Abstract
Migration of both uninfected and infected monocytes into the brain during acute HIV infection likely initiates metabolic changes that can be observed with magnetic resonance spectroscopy (MRS). Herein, we measured changes in brain metabolism during the first year of HIV infection and examined the relationship of these metabolite levels to CD16+ monocyte populations measured in the blood. MRS was performed on nine HIV+ subjects identified during acute HIV infection and nine seronegative control subjects. HIV+ subjects were examined within 90 days of an indeterminate Western blot, then again 2 and 6 months later, during early infection. Blood samples were collected for plasma viral RNA and monocyte subset quantification. HIV+ subjects were identified with acute viral ailment and did not display severe cognitive deficits such as dementia or minor cognitive motor disorder. Changes in lipid membrane metabolism (choline levels) in the frontal cortex and white matter were observed during the initial year of HIV infection. Greater numbers of CD16+ monocytes were associated with lower N-acetylaspartate levels and higher choline levels in the brain. These results suggest that HIV infection induces metabolic changes in the brain early during infection and that these changes may be related to monocyte dynamics in the periphery.
Keywords: HIV, CD14highCD16low, CD14lowCD16high, Choline, NAA, MRS
Introduction
Few magnetic resonance (MR) studies have examined the central nervous system (CNS) during the first year of infection (Ances et al. 2009; Lentz et al. 2009), even though crucial immunologic events predictive of end-stage disease are known to transpire during this time (Mellors et al. 1995; Schacker et al. 1998). Acute HIV infection is the time period immediately after infection when impairment and destruction of CD4+ T lymphocytes occur and viral levels are uncontrolled due to the host's insufficient immune response (Rosenberg et al. 2000). These events are similar to those that manifest during chronic infection when HIV-associated dementia is more likely to occur. Early infection is considered to be the initial year of infection after seroconversion occurs. Viral reservoirs, including the CNS, are thought to be established during these initial stages of infection. These reservoirs may require decades to clear with successful antiretroviral use (Finzi et al. 1999; Perelson et al. 1997), underscoring the importance of studies that examine early neuronal and glial injury.
Current theories of HIV neuropathogenesis focus on the activation of monocyte populations (CD16+) and monocyte-derived macrophages, being one of the initial cell types infected, that can traffic into the brain resulting in a cascade of neurotoxic events (Kaul and Lipton 2006). Monocyte populations expand in the blood at many points during the course of the disease and have been found in the brain after 2 weeks of infection with simian immunodeficiency virus (SIV) (Clay et al. 2007; Kim et al. 2009). Previous studies in SIV-infected rhesus macaques indicate that dynamic changes in metabolism occur during the initial months of infection and are related to changes in both plasma viral RNA and monocyte subsets (Greco et al. 2004; Ratai et al. 2009; Williams et al. 2005). Recently, we reported metabolic changes in the brain that were consistent with neuronal dysfunction, using MR spectroscopy (MRS) in HIV subjects identified with acute viral ailment in the absence of severe neurologic disease (Lentz et al. 2009). The following longitudinal study was undertaken in an effort to examine these subjects over the first year of HIV infection and to determine if metabolite changes were related to perturbations in monocyte populations.
Results
Immunologic changes during early infection
At study entry, plasma HIV RNA levels were detectable (mean ± SD, 4.98±0.96 log copies/mL; Table 1) and declined significantly as a function of time over the 6 months of this longitudinal study (RM-ANOVA, P=0.005). CD4+ and CD8+ T cell counts of subjects during early HIV infection did not change in a similar fashion over time within this HIV+ cohort (RM-ANOVA, P=0.24, P=0.19, respectively). However, therapy use at each study time point clearly had significant effects on viral RNA and trends towards significant effects on CD4+ T cell counts, but not CD8+ T cell counts (Table 2).
Table 1. Demographic and therapy details of HIV+ subjects enrolled.
| Acute/early subject ID | Sex | Age (years) | At study entry (baseline) | Days of therapy used during study | Therapies used during study | Days of therapy used during study | |||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| HIV RNA (copies/mL) | CD3+CD4+ (cells/mL) | CD3+CD8+ (cells/mL) | Days on therapy | ||||||
| 1 | M | 38 | 179,000 | 277 | 1,138 | 6 | 173 | Combivir | 173 |
| Kaletra | 173 | ||||||||
| 2 | M | 46 | 47,900 | 692 | 1,116 | 0 | 195 | Sustiva | 195 |
| Epivir | 179 | ||||||||
| Viread | 179 | ||||||||
| Truvada | 16 | ||||||||
| 3 | M | 38 | 437 | 587 | 611 | 23 | 168 | Trizivir | 168 |
| Sustiva | 168 | ||||||||
| 4 | M | 32 | 71,300 | 237 | 1,069 | 0 | 0 | None | – |
| 5 | M | 43 | 200,000 | 360 | 2,918 | 0 | 0 | None | – |
| 6 | M | 36 | 160,000 | 313 | 1,394 | 0 | 0 | None | – |
| 7 | M | 51 | >1,000,000 | 582 | 1,278 | 0 | 73 | Atripla | 73 |
| 8 | M | 32 | 277,000 | 336 | 815 | 0 | 72 | Atripla | 72 |
| 9 | M | 22 | 297,000 | 512 | 2,358 | 0 | 54 | Atripla | 54 |
Table 2. Effects of ART use on viral RNA and T cell populations.
