Abstract
Objective
Synovitis is associated with pain and other symptoms in patients with knee OA, and in patients with meniscal tears even in the absence of radiographic OA. Patients undergoing arthroscopic partial meniscectomy were followed for 2 years to determine whether synovitis predicts post-operative symptoms.
Design
Thirty-three patients scheduled for arthroscopy were recruited for this pilot study. Symptoms were assessed using a knee pain scale, the Lysholm score, and the SF-12® pre-operatively and at 16 weeks, 1 year and 2 years post-operatively. Synovial inflammation and hyperplasia were graded on surgical biopsies. Linear mixed effects models were tested to determine whether inflammation or hyperplasia is associated with outcome scores over time.
Results
Lysholm scores and SF-12® physical component sub-scores were worse pre-operatively in patients with inflammation (Lysholm: 52.42 [95%CI 42.37,62.47] vs. 72.38 [66.03,78.72], p<0.001; SF-12: 36.81 [28.26,45.37] vs 46.23 [40.14,52.32], p<0.05). Up to two-years post- operatively, patients with inflammation achieved mean scores similar to those without inflammation. As a result, the mean improvement in Lysholm scores was 13.01 [1.48–24.53] points higher than patients without inflammation, p = 0.03. 33% (4/12) of patients with inflammation still had fair-to-poor Lysholm scores two years after surgery compared to 7% (1/15, (p=0.14) without inflammation. No association between hyperplasia and symptoms was noted.
Conclusions
In this pilot study of patients undergoing partial meniscectomy, synovial inflammation was associated with worse pre-operative symptoms, but not with poorer outcomes in the first two years post-arthroscopy. Larger cohorts and longer follow-up should be pursued to confirm this relationship, and determine if the initial response is sustained.
Keywords: synovitis, osteoarthritis, meniscal tear, inflammation, arthroscopy
INTRODUCTION
Osteoarthritis is a disease in which multiple joint structures are compromised, including cartilage, meniscus, bone, and synovial membrane.1 Structural alterations seen on plain radiographs, specifically osteophytes and joint space narrowing, can be identified in approximately 34% of the United States population over age 65, but in only about half are structural changes accompanied by pain and dysfunction.2 Importantly, pain and related symptoms are associated with more rapid structural progression.3 Therefore understanding factors responsible for symptom development is essential to identify patients at risk of greater morbidity and disability, and to identify modifiable disease targets for therapeutic intervention.
Multiple investigators have demonstrated the association of patient reported symptoms such as pain with presence and severity of synovitis in patients with OA.4 Synovitis also is associated with objective measures of knee joint dysfunction including walking and stair climbing times.5 We have reported that the relationship between synovitis and symptoms applies to patients at risk for OA, but without radiographic signs of OA structural alterations, based on the analysis of a cohort of patients with meniscal tears undergoing meniscectomy.6
In young sports-participants, traumatic meniscal tears are among the most common knee joint derangements and these tears are associated with an increased risk of OA development.7 But the most common meniscal derangements are degenerative meniscal tears 8 which can occur in the setting of pre-existing OA and are also associated with risk of disease progression.7 Patients with meniscal tears, with or without concomitant OA, often seek surgical intervention due to a variety of symptoms (pain, locking, stiffness, inability to participate in specific activities). In fact, there are close to 1 million arthroscopic meniscal procedures performed in the US annually,9 although meniscal derangement itself has not consistently been associated with pain.10–12
The present longitudinal pilot study was designed to test the hypothesis that synovitis detected at the time of surgery predicts poorer symptomatic outcomes after arthroscopic meniscectomy. This study was conducted on patients with meniscal tears but with normal pre-surgical knee radiographs. Patient–reported knee symptoms were measured at three postoperative time points using the Lysholm score,13 Short form-12 (SF-12 ®) health surveys,14 and a visual analogue knee pain scale. The pre-operative characteristics and relationship with baseline Lysholm scores of these patients have been previously published.6 Here, we report analysis of the relationship between synovitis measured at the time of surgery, and knee symptoms using three outcome measures up to 2 years post-operatively.
METHODS
Patients
The study was approved by the Institutional Review Board (IRB) of the New England Baptist Hospital (NEBH), and all patients gave written, informed consent. Inclusion and exclusion criteria, as well as demographics and baseline characteristics, have been described in detail previously.6 Briefly, thirty-three patients with a history of knee injury and an MRI-confirmed meniscal tear scheduled for arthroscopic partial meniscectomy were recruited between September 2005 and April 2009. Patients with radiographic evidence of OA (osteophytes or joint space narrowing) were excluded. There were twenty-one males and twelve females, median age was 45 (IQR 40–53), and median BMI was 26.9 (IQR 24.7–28.1). The majority (twenty-five patients) had complex meniscal tears with multiple cleavage planes consistent with degenerative type tears, and only 20% had completely normal cartilage surfaces as indicated by an Outerbridge score15 of zero in all three knee compartments.
Synovial tissue collection and evaluation of synovitis
Knee synovial biopsies were obtained during surgery from three locations (suprapatellar pouch, medial and lateral gutters). Biopsies were taken from areas that appeared inflamed or thickened. When no inflammation was apparent, standard locations were used: femoral aspects of the gutters and the central supratrochlear region in the pouch. Biopsies from five patients were insufficient for analysis, so 28 patients contributed biopsies. Histologic features of synovitis16 were evaluated on formalin-fixed, paraffin-embedded, hematoxylin and eosin (H&E) stained synovial membrane sections. Inflammation was evaluated on a semi-quantitative scale 6 presented in Table 1, based on perivascular mononuclear cell aggregates. In addition, synovial lining hyperplasia, vascularity, fibrosis, and the presence of detritic fragments of bone and cartilage were graded independently (Table 1). The grading system was adapted from multiple sources16–19with input from an experienced musculoskeletal Pathologist (ED). Randomly chosen subsets of synovial specimens were scored by two independent readers (E.D., C.R.S.) for assessment of inter- and intra-reader reliability. However, the final reported scores reflect those of a single reader (the experienced musculoskeletal Pathologist, ED).
