did we measure small, dense LDL, which has been related to IR but is also strongly related to the presence of hypertriglyceridemia.16
The lipid measures evaluated in the present investigation included: total cholesterol, HDL-C, TG; and two lipid ratio measures, TG/HDL-C and total cholesterol/HDL-C. We did not evaluate apolipoproteins because they are not widely used as total cholesterol and HDL-C as indicators of IR. We evaluated the distribution of the lipid measures and the clinical covariates. Spearman correlation coefficients were estimated for the interrelations between the various lipid measurements, and with homeostasis model assessment insulin resistance.
Logistic regression was used to calculate age-adjusted prevalence across sex-specific tertiles of the lipid ratios. A corresponding p-value testing for a trend across the tertiles was also calculated. We constructed receiver-operating-characteristic curves (plots of sensitivity versus [1-specificity]), and calculated the areas under the curve. The area under the curve corresponds to the c statistic from the logistic regression models, and indicates the probability that the presence (or absence) of IR would be correctly identified based on the levels of the lipid measures in a randomly chosen subject pair (one with and one without IR). The area under the curve was used as an index of global test performance of lipid measures for identification of IR across the entire range of values, with an area under the curve of 0.5 indicating no discrimination ability. Conventionally, an area under the curve of 0.90 or more is considered excellent, values between 0.80–0.90 regarded as good, between 0.70 and 0.80 indicative of fair test performance, and values between 0.70–0.50 viewed as poor.
We used multivariable Cox proportional hazards regression to investigate the relations of the various lipid measures to CHD incidence, adjusting for age, sex, systolic blood pressure, anti-hypertensive treatment, diabetes, and current smoking (all defined at the baseline examination). Since the distribution of lipid variables varies by sex, we used sex-standardized lipid variables in analyses. This sex-standardization was accomplished by subtracting from each value the sex-specific mean and dividing by sex-specific standard deviation.
Cigarette smoking was defined by self-reported cigarette use within the year preceding the baseline examination. Diabetes was defined as a fasting blood glucose ≥ 126 mg/dL or use of insulin or oral hypoglycemic agents. The assumption of proportionality of hazards was confirmed by examining interactions of time-dependent covariates and survival time in Cox models. We estimated hazards ratios (HR) and their 95% confidence intervals (CI) for a standard deviation increment of each lipid measure (thereby facilitating comparisons of effect sizes for individual measures) and homeostasis model assessment-IR (log-transformed to normalize a skewed distribution), and for presence (versus absence) of IR.
We constructed 2 types of models hierarchically: A) Models adjusting for age, sex, systolic blood pressure, anti-hypertensive treatment, diabetes, current smoking and each individual lipid variable, or log homeostasis model assessment-IR, or IR. B) Models as above but simultaneously incorporating IR, to assess if adjustment for lipids attenuated the association of IR with CHD risk. We compared the performance of models incorporating individual lipid ratios with those incorporating IR using the model discrimination and calibration characteristics. Discrimination is the ability of a prediction model to separate those who experience a CHD event from those who do not. The C-statistic from a Cox models is conceptually analogous to the area under a receiver operating characteristic curve estimated for logistic models. Calibration evaluates the degree of correspondence between the predicted probability of CHD based on a model and the observed CHD incidence, and is typically evaluated with the Hosmer-Lemeshow χ2-statistic. Small values indicate a good calibration, while values exceeding 20 indicate significant lack of calibration (P<0.01). All analyses were performed with SAS version 9.1 (SAS Institute Inc, Cary, NC), and a two-tailed p value of <0.05 indicated statistical significance.
Results
The baseline characteristics of our study sample (overall and according to presence versus absence of IR) are displayed in Table 1. Participants with IR were older, more likely to be men, weigh more, with higher levels of blood pressure and a greater prevalence of hypertension. TG levels were higher and HDL-C levels lower in those with IR, resulting in higher TG/HDL-C ratios. The Total cholesterol/HDL-C ratio was also higher in individuals with IR.
Table 1.