| ART use | Viral RNA (log copies/mL) | CD3+CD4+ T cell (cells/mL) | CD3+CD8+ T cell (cells/mL) |
|---|---|---|---|
| At baseline | |||
| Yes (N=2) | 3.95±1.85 | 432±219 | 875±373 |
| No (N=7) | 5.28±0.43 | 433±165 | 1,564±773 |
| P value | <0.08 | 0.99 | 0.28 |
| At 2 months | |||
| Yes (N=4) | 2.26±0.99 | 715±218 | 671±146 |
| No (N=5) | 5.29±0.35 | 431±147 | 1,506±1,291 |
| P value | 0.0003 | 0.05 | 0.24 |
| At 6 months | |||
| Yes (N=5) | 2.05±0.48 | 734±354 | 879±332 |
| No (N=3) | 4.85±0.38 | 269±56 | 897±512 |
| P value | 0.0001 | 0.07 | 0.95 |
Values presented as mean ± SD
Baseline MRS measures of acute HIV and control subjects
Previously, we reported neuronal dysfunction (i.e., reductions in N-acetylaspartate and glutamate–glutamine concentrations) in the frontal cortex of eight of the nine acute/early HIV subjects reported herein (Lentz et al. 2009). Further analysis between the nine controls and the nine HIV+ subjects at baseline indicates that HIV+ subjects had lower choline (Cho) concentrations (−14%, P=0.04) and glutamate–glutamine concentrations (Glx, −11%, P=0.01) in the frontal cortex. The ratio of Cho to creatine (Cho/Cr) also showed trends toward a similar reduction (−9%, P=0.07). Metabolite levels in the white matter were similar between control and HIV+ subjects. In the basal ganglia, acute/early HIV infection appeared to induce changes in glial metabolism. Compared to seronegative control levels, elevated myo-inositol (MI) concentrations (+26%, P=0.05) and MI/Cr (+26%, P=0.04) were observed in the basal ganglia of HIV+ subjects, with trends towards lower Glx concentrations (−9%, P=0.09). Additional testing indicated that MI and MI/Cr in the basal ganglia were affected by age (MI, P<0.01; MI/Cr, P=0.01). Further analysis did not uncover age-associated differences for any other metabolites.
Changes in brain metabolism during the first year of infection
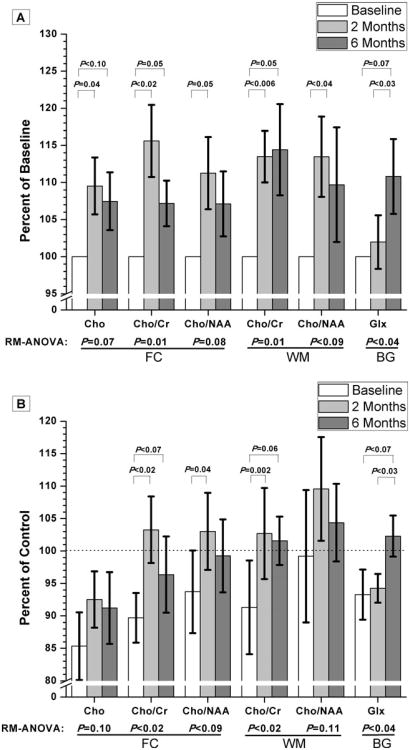
Subjects identified with acute HIV infection underwent imaging at study entry, as well as two and 6 months later (Fig. 1). Significant changes in metabolite concentrations and ratios analyzed as percent change over time from baseline values are shown in Fig. 2a. Increases in frontal gray matter Cho and Cho/Cr were observed after 2 months (+10% and +16%, respectively). After 6 months, Cho and Cho/Cr levels remained above baseline levels (+7% for both). In addition, an 11% increase in Cho/N-acetylaspartate (NAA) from baseline was seen in this brain region after 2 months. Within the white matter, Cho/Cr levels were elevated from baseline at both 2 and 6 months (+13% and +14%, respectively). Moreover, a 13% increase in Cho/NAA from baseline was observed in the white matter after 2 months. In the basal ganglia, Glx levels after 6 months were increased from that of baseline levels and as well as from 2-month levels (+11% and +9%, respectively). There was no change in basal ganglia Glx concentrations between 2 months and baseline. No other metabolites in these regions (NAA, MI, or creatine) were significantly changing over time. No significant therapy effects were found to contribute to these changes, but larger sampling is most likely necessary to verify this since previous MRS studies have demonstrated changes in brain metabolism due to antiretroviral therapy use (Chang et al. 1999b).
