Abstract
Introduction
Empty sella in MRI is an important finding associated with idiopathic intracranial hypertension (IIH). This study assesses the sensitivity and reproducibility of several morphological measures of the sella and pituitary gland to indentify the measure that best differentiates IIH from controls. Additionally, the study assesses reversal in gland compression following treatment.
Methods
Sagittal 3D-T1W sequence with 1 mm isotropic resolution was obtained from ten newly diagnosed IIH patients and 11 matched healthy controls. Follow-up MRI scans were obtained from eight patients at 1-week postlumbar puncture and acetazolamide treatment. 1D and 2D measures of absolute and normalized heights and crosssectional areas of the gland and sella were obtained to identify the measure that best differentiates IIH patients and controls.
Results
Overall area-based measurements had higher sensitivity than length with p<0.0001 for sella area compared with p=0.004 for normalized gland height. The gland crosssectional areas were similar in both cohorts (p=0.557), while the sella area was significantly larger in IIH, 200±24 versus 124±25 mm2, with the highest sensitivity and specificity, 100 % and 90.9 %, respectively. Absolute gland area was the most sensitive measure for assessing post treatment changes, with 100 % sensitivity and 50 % specificity. Average posttreatment gland area was 18 % larger (p=0.016). Yet, all eight patients remained within the empty sella range based on a normalized gland area threshold of 0.41.
Conclusions
Sellar area is larger in IIH, and it demonstrated highest sensitivity for differentiating IIH from control subjects, while absolute gland area was more sensitive for detecting post treatment changes.
Keywords: Idiopathic intracranial hypertension, Empty sella, Pituitary gland compression, MRI
Introduction
Empty sella refers to a magnetic resonance (MR) imaging sign whereby the pituitary gland appears compressed against the sella turcica forming a concave, “half-moon” shape of varying degrees [1–4]. An accepted possible cause for empty sella is increased intracranial pressure (ICP) leading to herniation of the subarachnoid space into the anterior portion of the sella turcica diaphragm, which fills with CSF [5]. Empty sella has been associated with idiopathic intracranial hypertension (IIH) [1–4, 6], yet its occurrence in IIH varies considerably in the literature, ranging from 2.5 % for totally empty sella in which the pituitary gland is not visible, and up to 94 % for partially empty sella that encompasses a wide range of pituitary height changes [6–10]. This could be in part due to lack of well-defined anatomical criteria for what constitutes empty sella. Furthermore, empty sella is a non-specific finding, as it is often found in healthy subjects [11–13] as well as in obesity [14], meningioma [15], pediatric nevoid basal cell carcinoma [16], and even following therapy for growth hormone deficiency [17]. Regardless of the limited specificity, finding of empty sella on MR images in conjunction with other imaging signs suggests the diagnosis of IIH at the exclusion of tumor, venous thrombosis, or infection [3, 6].
The degree of pituitary compression in IIH was quantitatively assessed by Yuh et al. from the ratio of pituitary area and sella turcica area, as measured by manual tracing on mid-sagittal T1-weighted MR images [6]. They found that 85 % of IIH patients had a significantly smaller ratio as compared with their control group [6]. While empty sella in IIH is well established, there is limited and inconsistent information regarding the reversibility of pituitary compression following treatment. Zagardo et al. reported the reappearance of normal sellar contents in two cases of IIH, following 2 weeks of treatment with acetazolamide in one patient and lumbo-peritoneal shunt in the other [18].
The present study aims to quantify pituitary gland compression in a homogenous cohort of newly diagnosed obese IIH patients in comparison with age, gender, and body mass index (BMI) matched healthy control subjects. Furthermore, this study also aims to quantify the effect of lumbar puncture (LP) and acetazolamide treatment on reversal of pituitary compression in IIH.