Table 1.
Synovial Histopathologic Grading (for H&E stained sections):
| 1) Mononuclear Inflammation: |
| Grade 0 = none |
| Grade 1 = mild (0–1 perivascular aggregates per field) |
| Grade 2 = moderate (>1 perivascular aggregate per field +/− focala interstitial infiltration) |
| Grade 3 = marked (both perivascular and widespreada interstitial aggregates) |
| 2) Vascularity: |
| Grade 0 = normal |
| Grade 1 = mildly increased |
| Grade 2 = markedly increased |
| 3) Fibrosis of sublining: |
| Grade 0 = absent |
| Grade 1 = focal |
| Grade 2 = widespread |
| 4) Detritus: |
| Grade 0 = absent |
| Grade 1 = small particulate |
| Grade 2 = large particulate |
| 5) Synovial Hyperplasiab |
| Grade 0 = Normal (up to 2 cell layers thick) |
| Grade 1 = Hyperplasia I (3–4 cells thick) |
| Grade 2 = Hyperplasia II (> 4 cell thick) |
Focal = present in < 50% of the field. Widespread = present in > 50% of the field.
Hyperplasia evaluated at high power (40X). All other features evaluated at low power (5–10X).
Outcome scores
Symptoms were assessed using three instruments. First, Lysholm questionnaires were utilized to assess knee-specific symptoms. The Lysholm questionnaire is a clinician-developed instrument measuring symptoms including pain, swelling, limp, locking, and instability, as well as functional disability (stair-climbing, squatting, use of supports). A summed score is reported on a scale of 0–100, where 100 = no symptoms/disability.13 Second, the SF-12 ® health survey was utilized to measure general physical and emotional health. Physical (PCS) and mental (MCS) component summary sub-scores were calculated and expressed as norm-based standardized scores.14, with 50 equal to the population mean and 10 the population standard deviation. Finally, patients were also asked to assess their knee pain on a scale from 0–10 (0 = no pain). All three instruments were administered pre-operatively and at 16 weeks, 1 year and 2 years post-operatively. Thirty-one patients completed the 2 year follow-up.
Measurement of chemokine transcript levels
mRNA levels of four chemokines and one chemokine receptor (IL-8, CCR7, CCL19, CCL21 and CCL5) were measured in twelve suprapatellar synovial biopsies by real-time PCR.6 These transcripts were chosen for analysis as they had been identified as differentially expressed in biopsies with or without synovial inflammatory infiltrates by microarray analysis6. Cycle threshold (Ct) values were normalized to GAPDH, and expression levels calculated20 relative to the mean of specimens without inflammation.
Statistical analyses
Inter- and intra-reader reliability of histopathology scores were reported as a weighted kappa statistic. Between-group differences in patient characteristics were evaluated with Mann-Whitney U-tests (for continuous variables) or Fisher’s Exact test (for categorical variables). We used repeated measures ANOVA to compare mean outcomes and change in outcomes in patients with or without synovial inflammation, or with or without hyperplasia at each time point. We then used a linear mixed effects model21 to study whether synovial inflammation or hyperplasia was associated independently with Lysholm scores over time, controlling for age (centered at 45), BMI (centered at 27), gender and cartilage integrity measured by the Outerbridge score. We adjusted for cartilage integrity assessed intra-operatively, since the presence of pre-existing OA change is a known risk factor for poor outcomes after partial meniscectomy22,23 and the majority of these patients had pre-radiographic cartilage disease despite normal radiographs. We tested whether mean Lysholm scores differed in patients with or without inflammation at each time point using contrast statements for the model. Associations between synovial chemokine transcript levels and outcome scores were tested using Spearman’s correlation.
RESULTS
Synovial histopathologic changes at the three different biopsy sites
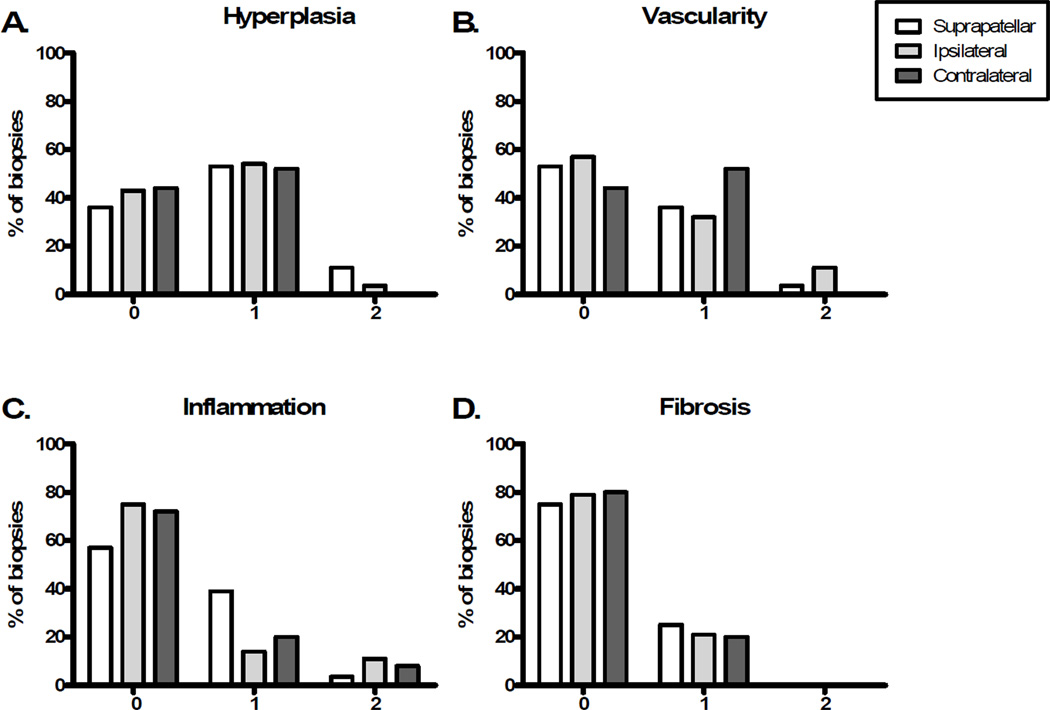
Histological features were assessed in synovial biopsies from the suprapatellar region and the medial and lateral gutters (described as ipsilateral or contralateral to the meniscal tear). Detritic cartilage and bone fragments were not identified in any patient. The distributions of other histopathologic features are depicted in Figure 1. Synovial lining hyperplasia was most common, observed in 64% of suprapatellar, 58% of ipsilateral, and 52% of contralateral biopsies. Increased vascularity was most often identified in the contralateral gutter (in 52% of patients). Fibrosis was the least common feature observed, occurring in 25% of suprapatellar biopsies, 21% of ipsilateral and 20% of contralateral biopsies. As previously reported, inflammation was identified in 43% of patients, most commonly in the suprapatellar location. 6 Scores are presented in Figure 1 in comparison to other features of synovitis.