Baseline Characteristics of Study Sample
| Characteristic | Whole Sample (N=3014) | Insulin Resistance | |
|---|---|---|---|
| Present (N=850) | Absent (N=2164) | ||
| Age, years | 54±10 | 56±9 | 53±10 |
| Women | 55 % | 42 % | 60 % |
| Systolic Blood Pressure (mm Hg) | 125±18 | 134±18 | 122±17 |
| Diastolic Blood pressure (mm Hg) | 75±10 | 79±10 | 73±10 |
| Hypertension | 31 % | 52 % | 22 % |
| Anti-hypertensive treatment | 16 % | 30 % | 11 % |
| Body mass index (kg/m2) | 27.3±4.9 | 30.8±5.4 | 25.9±4.0 |
| Current smokers | 19 % | 17 % | 20 % |
| Diabetes | 6 % | 17 % | 2 % |
| Total cholesterol (mg/dl | 204±36 | 208±36 | 202±36 |
| - men | 201±35 | 204±36 | 200±34 |
| - women | 206±37 | 213±36 | 204±37 |
| Low Density Lipoprotein Cholesterol (mg/dl) | 126±33 | 130±33 | 125±33 |
| - men | 129±31 | 129±33 | 129±31 |
| - women | 124±34 | 130±32 | 122±34 |
| High Density Lipoprotein Cholesterol (mg/dl) | 51±15 | 43±12 | 54±15 |
| - men | 44±11 | 39±9 | 46±11 |
| - women | 57±15 | 48±13 | 59±15 |
| Triglycerides, mg/dl (mg/dl) | 133±70 | 175±78 | 117±58 |
| - men | 142±75 | 177±81 | 122±63 |
| - women | 126±64 | 172±75 | 113±55 |
| Total cholesterol/High Density cholesterol (mg/dl) | 4.3±1.4 | 5.1±1.4 | 4.0±1.3 |
| - men | 4.9±1.4 | 5.4±1.4 | 4.6±1.3 |
| - women | 3.9±1.3 | 4.7±1.3 | 3.7±1.1 |
| Triglyceride/High Density Cholesterol (mg/dl) | 3.1±2.3 | 4.6±2.9 | 2.5±1.8 |
| - Men | 3.7±2.6 | 5.0±3.0 | 3.0±2.1 |
| - Women | 2.5±1.9 | 4.0±2.5 | 2.1±1.4 |
| Insulin Resistance* | 7.6±5.6 | 12.5±8.5 | 5.6±1.2 |
| - men | 8.1±4.8 | 12.2±6.1 | 5.8±1.1 |
| - women | 7.1±6.1 | 12.9±11.0 | 5.5±1.2 |
Values are mean ± standard deviation, unless otherwise noted. IR (insulin resistance) defined as *Top quartile of homeostasis model assessment-IR without diabetes.
All comparisons for participants with and without IR are statistically significant at P< 0.001, with the exception in men of total cholesterol (P-value of 0.04) and LDL cholesterol (P=0.89). Overall, there was also no significant different in % current smoking (p=0.07) and in men or women in % current smoking (p=0.24 and p=0.15, respectively).
The age and sex-adjusted correlations among the lipid variables are shown in Table 2. As expected, there is a strong reciprocal relationship between HDL-C and TG (r=−0.53) and each of these lipids was strongly related to the TG/HDL-C ratio. The total cholesterol was moderately correlated with the TG (r= 0.34) and with the TG/HDL-C ratio (0.23). The total cholesterol/HDL-C ratio was also highly correlated with the TG/HDL-C ratio (r= 0.81).
Table 2.
Age- and sex-adjusted Spearman Correlation Coefficients among Lipid Parameters
| TG | HDL-C | Total-C | LDL-C | TG/HDL-C | Total-C/HDL-C | HOMA-IR | |
|---|---|---|---|---|---|---|---|
| TG | 1.0 | −0.53 | 0.34 | 0.20 | 0.94 | 0.66 | 0.42 |
| HDL-C | -- | 1.0 | 0.05* | −0.12 | −0.78 | −0.82 | −0.37 |
| Total-C | -- | -- | 1.0 | 0.91 | 0.23 | 0.49 | 0.11 |
| LDL-C | -- | -- | -- | 1.0 | 0.20 | 0.61 | 0.10 |
| TG/HDL-C | -- | -- | -- | -- | 1.0 | 0.81 | 0.46 |
| Total-C/HDL-C | -- | -- | -- | -- | -- | 1.0 | 0.38 |
| HOMA-IR | -- | -- | -- | -- | -- | -- | 1.0 |
P-value 0.003. All other correlations P<0.0001.
In analyses evaluating correlations with IR, a highly significant and fairly strong relation of both the TG/HDL-C ratio (r=0.46) and TG (r=0.42) to IR was observed. HDL-C was inversely correlated with IR (r= −0.37), whereas TG was positively related (r=0.42). As anticipated by its correlation with TG/HDL-C, there was also a correlation of the total cholesterol/HDL-C ratio with IR (r=0.38).