Methods
Patients and study protocol
This study was approved by the local institutional review board and is in compliance with the Declaration of Helsinki. After obtaining written informed patient consent, ten consecutive young women newly diagnosed with IIH (mean± SD age 28.4±9.8 years; mean±SD BMI 35.2±3.9 kg/m2) and 11 healthy young women of matched age and BMI (mean±SD age 30.8±8.5 years; mean±SD BMI 36.6± 4.9 kg/m2) were enrolled for this study which included an MRI scan of the brain. A carefully matched group of control subjects were included in order to generate normative data and to establish pituitary compression in the IIH patient cohort. All study participants underwent a diagnostic vision exam by an ophthalmologist, and the papilledema grade was recorded for IIH patients. Following MRI, the patients underwent LP to confirm the diagnosis of IIH as per the modified Dandy criteria [19]. The mean opening pressure was 36.9±7.1 cm H2O (range, 26 to 47 cm H2O). The mean CSF volume withdrawn during LP was 13±3 mL (range, 9 to 18 mL). The mean closing pressure was 18.8±3 cm H2O (range, 13 to 22 cm H2O). All patients were then prescribed acetazolamide, a diuretic and inhibitor of carbonic anhydrase enzyme, suspected to restrain the CSF production [20]. All study participants also filled out a questionnaire to report symptoms including headache and vision impairments. A headache impact test score (range, 36 to 78) was calculated to quantify headache severity. None of the patients reported any endocrine-related problems. Eight out of ten patients returned for follow-up MRI scans and vision exams approximately 10 days post-LP and initiation of acetazolamide (range, 5–15 days).
Imaging and morphological analysis
MRI data was acquired using 1.5 T and 3 T scanners (Siemens, Erlangen, Germany). MR imaging protocol included a high-resolution, 3D T1-weighted magnetizationprepared rapid acquisition gradient echo (MPRAGE) sequence with the following imaging parameters: sagittal acquisition, TR of 1,700 ms (1,900 ms for 3 T), TE of 3.53 ms (4.7 ms for 3 T), TI of 1,100 ms, flip angle of 15°, slice thickness of 1 mm, FOV of 25.6×23.2 cm, acquisition matrix of 256×232 (i.e., isotropic resolution 1 mm) and pixel bandwidth of 130 KHz.
Length and area measurements of the pituitary gland and sellar compartment were obtained by manual delineation on the mid-sagittal MR images by two observers with different levels of experience. The observers were blinded to subject information. These data were used to estimate inter-observer variability. Data obtained by the more experienced observer were then used to assess the degree of pituitary compression, as well as the degree of primary regression following treatment in IIH patients.
Height measurements were performed along the direction of the pituitary stalk for improved consistency. Pituitary gland height was defined as the difference between two length measurements (H1–H2) where H1 was the total height of the sellar compartment (distance between the inferior edge of the optic chiasm and inferior edge of the sella turcica base) and H2 was the height of the pituitary stalk (distance between the inferior edge of the optic chiasm and superior edge of the pituitary gland. Pituitary height measurements for each subject were normalized to their respective sellar compartment heights to account for intersubject variability. Normalized pituitary height was defined as the ratio, (H1–H2)/H1.
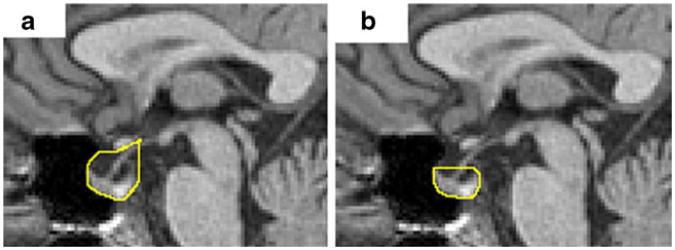
Cross-sectional area of the sella turcica (A1) was measured using two definitions. As per the first definition, the superior boundary of the sella was delineated along the inferior edge of the optic chiasm from the intersection with the pituitary stalk to the boundary with sphenoid sinus, as shown in Fig. 1a. Thus, the suprasellar cistern is included in the cross-sectional area measurement of the sella. This definition for the sellar compartment is based on well indentified anatomical landmarks on mid-sagittal MR images. The second definition is based on Yuh et al. where the upper extents of the pituitary gland were used as the superior boundary of the sella [6], as shown in Fig. 1b. Sellar area measured using the two methods were also compared to evaluate measurement reproducibility. Normalized ratio between the pituitary gland (A2) and the sellar compartment (A1) was obtained for the two definitions of the sellar compartment area for comparison.
Fig. 1.
