Abstract
The persistence of chronic immune activation and oxidative stress in human immunodeficiency virus (HIV)-infected, antiretroviral drug-treated individuals are major obstacles to fully preventing HIV disease progression. The immune modulator and antioxidant dimethyl fumarate (DMF) is effective in treating immune-mediated diseases and it also has potential applications to limiting HIV disease progression. Among the relevant effects of DMF and its active metabolite monomethyl fumarate (MMF) are induction of a Th1 → Th2 lymphocyte shift, inhibition of pro-inflammatory cytokine signaling, inhibition of NF-κB nuclear translocation, inhibition of dendritic cell maturation, suppression of lymphocyte and endothelial cell adhesion molecule expression, and induction of the Nrf2-dependent antioxidant response element (ARE) and effector genes. Associated with these effects are reduced lymphocyte and monocyte infiltration into psoriatic skin lesions in humans and immune-mediated demyelinating brain lesions in rodents, which confirms potent systemic and central nervous system (CNS) effects. In addition, DMF and MMF limit HIV infection in macrophages in vitro, albeit by unknown mechanisms. Finally, DMF and MMF also suppress neurotoxin production from HIV-infected macrophages, which drives CNS neurodegeneration. Thus, DMF might protect against systemic and CNS complications in HIV infection through its effective suppression of immune activation, oxidative stress, HIV replication, and macrophage-associated neuronal injury.
Keywords: monomethyl fumarate, fumaric acid esters, neuroprotection, HIV, inflammation, oxidative stress, immune activation, antioxidant response, Nrf2, NF-κB
I. INTRODUCTION
Fumaric acid was initially proposed for treatment of psoriasis by the German chemist Walter Schweckendiek in 1959.1 However, because fumaric acid is poorly absorbed after oral intake, developing a mixture of fumaric acid esters (FAEs) to achieve higher effective bioavailability after oral dosing was necessary. In 1994, an enteric-coated preparation of FAEs containing 120 mg of dimethyl fumarate (DMF) and three salts of monoethyl fumarate (MEF) was licensed in Germany under the trade name Fumaderm® for the treatment of psoriasis. After positive results in psoriasis treatment trials, research into the therapeutic potential of FAEs has greatly accelerated. Most recently, a DMF-containing formulation, BG-12, showed marked efficacy in Phase III multiple sclerosis clinical trials.2, 3 Furthermore, additional in vitro and in vivo studies over the past decade have explored the therapeutic potential of FAEs for the treatment of other inflammatory diseases. With the recent FDA approval of BG-12 (brand name Tecfidera™) on March 27th, 2013 for the treatment of multiple sclerosis, the clinical application of FAEs for treating inflammatory diseases is likely to further rapidly expand. This review will assess the understanding of the mechanisms of FAEs in modulating immune responses and antioxidant responses and their potential application for treating disorders of inflammation and associated oxidative stress. Among the potential uses of FAEs is the treatment of HIV infection and its associated complications, as inflammation and oxidative stress are central to HIV pathogenesis. Notably, as DMF and MMF have been shown to effectively suppress inflammatory responses in vivo in both systemic and CNS compartments, DMF formulation therapy could offer adjunctive protection against both systemic and CNS complications of HIV infection.
II. PHARMACOKINETICS
Within minutes after oral intake, DMF is rapidly hydrolyzed by esterases within the small intestine to form its biologically active metabolite, monomethyl fumarate (MMF).4 MMF, but not DMF, can be detected in serum after oral DMF ingestion. DMF is undetectable likely due its rapid hydrolysis. MMF is further metabolized through the tricarboxylic acid cycle to form H2O and carbon dioxide, which is excreted through respiration. There is no evidence for cytochrome P450-dependent metabolism. Small amounts of non-metabolized MMF are detectable in the urine and feces.5 In fasting healthy individuals, the half-life of MMF was estimated to be ~56 minutes and peak serum levels (mean 6 µM, range 3–10 µM) were observed at ~178 minutes (standard deviation 39 minutes) after 120 mg of oral DMF (and 95 mg of MEF).5 When these healthy individuals ingested DMF with meals, the peak MMF serum levels increased by more than 25% in 57% of the patients, but they decreased by an average of 69% in the remaining subjects; this demonstrates that food intake increases variability in serum MMF concentrations. In a smaller study of psoriasis patients, the average half-life of MMF was estimated to be ~47 minutes with peak serum levels of 11.5 µM observed at ~219 minutes post-intake (two tablets of Fumaderm®, 240 mg DMF and 190 mg MEF).6 In both of these pharmacokinetic studies, DMF was not detected in serum (<0.07 µM in healthy individuals). This suggests that MMF, but not DMF, is absorbed into the systemic circulation, and that MMF is the functional molecule in vivo that should be targeted for mechanistic studies in vitro. Nonetheless, Rostami-Yazdi et al. detected the mercapturic acid derivative of DMF in urine from individuals taking Fumaderm®, suggesting that DMF might indeed enter the circulation prior to being rapidly metabolized.7 Thus, DMF might express some biological activity in vivo, however transiently, and therefore defining the mechanisms of action of FAEs requires the study of both MMF and DMF to fully define the biological effects of oral DMF formulation therapy.
III. FAE THEARAPY SAFETY AND ADVERSE EVENTS
Oral DMF (240 mg taken 2–3 times daily) has been tested in several clinical trials in individuals with psoriasis,8, 9 multiple sclerosis,2, 3, 10 and other inflammatory diseases.11, 12 The recent phase III efficacy trials for multiple sclerosis demonstrated no increase rate of patient dropout in drug vs. placebo-treated patients, indicating excellent long-term tolerability.2, 3 The most common reported adverse events in these trials were flushing in ~33% of patients, gastrointestinal events (diarrhea, nausea, upper abdominal pain, and vomiting) in ~33% of patients, and pruritus and proteinuria in ~9% of patients. Flushing and gastrointestinal events decreased after the first month of therapy. Transient eosinophilia has also been reported in patients at 4–8 weeks post initiation of FAE therapy.13 DMF formulation treatment was associated with a persistent leukocytopenia (~11% reduction in white blood cell count) and particularly lymphocytopenia (~30% reduction in circulating T-lymphocytes). White-cell and lymphocyte counts decreased in patients over the first year of DMF therapy and eventually plateaued at a lower set-point, though the majority of patients’ cell counts remained within the normal range. White-cell counts less than 3.0 × 109 per liter and lymphocyte counts of less that 0.5 × 109 per liter (corresponding to the National Cancer Institute Common Toxicity Criteria grade 2 or higher leukocytopenia and grade 3 or higher lymphocytopenia, respectively) were seen in ~4–7% of patients in the oral DMF groups versus ~1% in patients in the placebo group.2, 3
Despite the reduced white blood cell and lymphocyte counts, an increase in risk for malignancy or infection, including opportunistic infections, has not been reported in any FAE clinical trials or retrospective studies during the 20 years of FAE use.8, 14 However, recent case reports of progressive multifocal leukoencephalopathy (PML) have been reported in patients taking combination formulations of DMF and other fumarate derivatives (Fumaderm® and Psorinovo).15–17 Significant confounding risk factors for PML were present in these patients (including sarcoidosis, cancer, and efalizumab use) and so the true relative risk, if any, for FAE-associated PML is difficult to assess. Notably, to date, there is no evidence that patients treated with oral DMF monotherapy (BG-12/Tecfidera™) are at increased risk for opportunistic infections, including PML.17 Nonetheless, the rare case reports of PML (180,000 patents-years of experience with Fumdaerm®), suggest that vigilance in prescribers is critical. Prior to these reports, precautions for use such as monitoring blood lymphocyte levels had been suggested. A reduction of FAE dose or discontinuation was recommended if the white blood cell or lymphocyte counts fall below 3.0 × 109 or 0.5 × 109 cells per liter, respectively.18 This recommendation is especially prudent, in view of these recent PML case reports.
IV. MODE OF ACTION IN DIFFERENT CELL LINEAGES: IMMUNOMODULATION AND ANTIOXIDANT RESPONSE
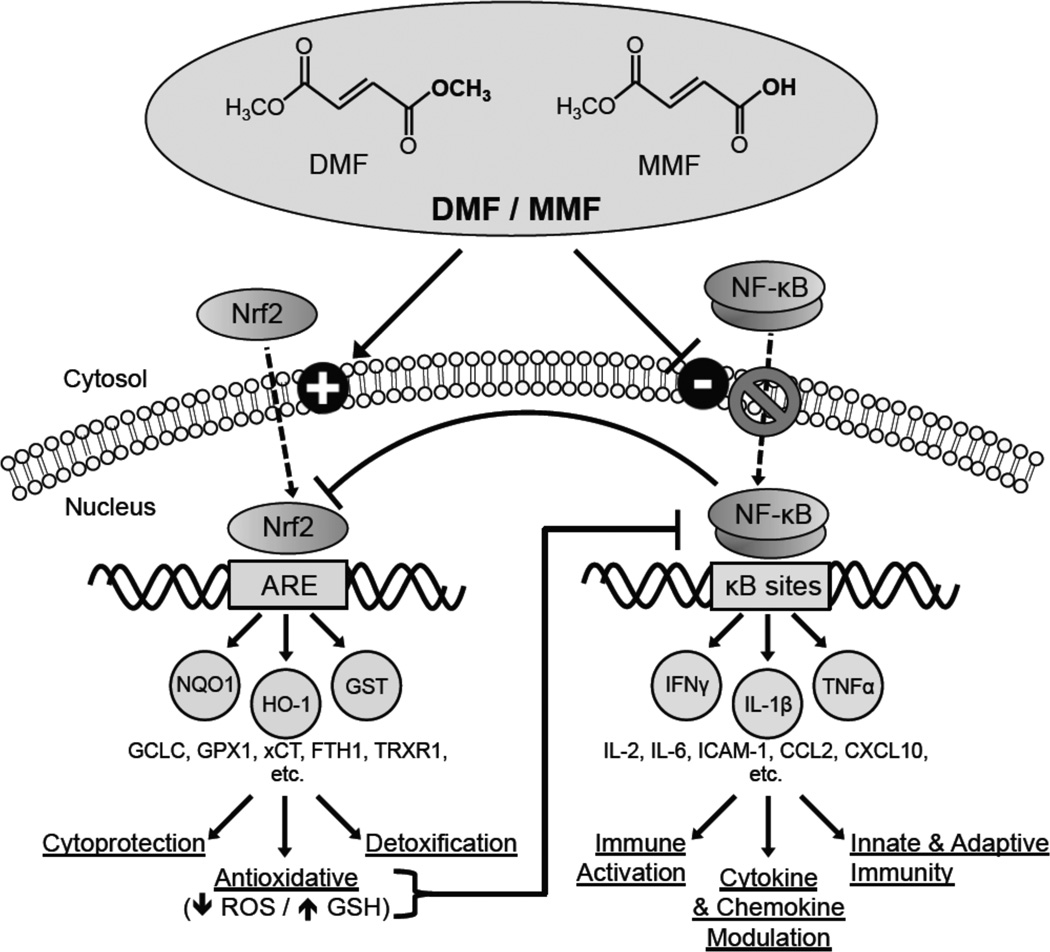
The effects of DMF and MMF on immune responses have thus far been only partially characterized. Several in vitro studies have demonstrated perturbation of nuclear factor κB (NF-κB) function through inhibition of NF-κB nuclear translocation and DNA binding (Figure 1). The NF-κB pathway plays a central role in regulating cytokine production, cellular activation, development, survival, and the innate and adaptive immune system among other roles (reviewed in19). NF-κB has been shown to induce TNFα, iNOS, IL-1, IL-2, IL-6, ICAM-1, and COX-2, among others.20 DMF and MMF also induce the nuclear factor erythroid-2 related factor-2 (Nrf2)-dependent antioxidant response element (ARE) pathway (Figure 1). The ARE response is a ubiquitous cytoprotective cellular stress response involving induction of multiple genes that protect cells from many forms of intracellular oxidative stress and injury (reviewed in21). Generally, oxidative stress occurs when cells are unable to detoxify injurious agents or repair damage resulting from reactive oxygen species, hydrogen peroxide, hydroxyl radicals, and other mediators of oxidative stress. Oxidative stress induces the translocation of Nrf2 to the nucleus where it binds to the ARE promoter element and activates gene transcription22–24 of hundreds of genes,23, 25, 26 including many antioxidant defense enzymes such as the sentinel cytoprotectant heme oxygenase-1 (HO-1),27 NAD(P)H quinone oxidoreductase-1 (NQO1),28 γ-glutamate cysteine ligase catalytic subunit (GCLC),29 glutathione S-transferase (GST),30 and the cysteine/glutamate transporter (xCT).31
Figure 1.
Effects of the fumaric acid esters (FAEs) DMF and MMF on the Nrf2-dependent antioxidant response and NF-κB pathways. Flat head on a line instead of an arrow head indicates a negative or inhibitory relationship. Abbreviations used: antioxidant response element (ARE), CC chemokine ligand (CCL), CXC chemokine ligand (CXCL), cysteine/glutamate transporter (xCT), dimethyl fumarate (DMF), ferritin heavy chain 1 (FTH1), γ-glutamate cysteine ligase catalytic subunit (GCLC), glutathione (GSH), glutathione peroxidase-1 (GPX1), glutathione S-transferase (GST), heme oxygenase-1 (HO-1), intercellular adhesion molecule (ICAM), interferon (IFN), interleukin (IL), monomethyl fumarate (MMF), NAD(P)H quinone oxidoreductase-1 (NQO1), nuclear factor E2-related factor 2 (Nrf2), nuclear factor κB (NF-κB), reactive oxygen species (ROS), thioredoxin reductase 1 (TrxR1), tumor necrosis factor (TNF).
Many studies have demonstrated complex regulatory interactions between the FAE-responsive Nrf2-dependent ARE and NF-κB pathways. ARE induction can inhibit the NF-κB pathway and thus indirectly modulate inflammatory cytokine and chemokine signaling.32 This inhibition of NF-κB likely occurs through ARE-driven reduction in oxidative stress, which has been shown to activate the NF-κB signaling pathway.33–36 Further supporting a role for Nrf2, several studies have demonstrated increased NF-κB activation and dysregulation of cytokines and chemokines in Nrf2−/− mice after inflammatory insults such as exposure to lipopolysaccharide (LPS)37 or TNFα,37 after infection with respiratory syncytial virus,38 and after traumatic brain injury.39 Direct pharmacologic activators of Nrf2 such as tert-butylhydroquinone, sulforaphane, and phenethyl isothiocyanate also attenuate NF-κB activation in vitro.40–43 Complicating the Nrf2 -NF-κB regulation, a reciprocal effect of NF-κB on Nrf2 function has been demonstrated.44 The NF-κB p65 subunit has been shown to repress Nrf2 signaling at the transcriptional level, through competition with Nrf2 for transcription co-activator CREB binding protein (CBP) and through recruitment of histone deacetylase 3, resulting in local hypoacetylation of the ARE promoter element and the consequent suppression of Nrf2 signaling.44 Thus, the Nrf2 and NF-κB pathways regulate each other through reciprocal inhibition (Figure 1). Additional studies are needed to further define the complex interactions between the NF-κB and Nrf2 pathways believed to be responsible for the effects of DMF and MMF. Through a combination of ARE induction and NF-κB signaling inhibition (Figure 1), and as yet undefined effects of other pathways, FAEs can promote an anti-inflammatory, anti-infiltrative, and anti-oxidative cellular state in multiple cell lineages, particularly immune cells. Notably, T-lymphocytes and cells of the monocyte lineage appear to be particularly sensitive to FAE exposure. The effects of DMF and MMF exposure on individual cell lineages is described in detail below and summarized in Table 1.
Table 1.
Effects of dimethyl fumarate (DMF) and monomethyl fumarate (MMF) on different cell lineages relevant to HIV infection.
| Cell Type | DMF/MMF | Cytokine/Receptor Expression changes |
Effects | References |
|---|---|---|---|---|
| T-cells | DMF | ↑ IL-4, IL-10 ↔ CCR4, IL-5 ↓ NF-κB DNA binding, INFγ, CXCR3 CXCR10, IL-6, TGFα, CD25, CLA | TH1 → Th2 shift ↓ endothelial rolling, firm adhesion, & tissue infiltration ↓ binding to E-selectin, P-selectin, and VCAM-1 | 47–51, 53 |
| MMF | ↑ IL-4, IL-5 ↔ NF-κB DNA binding, INFγ, IL-2 | Th1 → Th2 shift ↓ dendritic cell differentiation | ||
| PBMCs | DMF | ↓ CXCL8,CXCL9, CXCL10 | Th1 → Th2 shift ↓ macrophage inflammatory cytokine production ↓ leukocyte rolling | 47, 52, 53 |
| MMF | ↑ IL-4, IL-10, IL-5 ↔ INFγ, IL12, IL-2 | Th1 → Th2 shift | ||
| Monocyte/Macrophages | DMF | ↑ Nrf2 ↓ NF-κB nuclear translocation & DNA binding, TNFα, CCR2 | ↑ Antioxidant response, ↑ HO-1 ↑ superoxide anion ↓ CCL2-induced chemotaxis | 54, 57 |
| MMF | ↑ Nrf2 ↓ NF-κB nuclear translocation | ↑ Antioxidant response, ↑ HO-1 ↑ superoxide anion ↓ CCL2-induced chemotaxis | ||
| Dendritic Cells | DMF | ↑ IL-10 ↓ NF-κB activity (less free p65), IL-12, IL-6, IL-23 | ↑ Type II dendritic cells ↓Monocyte dendritic cell differentiation ↓ intracellular glutathione levels | 59–63 |
| MMF | ↓ NF-κB activity (less free p65), IL-12, IL-10, TNFα, IFNγ | ↑ Type II dendritic cells ↓Monocyte dendritic cell differentiation ↓ effectiveness in stimulating Th1 cytokine production in T cells | ||
| Endothelial cells | DMF | ↔NF-κB DNA binding ↓NF-κB nuclear entry, E-selectin, VCAM-1, ICAM-1 | ↓ TNFα indicated cytokine release ↓ leukocyte rolling and firm adhesion | 64–67 |
| MMF | ↔ NF-κB DNA binding, E-selectin, VCAM-1, ICAM-1 | ? | ||
| Microglia | DMF | ↑ Nrf2 expression ↓ IL-1β, IL-6, TNFα, iNOS | ↑ Antioxidiant response, ↑ HO-1 ↓ Nitrites | 55,56 |
| MMF | ↑ Nrf2 | ↑ Antioxidant response, ↑ HO-1 | ||
| Astrocytes | DMF | ↑Nrf2 expression, nuclear entry, and DNA binding ↓ IL-1β, IL-6, TNFα | ↑ Antioxidant response, ↑ HO-1 ↑ Viability after oxidative stress | 55, 69, 70 |
| MMF | ↑Nrf2 expression, nuclear entry, and DNA binding | ↑ Antioxidant response, ↑ HO-1 ↑ viability after oxidative stress (Nrf2-depdent) | ||
| Neurons | DMF | ↑Nrf2 nuclear entry ↔ NF-κB nuclear entry | ↑ Antioxidant response ↑ viability post oxidative & glutamate toxicity (Nrf2-dependent) | 69–71 |
| MMF | ↑Nrf2 nuclear entry | ↑ Antioxidant response ↑ viability post oxidative stress (Nrf2-dependent) ↔ viability post glutamate toxicity |
A. Effect of FAE in T-lymphocytes
Early studies of Fumaderm® for psoriasis treatment demonstrated a significant decline (~30%) in lymphocytes, particularly T-lymphocytes, in 94% of treated patients.45 Similar results were observed in recent Phase III Multiple Sclerosis trials with the DMF formulation BG-12 (240 mg of DMF 2–3 times daily).2, 3 However, the mechanism of DMF-induced lymphocytopenia is unknown. In vitro studies have demonstrated that DMF, but not MMF, induces apoptosis in human T-lymphocytes after 48 hours of exposure to a concentration of ~70 µM, which is 10-fold higher than the MMF serum levels achieved with oral DMF dosing.46 This in vitro apoptotic effect of DMF was not seen at ~7uM, the physiologic concentration achieved by its primary in vivo metabolite MMF. Because DMF is not detectable in vivo after oral dosing, the contribution of direct DMF cytotoxicity to this lymphocytopenia is questionable. Thus further studies of the effects of long term exposure of MMF on T-lymphocyte viability and proliferation are needed.
Analyses of the immunomodulatory effects of FAEs on human T-lymphocytes demonstrated that FAEs alter cytokine production, cytokine and chemokine receptor expression, and adhesion molecule expression. Both DMF and MMF increased the production of multiple Th2 cytokines (IL-4, IL-10)47–49 in T-lymphocytes. Furthermore, DMF, but not MMF, suppressed Th1 cytokine (IFN-γ) release and chemokine receptor (CXCR3) expression, as well as the skin-homing chemokine receptor CCR10.50 In contrast, DMF exposure decreased release of IL-6,50 a cytokine that inhibits Th1 polarization and promotes Th2 differentiation. The suppression of Th1 cytokine release and chemokine receptor expression is likely linked to DMF’s ability to inhibit NF-κB DNA binding in T-lymphocytes,51 an effect that is not induced by MMF in vitro. In line with these findings, DMF inhibited IFN-γ- and LPS-induced Th1 chemokine (CXCL9 and CXCL10) production in a dose dependent manner in human peripheral blood mononuclear cells (PBMC).52 Furthermore, DMF was able to increase the expression of Th2 cytokines in lymphocytes in multiple sclerosis patients and mice with herpes stromal keratitis (see section V. FAEs In Inflammatory Diseases for further discussion), suggesting that DMF treatment maintains the Th1 to Th2 shift in the setting of superimposed infections and other inflammatory states. The persistence of this Th1 to Th2 shift after DMF therapy is stopped has not been reported.
DMF has also been shown to reduce adhesion molecule expression (CD25, HLA-DR, and cutaneous lymphocyte-associated antigen) in treated T-lymphocytes, which can explain the decreased binding of treated lymphocytes to E-selectin, P-selectin, and VCAM-1.53 Such effects might also explain reduced leukocyte rolling and adhesion in DMF-treated mice.53 As many of these in vitro effects were either not studied or were not observed with MMF treatment, it is unknown if they occur in vivo in DMF-treated subjects. Furthermore, the roles for DMF and MMF in Nrf2-driven ARE activation in T-lymphocytes, beyond its associated NF-κB modulation, have not been studied. Thus, although limited in scope, these in vitro studies suggest that DMF, and MMF in part, promote a shift from a pro-inflammatory, IFN-γ-driven Th1 T-lymphocyte profile to an anti-inflammatory, IL-10-driven Th2 T-lymphocyte profile. Such a shift from a Th1 response (autoimmune responses and the killing intracellular parasites) to a Th2 response associated with eosinophilic responses and counteraction against Th1 responses could result in decreased T-lymphocyte activation and tissue infiltration in vivo.
B. Effect of FAE in B-Lymphocytes
Studies of the direct effects of FAE on B-Lymphocyte function have not been reported. However, there is evidence that disruption of NF-κB signaling can interfere with B-lymphocyte development and survival,19 suggesting FAE-mediated NF-κB modulation may alter Blymphocyte function.
C. Effect of FAE in Monocytes/Macrophages/Microglia
DMF and MMF express potent anti-inflammatory and antioxidant effects in monocytes, monocyte-derived macrophages, and microglia. In human macrophages, DMF and MMF decreased NF-κB nuclear translocation and DNA binding in response to TNFα exposure and reduced TNFα secretion in response to phytohaemagglutinin, suggesting a decreased state of activation.54 Furthermore, DMF and MMF suppressed CCL2-induced chemotaxis of human monocytes,54 which would likely result in decreased infiltration across endothelial surfaces into tissue. In LPS-activated microglia, DMF has been shown to decrease release of the pro-inflammatory cytokines IL-1β, IL-6, and TNFα,55 likely through decreased NF-κB signaling. DMF- and MMF-induced expression of Nrf2-dependent ARE proteins, including the ubiquitous cytoprotectant enzyme HO-1, was also reported in macrophages and microglia,54, 56 suggesting that FAEs can modulate the oxidative state within these cells. However, DMF- and MMF-treated monocytes expressed higher levels of reactive oxygen intermediates in the respiratory burst,57 a rapid release of free radicals that is essential step in immunological defense, particularly against bacteria and fungi. In contrast, induction of HO-1 can also decrease expression of reactive nitrogen species, another principal component of the respiratory burst, through suppression of the inducible nitric oxide synthase (iNOS) expression and associated nitric oxide induction.58 Overall, these data suggest that DMF and MMF have potent anti-inflammatory and anti-oxidative effects in macrophages and microglia and this further suggests that DMF formulations could be effective in treating both systemic and CNS inflammatory diseases that involve macrophage/microglial activation.
D. Effect of FAE in Dendritic Cells
Both DMF and MMF suppress monocyte-derived dendritic cell differentiation and cytokine production in vitro through suppression of both NF-κB and extracellular signal-regulated kinase 1 and 2 (ERK1/2) signaling.59, 60 In addition, MMF decreased IL-12, IL-10, TNFα, and IFN-γ secretion from dendritic cells, which resulted in decreased dendritic cell effectiveness in stimulating Th1 cytokine production in T-lymphocytes.61, 62 DMF exposure to dendritic cells also decreased inflammatory cytokine production (IL-12, IL-23, and IL-6), dendritic cell maturation, and effectiveness in generating activated T-lymphocytes60. These findings, coupled with reports demonstrating that DMF exposure increases the production of IL-10 by dendritic cells, suggest that DMF promotes the type II dendritic cell phenotype.63 In summary, FAE interference with dendritic cell differentiation and cytokine secretion further suppresses Th1 responses in addition to the direct effects of FAEs on T-lymphocytes and also promotes a type II dendritic cell response.
E. Effects of FAE in Endothelial Cells
Similar to DMF effects in T-lymphocytes, macrophages, and dendritic cells, DMF has been shown to decrease TNFα-induced NF-κB activation and associated activation of vascular endothelial cells.64, 65 DMF also decreased the expression of the adhesion molecules E-selectin, VCAM-1, and ICAM-1 on endothelial cells, resulting in decreased leukocyte rolling, firm adhesion, and diapedesis in vitro,66, 67 perhaps through inhibition of NF-κB activation. Notably, these effects were not seen with MMF, which might indicate that DMF formulations might not induce these effects after oral administration. Nonetheless, these results suggest a second mechanism (decreased adhesion and transendothelial migration) by which FAEs decrease immune cell migration into tissues, in addition to direct effects on chemotaxis by exposed lymphocytes and mononuclear phagocytes, which could further limit tissue inflammation.
F. Effects of FAE in CNS Cells
The effects of FAEs in the CNS could be expressed through modulation of macrophages, microglia, endothelial cells, astrocytes, neurons, oligodendrocytes, and choroid plexus cells. Among those cell types in which FAE effects have been examined are monocyte-derived macrophages, microglia, endothelial cells (previously discussed), astrocytes, neurons, and oligodendrocytes. No studies of DMF or MMF effects in choroid plexus-derived cells have been published. DMF has been shown to decrease IL-1β, IL-6, and TNFα release from astrocytes and microglia,55, 68 which could contribute to an anti-inflammatory effect in the CNS compartment. Furthermore, DMF and MMF, through Nrf2 activation, induced the expression of ARE effector proteins, including NAD(P)H quinone oxidoreductase-1 and HO-1, in microglia, astrocytes, and neurons.55, 69, 70 Such induction of antioxidant enzymes in astrocytes and neurons was shown to protect against oxidative injury and glutamate toxicity.69–71 Despite this protection in astrocytes and neurons, DMF and MMF did not protect against oxidative injury in the oligodendrocyte CG4 cell line.72 These studies and those mentioned above suggest a role for FAEs as both indirect neuroprotectants through anti-inflammatory and anti-oxidative effects in macrophages, microglia, astrocytes, and endothelial cells, and as direct neuroprotectants through induction of the ARE in neurons.
V. FAEs IN INFLAMMATORY DISEASES
A. Psoriasis
Psoriasis is a common (1–3% of the worldwide population), systemic T-lymphocyte-mediated chronic inflammatory skin disease characterized by cutaneous inflammation, increased epidermal proliferation, abnormal keratinization, and appearance of erythematous plaques (reviewed in73). Psoriasis is the disease for which FAEs have been most widely applied for therapeutic benefit. The efficacy of FAEs likely results from their potent anti-inflammatory and antioxidant effects.
1. Inflammation in Psoriasis
Early reports demonstrated persistent Th1 T-lymphocyte activation within psoriatic plaques,74–76 although natural killer T-lymphocytes were also implicated.77–79 Recent work has shown a significantly complicated picture of the inflammatory milieu in psoriasis plaques. Current models now incorporate an inflammatory axis that balances the Th1, Th2, Th17, and T-regulatory type responses as well as contributions by dendritic cells, natural killer T-lymphocytes, and macrophages (reviewed in80). Underscoring this central role of inflammation in the pathogenesis of psoriasis, numerous immunomodulatory therapies have shown efficacy in treating psoriasis, including corticosteroids,81 tacrolimus,82 pimecrolimus,83 methotrexate,84 and the TNFα inhibiting therapies etanercept,85 infliximab,86 and adalimumab.87
2. Oxidative Stress in Psoriasis
In addition to markers of immune activation, markers of oxidative stress are consistently elevated in psoriasis patients and coupled with antioxidant dysfunction. These patients have increased carbonylation (oxidative stress marker) of macromolecules in skin biopsies and cultured fibroblasts,88 increased plasma malondialdehyde (lipid peroxidation product),89 increased urine 8-hydroxydeoxyguanosine (a marker of DNA oxidation) and nitrate (a product of nitric oxide),90 increased lipid hydroperoxides,91 and lower serum antioxidants, including paraoxonase-1 and thiols.89, 91, 92 Furthermore, the 55 L/M allele of paraoxonase-1 (an antioxidant enzyme implicated in psoriasis that hydrolyses lipid peroxidases) is a risk factor for psoriasis and allele carriers have higher levels of oxidative stress and lower ARE activity.93 In summary, psoriasis is a chronic inflammatory skin diseases involving numerous immune axes, particularly various arms of the T-lymphocyte axis, and elevated oxidative stress and impaired antioxidant responses.