Figure 1. Distribution of synovial histology scores in the three biopsy sites.
(A) Lining hyperplasia, (B) Vascularity, (C) Inflammation , and (D) Fibrosis were graded on H&E stained sections as described in Materials and Methods. Twenty-eight patients provided synovial biopsies from all three sites. The medial and lateral gutter biopsies were described as ipsilateral or contralateral to the meniscal tear in each individual. 0–2 on the x-axes represents the histologic grade (Table 1).
Reliability of the grading scales
The inflammation score was previously reported to have acceptable inter- and intra-rater reliability (weighted kappas of 0.87 and 1.0 respectively). 6 Reliability of scores for other synovitis features was variable. Weighted kappas (inter- and intra-rater, respectively) for hyperplasia were 0.31 and 0.65, for vascularity 0.30 and 0.50, and for fibrosis 0.23 and 0.25. Reliability was most acceptable for inflammation and hyperplasia, and there was some correlation between these features (Spearman rho = 0.30, p = 0.01). Therefore, relationships between outcomes and these two variables were evaluated.
Characteristics of patients with or without synovial inflammation or lining hyperplasia
Patient characteristics stratified by presence of synovial inflammation and hyperplasia are presented in Table 2. Patients with synovial inflammation were significantly older, and had a shorter duration between injury and surgery than those without6 Characteristics (age, gender, BMI, Outerbridge scores, proportions of medial and lateral tears and side of injury) were similar in patients with and without hyperplasia.
Table 2.
Pt characteristics
| − Inflammation (n=16) |
+ Inflammation (n=12) |
P# | − Hyperplasia (n=10) |
+ Hyperplasia (n=18) |
P# | |
|---|---|---|---|---|---|---|
| Age* | 42.5 (30.2–51.8) |
54.0 (45.0–57.5) |
41.5 (28.2–55.2) |
47.0 ( 41.8–51.8) |
0.39 | |
|
Gender+ (Male, Female) |
10, 6 | 9, 3 | 0.69 | 7, 3 | 12, 6 | 1.00 |
| BMI* | 26.2 (21.9 – 28.9) |
26.6 (25.4–27.8) |
0.56 | 27.0 (21.0–30.3) |
26.4 (25.0–27.2) |
0.83 |
|
Outerbridge score* |
1 (0–2) | 2 (1–3) |
0.06 | 1 (0–2) |
2 (1–3) |
0.25 |
|
Injury to surgery (weeks)* |
16.5 (8.0–27.5) |
7 (3.2–18.0) |
0.04 | 8.5 (4.8–24.5) |
15.0 (6.8–24.5) |
0.58 |
|
Meniscal tear+ (Medial, Lateral) |
13, 3 | 10, 2 | 1.00 | 9,1 | 14, 4 | 1.00 |
|
Side of Injury+ (Left, Right) |
9, 7 | 6, 6 | 1.00 | 5, 5 | 10, 8 | 1.00 |
Medians and Interquartile ranges are presented for these age, BMI, and weeks between injury and surgery.
Absolute numbers are reported for gender, meniscal tear, and side of injury.
Age, BMI, Outerbridge scores, and weeks between injury and surgery were compared between groups using the Mann-Whitney test. Contingency tables were constructed for categorical variables (gender, side of injury, meniscal tear), and differences between groups compared using Fisher’s Exact test.
Post-operative outcome measures
As expected, Lysholm scores at each post-operative time-point improved from baseline levels (mean +/− SD improvement of 27+/−16 points at two years), and reductions in pain scale were observed (pre-operative mean +/− SD 3.7+/−2.3, 2 year mean 0.55 +/−0.95) in the group as a whole. SF-12® PCS sub-scores improved post-operatively (pre-operative mean 42.2+/−11.0, 2 year mean 57.0 +/−2.8), but the MCS sub-scores remained stable over time (55.6+/−9.2 pre-operatively, 54.2+/−6.9 at 2 years).