Delineated of the sella cross-sectional area using two definitions: a method 1: the superior boundary of the sella along the inferior edge of the optic chiasm until the intersection with the pituitary stalk, and b method 2: the superior boundary of the sella is at the level of the upper anterior and posterior extents of the pituitary gland
Statistical analysis
The degree of pituitary compression in IIH patients was assessed and compared with measurements obtained in the healthy subjects. A two-tailed Mann–Whitney U test for independent samples with 95 % confidence interval (CI) was applied to assess the significance of differences between the healthy cohort and the untreated patient group. Receiver operating characteristic (ROC) analysis was used to establish quantitative criterion for classifying presence of empty sella syndrome. A two-tailed Wilcoxon test for paired samples at alpha<0.05 was applied to assess differences between pre- and post-treatment measurements within the same patient. Intraclass correlation coefficient (ICC) was calculated to estimate reproducibility between the two observers. Statistical analyses were performed using statistical software (MedCalc v12.5.0, Mariakerke, Belgium).
Results
Healthy versus IIH
Apparent ‘empty sella’ by visual inspection was noted in all ten IIH patients and in one of the 11 healthy subjects. An example of the manually delineated height and area measurements from a healthy subject and an IIH patient, respectively, are shown in Fig. 2. Mean values of height and area measurements from the healthy and patient cohorts are summarized in Table 1. The mean and SD of the gland cross-sectional area for the control and the IIH cohorts were found to be similar, 57.20±14.45 and 62.02±16.74 mm2, respectively (p=0.557). On the other hand, absolute sellar area based on both methods demonstrated significantly larger sellar compartment area in the IIH cohort. The respective sellar compartment area based on method 1 which includes suprasellar cistern provided the strongest significant difference between the healthy controls and IIH patients 124± 25 mm2 versus 200±24 mm2, respectively, with a p value< 0.0001. ROC analysis demonstrated 100 % sensitivity and 90.9 % specificity with a threshold of 151 mm2. Sensitivity of the sellar area using method 2 was also 100 % but with a lower specificity of 72.2 %.
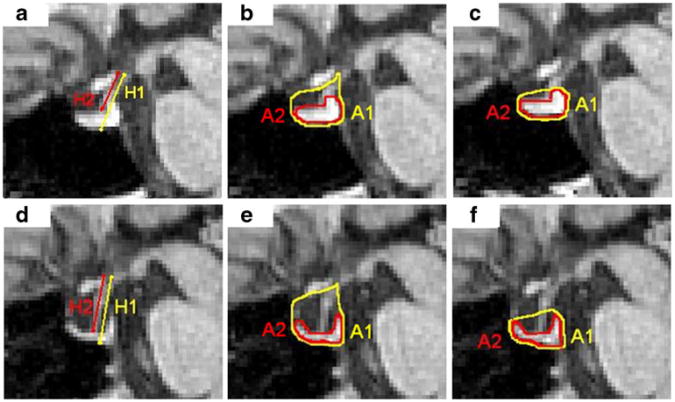
Fig. 2.
Delineated sella and pituitary gland height (first column) and area (second and third columns) measurements obtained from a healthy subject (top row) and IIH patient (bottom row). Sella area is delineated using method 1 (second column) and using method 2 (third column). H1 and A1 (yellow) represent the total height and area of the sellar compartment, respectively. (H1–H2) and A2 (red) represent the height and area of the pituitary gland, respectively, where H2 represents the height of the pituitary stalk
Table 1. Height and area measures of pituitary compression in newly diagnosed IIH patients and matched control subjects.