3. Clinical Trials of FAEs in Psoriasis
Before the roles for immune activation and oxidative stress in psoriasis were identified, the German chemist Walter Schweckendiek empirically developed an FAE therapy for psoriasis.1 Although unknown at that time, the anti-inflammatory effects through suppression of NF-κB activity and oxidative stress reducing effects through induction of the ARE suggest a role for FAEs in treating psoriasis. The first randomized, double-blind trial of FAE therapy for psoriasis was published by Nugteren-Huying et al. in 1990.94 This trial demonstrated an average reduction in the psoriatic skin lesion area by 68% in 39 psoriasis patients treated with a combination of DMF and MEF for 4 months, an effect significantly different from the groups treated with an MEF/octylhydrogen-fumarate combination or with a placebo. Since that initial trial, other larger and longer-term clinical trials (up to 3 years) have affirmed the effectiveness and safety of FAE therapy for patients with severe psoriasis.9, 13, 95–99 Combining DMF with other FAEs including MEF (Fumaderm®) showed greater efficacy and patient adherence to FAE therapy than DMF monotherapy.95, 97 The mechanism for this synergistic effect is unknown, but likely could be due to competitive metabolism.
4. FAE effects on keratinocytes: role in psoriasis
The mechanisms of action of FAE therapy in effectively treating psoriasis likely represent both direct effects on keratinocytes and the aforementioned immunomodulatory and anti-oxidative properties of DMF and MMF on other cell types. Keratinocytes play a major role in the inflammatory response in psoriasis through expression of chemokines and cytokines (reviewed in100). DMF treatment in co-cultured keratinocytes and T-lymphocytes increased the Th2 cytokine IL-10, while decreasing IFN-γ, IL-6, and TGFα.48 Furthermore, DMF inhibited the expression of the chemokines CXCL1, CXCL8, CXCL9, CXCL10, and CXCL11 in primary human keratinocytes.52 These immunomodulatory effects on keratinocytes have not been tested with MMF. However, DMF and to a lesser extent MMF have been shown to decrease keratinocyte proliferation.101, 102 In clinical studies, DMF treatment of psoriasis in patients resulted in improved epidermal hyperproliferation, epidermal thickness, and keratinocyte differentiation.98 Overall, these direct effects on keratinocytes suggest multiple mechanisms that modulate chemoattraction of macrophages, T-lymphocytes, and neutrophilic granulocytes and pathologic proliferation of keratinocytes.
5. FAEs reduce immune cell infiltration in psoriasis and other skin disorders
Psoriasis involves infiltration of the dermis by CD4+ T-lymphocytes and the epidermis by CD8+ T-lymphocytes, the majority of which are CD45RO+ (memory effector).103–106 Clinical studies have demonstrated that DMF treatment of psoriasis patients for sixteen weeks decreases CD3+CD4+CD45RO+ T-lymphocytes in the dermis and CD3+ CD8+ CD45RO+ T-lymphocytes in the epidermis.98 This FAE effect of decreased immune cell infiltration is supported by the demonstrations of FAE efficacy in decreasing immune cell infiltration in other inflammatory skin diseases. These include disseminated granuloma annulare11, 107 and recalcitrant cutaneous sarcoidosis,12 each of which involves large number of CD3+ lymphocytes and CD3+/CD68+ mononuclear phagocytes, each of which is suspected to result from unregulated Th1 reaction. These in vivo findings are consistent with in vitro studies of DMF that demonstrate decreased expression of adhesion molecules in endothelial cells66, 67 and lymphocytes53 and decreased CCL2-induced chemotaxis in macrophages54, resulting in decreased immune cell tissue infiltration into tissues. Of note, DMF also decreased T-lymphocyte expression of the chemokine CCR10,50 which is often expressed on skin-homing T-lymphocytes. In addition to decreased chemotaxis and homing in DMF-exposed macrophage and T-lymphocytes, induction of leukocytopenia and lymphocytopenia by DMF might also contribute to DMF efficacy in treating psoriasis.2, 3, 8–10
B. Multiple Sclerosis
Multiple sclerosis is the most common cause of neurological disability in young adults worldwide with approximately 2.1 million cases worldwide and 400,000 cases in the United States.108, 109 Epidemiologic data suggest that multiple sclerosis pathogenesis involves environmental influences occurring on a background of genetic susceptibility.110 In most patients, multiple sclerosis presents as a relapsing-remitting disease characterized by dynamic inflammatory demyelinating lesions in the CNS that are associated with sensory (~30%) or motor (~13%) disturbance of the limbs, partial or complete visual loss (~16%) and other signs and symptoms (reviewed in111). Multiple sclerosis is the first CNS disease successfully treated with FAEs. The efficacy of FAEs in multiple sclerosis likely results from their potent anti-inflammatory and antioxidant effects within the CNS compartment.
1. Inflammation and Degeneration in Multiple Sclerosis
Traditionally multiple sclerosis was considered to be an autoimmune, inflammatory disease of the CNS mediated by an aberrant T-lymphocyte attack against CNS elements, particularly myelin, resulting in degeneration of axons.112 This view was strongly supported by pathological, laboratory, radiological, genetic, epidemiological, and therapeutic data, all of which supported an autoimmune and inflammatory phenotype that is driven by Th1 type inflammation in the brain (reviewed in112). However, recently published studies have posited that multiple sclerosis is primarily a degenerative disorder, with a secondary immune response to myelin and other highly immunogenic debris (reviewed in113). Evidence for this includes reports demonstrating that myelin abnormalities might begin at the inner myelin sheath in areas outside focal inflammation;114, 115 patients in early stages of multiple sclerosis show little evidence of T-lymphocyte or B-lymphocyte infiltration in newly formed demyelinating lesions (although evidence of the innate immune response by macrophage infiltration and microglial activation is present, probably for clearing debris);116, 117 and the ineffectiveness of autologous hematopoietic stem-cell transplantation to halt the progression of demyelination, axonal degeneration, and brain atrophy despite reducing CNS inflammatory activity.118, 119 Regardless of the initial pathologic substrate, the inflammatory response present in relapsing-remitting multiple sclerosis plays a critical role in disease pathogenesis as evidenced by the success of numerous immune modulators, including interferon beta-1b100 and beta-1a,120, 121 glatiramer acetate,122, 123 fingolimod,81, 124 and teriflunomide125 among many others, in reducing and in some instances eliminating neuroinflammation and clinical relapses.
2. Clinical Trials of FAEs for Multiple Sclerosis Treatment
BG-12, an oral FAE formulation containing DMF, recently showed strong positive effects in two independent placebo-controlled phase III clinical trials (DEFINE and CONFIRM) involving more than 2600 relapsing-remitting multiple sclerosis patients.2, 3 Importantly, BG-12 improved clinical outcomes associated with suppression of brain inflammation in such patients when given twice daily or three times daily, likely due in part to the CNS penetrance of MMF.70 BG-12 therapy in both trials significantly reduced the proportion of patients who relapsed and the annualized relapse rate at 2 years by ~50%. Furthermore, BG-12 reduced the number of gadolinium-enhancing lesions (inflammation) and of new or enlarging T2-weighted hyperintense lesions (demyelination). In the DEFINE trial,2 BG-12 significantly reduced progression of disability by 38% with BG-12 twice daily and 34% with BG-12 thrice daily. In the CONFIRM trial,3 BG-12 also reduced progression of disability, but the trend was not significant. Given the positive results of these clinical trials BG-12 was approved for the treatment of multiple sclerosis by the FDA on March 27th, 2013 under the brand name Tecfidera™. These results show that the oral DMF formulation (BG-12/ Tecfidera™) is effective in modifying CNS inflammation and associated neurological dysfunction in treated patients and they suggest that DMF formulations should be considered for use in other neuroinflammatory diseases.
3. FAE Immune Modulation in Multiple Sclerosis
The balance between Th2 and Th1 responses plays a key role in multiple sclerosis pathogenesis. Multiple sclerosis lesions contain activated CD4+ and CD8+ T-lymphocytes, mononuclear phagocytes, and high expression of IFN-γ, TNFα, IL-1, and leukocyte and vascular adhesion molecules.126–128 A shift from a Th1 towards a Th2 cytokine profile, as induced by FAEs in T-lymphocytes and PBMCs in vitro,47–50, 52 could have a beneficial effect on the clinical course of the disease. This is supported by a recent study that showed that BG-12-treated multiple sclerosis patients showed increased intracellular expression of the Th2 cytokine IL-10 in CD4+ peripheral lymphocytes, with no change in IFN-γ expression.129 It has also been proposed that the mild leukocytopenia and lymphocytopenia observed in BG-12 treated individuals could contribute to BG-12 efficacy by reducing the number of circulating immune cells.130 A possible complimentary mechanism for the efficacy of BG-12 in multiple sclerosis is the decreased expression of adhesion molecules on endothelial cells66, 67 and lymphocytes53 and decreased CCL2-induced chemotaxis in macrophages54, resulting in decreased immune cell tissue infiltration into the brain. Each of these mechanisms is supported by studies of autoimmune encephalomyelitis (EAE), the murine model of multiple sclerosis. During chronic EAE, MMF significantly and DMF nonsignifcantly reduced the infiltration of T-lymphocytes in the spinal cord. Additionally, MMF, and to a lesser degree DMF, significantly reduced the infiltration of macrophages into the spinal cord.131 Thus, although the major beneficial effects of BG-12/Tecfidera™ in treating multiple sclerosis are likely associated primarily with the ability to reduce CNS inflammation, the reduction of levels of circulating T lymphocytes and reduction of T-lymphocyte and monocyte/macrophage chemotaxis might also contribute to the beneficial effects in multiple sclerosis.
4. FAE Antioxidant Response Induction in Multiple Sclerosis
The emerging and prominent role for oxidative stress in multiple sclerosis pathogenesis and the well studied protective effects of the ARE activation in cell of different lineages in the CNS highlight another probable mechanism of FAE efficacy in multiple sclerosis. Oxidative stress associated with mitochondrial dysfunction132 and excessive release of free radicals133 has been strongly associated with multiple sclerosis pathogenesis, perhaps contributing to increased CNS leukocyte invasion and activation, neurodegeneration, and oligodendrocyte damage. Free radicals include reactive oxygen species, reactive nitrogen species, and nitric oxide, which are mainly produced by macrophages and microglia.134, 135 In addition, myeloperoxidase activity, which produces the cytotoxic oxidizer hypochlorous acid and tyrosyl radicals catalyzed from H2O2, is elevated in macrophages and microglia in actively demyelinating white matter and cortical lesions in multiple sclerosis.136 These data suggest that a reduction in oxidative stress might reduce neuroinflammation and associated CNS damage, each of which could be suppressed by BG-12.
BG-12 is the first multiple sclerosis therapy shown to activate the Nrf2-dependent ARE pathway and thus reduce oxidative stress. Previous studies have shown that FAE induction of the ARE and its effector proteins, including HO-1, prevented oxidative-stress induced astrocyte and neuronal injury,69, 70, 137 decreased microglia nitric oxide burst,72 and prevented myelin loss within the CNS.70 In EAE studies, DMF treatment attenuated axonal loss and reduced astrocyte activation in wild-type mice, but not in Nrf2−/− knockout mice,70 strongly suggesting a role for Nrf2-dependent ARE activation in FAE-mediated neuroprotection. This finding is supported by in vitro data that demonstrated that Nrf2 knockdown removed the dose-dependent protective effect of DMF and MMF on astrocyte and neuron cell viability after toxic oxidative challenge.69 High concentrations (100 µM) of DMF and MMF have been shown to induce the ARE protein HO-1 in oligodendrocytes without evidence of drug cytotoxicity.138 However, whether FAEs are directly protective against oxidative injury or other insults in oligodendrocytes has not been reported. Despite this strong evidence linking ARE induction to protection during CNS oxidative and inflammatory injury in multiple sclerosis, the specific antioxidant pathways and effector proteins responsible for these protective effects are not known.
C. Other Inflammatory Diseases as Targets for FAEs
1. Huntington’s Disease
FAEs have been studied preclinically in other diseases linked to inflammation and oxidative stress. Huntington’s Disease (HD) is an autosomal dominant, progressive neurodegenerative genetic disorder, associated with an expanded trinucleotide (CAG)n repeat encoding a polyglutamine stretch in the N-terminus of the huntingtin protein.139 Oxidative stress, mitochondrial dysfunction, and other metabolic deficits have been shown to play central roles in the pathogenesis of HD.140 Furthermore, symptomatic CNS disease staging in HD model mice was associated with increased levels of brain oxygen radicals.141 Treatment of these HD model mice with DMF prolonged survival and preserved motor functions and neuronal morphology within the striatum and motor cortex,6 likely through the induction of the ARE. These findings implicate a central role for oxidative stress in HD pathology and have led to multiple clinical trials studying the efficacy of various antioxidants and modulators of the Nrf-2/ARE pathway,142 though FAEs have not yet been studied clinically in HD. The efficacy of FAEs in this model and in multiple sclerosis suggests a broader applicability to CNS disease states with oxidative stress components, regardless of cause.
2. Herpes Stromal Keratitis
Herpes Stromal Keratitis (HSK), the leading cause of infectious blindness in developed nations,143 is a disease characterized by an immune-based pathological reaction that is triggered by herpes simplex virus (HSV) infection of the cornea. In HSK, CD4+ Th1 T-lymphocytes are believed to be the principal disease mediators, as the Th1 cytokines IFN-γ and IL-2 were highly expressed in the HSK cornea144 and anti-IFN-γ and anti-IL-2 treatment decreased the incidence and severity of herpetic corneal disease.145–147 Neutrophils and antigen-presenting cells may also play central roles in HSK pathogenesis.148, 149 In mice exposed to HSV after a corneal scratch, DMF therapy reduced corneal infiltration of lymphocytes, polymorphonuclear leukocytes (PMNs), and macrophages and decreased the severity and incidence of stromal keratitis.150, 151 Additional studies of these mice demonstrated that DMF treatment increased the expression of Th2 cytokines IL-4 and IL-10 in lymphocytes cultured from the spleen, although no changes were seen in expression of the Th1 cytokines IFN-γ and IL-2.151 Thus, while Th1 cytokines are associated with HSK progression, Th2 cytokines are consistently associated with improvement.151–153 Even though DMF treatment improved outcomes in these HSK mouse studies, DMF had no effect on viral titers, HSV antibody production, or HSV antigen-specific T cell proliferation.151 These data demonstrate that FAEs have therapeutic efficacy in treating virally-induced inflammation and immune cell infiltration, and that these effects likely result from direct modulation of the immune response rather than interference with virus replication.
VI. FAEs AS ADJUNCTIVE THERAPY FOR HIV INFECTION
A. HIV Systemic Disease
Human immunodeficiency virus type 1 (HIV-1) infection of cells of the immune system (helper T-lymphocytes, macrophages, and dendritic cells) results in profound immunosuppression as well as chronic immune activation. Untreated HIV infection is characterized by high levels of viremia, low levels of CD4+ T-lymphocytes, and subsequent progressive failure of the immune system, resulting in life-threatening opportunistic infections and/or cancer and death. With the widespread use of highly active antiretroviral therapy (HAART) or combination antiretroviral therapy (cART) as such therapy is now called, the long-term survival of individuals living with HIV/AIDS has dramatically increased. As of 2006, the 6-year survival rate for cART-treated HIV infected individuals was 90%.154 Today, a 20 year-old individual with HIV infection and starting cART is now expected to live into his or her 60s.155
1. Persistent Systemic Immune Activation in HIV/AIDS: A Target For Adjunctive Immunosuppressive Therapy
Despite cART providing a significant reduction in morbidity and mortality in HIV-infected individuals, life expectancy is still reduced, particularly in those who begin cART after becoming immunosuppressed (CD4 T-lymphocyte counts < 200 cells/µl).156–160 A significant risk factor for increased mortality and end-organ diseases in cART-treated individuals is chronic immune activation,161 which is thought to result from the effects of increased microbial translocation across the damaged gastrointestinal tract (reviewed in162). In HIV-infected humans and simian immunodeficiency virus (SIV)-infected macaques microbial translocation across the gut results from several events at the gut mucosal barrier: i) severe depletion of mucosal CD4+ T lymphocytes, ii) mucosal immune activation and persistent inflammation, iii) structural damage to the intestinal epithelium, and iv) migration of enteric microbial products into the local mesenteric lymph nodes and extraintestinal sites through the portal and systemic circulation.162 Among translocated microbial products is lipopolysaccharide (LPS),162–164 which, upon entering the systemic circulation, interacts with LPS-binding protein (LBP) and then facilitates binding to the monocyte CD14 receptor and toll-like receptor 4 (TLR4). These events then lead to NF-κB activation, cytokine production, and associated immune activation events.165, 166
Enhanced immune activation is a consistent observation in both HIV-infected humans and SIV-infected macaques,167–175 and immune activation of T-lymphocytes and monocytes and associated inflammation are thought to drive HIV pathogenesis.163, 164, 176–184,185, 186,187, 188 Elevated plasma levels of IL-6, D-dimer, C-reactive protein (CRP), and fibrinogen are strongly associated with HIV/AIDS mortality.189, 190 Monocyte/macrophage activation is associated with elevated blood levels of LPS, soluble CD14 (sCD14), and numbers of CD14+/CD16+ monocytes.182, 191 CD16+ monocytes are more permissive for HIV infection than CD16− monocytes,192 and circulating CD14+/CD16+ monocytes levels are correlated with increased risk of disease progression.191 Elevated plasma LPS from gut bacteria translocation can increase the circulating CD16+ monocyte population.193 LPS is also thought to activate T-lymphocytes in HIV-infected individuals through interactions with TLR4, resulting in effector T-lymphocyte sequestration in lymphoid tissues and T-lymphocyte turnover.194 The increased T-lymphocyte turnover associated with generalized immune activation could increase the proliferative generation of new memory CD4+ T lymphocytes, which provide new targets for HIV replication and thus promote disease progression, as defined by the endpoints of death, AIDS, CD4+ T lymphocyte counts, and plasma viral load.162, 195 Depletion of both CD4+ and CD8+ T-lymphocyte populations, which is strongly associated with disease progression,196 occurs in late stages of disease and is associated with and proposed to be caused by immune activation.197, 198
In addition to increasing plasma levels of LPS, HIV can induce immune activation of monocyte/macrophages through direct effects of infection or through the release of factors that can interact with bystander uninfected monocyte/macrophages. For example, the HIV envelope glycoprotein gp120 is shed from virions and can transduce signals in monocytes when presented as a soluble protein or as a component of the virus particle even when presented on non-infectious virions (as in plasma in vivo).199, 200 Gp120 interacts with non-infected, bystander monocytes/macrophages and activates intracellular pathways that lead to monocyte/macrophage immune activation.199, 200 Other factors released from HIV-infected macrophages, such as TNFα and other proinflammatory cytokines, can also induce bystander cell immune activation.201–203
Thus, as systemic immune activation is driven by HIV replication, suppression of HIV replication with cART significantly dampens such immune activation; however this suppression is incomplete.162, 163, 178, 180, 181, 204–208 The persistence of immune activation despite suppression of HIV replication to undetectable levels remains a major obstacle to preventing further disease progression in cART-treated HIV infected individuals. Successful therapeutic targeting of persistent immune activation has now been proposed as a critical goal for further improving long-term survival, lowering risk of associated end-organ diseases, and improving quality of life in HIV-infected cART-treated individuals.162, 195
2. Systemic Oxidative Stress in HIV/AIDS: A Target For Adjunctive Antioxidant Therapy
HIV infection can induce systemic oxidative stress209–212 through both chronic immune activation213–215 and direct effects of HIV proteins.216–219 Increased oxidative stress in HIV-infected individuals was first suggested after confirmation of diminished levels of reduced glutathione in plasma, lymphocytes, PBMCs, and monocytes isolated from such individuals.220–222 These early findings were supported by later studies demonstrating reduced levels of thioredoxin (a thiol antioxidant) in HIV-infected cells223 and elevated serum levels of the lipid peroxidation products malondialdehyde224, 225 and lipid hydroperoxides226 in HIV-infected individuals. Moreover, the HIV proteins gp120,216, 219, 227–230 Vpr,218, 231 and Tat217, 219 have been directly implicated in the induction of cellular oxidative stress. Oxidative stress in turn can drive NF-κB-driven HIV replication232–234 and inflammatory cytokine release,232, 235–239 thus perpetuating chronic systemic immune activation and disease progression in HIV-infected individuals. Furthermore, in vivo markers of oxidative stress correlate with systemic disease progression in HIV-infected individuals240–242. One study showed that glutathione deficiency in CD4+ T-lymphocytes from HIV-infected patients was associated with markedly increased mortality.243 These findings implicate oxidative stress in HIV disease pathogenesis and mortality and highlight the potential for adjunctive antioxidant therapies to ameliorate disease progression in cART-treated patients.
3. FAEs as Candidate Adjunctive Therapy for Systemic HIV disease in cART-Treated Individuals
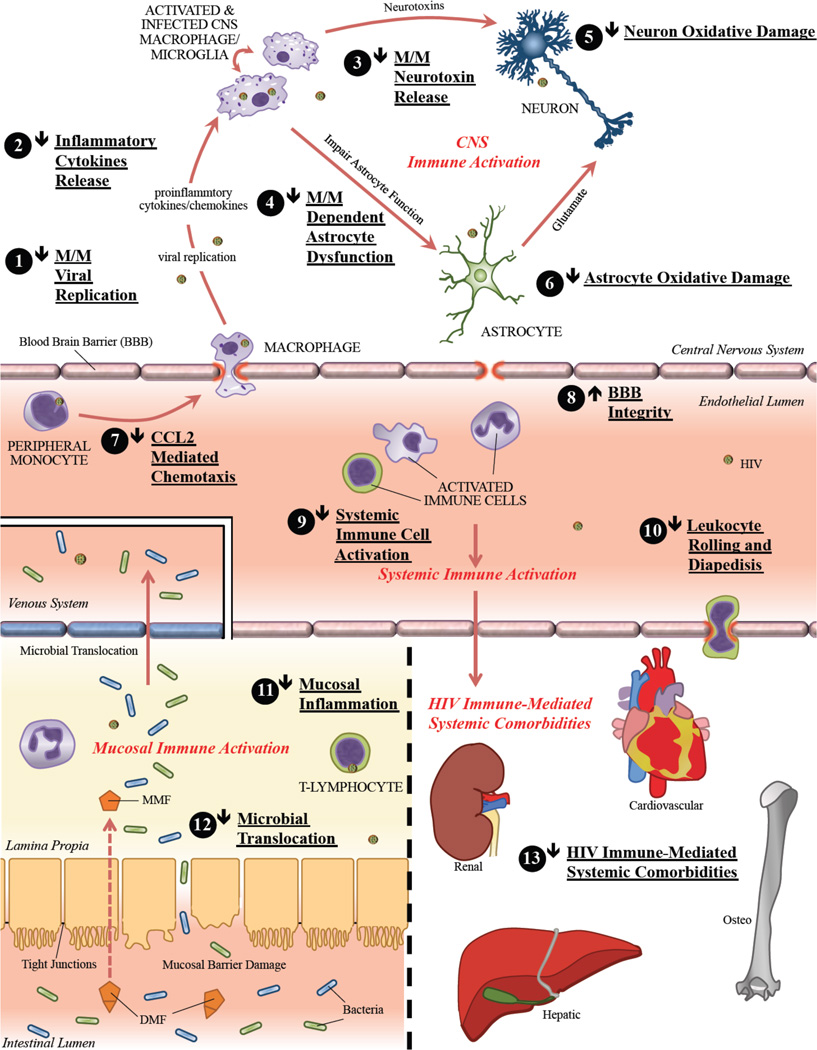
The FAEs, and specifically the oral DMF preparations (BG-12/Tecfidera™, Fumaderm®), are attractive candidates as adjunctive agents for suppressing chronic immune activation, oxidative stress, and associated HIV disease progression and comorbidities in cART-treated individuals (reviewed in Figure 2). To date, no in vivo or clinical studies have examined the efficacy of FAE therapy in HIV infection, however the dual anti-inflammatory and anti-oxidative mechanisms of FAEs suggest potential potent systemic efficacy. FAEs have been studied in in vitro models of HIV macrophage-mediated neurotoxicity where they have shown promising results. The CNS penetrance of MMF and the demonstrable suppression of CNS inflammation in BG-12-treated multiple sclerosis patients are especially appealing for targeting persistent CNS inflammation and oxidative stress in such individuals (see section VI.B. HIV CNS Disease).
Figure 2.
Potential effects of dimethyl fumarate (DMF) therapy on systemic and CNS HIV-disease pathogenesis. Oral DMF, primarily through its active in vivo metabolite monomethyl fumarate (MMF), might have potent anti-inflammatory and anti-oxidative effects (marked by numbers 1 – 13 in figure) in HIV-infected cART-treated individuals thereby limiting both systemic and CNS chronic immune activation and oxidative stress. This could result in decreased HIV-disease progression and associated comorbidities. Abbreviations used: monomethyl fumarate (MMF), dimethyl fumarate (DMF), blood brain barrier (BBB), macrophage/microglia (M/M)
a. FAEs as Suppressers of Systemic Immune Activation in HIV/AIDS
Through inhibition of the NF-κB signaling, which regulates cytokine and chemokine signaling, oral DMF therapy might provide an additional suppressive effect on the chronic immune activation in cART-treated patients. This prediction is supported by the efficacy of oral DMF-formulations (BG-12/Tecfidera™ and Fumaderm®) in limiting inflammation and immune cell infiltration in multiple sclerosis and psoriasis.2, 3, 8–10, 103–106, 129 Suppression of chronic immune activation will likely improve long-term survival, lower risk of associated end-organ diseases, and improve quality of life in HIV-infected cART-treated individuals.162, 195 The glucocorticoid prednisolone, a potent immunosuppressant, lowered systemic immune activation in both untreated and cART-treated patients244 and postponed CD4+ T-lymphocyte count decreases for a median of two years in untreated HIV-infected patients.245, 246 Prednisone, the precursor to prednisolone, inhibited monocyte TNFα production without affecting T-lymphocyte antigenic responses.247 However, the profound side effects of glucocorticoid therapy make its long-term use unattractive as adjunctive therapy. In a small retrospective study, addition of the immunosuppressant cyclosporine to cART therapy during primary HIV-1 infection restored CD4+ T-lymphocytes to normal levels (both percentage and absolute numbers) and adjunctively treated patients maintained higher CD4+ T-lymphocyte levels than those in patients taking cART alone.248 In this study, cyclosporine adjunctive therapy did not significantly affect virus-specific CD8+ or CD4+ T-lymphocyte responses or viral load.248 However, another study of the effects of cyclosporine treatment during acute and early HIV-infection in 48 subjects demonstrated no significant improvement in CD4+ T-lymphocyte counts or other markers of disease progression.249 Notably, cyclosporine has shown limited efficacy in multiple sclerosis,250–253 in direct contrast to the strong and consistent efficacy reported for the immune modulator BG-12/Tecfidera™,2, 3 suggesting DMF therapy may similarly prove more effective in HIV-infection. The central role of chronic immune activation in disease progression in cART-treated individuals coupled with these promising findings demonstrating a delayed disease progression with suppression of immune activation strongly supports further exploration of immunomodulatory therapy for the treatment of HIV infection. The anti-inflammatory effects of DMF in cell lineages relevant to HIV-infection coupled with its effectiveness in the chronic inflammatory diseases multiple sclerosis and psoriasis, makes it a promising adjunctive therapy for improving morbidity and mortality in cART-treated HIV-infected individuals.
b. FAEs as Possible Protectors of the Intestinal Mucosal Barrier in HIV/AIDS
Chronic immune inflammation is believed to occur primarily as a result of microbial translocation across the damaged gastrointestinal tract,162 as described above. Beyond direct anti-inflammatory effects on immune cells, FAE therapy might ameliorate chronic immune activation through decreasing microbial translocation by several effects: i) reversing or halting the depletion of mucosal CD4+ T-lymphocytes, ii) limiting mucosal immune activation and inflammation, and iii) preventing structural damage to the intestinal epithelium. Through inhibition of NF-κB activity in mucosal lymphocytes and other mucosal immune cells, FAEs could dampen mucosal immune cell activation thereby limiting mucosal inflammation. This decreased inflammation could reduce immune activation-induced T-lymphocyte apoptosis.254 Recent in vitro and in vivo mouse studies suggest that inducers of the Nrf2-dependent ARE can protect intestinal mucosa from inflammation-associated oxidative stress and injury and reduce the associated invasion of anaerobic bacteria into the mucosa.43, 255–259 Therefore, oral DMF through induction of the ARE might limit microbial translocation across the intestinal mucosa and through NF-κB inhibition promote gut immunity, thereby preventing the primary cause of chronic systemic inflammation in HIV-infected individuals.
c. FAEs as Suppressors of Systemic HIV Replication
The ability of DMF and MMF to modulate NF-κB signaling and cellular pathways of cytokine production, particularly of TNFα, suggests the possibility of associated effects on replication of HIV in immune cells. Increased nuclear translocation of NF-κB, through immune activation including TNFα stimulation,232, 260, 261 drives HIV gene expression from the HIV long terminal repeat (LTR).262, 263 Therefore, FAE-driven inhibition of NF-κB nuclear translocation and DNA binding might further limit HIV-replication in vivo. Potent inhibition of HIV replication by DMF and MMF pre-treatment in primary human monocyte-derived macrophages has been demonstrated in vitro.54 The effect of FAEs on HIV replication in T-lymphocytes, the primary targets of HIV infection, has not been reported. In addition, modification of the oxidative state of immune cells such as increasing glutathione has been shown to interfere with HIV particle assembly, infectivity, and release,233, 264 which suggests another possible inhibitory effect of FAEs on the HIV life cycle.
d. FAEs as Suppressors of Oxidative Stress in HIV/AIDS
In addition to markers of immune activation, markers of oxidative stress are consistently elevated and associated with disease progression in HIV-infected patients. This role of oxidative stress and glutathione depletion in HIV systemic disease pathogenesis and mortality served as the rationale for treatment of HIV-infected patients with various direct antioxidants, including N-acetylcysteine243, 265–268 and selenium.269–271 The largest selenium clinical trial was a randomized controlled trial of 262 HIV-infected patients (192 receiving ART) that reported that nine-months of selenium supplementation resulted in a significant increase in CD4+ T-lymphocyte count and a decrease in viral load.271 Similar effects on increasing CD4+ T-lymphocyte counts and decreased viral loads were seen in two of the N-acetylcysteine studies.267, 268 These trials suggest that antioxidant therapies have the potential to modulate HIV disease progression. However, numerous studies suggest that N-acetylcysteine,272–274 selenium,275 and other direct antioxidants276–278 actually suppress the endogenous Nrf2-driven ARE (a pro-oxidative effect), likely due to their direct suppression of oxidative stress, which serves as the main inducer of the Nrf2 translocation and subsequent ARE induction. Furthermore complicating their clinical usefulness, direct antioxidants are expended to elicit their antioxidant effects and thus are often short-lived and need to be replenished frequently.279 Therapies that induce sustained activation of the endogenous ARE might provide greater protection against oxidative stress than direct antioxidants due to sustained increased expression of multiple antioxidant effector proteins.