Fig. 1.
a Representative T2-weighted images of a HIV+ subject indicate the voxel positions in the frontal cortex, white matter (top left), and basal ganglia (top right) examined in both HIV+ and control subjects. b Representative single voxel proton MR spectra from the frontal cortex region of a HIV+ subject at study entry (left spectrum) and 6 months later (right spectrum) during early HIV infection. Six months spectrum was normalized to baseline creatine levels (3.044 ppm). Significant elevations in choline can be seen in this subject after 6 months
Fig. 2.
a Changes in brain metabolism which occur during the first year of infection are shown. Examined brain regions included gray matter in the frontal cortex (FC), white matter (WM), and basal ganglia (BG). All measures taken at later time points have been normalized using each HIV+ subject's baseline values. This analysis provides clarity with respect to percent changes in metabolite levels over time. Error bars represent SEM. b Additionally, all MRS values for subjects with early HIV infection have been normalized to control values (dashed line) so that meaningful comparisons between disease and control settings could be made. Error bars represent SEM
To better understand the nature of these elevations in metabolites over time, an additional analysis was performed, as shown in Fig. 2b, in which the data was normalized to mean metabolite values from the healthy controls. As stated previously, baseline levels of choline-related ratios in both the frontal cortex and white matter are shown to be lower than that of control levels. Within 2 months, choline-related metabolites in the frontal cortex and white matter appear to improve and are indistinguishable from control levels. Comparisons to control data also suggest that, after 6 months, Glx concentrations in the BG may be increasing back towards control levels.
Correlations between MRS metabolites and monocyte populations
Unfortunately, not all samples of peripheral blood mononuclear cell populations from each HIV+ subject at each time point were available (Table 3). A total of 16 samples from the nine HIV+ subjects enrolled over the study duration (six at baseline, six at 2 months, and four at 6 months) were obtained in order to examine the relationship between monocyte populations and measured brain metabolites (Table 4). The total number of monocytes was negatively correlated with Cr and NAA levels in the frontal cortex. Classical monocytes (CD14highCD16-) were positively correlated with MI and MI/Cr levels in the basal ganglia. CD14highCD16low monocytes positively correlated with all choline-related metabolites in the frontal cortex, as well as Glx/Cr in the basal ganglia. This phenotype was also negatively correlated with NAA in the basal ganglia, suggesting that higher levels of these monocytes are related to decreased neuroaxonal metabolism and function. The number of CD14lowCD16high monocytes was negatively correlated with NAA levels, yet positively correlated with Cho/Cr and Cho/NAA in the frontal cortex. This monocyte population was also positively correlated with all choline-related metabolites in the white matter and basal ganglia, and with Glx levels in the basal ganglia.
Table 3. Monocyte subpopulations examined during the initial year of HIV infection.
| Acute/early subject ID | Total monocyte population (cells/μL blood) | CD14highCD16-(cells/μL blood) | CD14highCD16low (cells/μL blood) | CD14lowCD16high (cells/μL blood) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||
| Baseline | 2 months | 6 months | Baseline | 2 months | 6 months | Baseline | 2 months | 6 months | Baseline | 2 months | 6 months | |
| 1 | 195 | 260 | 310 | – | 192 | 246 | – | 31 | 8 | – | 8 | 9 |
| 2 | 680 | 380 | 470 | 516 | 275 | 354 | 29 | 18 | 14 | 82 | 46 | 44 |
| 3 | 310 | 370 | 190 | 247 | 298 | 151 | 9 | 14 | 7 | 22 | 11 | 8 |
| 4 | 310 | 320 | 280 | 164 | 182 | 157 | 64 | 46 | 16 | 33 | 36 | 39 |
| 5 | 1,390 | 70 | 410 | 125 | 43 | – | 170 | 10 | – | 36 | 8 | – |
| 6 | 340 | 340 | 320 | 216 | 210 | – | 8 | 15 | – | 42 | 62 | – |
| 7 | 610 | 450 | 370 | 457 | – | – | 10 | – | – | 16 | – | – |
| 8 | – | 270 | 220 | – | – | – | – | – | – | – | – | – |
| 9 | 560 | 400 | – | – | – | – | – | – | – | – | – | – |
| Average | 549 | 318 | 321 | 438 | 200 | 227 | 48 | 22 | 11 | 39 | 29 | 25 |
En dashes indicate data unavailable for this time point
Table 4. Associations between brain metabolites and absolute numbers of monocyte populationsa.