Pre- and post-operative outcome scores in patients with or without synovial inflammation and hyperplasia
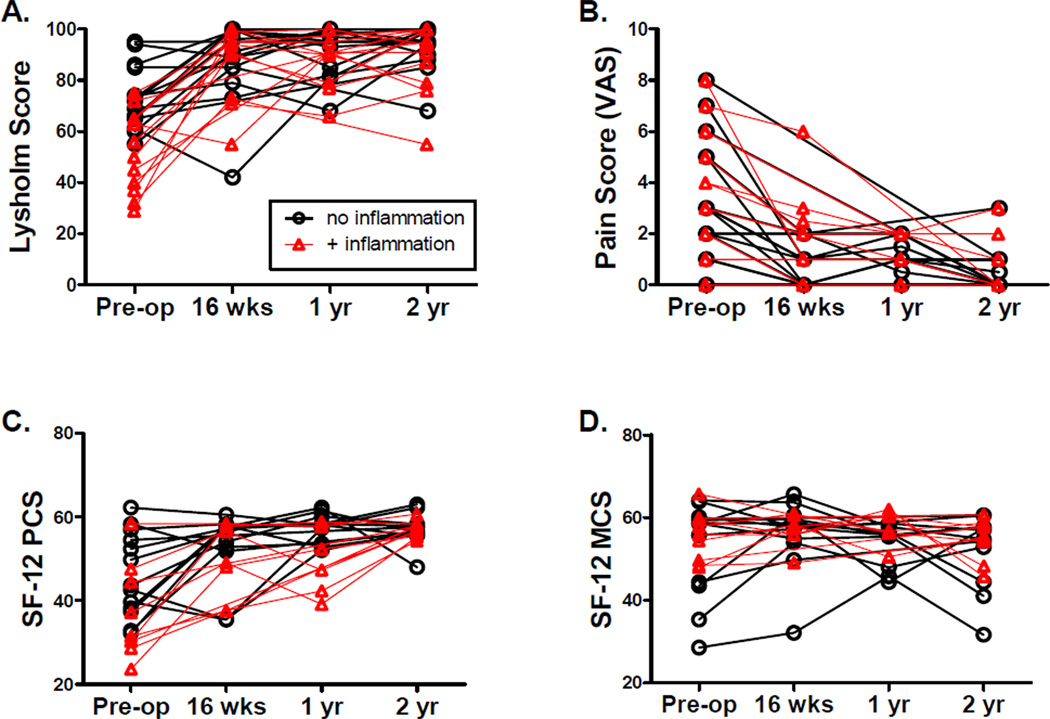
Outcome scores in individual patients at each time point are depicted graphically in Figure 2. In our previous cross-sectional analysis, we reported that patients with synovial inflammation in this cohort demonstrated lower Lysholm scores pre-operatively than those without, but no significant differences in pre-operative raw SF-12 scores or the knee pain scale were observed.6 For the current analysis, standardized norm-based SF-12® PCS and MCS scores were calculated. The norm-based SF-12® PCS scores were lower in patients with synovial inflammation than in those without inflammation pre-operatively (36.81 [28.26,45.37] vs. 46.23 [40.14,52.32], p<0.05, Table 3 and Figure 2C), consistent with the knee-specific Lysholm scale. But in longitudinal follow-up, mean Lysholm and SF-12® PCS scores did not differ significantly at any time point after surgery (Table 3, repeated measures ANOVA). No significant differences in mean SF-12® PCS or Lysholm scores were observed pre- or post-operatively comparing patients with or without hyperplasia (Table 3).
Figure 2. Pre- and post-operative symptom scores in patients undergoing meniscetomy over two years, stratified by presence or absence of synovial inflammation.
Questionnaires and surveys were administered to patients pre-operatively (pre-op), as well as at 16 weeks (wks), 1 year (yr) and 2 years post-operatively as described. Improvements in all measures except the SF-12® MCS subscores were observed after surgery. Patients with inflammation are indicated by red triangles, patients without are indicated by black circles. (A) Lysholm scores, (B) Pain scale, (C) SF-12® PCS subscores, (D) SF-12® MCS subscore.
Table 3.
Mean (95% CI*) Lysholm and SF-12®PCS scores over time in patients with and without inflammation or hyperplasia:
| − Inflammation (n=16 total) |
+ Inflammation (n=12 total) |
P# | − Hyperplasia (n=10 total) |
+ Hyperplasia (n=18 total) |
P# | ||
|---|---|---|---|---|---|---|---|
| Lysholm |
Pre op |
72.438 (66.03–78.72) (n=16) |
52.42 (42.37–62.47) (n=12) |
<0.001 | 69.6 (62.71–76.49) (n=10) |
60.6 (51.07–70.15) (n=18) |
>0.05 |
|
16 weeks |
87.71 (78.64–96.79) (n=14) |
86.30 (75.67–96.93) (n=10) |
>0.05 | 85 (65.45–104.5) (n=7) |
88 (81.59–94.41) (n=17) |
>0.05 | |
| 1 year | 91.33 (84.86–97.80) (n=12) |
86.56 (78.47–94.65) (n=9) |
>0.05 | 88.2 (78.14–98.26) (n=5) |
89.6 (83.67–95.58) (n=16) |
>0.05 | |
| 2 year | 93.27 (88.65–97.89) (n=15) |
87.75 (78.91–96.59) (n=12) |
>0.05 | 96.2 (92.24–100.4) (n=10) |
87.6 (81.13–94.16) (n=17) |
>0.05 | |
|
SF-12® (PCS) |
Pre op |
46.23 (40.14–52.32) (n=13) |
36.81 (28.26–45.37) (n=9) |
<0.05 | 48.00 (39.04–56.95) (n=7) |
39.75 (33.48–46.02) (n=15) |
>0.05 |
|
16 weeks |
52.98 (47.57–58.39) (n=12) |
49.56 (40.94–57.52) (n=7) |
>0.05 | 52.00 (40.09–63.90) (n=5) |
51.45 (46.46–56.45) (n=14) |
>0.05 | |
| 1 year | 57.40 (54.86–59.94) (n=10) |
49.55 (41.09–58.02) (n=6) |
>0.05 | 57.31 (51.65–62.97) (n=4) |
53.51 (48.86–58.16) (n=12) |
>0.05 | |
| 2 year | 57.04 (54.68–59.40) (n=12) |
56.94 (55.45–58.43) (n=9) |
>0.05 | 57.69 (55.22–60.17) (n=6) |
56.72 (54.90–58.53) (n=15) |
>0.05 | |
CI=Confidence interval).
Repeated-measures ANOVA. Due to missing data, P values were estimated (not exact) using unweighted means analysis.
SF-12® MCS scores and pain scores did not differ significantly at any time point between patients with or without inflammation (Figure 2B and 2D) or hyperplasia (data not shown).