The monoamine oxidase B inhibitor selegiline, which had been shown decrease oxygen free radicals and enhance the expression of antioxidant enzymes superoxide dismutase and catalase,280–282 has been used to attempt to increase dopaminergic neurotransmission and reduce neuronal injury caused by oxidative stress in Parkinson’s and Alzheimer’s Disease.283, 284 In addition, selegiline has been shown to activate the Nrf2 pathway in neuronal cell lines.285 Despite the success of selegiline in other neurocognitive diseases and the evidence that selegiline may induce the ARE and limit oxidative stress, a 24-week clinical trial of transdermal selegiline therapy (two doses) with a 24-week open-label extension in HIV-infected patients failed to show neurocognitive improvement or positive changes in brain or CSF biomarkers of oxidative stress.286–288 The reasons for the failure of selegiline therapy to achieve positive outcomes in this trial are unclear. The 24-week treatment duration was relatively brief and each treatment arm, including placebo, showed no neurocognitive deterioration over 24 weeks of open-label follow-up. Some investigators have suggested that augmenting dopaminergic signaling could enhance HIV infection of macrophages and promote neuropathological disease progression in simian immunodeficiency virus (SIV)-infected macaques.289, 290
Despite the clinical trial failure of transdermal selegiline, compelling evidence supports oxidative stress as a major driving force in HIV neuropathogenesis. Furthermore, the partial clinical efficacy of direct antioxidants (N-acetylcysteine, Selenium) strongly supports further study of ARE-inducing therapies such as DMF for disease suppression in HIV infected individuals.
e. Potential of FAEs in Treating Systemic HIV-Associated Comorbidities
Despite use of cART therapy, HIV-infected individuals have an increased risk of progressive dysfunction of major organs. Such HIV disease progression can involve the central nervous system (brain/cognition, see section VI.B HIV CNS Disease; spinal cord/myelopathy), peripheral nerves (neuropathy), heart (atherosclerosis, heart failure), liver (cirrhosis, hepatocellular carcinoma), kidney (nephropathy, glomerulonephritis/sclerosis, tubulointerstitial nephritis), and bone (osteopenia, osteoporosis) (reviewed in291–293). In these comorbidities, inflammation and possibly the associated oxidative stress are thought to play a significant role.
Cardiovascular Disease
HIV-infected patients have increased risk of atherosclerosis, myocardial infarction, heart failure, and other vascular diseases.294, 295 Increased serum levels of several markers of inflammation strongly predict cardiovascular disease and mortality.296–298 Although HIV-infected cART-treated individuals are at lower risk for cardiovascular disease than HIV-infected individuals not receiving cART, they are nonetheless at higher risk than the general population. Risk factors for cardiovascular disease in cART-treated individuals include low-level virus replication, chronic immune activation associated microbial translocation, monocyte activation, and oxidative stress (reviewed in299), each of which is a potential target of DMF therapy. Atherosclerosis in particular is driven by persistent inflammation, macrophage and T-lymphocyte infiltration, endothelial cell activation, and oxidative stress.300, 301 Furthermore, studies of transgenic mice expressing HIV Tat, gp120, and Nef suggest that expression of these HIV-1 proteins, independent of viral infection, can induce cardiac damage through oxidative stress.228 Such damage is associated with elevated cystine/cysteine ratios and altered glutathione metabolism in cardiac muscle.228 Similarly, cardiac tissue from Tatexpressing transgenic mice showed decreased glutathione levels, mitochondrial damage, and cardiomyopathy.302 Thus, the ability of DMF and MMF to decrease inflammation, immune cell activation and invasion, endothelial cell activation, and injury due to oxidative stress suggest that DMF formulations could decrease the elevated risk of cardiovascular events in cART-treated individuals.
Bone Disease: Osteopenia and Osteoporosis
A recent meta-analysis showed that the prevalence of osteopenia and osteoporosis in HIV-infected individuals was more than three times higher than that in uninfected controls.303 This could reflect effects of chronic inflammation and associated increased bone resorption304 as well as HIV-driven (and potentially cART-driven) increases in osteoclast activity.305 DMF therapy, through limiting systemic inflammation and viral replication, could potentially reduce these pro-osteoclast signals. One such signal, NF-κB activity, regulates osteoclast differentiation and the inflammatory cytokine TNFα that induces bone loss through stimulation of osteoclasts.306–308 Therefore, DMF therapy through inhibition of NF-κB and TNFα release from immune cells could have further efficacy in decreasing osteoclast activity.
Hepatic and Renal Disease
HIV-infected individuals also have increased risk of both kidney309–311 and liver311–313 disease associated with viral replication, low peripheral CD4+ T-lymphocyte count, and the use of certain antiretroviral drugs.314–319 Given these correlations among renal and liver disease, viral replication (despite cART), and T-lymphocyte counts, DMF therapy could potentially ameliorate HIV-associated liver and kidney disease.
In summary, DMF therapy in HIV-infected patients could reduce disease progression by limiting systemic immune activation and associated oxidative stress. Through induction of the Nrf2/ARE and inhibition of NF-κB, DMF therapy could systemically: i) reduce immune cell activation and cytokine release, ii) prevent leukocyte tissue infiltration; and iii) inhibit HIV infection and replication. Each of these effects has been demonstrated in published studies examining DMF. In addition, suggested possible effects of DMF therapy include the following: i) limiting intestinal mucosal barrier damage and associated microbial translocation, ii) reducing HIV-associated organ comorbidities, and iii) limiting oxidative stress (reviewed in Figure 2). Thus, there is much promise for the use of oral DMF formulations as adjunctive therapy for systemic disease progression and comorbidities in cART-treated HIV-infected individuals.
B. HIV CNS Disease
In addition to systemic inflammation and oxidative stress, HIV infection is also associated with persistent inflammation and oxidative stress within the CNS compartment. Because HIV infection is associated with neurocognitive dysfunction even with individuals who demonstrate complete or nearly complete suppression of HIV replication with cART, adjunctive therapy for neuroprotection is clearly needed in HIV-infected individuals. HIV infection causes varying degrees of cognitive, motor, and behavioral deficits collectively known as HIV-associated neurocognitive disorders (HAND) in up to 50% of cART treated individuals.320–324 HAND consists of three sub-classifications that span a neuropsychological spectrum of impairment, from asymptomatic neurocognitive impairment (ANI) and mild neurocognitive disorder (MND) to HIV-Associated Dementia (HAD), the most severe form of HAND. cART has decreased the prevalence of HAD,325–327 however, a significant proportion of patients on ART (30–50%) continue to develop neurocognitive impairments, despite reduced plasma viremia, improved immunological parameters, and decreased progression to AIDS.138, 326, 328–337 A recent study shows a significant increase in the prevalence of the milder forms of HAND in the cART era.330 The high prevalence of HAND and other neurologic manifestations of HIV-infection despite the introduction of cART underscores a critical need for adjunctive therapies that target the persistent inflammation and oxidative stress within the CNS compartment.338–343
1. Macrophages/Microglia are Central Mediators of HAND Neuropathogenesis
Systemic effects of HIV infection are likely driven by HIV replication in CD4+ T-lymphocytes, while CNS effects of infection are predominantly driven by HIV infection of macrophages/microglia. HIV entry into the CNS occurs early in infection,344–347 with subsequent productive replication in the brain occurring primarily within perivascular monocyte-derived macrophages (MDMs) and microglia, and restricted, non-productive replication in astrocytes.348–351 Persistent CNS HIV infection drives immune activation of resident macrophages/microglia, pervasive reactive astrocytosis, perivascular inflammation and infiltration of monocytic cells,352 which can result in HIV-encephalitis (HIVE), particularly in individuals not receiving cART.353 The introduction of cART has decreased morbidity/mortality of HIV infection, the severity of neurocognitive impairment and associated neuroinflammation in HAND, and the prevalence of encephalitis.354–359 Despite these beneficial effects of cART, neuroinflammation and neuropathological damage persists,354–358, 360, 361 and persistent systemic and CNS markers of monocyte/macrophage activation predict neurocognitive impairment,362–370 even in individuals with excellent virological suppression.371–377 Moreover, the clinical severity of HAND correlates more strongly with monocyte infiltration and MDM/microglia activation than with CNS viral antigen load or number of HIV-infected cells in the brain.377–381 In summary, current research highlights the central role of macrophages/ microglia and persistent neuroinflammation in HAND in individuals receiving cART.
2. Macrophages and Microglia Produce HAND-associated Neurotoxins
The neuropathogenesis of HAND has been associated with decreased CNS neuronal synaptic and dendritic density,380, 382, 383 neuronal apoptosis and necrosis,379, 384–386 and altered neuronal physiology,387–395 linked to the aforementioned inflammation and oxidative stress. This neuronal dysfunction is likely a consequence of neurotoxins released from HIV-infected MDM (HIV-MDM) and immune activated MDM/microglia and astrocytes.396–399 Our laboratory and others have demonstrated that a major component of HIV-MDM neurotoxins are small (<3 kDa), heat-stable, protease-resistant excitotoxins that act through N-methyl-D-aspartate-receptor (NMDAR) activation, a finding supported by studies in animal models of HIV neurotoxicity.400–402 Many candidate excitotoxins released from HIV-MDM have been described including glutamate,403 quinolinic acid,404, 405 NTox,406 platelet activating factor,407 TNFα,408 and the viral proteins gp120409, 410 and Tat402, 411, 412 among others. HIV-infected individuals express increased levels of glutamate,413–415 quinolinic acid,416, 417 platelet activation factor,407 TNFα,418 and other excitotoxins in their CSF, which correlate with increased severity of HAND and neuroinflammation. Many of these neurotoxins released from HIV-MDM also dysregulate astrocyte function, particularly glutamate homeostasis.
Proper functioning of glutamate uptake and metabolism by astrocytes is vital to prevent glutamate-dependent excitotoxicity as astrocytes are the major regulator of glutamate in the brain.419, 420 Astrocyte control of extracellular glutamate is regulated through multiple mechanisms including direct uptake of glutamate, Ca2+-dependent release of glutamate, and catabolism of glutamate by glutamine synthetase.419, 420 Each of these processes can be perturbed through the actions of HIV coat protein gp120,421–424 TNFα,425–428 arachidonic acid,421 prostaglandins,429 reactive oxygen species,430 and associated neuroinflammation,420 each of which is also associated with HIV replication within CNS macrophages and microglia. This further emphasizes the link between inflammation, oxidative stress, and neurodegeneration in HIV CNS infection. Accordingly, the identification of endogenous pathways that regulate HIV-MDM production of neurotoxins and factors that result in astrocyte dysfunction will provide novel therapeutic targets for ameliorating neurodegeneration and neurocognitive impairment in HAND.
3. Macrophage/Microglia-Associated Oxidative Stress in HAND
HIV infection can induce CNS oxidative stress235, 431–433 through chronic immune activation213–215 and direct effects of HIV proteins. The HIV viral proteins gp120,216, 219, 434 Vpr,218, 435 and Tat217, 219 have been directly implicated in the induction of oxidative stress in CNS-relevant cell types. Oxidative stress drives NF-κB-driven HIV replication,232–234 inflammatory cytokine release,232, 235–239 and neurotoxin production.236, 433, 436–440 Furthermore, in vivo markers of oxidative stress correlate with neurocognitive impairment235, 439, 441 as well as systemic disease progression240–242 in HIV-infected individuals. Protection against oxidative stress is provided by the ubiquitous ARE pathway, which is the endogenous cellular antioxidant pathway. The ARE response is driven by the Nrf2 transcription factor, and gp120-induced oxidative stress and inflammation in astrocytes was reported to be significantly higher when Nrf2 expression was suppressed by siRNA.236 The expression profiles of Nrf2 and its ARE effector proteins in the CNS of patients with HAND has not been reported. Finally, in addition to its role in HAND, oxidative stress has been implicated in the pathogenesis of other neurodegenerative disorders, including Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease,438, 442–450 which emphasizes the broad potential value of developing effective CNS therapies against oxidative stress.
4. FAE as Candidate Adjunctive Therapy for CNS Disease in cART-Treated Individuals
As discussed, the oral DMF formulation BG-12/Tecfidera™ decreased relapse rates and associated neuroinflammation in patients with multiple sclerosis in two independent Phase III clinical treatment trials.2, 3 These studies clearly demonstrate efficacy of oral DMF-treatment in the CNS and its efficacy against neuroinflammation and oxidative stress may provide important adjunctive therapy for HIV-infected patients to prevent and treat HAND.
a. FAEs as Inhibitors of HIV Viral Infection in Monocytes and Macrophages
Pretreatment with DMF and MMF decreased HIV infection and TNFα secretion in HIV monocyte-derived macrophages (HIV-MDM), likely due in part to inhibition of NF-κB nuclear translocation and DNA binding.54 It is well established that TNFα exposure increases HIV replication through increased nuclear translocation of NF-κB,260, 261 which drives HIV gene expression from the HIV long terminal repeat (LTR).262, 263 However, in addition to decreasing autocrine effects of TNFα secretion, there are other possible NF-κB modulating effects of DMF and MMF associated with oxidative stress in HIV-MDM. Oxidative stress has been shown to drive HIV replication,232–234 likely through NF-κB activation,33–36 and Nrf2 nuclear translocation and activation of the ARE have been shown to suppress NF-κB (described above).
DMF and MMF induce activation of the ARE and suppress HIV infection at concentrations that are consistent with CSF MMF concentrations in vivo (4.4 µM).70 However, inhibition of NF-κB may require higher DMF and MMF concentrations (>15 µM),54 which suggests other ARE-mediated, NF-κB-independent effects of DMF and MMF on HIV infection. These effects could include suppression of HIV entry into monocytes and macrophages and disruption of virion assembly and release from infected cells. It has been reported that antioxidants can decrease the stability of CXCR4 and CCR5 mRNA transcripts in human monocytes.451 Because CCR5 and CXCR4 are the major cellular co-receptors for HIV, such antioxidant effects of DMF and MMF could limit HIV entry into monocytes and macrophages. Furthermore high intracellular glutathione levels in macrophages were associated with defective HIV particle assembly and decreased virion infectivity and release.233 Thus, DMF and MMF activation of the ARE may limit HIV infection of monocytes and macrophages at several steps in the HIV life cycle (entry, replication, and particle assembly).
b. FAEs as Inhibitors of Macrophage Neurotoxin Release
One consequence of HIV infection and/or immune activation of macrophages and associated inflammation is the production and release of soluble neurotoxins. Beyond promoting HIV-replication, elevated TNFα levels associated with immune activation increase monocyte entry into the brain and drive associated inflammatory cascades; one consequence is the production and release of soluble neurotoxins from macrophages, microglia, and astrocytes.452 Activation of the ARE pathway decreases macrophage and microglia activation,54, 453–456 probably through ARE-mediated inhibition of NF-κB signaling. Work from our laboratory demonstrated that DMF and MMF treatment reduces neurotoxin release from HIV-MDM and associated neuronal death in an HIV neurotoxicity model system.54 We further demonstrated that the decrease in HIV-MDM neurotoxin release was partly dependent on the ability of DMF and MMF to induce the expression of the Nrf2/ARE effector and cytoprotective protein heme-oxygenase-1 (HO-1).54 HO-1 is the inducible, rate-limiting enzyme that degrades pro-oxidative and cytotoxic heme to bilirubin and carbon monoxide, each of which has well-documented antioxidant and antiinflammatory effects.54, 457–460 We showed that HIV-infection of MDM in vitro drastically decreases HO-1 protein expression and that induction of HO-1 in HIV-MDM lowered neurotoxin production independent of viral replication; in contrast, inhibition of HO-1 enzymatic activity in HIV-MDM increased neurotoxin production.54 These data strongly suggest that HO-1 suppression in HIV-MDM plays a pivotal role in macrophage-mediated neurotoxin production during HIV infection and that rescuing this suppression with DMF or MMF might suppress neurodegeneration in HAND.
c. FAEs as Modulators of Immune Cell CNS Infiltration and Blood Brain Barrier Integrity
In addition to suppression of MDM activation and neurotoxin production, DMF and MMF reduced monocyte chemotaxis driven by CCL2,54 thus potentially limiting transendothelial migration of monocytes into the CNS during HIV infection. Less monocyte trafficking into the CNS could limit HIV access to the brain and subsequent neuroinflammation. Complementing this effect, DMF can decrease endothelial cell adhesion molecule (ICAM-1, VCAM-1, and E-selectin) expression, thereby decreasing leukocyte rolling and diapedesis.66, 67 This could potentially further limit immune cell trafficking into the CNS and its complications in HAND. HIV infection also impairs blood brain barrier (BBB) permeability, possibly through actions of released viral proteins, induction of oxidative stress, or induction of systemic inflammation (reviewed in461). Each of these can disrupt the structural integrity of the BBB in part through breakdown of tight junctions, thereby increasing microbial invasion and immune cell infiltration.461 Recent studies of post-traumatic brain injury462 and subarachnoid hemorrhage463 in mice suggested that activation of the Nrf2/ARE antioxidant and detoxifying enzymes preserve BBB and tight junction integrity. Moreover, Nrf2/ARE activation in choroid plexus cells preserved the blood-CSF barrier after oxidative stress injury.464 Therefore, DMF therapy could further protect the CNS compartment more globally from microbe and immune cell invasion and other systemic insults through direct protection of the BBB and blood-CSF barrier through its antioxidants effects.
d. FAEs as Astrocyte and Neuron Protectants From Inflammation and Oxidative Stress
In addition to macrophages and microglia, astrocytes and neurons are also possible CNS targets for FAEs. DMF has been shown to decrease astrocyte activation and the release of inflammatory cytokines,69–71 which could further dampen CNS neuroinflammation. DMF treatment also decreased macrophage and microglia activation55 and associated release of TNFα release54 and likely other HIV-MDM factors421–430 through ARE induction, which have been shown to impair astrocyte glutamate homeostasis and promote glutamate-mediated excitotoxicity. TNFα in particular can suppress astrocyte glutamate scavenging through impairment of their high affinity glutamate transporters, which are critical for regulation of extracellular glutamate levels.420, 465 Furthermore, TNFα released into supernatants of HIV-infected macrophage cultures can induce nitric oxide synthase and nitric oxide production and release by exposed astrocytes;466 such a process could amplify CNS oxidative stress and associated injury during HIV infection. Although such possible effects of DMF and MMF on HIV-MDM-mediated astrocyte dysfunction have not been studied, DMF treatment has been shown to directly protect both astrocytes and neurons from oxidative injury and glutamate toxicity through Nrf2/ARE induction.69–71 Because glutamate mediated excitotoxic injury has been implicated in the pathogenesis of HAND, these studies suggest DMF might project neurons directly from HIV-mediated neurotoxicity as well as indirectly by preventing astrocyte dysfunction.
In summary, DMF therapy in HAND patients could have both direct and indirect neuroprotective effects through inhibition of NF-κB activity and Nrf2/ARE induction in different CNS cell types (reviewed in Figure 2). Direct effects could occur through induction of neuronal cytoprotective antioxidant responses. Indirect effects could occur through DMF/MMF actions in macrophages/microglia, astrocytes, endothelial cells, and choroid plexus cells. Confirmed macrophage effects of DMF and MMF54 include: i) suppressing neurotoxin production; ii) limiting HIV infection; iii) impairing monocyte chemotaxis; iv) inhibiting TNFα release; and v) inhibiting NF-κB nuclear translocation. Proposed astrocyte effects include: i) inhibiting release of TNFα and other proinflammatory cytokines; ii) preserving glutamate scavenging and glutamate homeostasis; iii) limiting production of nitric oxide and associated pro-oxidants. Additionally, indirect neuroprotective effects of DMF and MMF could also occur through actions in endothelial cells, which maintain the BBB. Confirmed effects in endothelial cells include: i) reducing adhesion molecule expression; and ii) promoting BBB structural integrity. Finally, DMF therapy could preserve the blood-CSF barrier integrity through actions in choroid plexus cells. Thus, there is much supporting evidence for the study of DMF as an adjunctive therapy for neuroprotection against HAND in cART-treated HIV-infected individuals.
C. Safety of FAE Immunomodulatory Therapy in HIV-infected Individuals
The clinical safety of long-term use of oral FAE therapy in humans is supported by a lack of clear evidence of serious adverse events after more than 17 years of its widespread use for the treatment of psoriasis, and its recent use in Phase III multiple sclerosis treatment trials involving more than 2600 patients (see section III. Safety and Adverse Events for long term clinical safety data and recent PML case reports). Nonetheless, its use in HIV-infected individuals has not been established, and the potential risks of this application must be considered. Among these concerns is the possibility that immunosuppressive FAE therapy in HIV-infected patients would increase their risk for opportunistic infections, malignancy, acceleration of HIV infection, and mortality.467, 468 However, potent immunosuppressive therapy has been used successfully in HIV-infected individuals undergoing organ transplantation for nearly two decades, with no significant increase in risk for these complications in comparison with such complications in HIV-negative transplant recipients.469–472 Several studies have reported that HIV viral loads and CD4+ T-lymphocyte counts were well-maintained after kidney and liver transplantation in HIV-infected patients.473–475 Moreover, the largest prospective study of cART-treated HIV-infected recipients of kidney transplantation (n = 150) receiving standard immunosuppressive therapy reported one and three year survival rates to be 94.6% and 88.2%475, respectively, which is not significantly different from current kidney transplant survival rates as reported by the U.S. Scientific Registry of Transplant Recipients.476 Furthermore, the increased risk of de novo or recurrent malignancy in HIV-infected patients undergoing solid-organ transplantation and subsequent immunosuppressive therapy was low.477
Other immunosuppressive drugs including prednisolone244, 245 and cyclosporine248, 249 have also been studied in clinical trials in cART-treated HIV-infected patients and these trials revealed no increased risk for opportunistic infections, increased viral load, or disease progression. These studies demonstrate that potent immunosuppressive regimens are generally safely used in cART-treated HIV-infected individuals and they further suggest that DMF formulations could also be safely used in HIV-infected cART-treated individuals.
VII. CONCLUSIONS
DMF formulation therapy reduces inflammation, immune activation, and oxidative stress in numerous cell lineages in vitro, particularly cells of the immune system, and such therapy has potent clinical efficacy in psoriasis and multiple sclerosis, likely through limiting inflammation and associated oxidative stress and immune cell infiltration in these diseases. Based on these effects, this review proposes DMF as a potential adjunctive therapeutic in cART-treated HIV-infected individuals for improving morbidity and mortality by ameliorating immune activation and associated microbial translocation, systemic oxidative stress, and HIV-associated comorbidities, particularly HIV-associated neurocognitive disorder (HAND).
ACKNOWELDGMENTS
This work was supported by National Institute of Health R01 grants MH-095671, NS-043994, and NS-072005 and T32 grant AI-007632.
Abbreviation List
- AIDS
acquired immunodeficiency syndrome
- ARE
antioxidant response element
- BBB
blood brain barrier
- cART
combination antiretroviral therapy
- CCL
CC chemokine ligand
- CCR
CC chemokine receptor
- CD
cluster of differentiation
- CNS
central nervous system
- CXCL
CXC chemokine ligand
- CXCR
CXC chemokine receptor
- DMF
dimethyl fumarate
- FAEs
fumaric acid esters
- HAND
HIV-associated neurocognitive disorder
- HIV
human immunodeficiency virus
- HO-1
heme oxygenase-1
- HD
Huntington’s Disease
- HSK
Herpe Stromal Keratitis
- HSV
herpes simplex virus
- ICAM
intercellular adhesion molecule
- IFN
interferon
- IL
interleukin
- LPS
lipopolysaccharide
- MDM
monocyte-derived macrophages
- MEF
monoethyl fumrate
- MMF
monomotheyl fumarate
- Nrf2
nuclear factor erythroid-2 related factor-2
- NF-κB
nuclear factor κB
- PBMC
peripheral blood mononuclear cells
- TNF
tumor necrosis factor
Footnotes
Disclosures
D.L.K. has served as a paid consultant to Teva Neurosciences and Biogen Idec, Inc. and serves on the advisory committee to the National Institutes of Health/National Institute of Mental Health National NeuroAIDS Tissue Consortium.
The other author has no financial conflicts of interest.
Contributor Information
Alexander J. Gill, Email: agill@mail.med.upenn.edu.
Dennis L. Kolson, Email: kolsond@mail.med.upenn.edu.