| Total monocytes | CD14highCD16- | CD14highCD16low | CD14lowCD16hig | |
|---|---|---|---|---|
| Frontal cortex (gray matter) | ||||
| Cho |
Rs=0.43 P=0.09 |
|||
| Cho/Cr |
Rs=0.44 P=0.09 |
Rs=0.43 P<0.10 |
||
| Cho/NAA |
Rs=0.56 P=0.02 |
Rs=0.43 P<0.10 |
||
| Cr |
Rs=−0.34 P=0.09 |
|||
| NAA |
Rs= −0.40 P=0.04 |
Rs= −0.48 P=0.06 |
||
| White matter | ||||
| Cho |
Rs=0.74 P=0.001 |
|||
| Cho/Cr |
Rs=0.70 P=0.002 |
|||
| Cho/NAA |
Rs=0.68 P<0.004 |
|||
| Basal ganglia | ||||
| Cho |
Rs=0.47 P<0.07 |
|||
| Cho/Cr |
Rs=0.60 P=0.01 |
|||
| Cho/NAA |
Rs=0.60 P=0.01 |
|||
| MI |
Rs=0.44 P=0.09 |
|||
| MI/Cr |
Rs=0.49 P=0.05 |
|||
| NAA |
Rs= −0.56 P=0.02 |
|||
| Glx |
Rs=0.55 P<0.03 |
|||
| Glx/Cr |
Rs=0.56 P=0.02 |
|||
Spearman rank coefficient (Rs) indicates the strength and direction of the relationship and the P value indicates significance
Discussion
Early HIV infection of the CNS has been demonstrated by many CSF studies (Gray et al. 1996), though it is believed most people identified during this time do not suffer from severe neurologic disease, which appears in occasional case reports (Mogensen et al. 2007; Yoshizawa et al. 2007). Reports on the early prevalence of cognitive impairment and immunologic events related to CNS disease over the first year of infection are relatively recent and few (Moore et al. 2010; Schnell et al. 2010). Presently, we have shown that changes in brain metabolism, particularly in choline concentrations and related ratios, occur during the first year of HIV infection after seroconversion. Increases in choline-containing compounds, as measured by in vivo MRS in the setting of chronic HIV infection, are typically thought to represent increased lipid membrane turnover due to glial activation. It is possible that these elevations represent a response to the virus, as supported by pathology observed during early HIV and SIV infection indicating the occurrence of oligodendrocyte (lipid) damage, as well as astrocyte and microglial activation (Chakrabarti et al. 1991; Gray et al. 1996; Kim et al. 2005). Choline levels within the brain are typically believed to be elevated in a nearly global fashion in patients with AIDS (Meyerhoff et al. 1999; Mohamad et al. 2007) and elevations in choline have been observed during both neurologically asymptomatic and symptomatic stages of chronic HIV infection (Chang et al. 1999a; Meyerhoff et al. 1999). It is possible that these choline elevations could begin as early as the first year of infection.
Alternatively, previous SIV-related imaging studies have also indicated that dynamic and transient changes in choline-containing metabolites occur during acute/early SIV infection (Greco et al. 2004). Specifically, within weeks of a large Cho/Cr elevation (∼20%), choline levels within the brain dramatically reduce to levels well below pre-infection (−18% at 4 weeks post-infection). In the current study, when the levels of choline and similar metabolite ratios were normalized to those of seronegative controls, as shown in Fig. 2b, HIV+ subjects were found to have lower levels of choline at study entry. Therefore, elevations in the levels of choline-containing metabolites in the frontal cortex and white matter observed over the course of this study may be reflective of choline reductions returning to healthy control levels. Decreases in choline levels below baseline have rarely been reported in diseases not involving necrosis or hepatic encephalopathy (Groeschel et al. 2006; Kreis et al. 1992; Ross et al. 1994). Despite the many metabolic changes that we observed in SiV-infected animals, no structural or signal abnormalities indicative of necrosis were detected by MRI in any animal (or the acute HIV+ subjects) at any time point. Cross-sectional studies in our SIV macaque models indicated that the expected relationship between choline and glial fibrillary associated protein levels (increased expression of which is observed with astrogliosis) during this time period was not observed (Kim et al. 2005). Further studies using animal models are ongoing to better understand the changing nature of choline and its relationship to biologic changes such as microgliosis and monocyte infiltration which occur in the setting of early HIV infection.
