Abstract
Photodynamic therapy relies on the interaction between light, oxygen and a photosensitizing agent. Its medical significance relates to the ability of certain agents, usually based on porphyrin or phthalocyanine structures, to localize somewhat selectively in neoplastic cells and their vasculature. Subsequent irradiation, preferably at a sufficiently high wavelength to have a significant pathway through tissues, results in a photophysical reaction whereby the excited state of the photosensitizing agent transfers energy to molecular oxygen and results in the formation of reactive oxygen species. Analogous reactive nitrogen species are also formed. These contain both nitrogen and oxygen atoms. The net result is both direct tumor cell death and a shutdown of the tumor vasculature. Other processes may also occur that promote the anti-tumor response but these are outside the scope of this review.
Keywords: apoptosis, autophagy, photodynamic therapy, reactive oxygen species, reactive nitrogen species
1. Introduction
In the context of photodynamic therapy (PDT), singlet oxygen (1O2) was identified as a major phototoxic element in the anti-tumor effects of hematoporphyrin derivative (HPD) in 1976.[1] HPD was the first of the major clinically useful photosensitizers. Since oxygen is required for 1O2 formation, it was not surprising that hypoxic regions of tumors were insensitive to PDT.[2] As one of the effects of PDT is vascular shutdown, adjustments in irradiation protocols that lead to only periodic interruptions of blood flow can promote efficacy.[3] It is generally considered that 1O2 is the predominant factor in photokilling, although this may not be true for all photosensitizing agents. Foote proposed a distinction between ‘type I’ and ‘type II’ photochemistry.[4] In the former, a reaction between the activated state of the photosensitizer and substrate or solvent yields radicals or radical ions. In the latter, reaction occurs at oxygen to form either 1O2 or other reactive oxygen species (ROS).
Another view was expressed by Vidòczy, who proposed that Foote's definitions might be applicable only to PDT.[5] Rodgers provided yet another view, suggesting that the distinction was actually between electron transfer and energy transfer reactions.[6] In the case of the porphyrin/phthalocyanine structures commonly used in PDT, O2 is the only biological molecule with a 1Δg state that can behave as an energy acceptor. PDT effects are further complicated by ROS downstream from 1O2, as summarized by Girotti.[7] These include the superoxide anion radical (•O2–), the OH radical (•OH), hydrogen peroxide (H2O2) and lipid peroxides. In his review, Girotti considers •O2– to be a Type I product. Crosstalk among the species adds further complication, e.g., ascorbate can interact with 1O2 to produce H2O2.[8] Moreover, reactive nitrogen species (RNS) can be formed as a byproduct of interactions between nitric oxide and ROS.[9]
2. ROS and RNS Detection
Identification of the different ROS associated with photodynamic action is not a simple matter. An unambiguous method is electron spin resonance (ESR) spectroscopy, but this technique does not readily lend itself to studies in biological cultures, and the necessary equipment is not found in most laboratories. One common approach involves the use of fluorescent probes that are supposed to light up when confronted with specific reactive species. Considerable specificity is often claimed for these probes, but they are generally not nearly as selective as advertised.
Among the more common probes for ROS is reduced dichlorofluorescein (H2DCF). Oxidation to DCF yields a green fluorescence. This agent is often provided as the diacetate to promote cellular uptake, with the acetates later cleaved by the action of intracellular esterases. The specificity of H2DCF is quite broad, with a spectrum that includes H2O2, •OH, •O2–, ONOO–, OCl– and 1O2. Self-oxidation is also observed in the presence of light.[10] Our experience with this and other probes is described later in this review.
Dihydrorhodamine 123 (DHR) is often used as a probe for H2O2,[11] but is also sensitive to •O2–[12] and the peroxynitrite anion (ONOO–).[13] Dihydroethidium (DHE) is often claimed to be a selective fluorescent probe for •O2– formation,[14] but the fluorescence emission spectrum must be monitored to distinguish between the probe oxidation product produced by superoxide and that produced by the OH radical. HPLC analysis is sometimes needed to confirm the identity of the ROS.[15] Tang's group described a naphthofluorescein derivative that fluoresces in the presence of •O2–.[16] Use of this probe might be compromised by the fact that naphthofluoresceins are nonpolar and difficult to work with in aqueous environments. Moreover, fluorescence emission from naphthofluorescein is highly pH dependent with a pKa of ~7.5. This will complicate fluorescence measurements, especially if the probe accumulates in subcellular regions of low pH. The sensitivity of Tang's test for •OH also appears suboptimal since it relies on production of •OH via the Fenton reaction which is quite inefficient. Maeda's approach to superoxide detection resulted in a substantially greater response to •O2– than to •OH, using a probe based on a nitrobenzenesulfonyl ester structure.[17] This reagent also becomes fluorescent in the presence of thiol-containing compounds. Although this is noted in the report, in a critical test only a 50 μM concentration of glutathione (GSH) was used, which is perhaps 1% of the expected intracellular GSH concentration.