| Measurement | Controls (n=11) (mean±SD) | IIH patients (n=10) (mean±SD) | p value |
|---|---|---|---|
| H1 (mm) | 14.83±2.44 | 16.27±2.69 | 0.173 |
| H2 (mm) | 9.94±1.82 | 12.46±2.11 | 0.009* |
| H1–H2 (mm) | 4.89±1.36 | 3.81±1.30 | 0.067 |
| (H1–H2)/H1 | 0.33±0.06 | 0.23±0.06 | 0.004* |
| M1_A1 (mm2) | 124.15±24.63 | 200.39±24.18 | <0.0001* |
| M2_A1 (mm2) | 71.97±22.25 | 116.09±25.01 | 0.001* |
| A2 (mm2) | 57.20±14.45 | 62.02±16.74 | 0.557 |
| A2/M1_A1 | 0.47±0.11 | 0.31 ±0.07 | 0.003* |
| A2/M2_A1 | 0.82±0.15 | 0.54±0.11 | 0.0004* |
p value<0.05, statistically significant
H1 height of sellar compartment, H2 height of pituitary stalk, H1–H2 height of pituitary gland, A1 area of sellar compartment, A2 area of pituitary gland, A2/A1 pituitary gland fraction of the sella, M1_A1 method 1: superior sellar boundary along inferior edge of optic chiasm and anteroposteriorly bound by the sphenoid bone, M2_A1 method 2: superior sellar boundary at the level of the upper limits of the pituitary gland, as illustrated by Yuh et al. [6], A2/M1_A1 and A2/M2_A1 pituitary gland fraction of the sella calculated using sella area measured by methods M1 and M2, respectively
Consequently, due to the larger sellar compartment area, both normalized height and normalized area measurements of the pituitary gland were significantly smaller in the IIH patient cohort as compared with the healthy subject group. Mean and SD values of normalized pituitary gland height in control subjects and IIH patients were 0.33±0.06 and 0.23± 0.06, respectively (p=0.004), while the mean and SD for pituitary stalk height in controls and IIH patients were 9.94± 1.82 and 12.46±2.11 mm, respectively (p=0.009). Mean and SD values of the pituitary gland normalized for area per method 1 in control subjects and IIH patients were 0.47± 0.11 and 0.31±0.07, respectively (p=0.003). Inter-observer reproducibility of the sellar area measurement based on the ICC was 0.985 (95 % CI=0.965 to 0.994).
Effect of treatment
Seven out of eight patients who returned for a follow-up visit 1 week post-LP and initiation of acetazolamide showed an increase in absolute pituitary area (average of 18 %; range, 1.2 % to 42.3 %). Both absolute and normalized area measurements of pre- and post-1 week follow-up studies are listed in Table 2. The increase in absolute gland area showed stronger statistical significance (p=0.016) compared with normalized gland area (p=0.031), while changes in absolute height (p=0.945) and normalized height (p=0.945) of the gland were not statistically significant, as shown in Table 3. This indicates that absolute gland area, rather than normalized area, is the more sensitive measure of change for comparing patients before and after treatment. ROC analysis demonstrated that absolute gland area achieved 100 % sensitivity and 50 % specificity. Figure 3 shows sagittal MRI images that demonstrate changes in empty sella appearance at 1 week. No significant association was found between the relative increase in gland area and improvements in headache score (r=0.276), visual impairment (r=−0.235), or papilledema grade (r=0.235).
Table 2. Change in absolute and normalized pituitary gland area between pre-treatment and post-1 week follow-up visit.
| IIH patient (n=8) | Sella area (A1) (mm2) | Gland area (A2) (mm2) | Normalized gland area (A2/A1) | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Pre | Post-1 week | Pre | Post-1 week | Pre | Post-1 week | |
| P2 | 212.0 | 218.0 | 56.3 | 56.0 | 0.27 | 0.26 |
| P3 | 198.0 | 206.0 | 52.8 | 68.5 | 0.27 | 0.33 |
| P4 | 216.0 | 221.0 | 48.5 | 69.0 | 0.22 | 0.31 |
| P6 | 183.0 | 175.0 | 49.3 | 53.0 | 0.27 | 0.30 |
| P7 | 182.0 | 185.0 | 64.3 | 65.0 | 0.35 | 0.35 |
| P9 | 224.0 | 227.0 | 58.0 | 65.0 | 0.26 | 0.29 |
| P11 | 216.9 | 219.1 | 82.8 | 85.7 | 0.38 | 0.39 |
| P12 | 152.0 | 146.0 | 41.8 | 54.5 | 0.27 | 0.37 |
| Mean | 197.9 | 199.6 | 56.7 | 64.6 | 0.29 | 0.33 |
| SD | 24.4 | 28.4 | 12.6 | 10.6 | 0.05 | 0.05 |
| p value | 0.547 | 0.016* | 0.031* | |||
p value<0.05, statistically significant
IIH idiopathic intracranial hypertension, Pre before lumbar puncture and acetazolamide, P1w 1 week post-lumbar puncture and acetazolamide, A1 area of sella turcica measured on mid-sagittal slice using method 1, A2 area of pituitary gland measured on mid-sagittal slice, A2/A1 gland-occupied fraction of the sella
Table 3. Comparing absolute and normalized measurements of pituitary gland height and area in IIH patients (n=8) before and 1 week after LP and acetazolamide treatment.