While we have previously reported differences in neuronal metabolism (NAA and Glx levels) measured in the frontal cortex of these subjects (Lentz et al. 2009), examination of data collected from the basal ganglia indicates that elevations in MI and MI/Cr are observed in the acute/early HIV-infected population when compared to the seronegative controls. Elevations in MI are believed to be related to microglial activation which has been observed in both the settings of normal aging and chronic HIV infection (Chang et al. 2004; Gruber et al. 2008). Our analyses concur with these ideas, but failed to find a cross effect, indicating that within this cohort, age did not have an additive effect with disease at this stage of infection. However, changes in either MI or MI/Cr were not observed over the course of the study, suggesting that persistent microgliosis may be occurring during this time.
Since monocytes are one of the initial cell types infected by HIV and are capable of crossing the blood–brain barrier, monocyte expansion and translocation in the central nervous system could be a primary avenue for establishing viral reservoirs and immunological environments that favor glial response and neuronal injury (Kaul and Lipton 2006). Support for this relationship was revealed in the consistent association between choline-containing metabolites and levels of non-classical CD16+ monocyte populations. Specifically, the higher the concentrations of choline-related metabolites in the brain, the larger the number of activated monocytes were found circulating in the periphery. This association is significant in the white matter and basal ganglia and shows trends for significance in the frontal cortex. In addition, lower NAA levels (a MRS marker of neuronal integrity) in both the basal ganglia and frontal cortex were associated with higher levels of these activated monocytes. Correlative analysis cannot determine cause or effect, but the analysis does support the proposed hypothesis that changes in brain metabolism are related to changes in the number of activated monocytes in the periphery. As to their role, either protective or inducing neuronal injury, further studies examining the amount of virus in these cell populations are necessary.
A notable constraint in this study included the limited ability of the neurologic assessment to detect more subtle neurocognitive injury. While the subjects recruited in this study did not exhibit symptoms of more severe neurologic disease, results from this MRS study imply that brain function is being affected during the initial year of infection. It is indeed possible that a more sensitive neurocognitive battery will be able to detect early changes in cognition and provide critical insight into the relationship between changes in brain metabolism and early cognitive impairment.
Other limitations of this study included inconsistencies in therapy use among the subjects as well as the small cohort size. As expected, therapy clearly affects viral kinetics and CD4+ T cell population dynamics during early HIV infection (Perelson et al. 1997). Therefore, antiretroviral therapies could affect brain metabolism and monocyte infection or expansion at this stage. Currently, the advantages of initiating therapy during acute/early infection are highly debated (Kaufmann and Walker 2007; Rosenberg et al. 2000). Therefore, the use of imaging techniques, such as MRS, that are capable of detecting early neuronal dysfunction and changes in lipid membrane metabolism provides an ideal means for the assessment of early therapy effectiveness on the CNS. It is possible that metabolic changes observed in this study could be attributed to therapy use. However, our preliminary analysis did not reveal any significant therapy effect, perhaps due to the small cohort sizes. In order to further examine the relationship between immunological changes and metabolic changes, future studies with larger cohorts undergoing comparable therapy regimens are needed.
In conclusion, these findings suggest that brain metabolism is changing during the initial year of infection and that alterations in the brain due to HIV infection are more visible than previously thought. These results also suggest that monocyte levels are related to neuronal dysfunction and increased lipid metabolism occurring during early infection.
Methods
Subjects
Nine HIV+ subjects (mean age ± SD=38±9 years) were identified from a well-established acute/early HIV infection cohort (Kassutto et al. 2006). Subjects were screened (self-report/questionnaire) to exclude individuals with current or past neurologic disorders such as stroke, unstable or severe intercurrent medical conditions, or magnetic resonance contraindications. All were identified during acute HIV infection, defined as having detectable plasma HIV RNA and a positive ELISA, with a negative or indeterminate Western blot (Lentz et al. 2009). A neurologist with expertise in HIV-related neurologic disease evaluated all subjects at study entry. This evaluation included a brief peripheral neuropathy screening devised by the AIDS Clinical Trials Group, clinical staging based on the Memorial Sloan Kettering scale; the HIV Dementia Scale; and a series of comprehensive sensory, motor, reflex, and gait examinations (Power et al. 1995). HIV+ subjects presented with symptoms of acute viral ailment, but not the severe neurologic symptoms described in previous case studies (Mogensen et al. 2007; Yoshizawa et al. 2007). All subjects had blood samples taken within 24–48 h of imaging. Subjects were imaged within 90 days (median= 23 days) of an indeterminate Western blot, while they still had detectable plasma viral RNA levels (median=179,000 copies of RNA/mL). Thus, subjects underwent initial imaging during acute infection, or at the latest, during the onset of early HIV infection. Early infection is defined as the time in which seroconversion has occurred and viral set point is occurring, before progressing into the asymptomatic stage (>1 year of infection). All HIV+ subjects were re-examined 2 months later during the course of early infection, and eight of the nine HIV+ subjects were examined 6 months after the initial imaging session. Two were taking combined antiretroviral therapy before the initial imaging session (Table 1). Nine HIV-seronegative healthy control subjects (mean age ± SD=31±5 years) were also enrolled and underwent all portions of this study. All subjects were verbally screened for drug and alcohol abuse but no toxicology screening was performed. All nine HIV+ subjects enrolled reported potential infection through sexual encounters, mostly men who have sex with men, and were not chronic drug and alcohol abusers. This study was approved by the collaborating institutions' Institutional Review Boards. Subject consent was received from those enrolled.