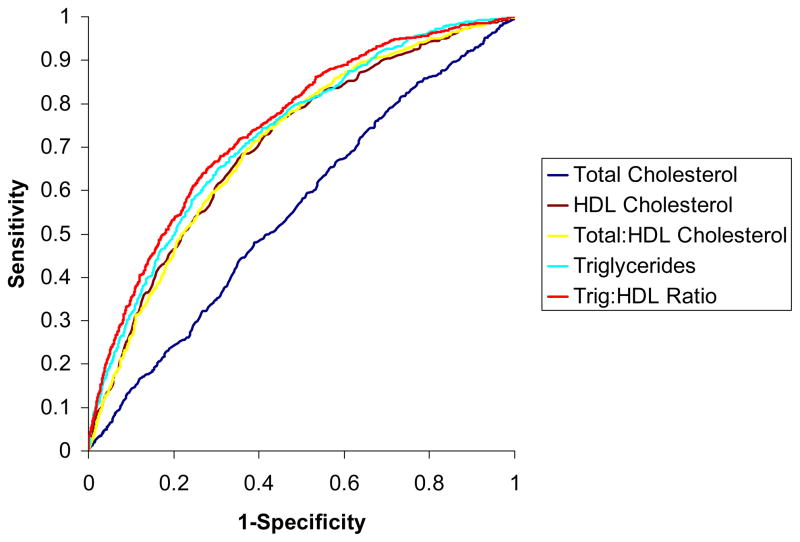
Figure 1 displays the receiver-operating-character curves for predicting IR with individual lipid measures and the two lipid ratios. The area under the curve for predicting IR was highest for the TG/HDL-C ratio, 0.745 (95% CI 0.726–0.764), being higher than the corresponding value for TG alone (0.727, 95% CI 0.708–0.747, p<0.0001 for comparison). The area under the curve for total cholesterol/HDL-C ratio was 0.707 (95% CI 0.687–0.727), and this was lower than that for the TG/HDL-C ratio noted above (p<0.0001 for comparison). The area under the curve for HDL-C was similar to that of total cholesterol/HDL-C ratio (0.705, 95% CI 0.684–0.725).
Figure 1.
ROC curves for predicting IR from lipid variables (red: TG/HDL-C ratio; yellow: TG; light blue: total cholesterol/HDL-C ratio; brown: HDL-C; navy blue: total cholesterol).
On follow-up (mean 6.4 years), 112 individuals experienced a first CHD event. Table 3A displays the results of analyses evaluating the relations of individual lipid measures and IR to CHD risk. The lipid measures (except for total cholesterol) and both log homeostasis model assessment-IR and IR were associated with CHD risk. The models incorporating the different variables were all comparable in terms of discrimination. All calibration statistics were <20, indicating good calibration. Of note, IR was associated with a marked increase in CHD risk (adjusted-hazards ratio 2.71, 95% CI 1.79–4.11).
Table 3.
Association of Insulin Resistance and Lipid Measures with Incidence of a first CHD event. 7 year follow-up, 5 year prediction, 112 CHD events
| Lipid | Hazards ratio per SD (95% CI) | P | Discrimination Overall C (95% CI) | Calibration (χ2) |
|---|---|---|---|---|
| A. Models with Individual variables | ||||
| IR (vs. No IR) | 2.71 (1.79–4.11) | <0.0001 | 0.80 (0.76–0.85) | 10.8 |
| Log HOMA-IR | 2.32 (1.55–3.48) | <0.0001 | 0.80 (0.75–0.84) | 19.8 |
| Total cholesterol | 1.18 (0.98–1.42) | 0.08 | 0.80 (0.76–0.84) | 9.8 |
| HDL-C cholesterol | 0.64 (0.51–0.80) | 0.0001 | 0.80 (0.76–0.85) | 6.6 |
| TG | 1.42 (1.21–1.67) | <0.0001 | 0.80 (0.76–0.84) | 8.2 |
| Total cholesterol/HDL-C | 1.42 (1.21–1.68) | <0.0001 | 0.80 (0.76–0.85) | 13.4 |
| TG/HDL-C | 1.39 (1.20–1.60) | <0.0001 | 0.80 (0.76–0.84) | 10.1 |
| B. Models with Individual lipid variables/ratios & IR | ||||
| IR (vs. No IR) | 2.68 (1.77–4.06) | <0.0001 | 0.81 (0.76–0.85) | 13.9 |
| Total cholesterol | 1.16 (0.97–1.40) | 0.11 | ||
| IR (vs. No IR) | 2.25 (1.46–3.47) | 0.0002 | 0.81 (0.77–0.85) | 17.7 |
| HDL-C | 0.72 (0.57–0.91) | 0.01 | ||
| IR (vs. No IR) | 2.28 (1.48–3.51) | 0.0002 | 0.81 (0.76–0.85) | 11.0 |
| TG | 1.30 (1.10–1.53) | 0.002 | ||
| IR (vs. No IR) | 2.32 (1.51–3.55) | 0.0001 | 0.81 (0.77–0.85) | 7.0 |
| Total cholesterol/HDL-C | 1.32 (1.11–1.57) | 0.002 | ||
| IR (vs. No IR) | 2.26 (1.46–3.49) | 0.0003 | 0.81 (0.76–0.85) | 9.9 |
| TG/HDL-C cholesterol | 1.26 (1.09–1.47) | 0.003 | ||
Models adjusted for the following covariates: age, sex, systolic blood pressure, anti-hypertensive treatment, d