Change in symptom scores over time
In unadjusted analysis, Lysholm scores in patients with inflammation improved more between baseline and 16 weeks (Table 4). Mean improvement in patients with inflammation was 35.10 [20.23,49.97] points compared with 16.43 [6.56–26.30] points in patients without inflammation ( p<0.05, difference = 18.67 [2.19, 35.15] repeated measures ANOVA). In patients without inflammation, mean scores continued to improve from baseline at 1 and 2 years, but stabilized in those with inflammation after 16 weeks so that differences between these two groups at 1 and 2 years were no longer statistically significant (Table 4). A similar pattern was observed when comparing patients with and without hyperplasia (greater improvement between baseline and 16 weeks in patients with hyperplasia), although these differences were not statistically significant (Table 4).
Table 4.
Mean change (95%CI*) from pre-operative Lysholm and SF-12® PCS scores at three post-operative time points in patients with and without inflammation or hyperplasia:
| − Inflammation (n=16 total) |
+ Inflammation (n=12 total) |
P# | − Hyperplasia (n=10 total) |
+ Hyperplasia (n=18 total) |
P# | ||
|---|---|---|---|---|---|---|---|
| Lysholm | Δ 16 weeks |
16.43 (6.56–26.30) (n=14) |
35.10 (20.23–49.97) (n=10) |
<0.05 | 18.71 (0.10–37.33) (n=7) |
26.47 (15.72–37.22) (n=17) |
>0.05 |
| Δ 1 years |
20.75 (11.43–30.07) (n=12) |
35.44 (25.48–45.41) (n=9) |
>0.05 | 21.60 (14.02–29.18) (n=5) |
28.75 (19.54–37.96) (n=16) |
>0.05 | |
| Δ 2 years |
20.93 (11.93–29.93) (n=15) |
35.33 (26.27–44.39) (n=12) |
>0.05 | 26.60 (18.52–34.68) (n=10) |
27.76 (17.68–37.95) (n=17) |
>0.05 | |
|
SF-12® (PCS) |
Δ 16 weeks |
7.44 (0.08–14.81) (n=12) |
12.54 (3.45–21.64) (n=7) |
>0.05 | 4.58 (−11.34–20.50) (n=5) |
11.02 (5.10–16.93) (n=14) |
>0.05 |
| Δ 1 years |
14.82 (9.36–20.28) (n=10) |
11.99 (−2.55–26.52) (n=6) |
>0.05 | 12.61 (−1.31–26.54) (n=4) |
14.14 (7.29–20.99) (n=12) |
>0.05 | |
| Δ 2 years |
10.27 (3.76–16.78) (n=12) |
20.12 (12.45–27.80) (n=9) |
>0.05 | 8.31 (−1.10–17.73) (n=6) |
16.96 (10.81–23.12) (n=15) |
>0.05 | |
CI=Confidence interval.
Repeated-measures ANOVA. Due to missing data, P values were estimated (not exact) using unweighted means analysis.
Improvements in SF-12® PCS sub-scores indicated by positive changes from baseline to each post-operative time point were observed in patients with and without inflammation, and patients with and without hyperplasia. Again, patients with inflammation and hyperplasia appeared to improve to a slightly greater extent (Table 4), but differences between groups in this unadjusted analysis were not statistically significant.
We next used a linear mixed effects model (Table 5) with a correlation structure accounting for baseline Lysholm scores to test whether the relationship between synovial inflammation and change in symptoms over time was independent of age, BMI, gender, or cartilage Outerbridge score. For this adjusted analysis, we focused on the knee-specific metric (Lysholm). This approach confirmed the independent association of inflammation with lower Lysholm scores at baseline (estimate = −20.8 [−31.66, −9.90], p=0.001, Table 5). Consistent with the unadjusted analysis, contrast statements for the model demonstrated that patients with pre-operative inflammation achieved mean Lysolm scores similar to those without inflammation at all postoperative time points (two-year difference in mean scores = −7.78[−18.73,3.17], p=0.17, Table 5). As a result, their 2-year post-operative improvement in Lysholm scores were 13.01 [1.48,24.53] points higher than patients without inflammation (p = 0.03, Table 5). The same approach revealed no independent association of hyperplasia with baseline Lysholm scores (estimate = −4.90±5.6, p=0.40), and no differences in mean score or change from baseline at any post-operative time point comparing patients with and without hyperplasia (data not shown).
Table 5.
Mixed effects model: Association of Inflammation with Lysholm Scores
| Timepoint | Estimate | Std. Error | 95% CI | P-value | |
|---|---|---|---|---|---|
| aIntercept | 0 (pre-op) | 64.85 | 4.89 | 55.26, 74.43 | <0.0001 |
| bSynovial inflammation | 0 | −20.78 | 5.55 | −31.66, −9.90 | 0.001 |
| bGender (male) | 0 | 8.52 | 4.15 | 0.39, 16.66 | 0.05 |
| bBMI | 0 | −0.579 | 0.36 | −1.28, 0.12 | 0.12 |
| bOuterbridge score | 0 | 0.605 | 1.44 | −2.21, 3.42 | 0.68 |
| bAge | 0 | 0.228 | 0.20 | −0.16, 0.62 | 0.28 |
|
cChange in Lysholm (no infl.) |
0 to16 weeks | 16.44 | 3.97 | 8.66, 24.22 | 0.0001 |
| 0 to1 year | 20.01 | 4.18 | 11.82, 28.20 | <0.0001 | |
| 0 to 2 years | 22.32 | 3.97 | 14.54, 30.10 | <0.0001 | |
|
drDifference, change in Lysholm (infl. vs. no infl.) |
0 to16 weeks | f+18.44 | 6.08 | 6.53, 30.36 | 0.004 |
| 0 to 1 year | +14.44 | 6.33 | 2.04, 26.85 | 0.03 | |
| 0 to 2 years | +13.01 | 5.88 | 1.48, 24.53 | 0.03 | |
|
eDifference, mean Lysholm (infl. vs. no infl.) |
16 weeks | f−2.34 | 5.73 | −13.57, 8.89 | 0.69 |
| 1 year | −6.34 | 5.97 | −18.04, 5.36 | 0.29 | |
| 2 years | −7.78 | 5.59 | −18.73, 3.17 | 0.17 | |
Intercept of the model, which can be interpreted as the adjusted mean Lysholm score in patients without inflammation pre-operatively (time 0).