| Measurement | Pre (n=8) (mean±SD) | Post-1 week (n=8) (mean±SD) | p value |
|---|---|---|---|
| (H1–H2) (mm) | 3.43±1.10 | 3.58±1.35 | 0.945 |
| (H1–H2)/H1 | 0.22±0.06 | 0.23±0.07 | 0.945 |
| A1 (mm2) | 197.9±24.4 | 199.6±28.4 | 0.547 |
| A2 (mm2) | 56.7±12.6 | 64.6±10.6 | 0.016* |
| A2/A1 | 0.29±0.05 | 0.33±0.05 | 0.031* |
p value<0.05, statistically significant
IIH idiopathic intracranial hypertension, H1 height of sellar compartment, H2 height of pituitary stalk, A1 area of sellar compartment measured on mid-sagittal slice using method 1, A2 area of pituitary gland measured on mid-sagittal slice, A2/A1 pituitary gland-occupied fraction of the sella
Fig. 3.
Example of sagittal MRI images demonstrating the changes in empty sella appearance after treatment in a patient with IIH. The delineated gland area is shown in the bottom row. a, c Pre-treatment; b, d 1 week post-LP and acetazolamide treatment
Discussion
The present study evaluates the sensitivity of 1D and 2D measures of pituitary compression, namely height and area measurements on mid-sagittal MR images, for identification of partially empty sella in IIH. Based on the results summarized in Table 1, it is evident that area-based measurements provide higher sensitivity sella compared with length-based measurements. The most significant finding of the study is the importance of the sellar area as an indicator of IIH. This is evident since the pituitary gland area measurements were similar in the patients and control cohorts, 62.02±16.74 and 57.20±14.45 mm2, respectively, with p=0.56. This suggests that the pituitary gland in partially empty sella is rather deformed but not compressed. Furthermore, the appearance of partially empty sella is primarily due to the increased sellar compartment and secondary to the deformation in the pituitary gland shape. The sellar measurement which includes the suprasellar cistern provides the strongest sensitivity for differentiating between the patients and control subjects (p<0.0001). ROC analysis suggests that sellar areas with a value of 151 mm2 measured with method 1 are the most reliable threshold to distinguish IIH patients among obese subjects. This finding of enlarged sella turcica and the suprasellar cistern is consistent with a recent observation that obesity-related IIH is associated with larger intracranial volume and larger intracranial CSF volume that accumulates in the extraventricular spaces [21].
The finding of empty or partially empty sella in obesityrelated IIH can be explained as a phenomenon of remodeling of the sellar cavity under the influence of chronically elevated CSF pressure and pressure pulsation resulting with widening and enlargement of the sella turcica and effacement of the pituitary gland, thus producing the typical appearance of an empty sella [5]. Butros et al. recently hypothesized that osseous erosions are caused in IIH by a similar mechanism, leading to CSF leak as a result of osseous dehiscences and arachnoid herniation. They found empty sella in 65.9 % of their IIH patients and also reported significant widening of the foramen ovale at the skull base in IIH patients compared with age-, gender-matched controls [22].
Normalized pituitary gland areas using both definitions of the sellar area demonstrated significant differences between the control and IIH cohorts. The normalized ratio using method 1, which include the suprasellar cistern, were consequently smaller compared with method 2, 0.47±0.11 and 0.31±0.07, versus 0.82±0.15 and 0.54±0.11, respectively, as expected. The normalized gland areas obtained using method 2 are in good agreement with the values of 0.83 and 0.44 reported by Yuh et al. [6]. The most unexpected finding, however, is the fact that the absolute sellar area, which includes the suprasellar cistern, provides the strongest differentiation between the IIH and control cohorts compared with the measurements of normalized gland area.
The definition of the sellar area using method 1 demonstrated slightly higher inter-observer consistency based on smaller relative difference compared with method 2. This can be due to the well-defined markers of the superior boundaries in method one while the sellar definition based on method 2 is dependent on the gland shape. Moreover, the coefficient of variation (COV) (SD/mean) calculated for the sella area measured using method 1 is smaller when compared with method 2. Using method one, the COV obtained for control and patient groups were 0.198 and 0.121 respectively; whereas using method two, the COV obtained for the control and patient groups were 0.317 and 0.215, respectively. This shows greater sensitivity of the sellar area measurements with method 1.