Sites of photodamage can be assessed by fluorescent probes specific for mitochondrial membrane potential (MitoTracker Orange), lysosomal integrity (LysoTracker and LysoSensor), the endoplasmic reticulum (ER-Trackers) or membrane integrity (propidium iodide or trimethylamino-diphenylhexatriene).[18] There is also a probe said to report on lipid oxidation: C-11-BODIPY,[20] which appears to provide qualitative rather than quantitative data.
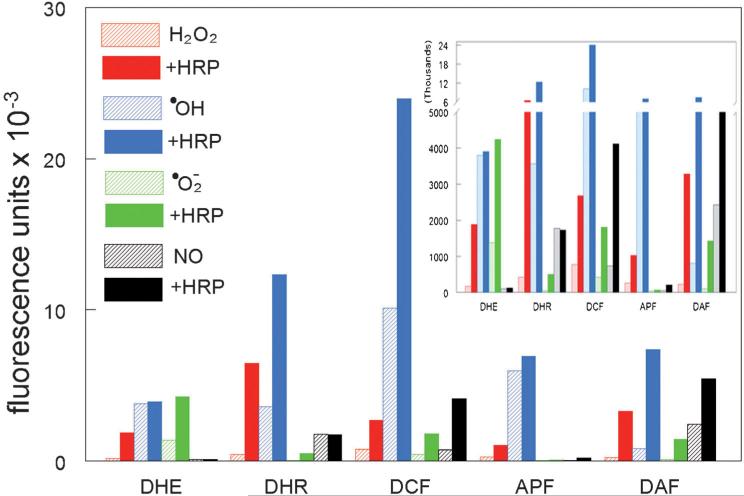
Of the probes we have examined, the most specific appears to be aminophenoxyxanthene benzoic acid (APF),[21] a product developed for detection of •OH.[10] APF can, however, also respond to 1O2.[21] A summary of studies relating to probe specificity in a cell-free system is shown in Figure 1. Procedures generally followed the experimental approach outlined by Setsukinai et al.,[10] except that Fe(NH4)SO4 was substituted for Fe(ClO4)2 in the Fenton reaction. Horseradish peroxidase (10 μgmL–1) was present where specified. It is noteworthy that most of the probes were highly responsive to •OH and that addition of horseradish peroxidase markedly promoted probe oxidation in most cases. Included in this survey was the probe 4-amino-5-methylamino-2′,7′-difluorofluorescein (DAF). Although intended as a probe for •NO,[22] DAF can also be oxidized by •OH. A probe for 1O2 has recently appeared designated as ‘Singlet Oxygen Sensor Green’ (SOSG). This is said to be cell impermeable, but recent reports have indicated that SOSG can be accumulated by cells and that it can yield 1O2 upon irradiation.[23]
Figure 1.
Fluorescence exhibited by five fluorescent probes (5 μM) upon treatment with different reactive species, as described by Setsukinai et al.[10] Horseradish peroxidase (10 μgmL–1) was present where specified. The numbers shown represent the mean fluorescence emission intensity upon excitation at 490–510 nm. In four replicate determinations, the variation was less than ±3% of these values. Inset: the same data plotted on a different scale so as to better illustrate the lower fluorescence intensities.
In addition to the report by Girotti cited above,[7] there are many reviews on the general topic of fluorescent probes.[24] In the context of PDT, some additional factors need to be considered. The photosensitizers also fluoresce, so care needs to be taken that the fluorescence of the photosensitizer is not confused with the fluorescence of the probe.[25] Studies in cell culture or cell-free systems will necessarily neglect important elements of PDT, such as the effects of NO on vascular elements during PDT.[26] A final caution is the occasional appearance of spontaneous fluorescence that can mimic the effects of probe oxidation without actually involving ROS formation. An example is the fluorescence that is observed when the proapoptotic drug HA14-1 interacts with albumin in culture media.[27] The resulting fluorescence emission spectrum is an almost exact duplicate of that of DCF, and was interpreted in one study as evidence that adding HA14-1 to growth media initiated production of ROS.[28]
3. Effects of ROS Formed during PDT
PDT is intended to kill malignant cells; this can take the form of either apoptotic or necrotic death.[29] The latter often occurs when PDT conditions are sufficient to cause massive plasma membrane and organelle membrane damage, and destroy/inactivate the enzymatic processes (e.g., caspases) involved in the apoptotic program.[30] In PDT protocols the apoptotic program is commonly initiated by photosensitizers that accumulate in, and subsequently cause photodamage to, organelles such as mitochondria, lysosomes, and the endoplasmic reticulum (ER). In particular, photosensitizing agents that target mitochondria and the ER upon illumination often photodamage organelle-associated anti-apoptotic members of the Bcl-2 family (i.e., Bcl-2, Bcl-XL),[29,31] inhibit the activity of the ER SERCA2 pump,[31,32] and activate Bax.[32a] Such effects result in the release of ER calcium stores, Bax-mediated permeabilization of mitochondria, release of pro-apoptotic mitochondrial factors such as cytochrome c and apoptosis-inducing factor, and the subsequent activation of procaspases that selectively cleave certain proteins and results in the apoptotic phenotype. Photodamage to late endosomes/lysosomes can cause their permeabilization and the release of lysosomal hydrolases.[33] Depending upon the extent of their release such hydrolases can cause either necrosis or apoptosis. With regards to the latter, apoptosis is mediated by lysosomal cathepsin-mediated cleavage of the pro-apoptotic protein Bid, and subsequent permeabilization of mitochondria by the cleavage product tBid.[33b]