Variables included, and association with baseline (pre-operative) Lysholm scores.
Mean change in Lysholm scores in patients without inflammation, adjusted for gender, BMI, outerbridge score and age, over three time periods indicated.
Difference in Lysholm change from baseline between patients with inflammation and those without over three time periods, adjusting for variables as above.
Difference between adjusted mean Lysholm scores in patients with or without inflammation, tested by contrast statements for the model.
+ indicates higher, and − indicates lower, in patients with inflammation compared to those without.
We next stratified two-year Lysholm scores using previously described thresholds.24 Outcomes after surgery using this scale have been interpreted as excellent between 95 and 100, good between 84–94, fair between 65–83, and poor below 65. All patients with synovial inflammation had scores in the fair to poor range at baseline compared to 75% of patients without inflammation (Table 6 and Figure 2). Two years post-surgery, four patients (33%) with inflammation scored in the fair to poor range, compared to only 1 (7%) of patients without inflammation (p=0.14, Fisher’s Exact test).
Table 6.
Pre-operative and 2-year post-operative Lysholm score in patients with and without inflammation:
| Pre-operative | 2 years post-operative | |||
|---|---|---|---|---|
| Lysholm scores | − Inflammation (n=16) |
+ Inflammation (n=12) |
− Inflammation (n=15*) |
+ Inflammation (n=12) |
|
Good / Excellent (≥84) |
4 (25) # | 0 (0) # | 14 (93) # | 8 (67) # |
| Fair/ Poor(≤83) | 12(75) | 12 (100) | 1 (7) | 4 (33) |
Numbers of patients (percent of patients).
One patient in this group did not complete 2 year follow-up.
CCL-19 and CCR7 mRNA relative expression levels were also associated with Lysholm scores
We previously reported that CCL19 and CCR7 mRNA expression was associated with synovial inflammation, and higher levels correlated with worse pre-operative Lysholm scores. 6 In follow-up, synovial relative expression levels of CCL19 and CCR7 were associated with greater post-operative improvements in Lysholm score, similar to associations observed with histologic inflammation. Significant associations were observed between expression levels and change in Lysholm scores pre- to post-operatively at the 16 week (CCL19: Spearman’s ρ=0.708, p=0.050, CCR7: ρ =0.704, p=0.016) and 2 year time points (CCL19: ρ =0.850, p=0.004; CCR7: ρ =0.790, p=0.002).
DISCUSSION
This longitudinal pilot study was designed to test whether the presence of synovitis predicted worse post-operative symptoms in patients undergoing arthroscopic meniscectomy. We previously reported that synovial inflammation (defined by the presence of perivascular mononuclear cell infiltrates) is associated with pre-operative symptoms using the Lysholm scale, a commonly used measure of patient-reported knee symptoms6. In the current analysis, similar associations between inflammation and pre-operative symptoms were observed using a more general measure of physical health, the standardized PCS sub-score of the SF-12® survey, strengthening our initial findings. In unadjusted longitudinal analyses, both patients with or without synovial inflammation demonstrated improvements in knee symptoms measured by the Lysholm score, and achieved similar mean scores post-operatively up to two years (Table 3). As they had started with lower scores, post-operative improvement in Lysholm scores was greater at 16 weeks in patients with inflammation compared to the patients without inflammation (Table 4). Adjustment for other factors confirmed similar mean scores and greater improvement in patients with inflammation compared to those without up to two years post-operatively. These findings suggest that the presence of inflammation did not preclude improvement in the first two years after surgical intervention.
Multiple studies of outcomes from meniscal arthroscopy have documented the long-term risk of developing structural and symptomatic OA, and estimates suggest up to 50% of patients have radiographic knee OA 10–20 years after undergoing meniscectomy25. Short-term (3 month) prospective analysis of symptomatic outcomes has also been reported 23. To our knowledge, this is the first longitudinal analysis to specifically study the relationship between synovial histology and symptomatic outcomes after meniscal arthroscopy. As most patients did well within the two-years of follow-up, it is likely that the timeframe of this pilot study is inadequate to capture sufficient numbers of patients with progressive symptoms. Our finding that a greater proportion of patients with inflammation (33% vs. 7% without inflammation) had scores in the fair to poor range (< 84) at the two-year post-operative time point suggests that outcomes may change in the longer follow-up period. Although not statistically significant in this small study, future work on validation of these findings in larger populations and longer time periods is necessary.
Most histopathologic synovitis scoring systems that have been applied to OA and knee injury combine various features of synovitis (i.e. lining hyperplasia, inflammatory cell infiltration, vascularity, fibrosis) into a single summated score.16–17 Summated scores have shown to be useful in distinguishing different forms of arthritis and in monitoring disease progression.26– 28 We chose to evaluate synovitis features independently for two main reasons; first, some synovial changes have been reported to vary according to the stage of OA16 and have not yet been evaluated in patients presenting for meniscal arthroscopy; and secondly, individual synovial changes may not reflect the same cellular and molecular processes, or have the same relationship with symptoms. This analysis revealed a number of interesting, and previously unreported findings.
First, comparison of five features at three different anatomic sites demonstrated that no feature of synovitis was preferentially found on the side of the meniscal tear, suggesting that the synovial reaction in these patients can be a joint-wide phenomenon (Figure 1). Fibrosis was the least common abnormality identified in the meniscectomy patients. Detritic fragments of bone and cartilage, often identified in advanced OA patients16 were not found in any patient in this study. Our previously reported inflammation score was the most reliable, likely due to easy recognition of perivascular aggregates on H&E staining. The hyperplasia scale was the second most reliable indicator, although both inter- and intra-rater reliability were lower than the inflammation score. The lower reliability of the hyperplasia, vascularity and fibrosis scores may relate to the limitations with which these features can be recognized on H&E stained sections, and the more subjective nature of their grading scales (i.e. mild, moderate or marked). And at least with regards to fibrosis, the low kappas may reflect high chance agreement in the setting of the low prevalence of this particular feature.