Magnetic resonance studies
All imaging experiments were conducted on a 1.5-T Signa scanner (General Electric, Milwaukee, WI, USA), with a standard GE head coil. Imaging sequences included a three-plane localizer, sagittal T1, and axial T2 series described previously (Lentz et al. 2009). Proton magnetic resonance spectroscopy was acquired with the GE pulse sequence PROBE-P; a PRESS sequence with CHESS water suppression using standard clinical parameters (TE/TR 35/3,000 ms, 20×20×15 mm3 voxels, 128 acquisitions, spectral width 2,500 Hz, 2,048 points). Three voxels were selected for spectroscopy and prescribed from the axial T2 images (Fig. 1). Frontal cortical gray matter voxel was centered on the superior frontal gyrus along the longitudinal fissure and the basal ganglia on the caudate/putamen. Spectroscopic data were processed using LCModel 6.2, yielding absolute concentrations of N-acetylaspartate (being the sum of N-acetylaspartate + N-acetylaspartyl-glutamate), myo-inositol, choline, glutamate + glutamine (the overlapping resonances known as Glx), and creatine. Ratios with respect to creatine were also examined. Differences in gray and white matter water content were taken into consideration during spectral analysis. A phantom with known concentrations of NAA, Cho, Cr, and MI was used for quality assurance between imaging sessions.
Immunologic and virologic assessment
All HIV+ subjects had blood drawn for complete blood count and HIV RNA quantification (Amplicor Monitor, Roche, Indianapolis, IN, USA) performed in the microbiology laboratories at MGH. HIV antibody testing was performed to confirm seronegative status of control subjects.
Flow cytometric immunophenotyping of monocyte populations
Peripheral blood mononuclear cells of HIV+ subjects were isolated from heparinized blood by Ficoll gradient centrifugation, and were stained with fluorescein isothiocyanate-conjugated anti-CD14 (clone M5E2; PharMingen), phycoerythrin-conjugated anti-CD16 (3G8; PharMingen), peridinium chlorophyll protein-conjugated anti-HLA-DR (L243; Becton Dickinson), and APC-conjugated anti-CD3 (SK7; Becton Dickinson). Monocyte populations were assessed using forward-versus-side scatter profiles and a gate that excluded CD3+ T lymphocytes. HLA-DR-positive monocytes were fractionated into three different subsets based on CD14 and CD16 expression: CD14highCD16-, CD14highCD16low, and CD14lowCD16high monocyte populations. These monocyte populations have been shown to be phenotypically and functionally distinct and have demonstrated differential contributions to HIV infection and neuropathogenesis. The percentage and absolute count of these populations was assessed as previously described (Ellery et al. 2007; Kim et al. 2009; Williams et al. 2005).
Statistical analysis
All statistics were performed using JMP (Version 8.0, SAS Institute Inc., Cary, NC, USA). Differences in metabolites between control (nine subjects) and early HIV infection (nine subjects) cohorts were determined using a Student's t test. Age effects on each metabolite, as well as the interaction effect of age and serostatus, were examined using a mixed effects model. Longitudinal changes in immunological variables and normalized metabolite values were assessed by performing repeated measures analysis of variance (RM-ANOVA) on data obtained from the 8 subjects that underwent repeated measures at all time points. Normalization of MRS data was performed using two methods: (1) MRS data of HIV+ subjects at all time points were normalized to each subject's baseline levels to best illustrate metabolic changes that occur within the first months of HIV infection. (2) Additionally, the MRS data was normalized to the mean metabolite values of healthy, seronegative control subjects to make meaningful comparisons between disease and control settings over time. These normalizations were reported as either “percent of baseline” or “percent of control”, respectively. In either analysis, if the time component of the RM-ANOVA was found to be significant, or exhibited a trend towards significance, then matched-paired t tests were used to isolate differences between time points. Spearman rank correlations were also used to determine associations between the absolute number of monocytes in each subset and metabolites (using non-normalized values). Log transformations of the viral load were used in all analyses. Reported values are mean ± standard deviation unless specified otherwise. Significance was deemed to be P<0.05 and reported trends were limited to P≤0.10.