Table 3B displays the results of analyses incorporating IR along with the individual lipid measures. In these models, IR and the individual lipid variables remained associated with CHD risk. Although the point estimate of the hazards ratio for IR decreased slightly upon adjustment for lipid variables, IR was associated with an approximate 2.7-fold risk of CHD for total cholesterol and a 2.3-fold risk of CHD for the other lipid variables (relative to those without IR) in each of the models. The models were also comparable in terms of their discrimination. All calibration statistics were <20, indicating good calibration. The impact of IR on CHD risk was only moderately reduced when adjusted for TG/HDL-C (16.6%) or total cholesterol/HDL-C (14.4%). Conversely, the impact of the TG/HDL ratio on CHD risk was reduced only 10.7% on adjustment for IR, and the impact of total cholesterol/HDL-C on CHD risk was reduced by a mere 7% on adjustment for IR (Table 3).
Discussion
Our principal findings are 3-fold. First, cross-sectional analyses suggested that, of the several candidate lipid markers evaluated, the TG/HDL-C ratio was the best correlate of IR. However, the performance of this ratio for predicting IR was only fair, as reflected by the area under the curve of 0.745. Second, longitudinal analyses demonstrated that even after adjustment for lipid variables (including the TG/HDL-C ratio), IR remained significantly and strongly associated with CHD risk. These prospective analyses complement our cross-sectional observations and suggest that lipid variables (including the TG/HDL-C ratio) are imperfect surrogates of IR. Third, the total cholesterol/HDL-C ratio was almost as powerful a predictor of CHD risk as the TG/HDL-C ratio. Since the risk factors adjusted for in multivariable analysis include both diabetes and IR, the association of TG/HDL-C on CHD risk does not appear to be predominantly attributable to IR.
We noted a modest correlation between the total cholesterol/HDL-C ratio and IR, confirming observations reported by other investigators.3,7–9 However, Reaven et al reported a higher correlation of the TG/HDL-C ratio with IR (0.60) compared to the present study.3 The area under the curve for predicting IR with the TG/HDL-C ratio was also slightly higher in that report,3 although the 95% CI overlapped with that observed in our investigation. Our investigation, therefore, extends prior observations made on samples of more modest size3,7–9 to a large community-based sample with a wide range of HDL-C and TG values.
The association of IR with CHD risk in our study complements prior reports that high values of plasma insulin alone, a surrogate marker for IR, appear to identify a subset of persons at particularly high risk for an atherosclerotic cardiovascular event.1,17,18 In a previous Framingham Study report, measures of IR were examined in relation to cardiovascular disease incidence. IR (as defined by homeostasis model assessment and the Gutt insulin sensitivity index) was found to be independently related to incident CVD, imposing a similar 2-fold age-and sex-adjusted hazard ratio.19
The association of the TG/HDL-C ratio with CHD risk in our investigation confirms prior reports that have noted that the combination of a high TG and low HDL-C (referred to as atherogenic dyslipidemia20–22) is a powerful risk factor for CHD risk.23,24 There is evidence from one population study that a high TG/HDL-C ratio might better predict CHD in men than conventional risk factors such as hypertension, smoking and physical activity.23 However, our investigation demonstrates that with IR a high TG/HDL-C ratio is as strong a lipid predictor of CHD as the widely used total cholesterol/HDL-C ratio. Evaluation of effect sizes (standardized hazards ratios), model discrimination and calibration demonstrate similar performance of these two lipid ratios for CHD prediction in the presence of IR.