In unadjusted analyses, inflammation but not hyperplasia was associated with worse pre-operative SF-12® PCS scores, consistent with previous findings using the Lysholm scale. This suggests that only specific features of synovitis (i.e. the mononuclear cell infiltrates) may be related to symptoms in these patients. Alternatively, the lower reliability of the hyperplasia score may be partly responsible for the lack of association with outcomes. However, identifying the aspects of synovial histopathology that are most easily and reliably measured will simplify and focus efforts to develop predictive tools and markers.
We had previously demonstrated that synovial inflammation was associated with increased mRNA expression of a set of chemokines involved in leukocyte recruitment, and levels of the chemokine /receptor pair CCL19 and CCR7 were associated with worse symptoms at baseline. 6 Here we demonstrate that expression levels of CCL19 and CCR7 also correlated with greater symptomatic improvements after surgery, similar to the associations seen with histologic inflammation (Table 3). In this analysis, we utilized GAPDH as a reference gene, which has been demonstrated by others to be regulated in other settings involving inflammation 29. We did not have sufficient quantities of RNA to analyze additional reference genes, however we observed no significant difference between mean GAPDH Ct values comparing synovial biopsies from patients with or without inflammation (data not shown). Numbers of specimens available for chemokine analysis were also not sufficient to pursue adjusted analyses, but the similar observations made using both cellular and molecular measures of inflammation strengthen our results, and suggest these mediators may be surrogate markers of synovial inflammation in these patients.
Limitations of this pilot study include the small numbers and limited duration of follow-up. In addition, the Lysholm score evaluates both patient–reported symptoms and functional disability on a single scale, and minimal clinically important differences are not known. However, our findings are strengthened by the similar relationships found using a second, more general instrument, the SF-12® PCS summary. We did not find evidence of a relationship between inflammation and a simple pain scale in this study. This may be due to the small numbers of patients in this study, but may also reflect a more complex symptom set in this population of patients that was captured by the other outcome measures utilized. Finally, the lack of data on OA radiographic progression makes extending our results to the risk of structural OA progression impossible in this cohort. Of note, the majority (80%) of patients in this cohort already had evidence of pre-radiographic disease based on intra-operative inspection, and progressive joint symptoms rather than structural changes prompt patients to seek care. Further studies are needed to determine if there is a relationship between inflammation, subclinical cartilage pathology, and development of structural disease progression.
In summary, in patients undergoing partial meniscectomy, inflammation was associated with worse pre-operative symptoms measured by two outcome scores, the knee-specific Lysholm scale and the more general SF-12® PCS subscore. In the first two-years following surgery, patients with synovial inflammation achieved similar mean scores post-operatively compared to those without inflammation. By two years post-surgery, 33% (4 of 12) of patients with synovial inflammation had Lysholm scores in the fair to poor range, compared with 7 % (1 of 15) of those without inflammation. Results suggest good two year outcomes post-meniscectomy, but cannot rule-out a relationship between synovial inflammation and progressive knee symptoms and should be confirmed with longer follow-up and larger cohorts.
Acknowledgements
The authors would like to acknowledge the following individuals: Fae Williams for clinical coordination and collection of outcome data, Paul Weitzel for assistance with recruitment of patients, Mark Kosinski for scoring of the SF-12® surveys, and Jeremy Z. Fields for editing of the final draft for clarity.
Funding:
Funding for this study was obtained from The New England Baptist Hospital Bone and Joint Institute, Boston, MA. Additionally, Carla R. Scanzello was supported by the National Institute of Arthritis, Musculoskeletal and Skin Diseases (K08AR057859), and a career development award jointly funded by The Atlantic Philanthropies, American College of Rheumatology Research and Education Fund, John A. Hartford Foundation, and the Association of Specialty Professors. Steven R. Goldring was supported by the American College of Rheumatology Research and Education Fund, Within Our Reach. These funding agencies and sponsors played no role in the study design, data collection, analysis interpretation of data, writing of the report, or decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions:
Conception and Design: JNK, SRG, JCR, BM. Acquisition of Data: CRS, ASA, ED, VK, EUA, BHS. Analysis and Interpretation of data: CRS, ASA, ED, KBR, JNK, SRG, BM. Drafting the article: CRS. Revising the article for important intellectual content: ASA, ED, KBR, VK, EUA, BHS, JNK, SRG, JCR, BM. Final approval of this version: CRS, ASA, ED, KBR, VK, EUA, BHS, JNK, SRG, JCR, BM.
Conflict of Interest Statement:
Rush University and the Hospital for Special Surgery have filed an international patent application for biomarkers in osteoarthritis; Dr. Scanzello and Goldring are named inventors on the application.
CRS has received consultant fees from Cinkate, Corp.
BM owns stock in Parcus, Inc. and Conformis, Inc.
SRG has received consultant fees and honoraria from Fidia, Bone Therapeutics, Pfizer and Abbott.
ASA, ED, KBR, VK, EUA, BHS, JNK, JCR: No competing interests identified.