References
- 1.Schweckendiek W. Treatment of psoriasis vulgaris. Med Monatsschr. 1959 Feb;13(2):103–104. [PubMed] [Google Scholar]
- 2.Gold R, Kappos L, Arnold DL, Bar-Or A, Giovannoni G, Selmaj K, Tornatore C, Sweetser MT, Yang M, Sheikh SI, Dawson KT. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med. 2012 Sep 20;367(12):1098–1107. doi: 10.1056/NEJMoa1114287. [DOI] [PubMed] [Google Scholar]
- 3.Fox RJ, Miller DH, Phillips JT, Hutchinson M, Havrdova E, Kita M, Yang M, Raghupathi K, Novas M, Sweetser MT, Viglietta V, Dawson KT. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med. 2012 Sep 20;367(12):1087–1097. doi: 10.1056/NEJMoa1206328. [DOI] [PubMed] [Google Scholar]
- 4.Werdenberg D, Joshi R, Wolffram S, Merkle HP, Langguth P. Presystemic metabolism and intestinal absorption of antipsoriatic fumaric acid esters. Biopharm Drug Dispos. 2003 Sep;24(6):259–273. doi: 10.1002/bdd.364. [DOI] [PubMed] [Google Scholar]
- 5.Litjens NH, Burggraaf J, van Strijen E, van Gulpen C, Mattie H, Schoemaker RC, van Dissel JT, Thio HB, Nibbering PH. Pharmacokinetics of oral fumarates in healthy subjects. Br J Clin Pharmacol. 2004 Oct;58(4):429–432. doi: 10.1111/j.1365-2125.2004.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rostami-Yazdi M, Clement B, Mrowietz U. Pharmacokinetics of anti-psoriatic fumaric acid esters in psoriasis patients. Arch Dermatol Res. 2010 Sep;302(7):531–538. doi: 10.1007/s00403-010-1061-4. [DOI] [PubMed] [Google Scholar]
- 7.Rostami-Yazdi M, Clement B, Schmidt TJ, Schinor D, Mrowietz U. Detection of metabolites of fumaric acid esters in human urine: implications for their mode of action. J Invest Dermatol. 2009 Jan;129(1):231–234. doi: 10.1038/jid.2008.197. [DOI] [PubMed] [Google Scholar]
- 8.Hoefnagel JJ, Thio HB, Willemze R, Bouwes Bavinck JN. Long-term safety aspects of systemic therapy with fumaric acid esters in severe psoriasis. Br J Dermatol. 2003 Aug;149(2):363–369. doi: 10.1046/j.1365-2133.2003.05433.x. [DOI] [PubMed] [Google Scholar]
- 9.Reich K, Thaci D, Mrowietz U, Kamps A, Neureither M, Luger T. Efficacy and safety of fumaric acid esters in the long-term treatment of psoriasis--a retrospective study (FUTURE) J Dtsch Dermatol Ges. 2009 Jul;7(7):603–611. doi: 10.1111/j.1610-0387.2009.07120.x. [DOI] [PubMed] [Google Scholar]
- 10.Kappos L, Gold R, Miller DH, Macmanus DG, Havrdova E, Limmroth V, Polman CH, Schmierer K, Yousry TA, Yang M, Eraksoy M, Meluzinova E, Rektor I, Dawson KT, Sandrock AW, O'Neill GN. Efficacy and safety of oral fumarate in patients with relapsing-remitting multiple sclerosis: a multicentre, randomised, double-blind, placebo-controlled phase IIb study. Lancet. 2008 Oct 25;372(9648):1463–1472. doi: 10.1016/S0140-6736(08)61619-0. [DOI] [PubMed] [Google Scholar]
- 11.Weber HO, Borelli C, Rocken M, Schaller M. Treatment of disseminated granuloma annulare with low-dose fumaric acid. Acta Derm Venereol. 2009;89(3):295–298. doi: 10.2340/00015555-0647. [DOI] [PubMed] [Google Scholar]
- 12.Nowack U, Gambichler T, Hanefeld C, Kastner U, Altmeyer P. Successful treatment of recalcitrant cutaneous sarcoidosis with fumaric acid esters. BMC Dermatol. 2002 Dec 24;2:15. doi: 10.1186/1471-5945-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mrowietz U, Christophers E, Altmeyer P. Treatment of psoriasis with fumaric acid esters: results of a prospective multicentre study. German Multicentre Study. Br J Dermatol. 1998 Mar;138(3):456–460. doi: 10.1046/j.1365-2133.1998.02124.x. [DOI] [PubMed] [Google Scholar]
- 14.Mrowietz U, Christophers E, Altmeyer P. Treatment of severe psoriasis with fumaric acid esters: scientific background and guidelines for therapeutic use. The German Fumaric Acid Ester Consensus Conference. Br J Dermatol. 1999 Sep;141(3):424–429. doi: 10.1046/j.1365-2133.1999.03034.x. [DOI] [PubMed] [Google Scholar]
- 15.van Oosten BW, Killestein J, Barkhof F, Polman CH, Wattjes MP. PML in a patient treated with dimethyl fumarate from a compounding pharmacy. N Engl J Med. 2013 Apr 25;368(17):1658–1659. doi: 10.1056/NEJMc1215357. [DOI] [PubMed] [Google Scholar]
- 16.Ermis U, Weis J, Schulz JB. PML in a patient treated with fumaric acid. N Engl J Med. 2013 Apr 25;368(17):1657–1658. doi: 10.1056/NEJMc1211805. [DOI] [PubMed] [Google Scholar]
- 17.Sweetser MT, Dawson KT, Bozic C. Manufacturer's response to case reports of PML. N Engl J Med. 2013 Apr 25;368(17):1659–1661. doi: 10.1056/NEJMc1300283. [DOI] [PubMed] [Google Scholar]
- 18.Mrowietz U, Altmeyer P, Bieber T, Rocken M, Schopf RE, Sterry W. Treatment of psoriasis with fumaric acid esters (Fumaderm) J Dtsch Dermatol Ges. 2007 Aug;5(8):716–717. doi: 10.1111/j.1610-0387.2007.06346.x. [DOI] [PubMed] [Google Scholar]
- 19.Hayden MS, Ghosh S. NF-kappaB in immunobiology. Cell Res. 2011 Feb;21(2):223–244. doi: 10.1038/cr.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999 Nov 22;18(49):6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 21.Singh S, Vrishni S, Singh BK, Rahman I, Kakkar P. Nrf2-ARE stress response mechanism: a control point in oxidative stress-mediated dysfunctions and chronic inflammatory diseases. Free Radic Res. 2010 Nov;44(11):1267–1288. doi: 10.3109/10715762.2010.507670. [DOI] [PubMed] [Google Scholar]
- 22.Itoh K, Wakabayashi N, Katoh Y, Ishii T, O'Connor T, Yamamoto M. Keap1 regulates both cytoplasmic-nuclear shuttling and degradation of Nrf2 in response to electrophiles. Genes Cells. 2003 Apr;8(4):379–391. doi: 10.1046/j.1365-2443.2003.00640.x. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi M, Yamamoto M. Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv Enzyme Regul. 2006;46:113–140. doi: 10.1016/j.advenzreg.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Itoh K, Tong KI, Yamamoto M. Molecular mechanism activating Nrf2-Keap1 pathway in regulation of adaptive response to electrophiles. Free Radic Biol Med. 2004 May 15;36(10):1208–1213. doi: 10.1016/j.freeradbiomed.2004.02.075. [DOI] [PubMed] [Google Scholar]
- 25.Lee JM, Calkins MJ, Chan K, Kan YW, Johnson JA. Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. J Biol Chem. 2003 Apr 4;278(14):12029–12038. doi: 10.1074/jbc.M211558200. [DOI] [PubMed] [Google Scholar]
- 26.Fourtounis J, Wang IM, Mathieu MC, Claveau D, Loo T, Jackson AL, Peters MA, Therien AG, Boie Y, Crackower MA. Gene expression profiling following NRF2 and KEAP1 siRNA knockdown in human lung fibroblasts identifies CCL11/Eotaxin-1 as a novel NRF2 regulated gene. Respir Res. 2012;13:92. doi: 10.1186/1465-9921-13-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alam J, Stewart D, Touchard C, Boinapally S, Choi AM, Cook JL. Nrf2, a Cap'n'Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J Biol Chem. 1999 Sep 10;274(37):26071–26078. doi: 10.1074/jbc.274.37.26071. [DOI] [PubMed] [Google Scholar]
- 28.Bloom DA, Jaiswal AK. Phosphorylation of Nrf2 at Ser40 by protein kinase C in response to antioxidants leads to the release of Nrf2 from INrf2, but is not required for Nrf2 stabilization/accumulation in the nucleus and transcriptional activation of antioxidant response element-mediated NAD(P)H:quinone oxidoreductase-1 gene expression. J Biol Chem. 2003 Nov 7;278(45):44675–44682. doi: 10.1074/jbc.M307633200. [DOI] [PubMed] [Google Scholar]
- 29.Wild AC, Moinova HR, Mulcahy RT. Regulation of gamma-glutamylcysteine synthetase subunit gene expression by the transcription factor Nrf2. J Biol Chem. 1999 Nov 19;274(47):33627–33636. doi: 10.1074/jbc.274.47.33627. [DOI] [PubMed] [Google Scholar]
- 30.Hayes JD, Chanas SA, Henderson CJ, McMahon M, Sun C, Moffat GJ, Wolf CR, Yamamoto M. The Nrf2 transcription factor contributes both to the basal expression of glutathione S-transferases in mouse liver and to their induction by the chemopreventive synthetic antioxidants, butylated hydroxyanisole and ethoxyquin. Biochem Soc Trans. 2000 Feb;28(2):33–41. doi: 10.1042/bst0280033. [DOI] [PubMed] [Google Scholar]
- 31.Sasaki H, Sato H, Kuriyama-Matsumura K, Sato K, Maebara K, Wang H, Tamba M, Itoh K, Yamamoto M, Bannai S. Electrophile response element-mediated induction of the cystine/glutamate exchange transporter gene expression. J Biol Chem. 2002 Nov 22;277(47):44765–44771. doi: 10.1074/jbc.M208704200. [DOI] [PubMed] [Google Scholar]
- 32.Wakabayashi N, Slocum SL, Skoko JJ, Shin S, Kensler TW. When NRF2 talks, who's listening? Antioxid Redox Signal. 2010 Dec 1;13(11):1649–1663. doi: 10.1089/ars.2010.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin W, Wang H, Yan W, Xu L, Wang X, Zhao X, Yang X, Chen G, Ji Y. Disruption of Nrf2 enhances upregulation of nuclear factor-kappaB activity, proinflammatory cytokines, and intercellular adhesion molecule-1 in the brain after traumatic brain injury. Mediators Inflamm. 2008;2008:725174. doi: 10.1155/2008/725174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vollgraf U, Wegner M, Richter-Landsberg C. Activation of AP-1 and nuclear factor-kappaB transcription factors is involved in hydrogen peroxide-induced apoptotic cell death of oligodendrocytes. J Neurochem. 1999 Dec;73(6):2501–2509. doi: 10.1046/j.1471-4159.1999.0732501.x. [DOI] [PubMed] [Google Scholar]
- 35.Rahman I, Gilmour PS, Jimenez LA, MacNee W. Oxidative stress and TNF-alpha induce histone acetylation and NF-kappaB/AP-1 activation in alveolar epithelial cells: potential mechanism in gene transcription in lung inflammation. Mol Cell Biochem. 2002 May-Jun;234–235(1–2):239–248. [PubMed] [Google Scholar]
- 36.Bonello S, Zahringer C, BelAiba RS, Djordjevic T, Hess J, Michiels C, Kietzmann T, Gorlach A. Reactive oxygen species activate the HIF-1alpha promoter via a functional NFkappaB site. Arterioscler Thromb Vasc Biol. 2007 Apr;27(4):755–761. doi: 10.1161/01.ATV.0000258979.92828.bc. [DOI] [PubMed] [Google Scholar]
- 37.Thimmulappa RK, Lee H, Rangasamy T, Reddy SP, Yamamoto M, Kensler TW, Biswal S. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J Clin Invest. 2006 Apr;116(4):984–995. doi: 10.1172/JCI25790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cho HY, Imani F, Miller-DeGraff L, Walters D, Melendi GA, Yamamoto M, Polack FP, Kleeberger SR. Antiviral activity of Nrf2 in a murine model of respiratory syncytial virus disease. Am J Respir Crit Care Med. 2009 Jan 15;179(2):138–150. doi: 10.1164/rccm.200804-535OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jin W, Zhu L, Guan Q, Chen G, Wang QF, Yin HX, Hang CH, Shi JX, Wang HD. Influence of Nrf2 genotype on pulmonary NF-kappaB activity and inflammatory response after traumatic brain injury. Ann Clin Lab Sci. 2008 Summer;38(3):221–227. [PubMed] [Google Scholar]
- 40.Jeong WS, Kim IW, Hu R, Kong AN. Modulatory properties of various natural chemopreventive agents on the activation of NF-kappaB signaling pathway. Pharm Res. 2004 Apr;21(4):661–670. doi: 10.1023/b:pham.0000022413.43212.cf. [DOI] [PubMed] [Google Scholar]
- 41.Xu C, Shen G, Chen C, Gelinas C, Kong AN. Suppression of NF-kappaB and NF-kappaB-regulated gene expression by sulforaphane and PEITC through IkappaBalpha, IKK pathway in human prostate cancer PC-3 cells. Oncogene. 2005 Jun 30;24(28):4486–4495. doi: 10.1038/sj.onc.1208656. [DOI] [PubMed] [Google Scholar]
- 42.Khodagholi F, Tusi SK. Stabilization of Nrf2 by tBHQ prevents LPS-induced apoptosis in differentiated PC12 cells. Mol Cell Biochem. 2011 Aug;354(1–2):97–112. doi: 10.1007/s11010-011-0809-2. [DOI] [PubMed] [Google Scholar]
- 43.Jin W, Ni H, Dai Y, Wang H, Lu T, Wu J, Jiang J, Liang W. Effects of tert-butylhydroquinone on intestinal inflammatory response and apoptosis following traumatic brain injury in mice. Mediators Inflamm. 2010;2010:502–564. doi: 10.1155/2010/502564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu GH, Qu J, Shen X. NF-kappaB/p65 antagonizes Nrf2-ARE pathway by depriving CBP from Nrf2 and facilitating recruitment of HDAC3 to MafK. Biochim Biophys Acta. 2008 May;1783(5):713–727. doi: 10.1016/j.bbamcr.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 45.Altmeyer P, Hartwig R, Matthes U. Efficacy and safety profile of fumaric acid esters in oral long-term therapy with severe treatment refractory psoriasis vulgaris. A study of 83 patients. Hautarzt. 1996 Mar;47(3):190–196. doi: 10.1007/s001050050401. [DOI] [PubMed] [Google Scholar]
- 46.Treumer F, Zhu K, Glaser R, Mrowietz U. Dimethylfumarate is a potent inducer of apoptosis in human T cells. J Invest Dermatol. 2003 Dec;121(6):1383–1388. doi: 10.1111/j.1523-1747.2003.12605.x. [DOI] [PubMed] [Google Scholar]
- 47.de Jong R, Bezemer AC, Zomerdijk TP, van de Pouw-Kraan T, Ottenhoff TH, Nibbering PH. Selective stimulation of T helper 2 cytokine responses by the anti-psoriasis agent monomethylfumarate. Eur J Immunol. 1996 Sep;26(9):2067–2074. doi: 10.1002/eji.1830260916. [DOI] [PubMed] [Google Scholar]
- 48.Ockenfels HM, Schultewolter T, Ockenfels G, Funk R, Goos M. The antipsoriatic agent dimethylfumarate immunomodulates T-cell cytokine secretion and inhibits cytokines of the psoriatic cytokine network. Br J Dermatol. 1998 Sep;139(3):390–395. doi: 10.1046/j.1365-2133.1998.02400.x. [DOI] [PubMed] [Google Scholar]
- 49.Zoghi S, Amirghofran Z, Nikseresht A, Ashjazadeh N, Kamali-Sarvestani E, Rezaei N. Cytokine secretion pattern in treatment of lymphocytes of multiple sclerosis patients with fumaric acid esters. Immunol Invest. 2011;40(6):581–596. doi: 10.3109/08820139.2011.569626. [DOI] [PubMed] [Google Scholar]
- 50.Moed H, Stoof TJ, Boorsma DM, von Blomberg BM, Gibbs S, Bruynzeel DP, Scheper RJ, Rustemeyer T. Identification of anti-inflammatory drugs according to their capacity to suppress type-1 and type-2 T cell profiles. Clin Exp Allergy. 2004 Dec;34(12):1868–1875. doi: 10.1111/j.1365-2222.2004.02124.x. [DOI] [PubMed] [Google Scholar]
- 51.Gerdes S, Shakery K, Mrowietz U. Dimethylfumarate inhibits nuclear binding of nuclear factor kappaB but not of nuclear factor of activated T cells and CCAAT/enhancer binding protein beta in activated human T cells. Br J Dermatol. 2007 May;156(5):838–842. doi: 10.1111/j.1365-2133.2007.07779.x. [DOI] [PubMed] [Google Scholar]
- 52.Stoof TJ, Flier J, Sampat S, Nieboer C, Tensen CP, Boorsma DM. The antipsoriatic drug dimethylfumarate strongly suppresses chemokine production in human keratinocytes and peripheral blood mononuclear cells. Br J Dermatol. 2001 Jun;144(6):1114–1120. doi: 10.1046/j.1365-2133.2001.04220.x. [DOI] [PubMed] [Google Scholar]
- 53.Rubant SA, Ludwig RJ, Diehl S, Hardt K, Kaufmann R, Pfeilschifter JM, Boehncke WH. Dimethylfumarate reduces leukocyte rolling in vivo through modulation of adhesion molecule expression. J Invest Dermatol. 2008 Feb;128(2):326–331. doi: 10.1038/sj.jid.5700996. [DOI] [PubMed] [Google Scholar]
- 54.Cross SA, Cook DR, Chi AW, Vance PJ, Kolson LL, Wong BJ, Jordan-Sciutto KL, Kolson DL. Dimethyl fumarate, an immune modulator and inducer of the antioxidant response, suppresses HIV replication and macrophage-mediated neurotoxicity: a novel candidate for HIV neuroprotection. J Immunol. 2011 Nov 15;187(10):5015–5025. doi: 10.4049/jimmunol.1101868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilms H, Sievers J, Rickert U, Rostami-Yazdi M, Mrowietz U, Lucius R. Dimethylfumarate inhibits microglial and astrocytic inflammation by suppressing the synthesis of nitric oxide, IL-1beta, TNF-alpha and IL-6 in an in-vitro model of brain inflammation. J Neuroinflammation. 2010;7:30. doi: 10.1186/1742-2094-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Graber DJ, Hickey WF, Stommel EW, Harris BT. Anti-inflammatory efficacy of dexamethasone and Nrf2 activators in the CNS using brain slices as a model of acute injury. J Neuroimmune Pharmacol. 2012 Mar;7(1):266–278. doi: 10.1007/s11481-011-9338-8. [DOI] [PubMed] [Google Scholar]
- 57.Zhu K, Mrowietz U. Enhancement of antibacterial superoxide-anion generation in human monocytes by fumaric acid esters. Arch Dermatol Res. 2005 Oct;297(4):170–176. doi: 10.1007/s00403-005-0598-0. [DOI] [PubMed] [Google Scholar]
- 58.Srisook K, Cha YN. Super-induction of HO-1 in macrophages stimulated with lipopolysaccharide by prior depletion of glutathione decreases iNOS expression and NO production. Nitric Oxide. 2005 Mar;12(2):70–79. doi: 10.1016/j.niox.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 59.Zhu K, Mrowietz U. Inhibition of dendritic cell differentiation by fumaric acid esters. J Invest Dermatol. 2001 Feb;116(2):203–208. doi: 10.1046/j.1523-1747.2001.01159.x. [DOI] [PubMed] [Google Scholar]
- 60.Peng H, Guerau-de-Arellano M, Mehta VB, Yang Y, Huss DJ, Papenfuss TL, Lovett-Racke AE, Racke MK. Dimethyl fumarate inhibits dendritic cell maturation via nuclear factor kappaB (NF-kappaB) and extracellular signal-regulated kinase 1 and 2 (ERK1/2) and mitogen stress-activated kinase 1 (MSK1) signaling. J Biol Chem. 2012 Aug 10;287(33):28017–28026. doi: 10.1074/jbc.M112.383380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Litjens NH, Rademaker M, Ravensbergen B, Rea D, van der Plas MJ, Thio B, Walding A, van Dissel JT, Nibbering PH. Monomethylfumarate affects polarization of monocyte-derived dendritic cells resulting in down-regulated Th1 lymphocyte responses. Eur J Immunol. 2004 Feb;34(2):565–575. doi: 10.1002/eji.200324174. [DOI] [PubMed] [Google Scholar]
- 62.Litjens NH, Rademaker M, Ravensbergen B, Thio HB, van Dissel JT, Nibbering PH. Effects of monomethylfumarate on dendritic cell differentiation. Br J Dermatol. 2006 Feb;154(2):211–217. doi: 10.1111/j.1365-2133.2005.07002.x. [DOI] [PubMed] [Google Scholar]
- 63.Ghoreschi K, Bruck J, Kellerer C, Deng C, Peng H, Rothfuss O, Hussain RZ, Gocke AR, Respa A, Glocova I, Valtcheva N, Alexander E, Feil S, Feil R, Schulze-Osthoff K, Rupec RA, Lovett-Racke AE, Dringen R, Racke MK, Rocken M. Fumarates improve psoriasis and multiple sclerosis by inducing type II dendritic cells. The Journal of experimental medicine. 2011 Oct 24;208(11):2291–2303. doi: 10.1084/jem.20100977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Loewe R, Pillinger M, de Martin R, Mrowietz U, Groger M, Holnthoner W, Wolff K, Wiegrebe W, Jirovsky D, Petzelbauer P. Dimethylfumarate inhibits tumor-necrosis-factor-induced CD62E expression in an NF-kappa B-dependent manner. J Invest Dermatol. 2001 Dec;117(6):1363–1368. doi: 10.1046/j.0022-202x.2001.01576.x. [DOI] [PubMed] [Google Scholar]
- 65.Loewe R, Holnthoner W, Groger M, Pillinger M, Gruber F, Mechtcheriakova D, Hofer E, Wolff K, Petzelbauer P. Dimethylfumarate inhibits TNF-induced nuclear entry of NF-kappa B/p65 in human endothelial cells. J Immunol. 2002 May 1;168(9):4781–4787. doi: 10.4049/jimmunol.168.9.4781. [DOI] [PubMed] [Google Scholar]
- 66.Vandermeeren M, Janssens S, Borgers M, Geysen J. Dimethylfumarate is an inhibitor of cytokine-induced E-selectin, VCAM-1, and ICAM-1 expression in human endothelial cells. Biochem Biophys Res Commun. 1997 May 8;234(1):19–23. doi: 10.1006/bbrc.1997.6570. [DOI] [PubMed] [Google Scholar]
- 67.Wallbrecht K, Drick N, Hund AC, Schon MP. Downregulation of endothelial adhesion molecules by dimethylfumarate, but not monomethylfumarate, and impairment of dynamic lymphocyte-endothelial cell interactions. Exp Dermatol. 2011 Dec;20(12):980–985. doi: 10.1111/j.1600-0625.2011.01376.x. [DOI] [PubMed] [Google Scholar]
- 68.Wierinckx A, Breve J, Mercier D, Schultzberg M, Drukarch B, Van Dam AM. Detoxication enzyme inducers modify cytokine production in rat mixed glial cells. J Neuroimmunol. 2005 Sep;166(1–2):132–143. doi: 10.1016/j.jneuroim.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 69.Scannevin RH, Chollate S, Jung MY, Shackett M, Patel H, Bista P, Zeng W, Ryan S, Yamamoto M, Lukashev M, Rhodes KJ. Fumarates promote cytoprotection of central nervous system cells against oxidative stress via the nuclear factor (erythroid-derived 2)-like 2 pathway. J Pharmacol Exp Ther. 2012 Apr;341(1):274–284. doi: 10.1124/jpet.111.190132. [DOI] [PubMed] [Google Scholar]
- 70.Linker RA, Lee DH, Ryan S, van Dam AM, Conrad R, Bista P, Zeng W, Hronowsky X, Buko A, Chollate S, Ellrichmann G, Bruck W, Dawson K, Goelz S, Wiese S, Scannevin RH, Lukashev M, Gold R. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain. 2011 Mar;134(Pt 3):678–692. doi: 10.1093/brain/awq386. [DOI] [PubMed] [Google Scholar]
- 71.Albrecht P, Bouchachia I, Goebels N, Henke N, Hofstetter HH, Issberner A, Kovacs Z, Lewerenz J, Lisak D, Maher P, Mausberg AK, Quasthoff K, Zimmermann C, Hartung HP, Methner A. Effects of dimethyl fumarate on neuroprotection and immunomodulation. J Neuroinflammation. 2012;9:163. doi: 10.1186/1742-2094-9-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moharregh-Khiabani D, Blank A, Skripuletz T, Miller E, Kotsiari A, Gudi V, Stangel M. Effects of fumaric acids on cuprizone induced central nervous system de- and remyelination in the mouse. PLoS One. 2010;5(7):e11769. doi: 10.1371/journal.pone.0011769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Naldi L, Gambini D. The clinical spectrum of psoriasis. Clin Dermatol. 2007 Nov-Dec;25(6):510–518. doi: 10.1016/j.clindermatol.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 74.Uyemura K, Yamamura M, Fivenson DF, Modlin RL, Nickoloff BJ. The cytokine network in lesional and lesion-free psoriatic skin is characterized by a T-helper type 1 cell-mediated response. J Invest Dermatol. 1993 Nov;101(5):701–705. doi: 10.1111/1523-1747.ep12371679. [DOI] [PubMed] [Google Scholar]
- 75.Schlaak JF, Buslau M, Jochum W, Hermann E, Girndt M, Gallati H, Meyer zum Buschenfelde KH, Fleischer B. T cells involved in psoriasis vulgaris belong to the Th1 subset. J Invest Dermatol. 1994 Feb;102(2):145–149. doi: 10.1111/1523-1747.ep12371752. [DOI] [PubMed] [Google Scholar]
- 76.Austin LM, Ozawa M, Kikuchi T, Walters IB, Krueger JG. The majority of epidermal T cells in Psoriasis vulgaris lesions can produce type 1 cytokines, interferon-gamma, interleukin-2, and tumor necrosis factor-alpha, defining TC1 (cytotoxic T lymphocyte) and TH1 effector populations: a type 1 differentiation bias is also measured in circulating blood T cells in psoriatic patients. J Invest Dermatol. 1999 Nov;113(5):752–759. doi: 10.1046/j.1523-1747.1999.00749.x. [DOI] [PubMed] [Google Scholar]
- 77.Nickoloff BJ, Wrone-Smith T, Bonish B, Porcelli SA. Response of murine and normal human skin to injection of allogeneic blood-derived psoriatic immunocytes: detection of T cells expressing receptors typically present on natural killer cells, including CD94, CD158, and CD161. Arch Dermatol. 1999 May;135(5):546–552. doi: 10.1001/archderm.135.5.546. [DOI] [PubMed] [Google Scholar]
- 78.Luszczek W, Manczak M, Cislo M, Nockowski P, Wisniewski A, Jasek M, Kusnierczyk P. Gene for the activating natural killer cell receptor, KIR2DS1, is associated with susceptibility to psoriasis vulgaris. Hum Immunol. 2004 Jul;65(7):758–766. doi: 10.1016/j.humimm.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 79.Bonish B, Jullien D, Dutronc Y, Huang BB, Modlin R, Spada FM, Porcelli SA, Nickoloff BJ. Overexpression of CD1d by keratinocytes in psoriasis and CD1d-dependent IFN-gamma production by NK-T cells. J Immunol. 2000 Oct 1;165(7):4076–4085. doi: 10.4049/jimmunol.165.7.4076. [DOI] [PubMed] [Google Scholar]
- 80.Nickoloff BJ, Qin JZ, Nestle FO. Immunopathogenesis of psoriasis. Clin Rev Allergy Immunol. 2007 Oct;33(1–2):45–56. doi: 10.1007/s12016-007-0039-2. [DOI] [PubMed] [Google Scholar]
- 81.Castela E, Archier E, Devaux S, Gallini A, Aractingi S, Cribier B, Jullien D, Aubin F, Bachelez H, Joly P, Le Maitre M, Misery L, Richard MA, Paul C, Ortonne JP. Topical corticosteroids in plaque psoriasis: a systematic review of efficacy and treatment modalities. J Eur Acad Dermatol Venereol. 2012 May;26(Suppl 3):36–46. doi: 10.1111/j.1468-3083.2012.04522.x. [DOI] [PubMed] [Google Scholar]
- 82.Lebwohl M, Freeman AK, Chapman MS, Feldman SR, Hartle JE, Henning A. Tacrolimus ointment is effective for facial and intertriginous psoriasis. J Am Acad Dermatol. 2004 Nov;51(5):723–730. doi: 10.1016/j.jaad.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 83.Gribetz C, Ling M, Lebwohl M, Pariser D, Draelos Z, Gottlieb AB, Zaias N, Chen DM, Parneix-Spake A, Hultsch T, Menter A. Pimecrolimus cream 1% in the treatment of intertriginous psoriasis: a double-blind, randomized study. J Am Acad Dermatol. 2004 Nov;51(5):731–738. doi: 10.1016/j.jaad.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 84.Gottlieb SL, Gilleaudeau P, Johnson R, Estes L, Woodworth TG, Gottlieb AB, Krueger JG. Response of psoriasis to a lymphocyte-selective toxin (DAB389IL-2) suggests a primary immune, but not keratinocyte, pathogenic basis. Nat Med. 1995 May;1(5):442–447. doi: 10.1038/nm0595-442. [DOI] [PubMed] [Google Scholar]
- 85.Leonardi CL, Powers JL, Matheson RT, Goffe BS, Zitnik R, Wang A, Gottlieb AB. Etanercept as monotherapy in patients with psoriasis. N Engl J Med. 2003 Nov 20;349(21):2014–2022. doi: 10.1056/NEJMoa030409. [DOI] [PubMed] [Google Scholar]
- 86.Reich K, Nestle FO, Papp K, Ortonne JP, Evans R, Guzzo C, Li S, Dooley LT, Griffiths CE. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trial. Lancet. 2005 Oct 15–21;366(9494):1367–1374. doi: 10.1016/S0140-6736(05)67566-6. [DOI] [PubMed] [Google Scholar]
- 87.Leonardi C, Langley RG, Papp K, Tyring SK, Wasel N, Vender R, Unnebrink K, Gupta SR, Valdecantos WC, Bagel J. Adalimumab for treatment of moderate to severe chronic plaque psoriasis of the hands and feet: efficacy and safety results from REACH, a randomized, placebo-controlled, double-blind trial. Arch Dermatol. 2011 Apr;147(4):429–436. doi: 10.1001/archdermatol.2010.384. [DOI] [PubMed] [Google Scholar]
- 88.Dimon-Gadal S, Gerbaud P, Therond P, Guibourdenche J, Anderson WB, Evain-Brion D, Raynaud F. Increased oxidative damage to fibroblasts in skin with and without lesions in psoriasis. J Invest Dermatol. 2000 May;114(5):984–989. doi: 10.1046/j.1523-1747.2000.00962.x. [DOI] [PubMed] [Google Scholar]
- 89.Relhan V, Gupta SK, Dayal S, Pandey R, Lal H. Blood thiols and malondialdehyde levels in psoriasis. J Dermatol. 2002 Jul;29(7):399–403. doi: 10.1111/j.1346-8138.2002.tb00293.x. [DOI] [PubMed] [Google Scholar]
- 90.Nakai K, Yoneda K, Maeda R, Munehiro A, Fujita N, Yokoi I, Moriue J, Moriue T, Kosaka H, Kubota Y. Urinary biomarker of oxidative stress in patients with psoriasis vulgaris and atopic dermatitis. J Eur Acad Dermatol Venereol. 2009 Dec;23(12):1405–1408. doi: 10.1111/j.1468-3083.2009.03327.x. [DOI] [PubMed] [Google Scholar]
- 91.Ferretti G, Bacchetti T, Campanati A, Simonetti O, Liberati G, Offidani A. Correlation between lipoprotein(a) and lipid peroxidation in psoriasis: role of the enzyme paraoxonase-1. Br J Dermatol. 2012 Jan;166(1):204–207. doi: 10.1111/j.1365-2133.2011.10539.x. [DOI] [PubMed] [Google Scholar]