Macroautophagy (hereafter referred to as autophagy) is a process whereby the cytosol and entire organelles become encapsulated in a double-membrane cytosolic vesicle termed the autophagosome. Eventual fusion of the autophagosome with the lysosome forms an autolysosome and results in the proteolytic breakdown and recycling of what was the autophagosome and its contents.[34] Autophagy is induced by different types of stress, and appears to play a major role in maintaining cellular homeostasis. While autophagy is generally considered to be a survival pathway, it can also be a death mode if the process becomes excessive. Recent studies have documented the induction of autophagy in a variety of cell types following light activation of the photosensitizers mTHPC, Pc 4, hypericin, NPe6, CPO and BPD.[31] The contribution of autophagy to the eventual fate of cells in PDT protocols is variable. In some situations it appears to support cell survival,[31,35] whereas in others it seems to contribute to cell death.[31,36]
Although type II PDT reactions produce predominantly 1O2, a variety of secondary ROS species including •O2–, H2O2, and •OH are also generated. Two important questions are whether the different ROS species have similar effects, and to what extent they contribute to the cytotoxicity observed in PDT protocols. Although an early study by Weishaupt et al. did not characterize the role of putative secondary ROS species in HPD-sensitized cells, trapping studies with 1,3-diphenylisobenzofuran clearly indicated that rapid removal of 1O2 markedly attenuated HPD cytotoxicity.[37] Similarly, studies by Kochevar et al. demonstrated that the 1O2 generated by photoactivation of rose bengal could induce the apoptosis of cultured cells, whereas radical species derived from rose bengal could not.[38] Interestingly, rose bengal radicals, but not 1O2, were very efficient at inducing lipid peroxidation. With regard to a role for •O2– in PDT, treatment with 2-methoxyestradiol has been reported to increase the efficacy of PDT in tumor-bearing mice, an effect attributed to its inhibition of superoxide dismutase (SOD).[39] Inhibition of SOD activity would indeed be expected to result in higher levels of •O2– . However, Fridovich has demonstrated that 2-methoxyestradiol does promote •O2– formation but not by a mechanism involving SOD inhibition.[40] Price et al. have reported the generation of H2O2 following the irradiation of cultures preloaded with the photosensitizer BPD.[41] In these studies the cytotoxicity of BPD-PDT was enhanced through the inhibition of catalase using 3-aminotriazole, which facilitates the accumulation of H2O2. Conversely, the cytotoxicity of BPD-PDT was significantly reduced in cells in which peroxisomal levels of catalase had been augmented.[41] The mechanism by which PDT induces autophagy is not known. However, several non-PDT studies have shown that exogenously added •O2– or H2O2 induces autophagy in cultured cells.[42] Detailed mechanistic studies by Chen have suggested that •O2– is principally responsible for the induction of autophagy,[42a] but in this report it was assumed that reduced DCF is specific for hydrogen peroxide and DHE for the superoxide radical. As was demonstrated above, this is an oversimplification of the response pattern of these probes.
A new photosensitizer termed WST11 has been developed[43] that has the unusual property of producing mainly •O2– and •OH but not 1O2 upon irradiation.[43b] It has been reported that both •O2– and H2O2 can lead to the initiation of autophagy.[42] Formation of H2O2 from •O2– can occur spontaneously and is also catalyzed by SOD. In preliminary studies, we observed that irradiation of WST11-treated murine hepatoma 1c1c7 cells elicited an autophagic response. This effect can also occur when the photosensitizing agent is BPD,[35b] which is known to produce significant levels of 1O2 upon irradiation.[44] However, while autophagy appears to serve a cytoprotective function when elicited by BPD,[35b] the same effect was not observed with WST11. These results will be reported in detail elsewhere. Although WST11 is currently being used to target the tumor vasculature,[43] the efficacy of an agent that does not produce significant levels of singlet oxygen is a novel result that may suggest new pathways for drug development.