The large community-based sample and evaluation of both cross-sectional and longitudinal analyses strengthen the present investigation. The amount of data was not large enough for sex-specific evaluation of the lipid-IR relations. The use of homeostasis model assessment-IR for assessment of insulin resistance may be viewed a limitation of the present investigation. The hyperinsulinemic euglycemic clamp is the most accurate method of examining IR. However, estimates of IR using homeostasis model assessment-IR correlate well with results of clamp studies,14 and are more convenient and economical to perform in an epidemiological setting. Our sample consists predominantly of middle-aged whites of European descent, limiting the generalizability of our findings to other age groups and ethnicities. As noted previously, the TG/HDL-C ratio may perform poorly for identifying IR in African-Americans.10
Acknowledgments
This work was supported through National Institutes of Health/National Heart, Lung, and Blood Institute Contract N01-HC-25195 and 2K24 HL04334 (Dr. Vasan). Dr. Kannel is supported by the Visiting Scientist Program which is supported by Astra Zeneca.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Despres JP, Lamarche B, Mauriege P, Cantin B, Dagenais GR, Moorjani S, Lupien PJ. Hyperinsulinemia as an Independent Risk Factor for Ischemic Heart Disease. N Engl J Med. 1996;334:952–958. doi: 10.1056/NEJM199604113341504. [DOI] [PubMed] [Google Scholar]
- 2.Haffner SM, Mykkanen L, Festa A, Burke JP, Stern MP. Insulin-resistant prediabetic subjects have more atherogenic risk factors than insulin-sensitive prediabetic subjects: implications for preventing coronary heart disease during the prediabetic state. Circulation. 2000;101:975–980. doi: 10.1161/01.cir.101.9.975. [DOI] [PubMed] [Google Scholar]
- 3.McLaughlin T, Abbasi F, Cheal K, Chu J, Lamendola C, Reaven G. Use of Metabolic Markers To Identify Overweight Individuals Who Are Insulin Resistant. Ann Intern Med. 2003;139:802–809. doi: 10.7326/0003-4819-139-10-200311180-00007. [DOI] [PubMed] [Google Scholar]
- 4.Borggreve SE, de Vries R, Dullaart RPF. Alterations in high-density lipoprotein metabolism and reverse cholesterol transport in insulin resistance and type 2 diabetes mellitus: role of lipolytic enzymes, lecithin:cholesterol acyltransferase and lipid transfer proteins. European Journal of Clinical Investigation. 2003;33:1051–1069. doi: 10.1111/j.1365-2362.2003.01263.x. [DOI] [PubMed] [Google Scholar]
- 5.Olefsky JM, Farquhar JW, Reaven GM. Reappraisal of the role of insulin in hypertriglyceridemia. Am J Med. 1974;57:551–560. doi: 10.1016/0002-9343(74)90006-0. [DOI] [PubMed] [Google Scholar]
- 6.Vinik AI. The metabolic basis of atherogenic dyslipidemia. Clinical Cornerstone. 2005;7:27–35. doi: 10.1016/s1098-3597(05)80065-1. [DOI] [PubMed] [Google Scholar]
- 7.McLaughlin T, Reaven G, Abbasi F, Lamendola C, Saad M, Waters D, Simon J, Krauss RM. Is There a Simple Way to Identify Insulin-Resistant Individuals at Increased Risk of Cardiovascular Disease? The Am J Cardiol. 2005;96:399–404. doi: 10.1016/j.amjcard.2005.03.085. [DOI] [PubMed] [Google Scholar]
- 8.Bovet P, Faeh D, Gabriel A, Tappy L. The Prediction of Insulin Resistance With Serum Triglyceride and High-Density Lipoprotein Cholesterol Levels in an East African Population. Arch Intern Med. 2006;166:1236–123a. doi: 10.1001/archinte.166.11.1236-b. [DOI] [PubMed] [Google Scholar]
- 9.Brehm A, Pfeiler G, Pacini G, Vierhapper H, Roden M. Relationship between Serum Lipoprotein Ratios and Insulin Resistance in Obesity. Clin Chem. 2004;50:2316–2322. doi: 10.1373/clinchem.2004.037556. [DOI] [PubMed] [Google Scholar]