- 92.Coaccioli S, Panaccione A, Biondi R, Sabatini C, Landucci P, Del Giorno R, Fantera M, Monno Mondo A, Di Cato L, Paladini A, Fatati G, Puxeddu A. Evaluation of oxidative stress in rheumatoid and psoriatic arthritis and psoriasis. Clin Ter. 2009;160(6):467–472. [PubMed] [Google Scholar]
- 93.Asefi M, Vaisi-Raygani A, Bahrehmand F, Kiani A, Rahimi Z, Nomani H, Ebrahimi A, Tavilani H, Pourmotabbed T. Paraoxonase 1 (PON1) 55 polymorphism, lipid profiles and psoriasis. Br J Dermatol. 2012 Dec;167(6):1279–1286. doi: 10.1111/j.1365-2133.2012.11170.x. [DOI] [PubMed] [Google Scholar]
- 94.Nugteren-Huying WM, van der Schroeff JG, Hermans J, Suurmond D. Fumaric acid therapy for psoriasis: a randomized, double-blind, placebo-controlled study. J Am Acad Dermatol. 1990 Feb;22(2 Pt 1):311–312. doi: 10.1016/s0190-9622(08)80766-9. [DOI] [PubMed] [Google Scholar]
- 95.Kolbach DN, Nieboer C. Fumaric acid therapy in psoriasis: results and side effects of 2 years of treatment. J Am Acad Dermatol. 1992 Nov;27(5 Pt 1):769–771. doi: 10.1016/s0190-9622(08)80228-9. [DOI] [PubMed] [Google Scholar]
- 96.Altmeyer PJ, Matthes U, Pawlak F, Hoffmann K, Frosch PJ, Ruppert P, Wassilew SW, Horn T, Kreysel HW, Lutz G, Barth J, Rietzschel I, Joshi RK. Antipsoriatic effect of fumaric acid derivatives. Results of a multicenter double-blind study in 100 patients. J Am Acad Dermatol. 1994 Jun;30(6):977–981. doi: 10.1016/s0190-9622(94)70121-0. [DOI] [PubMed] [Google Scholar]
- 97.Nieboer C, de Hoop D, Langendijk PN, van Loenen AC, Gubbels J. Fumaric acid therapy in psoriasis: a double-blind comparison between fumaric acid compound therapy and monotherapy with dimethylfumaric acid ester. Dermatologica. 1990;181(1):33–37. doi: 10.1159/000247856. [DOI] [PubMed] [Google Scholar]
- 98.Bovenschen HJ, Langewouters AM, van de Kerkhof PC. Dimethylfumarate for psoriasis: Pronounced effects on lesional T-cell subsets, epidermal proliferation and differentiation, but not on natural killer T cells in immunohistochemical study. Am J Clin Dermatol. 2010;11(5):343–350. doi: 10.2165/11533240-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 99.Wain EM, Darling MI, Pleass RD, Barker JN, Smith CH. Treatment of severe, recalcitrant, chronic plaque psoriasis with fumaric acid esters: a prospective study. Br J Dermatol. 2010 Feb 1;162(2):427–434. doi: 10.1111/j.1365-2133.2009.09267.x. [DOI] [PubMed] [Google Scholar]
- 100.Homey B, Meller S. Chemokines and other mediators as therapeutic targets in psoriasis vulgaris. Clin Dermatol. 2008 Sep-Oct;26(5):539–545. doi: 10.1016/j.clindermatol.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 101.Thio HB, Zomerdijk TP, Oudshoorn C, Kempenaar J, Nibbering PH, van der Schroeff JG, Ponec M. Fumaric acid derivatives evoke a transient increase in intracellular free calcium concentration and inhibit the proliferation of human keratinocytes. Br J Dermatol. 1994 Dec;131(6):856–861. doi: 10.1111/j.1365-2133.1994.tb08589.x. [DOI] [PubMed] [Google Scholar]
- 102.Sebok B, Bonnekoh B, Geisel J, Mahrle G. Antiproliferative and cytotoxic profiles of antipsoriatic fumaric acid derivatives in keratinocyte cultures. Eur J Pharmacol. 1994 Jan 3;270(1):79–87. doi: 10.1016/0926-6917(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 103.Paukkonen K, Naukkarinen A, Horsmanheimo M. The development of manifest psoriatic lesions is linked with the invasion of CD8 + T cells and CD11c + macrophages into the epidermis. Arch Dermatol Res. 1992;284(7):375–379. doi: 10.1007/BF00372065. [DOI] [PubMed] [Google Scholar]
- 104.Cabrijan L, Lipozencic J, Batinac T, Peternel S, Pastar Z. The role of CD4 and CD8 lymphocytes and macrophages in psoriasis vulgaris. Acta Dermatovenerol Croat. 2009;17(3):162–165. [PubMed] [Google Scholar]
- 105.Ramirez-Bosca A, Martinez-Ojeda L, Valcuende-Cavero F, Castells-Rodellas A. A study of local immunity in psoriasis. Br J Dermatol. 1988 Nov;119(5):587–595. doi: 10.1111/j.1365-2133.1988.tb03469.x. [DOI] [PubMed] [Google Scholar]
- 106.Bovenschen HJ, Seyger MM, Van de Kerkhof PC. Plaque psoriasis vs. atopic dermatitis and lichen planus: a comparison for lesional T-cell subsets, epidermal proliferation and differentiation. Br J Dermatol. 2005 Jul;153(1):72–78. doi: 10.1111/j.1365-2133.2005.06538.x. [DOI] [PubMed] [Google Scholar]
- 107.Eberlein-Konig B, Mempel M, Stahlecker J, Forer I, Ring J, Abeck D. Disseminated granuloma annulare--treatment with fumaric acid esters. Dermatology. 2005;210(3):223–226. doi: 10.1159/000083514. [DOI] [PubMed] [Google Scholar]
- 108.Rosati G. The prevalence of multiple sclerosis in the world: an update. Neurol Sci. 2001 Apr;22(2):117–139. doi: 10.1007/s100720170011. [DOI] [PubMed] [Google Scholar]
- 109.Pugliatti M, Sotgiu S, Rosati G. The worldwide prevalence of multiple sclerosis. Clin Neurol Neurosurg. 2002 Jul;104(3):182–191. doi: 10.1016/s0303-8467(02)00036-7. [DOI] [PubMed] [Google Scholar]
- 110.Hogancamp WE, Rodriguez M, Weinshenker BG. The epidemiology of multiple sclerosis. Mayo Clin Proc. 1997 Sep;72(9):871–878. doi: 10.4065/72.9.871. [DOI] [PubMed] [Google Scholar]
- 111.Stüve O, Oksenberg J. Multiple Sclerosis Overview. In: Pagon R, Bird T, Dolan C, et al., editors. GeneReviews™ [Internet] Seattle (WA): University of Washington, Seattle; 2006. Jan 10, (Updated 2010 May 11). [Google Scholar]
- 112.Weiner HL. Multiple sclerosis is an inflammatory T-cell-mediated autoimmune disease. Arch Neurol. 2004 Oct;61(10):1613–1615. doi: 10.1001/archneur.61.10.1613. [DOI] [PubMed] [Google Scholar]
- 113.Stys PK, Zamponi GW, van Minnen J, Geurts JJ. Will the real multiple sclerosis please stand up? Nat Rev Neurosci. 2012 Jul;13(7):507–514. doi: 10.1038/nrn3275. [DOI] [PubMed] [Google Scholar]
- 114.Rodriguez M, Scheithauer B. Ultrastructure of multiple sclerosis. Ultrastruct Pathol. 1994 Jan-Apr;18(1–2):3–13. doi: 10.3109/01913129409016267. [DOI] [PubMed] [Google Scholar]
- 115.Aboul-Enein F, Rauschka H, Kornek B, Stadelmann C, Stefferl A, Bruck W, Lucchinetti C, Schmidbauer M, Jellinger K, Lassmann H. Preferential loss of myelin-associated glycoprotein reflects hypoxia-like white matter damage in stroke and inflammatory brain diseases. J Neuropathol Exp Neurol. 2003 Jan;62(1):25–33. doi: 10.1093/jnen/62.1.25. [DOI] [PubMed] [Google Scholar]
- 116.Barnett MH, Prineas JW. Relapsing and remitting multiple sclerosis: pathology of the newly forming lesion. Ann Neurol. 2004 Apr;55(4):458–468. doi: 10.1002/ana.20016. [DOI] [PubMed] [Google Scholar]
- 117.Henderson AP, Barnett MH, Parratt JD, Prineas JW. Multiple sclerosis: distribution of inflammatory cells in newly forming lesions. Ann Neurol. 2009 Dec;66(6):739–753. doi: 10.1002/ana.21800. [DOI] [PubMed] [Google Scholar]
- 118.Inglese M, Mancardi GL, Pagani E, Rocca MA, Murialdo A, Saccardi R, Comi G, Filippi M. Brain tissue loss occurs after suppression of enhancement in patients with multiple sclerosis treated with autologous haematopoietic stem cell transplantation. J Neurol Neurosurg Psychiatry. 2004 Apr;75(4):643–644. [PMC free article] [PubMed] [Google Scholar]
- 119.Metz I, Lucchinetti CF, Openshaw H, Garcia-Merino A, Lassmann H, Freedman MS, Atkins HL, Azzarelli B, Kolar OJ, Bruck W. Autologous haematopoietic stem cell transplantation fails to stop demyelination and neurodegeneration in multiple sclerosis. Brain. 2007 May;130(Pt 5):1254–1262. doi: 10.1093/brain/awl370. [DOI] [PubMed] [Google Scholar]
- 120.Jacobs LD, Cookfair DL, Rudick RA, Herndon RM, Richert JR, Salazar AM, Fischer JS, Goodkin DE, Granger CV, Simon JH, Alam JJ, Bartoszak DM, Bourdette DN, Braiman J, Brownscheidle CM, Coats ME, Cohan SL, Dougherty DS, Kinkel RP, Mass MK, Munschauer FE, 3rd, Priore RL, Pullicino PM, Scherokman BJ, Guttman BW, Whitham RH. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG) Ann Neurol. 1996 Mar;39(3):285–294. doi: 10.1002/ana.410390304. [DOI] [PubMed] [Google Scholar]
- 121.Reichard O, Norkrans G, Fryden A, Braconier JH, Sonnerborg A, Weiland O. Randomised, double-blind, placebo-controlled trial of interferon alpha-2b with and without ribavirin for chronic hepatitis C. The Swedish Study Group. Lancet. 1998 Jan 10;351(9096):83–87. doi: 10.1016/s0140-6736(97)06088-1. [DOI] [PubMed] [Google Scholar]
- 122.Johnson KP, Brooks BR, Cohen JA, Ford CC, Goldstein J, Lisak RP, Myers LW, Panitch HS, Rose JW, Schiffer RB. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo-controlled trial. The Copolymer 1 Multiple Sclerosis Study Group. Neurology. 1995 Jul;45(7):1268–1276. doi: 10.1212/wnl.45.7.1268. [DOI] [PubMed] [Google Scholar]
- 123.Comi G, Filippi M, Wolinsky JS. European/Canadian multicenter, double-blind, randomized, placebo-controlled study of the effects of glatiramer acetate on magnetic resonance imaging--measured disease activity and burden in patients with relapsing multiple sclerosis. European/Canadian Glatiramer Acetate Study Group. Ann Neurol. 2001 Mar;49(3):290–297. [PubMed] [Google Scholar]
- 124.Cohen JA, Barkhof F, Comi G, Hartung HP, Khatri BO, Montalban X, Pelletier J, Capra R, Gallo P, Izquierdo G, Tiel-Wilck K, de Vera A, Jin J, Stites T, Wu S, Aradhye S, Kappos L. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010 Feb 4;362(5):402–415. doi: 10.1056/NEJMoa0907839. [DOI] [PubMed] [Google Scholar]
- 125.O'Connor P, Wolinsky JS, Confavreux C, Comi G, Kappos L, Olsson TP, Benzerdjeb H, Truffinet P, Wang L, Miller A, Freedman MS. Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med. 2011 Oct 6;365(14):1293–1303. doi: 10.1056/NEJMoa1014656. [DOI] [PubMed] [Google Scholar]
- 126.Lassmann H, Ransohoff RM. The CD4-Th1 model for multiple sclerosis: a critical [correction of crucial] re-appraisal. Trends Immunol. 2004 Mar;25(3):132–137. doi: 10.1016/j.it.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 127.Ffrench-Constant C. Pathogenesis of multiple sclerosis. Lancet. 1994 Jan 29;343(8892):271–275. doi: 10.1016/s0140-6736(94)91118-5. [DOI] [PubMed] [Google Scholar]
- 128.Nylander A, Hafler DA. Multiple sclerosis. J Clin Invest. 2012 Apr 2;122(4):1180–1188. doi: 10.1172/JCI58649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Schimrigk S, Brune N, Hellwig K, Lukas C, Bellenberg B, Rieks M, Hoffmann V, Pohlau D, Przuntek H. Oral fumaric acid esters for the treatment of active multiple sclerosis: an open-label, baseline-controlled pilot study. Eur J Neurol. 2006 Jun;13(6):604–610. doi: 10.1111/j.1468-1331.2006.01292.x. [DOI] [PubMed] [Google Scholar]
- 130.Confavreux C, Vukusic S. Non-specific immunosuppressants in the treatment of multiple sclerosis. Clin Neurol Neurosurg. 2004 Jun;106(3):263–269. doi: 10.1016/j.clineuro.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 131.Schilling S, Goelz S, Linker R, Luehder F, Gold R. Fumaric acid esters are effective in chronic experimental autoimmune encephalomyelitis and suppress macrophage infiltration. Clin Exp Immunol. 2006 Jul;145(1):101–107. doi: 10.1111/j.1365-2249.2006.03094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Witte ME, Bo L, Rodenburg RJ, Belien JA, Musters R, Hazes T, Wintjes LT, Smeitink JA, Geurts JJ, De Vries HE, van der Valk P, van Horssen J. Enhanced number and activity of mitochondria in multiple sclerosis lesions. J Pathol. 2009 Oct;219(2):193–204. doi: 10.1002/path.2582. [DOI] [PubMed] [Google Scholar]
- 133.van Horssen J, Witte ME, Schreibelt G, de Vries HE. Radical changes in multiple sclerosis pathogenesis. Biochim Biophys Acta. 2011 Feb;1812(2):141–150. doi: 10.1016/j.bbadis.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 134.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007 Jan;87(1):245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 135.Liu JS, Zhao ML, Brosnan CF, Lee SC. Expression of inducible nitric oxide synthase and nitrotyrosine in multiple sclerosis lesions. Am J Pathol. 2001 Jun;158(6):2057–2066. doi: 10.1016/S0002-9440(10)64677-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gray E, Thomas TL, Betmouni S, Scolding N, Love S. Elevated myeloperoxidase activity in white matter in multiple sclerosis. Neurosci Lett. 2008 Oct 24;444(2):195–198. doi: 10.1016/j.neulet.2008.08.035. [DOI] [PubMed] [Google Scholar]
- 137.Lin SX, Lisi L, Dello Russo C, Polak PE, Sharp A, Weinberg G, Kalinin S, Feinstein DL. The anti-inflammatory effects of dimethyl fumarate in astrocytes involve glutathione and haem oxygenase-1. ASN Neuro. 2011;3(2):e00055. doi: 10.1042/AN20100033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Thiessen A, Schmidt MM, Dringen R. Fumaric acid dialkyl esters deprive cultured rat oligodendroglial cells of glutathione and upregulate the expression of heme oxygenase 1. Neurosci Lett. 2010 May 7;475(1):56–60. doi: 10.1016/j.neulet.2010.03.048. [DOI] [PubMed] [Google Scholar]
- 139.Ross CA, Tabrizi SJ. Huntington's disease: from molecular pathogenesis to clinical treatment. Lancet Neurol. 2011 Jan;10(1):83–98. doi: 10.1016/S1474-4422(10)70245-3. [DOI] [PubMed] [Google Scholar]
- 140.Chen CM. Mitochondrial dysfunction, metabolic deficits, and increased oxidative stress in Huntington's disease. Chang Gung Med J. 2011 Mar-Apr;34(2):135–152. [PubMed] [Google Scholar]
- 141.Ellrichmann G, Petrasch-Parwez E, Lee DH, Reick C, Arning L, Saft C, Gold R, Linker RA. Efficacy of fumaric acid esters in the R6/2 and YAC128 models of Huntington's disease. PLoS One. 2011;6(1):e16172. doi: 10.1371/journal.pone.0016172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Johri A, Beal MF. Antioxidants in Huntington's disease. Biochim Biophys Acta. 2012 May;1822(5):664–674. doi: 10.1016/j.bbadis.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Farooq AV, Shukla D. Herpes simplex epithelial and stromal keratitis: an epidemiologic update. Surv Ophthalmol. 2012 Sep;57(5):448–462. doi: 10.1016/j.survophthal.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Niemialtowski MG, Rouse BT. Predominance of Th1 cells in ocular tissues during herpetic stromal keratitis. J Immunol. 1992 Nov 1;149(9):3035–3039. [PubMed] [Google Scholar]
- 145.Hendricks RL, Tumpey TM, Finnegan A. IFN-gamma and IL-2 are protective in the skin but pathologic in the corneas of HSV-1-infected mice. J Immunol. 1992 Nov 1;149(9):3023–3028. [PubMed] [Google Scholar]
- 146.Tang W, Chang Y, Xu LF, Zheng ZC, Liu XY. Studies on the PEGylation of Protein at a Specific Site: Sulfhydryl-PEGylation of 97Cys-IFN-gamma. Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao (Shanghai) 1996;28(3):312–315. [PubMed] [Google Scholar]
- 147.Tang Q, Chen W, Hendricks RL. Proinflammatory functions of IL-2 in herpes simplex virus corneal infection. J Immunol. 1997 Feb 1;158(3):1275–1283. [PubMed] [Google Scholar]
- 148.Inoue Y. Immunological aspects of herpetic stromal keratitis. Semin Ophthalmol. 2008 Jul-Aug;23(4):221–227. doi: 10.1080/08820530802111390. [DOI] [PubMed] [Google Scholar]
- 149.Hawthorne KM, Dana R, Chodosh J. Delayed type hypersensitivity in the pathogenesis of recurrent herpes stromal keratitis. Semin Ophthalmol. 2011 Jul-Sep;26(4–5):246–250. doi: 10.3109/08820538.2011.588659. [DOI] [PubMed] [Google Scholar]
- 150.Heiligenhaus A, Li H, Wasmuth S, Bauer D. Influence of dimethylfumarate on experimental HSV-1 necrotizing keratitis. Graefes Arch Clin Exp Ophthalmol. 2004 Oct;242(10):870–877. doi: 10.1007/s00417-004-0933-8. [DOI] [PubMed] [Google Scholar]
- 151.Heiligenhaus A, Li H, Schmitz A, Wasmuth S, Bauer D. Improvement of herpetic stromal keratitis with fumaric acid derivate is associated with systemic induction of T helper 2 cytokines. Clin Exp Immunol. 2005 Oct;142(1):180–187. doi: 10.1111/j.1365-2249.2005.02896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Heiligenhaus A, Bauer D, Zheng M, Mrzyk S, Steuhl KP. CD4+ T-cell type 1 and type 2 cytokines in the HSV-1 infected cornea. Graefes Arch Clin Exp Ophthalmol. 1999 May;237(5):399–406. doi: 10.1007/s004170050251. [DOI] [PubMed] [Google Scholar]
- 153.Heiligenhaus A, Jayaraman S, Soukiasian S, Dorf M, Foster CS. Glycoprotein D (5–23) specific Th2-T-cell line induces HSV-1 keratitis. Ophthalmologe. 1995 Aug;92(4):484–491. [PubMed] [Google Scholar]
- 154.Crum NF, Riffenburgh RH, Wegner S, Agan BK, Tasker SA, Spooner KM, Armstrong AW, Fraser S, Wallace MR. Comparisons of causes of death and mortality rates among HIV-infected persons: analysis of the pre-, early, and late HAART (highly active antiretroviral therapy) eras. Journal of acquired immune deficiency syndromes. 2006 Feb 1;41(2):194–200. doi: 10.1097/01.qai.0000179459.31562.16. [DOI] [PubMed] [Google Scholar]
- 155.Broder S. The development of antiretroviral therapy and its impact on the HIV-1/AIDS pandemic. Antiviral Res. Jan;85(1):1–18. doi: 10.1016/j.antiviral.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rajasuriar R, Khoury G, Kamarulzaman A, French MA, Cameron PU, Lewin SR. Persistent immune activation in chronic HIV infection: do any interventions work? AIDS. Jan 16; doi: 10.1097/QAD.0b013e32835ecb8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 62:141–155. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bhaskaran K, Hamouda O, Sannes M, Boufassa F, Johnson AM, Lambert PC, Porter K. Changes in the risk of death after HIV seroconversion compared with mortality in the general population. JAMA. 2008 Jul 2;300(1):51–59. doi: 10.1001/jama.300.1.51. [DOI] [PubMed] [Google Scholar]
- 159.Losina E, Schackman BR, Sadownik SN, Gebo KA, Walensky RP, Chiosi JJ, Weinstein MC, Hicks PL, Aaronson WH, Moore RD, Paltiel AD, Freedberg KA. Racial and sex disparities in life expectancy losses among HIV-infected persons in the united states: impact of risk behavior, late initiation, and early discontinuation of antiretroviral therapy. Clin Infect Dis. 2009 Nov 15;49(10):1570–1578. doi: 10.1086/644772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008 Jul 26;372(9635):293–299. doi: 10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hunt PW. Role of immune activation in HIV pathogenesis. Curr HIV/AIDS Rep. 2007 Feb;4(1):42–47. doi: 10.1007/s11904-007-0007-8. [DOI] [PubMed] [Google Scholar]
- 162.Marchetti G, Tincati C, Silvestri G. Microbial Translocation in the Pathogenesis of HIV Infection and AIDS. Clin Microbiol Rev. Jan;26(1):2–18. doi: 10.1128/CMR.00050-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, Blazar BR, Rodriguez B, Teixeira-Johnson L, Landay A, Martin JN, Hecht FM, Picker LJ, Lederman MM, Deeks SG, Douek DC. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006 Dec;12(12):1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 164.Ancuta P, Kamat A, Kunstman KJ, Kim EY, Autissier P, Wurcel A, Zaman T, Stone D, Mefford M, Morgello S, Singer EJ, Wolinsky SM, Gabuzda D. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS One. 2008;3(6):e2516. doi: 10.1371/journal.pone.0002516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hamann L, Alexander C, Stamme C, Zahringer U, Schumann RR. Acute-phase concentrations of lipopolysaccharide (LPS)-binding protein inhibit innate immune cell activation by different LPS chemotypes via different mechanisms. Infect Immun. 2005 Jan;73(1):193–200. doi: 10.1128/IAI.73.1.193-200.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Freudenberg MA, Tchaptchet S, Keck S, Fejer G, Huber M, Schutze N, Beutler B, Galanos C. Lipopolysaccharide sensing an important factor in the innate immune response to Gram-negative bacterial infections: benefits and hazards of LPS hypersensitivity. Immunobiology. 2008;213(3–4):193–203. doi: 10.1016/j.imbio.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 167.Cotton MF, Ikle DN, Rapaport EL, Marschner S, Tseng PO, Kurrle R, Finkel TH. Apoptosis of CD4+ and CD8+ T cells isolated immediately ex vivo correlates with disease severity in human immunodeficiency virus type 1 infection. Pediatr Res. 1997;42(5):656–664. doi: 10.1203/00006450-199711000-00018. [DOI] [PubMed] [Google Scholar]
- 168.Gougeon ML, Lecoeur H, Dulioust A, Enouf MG, Crouvoiser M, Goujard C, Debord T, Montagnier L. Programmed cell death in peripheral lymphocytes from HIV-infected persons: increased susceptibility to apoptosis of CD4 and CD8 T cells correlates with lymphocyte activation and with disease progression. J Immunol. 1996;156(9):3509–3520. [PubMed] [Google Scholar]
- 169.Ross TM. Using death to one's advantage: HIV modulation of apoptosis. Leukemia. 2001;15(3):332–341. doi: 10.1038/sj.leu.2402028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chakrabarti LA, Lewin SR, Zhang L, Gettie A, Luckay A, Martin LN, Skulsky E, Ho DD, Cheng-Mayer C, Marx PA. Normal T-cell turnover in sooty mangabeys harboring active simian immunodeficiency virus infection. J Virol. 2000;74(3):1209–1223. doi: 10.1128/jvi.74.3.1209-1223.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Davis IC, Girard M, Fultz PN. Loss of CD4+ T cells in human immunodeficiency virus type 1-infected chimpanzees is associated with increased lymphocyte apoptosis. J Virol. 1998;72(6):4623–4632. doi: 10.1128/jvi.72.6.4623-4632.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bostik P, Mayne AE, Villinger F, Greenberg KP, Powell JD, Ansari AA. Relative resistance in the development of T cell anergy in CD4+ T cells from simian immunodeficiency virus disease-resistant sooty mangabeys. J Immunol. 2001;166(1):506–516. doi: 10.4049/jimmunol.166.1.506. [DOI] [PubMed] [Google Scholar]
- 173.Villinger F, Folks TM, Lauro S, Powell JD, Sundstrom JB, Mayne A, Ansari AA. Immunological and virological studies of natural SIV infection of disease-resistant nonhuman primates. Immunol Lett. 1996;51(1–2):59–68. doi: 10.1016/0165-2478(96)02556-4. [DOI] [PubMed] [Google Scholar]
- 174.Silvestri G, Fedanov A, Germon S, Kozyr N, Kaiser WJ, Garber DA, McClure H, Feinberg MB, Staprans SI. Divergent host responses during primary simian immunodeficiency virus SIVsm infection of natural sooty mangabey and nonnatural rhesus macaque hosts. J Virol. 2005 Apr;79(7):4043–4054. doi: 10.1128/JVI.79.7.4043-4054.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Silvestri G, Sodora DL, Koup RA, Paiardini M, O'Neil SP, McClure HM, Staprans SI, Feinberg MB. Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity. 2003 Mar;18(3):441–452. doi: 10.1016/s1074-7613(03)00060-8. [DOI] [PubMed] [Google Scholar]
- 176.Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, Nguyen PL, Khoruts A, Larson M, Haase AT, Douek DC. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004 Sep 20;200(6):749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Brenchley JM, Price DA, Douek DC. HIV disease: fallout from a mucosal catastrophe? Nat Immunol. 2006 Mar;7(3):235–239. doi: 10.1038/ni1316. [DOI] [PubMed] [Google Scholar]
- 178.Wallet MA, Rodriguez CA, Yin L, Saporta S, Chinratanapisit S, Hou W, Sleasman JW, Goodenow MM. Microbial translocation induces persistent macrophage activation unrelated to HIV-1 levels or T-cell activation following therapy. AIDS. Jun 1;24(9):1281–1290. doi: 10.1097/QAD.0b013e328339e228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Eden A, Price RW, Spudich S, Fuchs D, Hagberg L, Gisslen M. Immune activation of the central nervous system is still present after >4 years of effective highly active antiretroviral therapy. J Infect Dis. 2007 Dec 15;196(12):1779–1783. doi: 10.1086/523648. [DOI] [PubMed] [Google Scholar]
- 180.Marchetti G, Bellistri GM, Borghi E, Tincati C, Ferramosca S, La Francesca M, Morace G, Gori A, Monforte AD. Microbial translocation is associated with sustained failure in CD4+ T-cell reconstitution in HIV-infected patients on long-term highly active antiretroviral therapy. AIDS. 2008 Oct 1;22(15):2035–2038. doi: 10.1097/QAD.0b013e3283112d29. [DOI] [PubMed] [Google Scholar]
- 181.Jiang W, Lederman MM, Hunt P, Sieg SF, Haley K, Rodriguez B, Landay A, Martin J, Sinclair E, Asher AI, Deeks SG, Douek DC, Brenchley JM. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J Infect Dis. 2009 Apr 15;199(8):1177–1185. doi: 10.1086/597476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Pulliam L, Gascon R, Stubblebine M, McGuire D, McGrath MS. Unique monocyte subset in patients with AIDS dementia. Lancet. 1997 Mar 8;349(9053):692–695. doi: 10.1016/S0140-6736(96)10178-1. [DOI] [PubMed] [Google Scholar]
- 183.Reingold J, Wanke C, Kotler D, Lewis C, Tracy R, Heymsfield S, Tien P, Bacchetti P, Scherzer R, Grunfeld C, Shlipak M. Association of HIV infection and HIV/HCV coinfection with C-reactive protein levels: the fat redistribution and metabolic change in HIV infection (FRAM) study. J Acquir Immune Defic Syndr. 2008 Jun 1;48(2):142–148. doi: 10.1097/QAI.0b013e3181685727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Neuhaus J, Jacobs DR, Jr, Baker JV, Calmy A, Duprez D, La Rosa A, Kuller LH, Pett SL, Ristola M, Ross MJ, Shlipak MG, Tracy R, Neaton JD. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. Jun 15;201(12):1788–1795. doi: 10.1086/652749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Roberts ES, Masliah E, Fox HS. CD163 identifies a unique population of ramified microglia in HIV encephalitis (HIVE) J Neuropathol Exp Neurol. 2004 Dec;63(12):1255–1264. doi: 10.1093/jnen/63.12.1255. [DOI] [PubMed] [Google Scholar]
- 186.Roberts ES, Zandonatti MA, Watry DD, Madden LJ, Henriksen SJ, Taffe MA, Fox HS. Induction of pathogenic sets of genes in macrophages and neurons in NeuroAIDS. Am J Pathol. 2003 Jun;162(6):2041–2057. doi: 10.1016/S0002-9440(10)64336-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Fischer-Smith T, Tedaldi EM, Rappaport J. CD163/CD16 coexpression by circulating monocytes/macrophages in HIV: potential biomarkers for HIV infection and AIDS progression. AIDS Res Hum Retroviruses. 2008 Mar;24(3):417–421. doi: 10.1089/aid.2007.0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hazenberg MD, Otto SA, van Benthem BH, Roos MT, Coutinho RA, Lange JM, Hamann D, Prins M, Miedema F. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS. 2003 Sep 5;17(13):1881–1888. doi: 10.1097/00002030-200309050-00006. [DOI] [PubMed] [Google Scholar]
- 189.Tien PC, Choi AI, Zolopa AR, Benson C, Tracy R, Scherzer R, Bacchetti P, Shlipak M, Grunfeld C. Inflammation and Mortality in HIV-Infected Adults: Analysis of the FRAM Study Cohort. J Acquir Immune Defic Syndr. 2010 Jun 25;55(3):316–322. doi: 10.1097/QAI.0b013e3181e66216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, Ledergerber B, Lundgren J, Neuhaus J, Nixon D, Paton NI, Neaton JD. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008 Oct 21;5(10):e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Han J, Wang B, Han N, Zhao Y, Song C, Feng X, Mao Y, Zhang F, Zhao H, Zeng H. CD14(high)CD16(+) rather than CD14(low)CD16(+) monocytes correlate with disease progression in chronic HIV-infected patients. J Acquir Immune Defic Syndr. 2009 Dec;52(5):553–559. doi: 10.1097/qai.0b013e3181c1d4fe. [DOI] [PubMed] [Google Scholar]