4. Chemistry of Nitric Oxide and RNS
Nitric oxide (nitrogen monoxide, •NO) is a small, nonpolar, highly diffusible, and relatively stable free radical. It is an important signaling molecule and influences a diverse range of physiological processes including vasodilation, neurotransmission, angiogenesis, tumor metastasis, and antimicrobial and antitumor activities. It is generated by a family of enzymes termed the nitric oxide synthases (NOS). This family includes two constitutively expressed and calcium/calmodulin-dependent isoforms that were initially identified in neuronal tissue (nNOS) and endothelial cells (eNOS). A third isoform is neither calcium-dependent nor constitutively expressed, but can be induced by a variety of stressors, and is therefore termed inducible NOS (iNOS). Isoform expression and tissue activity/content vary among cell types, and can be influenced by a variety of factors.
Although reasonably stable for a radical species, nitric oxide is not inert. In particular, it reacts with •O2– at diffusion-controlled rates (~4–16 × 109 M–1s–1) to form ONOO– .[45] This rate is several-fold faster than that of the enzymatic dismutation of •O2– by SOD.[46] Hence, •NO is a far better scavenger of •O2– than SOD. Because of its reactivity, many of the cytotoxic properties of •NO are often attributed to ONOO–. However, at physiological pH a considerable percentage of ONOO– is in equilibrium with peroxynitrous acid (ONOOH; pKa = 6.8). Both species can participate in one- and two-electron oxidation reactions with susceptible biomolecules.[46,47] For example, CO2 readily reacts with ONOO– to form carbonate (CO3•–) and nitrogen dioxide (•NO2) radicals, both of which are potent, short-lived oxidants.[47] In the case of ONOOH, this species can facilitate direct two-electron oxidation of thiols to produce NO2– and sulfenic acid derivatives, which are subsequently converted to disulfides (e.g., glutathione and cysteine disulfides). Alternatively, ONOOH can undergo homolytic fission to generate •OH and •NO2 radicals, which play a role in the initiation of lipid peroxidation. In addition, •NO2 can participate in the diffusion-controlled nitration of proteins, lipids and DNA.[47]
5. Generation of Nitric Oxide in PDT Protocols
Numerous investigators have used a variety of techniques to document increased generation or accumulation of RNS following illumination of cultured cells or tissues that had been preloaded with the photosensitizers Photofrin,[48] 2-butylamino-2-demethoxyhypocrellin B,[49] aminolevulinic acid (ALA),[50] or Verteporfin.[51] The methods used for assessing RNS generation in the above studies included: 1) monitoring of the fluorescence generated following •NO oxidation of 4,5-diaminofluorescein;[49,50a,b] 2) measurement of the •NO metabolites nitrite or nitrate;[48,50c,51] 3) ESR spin-trapping of •NO;[49] and 4) immunological detection of protein-associated nitrotyrosines.[52] In many of these studies, increased production of RNS was preceded or accompanied by an increased cytosolic calcium concentration, which would favor the activation of constitutive NOS isoforms, as well as modest increases in constitutive NOS isoform content and/or dramatic increases in iNOS content.
Both in vitro and in vivo studies suggest that endogenously generated nitric oxide may play a protective role in PDT. Specifically, in vivo suppression of NOS activities with the inhibitors N-nitro-l-arginine (l-NNA) or N-nitro-l-arginine methyl ester (l-NAME) markedly increased Photofrin-PDT-mediated cures of xenografted tumor lines that constitutively expressed high levels of •NO (i.e., RIF-1 and SCCVII cells), but had only marginal effects on xenografts consisting of tumors cells having low levels of •NO (i.e., EMTS and FsaR cells).[48,53] The enhancement of tumor cures by pretreatment or cotreatment with NOS inhibitors correlated with reduced xenograft blood flow and increased vascular leakage.[48] Similarly, Reeves et al. reported that pretreatment of RIF-1 or EMTS xenograft-bearing mice with l-NNA or l-NAME potentiated ALA-PDT-induced damage to tumor and normal microvasculature, and also increased macromolecular leakage in the RIF-1 tumors.[54] Presumably, given the ability of •NO to easily diffuse across membranes, elevated levels of tumor •NO may offset the effects of PDT on tumor vasculature by maintaining sufficient vasodilation to ensure adequate blood supply to the tumor. As for the protective effects of •NO on macromolecular leakage, permeabilization of membranes often occurs as a consequence of membrane lipid peroxidation. •NO is quite facile at interacting with other radicals to generate a non–free radical adduct.[46] In effect, such radical-radical termination reactions would stop radical propagation reactions, such as those occurring during membrane lipid peroxidation. Indeed, Niziolek et al. have documented the ability of •NO to terminate lipid chain peroxidation induced by protoporphyrin IX–PDT in both model membranes and cultured cells.[55]