- 10.Sumner AE, Finley KB, Genovese DJ, Criqui MH, Boston RC. Fasting Triglyceride and the Triglyceride-HDL Cholesterol Ratio Are Not Markers of Insulin Resistance in African Americans. Arch Intern Med. 2005;165:1395–1400. doi: 10.1001/archinte.165.12.1395. [DOI] [PubMed] [Google Scholar]
- 11.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 12.McNamara JR, Schaefer EJ. Automated enzymatic standardized lipid analyses for plasma and lipoprotein fractions. Clin Chim Acta. 1987;166:1–8. doi: 10.1016/0009-8981(87)90188-4. [DOI] [PubMed] [Google Scholar]
- 13.Warnick GR, Benderson J, Albers JJ. Dextran sulfate-Mg2+ precipitation procedure for quantitation of high- density-lipoprotein cholesterol. Clin Chem. 1982;28:1379–1388. [PubMed] [Google Scholar]
- 14.Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, Monauni T, Muggeo M. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23:57–63. doi: 10.2337/diacare.23.1.57. [DOI] [PubMed] [Google Scholar]
- 15.Kannel WB, Wolf PA, Garrison RJ, editors. Framingham Heart Study, 30 year follow-up. Bethesda, MD: 2001. Section 34: Some risk factors related to the annual incidence of cardiovascular disease and death in pooled repeated biennial measurements. [Google Scholar]
- 16.McNamara JR, Campos H, Ordovas JM, Peterson J, Wilson PW, Schaefer EJ. Effect of gender, age, and lipid status on low density lipoprotein subfraction distribution. Results from the Framingham Offspring Study. Arteriosclerosis. 1987;7:483–490. doi: 10.1161/01.atv.7.5.483. [DOI] [PubMed] [Google Scholar]
- 17.Lempiainen P, Mykkanen L, Pyorala K, Laakso M, Kuusisto J. Insulin Resistance Syndrome Predicts Coronary Heart Disease Events in Elderly Nondiabetic Men. Circulation. 1999;100:123–128. doi: 10.1161/01.cir.100.2.123. [DOI] [PubMed] [Google Scholar]
- 18.Zavaroni I, Bonora E, Pagliara M, Dall’Aglio E, Luchetti L, Buonanno G, Bonati PA, Bergonzani M, Gnudi L, Passeri M. Risk factors for coronary artery disease in healthy persons with hyperinsulinemia and normal glucose tolerance. N Engl J Med. 1989;320:702–706. doi: 10.1056/NEJM198903163201105. [DOI] [PubMed] [Google Scholar]
- 19.Rutter MK, Meigs JB, Sullivan LM, D’Agostino RB, Sr, Wilson PW. Insulin Resistance, the Metabolic Syndrome, and Incident Cardiovascular Events in the Framingham Offspring Study. Diabetes. 2005;54:3252–3257. doi: 10.2337/diabetes.54.11.3252. [DOI] [PubMed] [Google Scholar]
- 20.Dobiasova M, Frohlich J. The plasma parameter log (TG/HDL-C) as an atherogenic index: correlation with lipoprotein particle size and esterification rate inapob-lipoprotein-depleted plasma (FERHDL) Clin Biochem. 2001;34:583–588. doi: 10.1016/s0009-9120(01)00263-6. [DOI] [PubMed] [Google Scholar]
- 21.Dobiasova M. Atherogenic Index of Plasma [Log(Triglycerides/HDL-Cholesterol)]: Theoretical and Practical Implications. Clin Chem. 2004;50:1113–1115. doi: 10.1373/clinchem.2004.033175. [DOI] [PubMed] [Google Scholar]
- 22.Grundy SM. Atherogenic dyslipidemia associated with metabolic syndrome and insulin resistance. Clin Cornerstone. 2006;8 (Suppl 1):S21–S27. doi: 10.1016/s1098-3597(06)80005-0. [DOI] [PubMed] [Google Scholar]
- 23.Gaziano JM, Hennekens CH, O’Donnell CJ, Breslow JL, Buring JE. Fasting Triglycerides, High-Density Lipoprotein, and Risk of Myocardial Infarction. Circulation. 1997;96:2520–2525. doi: 10.1161/01.cir.96.8.2520. [DOI] [PubMed] [Google Scholar]
- 24.Jeppesen J, Hein HO, Suadicani P, Gyntelberg F. Low Triglycerides-High High-Density Lipoprotein Cholesterol and Risk of Ischemic Heart Disease. Arch Intern Med. 2001;161:361–366. doi: 10.1001/archinte.161.3.361. [DOI] [PubMed] [Google Scholar]