- 192.Ellery PJ, Tippett E, Chiu YL, Paukovics G, Cameron PU, Solomon A, Lewin SR, Gorry PR, Jaworowski A, Greene WC, Sonza S, Crowe SM. The CD16+ monocyte subset is more permissive to infection and preferentially harbors HIV-1 in vivo. J Immunol. 2007 May 15;178(10):6581–6589. doi: 10.4049/jimmunol.178.10.6581. [DOI] [PubMed] [Google Scholar]
- 193.Wang H, Sun J, Goldstein H. Human immunodeficiency virus type 1 infection increases the in vivo capacity of peripheral monocytes to cross the blood-brain barrier into the brain and the in vivo sensitivity of the blood-brain barrier to disruption by lipopolysaccharide. J Virol. 2008 Aug;82(15):7591–7600. doi: 10.1128/JVI.00768-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Funderburg N, Luciano AA, Jiang W, Rodriguez B, Sieg SF, Lederman MM. Toll-like receptor ligands induce human T cell activation and death, a model for HIV pathogenesis. PLoS One. 2008;3(4):e1915. doi: 10.1371/journal.pone.0001915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Douek DC. Disrupting T-cell homeostasis: how HIV-1 infection causes disease. AIDS Rev. 2003 Jul-Sep;5(3):172–177. [PubMed] [Google Scholar]
- 196.Kovacs JA, Lempicki RA, Sidorov IA, Adelsberger JW, Herpin B, Metcalf JA, Sereti I, Polis MA, Davey RT, Tavel J, Falloon J, Stevens R, Lambert L, Dewar R, Schwartzentruber DJ, Anver MR, Baseler MW, Masur H, Dimitrov DS, Lane HC. Identification of dynamically distinct subpopulations of T lymphocytes that are differentially affected by HIV. J Exp Med. 2001 Dec 17;194(12):1731–1741. doi: 10.1084/jem.194.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sousa AE, Carneiro J, Meier-Schellersheim M, Grossman Z, Victorino RM. CD4 T cell depletion is linked directly to immune activation in the pathogenesis of HIV-1 and HIV-2 but only indirectly to the viral load. J Immunol. 2002 Sep 15;169(6):3400–3406. doi: 10.4049/jimmunol.169.6.3400. [DOI] [PubMed] [Google Scholar]
- 198.Deeks SG, Kitchen CM, Liu L, Guo H, Gascon R, Narvaez AB, Hunt P, Martin JN, Kahn JO, Levy J, McGrath MS, Hecht FM. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood. 2004 Aug 15;104(4):942–947. doi: 10.1182/blood-2003-09-3333. [DOI] [PubMed] [Google Scholar]
- 199.Rychert J, Strick D, Bazner S, Robinson J, Rosenberg E. Detection of HIV gp120 in Plasma During Early HIV Infection Is Associated with Increased Proinflammatory and Immunoregulatory Cytokines. AIDS Res Hum Retroviruses. 2010 Aug 19;26(10):1139–1145. doi: 10.1089/aid.2009.0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kong LY, Wilson BC, McMillian MK, Bing G, Hudson PM, Hong JS. The effects of the HIV-1 envelope protein gp120 on the production of nitric oxide and proinflammatory cytokines in mixed glial cell cultures. Cell Immunol. 1996 Aug 25;172(1):77–83. doi: 10.1006/cimm.1996.0217. [DOI] [PubMed] [Google Scholar]
- 201.Herbein G, Gras G, Khan KA, Abbas W. Macrophage signaling in HIV-1 infection. Retrovirology. 2010;7:34. doi: 10.1186/1742-4690-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Molina JM, Schindler R, Ferriani R, Sakaguchi M, Vannier E, Dinarello CA, Groopman JE. Production of cytokines by peripheral blood monocytes/macrophages infected with human immunodeficiency virus type 1 (HIV-1) The Journal of infectious diseases. 1990 May;161(5):888–893. doi: 10.1093/infdis/161.5.888. [DOI] [PubMed] [Google Scholar]
- 203.Finnegan A, Roebuck KA, Nakai BE, Gu DS, Rabbi MF, Song S, Landay AL. IL-10 cooperates with TNF-alpha to activate HIV-1 from latently and acutely infected cells of monocyte/macrophage lineage. J Immunol. 1996 Jan 15;156(2):841–851. [PubMed] [Google Scholar]
- 204.Marchetti G, Gori A, Casabianca A, Magnani M, Franzetti F, Clerici M, Perno CF, Monforte A, Galli M, Meroni L. Comparative analysis of T-cell turnover and homeostatic parameters in HIV-infected patients with discordant immune-virological responses to HAART. AIDS. 2006 Aug 22;20(13):1727–1736. doi: 10.1097/01.aids.0000242819.72839.db. [DOI] [PubMed] [Google Scholar]
- 205.Hunt PW, Martin JN, Sinclair E, Bredt B, Hagos E, Lampiris H, Deeks SG. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003 May 15;187(10):1534–1543. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]
- 206.Gazzola L, Tincati C, Bellistri GM, Monforte A, Marchetti G. The absence of CD4+ T cell count recovery despite receipt of virologically suppressive highly active antiretroviral therapy: clinical risk, immunological gaps, and therapeutic options. Clin Infect Dis. 2009 Feb 1;48(3):328–337. doi: 10.1086/595851. [DOI] [PubMed] [Google Scholar]
- 207.Gori A, Tincati C, Rizzardini G, Torti C, Quirino T, Haarman M, Ben Amor K, van Schaik J, Vriesema A, Knol J, Marchetti G, Welling G, Clerici M. Early impairment of gut function and gut flora supporting a role for alteration of gastrointestinal mucosa in human immunodeficiency virus pathogenesis. J Clin Microbiol. 2008 Feb;46(2):757–758. doi: 10.1128/JCM.01729-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Tincati C, Biasin M, Bandera A, Violin M, Marchetti G, Piacentini L, Vago GL, Balotta C, Moroni M, Franzetti F, Clerici M, Gori A. Early initiation of highly active antiretroviral therapy fails to reverse immunovirological abnormalities in gut-associated lymphoid tissue induced by acute HIV infection. Antivir Ther. 2009;14(3):321–330. [PubMed] [Google Scholar]
- 209.Perl A, Banki K. Genetic and metabolic control of the mitochondrial transmembrane potential and reactive oxygen intermediate production in HIV disease. Antioxid Redox Signal. 2000 Fall;2(3):551–573. doi: 10.1089/15230860050192323. [DOI] [PubMed] [Google Scholar]
- 210.Elbim C, Pillet S, Prevost MH, Preira A, Girard PM, Rogine N, Hakim J, Israel N, Gougerot-Pocidalo MA. The role of phagocytes in HIV-related oxidative stress. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2001 Feb;20(3):99–109. doi: 10.1016/s1386-6532(00)00133-5. [DOI] [PubMed] [Google Scholar]
- 211.Allard JP, Aghdassi E, Chau J, Salit I, Walmsley S. Oxidative stress and plasma antioxidant micronutrients in humans with HIV infection. The American journal of clinical nutrition. 1998 Jan;67(1):143–147. doi: 10.1093/ajcn/67.1.143. [DOI] [PubMed] [Google Scholar]
- 212.Repetto M, Reides C, Gomez Carretero ML, Costa M, Griemberg G, Llesuy S. Oxidative stress in blood of HIV infected patients. Clinica chimica acta; international journal of clinical chemistry. 1996 Nov 29;255(2):107–117. doi: 10.1016/0009-8981(96)06394-2. [DOI] [PubMed] [Google Scholar]
- 213.Valcour V, Shiramizu B. HIV-associated dementia, mitochondrial dysfunction, and oxidative stress. Mitochondrion. 2004 Jul;4(2–3):119–129. doi: 10.1016/j.mito.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 214.Mollace V, Nottet HS, Clayette P, Turco MC, Muscoli C, Salvemini D, Perno CF. Oxidative stress and neuroAIDS: triggers, modulators and novel antioxidants. Trends in neurosciences. 2001 Jul;24(7):411–416. doi: 10.1016/s0166-2236(00)01819-1. [DOI] [PubMed] [Google Scholar]
- 215.Aukrust P, Svardal AM, Muller F, Lunden B, Berge RK, Froland SS. Decreased levels of total and reduced glutathione in CD4+ lymphocytes in common variable immunodeficiency are associated with activation of the tumor necrosis factor system: possible immunopathogenic role of oxidative stress. Blood. 1995 Aug 15;86(4):1383–1391. [PubMed] [Google Scholar]
- 216.Ronaldson PT, Bendayan R. HIV-1 viral envelope glycoprotein gp120 produces oxidative stress and regulates the functional expression of multidrug resistance protein-1 (Mrp1) in glial cells. J Neurochem. 2008 Aug;106(3):1298–1313. doi: 10.1111/j.1471-4159.2008.05479.x. [DOI] [PubMed] [Google Scholar]
- 217.Turchan-Cholewo J, Dimayuga FO, Gupta S, Keller JN, Knapp PE, Hauser KF, Bruce-Keller AJ. Morphine and HIV-Tat increase microglial-free radical production and oxidative stress: possible role in cytokine regulation. J Neurochem. 2009 Jan;108(1):202–215. doi: 10.1111/j.1471-4159.2008.05756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Deshmane SL, Mukerjee R, Fan S, Del Valle L, Michiels C, Sweet T, Rom I, Khalili K, Rappaport J, Amini S, Sawaya BE. Activation of the oxidative stress pathway by HIV-1 Vpr leads to induction of hypoxia-inducible factor 1alpha expression. J Biol Chem. 2009 Apr 24;284(17):11364–11373. doi: 10.1074/jbc.M809266200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Price TO, Ercal N, Nakaoke R, Banks WA. HIV-1 viral proteins gp120 and Tat induce oxidative stress in brain endothelial cells. Brain Res. 2005 May 31;1045(1–2):57–63. doi: 10.1016/j.brainres.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 220.Eck HP, Gmunder H, Hartmann M, Petzoldt D, Daniel V, Droge W. Low concentrations of acid-soluble thiol (cysteine) in the blood plasma of HIV-1-infected patients. Biol Chem Hoppe Seyler. 1989 Feb;370(2):101–108. doi: 10.1515/bchm3.1989.370.1.101. [DOI] [PubMed] [Google Scholar]
- 221.de Quay B, Malinverni R, Lauterburg BH. Glutathione depletion in HIV-infected patients: role of cysteine deficiency and effect of oral N-acetylcysteine. AIDS. 1992 Aug;6(8):815–819. [PubMed] [Google Scholar]
- 222.Malvy DJ, Richard MJ, Arnaud J, Favier A, Amedee-Manesme O. Relationship of plasma malondialdehyde, vitamin E and antioxidant micronutrients to human immunodeficiency virus-1 seropositivity. Clinica chimica acta; international journal of clinical chemistry. 1994 Jan 14;224(1):89–94. doi: 10.1016/0009-8981(94)90124-4. [DOI] [PubMed] [Google Scholar]
- 223.Masutani H, Naito M, Takahashi K, Hattori T, Koito A, Takatsuki K, Go T, Nakamura H, Fujii S, Yoshida Y, Okuma M, Junji Y. Dysregulation of adult T-cell leukemia-derived factor (ADF)/thioredoxin in HIV infection: loss of ADF high-producer cells in lymphoid tissues of AIDS patients. AIDS research and human retroviruses. 1992 Sep;8(9):1707–1715. doi: 10.1089/aid.1992.8.1707. [DOI] [PubMed] [Google Scholar]
- 224.Sonnerborg A, Carlin G, Akerlund B, Jarstrand C. Increased production of malondialdehyde in patients with HIV infection. Scand J Infect Dis. 1988;20(3):287–290. doi: 10.3109/00365548809032453. [DOI] [PubMed] [Google Scholar]
- 225.Revillard JP, Vincent CM, Favier AE, Richard MJ, Zittoun M, Kazatchkine MD. Lipid peroxidation in human immunodeficiency virus infection. Journal of acquired immune deficiency syndromes. 1992;5(6):637–638. [PubMed] [Google Scholar]
- 226.McLemore JL, Beeley P, Thorton K, Morrisroe K, Blackwell W, Dasgupta A. Rapid automated determination of lipid hydroperoxide concentrations and total antioxidant status of serum samples from patients infected with HIV: elevated lipid hydroperoxide concentrations and depleted total antioxidant capacity of serum samples. Am J Clin Pathol. 1998 Mar;109(3):268–273. doi: 10.1093/ajcp/109.3.268. [DOI] [PubMed] [Google Scholar]
- 227.Foga IO, Nath A, Hasinoff BB, Geiger JD. Antioxidants and dipyridamole inhibit HIV-1 gp120-induced free radical-based oxidative damage to human monocytoid cells. J Acquir Immune Defic Syndr Hum Retrovirol. 1997 Dec 1;16(4):223–229. doi: 10.1097/00042560-199712010-00001. [DOI] [PubMed] [Google Scholar]
- 228.Otis JS, Ashikhmin YI, Brown LA, Guidot DM. Effect of HIV-1-related protein expression on cardiac and skeletal muscles from transgenic rats. AIDS Res Ther. 2008;5:8. doi: 10.1186/1742-6405-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Yu QR, Zhang ZP, Zhang H, Lin HT, Li XM, Bai L, Cai WB. Inducible nitric oxide synthase is involved in the oxidation stress induced by HIV-1 gp120 in human retina pigment epithelial cells. Chin Med J (Engl) 2008 Dec 20;121(24):2578–2583. [PubMed] [Google Scholar]
- 230.Radrizzani M, Accornero P, Delia D, Kurrle R, Colombo MP. Apoptosis induced by HIV-gp120 in a Th1 clone involves the generation of reactive oxygen intermediates downstream CD95 triggering. FEBS letters. 1997 Jul 7;411(1):87–92. doi: 10.1016/s0014-5793(97)00672-8. [DOI] [PubMed] [Google Scholar]
- 231.Stromajer-Racz T, Gazdag Z, Belagyi J, Vagvolgyi C, Zhao RY, Pesti M. Oxidative stress induced by HIV-1 F34IVpr in Schizosaccharomyces pombe is one of its multiple functions. Exp Mol Pathol. 2010 Feb;88(1):38–44. doi: 10.1016/j.yexmp.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 232.Poli G, Fauci AS. The effect of cytokines and pharmacologic agents on chronic HIV infection. AIDS research and human retroviruses. 1992 Feb;8(2):191–197. doi: 10.1089/aid.1992.8.191. [DOI] [PubMed] [Google Scholar]
- 233.Palamara AT, Perno CF, Aquaro S, Bue MC, Dini L, Garaci E. Glutathione inhibits HIV replication by acting at late stages of the virus life cycle. AIDS Res Hum Retroviruses. 1996 Nov 1;12(16):1537–1541. doi: 10.1089/aid.1996.12.1537. [DOI] [PubMed] [Google Scholar]
- 234.Israel N, Gougerot-Pocidalo MA. Oxidative stress in human immunodeficiency virus infection. Cellular and molecular life sciences : CMLS. 1997 Dec;53(11–12):864–870. doi: 10.1007/s000180050106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Roc AC, Ances BM, Chawla S, Korczykowski M, Wolf RL, Kolson DL, Detre JA, Poptani H. Detection of human immunodeficiency virus induced inflammation and oxidative stress in lenticular nuclei with magnetic resonance spectroscopy despite antiretroviral therapy. Arch Neurol. 2007 Sep;64(9):1249–1257. doi: 10.1001/archneur.64.9.noc60125. [DOI] [PubMed] [Google Scholar]
- 236.Reddy PV, Agudelo M, Atluri VS, Nair MP. Inhibition of nuclear factor erythroid 2-related factor 2 exacerbates HIV-1 gp120-induced oxidative and inflammatory response: role in HIV associated neurocognitive disorder. Neurochemical research. 2012 Aug;37(8):1697–1706. doi: 10.1007/s11064-012-0779-0. [DOI] [PubMed] [Google Scholar]
- 237.Fuchs J, Oelke N, Imhof M, Ochsendorf F, Schofer H, Oromek G, Alaoui-Youssefi A, Emerit I. Multiparameter analysis of clastogenic factors, pro-oxidant cytokines, and inflammatory markers in HIV-1-infected patients with asymptomatic disease, opportunistic infections, and malignancies. Mol Med. 1998 May;4(5):333–343. [PMC free article] [PubMed] [Google Scholar]
- 238.Toborek M, Lee YW, Pu H, Malecki A, Flora G, Garrido R, Hennig B, Bauer HC, Nath A. HIV-Tat protein induces oxidative and inflammatory pathways in brain endothelium. J Neurochem. 2003 Jan;84(1):169–179. doi: 10.1046/j.1471-4159.2003.01543.x. [DOI] [PubMed] [Google Scholar]
- 239.Driscoll KE. TNFalpha and MIP-2: role in particle-induced inflammation and regulation by oxidative stress. Toxicology letters. 2000 Mar 15;112–113:177–183. doi: 10.1016/s0378-4274(99)00282-9. [DOI] [PubMed] [Google Scholar]
- 240.Wanchu A, Rana SV, Pallikkuth S, Sachdeva RK. Short communication: oxidative stress in HIV-infected individuals: a cross-sectional study. AIDS research and human retroviruses. 2009 Dec;25(12):1307–1311. doi: 10.1089/aid.2009.0062. [DOI] [PubMed] [Google Scholar]
- 241.Ibeh BO, Emeka-Nwabunnia IK. Increased oxidative stress condition found in different stages of HIV disease in patients undergoing antiretroviral therapy in Umuahia (Nigeria) Immunopharmacology and immunotoxicology. 2012 Dec;34(6):1060–1066. doi: 10.3109/08923973.2012.681327. [DOI] [PubMed] [Google Scholar]
- 242.Suresh DR, Annam V, Pratibha K, Prasad BV. Total antioxidant capacity--a novel early bio-chemical marker of oxidative stress in HIV infected individuals. Journal of biomedical science. 2009;16:61. doi: 10.1186/1423-0127-16-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Herzenberg LA, De Rosa SC, Dubs JG, Roederer M, Anderson MT, Ela SW, Deresinski SC. Glutathione deficiency is associated with impaired survival in HIV disease. Proc Natl Acad Sci U S A. 1997 Mar 4;94(5):1967–1972. doi: 10.1073/pnas.94.5.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Kasang C, Ulmer A, Donhauser N, Schmidt B, Stich A, Klinker H, Kalluvya S, Koutsilieri E, Rethwilm A, Scheller C. HIV patients treated with low-dose prednisolone exhibit lower immune activation than untreated patients. BMC Infect Dis. 2012;12:14. doi: 10.1186/1471-2334-12-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Andrieu JM, Lu W. Long-term clinical, immunologic and virologic impact of glucocorticoids on the chronic phase of HIV infection. BMC Med. 2004 May 5;2:17. doi: 10.1186/1741-7015-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Andrieu JM, Lu W, Levy R. Sustained increases in CD4 cell counts in asymptomatic human immunodeficiency virus type 1-seropositive patients treated with prednisolone for 1 year. The Journal of infectious diseases. 1995 Mar;171(3):523–530. doi: 10.1093/infdis/171.3.523. [DOI] [PubMed] [Google Scholar]
- 247.Wallis RS, Kalayjian R, Jacobson JM, Fox L, Purdue L, Shikuma CM, Arakaki R, Snyder S, Coombs RW, Bosch RJ, Spritzler J, Chernoff M, Aga E, Myers L, Schock B, Lederman MM. A study of the immunology, virology, and safety of prednisone in HIV-1-infected subjects with CD4 cell counts of 200 to 700 mm(−3) Journal of acquired immune deficiency syndromes. 2003 Mar 1;32(3):281–286. doi: 10.1097/00126334-200303010-00006. [DOI] [PubMed] [Google Scholar]
- 248.Rizzardi GP, Harari A, Capiluppi B, Tambussi G, Ellefsen K, Ciuffreda D, Champagne P, Bart PA, Chave JP, Lazzarin A, Pantaleo G. Treatment of primary HIV-1 infection with cyclosporin A coupled with highly active antiretroviral therapy. J Clin Invest. 2002 Mar;109(5):681–688. doi: 10.1172/JCI14522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Markowitz M, Vaida F, Hare CB, Boden D, Mohri H, Hecht FM, Kalayjian RC, Conrad A, Mildvan D, Aberg J, Hogan C, Kilby JM, Balfour HH, Jr, Schafer K, Richman D, Little S. The virologic and immunologic effects of cyclosporine as an adjunct to antiretroviral therapy in patients treated during acute and early HIV-1 infection. The Journal of infectious diseases. 2010 May 1;201(9):1298–1302. doi: 10.1086/651664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Zhao GJ, Li DK, Wolinsky JS, Koopmans RA, Mietlowski W, Redekop WK, Riddehough A, Cover K, Paty DW. Clinical and magnetic resonance imaging changes correlate in a clinical trial monitoring cyclosporine therapy for multiple sclerosis. The MS Study Group. J Neuroimaging. 1997 Jan;7(1):1–7. doi: 10.1111/jon1997711. [DOI] [PubMed] [Google Scholar]
- 251.Efficacy and toxicity of cyclosporine in chronic progressive multiple sclerosis: a randomized, double-blinded, placebo-controlled clinical trial. The Multiple Sclerosis Study Group. Ann Neurol. 1990 Jun;27(6):591–605. doi: 10.1002/ana.410270603. [DOI] [PubMed] [Google Scholar]
- 252.Steck AJ, Regli F, Ochsner F, Gauthier G. Cyclosporine versus azathioprine in the treatment of multiple sclerosis: 12-month clinical and immunological evaluation. Eur Neurol. 1990;30(4):224–228. doi: 10.1159/000117351. [DOI] [PubMed] [Google Scholar]
- 253.Rudge P, Koetsier JC, Mertin J, Mispelblom Beyer JO, Van Walbeek HK, Clifford Jones R, Harrison J, Robinson K, Mellein B, Poole T, Stokvis JC, Timonen P. Randomised double blind controlled trial of cyclosporin in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1989 May;52(5):559–565. doi: 10.1136/jnnp.52.5.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Cummins NW, Badley AD. Mechanisms of HIV-associated lymphocyte apoptosis: 2010. Cell Death Dis. 2010;1:e99. doi: 10.1038/cddis.2010.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Theiss AL, Vijay-Kumar M, Obertone TS, Jones DP, Hansen JM, Gewirtz AT, Merlin D, Sitaraman SV. Prohibitin is a novel regulator of antioxidant response that attenuates colonic inflammation in mice. Gastroenterology. 2009 Jul;137(1):199–208. doi: 10.1053/j.gastro.2009.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Yanaka A, Sato J, Ohmori S. Sulforaphane protects small intestinal mucosa from aspirin/NSAID-induced injury by enhancing host defense systems against oxidative stress and by inhibiting mucosal invasion of anaerobic enterobacteria. Curr Pharm Des. 2013;19(1):157–162. doi: 10.2174/13816128130120. [DOI] [PubMed] [Google Scholar]
- 257.Yeh CT, Chiu HF, Yen GC. Protective effect of sulforaphane on indomethacin-induced cytotoxicity via heme oxygenase-1 expression in human intestinal Int 407 cells. Mol Nutr Food Res. 2009 Sep;53(9):1166–1176. doi: 10.1002/mnfr.200800558. [DOI] [PubMed] [Google Scholar]
- 258.Cheng YT, Wu CH, Ho CY, Yen GC. Catechin protects against ketoprofen-induced oxidative damage of the gastric mucosa by up-regulating Nrf2 in vitro and in vivo. J Nutr Biochem. 2013 Feb;24(2):475–483. doi: 10.1016/j.jnutbio.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 259.Jin W, Wang HD, Hu ZG, Yan W, Chen G, Yin HX. Transcription factor Nrf2 plays a pivotal role in protection against traumatic brain injury-induced acute intestinal mucosal injury in mice. J Surg Res. 2009 Dec;157(2):251–260. doi: 10.1016/j.jss.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 260.Poli G, Kinter A, Justement JS, Kehrl JH, Bressler P, Stanley S, Fauci AS. Tumor necrosis factor alpha functions in an autocrine manner in the induction of human immunodeficiency virus expression. Proc Natl Acad Sci U S A. 1990 Jan;87(2):782–785. doi: 10.1073/pnas.87.2.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Butera ST, Roberts BD, Folks TM. Regulation of HIV-1 expression by cytokine networks in a CD4+ model of chronic infection. J Immunol. 1993 Jan 15;150(2):625–634. [PubMed] [Google Scholar]
- 262.Gatignol A, Buckler-White A, Berkhout B, Jeang KT. Characterization of a human TAR RNA-binding protein that activates the HIV-1 LTR. Science. 1991 Mar 29;251(5001):1597–1600. doi: 10.1126/science.2011739. [DOI] [PubMed] [Google Scholar]
- 263.Duh EJ, Maury WJ, Folks TM, Fauci AS, Rabson AB. Tumor necrosis factor alpha activates human immunodeficiency virus type 1 through induction of nuclear factor binding to the NF-kappa B sites in the long terminal repeat. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5974–5978. doi: 10.1073/pnas.86.15.5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Davis DA, Yusa K, Gillim LA, Newcomb FM, Mitsuya H, Yarchoan R. Conserved cysteines of the human immunodeficiency virus type 1 protease are involved in regulation of polyprotein processing and viral maturation of immature virions. Journal of virology. 1999 Feb;73(2):1156–1164. doi: 10.1128/jvi.73.2.1156-1164.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Look MP, Rockstroh JK, Rao GS, Barton S, Lemoch H, Kaiser R, Kupfer B, Sudhop T, Spengler U, Sauerbruch T. Sodium selenite and N-acetylcysteine in antiretroviral-naive HIV-1-infected patients: a randomized, controlled pilot study. Eur J Clin Invest. 1998 May;28(5):389–397. doi: 10.1046/j.1365-2362.1998.00301.x. [DOI] [PubMed] [Google Scholar]
- 266.De Rosa SC, Zaretsky MD, Dubs JG, Roederer M, Anderson M, Green A, Mitra D, Watanabe N, Nakamura H, Tjioe I, Deresinski SC, Moore WA, Ela SW, Parks D, Herzenberg LA. N-acetylcysteine replenishes glutathione in HIV infection. Eur J Clin Invest. 2000 Oct;30(10):915–929. doi: 10.1046/j.1365-2362.2000.00736.x. [DOI] [PubMed] [Google Scholar]
- 267.Treitinger A, Spada C, Masokawa IY, Verdi JC, Van Der Sander Silveira M, Luis MC, Reis M, Ferreira SI, Abdalla DS. Effect of N-acetyl-L-cysteine on lymphocyte apoptosis, lymphocyte viability, TNF-alpha and IL-8 in HIV-infected patients undergoing anti-retroviral treatment. Braz J Infect Dis. 2004 Oct;8(5):363–371. doi: 10.1590/s1413-86702004000500005. [DOI] [PubMed] [Google Scholar]
- 268.Spada C, Treitinger A, Reis M, Masokawa IY, Verdi JC, Luiz MC, Silveira MV, Michelon CM, Avila-Junior S, Gill D, Ostrowskyl S. The effect of N-acetylcysteine supplementation upon viral load, CD4, CD8, total lymphocyte count and hematocrit in individuals undergoing antiretroviral treatment. Clin Chem Lab Med. 2002 May;40(5):452–455. doi: 10.1515/CCLM.2002.077. [DOI] [PubMed] [Google Scholar]
- 269.Constans J, Delmas-Beauvieux MC, Sergeant C, Peuchant E, Pellegrin JL, Pellegrin I, Clerc M, Fleury H, Simonoff M, Leng B, Conri C. One-year antioxidant supplementation with beta-carotene or selenium for patients infected with human immunodeficiency virus: a pilot study. Clin Infect Dis. 1996 Sep;23(3):654–656. doi: 10.1093/clinids/23.3.654. [DOI] [PubMed] [Google Scholar]
- 270.Burbano X, Miguez-Burbano MJ, McCollister K, Zhang G, Rodriguez A, Ruiz P, Lecusay R, Shor-Posner G. Impact of a selenium chemoprevention clinical trial on hospital admissions of HIV-infected participants. HIV Clin Trials. 2002 Nov-Dec;3(6):483–491. doi: 10.1310/A7LC-7C9V-EWKF-2Y0H. [DOI] [PubMed] [Google Scholar]
- 271.Hurwitz BE, Klaus JR, Llabre MM, Gonzalez A, Lawrence PJ, Maher KJ, Greeson JM, Baum MK, Shor-Posner G, Skyler JS, Schneiderman N. Suppression of human immunodeficiency virus type 1 viral load with selenium supplementation: a randomized controlled trial. Arch Intern Med. 2007 Jan 22;167(2):148–154. doi: 10.1001/archinte.167.2.148. [DOI] [PubMed] [Google Scholar]
- 272.Yi JH, Hazell AS. N-acetylcysteine attenuates early induction of heme oxygenase-1 following traumatic brain injury. Brain Res. 2005 Feb 1;1033(1):13–19. doi: 10.1016/j.brainres.2004.10.055. [DOI] [PubMed] [Google Scholar]
- 273.Romanque P, Cornejo P, Valdes S, Videla LA. Thyroid hormone administration induces rat liver Nrf2 activation: suppression by N-acetylcysteine pretreatment. Thyroid. 2011 Jun;21(6):655–662. doi: 10.1089/thy.2010.0322. [DOI] [PubMed] [Google Scholar]
- 274.Chen F, Lewis W, Hollander JM, Baseler W, Finkel MS. N-acetylcysteine reverses cardiac myocyte dysfunction in HIV-Tat proteinopathy. J Appl Physiol. 2012 Jul;113(1):105–113. doi: 10.1152/japplphysiol.00068.2012. [DOI] [PubMed] [Google Scholar]
- 275.Burk RF, Hill KE, Nakayama A, Mostert V, Levander XA, Motley AK, Johnson DA, Johnson JA, Freeman ML, Austin LM. Selenium deficiency activates mouse liver Nrf2-ARE but vitamin E deficiency does not. Free Radic Biol Med. 2008 Apr 15;44(8):1617–1623. doi: 10.1016/j.freeradbiomed.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Wagner AE, Boesch-Saadatmandi C, Breckwoldt D, Schrader C, Schmelzer C, Doring F, Hashida K, Hori O, Matsugo S, Rimbach G. Ascorbic acid partly antagonizes resveratrol mediated heme oxygenase-1 but not paraoxonase-1 induction in cultured hepatocytes - role of the redox-regulated transcription factor Nrf2. BMC Complement Altern Med. 2011;11:1. doi: 10.1186/1472-6882-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Tarumoto T, Nagai T, Ohmine K, Miyoshi T, Nakamura M, Kondo T, Mitsugi K, Nakano S, Muroi K, Komatsu N, Ozawa K. Ascorbic acid restores sensitivity to imatinib via suppression of Nrf2-dependent gene expression in the imatinib-resistant cell line. Exp Hematol. 2004 Apr;32(4):375–381. doi: 10.1016/j.exphem.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 278.Yubero-Serrano EM, Gonzalez-Guardia L, Rangel-Zuniga O, Delgado-Casado N, Delgado-Lista J, Perez-Martinez P, Garcia-Rios A, Caballero J, Marin C, Gutierrez-Mariscal FM, Tinahones FJ, Villalba JM, Tunez I, Perez-Jimenez F, Lopez-Miranda J. Postprandial antioxidant gene expression is modified by Mediterranean diet supplemented with coenzyme Q(10) in elderly men and women. Age (Dordr) 2013 Feb;35(1):159–170. doi: 10.1007/s11357-011-9331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Dinkova-Kostova AT, Cheah J, Samouilov A, Zweier JL, Bozak RE, Hicks RJ, Talalay P. Phenolic Michael reaction acceptors: combined direct and indirect antioxidant defenses against electrophiles and oxidants. Med Chem. 2007 May;3(3):261–268. doi: 10.2174/157340607780620680. [DOI] [PubMed] [Google Scholar]