Although the above studies suggest that •NO may protect tumor vasculature from damage in PDT protocols, additional studies indicate that •NO may also directly protect tumor cells. For example, knockdown of iNOS expression with siRNA and inclusion of NOS inhibitors or •NO scavengers have been reported to enhance ALA-PDT-mediated killing of COH-BRI breast cancer cells.[56] Conversely, supplementation of COH-BRI cultures with nonlethal concentrations of the •NO generator spermine NONOate suppressed ALA-PDT-induced necrosis.[55] Similarly, Gomes et al. reported that incubation of human lymphoblastoid cells with •NO donors ((Z)-1-[(2-amino-ethyl)-N-(2-ammonioethyl)amino]diazen-1-ium-1,2-dio-late or S-nitroso-N-acetylpenicillamine) dramatically suppressed PDT-induced apoptosis when bisulfonated aluminum phthalocyanine was used as a photosensitizer.[57]
Although there is a body of literature that indicates •NO is protective in PDT protocols, several reports suggest the opposite. The approaches employed in these latter studies assessed the effects of either reducing or increasing cellular •NO content on PDT-induced toxicity. With regard to the former, Lu et al. demonstrated that addition of an NOS inhibitor or •NO scavenger to MCF7 cultures suppressed the cytotoxicity of the photosensitizer 2-butylamino-2-demethoxyhypocrellin B in PDT protocols.[49] As for the latter approach, Yamamoto et al. reported that overexpression of iNOS in HEK293T cells (via transfection) markedly elevated basal •NO levels and enhanced ALA-PDT-mediated cell killing.[50b] A similar potentiation of ALA-PDT-mediated killing was observed in RAW264.7 cultures in which cytokines had been used to induce iNOS prior to illumination.[50b] In this study, the inclusion of an •NO scavenger suppressed cell killing, thus confirming the contribution of •NO to the observed cytotoxicity. Although the RNS responsible for the cytotoxicity observed in the above studies were not identified, there is ample experimental precedent for assuming that it was not •NO, but rather peroxynitrite and radicals derived therefrom. Szabo et al. have published a comprehensive review on how peroxynitrite and its derivative radicals can contribute to the development of necrosis and apoptosis.[47]
6. Nitric Oxide and PDT-Induced Autophagy
We are currently unaware of any study that has investigated the relationship between PDT-induced increases in •NO and the induction of autophagy. However, the addition of exogenous •NO donors to cultured osteoblasts,[58] human mammary tumor cell lines,[59] and cardiomyocytes[60] resulted in the induction of autophagy. Although the above studies used an exogenous source of •NO, endogenously generated •NO may also contribute to the induction of autophagy. Yuan et al. reported that pharmacological inhibition of NOS suppressed LPS and tumor necrosis factor–mediated induction of autophagy in cardiomyocytes.[60] Given these results and the ability of PDT to induce iNOS, it is conceivable that •NO may contribute to the induction of autophagy in PDT protocols.
7. Summary and Outlook
Reactive oxygen and nitrogen species mediate the phototoxic effects of photodynamic therapy. Depending on the localization of the photosensitizing agent, the cell phenotype and the reactive species produced, the immediate effects can include apoptosis, autophagy, necrosis or combinations thereof. Vascular shutdown can also occur when in vivo systems are involved. The reactive species that are generated upon irradiation of photosensitizers can vary and the effects will depend on the localization site(s) of the agent. Apoptosis is a common response and has several advantages, including that the PDT dose needed is often small since the apoptotic process can be triggered by a relatively low PDT dose. Autophagy can serve both as a cytoprotective response and as a death mechanism, depending on several variables. It is not yet entirely clear which pathways to cell death are more effective. Moreover, the identification of reactive species is not a simple matter, and the information provided by most fluorescent probes is ambiguous. As new photosensitizing agents are developed, an examination of their localization patterns and photochemistry may aid in the selection of optimal agents for tumor eradication.
Acknowledgements
We thank the NIH (CA R01-23378 and ES090302) for support of work carried out at Wayne State University.
Biography
 David Kessel has been involved in studies relating to photodynamic therapy since 1976. He is the immediate Past President of the International Photodynamic Association and a Councilor and Historian for the American Society for Photobiology. His current research deals with modes of cell death after PDT.
David Kessel has been involved in studies relating to photodynamic therapy since 1976. He is the immediate Past President of the International Photodynamic Association and a Councilor and Historian for the American Society for Photobiology. His current research deals with modes of cell death after PDT.