Yuh et al. suggested that a compressed pituitary gland in IIH may re-expand after normalization of the ICP [6]. They further hypothesized that empty sella syndrome in IIH patients is due to a prolonged period of elevated ICP [6]. In our IIH cohort, the gland area demonstrated a modest increase of 18 % at 1 week after LP and initiation of acetazolamide in all but one IIH patient. The normalized gland areas post-1 week follow-up were still within the empty sella range in all eight patients, based on a threshold value of 0.41. The increase in gland area is likely primarily due to shape deformation and not due to decompression. A full reversal of the partially empty sella appearance may not be achieved in obesity-related IIH even after a long follow-up period since the primary reason for the appearance is the enlargement of the sellar compartment, which is not likely to be reversed by treatment.
This study has several limitations including small sample size for the IIH patient and control groups and a small number of subjects who had a follow-up scan. Regardless of the small sample size, strong statistical significances in several of the area and length measures were demonstrated. Another limitation was the use of two different scanners (1.5 T and 3 T). However, there should not be much in the way of differences due to field strength on this basis as geometrical distortion and image contrast were similar, and therefore, it is not expected to bias the manual delineation. Finally, regarding the clinical applicability of area-based measurements, it may be argued that 1D measurement is more easily performed and preferred over 2D measurement in clinical practice. However, the higher sensitivity of the area-based measures may justify the additional effort required for the area-based measurement.
Conclusions
While gland cross-sectional area is similar between obesityrelated IIH and control cohorts, sellar compartment crosssectional area is significantly enlarged in IIH. This contributes to the partially sellar appearance seen in IIH. The absolute sellar area (A1) which includes the suprasellar cistern demonstrates the strongest differentiation power among length and area measurements of the pituitary gland and sella turcica. In contrast, the gland area is the more sensitive measure for assessing changes in the pituitary gland following treatment.
Acknowledgments
This study is supported in part by NIH grant R01 NS052122. The authors thank Ms. Lauren S. Meshkov for her participation in the initial phase of the study.
Footnotes
Conflict of interest NA is a shareholder in Alperin Noninvasive Diagnostics, Inc.
Contributor Information
Sudarshan Ranganathan, Department of Radiology, University of Miami, Miami, FL, USA.
Sang H. Lee, Department of Radiology, University of Miami, Miami, FL, USA
Adam Checkver, Department of Radiology, University of Miami, Miami, FL, USA.
Evelyn Sklar, Department of Radiology, University of Miami, Miami, FL, USA.
Byron L. Lam, Bascom Palmer Eye Institute, Department of Ophthalmology, University of Miami, Miami, FL, USA
Gary H. Danton, Department of Radiology, University of Miami, Miami, FL, USA
Noam Alperin, Email: nalperin@med.miami.edu, Department of Radiology, University of Miami, Miami, FL, USA; Department of Radiology, Professional Arts Center, University of Miami Leonard M. Miller School of Medicine, 1150 NW 14th Street, Suite 713 (M-869), Miami, FL 33136, USA.
References
- 1.Kaufman B. The “empty” sella turcica—a manifestation of the intrasellar subarachnoid space. Radiology. 1968;90:931–941. doi: 10.1148/90.5.931. [DOI] [PubMed] [Google Scholar]
- 2.Foley KM, Posner JB. Does pseudotumor cerebri cause the empty sella syndrome? Neurology. 1975;25:565–569. doi: 10.1212/wnl.25.6.565. [DOI] [PubMed] [Google Scholar]
- 3.Wessel K, Thron A, Linden D, Petersen D, Dichgans J. Pseudotumor cerebri: clinical and neuroradiological findings. Eur Arch Psychiatry Neurol Sci. 1987;237:54–60. doi: 10.1007/BF00385667. [DOI] [PubMed] [Google Scholar]