- 280.Carrillo MC, Kitani K, Kanai S, Sato Y, Miyasaka K, Ivy GO. (−)deprenyl increases activities of superoxide dismutase and catalase in certain brain regions in old male mice. Life Sci. 1994;54(14):975–981. doi: 10.1016/0024-3205(94)00499-4. [DOI] [PubMed] [Google Scholar]
- 281.Matsui Y, Kumagae Y. Monoamine oxidase inhibitors prevent striatal neuronal necrosis induced by transient forebrain ischemia. Neurosci Lett. 1991 May 27;126(2):175–178. doi: 10.1016/0304-3940(91)90547-7. [DOI] [PubMed] [Google Scholar]
- 282.Ansari KS, Yu PH, Kruck TP, Tatton WG. Rescue of axotomized immature rat facial motoneurons by R(−)-deprenyl: stereospecificity and independence from monoamine oxidase inhibition. J Neurosci. 1993 Sep;13(9):4042–4053. doi: 10.1523/JNEUROSCI.13-09-04042.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Palhagen S, Heinonen E, Hagglund J, Kaugesaar T, Maki-Ikola O, Palm R. Selegiline slows the progression of the symptoms of Parkinson disease. Neurology. 2006 Apr 25;66(8):1200–1206. doi: 10.1212/01.wnl.0000204007.46190.54. [DOI] [PubMed] [Google Scholar]
- 284.Sano M, Ernesto C, Thomas RG, Klauber MR, Schafer K, Grundman M, Woodbury P, Growdon J, Cotman CW, Pfeiffer E, Schneider LS, Thal LJ. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer's disease. The Alzheimer's Disease Cooperative Study. N Engl J Med. 1997 Apr 24;336(17):1216–1222. doi: 10.1056/NEJM199704243361704. [DOI] [PubMed] [Google Scholar]
- 285.Nakaso K, Nakamura C, Sato H, Imamura K, Takeshima T, Nakashima K. Novel cytoprotective mechanism of anti-parkinsonian drug deprenyl: PI3K and Nrf2-derived induction of antioxidative proteins. Biochem Biophys Res Commun. 2006 Jan 20;339(3):915–922. doi: 10.1016/j.bbrc.2005.11.095. [DOI] [PubMed] [Google Scholar]
- 286.Schifitto G, Yiannoutsos CT, Ernst T, Navia BA, Nath A, Sacktor N, Anderson C, Marra CM, Clifford DB. Selegiline and oxidative stress in HIV-associated cognitive impairment. Neurology. 2009 Dec 8;73(23):1975–1981. doi: 10.1212/WNL.0b013e3181c51a48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Schifitto G, Zhang J, Evans SR, Sacktor N, Simpson D, Millar LL, Hung VL, Miller EN, Smith E, Ellis RJ, Valcour V, Singer E, Marra CM, Kolson D, Weihe J, Remmel R, Katzenstein D, Clifford DB. A multicenter trial of selegiline transdermal system for HIV-associated cognitive impairment. Neurology. 2007 Sep 25;69(13):1314–1321. doi: 10.1212/01.wnl.0000268487.78753.0f. [DOI] [PubMed] [Google Scholar]
- 288.Evans SR, Yeh TM, Sacktor N, Clifford DB, Simpson D, Miller EN, Ellis RJ, Valcour V, Marra CM, Millar L, Schifitto G. Selegiline transdermal system (STS) for HIV-associated cognitive impairment: open-label report of ACTG 5090. HIV Clin Trials. 2007 Nov-Dec;8(6):437–446. doi: 10.1310/hct0806-437. [DOI] [PubMed] [Google Scholar]
- 289.Czub S, Koutsilieri E, Sopper S, Czub M, Stahl-Hennig C, Muller JG, Pedersen V, Gsell W, Heeney JL, Gerlach M, Gosztonyi G, Riederer P, ter Meulen V. Enhancement of central nervous system pathology in early simian immunodeficiency virus infection by dopaminergic drugs. Acta neuropathologica. 2001 Feb;101(2):85–91. doi: 10.1007/s004010000313. [DOI] [PubMed] [Google Scholar]
- 290.Gaskill PJ, Calderon TM, Luers AJ, Eugenin EA, Javitch JA, Berman JW. Human immunodeficiency virus (HIV) infection of human macrophages is increased by dopamine: a bridge between HIV-associated neurologic disorders and drug abuse. Am J Pathol. 2009 Sep;175(3):1148–1159. doi: 10.2353/ajpath.2009.081067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Phillips AN, Neaton J, Lundgren JD. The role of HIV in serious diseases other than AIDS. AIDS. 2008 Nov 30;22(18):2409–2418. doi: 10.1097/QAD.0b013e3283174636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Deeks SG, Phillips AN. HIV infection, antiretroviral treatment, ageing, and non-AIDS related morbidity. Bmj. 2009;338:a3172. doi: 10.1136/bmj.a3172. [DOI] [PubMed] [Google Scholar]
- 293.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 2011;62:141–155. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007 Jul;92(7):2506–2512. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Currier JS, Taylor A, Boyd F, Dezii CM, Kawabata H, Burtcel B, Maa JF, Hodder S. Coronary heart disease in HIV-infected individuals. Journal of acquired immune deficiency syndromes. 2003 Aug 1;33(4):506–512. doi: 10.1097/00126334-200308010-00012. [DOI] [PubMed] [Google Scholar]
- 296.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997 Apr 3;336(14):973–979. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 297.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000 Mar 23;342(12):836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 298.Pai JK, Pischon T, Ma J, Manson JE, Hankinson SE, Joshipura K, Curhan GC, Rifai N, Cannuscio CC, Stampfer MJ, Rimm EB. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med. 2004 Dec 16;351(25):2599–2610. doi: 10.1056/NEJMoa040967. [DOI] [PubMed] [Google Scholar]
- 299.Hsue PY, Deeks SG, Hunt PW. Immunologic basis of cardiovascular disease in HIV-infected adults. The Journal of infectious diseases. 2012 Jun;205(Suppl 3):S375–S382. doi: 10.1093/infdis/jis200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Hulsmans M, Holvoet P. The vicious circle between oxidative stress and inflammation in atherosclerosis. J Cell Mol Med. 2010 Jan;14(1–2):70–78. doi: 10.1111/j.1582-4934.2009.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005 Apr 21;352(16):1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 302.Raidel SM, Haase C, Jansen NR, Russ RB, Sutliff RL, Velsor LW, Day BJ, Hoit BD, Samarel AM, Lewis W. Targeted myocardial transgenic expression of HIV Tat causes cardiomyopathy and mitochondrial damage. Am J Physiol Heart Circ Physiol. 2002 May;282(5):H1672–H1678. doi: 10.1152/ajpheart.00955.2001. [DOI] [PubMed] [Google Scholar]
- 303.Brown TT, Qaqish RB. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS. 2006 Nov 14;20(17):2165–2174. doi: 10.1097/QAD.0b013e32801022eb. [DOI] [PubMed] [Google Scholar]
- 304.Aukrust P, Haug CJ, Ueland T, Lien E, Muller F, Espevik T, Bollerslev J, Froland SS. Decreased bone formative and enhanced resorptive markers in human immunodeficiency virus infection: indication of normalization of the bone-remodeling process during highly active antiretroviral therapy. J Clin Endocrinol Metab. 1999 Jan;84(1):145–150. doi: 10.1210/jcem.84.1.5417. [DOI] [PubMed] [Google Scholar]
- 305.Fakruddin JM, Laurence J. HIV envelope gp120-mediated regulation of osteoclastogenesis via receptor activator of nuclear factor kappa B ligand (RANKL) secretion and its modulation by certain HIV protease inhibitors through interferon-gamma/RANKL cross-talk. J Biol Chem. 2003 Nov 28;278(48):48251–48258. doi: 10.1074/jbc.M304676200. [DOI] [PubMed] [Google Scholar]
- 306.Lam J, Abu-Amer Y, Nelson CA, Fremont DH, Ross FP, Teitelbaum SL. Tumour necrosis factor superfamily cytokines and the pathogenesis of inflammatory osteolysis. Ann Rheum Dis. 2002 Nov;61(Suppl 2):ii82–ii83. doi: 10.1136/ard.61.suppl_2.ii82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Romas E, Gillespie MT, Martin TJ. Involvement of receptor activator of NFkappaB ligand and tumor necrosis factor-alpha in bone destruction in rheumatoid arthritis. Bone. 2002 Feb;30(2):340–346. doi: 10.1016/s8756-3282(01)00682-2. [DOI] [PubMed] [Google Scholar]
- 308.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003 May 15;423(6937):337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 309.Islam FM, Wu J, Jansson J, Wilson DP. Relative risk of renal disease among people living with HIV: a systematic review and meta-analysis. BMC Public Health. 2012;12:234. doi: 10.1186/1471-2458-12-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Maggi P, Bartolozzi D, Bonfanti P, Calza L, Cherubini C, Di Biagio A, Marcotullio S, Montella F, Montinaro V, Mussini C, Narciso P, Rusconi S, Vescini F. Renal complications in HIV disease: between present and future. AIDS Rev. 2012 Jan-Mar;14(1):37–53. [PubMed] [Google Scholar]
- 311.Mocroft A, Reiss P, Gasiorowski J, Ledergerber B, Kowalska J, Chiesi A, Gatell J, Rakhmanova A, Johnson M, Kirk O, Lundgren J. Serious fatal and nonfatal non-AIDS-defining illnesses in Europe. Journal of acquired immune deficiency syndromes. 2010 Oct;55(2):262–270. doi: 10.1097/QAI.0b013e3181e9be6b. [DOI] [PubMed] [Google Scholar]
- 312.Smit C, Geskus R, Walker S, Sabin C, Coutinho R, Porter K, Prins M. Effective therapy has altered the spectrum of cause-specific mortality following HIV seroconversion. AIDS. 2006 Mar 21;20(5):741–749. doi: 10.1097/01.aids.0000216375.99560.a2. [DOI] [PubMed] [Google Scholar]
- 313.Palella FJ, Jr, Baker RK, Moorman AC, Chmiel JS, Wood KC, Brooks JT, Holmberg SD. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. Journal of acquired immune deficiency syndromes. 2006 Sep;43(1):27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- 314.Kalayjian RC, Franceschini N, Gupta SK, Szczech LA, Mupere E, Bosch RJ, Smurzynski M, Albert JM. Suppression of HIV-1 replication by antiretroviral therapy improves renal function in persons with low CD4 cell counts and chronic kidney disease. AIDS. 2008 Feb 19;22(4):481–487. doi: 10.1097/QAD.0b013e3282f4706d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Kalayjian RC, Lau B, Mechekano RN, Crane HM, Rodriguez B, Salata RA, Krishnasami Z, Willig JH, Martin JN, Moore RD, Eron JJ, Kitahata MM. Risk factors for chronic kidney disease in a large cohort of HIV-1 infected individuals initiating antiretroviral therapy in routine care. AIDS. 2012 Sep 24;26(15):1907–1915. doi: 10.1097/QAD.0b013e328357f5ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Flandre P, Pugliese P, Cuzin L, Bagnis CI, Tack I, Cabie A, Poizot-Martin I, Katlama C, Brunet-Francois C, Yazdanpanah Y, Dellamonica P. Risk factors of chronic kidney disease in HIV-infected patients. Clin J Am Soc Nephrol. 2011 Jul;6(7):1700–1707. doi: 10.2215/CJN.09191010. [DOI] [PubMed] [Google Scholar]
- 317.El-Sadr WM, Lundgren J, Neaton JD, Gordin F, Abrams D, Arduino RC, Babiker A, Burman W, Clumeck N, Cohen CJ, Cohn D, Cooper D, Darbyshire J, Emery S, Fatkenheuer G, Gazzard B, Grund B, Hoy J, Klingman K, Losso M, Markowitz N, Neuhaus J, Phillips A, Rappoport C. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006 Nov 30;355(22):2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 318.Weber R, Sabin CA, Friis-Moller N, Reiss P, El-Sadr WM, Kirk O, Dabis F, Law MG, Pradier C, De Wit S, Akerlund B, Calvo G, Monforte A, Rickenbach M, Ledergerber B, Phillips AN, Lundgren JD. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006 Aug 14–28;166(15):1632–1641. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 319.Forrester JE, Rhee MS, McGovern BH, Sterling RK, Knox TA, Terrin N. The association of HIV viral load with indirect markers of liver injury. J Viral Hepat. 2012 Feb;19(2):e202–e211. doi: 10.1111/j.1365-2893.2011.01529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007 Oct 30;69(18):1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Grant I. Neurocognitive disturbances in HIV. Int Rev Psychiatry. 2008 Feb;20(1):33–47. doi: 10.1080/09540260701877894. [DOI] [PubMed] [Google Scholar]
- 322.Gannon P, Khan MZ, Kolson DL. Current understanding of HIV-associated neurocognitive disorders pathogenesis. Curr Opin Neurol. 2011 Jun;24(3):275–283. doi: 10.1097/WCO.0b013e32834695fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Ellis RJ, Deutsch R, Heaton RK, Marcotte TD, McCutchan JA, Nelson JA, Abramson I, Thal LJ, Atkinson JH, Wallace MR, Grant I. Neurocognitive impairment is an independent risk factor for death in HIV infection. San Diego HIV Neurobehavioral Research Center Group. Arch Neurol. 1997 Apr;54(4):416–424. doi: 10.1001/archneur.1997.00550160054016. [DOI] [PubMed] [Google Scholar]
- 324.Mayeux R, Stern Y, Tang MX, Todak G, Marder K, Sano M, Richards M, Stein Z, Ehrhardt AA, Gorman JM. Mortality risks in gay men with human immunodeficiency virus infection and cognitive impairment. Neurology. 1993 Jan;43(1):176–182. doi: 10.1212/wnl.43.1_part_1.176. [DOI] [PubMed] [Google Scholar]
- 325.Dore GJ, McDonald A, Li Y, Kaldor JM, Brew BJ. Marked improvement in survival following AIDS dementia complex in the era of highly active antiretroviral therapy. AIDS. 2003 Jul 4;17(10):1539–1545. doi: 10.1097/00002030-200307040-00015. [DOI] [PubMed] [Google Scholar]
- 326.Robertson KR, Smurzynski M, Parsons TD, Wu K, Bosch RJ, Wu J, McArthur JC, Collier AC, Evans SR, Ellis RJ. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS. 2007 Sep 12;21(14):1915–1921. doi: 10.1097/QAD.0b013e32828e4e27. [DOI] [PubMed] [Google Scholar]
- 327.Sacktor N, McDermott MP, Marder K, Schifitto G, Selnes OA, McArthur JC, Stern Y, Albert S, Palumbo D, Kieburtz K, De Marcaida JA, Cohen B, Epstein L. HIV-associated cognitive impairment before and after the advent of combination therapy. J Neurovirol. 2002 Apr;8(2):136–142. doi: 10.1080/13550280290049615. [DOI] [PubMed] [Google Scholar]
- 328.Tozzi V, Balestra P, Bellagamba R, Corpolongo A, Salvatori MF, Visco-Comandini U, Vlassi C, Giulianelli M, Galgani S, Antinori A, Narciso P. Persistence of neuropsychologic deficits despite long-term highly active antiretroviral therapy in patients with HIV-related neurocognitive impairment: prevalence and risk factors. Journal of acquired immune deficiency syndromes. 2007 Jun 1;45(2):174–182. doi: 10.1097/QAI.0b013e318042e1ee. [DOI] [PubMed] [Google Scholar]
- 329.Simioni S, Cavassini M, Annoni JM, Rimbault Abraham A, Bourquin I, Schiffer V, Calmy A, Chave JP, Giacobini E, Hirschel B, Du Pasquier RA. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS. 2010 Jun 1;24(9):1243–1250. doi: 10.1097/QAD.0b013e3283354a7b. [DOI] [PubMed] [Google Scholar]
- 330.Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011 Feb;17(1):3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Heaton RK, Clifford DB, Franklin DR, Jr, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010 Dec 7;75(23):2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Mothobi NZ, Brew BJ. Neurocognitive dysfunction in the highly active antiretroviral therapy era. Curr Opin Infect Dis. 2012 Feb;25(1):4–9. doi: 10.1097/QCO.0b013e32834ef586. [DOI] [PubMed] [Google Scholar]
- 333.Kusdra L, McGuire D, Pulliam L. Changes in monocyte/macrophage neurotoxicity in the era of HAART: implications for HIV-associated dementia. AIDS. 2002 Jan 4;16(1):31–38. doi: 10.1097/00002030-200201040-00005. [DOI] [PubMed] [Google Scholar]
- 334.Ances BM, Ellis RJ. Dementia and neurocognitive disorders due to HIV-1 infection. Seminars in neurology. 2007 Feb;27(1):86–92. doi: 10.1055/s-2006-956759. [DOI] [PubMed] [Google Scholar]
- 335.Cysique LA, Brew BJ. Neuropsychological functioning and antiretroviral treatment in HIV/AIDS: a review. Neuropsychology review. 2009 Jun;19(2):169–185. doi: 10.1007/s11065-009-9092-3. [DOI] [PubMed] [Google Scholar]
- 336.Joska JA, Gouse H, Paul RH, Stein DJ, Flisher AJ. Does highly active antiretroviral therapy improve neurocognitive function? A systematic review. J Neurovirol. 2010 Mar;16(2):101–114. doi: 10.3109/13550281003682513. [DOI] [PubMed] [Google Scholar]
- 337.Price RW, Spudich S. Antiretroviral therapy and central nervous system HIV type 1 infection. The Journal of infectious diseases. 2008 May 15;197(Suppl 3):S294–S306. doi: 10.1086/533419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Morgello S, Estanislao L, Simpson D, Geraci A, DiRocco A, Gerits P, Ryan E, Yakoushina T, Khan S, Mahboob R, Naseer M, Dorfman D, Sharp V. HIV-associated distal sensory polyneuropathy in the era of highly active antiretroviral therapy: the Manhattan HIV Brain Bank. Arch Neurol. 2004 Apr;61(4):546–551. doi: 10.1001/archneur.61.4.546. [DOI] [PubMed] [Google Scholar]
- 339.Skopelitis EE, Kokotis PI, Kontos AN, Panayiotakopoulos GD, Konstantinou K, Kordossis T, Karandreas N. Distal sensory polyneuropathy in HIV-positive patients in the HAART era: an entity underestimated by clinical examination. Int J STD AIDS. 2006 Jul;17(7):467–472. doi: 10.1258/095646206777689062. [DOI] [PubMed] [Google Scholar]
- 340.Shurie JS, Deribew A. Assessment of the prevalence of distal symmetrical polyneuropathy and its risk factors among HAART-treated and untreated HIV infected individuals. Ethiop Med J. 2010 Apr;48(2):85–93. [PubMed] [Google Scholar]
- 341.Bing EG, Burnam MA, Longshore D, Fleishman JA, Sherbourne CD, London AS, Turner BJ, Eggan F, Beckman R, Vitiello B, Morton SC, Orlando M, Bozzette SA, Ortiz-Barron L, Shapiro M. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Archives of general psychiatry. 2001 Aug;58(8):721–728. doi: 10.1001/archpsyc.58.8.721. [DOI] [PubMed] [Google Scholar]
- 342.Dube B, Benton T, Cruess DG, Evans DL. Neuropsychiatric manifestations of HIV infection and AIDS. Journal of psychiatry & neuroscience : JPN. 2005 Jul;30(4):237–246. [PMC free article] [PubMed] [Google Scholar]
- 343.Nel A, Kagee A. Common mental health problems and antiretroviral therapy adherence. AIDS care. 2011 Nov;23(11):1360–1365. doi: 10.1080/09540121.2011.565025. [DOI] [PubMed] [Google Scholar]
- 344.Chiodi F, Keys B, Albert J, Hagberg L, Lundeberg J, Uhlen M, Fenyo EM, Norkrans G. Human immunodeficiency virus type 1 is present in the cerebrospinal fluid of a majority of infected individuals. J Clin Microbiol. 1992 Jul;30(7):1768–1771. doi: 10.1128/jcm.30.7.1768-1771.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Koenig S, Gendelman HE, Orenstein JM, Dal Canto MC, Pezeshkpour GH, Yungbluth M, Janotta F, Aksamit A, Martin MA, Fauci AS. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986 Sep 5;233(4768):1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- 346.Gartner S. HIV infection and dementia. Science. 2000 Jan 28;287(5453):602–604. doi: 10.1126/science.287.5453.602. [DOI] [PubMed] [Google Scholar]
- 347.Persidsky Y, Zheng J, Miller D, Gendelman HE. Mononuclear phagocytes mediate bloodbrain barrier compromise and neuronal injury during HIV-1-associated dementia. J Leukoc Biol. 2000 Sep;68(3):413–422. [PubMed] [Google Scholar]
- 348.Williams KC, Corey S, Westmoreland SV, Pauley D, Knight H, deBakker C, Alvarez X, Lackner AA. Perivascular macrophages are the primary cell type productively infected by simian immunodeficiency virus in the brains of macaques: implications for the neuropathogenesis of AIDS. The Journal of experimental medicine. 2001 Apr 16;193(8):905–915. doi: 10.1084/jem.193.8.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005 Jan;5(1):69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- 350.Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIVassociated dementia. Nature. 2001 Apr 19;410(6831):988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- 351.Morner A, Thomas JA, Bjorling E, Munson PJ, Lucas SB, McKnight A. Productive HIV-2 infection in the brain is restricted to macrophages/microglia. AIDS. 2003 Jul 4;17(10):1451–1455. doi: 10.1097/00002030-200307040-00005. [DOI] [PubMed] [Google Scholar]
- 352.Gelman BBM DJ. HIV-1 Neuropathology. In: Gendelman HEG I, Everall IP, Fox HS, Gelbard HA, Lipton SA, Swindells S, editors. The Neurology of AIDS. New York: Oxford University Press; 2012. pp. 518–535. [Google Scholar]
- 353.Wiestler OD, Leib SL, Brustle O, Spiegel H, Kleihues P. Neuropathology and pathogenesis of HIV encephalopathies. Acta histochemica Supplementband. 1992;42:107–114. [PubMed] [Google Scholar]
- 354.Bell JE. An update on the neuropathology of HIV in the HAART era. Histopathology. 2004 Dec;45(6):549–559. doi: 10.1111/j.1365-2559.2004.02004.x. [DOI] [PubMed] [Google Scholar]
- 355.Morgello S, Mahboob R, Yakoushina T, Khan S, Hague K. Autopsy findings in a human immunodeficiency virus-infected population over 2 decades: influences of gender, ethnicity, risk factors, and time. Archives of pathology & laboratory medicine. 2002 Feb;126(2):182–190. doi: 10.5858/2002-126-0182-AFIAHI. [DOI] [PubMed] [Google Scholar]
- 356.Langford TD, Letendre SL, Larrea GJ, Masliah E. Changing patterns in the neuropathogenesis of HIV during the HAART era. Brain pathology. 2003 Apr;13(2):195–210. doi: 10.1111/j.1750-3639.2003.tb00019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Anthony IC, Bell JE. The Neuropathology of HIV/AIDS. Int Rev Psychiatry. 2008 Feb;20(1):15–24. doi: 10.1080/09540260701862037. [DOI] [PubMed] [Google Scholar]
- 358.Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. Influence of HAART on HIV-related CNS disease and neuroinflammation. J Neuropathol Exp Neurol. 2005 Jun;64(6):529–536. doi: 10.1093/jnen/64.6.529. [DOI] [PubMed] [Google Scholar]
- 359.Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. Accelerated Tau deposition in the brains of individuals infected with human immunodeficiency virus-1 before and after the advent of highly active anti-retroviral therapy. Acta neuropathologica. 2006 Jun;111(6):529–538. doi: 10.1007/s00401-006-0037-0. [DOI] [PubMed] [Google Scholar]
- 360.Masliah E, DeTeresa RM, Mallory ME, Hansen LA. Changes in pathological findings at autopsy in AIDS cases for the last 15 years. AIDS. 2000 Jan 7;14(1):69–74. doi: 10.1097/00002030-200001070-00008. [DOI] [PubMed] [Google Scholar]
- 361.Silva AC, Rodrigues BS, Micheletti AM, Tostes S, Jr, Meneses AC, Silva-Vergara ML, Adad SJ. Neuropathology of AIDS: An Autopsy Review of 284 Cases from Brazil Comparing the Findings Pre- and Post-HAART (Highly Active Antiretroviral Therapy) and Pre- and Postmortem Correlation. AIDS research and treatment. 2012;2012:186850. doi: 10.1155/2012/186850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Backe E, Schwarting R, Gerdes J, Ernst M, Stein H. Ber-MAC3: new monoclonal antibody that defines human monocyte/macrophage differentiation antigen. J Clin Pathol. 1991 Nov;44(11):936–945. doi: 10.1136/jcp.44.11.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Buechler C, Ritter M, Orso E, Langmann T, Klucken J, Schmitz G. Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro- and antiinflammatory stimuli. J Leukoc Biol. 2000 Jan;67(1):97–103. [PubMed] [Google Scholar]
- 364.Hintz KA, Rassias AJ, Wardwell K, Moss ML, Morganelli PM, Pioli PA, Givan AL, Wallace PK, Yeager MP, Guyre PM. Endotoxin induces rapid metalloproteinase-mediated shedding followed by up-regulation of the monocyte hemoglobin scavenger receptor CD163. J Leukoc Biol. 2002 Oct;72(4):711–717. [PubMed] [Google Scholar]
- 365.Weaver LK, Pioli PA, Wardwell K, Vogel SN, Guyre PM. Up-regulation of human monocyte CD163 upon activation of cell-surface Toll-like receptors. J Leukoc Biol. 2007 Mar;81(3):663–671. doi: 10.1189/jlb.0706428. [DOI] [PubMed] [Google Scholar]
- 366.Weaver LK, Hintz-Goldstein KA, Pioli PA, Wardwell K, Qureshi N, Vogel SN, Guyre PM. Pivotal advance: activation of cell surface Toll-like receptors causes shedding of the hemoglobin scavenger receptor CD163. J Leukoc Biol. 2006 Jul;80(1):26–35. doi: 10.1189/jlb.1205756. [DOI] [PubMed] [Google Scholar]
- 367.Timmermann M, Hogger P. Oxidative stress and 8-iso-prostaglandin F(2alpha) induce ectodomain shedding of CD163 and release of tumor necrosis factor-alpha from human monocytes. Free Radic Biol Med. 2005 Jul 1;39(1):98–107. doi: 10.1016/j.freeradbiomed.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 368.Neaton JD, Neuhaus J, Emery S. Soluble biomarkers and morbidity and mortality among people infected with HIV: summary of published reports from 1997 to 2010. Curr Opin HIV AIDS. 2010 Nov;5(6):480–490. doi: 10.1097/COH.0b013e32833ed75d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Nixon DE, Landay AL. Biomarkers of immune dysfunction in HIV. Curr Opin HIV AIDS. 2010 Nov;5(6):498–503. doi: 10.1097/COH.0b013e32833ed6f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Lyons JL, Uno H, Ancuta P, Kamat A, Moore DJ, Singer EJ, Morgello S, Gabuzda D. Plasma sCD14 is a biomarker associated with impaired neurocognitive test performance in attention and learning domains in HIV infection. Journal of acquired immune deficiency syndromes. 2011 Aug 15;57(5):371–379. doi: 10.1097/QAI.0b013e3182237e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Burdo TH, Lo J, Abbara S, Wei J, DeLelys ME, Preffer F, Rosenberg ES, Williams KC, Grinspoon S. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. The Journal of infectious diseases. 2011 Oct 15;204(8):1227–1236. doi: 10.1093/infdis/jir520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Burdo TH, Lentz MR, Autissier P, Krishnan A, Halpern E, Letendre S, Rosenberg ES, Ellis RJ, Williams KC. Soluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after anti-retroviral therapy. The Journal of infectious diseases. 2011 Jul 1;204(1):154–163. doi: 10.1093/infdis/jir214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Burdo TH, Soulas C, Orzechowski K, Button J, Krishnan A, Sugimoto C, Alvarez X, Kuroda MJ, Williams KC. Increased monocyte turnover from bone marrow correlates with severity of SIV encephalitis and CD163 levels in plasma. PLoS Pathog. 2010 Apr;6(4):e1000842. doi: 10.1371/journal.ppat.1000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Burdo TWA, Woods S, :etendre S, Ellis R, Williams K. Elevated sCD163 is a Marker of Neurocognitive Impairment in HIV-infected Individuals on Effective ART. AIDS. 2013;27(9):1387–1395. doi: 10.1097/QAD.0b013e32836010bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Garvey LPN, Politis M, Ramlackhansingh A, Taylor-Robinson S, Brooks D, Winston A. Microglial Cell Activation is visualized with 11C-[R]-PK11195-PET scans in neuro-asymptomatic HIV infected subjects on effective antiretroviral therapy Conference on Retroviruses and Opportunistic Infections [Oral Abstract] 2012 In press. [Google Scholar]
- 376.Kamat A, Lyons JL, Misra V, Uno H, Morgello S, Singer EJ, Gabuzda D. Monocyte activation markers in cerebrospinal fluid associated with impaired neurocognitive testing in advanced HIV infection. Journal of acquired immune deficiency syndromes. 2012 Jul 1;60(3):234–243. doi: 10.1097/QAI.0b013e318256f3bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Nolting T, Lindecke A, Hartung HP, Koutsilieri E, Maschke M, Husstedt IW, Sopper S, Stuve O, Arendt G German Competence Network HA. Cytokine levels in CSF and neuropsychological performance in HIV patients. J Neurovirol. 2012 Jun;18(3):157–161. doi: 10.1007/s13365-012-0091-4. [DOI] [PubMed] [Google Scholar]