References
- 1.Dougherty TJ, Gomer CJ, Weishaupt KR. Cancer Res. 1976;36:2330–2333. [PubMed] [Google Scholar]
- 2.Mitchell JB, McPherson S, DeGraff W, Gamson J, Zabell A, Russo A. Cancer Res. 1985;45:2008–2011. [PubMed] [Google Scholar]
- 3.Henderson BW, Fingar V. Photochem. Photobiol. 1989;49:299–304. doi: 10.1111/j.1751-1097.1989.tb04110.x. [DOI] [PubMed] [Google Scholar]
- 4.Foote CS. Photochem. Photobiol. 1991;34:659. doi: 10.1111/j.1751-1097.1991.tb02071.x. [DOI] [PubMed] [Google Scholar]
- 5.Vidóczy T. J. Photochem. Photobiol., B. 1992;14:139–142. [Google Scholar]
- 6.Rodgers MAJ. J. Photochem. Photobiol., B. 1993;18:296–298. doi: 10.1016/1011-1344(93)80080-s. [DOI] [PubMed] [Google Scholar]
- 7.Girotti A. J. Photochem. Photobiol., B. 2001;63:103–113. doi: 10.1016/s1011-1344(01)00207-x. [DOI] [PubMed] [Google Scholar]
- 8.Kramarenko GG, Hummel SG, Martin SM, Buettner GR. Photochem. Photobiol. 2006;82:1634–1637. doi: 10.1562/2006-01-12-RN-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubbo H, Radi R, Trujillo M, Kalyanaraman B, Barnes S, Freeman BA. J. Biol. Chem. 1994;269:26066–26075. [PubMed] [Google Scholar]
- 10.Setsukinai K, Urano Y, Kakinuma K, Majima HJ, Nagano T. J. Biol. Chem. 2003;278:3170–3175. doi: 10.1074/jbc.M209264200. [DOI] [PubMed] [Google Scholar]
- 11.Qin Y, Lu M, Gong X. Cell Biol. Int. 2008;32:224–228. doi: 10.1016/j.cellbi.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 12.Henderson LM, Chappell JB. Eur. J. Biochem. 1993;217:973–980. doi: 10.1111/j.1432-1033.1993.tb18328.x. [DOI] [PubMed] [Google Scholar]
- 13.Crow JP. Nitric Oxide. 1997;1:145–157. doi: 10.1006/niox.1996.0113. [DOI] [PubMed] [Google Scholar]
- 14.Zhao H, Kalivendi S, Zhang H, Joseph J, Nithipatikom K, Vásquez-Vivar J, Kalyanaraman B. Free Radical Biol. Med. 2003;34:1359–1368. doi: 10.1016/s0891-5849(03)00142-4. [DOI] [PubMed] [Google Scholar]
- 15.Zielonka J, Kalyanaraman B. Free Radical Biol. Med. 2010;48:983–1001. doi: 10.1016/j.freeradbiomed.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu K, Liu X, Tang B. ChemBioChem. 2007;8:453–458. doi: 10.1002/cbic.200600392. [DOI] [PubMed] [Google Scholar]
- 17.Maeda H, Yamamoto K, Kohno I, Hafsi L, Itoh N, Nakagawa S, Kanagawa N, Suzuki K, Uno T. Chemistry. 2007;3:1946–1954. doi: 10.1002/chem.200600522. [DOI] [PubMed] [Google Scholar]
- 18.Kessel D. J. Porphyrins Phthalocyanines. 2004;8:1009–1014. [Google Scholar]
- 19.Kessel D, Luo Y. J. Photochem. Photobiol., B. 1998;42:89–95. doi: 10.1016/s1011-1344(97)00127-9. [DOI] [PubMed] [Google Scholar]
- 20.Drummen GP, van Liebergen LC, Op den Kamp JA, Post JA. Free Radical Biol. Med. 2002;33:473–490. doi: 10.1016/s0891-5849(02)00848-1. [DOI] [PubMed] [Google Scholar]
- 21.Price M, Reiners JJ, Jr., Santiago AM, Kessel D. Photo-chem. Photobiol. 2009;85:1177–1181. doi: 10.1111/j.1751-1097.2009.00555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McQuade LE, Lippard SJ. Curr. Opin. Chem. Biol. 2010;14:43–49. doi: 10.1016/j.cbpa.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 23.a Gollmer A, Arnbjerg J, Blaikie FH, Pedersen BW, Breitenbach T, Daasbjerg K, Glasius M, Ogilby PR. Photo-chem. Photobiol. 2011;87:671–679. doi: 10.1111/j.1751-1097.2011.00900.x. [DOI] [PubMed] [Google Scholar]; b Ragàs X, Jiménez-Banzo A, Sánchez-García D, Batllor X, Nonell S. Chem. Commun. 2009;28:2920–2922. doi: 10.1039/b822776d. [DOI] [PubMed] [Google Scholar]