REFERENCES
- 1.Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012;64:1697–1707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yusuf E, Bijsterbosch J, Slagboom PE, Kroon HM, Rosendaal FR, Huizinga TW, et al. Association between several clinical and radiological determinants with long-term clinical progression and good prognosis of lower limb osteoarthritis. PLoS ONE. 2011;6:e25426–e25426. doi: 10.1371/journal.pone.0025426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scanzello CR, Goldring SR. The role of synovitis in osteoarthritis pathogenesis. Bone. 2012;51(2):249–257. doi: 10.1016/j.bone.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sowers M, Karvonen-Gutierrez CA, Jacobson JA, Jiang Y, Yosef M. Associations of anatomical measures from MRI with radiographically defined knee osteoarthritis score, pain, and physical functioning. J Bone Joint Surg Am. 2011;93:241–251. doi: 10.2106/JBJS.I.00667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scanzello CR, McKeon B, Swaim BH, DiCarlo E, Asomugha EU, Kanda V, et al. Synovial inflammation in patients undergoing arthroscopic meniscectomy: molecular characterization and relationship to symptoms. Arthritis Rheum. 2011;63:391–400. doi: 10.1002/art.30137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Englund M, Guermazi A, Roemer FW, Aliabadi P, Yang M, Lewis CE, et al. Meniscal tear in knees without surgery and the development of radiographic osteoarthritis among middle-aged and elderly persons: The Multicenter Osteoarthritis Study. Arthritis Rheum. 2009;60:831–839. doi: 10.1002/art.24383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noble J. Lesions of the menisci. Autopsy incidence in adults less than fifty-five years old. J Bone Joint Surg Am. 1977;59:480–3. [PubMed] [Google Scholar]
- 9.Cullen KA, Hall MJ, Golosinskiy A. Hyattsville, MD: U.S. Dept. of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistic; 2009. National Center for Health Statistics (U.S.). Ambulatory surgery in the United States, 2006. [PubMed] [Google Scholar]
- 10.Bhattacharyya T, Gale D, Dewire P, et al. The clinical importance of meniscal tears demonstrated by magnetic resonance imaging in osteoarthritis of the knee. J Bone Joint Surg Am. 2003;85-A(1):4–9. doi: 10.2106/00004623-200301000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Englund M, Niu J, Guermazi A, et al. Effect of meniscal damage on the development of frequent knee pain, aching, or stiffness. Arthritis Rheum. 2007;56(12):4048–4054. doi: 10.1002/art.23071. [DOI] [PubMed] [Google Scholar]
- 12.Englund M, Guermazi A, Gale D, Hunter DJ, Aliabadi P, Clancy M, et al. Incidental meniscal findings on knee MRI in middle-aged and elderly persons. N Engl J Med. 2008;359:1108–1115. doi: 10.1056/NEJMoa0800777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med. 1982;10:150–154. doi: 10.1177/036354658201000306. [DOI] [PubMed] [Google Scholar]
- 14.Ware J, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Outerbridge RE. Further Studies on the Etiology of Chondromalacia Patellae. J Bone Joint Surg Br. 1964;46:179–190. [PubMed] [Google Scholar]
- 16.Oehler S, Neureiter D, Meyer-Scholten C, Aigner T. Subtyping of osteoarthritic synoviopathy. Clin Exp Rheumatol. 2002;20:633–640. [PubMed] [Google Scholar]
- 17.Krenn V, Morawietz L, Häupl T, Neidel J, Petersen I, Konig A. Grading of chronic synovitis--a histopathological grading system for molecular and diagnostic pathology. Pathol Res Pract. 2002;198:317–325. doi: 10.1078/0344-0338-5710261. [DOI] [PubMed] [Google Scholar]
- 18.Loeuille D, Chary-Valckenaere I, Champigneulle J, Rat AC, Toussaint F, Pinzano-Watrin A, et al. Macroscopic and microscopic features of synovial membrane inflammation in the osteoarthritic knee: correlating magnetic resonance imaging findings with disease severity. Arthritis Rheum. 2005;52:3492–3501. doi: 10.1002/art.21373. [DOI] [PubMed] [Google Scholar]
- 19.Haywood L, McWilliams DF, Pearson CI, Gill SE, Ganesan A, Wilson D, et al. Inflammation and angiogenesis in osteoarthritis. Arthritis Rheum. 2003;48:2173–2177. doi: 10.1002/art.11094. [DOI] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 22.Fabricant PD, Jokl P. Surgical outcomes after arthroscopic partial meniscectomy. J Am Acad Orthop Surg. 2007;15:647–653. doi: 10.5435/00124635-200711000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Roos EM, Roos HP, Ryd L, Lohmander LS. Substantial disability 3 months after arthroscopic partial meniscectomy: a prospective study of patient-relevant outcomes. J Arth Rel Surg. 2000;16:619–626. doi: 10.1053/jars.2000.4818. [DOI] [PubMed] [Google Scholar]
- 24.Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res. 1985;(198):43–49. [PubMed] [Google Scholar]
- 25.Lohmander LS, Englund M, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries. Am J Sports Med. 2007;35:1756–1769. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- 26.Krenn V, Morawietz L, Burmester GR, Kinne RW, Mueller-Ladner U, Muller B, et al. Synovitis score: discrimination between chronic low-grade and high-grade synovitis. Histopathology. 2006;49:358–364. doi: 10.1111/j.1365-2559.2006.02508.x. [DOI] [PubMed] [Google Scholar]
- 27.Pessler F, Dai L, Diaz Torne C, Gomez-Vaquero C, Paessler ME, Zheng DH, et al. The synovitis of "non-inflammatory" orthopaedic arthropathies: a quantitative histological and immunohistochemical analysis. Ann Rheum Dis. 2008;67:1184–1187. doi: 10.1136/ard.2008.087775. [DOI] [PubMed] [Google Scholar]
- 28.Slansky E, Li J, Häupl T, Morawietz L, Krenn V, Pessler F. Quantitative determination of the diagnostic accuracy of the synovitis score and its components. Histopathology. 2010;57:436–443. doi: 10.1111/j.1365-2559.2010.03641.x. [DOI] [PubMed] [Google Scholar]
- 29.Della Beffa C, Klawonn F, Menetski JP, et al. Evaluation of glyceraldehyde-3-phosphate, prolylpeptidyl isomerase A, and a set of stably expressed genes as reference mRNAs in urate crystal inflammation. BMC Res Notes. 2011;4:443. doi: 10.1186/1756-0500-4-443. [DOI] [PMC free article] [PubMed] [Google Scholar]