- 378.Glass JD, Fedor H, Wesselingh SL, McArthur JC. Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Ann Neurol. 1995 Nov;38(5):755–762. doi: 10.1002/ana.410380510. [DOI] [PubMed] [Google Scholar]
- 379.Kaul M, Zheng J, Okamoto S, Gendelman HE, Lipton SA. HIV-1 infection and AIDS: consequences for the central nervous system. Cell Death Differ. 2005 Aug;12(Suppl 1):878–892. doi: 10.1038/sj.cdd.4401623. [DOI] [PubMed] [Google Scholar]
- 380.Masliah E, Heaton RK, Marcotte TD, Ellis RJ, Wiley CA, Mallory M, Achim CL, McCutchan JA, Nelson JA, Atkinson JH, Grant I. Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. HNRC Group. The HIV Neurobehavioral Research Center. Ann Neurol. 1997 Dec;42(6):963–972. doi: 10.1002/ana.410420618. [DOI] [PubMed] [Google Scholar]
- 381.Wesselingh SL, Takahashi K, Glass JD, McArthur JC, Griffin JW, Griffin DE. Cellular localization of tumor necrosis factor mRNA in neurological tissue from HIV-infected patients by combined reverse transcriptase/polymerase chain reaction in situ hybridization and immunohistochemistry. J Neuroimmunol. 1997 Apr;74(1–2):1–8. doi: 10.1016/s0165-5728(96)00160-9. [DOI] [PubMed] [Google Scholar]
- 382.Masliah E, Ge N, Morey M, DeTeresa R, Terry RD, Wiley CA. Cortical dendritic pathology in human immunodeficiency virus encephalitis. Lab Invest. 1992 Mar;66(3):285–291. [PubMed] [Google Scholar]
- 383.Wiley CA, Masliah E, Morey M, Lemere C, DeTeresa R, Grafe M, Hansen L, Terry R. Neocortical damage during HIV infection. Ann Neurol. 1991 Jun;29(6):651–657. doi: 10.1002/ana.410290613. [DOI] [PubMed] [Google Scholar]
- 384.Gelbard HA, James HJ, Sharer LR, Perry SW, Saito Y, Kazee AM, Blumberg BM, Epstein LG. Apoptotic neurons in brains from paediatric patients with HIV-1 encephalitis and progressive encephalopathy. Neuropathol Appl Neurobiol. 1995 Jun;21(3):208–217. doi: 10.1111/j.1365-2990.1995.tb01052.x. [DOI] [PubMed] [Google Scholar]
- 385.Petito CK, Roberts B. Evidence of apoptotic cell death in HIV encephalitis. Am J Pathol. 1995 May;146(5):1121–1130. [PMC free article] [PubMed] [Google Scholar]
- 386.Adamson DC, Dawson TM, Zink MC, Clements JE, Dawson VL. Neurovirulent simian immunodeficiency virus infection induces neuronal, endothelial, and glial apoptosis. Mol Med. 1996 Jul;2(4):417–428. [PMC free article] [PubMed] [Google Scholar]
- 387.Gelman BB, Soukup VM, Schuenke KW, Keherly MJ, Holzer C, 3rd, Richey FJ, Lahart CJ. Acquired neuronal channelopathies in HIV-associated dementia. J Neuroimmunol. 2004 Dec;157(1–2):111–119. doi: 10.1016/j.jneuroim.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 388.Hu D, Liu J, Xiong H. Enhancement of neuronal outward delayed rectifier K+ current by human monocyte-derived macrophages. Glia. 2009 Nov 1;57(14):1492–1500. doi: 10.1002/glia.20865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Hu D, Liu J, Keblesh J, Xiong H. Involvement of the 4-aminopyridine-sensitive transient A-type K+ current in macrophage-induced neuronal injury. Eur J Neurosci. 2010 Jan;31(2):214–222. doi: 10.1111/j.1460-9568.2009.07063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Xiong H, Zeng YC, Zheng J, Thylin M, Gendelman HE. Soluble HIV-1 infected macrophage secretory products mediate blockade of long-term potentiation: a mechanism for cognitive dysfunction in HIV-1-associated dementia. J Neurovirol. 1999 Oct;5(5):519–528. doi: 10.3109/13550289909045381. [DOI] [PubMed] [Google Scholar]
- 391.Dong J, Xiong H. Human immunodeficiency virus type 1 gp120 inhibits long-term potentiation via chemokine receptor CXCR4 in rat hippocampal slices. J Neurosci Res. 2006 Feb 15;83(3):489–496. doi: 10.1002/jnr.20745. [DOI] [PubMed] [Google Scholar]
- 392.Behnisch T, Francesconi W, Sanna PP. HIV secreted protein Tat prevents long-term potentiation in the hippocampal CA1 region. Brain Res. 2004 Jun 25;1012(1–2):187–189. doi: 10.1016/j.brainres.2004.03.037. [DOI] [PubMed] [Google Scholar]
- 393.Anderson ER, Boyle J, Zink WE, Persidsky Y, Gendelman HE, Xiong H. Hippocampal synaptic dysfunction in a murine model of human immunodeficiency virus type 1 encephalitis. Neuroscience. 2003;118(2):359–369. doi: 10.1016/s0306-4522(02)00925-9. [DOI] [PubMed] [Google Scholar]
- 394.Gelman BB, Nguyen TP. Synaptic proteins linked to HIV-1 infection and immunoproteasome induction: proteomic analysis of human synaptosomes. J Neuroimmune Pharmacol. 2010 Mar;5(1):92–102. doi: 10.1007/s11481-009-9168-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci. 2007 Jan;8(1):33–44. doi: 10.1038/nrn2040. [DOI] [PubMed] [Google Scholar]
- 396.Deshpande M, Zheng J, Borgmann K, Persidsky R, Wu L, Schellpeper C, Ghorpade A. Role of activated astrocytes in neuronal damage: potential links to HIV-1-associated dementia. Neurotoxicity research. 2005;7(3):183–192. doi: 10.1007/BF03036448. [DOI] [PubMed] [Google Scholar]
- 397.Li W, Galey D, Mattson MP, Nath A. Molecular and cellular mechanisms of neuronal cell death in HIV dementia. Neurotoxicity research. 2005 Oct;8(1–2):119–134. doi: 10.1007/BF03033824. [DOI] [PubMed] [Google Scholar]
- 398.Cisneros IE, Ghorpade A. HIV-1, methamphetamine and astrocyte glutamate regulation: combined excitotoxic implications for neuro-AIDS. Current HIV research. 2012 Jul;10(5):392–406. doi: 10.2174/157016212802138832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Fan Y, Zou W, Green LA, Kim BO, He JJ. Activation of Egr-1 expression in astrocytes by HIV-1 Tat: new insights into astrocyte-mediated Tat neurotoxicity. J Neuroimmune Pharmacol. 2011 Mar;6(1):121–129. doi: 10.1007/s11481-010-9217-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Toggas SM, Masliah E, Mucke L. Prevention of HIV-1 gp120-induced neuronal damage in the central nervous system of transgenic mice by the NMDA receptor antagonist memantine. Brain Res. 1996 Jan 15;706(2):303–307. doi: 10.1016/0006-8993(95)01197-8. [DOI] [PubMed] [Google Scholar]
- 401.Anderson ER, Gendelman HE, Xiong H. Memantine protects hippocampal neuronal function in murine human immunodeficiency virus type 1 encephalitis. J Neurosci. 2004 Aug 11;24(32):7194–7198. doi: 10.1523/JNEUROSCI.1933-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Meisner F, Scheller C, Kneitz S, Sopper S, Neuen-Jacob E, Riederer P, ter Meulen V, Koutsilieri E. Memantine upregulates BDNF and prevents dopamine deficits in SIV-infected macaques: a novel pharmacological action of memantine. Neuropsychopharmacology. 2008 Aug;33(9):2228–2236. doi: 10.1038/sj.npp.1301615. [DOI] [PubMed] [Google Scholar]
- 403.Jiang ZG, Piggee C, Heyes MP, Murphy C, Quearry B, Bauer M, Zheng J, Gendelman HE, Markey SP. Glutamate is a mediator of neurotoxicity in secretions of activated HIV-1-infected macrophages. J Neuroimmunol. 2001 Jul 2;117(1–2):97–107. doi: 10.1016/s0165-5728(01)00315-0. [DOI] [PubMed] [Google Scholar]
- 404.Heyes MP, Jordan EK, Lee K, Saito K, Frank JA, Snoy PJ, Markey SP, Gravell M. Relationship of neurologic status in macaques infected with the simian immunodeficiency virus to cerebrospinal fluid quinolinic acid and kynurenic acid. Brain Res. 1992 Jan 20;570(1–2):237–250. doi: 10.1016/0006-8993(92)90587-y. [DOI] [PubMed] [Google Scholar]
- 405.Heyes MP, Brew BJ, Martin A, Price RW, Salazar AM, Sidtis JJ, Yergey JA, Mouradian MM, Sadler AE, Keilp J, Rubinow D, Markey SP. Quinolinic acid in cerebrospinal fluid and serum in HIV-1 infection: relationship to clinical and neurological status. Ann Neurol. 1991 Feb;29(2):202–209. doi: 10.1002/ana.410290215. [DOI] [PubMed] [Google Scholar]
- 406.Giulian D, Yu J, Li X, Tom D, Li J, Wendt E, Lin SN, Schwarcz R, Noonan C. Study of receptor-mediated neurotoxins released by HIV-1-infected mononuclear phagocytes found in human brain. J Neurosci. 1996 May 15;16(10):3139–3153. doi: 10.1523/JNEUROSCI.16-10-03139.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Gelbard HA, Nottet HS, Swindells S, Jett M, Dzenko KA, Genis P, White R, Wang L, Choi YB, Zhang D, Lipton SA, Tourtellotte WW, Epstein LG, Gendelman HE. Platelet-activating factor: a candidate human immunodeficiency virus type 1-induced neurotoxin. Journal of virology. 1994 Jul;68(7):4628–4635. doi: 10.1128/jvi.68.7.4628-4635.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Achim CL, Heyes MP, Wiley CA. Quantitation of human immunodeficiency virus, immune activation factors, and quinolinic acid in AIDS brains. J Clin Invest. 1993 Jun;91(6):2769–2775. doi: 10.1172/JCI116518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Pulliam L, West D, Haigwood N, Swanson RA. HIV-1 envelope gp120 alters astrocytes in human brain cultures. AIDS research and human retroviruses. 1993 May;9(5):439–444. doi: 10.1089/aid.1993.9.439. [DOI] [PubMed] [Google Scholar]
- 410.Lipton SA. Requirement for macrophages in neuronal injury induced by HIV envelope protein gp120. Neuroreport. 1992 Oct;3(10):913–915. doi: 10.1097/00001756-199210000-00023. [DOI] [PubMed] [Google Scholar]
- 411.Nath A. Human immunodeficiency virus (HIV) proteins in neuropathogenesis of HIV dementia. The Journal of infectious diseases. 2002 Dec 1;186(Suppl 2):S193–S198. doi: 10.1086/344528. [DOI] [PubMed] [Google Scholar]
- 412.Maragos WF, Tillman P, Jones M, Bruce-Keller AJ, Roth S, Bell JE, Nath A. Neuronal injury in hippocampus with human immunodeficiency virus transactivating protein, Tat. Neuroscience. 2003;117(1):43–53. doi: 10.1016/s0306-4522(02)00713-3. [DOI] [PubMed] [Google Scholar]
- 413.Ferrarese C, Aliprandi A, Tremolizzo L, Stanzani L, De Micheli A, Dolara A, Frattola L. Increased glutamate in CSF and plasma of patients with HIV dementia. Neurology. 2001 Aug 28;57(4):671–675. doi: 10.1212/wnl.57.4.671. [DOI] [PubMed] [Google Scholar]
- 414.Espey MG, Basile AS, Heaton RK, Ellis RJ. Increased glutamate in CSF and plasma of patients with HIV dementia. Neurology. 2002 May 14;58(9):1439. doi: 10.1212/wnl.58.9.1439. [DOI] [PubMed] [Google Scholar]
- 415.Espey MG, Ellis RJ, Heaton RK, Basile AS. Relevance of glutamate levels in the CSF of patients with HIV-1-associated dementia complex. Neurology. 1999 Sep 22;53(5):1144–1145. doi: 10.1212/wnl.53.5.1144. [DOI] [PubMed] [Google Scholar]
- 416.Martin A, Heyes MP, Salazar AM, Kampen DL, Williams J, Law WA, Coats ME, Markey SP. Progressive slowing of reaction time and increasing cerebrospinal fluid concentrations of quinolinic acid in HIV-infected individuals. J Neuropsychiatry Clin Neurosci. 1992 Summer;4(3):270–279. doi: 10.1176/jnp.4.3.270. [DOI] [PubMed] [Google Scholar]
- 417.Heyes MP, Brew BJ, Saito K, Quearry BJ, Price RW, Lee K, Bhalla RB, Der M, Markey SP. Inter-relationships between quinolinic acid, neuroactive kynurenines, neopterin and beta 2-microglobulin in cerebrospinal fluid and serum of HIV-1-infected patients. J Neuroimmunol. 1992 Sep;40(1):71–80. doi: 10.1016/0165-5728(92)90214-6. [DOI] [PubMed] [Google Scholar]
- 418.Ciardi M, Sharief MK, Thompson EJ, Salotti A, Vullo V, Sorice F, Cirelli A. High cerebrospinal fluid and serum levels of tumor necrosis factor-alpha in asymptomatic HIV-1 seropositive individuals. Correlation with interleukin-2 and soluble IL-2 receptor. J Neurol Sci. 1994 Sep;125(2):175–179. doi: 10.1016/0022-510x(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 419.Schousboe A, Waagepetersen HS. Role of astrocytes in glutamate homeostasis: implications for excitotoxicity. Neurotoxicity research. 2005 Nov;8(3–4):221–225. doi: 10.1007/BF03033975. [DOI] [PubMed] [Google Scholar]
- 420.Vesce S, Rossi D, Brambilla L, Volterra A. Glutamate release from astrocytes in physiological conditions and in neurodegenerative disorders characterized by neuroinflammation. Int Rev Neurobiol. 2007;82:57–71. doi: 10.1016/S0074-7742(07)82003-4. [DOI] [PubMed] [Google Scholar]
- 421.Dreyer EB, Lipton SA. The coat protein gp120 of HIV-1 inhibits astrocyte uptake of excitatory amino acids via macrophage arachidonic acid. Eur J Neurosci. 1995 Dec 1;7(12):2502–2507. doi: 10.1111/j.1460-9568.1995.tb01048.x. [DOI] [PubMed] [Google Scholar]
- 422.Kort JJ. Impairment of excitatory amino acid transport in astroglial cells infected with the human immunodeficiency virus type 1. AIDS research and human retroviruses. 1998 Oct 10;14(15):1329–1339. doi: 10.1089/aid.1998.14.1329. [DOI] [PubMed] [Google Scholar]
- 423.Vesce S, Bezzi P, Rossi D, Meldolesi J, Volterra A. HIV-1 gp120 glycoprotein affects the astrocyte control of extracellular glutamate by both inhibiting the uptake and stimulating the release of the amino acid. FEBS Lett. 1997 Jul 7;411(1):107–109. doi: 10.1016/s0014-5793(97)00674-1. [DOI] [PubMed] [Google Scholar]
- 424.Visalli V, Muscoli C, Sacco I, Sculco F, Palma E, Costa N, Colica C, Rotiroti D, Mollace V. N-acetylcysteine prevents HIV gp 120-related damage of human cultured astrocytes: correlation with glutamine synthase dysfunction. BMC Neurosci. 2007;8:106. doi: 10.1186/1471-2202-8-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Fine SM, Angel RA, Perry SW, Epstein LG, Rothstein JD, Dewhurst S, Gelbard HA. Tumor necrosis factor alpha inhibits glutamate uptake by primary human astrocytes. Implications for pathogenesis of HIV-1 dementia. J Biol Chem. 1996 Jun 28;271(26):15303–15306. doi: 10.1074/jbc.271.26.15303. [DOI] [PubMed] [Google Scholar]
- 426.Sitcheran R, Gupta P, Fisher PB, Baldwin AS. Positive and negative regulation of EAAT2 by NF-kappaB: a role for N-myc in TNFalpha-controlled repression. EMBO J. 2005 Feb 9;24(3):510–520. doi: 10.1038/sj.emboj.7600555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Cali C, Bezzi P. CXCR4-mediated glutamate exocytosis from astrocytes. J Neuroimmunol. 2010 Jul 27;224(1–2):13–21. doi: 10.1016/j.jneuroim.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 428.Zou J, Wang YX, Dou FF, Lu HZ, Ma ZW, Lu PH, Xu XM. Glutamine synthetase down-regulation reduces astrocyte protection against glutamate excitotoxicity to neurons. Neurochem Int. 2010 Mar;56(4):577–584. doi: 10.1016/j.neuint.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini BL, Pozzan T, Volterra A. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature. 1998 Jan 15;391(6664):281–285. doi: 10.1038/34651. [DOI] [PubMed] [Google Scholar]
- 430.Sorg O, Horn TF, Yu N, Gruol DL, Bloom FE. Inhibition of astrocyte glutamate uptake by reactive oxygen species: role of antioxidant enzymes. Mol Med. 1997 Jul;3(7):431–440. [PMC free article] [PubMed] [Google Scholar]
- 431.Haughey NJ, Cutler RG, Tamara A, McArthur JC, Vargas DL, Pardo CA, Turchan J, Nath A, Mattson MP. Perturbation of sphingolipid metabolism and ceramide production in HIV-dementia. Ann Neurol. 2004 Feb;55(2):257–267. doi: 10.1002/ana.10828. [DOI] [PubMed] [Google Scholar]
- 432.Bandaru VV, McArthur JC, Sacktor N, Cutler RG, Knapp EL, Mattson MP, Haughey NJ. Associative and predictive biomarkers of dementia in HIV-1-infected patients. Neurology. 2007 May 1;68(18):1481–1487. doi: 10.1212/01.wnl.0000260610.79853.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Turchan J, Pocernich CB, Gairola C, Chauhan A, Schifitto G, Butterfield DA, Buch S, Narayan O, Sinai A, Geiger J, Berger JR, Elford H, Nath A. Oxidative stress in HIV demented patients and protection ex vivo with novel antioxidants. Neurology. 2003 Jan 28;60(2):307–314. doi: 10.1212/01.wnl.0000042048.85204.3d. [DOI] [PubMed] [Google Scholar]
- 434.Viviani B, Corsini E, Binaglia M, Galli CL, Marinovich M. Reactive oxygen species generated by glia are responsible for neuron death induced by human immunodeficiency virus-glycoprotein 120 in vitro. Neuroscience. 2001;107(1):51–58. doi: 10.1016/s0306-4522(01)00332-3. [DOI] [PubMed] [Google Scholar]
- 435.Ferrucci A, Nonnemacher MR, Cohen EA, Wigdahl B. Extracellular human immunodeficiency virus type 1 viral protein R causes reductions in astrocytic ATP and glutathione levels compromising the antioxidant reservoir. Virus Res. 2012 Aug;167(2):358–369. doi: 10.1016/j.virusres.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Shi B, Raina J, Lorenzo A, Busciglio J, Gabuzda D. Neuronal apoptosis induced by HIV-1 Tat protein and TNF-alpha: potentiation of neurotoxicity mediated by oxidative stress and implications for HIV-1 dementia. J Neurovirol. 1998 Jun;4(3):281–290. doi: 10.3109/13550289809114529. [DOI] [PubMed] [Google Scholar]
- 437.Tian C, Sun L, Jia B, Ma K, Curthoys N, Ding J, Zheng J. Mitochondrial glutaminase release contributes to glutamate-mediated neurotoxicity during human immunodeficiency virus-1 infection. J Neuroimmune Pharmacol. 2012 Sep;7(3):619–628. doi: 10.1007/s11481-012-9364-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Velazquez I, Plaud M, Wojna V, Skolasky R, Laspiur JP, Melendez LM. Antioxidant enzyme dysfunction in monocytes and CSF of Hispanic women with HIV-associated cognitive impairment. J Neuroimmunol. 2009 Jan 3;206(1–2):106–111. doi: 10.1016/j.jneuroim.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Zhang Y, Wang M, Li H, Zhang H, Shi Y, Wei F, Liu D, Liu K, Chen D. Accumulation of nuclear and mitochondrial DNA damage in the frontal cortex cells of patients with HIV-associated neurocognitive disorders. Brain Res. 2012 Jun 6;1458:1–11. doi: 10.1016/j.brainres.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 440.Mattson MP, Haughey NJ, Nath A. Cell death in HIV dementia. Cell Death Differ. 2005 Aug;12(Suppl 1):893–904. doi: 10.1038/sj.cdd.4401577. [DOI] [PubMed] [Google Scholar]
- 441.Sacktor N, Haughey N, Cutler R, Tamara A, Turchan J, Pardo C, Vargas D, Nath A. Novel markers of oxidative stress in actively progressive HIV dementia. J Neuroimmunol. 2004 Dec;157(1–2):176–184. doi: 10.1016/j.jneuroim.2004.08.037. [DOI] [PubMed] [Google Scholar]
- 442.van Muiswinkel FL, Kuiperij HB. The Nrf2-ARE Signalling pathway: promising drug target to combat oxidative stress in neurodegenerative disorders. Curr Drug Targets CNS Neurol Disord. 2005 Jun;4(3):267–281. doi: 10.2174/1568007054038238. [DOI] [PubMed] [Google Scholar]
- 443.Droge W, Schipper HM. Oxidative stress and aberrant signaling in aging and cognitive decline. Aging Cell. 2007 Jun;6(3):361–370. doi: 10.1111/j.1474-9726.2007.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Mancuso M, Coppede F, Migliore L, Siciliano G, Murri L. Mitochondrial dysfunction, oxidative stress and neurodegeneration. J Alzheimers Dis. 2006 Sep;10(1):59–73. doi: 10.3233/jad-2006-10110. [DOI] [PubMed] [Google Scholar]
- 445.Halliwell B. Oxidative stress and neurodegeneration: where are we now? J Neurochem. 2006 Jun;97(6):1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- 446.Behl C. Oxidative stress in Alzheimer's disease: implications for prevention and therapy. Subcell Biochem. 2005;38:65–78. doi: 10.1007/0-387-23226-5_3. [DOI] [PubMed] [Google Scholar]
- 447.Jenner P. Oxidative stress in Parkinson's disease. Ann Neurol. 2003;53(Suppl 3):S26–S38. doi: 10.1002/ana.10483. [DOI] [PubMed] [Google Scholar]
- 448.Browne SE, Beal MF. Oxidative damage in Huntington's disease pathogenesis. Antioxid Redox Signal. 2006 Nov-Dec;8(11–12):2061–2073. doi: 10.1089/ars.2006.8.2061. [DOI] [PubMed] [Google Scholar]
- 449.Laspiur JP, Anderson ER, Ciborowski P, Wojna V, Rozek W, Duan F, Mayo R, Rodriguez E, Plaud-Valentin M, Rodriguez-Orengo J, Gendelman HE, Melendez LM. CSF proteomic fingerprints for HIV-associated cognitive impairment. J Neuroimmunol. 2007 Dec;192(1–2):157–170. doi: 10.1016/j.jneuroim.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Melendez LM, Colon K, Rivera L, Rodriguez-Franco E, Toro-Nieves D. Proteomic analysis of HIV-infected macrophages. J Neuroimmune Pharmacol. 2011 Mar;6(1):89–106. doi: 10.1007/s11481-010-9253-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Saccani A, Saccani S, Orlando S, Sironi M, Bernasconi S, Ghezzi P, Mantovani A, Sica A. Redox regulation of chemokine receptor expression. Proc Natl Acad Sci U S A. 2000 Mar 14;97(6):2761–2766. doi: 10.1073/pnas.97.6.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Fiala M, Looney DJ, Stins M, Way DD, Zhang L, Gan X, Chiappelli F, Schweitzer ES, Shapshak P, Weinand M, Graves MC, Witte M, Kim KS. TNF-alpha opens a paracellular route for HIV-1 invasion across the blood-brain barrier. Mol Med. 1997 Aug;3(8):553–564. [PMC free article] [PubMed] [Google Scholar]
- 453.Koh K, Kim J, Jang YJ, Yoon K, Cha Y, Lee HJ, Kim J. Transcription factor Nrf2 suppresses LPS-induced hyperactivation of BV-2 microglial cells. J Neuroimmunol. 2011 Apr;233(1–2):160–167. doi: 10.1016/j.jneuroim.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 454.Brandenburg LO, Kipp M, Lucius R, Pufe T, Wruck CJ. Sulforaphane suppresses LPS-induced inflammation in primary rat microglia. Inflammation Research. 2010 Jun;59(6):443–450. doi: 10.1007/s00011-009-0116-5. [DOI] [PubMed] [Google Scholar]
- 455.Kuhn AM, Tzieply N, Schmidt MV, von Knethen A, Namgaladze D, Yamamoto M, Brune B. Antioxidant signaling via Nrf2 counteracts lipopolysaccharide-mediated inflammatory responses in foam cell macrophages. Free Radic Biol Med. 2011 May 15;50(10):1382–1391. doi: 10.1016/j.freeradbiomed.2011.02.036. [DOI] [PubMed] [Google Scholar]
- 456.Rojo AI, Innamorato NG, Martin-Moreno AM, De Ceballos ML, Yamamoto M, Cuadrado A. Nrf2 regulates microglial dynamics and neuroinflammation in experimental Parkinson's disease. Glia. 2010 Apr;58(5):588–598. doi: 10.1002/glia.20947. [DOI] [PubMed] [Google Scholar]
- 457.Nath KA. Heme oxygenase-1: a provenance for cytoprotective pathways in the kidney and other tissues. Kidney international. 2006 Aug;70(3):432–443. doi: 10.1038/sj.ki.5001565. [DOI] [PubMed] [Google Scholar]
- 458.Pae HO, Lee YC, Chung HT. Heme oxygenase-1 and carbon monoxide: emerging therapeutic targets in inflammation and allergy. Recent patents on inflammation & allergy drug discovery. 2008 Nov;2(3):159–165. doi: 10.2174/187221308786241929. [DOI] [PubMed] [Google Scholar]
- 459.An L, Liu CT, Yu MJ, Chen ZH, Guo XG, Peng W, Wang JF, Fang XQ, Gao YH, Yu SY. Heme oxygenase-1 system, inflammation and ventilator-induced lung injury. Eur J Pharmacol. 2012 Feb 29;677(1–3):1–4. doi: 10.1016/j.ejphar.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 460.Paine A, Eiz-Vesper B, Blasczyk R, Immenschuh S. Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochemical pharmacology. 2010 Dec 15;80(12):1895–1903. doi: 10.1016/j.bcp.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 461.Spindler KR, Hsu TH. Viral disruption of the blood-brain barrier. Trends Microbiol. 2012 Jun;20(6):282–290. doi: 10.1016/j.tim.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Zhao J, Moore AN, Redell JB, Dash PK. Enhancing expression of Nrf2-driven genes protects the blood brain barrier after brain injury. J Neurosci. 2007 Sep 19;27(38):10240–10248. doi: 10.1523/JNEUROSCI.1683-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Wang Z, Ma C, Meng CJ, Zhu GQ, Sun XB, Huo L, Zhang J, Liu HX, He WC, Shen XM, Shu Z, Chen G. Melatonin activates the Nrf2-ARE pathway when it protects against early brain injury in a subarachnoid hemorrhage model. J Pineal Res. 2012 Sep;53(2):129–137. doi: 10.1111/j.1600-079X.2012.00978.x. [DOI] [PubMed] [Google Scholar]
- 464.Xiang J, Alesi GN, Zhou N, Keep RF. Protective effects of isothiocyanates on blood-CSF barrier disruption induced by oxidative stress. Am J Physiol Regul Integr Comp Physiol. 2012 Jul 1;303(1):R1–R7. doi: 10.1152/ajpregu.00518.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Rossi D, Volterra A. Astrocytic dysfunction: insights on the role in neurodegeneration. Brain Res Bull. 2009 Oct 28;80(4–5):224–232. doi: 10.1016/j.brainresbull.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 466.Hori K, Burd PR, Furuke K, Kutza J, Weih KA, Clouse KA. Human immunodeficiency virus-1-infected macrophages induce inducible nitric oxide synthase and nitric oxide (NO) production in astrocytes: astrocytic NO as a possible mediator of neural damage in acquired immunodeficiency syndrome. Blood. 1999 Mar 15;93(6):1843–1850. [PubMed] [Google Scholar]
- 467.Carlson L. Clinical management of the HIV-positive kidney transplant recipient. Nephrol Nurs J. 2008 Nov-Dec;35(6):559–567. [PubMed] [Google Scholar]
- 468.Rubin RH, Tolkoff-Rubin NE. The problem of human immunodeficiency virus (HIV) infection and transplantation. Transpl Int. 1988 Apr;1(1):36–42. doi: 10.1007/BF00337847. [DOI] [PubMed] [Google Scholar]
- 469.Huprikar S. Solid organ transplantation in HIV-infected individuals: an update. Rev Med Virol. 2009 Nov;19(6):317–323. doi: 10.1002/rmv.620. [DOI] [PubMed] [Google Scholar]
- 470.Norman SP, Kommareddi M, Kaul DR. Update on kidney transplantation in HIV-infected recipients. AIDS Rev. 2012 Jul;14(3):195–207. [PubMed] [Google Scholar]
- 471.Qiu J, Terasaki PI, Waki K, Cai J, Gjertson DW. HIV-positive renal recipients can achieve survival rates similar to those of HIV-negative patients. Transplantation. 2006 Jun 27;81(12):1658–1661. doi: 10.1097/01.tp.0000226074.97314.e0. [DOI] [PubMed] [Google Scholar]
- 472.Locke JE, Montgomery RA, Warren DS, Subramanian A, Segev DL. Renal transplant in HIV-positive patients: long-term outcomes and risk factors for graft loss. Arch Surg. 2009 Jan;144(1):83–86. doi: 10.1001/archsurg.2008.508. [DOI] [PubMed] [Google Scholar]
- 473.Kidney and liver organ transplantation in persons with human immunodeficiency virus: An Evidence-Based Analysis. Ont Health Technol Assess Ser. 2010;10(4):1–56. [PMC free article] [PubMed] [Google Scholar]
- 474.Roland ME, Barin B, Carlson L, Frassetto LA, Terrault NA, Hirose R, Freise CE, Benet LZ, Ascher NL, Roberts JP, Murphy B, Keller MJ, Olthoff KM, Blumberg EA, Brayman KL, Bartlett ST, Davis CE, McCune JM, Bredt BM, Stablein DM, Stock PG. HIV-infected liver and kidney transplant recipients: 1- and 3-year outcomes. Am J Transplant. 2008 Feb;8(2):355–365. doi: 10.1111/j.1600-6143.2007.02061.x. [DOI] [PubMed] [Google Scholar]
- 475.Stock PG, Barin B, Murphy B, Hanto D, Diego JM, Light J, Davis C, Blumberg E, Simon D, Subramanian A, Millis JM, Lyon GM, Brayman K, Slakey D, Shapiro R, Melancon J, Jacobson JM, Stosor V, Olson JL, Stablein DM, Roland ME. Outcomes of kidney transplantation in HIV-infected recipients. N Engl J Med. 2010 Nov 18;363(21):2004–2014. doi: 10.1056/NEJMoa1001197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.OPTN / SRTR, editor. Department of Health and Human Services HRaSA, Healthcare Systems Bureau, Division of Transplantation. Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR) Rockville, MD: 2011. 2010 Annual Data Report; pp. 33–35. [Google Scholar]
- 477.Nissen NN, Barin B, Stock PG. Malignancy in the HIV-infected patients undergoing liver and kidney transplantation. Curr Opin Oncol. 2012 Sep;24(5):517–521. doi: 10.1097/CCO.0b013e328355e0d7. [DOI] [PMC free article] [PubMed] [Google Scholar]