- 24.a Wardman P. Free Radical Biol. Med. 2007;43:995–1022. doi: 10.1016/j.freeradbiomed.2007.06.026. [DOI] [PubMed] [Google Scholar]; b Tarpey MM, Fridovich I. Circ. Res. 2001;89:224–236. doi: 10.1161/hh1501.094365. [DOI] [PubMed] [Google Scholar]; c Gomes A, Fernandes E, Lima JLFC. J. Biochem. Biophys. Methods. 2005;65:45–80. doi: 10.1016/j.jbbm.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Price M, Kessel D. J. Biomed. Opt. 2010;15:051605. doi: 10.1117/1.3484258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reeves KJ, Reed MW, Brown NJ. J. Photochem. Photobiol., B. 2009;95:141–147. doi: 10.1016/j.jphotobiol.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 27.Kessel D, Price M, Reiners JJ., Jr. Photochem. Photobiol. 2008;84:1272–1276. doi: 10.1111/j.1751-1097.2008.00371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doshi JM, Tian D, Xing C. Mol. Pharmaceutics. 2007;4:919–928. doi: 10.1021/mp7000846. [DOI] [PubMed] [Google Scholar]
- 29.a Oleinick NL, Morris RL, Belichenko I. Photochem. Photobiol. Sci. 2002;1:1–21. doi: 10.1039/b108586g. [DOI] [PubMed] [Google Scholar]; b Kim HR, Luo Y, Li G, Kessel D. Cancer Res. 1999;59:3429–3432. [PMC free article] [PubMed] [Google Scholar]
- 30.Kessel D, Luo Y. Photochem. Photobiol. 1997;66:479–483. doi: 10.1111/j.1751-1097.1997.tb03176.x. [DOI] [PubMed] [Google Scholar]
- 31.Reiners JJ, Jr., Agostinis P, Berg K, Oleinick NL, Kessel D. Autophagy. 2010;6:7–18. doi: 10.4161/auto.6.1.10220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.a Buytaert E, Callewaert G, Hendrickx N, Scorrano L, Hartmann D, Missiaen L, Vandenheede JR, Heirman I, Grooten J, Agostinis P. FASEB J. 2006;20:756–758. doi: 10.1096/fj.05-4305fje. [DOI] [PubMed] [Google Scholar]; b Buytaert E, Callewaert G, Vandenheede JR, Agostinis P. Autophagy. 2006;2:238–240. doi: 10.4161/auto.2730. [DOI] [PubMed] [Google Scholar]
- 33.a Kessel D, Luo Y, Mathieu P, Reiners JJ., Jr. Photochem. Photobiol. 2000;71:196–200. doi: 10.1562/0031-8655(2000)071<0196:dotart>2.0.co;2. [DOI] [PubMed] [Google Scholar]; b Reiners JJ, Jr., Caruso JA, Mathieu P, Chelladurai B, Yin XM, Kessel D. Cell Death Differ. 2002;9:934–944. doi: 10.1038/sj.cdd.4401048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klionsky DJ. Semin. Cell Dev. Biol. 2010;21:663. doi: 10.1016/j.semcdb.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 35.a Kessel D, Arroyo AS. Photochem. Photobiol. Sci. 2007;6:1290–1295. doi: 10.1039/b707953b. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Andrzejak M, Price M, Kessel D. Autophagy. 2011;7:979–984. doi: 10.4161/auto.7.9.15865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xue LY, Chiu SM, Oleinick NL. Autophagy. 2010;6:248–255. doi: 10.4161/auto.6.2.11077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weishaupt KR, Gomer CJ, Dougherty TJ. Cancer Res. 1976;36:2326–2329. [PubMed] [Google Scholar]
- 38.Kochevar IE, Lynch MC, Zhuang S, Lambert CR. Photochem. Photobiol. 2000;72:548–553. doi: 10.1562/0031-8655(2000)072<0548:sobnor>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 39.Gołąb J, Nowis D, Skrzycki M, Czeczot H, Barańczyk-Kuźma A, Wilczyński GM, Makowski M, Mróz P, Kozar K, Kamiński R, Jalili A, Kopeć M, Grzela T, Jakóbisiak M. J. Biol. Chem. 2003;278:407–414. doi: 10.1074/jbc.M209125200. [DOI] [PubMed] [Google Scholar]
- 40.Kachadourian R, Liochev SI, Cabelli DE, Patel MN, Fridovich I, Day BJ. Arch. Biochem. Biophys. 2001;392:349–353. doi: 10.1006/abbi.2001.2455. [DOI] [PubMed] [Google Scholar]
- 41.Price M, Terlecky SR, Kessel D. Photochem. Photobiol. 2009;85:1491–1496. doi: 10.1111/j.1751-1097.2009.00589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.a Chen Y, Azad MB, Gibson SB. Cell Death Differ. 2009;16:1040–1052. doi: 10.1038/cdd.2009.49. [DOI] [PubMed] [Google Scholar]; b Azad MB, Chen Y, Gibson SB. Antioxid. Redox Signaling. 2009;11:777–790. doi: 10.1089/ars.2008.2270. [DOI] [PubMed] [Google Scholar]; c Scherz-Shouval R, Shvets E, Elazar Z. Autophagy. 2007;3:371–373. doi: 10.4161/auto.4214. [DOI] [PubMed] [Google Scholar]