- 4.Berke JP, Buxton LF, Kokmen E. The “empty” sella. Neuroradiology. 1975;25:1137–1143. doi: 10.1212/wnl.25.12.1137. [DOI] [PubMed] [Google Scholar]
- 5.Neelon FA, Goree JA, Lebovitz HE. The primary empty sella: clinical and radiographic characteristics and endocrine function. Med (Baltimore) 1973;52:73–92. [PubMed] [Google Scholar]
- 6.Yuh WT, Zhu M, Taoka T, et al. MR imaging of pituitary morphology in idiopathic intracranial hypertension. J Magn Reson Imaging. 2000;12:808–813. doi: 10.1002/1522-2586(200012)12:6<808::aid-jmri3>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 7.Gibby WA, Cohen MS, Goldberg HI, Sergott RC. Pseudotumor cerebri: CT findings and correlation with vision loss. AJR Am J Roentgenol. 1993;160:143–146. doi: 10.2214/ajr.160.1.8416612. [DOI] [PubMed] [Google Scholar]
- 8.Brodsky MC, Vaphiades M. Magnetic resonance imaging in pseudotumor cerebri. Ophthalmology. 1998;105:1686–1693. doi: 10.1016/S0161-6420(98)99039-X. [DOI] [PubMed] [Google Scholar]
- 9.Weisberg LA. Computed tomography in benign intracranial hypertension. Neurology. 1985;35:1075–1078. doi: 10.1212/wnl.35.7.1075. [DOI] [PubMed] [Google Scholar]
- 10.Degnan AJ, Levy LM. Pseudotumor cerebri: brief review of clinical syndrome and imaging findings. AJNR Am J Neuroradiol. 2011;32:1986–1993. doi: 10.3174/ajnr.A2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sage MR, Blumbergs PC. Primary empty sella turcica: a radiological-anatomical correlation. Australas Radiol. 2000;44:341–348. doi: 10.1046/j.1440-1673.2000.00828.x. [DOI] [PubMed] [Google Scholar]
- 12.Sage MR, Blumbergs PC, Fowler GW. The diaphragma sellae: its relationship to normal sellar variations in frontal radiographic projections. Radiology. 1982;145:699–701. doi: 10.1148/radiology.145.3.7146398. [DOI] [PubMed] [Google Scholar]
- 13.Bergland RM, Ray BS, Torack RM. Anatomical variations in the pituitary gland and adjacent structures in 225 human autopsy cases. J Neurosurg. 1968;28:93–99. doi: 10.3171/jns.1968.28.2.0093. [DOI] [PubMed] [Google Scholar]
- 14.Quintos JB, Shah A, Castells S. Transient pituitary dysfunction, empty sella, pseudotumor cerebri in a morbidly obese adolescent. Pediatr Endocrinol Rev Suppl. 2006;4:576–578. [PubMed] [Google Scholar]
- 15.Kim JH, Ko JH, Kim HW, Ha HG, Jung CK. Analysis of empty sella secondary to the brain tumors. J Korean Neurosurg Soc. 2009;46:355–359. doi: 10.3340/jkns.2009.46.4.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takanashi J, Suzuki H, Nagasawa K, Kobayashi K. Empty sella in children as a key for diagnosis. Brain Dev. 2001;23:422–423. doi: 10.1016/s0387-7604(01)00254-6. [DOI] [PubMed] [Google Scholar]
- 17.Francois I, Casteels I, Silberstein J, Casaer P, de Zegher F. Empty sella, growth hormone deficiency and pseudotumor cerebri: effect of initiation, withdrawal, and resumption of growth hormone therapy. Eur J Pediatr. 1997;156:69–70. doi: 10.1007/s004310050556. [DOI] [PubMed] [Google Scholar]
- 18.Zagardo MT, Cail WS, Kelman SE, Rothman MI. Reversible empty sella in idiopathic intracranial hypertension: an indicator of successful therapy? AJNR Am J Neuroradiol. 1996;17:1953–1956. [PMC free article] [PubMed] [Google Scholar]
- 19.Friedman DI, Jacobson DM. Diagnostic criteria for idiopathic intracranial hypertension. Neurology. 2002;59:1492–1495. doi: 10.1212/01.wnl.0000029570.69134.1b. [DOI] [PubMed] [Google Scholar]
- 20.McComb JG. Recent research into the nature of cerebrospinal fluid formation and absorption. J Neurosurg. 1983;59:369–383. doi: 10.3171/jns.1983.59.3.0369. [DOI] [PubMed] [Google Scholar]
- 21.Alperin N, Ranganathan S, Bagci AM, et al. MRI evidence of impaired CSF homeostasis in obesity-associated idiopathic intracranial hypertension. AJNR Am J Neuroradiol. 2013;34:29–34. doi: 10.3174/ajnr.A3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butros SR, Goncalves LF, Thompson D, Agarwal A, Lee HK. Imaging features if idiopathic intracranial hypertension, including a new finding: widening of the foramen ovale. Acta Radiol. 2012;53:682–688. doi: 10.1258/ar.2012.110705. [DOI] [PubMed] [Google Scholar]