Acknowledgments
This work was supported by NIH grants K25 NS051129 (MRL), R01 NS040237 (KW), R01 NS037654 (KW), U19MH81835 (KW), NS050041 (RGG), AI040873 (ESR), and the National Center for Research Resources (P41 RR14075).
Footnotes
Disclosure: The authors report no conflicts of interest.
Contributor Information
Margaret R. Lentz, Email: mlentz@nmr.mgh.harvard.edu, Department of Neuroradiology and the A. A. Martinos Center, for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, USA.
Woong-Ki Kim, Department of Microbiology and Molecular Cell Biology, Eastern Virginia Medical School, Norfolk, VA, USA.
Hyun Kim, Department of Neuroradiology and the A. A. Martinos Center, for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, USA.
Caroline Soulas, Biology Department, Boston College, Boston, MA, USA.
Vallent Lee, Department of Neuroradiology and the A. A. Martinos Center, for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, USA.
Nagagopal Venna, Department of Neurology, Massachusetts General Hospital, Boston, MA, USA.
Elkan F. Halpern, Department of Neuroradiology and the A. A. Martinos Center, for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, USA
Eric S. Rosenberg, Departments of Medicine and Pathology, Massachusetts General Hospital, Boston, MA, USA
Kenneth Williams, Biology Department, Boston College, Boston, MA, USA.
R. G. González, Department of Neuroradiology and the A. A. Martinos Center, for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, USA
References
- Ances BM, Sisti D, et al. Resting cerebral blood flow: a potential biomarker of the effects of HIV in the brain. Neurology. 2009;73:702–708. doi: 10.1212/WNL.0b013e3181b59a97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti L, Hurtrel M, et al. Early viral replication in the brain of SIV-infected rhesus monkeys. Am J Pathol. 1991;139:1273–1280. [PMC free article] [PubMed] [Google Scholar]
- Chang L, Ernst T, et al. Cerebral metabolite abnormalities correlate with clinical severity of HIV-1 cognitive motor complex. Neurology. 1999a;52:100–108. doi: 10.1212/wnl.52.1.100. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, et al. Highly active antiretroviral therapy reverses brain metabolite abnormalities in mild HIV dementia. Neurology. 1999b;53:782–789. doi: 10.1212/wnl.53.4.782. [DOI] [PubMed] [Google Scholar]
- Chang L, Lee PL, et al. A multicenter in vivo proton-MRS study of HIV-associated dementia and its relationship to age. Neuroimage. 2004;23:1336–1347. doi: 10.1016/j.neuroimage.2004.07.067. [DOI] [PubMed] [Google Scholar]
- Clay CC, Rodrigues DS, et al. Neuroinvastion of fluorescein-positive monocytes in acute simian immunodeficiency virus infection. J Virol. 2007;81:12040–12048. doi: 10.1128/JVI.00133-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellery PJ, Tippett E, et al. The CD16+ monocyte subset is more permissive to infection and preferentially harbors HIV-1 in vivo. J Immunol. 2007;178:6581–6589. doi: 10.4049/jimmunol.178.10.6581. [DOI] [PubMed] [Google Scholar]
- Finzi D, Blankson J, et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5:512–517. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- Gray F, Scaravillio F, et al. Neuropathology of early HIV-1 infection. Brain Pathol. 1996;6:1–15. doi: 10.1111/j.1750-3639.1996.tb00775.x. [DOI] [PubMed] [Google Scholar]
- Greco JB, Westmoreland SV, et al. In vivo 1 H MRS of brain injury and repair during acute SIV infection in the macaque model of neuroAIDS. Magn Reson Med. 2004;51:1108–1114. doi: 10.1002/mrm.20073. [DOI] [PubMed] [Google Scholar]
- Groeschel S, Brockmann K, et al. Magnetic resonance imaging and proton magnetic resonance spectroscopy of megalencephaly and dilated Virchow-Robin spaces. Pediatr Neurol. 2006;34:35–40. doi: 10.1016/j.pediatrneurol.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Gruber S, Pinker K, et al. Metabolic changes in the normal ageing brain: consistent findings from short and long echo time proton spectroscopy. Eur J Radiol. 2008;68:320–327. doi: 10.1016/j.ejrad.2007.08.038. [DOI] [PubMed] [Google Scholar]
- Kassutto S, Maghsoudi K, et al. Longitudinal analysis of clinical markers following antiretroviral therapy initiated during acute or early HIV type 1 infection. Clin Infect Dis. 2006;42:1024–1031. doi: 10.1086/500410. [DOI] [PubMed] [Google Scholar]