- 43.a Mazor O, Brandis A, Plaks V, Neumark E, Rose-nbach-Belkin V, Salomon Y, Scherz A. Photochem. Photo-biol. 2005;81:342–351. doi: 10.1562/2004-06-14-RA-199. [DOI] [PubMed] [Google Scholar]; b Ashur I, Goldschmidt R, Pinkas I, Salomon Y, Szewczyk G, Sarna T, Scherz A. J. Phys. Chem. A. 2009;113:8027–8037. doi: 10.1021/jp900580e. [DOI] [PubMed] [Google Scholar]
- 44.Lee S, Isabelle ME, Gabally-Kinney KL, Pogue BW, Davis SJ. Biomed. Opt. Express. 2011;2:1233–1242. doi: 10.1364/BOE.2.001233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.a Goldstein S, Czapski G. Free Radical Biol. Med. 1995;19:505–510. doi: 10.1016/0891-5849(95)00034-u. [DOI] [PubMed] [Google Scholar]; b Kissner R, Nauser T, Bugnon P, Lye PG, Kippenol WH. Chem. Res. Toxicol. 1997;10:1285–1292. doi: 10.1021/tx970160x. [DOI] [PubMed] [Google Scholar]
- 46.Ferrer-Sueta G, Radi R. ACS Chem. Biol. 2009;4:161–177. doi: 10.1021/cb800279q. [DOI] [PubMed] [Google Scholar]
- 47.Szabo C, Ischiropoulos H, Radi R. Nat. Rev. Drug Discov. 2007;6:662–680. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- 48.Korbelik M, Parkins CS, Shibuya H, Cecic I, Stratford MRL, Chaplin DJ. Br. J. Cancer. 2000;82:1835–1843. doi: 10.1054/bjoc.2000.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu Z, Tao Y, Shou Z, Zhang J, Li C, Ou L, Zhao B. Free Radical Biol. Med. 2006;41:1590–1605. doi: 10.1016/j.freeradbiomed.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 50.a Bhowmick R, Girotti AW. Photochem. Photobiol. 2011;87:378–386. doi: 10.1111/j.1751-1097.2010.00877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Yamamoto F, Ohgari Y, Yamaki N, Kitajima S, Shimokawa O, Matsui H, Taketani S. Biochem. Biophys. Res. Commun. 2007;353:541–546. doi: 10.1016/j.bbrc.2006.12.007. [DOI] [PubMed] [Google Scholar]; c Di Venosa G, Perotti C, Fukuda H, Batlle A, Casas A. J. Photochem. Photobiol., B. 2005;80:195–202. doi: 10.1016/j.jphotobiol.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 51.Turkuoglu P, Ozturkmen C, Ilhan N, Kurt J, Aydemir O, Celiker U, Ibrahim MA, Rashid A. Indian J. Ophthalmol. 2011;59:5–8. doi: 10.4103/0301-4738.73705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frank J, Lambert C, Biesalski IHK, Thews O, Vaupel P, Kelleher DK. Int. J. Cancer. 2003;107:941–948. doi: 10.1002/ijc.11507. [DOI] [PubMed] [Google Scholar]
- 53.Henderson BW, Sitnik-Busch TM, Vaughan LA. Photochem. Photobiol. 1990;70:64–71. [PubMed] [Google Scholar]
- 54.a Reeves KJ, Reed MW, Brown NJ. J. Photochem. Photobiol., B. 2009;95:141–147. doi: 10.1016/j.jphotobiol.2009.02.005. [DOI] [PubMed] [Google Scholar]; b Reeves KJ, Reed MW, Brown NJ. J. Photochem. Photobiol., B. 2010;101:224–232. doi: 10.1016/j.jphotobiol.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 55.Niziolek M, Korytowski W, Girotti AW. Free Radical Biol. Med. 2005;40:1323–1331. doi: 10.1016/j.freeradbiomed.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 56.Bhowmick R, Girotti AW. Free Radical Biol. Med. 2010;48:1296–1301. doi: 10.1016/j.freeradbiomed.2010.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gomes ER, Almeida RD, Carvalho AP, Duarte CB. Photochem. Photobiol. 2002;76:423–430. doi: 10.1562/0031-8655(2002)076<0423:nomtcd>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 58.Son MJ, Lee SB, Byun YJ, Lee HO, Kim HS, Kwon OJ, Jeong SW. J. Biochem. Mol. Toxicol. 2010;24:313–322. doi: 10.1002/jbt.20340. [DOI] [PubMed] [Google Scholar]
- 59.McMurtry V, Saavedra JE, Nieves-Alicea R, Simeone AM, Keefer LK, Tari AM. Int. J. Oncol. 2011;38:963–971. doi: 10.3892/ijo.2011.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yuan H, Perry CN, Huang C, Iwai-Kanai E, Carreira RS, Glembotski CC, Gottieb RA. Am. J. Physiol. Heart Circ. Physiol. 2009;296:H470–479. doi: 10.1152/ajpheart.01051.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]