- Kaufmann DE, Walker BD. Treatment interruption to boost specific HIV immunity in acute infection. Curr Opin HIV AIDS. 2007;2:21–25. doi: 10.1097/COH.0b013e3280119275. [DOI] [PubMed] [Google Scholar]
- Kaul M, Lipton SA. Mechanisms of neuroimmunity and neurodegeneration associated with HIV-1 infection and AIDS. J Neuroimmune Pharmacol. 2006;1:138–151. doi: 10.1007/s11481-006-9011-9. [DOI] [PubMed] [Google Scholar]
- Kim JP, Lentz MR, et al. Relationships between astrogliosis and 1H MR spectroscopic measures of brain choline/creatine and myo-inositol/creatine in a primate model. AJNR Am J Neuroradiol. 2005;26:752–759. [PMC free article] [PubMed] [Google Scholar]
- Kim WK, Sun Y, et al. Monocyte heterogeneity underlying phenotypic changes in monocytes according to SIV disease stage. J Leukoc Biol. 2009;87:557–567. doi: 10.1189/jlb.0209082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreis R, Ross BD, et al. Metabolic disorders of the brain in chronic hepatic encephalopathy detected with H-1 MR spectroscopy. Radiology. 1992;182:19–27. doi: 10.1148/radiology.182.1.1345760. [DOI] [PubMed] [Google Scholar]
- Lentz MR, Kim WK, et al. Changes in MRS neuronal markers and T cell phenotypes observed during early HIV infection. Neurology. 2009;72:1465–1472. doi: 10.1212/WNL.0b013e3181a2e90a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellors JW, Kingsley LA, et al. Quantitation of HIV-1 RNA in plasma predicts outcome after seroconversion. Ann Intern Med. 1995;122:573–579. doi: 10.7326/0003-4819-122-8-199504150-00003. [DOI] [PubMed] [Google Scholar]
- Meyerhoff DJ, Bloomer C, et al. Elevated subcortical choline metabolites in cognitively and clinically asymptomatic HIV+ patients. Neurology. 1999;52:995–1003. doi: 10.1212/wnl.52.5.995. [DOI] [PubMed] [Google Scholar]
- Mogensen TH, Marinovskij E, et al. Acute demyelinizating encephalomyelitis (ADEM) as initial presentation of primary HIV infection. Scand J Infect Dis. 2007;39:630–634. doi: 10.1080/00365540601137379. [DOI] [PubMed] [Google Scholar]
- Mohamad MSN, Sacktor NC, et al. ISMRM. Vol. 15. Berlin: 2007. 3 Tesla MR Spectroscopy Reveals Decreased Glutamate and Glutamine levels in frontal white matter in HIV-associated dementia. [Google Scholar]
- Moore D, Letendre S, et al. HIV-associated Neurocognitive Disorder in Acute and Early HIV Infection; 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA. 2010. [Google Scholar]
- Perelson AS, Essunger P, et al. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature. 1997;387:188–191. doi: 10.1038/387188a0. [DOI] [PubMed] [Google Scholar]
- Power C, Selnes OA, et al. HIV dementia scale: a rapid screening test. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;8:273–278. doi: 10.1097/00042560-199503010-00008. [DOI] [PubMed] [Google Scholar]
- Ratai EM, Pilkenton SJ, et al. In vivo proton magnetic resonance spectroscopy reveals region specific metabolic responses to SIV infection in the macaque brain. BMC Neurosci. 2009;10:63. doi: 10.1186/1471-2202-10-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg ES, Altfeld M, et al. Immune control of HIV-1 after early treatment of acute infection. Nature. 2000;407:523–526. doi: 10.1038/35035103. [DOI] [PubMed] [Google Scholar]
- Ross BD, Jacobson S, et al. Subclinical hepatic encephalopathy: proton MR spectroscopic abnormalities. Radiology. 1994;193:457–463. doi: 10.1148/radiology.193.2.7972763. [DOI] [PubMed] [Google Scholar]
- Schacker TW, Hughes JP, et al. Biological and virologic characteristics of primary HIV infection. Ann Intern Med. 1998;128:613–620. doi: 10.7326/0003-4819-128-8-199804150-00001. [DOI] [PubMed] [Google Scholar]
- Schnell G, Price RW, et al. Compartmentalization and clonal amplification of HIV-1 variants in the cerebrospinal fluid during primary infection. J Virol. 2010;84:2395–2407. doi: 10.1128/JVI.01863-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K, Westmoreland S, et al. Magnetic resonance spectroscopy reveals that activated monocytes contribute to neuronal injury in SIV neuroAIDS. J Clin Invest. 2005;115:2534–2545. doi: 10.1172/JCI22953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizawa T, Motonishi S, et al. Case of acute primary HIV infection with menigoencephalitis demonstrating high signal intensity of the bilateral globus pallidus in T2-weighted MRI. Rinsho Shinkeigaku. 2007;47:597–600. [PubMed] [Google Scholar]