Abstract
Purpose
Concerns regarding neurocognitive toxicity of whole-brain radiotherapy (WBRT) have motivated development of alternative, dose-intensive chemotherapeutic strategies as consolidation in primary CNS lymphoma (PCNSL). We performed a multicenter study of high-dose consolidation, without WBRT, in PCNSL. Objectives were to determine: one, rate of complete response (CR) after remission induction therapy with methotrexate, temozolomide, and rituximab (MT-R); two, feasibility of a two-step approach using high-dose consolidation with etoposide plus cytarabine (EA); three, progression-free survival (PFS); and four, correlation between clinical and molecular prognostic factors and outcome.
Patients and Methods
Forty-four patients with newly diagnosed PCNSL were treated with induction MT-R, and patients who achieved CR received EA consolidation. We performed a prospective analysis of molecular prognostic biomarkers in PCNSL in the setting of a clinical trial.
Results
The rate of CR to MT-R was 66%. The overall 2-year PFS was 0.57, with median follow-up of 4.9 years. The 2-year time to progression was 0.59, and for patients who completed consolidation, it was 0.77. Patients age > 60 years did as well as younger patients, and the most significant clinical prognostic variable was treatment delay. High BCL6 expression correlated with shorter survival.
Conclusion
CALGB 50202 demonstrates for the first time to our knowledge that dose-intensive consolidation for PCNSL is feasible in the multicenter setting and yields rates of PFS and OS at least comparable to those of regimens involving WBRT. On the basis of these encouraging results, an intergroup study has been activated comparing EA consolidation with myeloablative chemotherapy in this randomized trial in PCNSL, in which neither arm involves WBRT.
INTRODUCTION
Primary CNS lymphoma (PCNSL) is typically an aggressive non-Hodgkin lymphoma, usually of large B-cell histology, which historically has been considered to be associated with a significantly worse prognosis than systemic lymphomas of the same histology, if not incurable. To date, there is no standardized approach to the treatment of PCNSL.1 Although there is consensus that high-dose methotrexate (HD-MTX) is the cornerstone of treatment, the median progression-free survival (PFS) with HD-MTX as monotherapy is modest, at only 12 to 13 months.2,3 The Radiation Therapy Oncology Group demonstrated that a combined-modality approach using HD-MTX–based chemotherapy followed by whole-brain radiotherapy (WBRT) markedly extends median PFS to 24 months4; however, concerns regarding the irreversible neurocognitive effects of brain irradiation,5 even at reduced doses,6 have prompted the development of alternative consolidative strategies.
In CALGB (Cancer and Leukemia Group B) 50202, we asked the question of whether it is possible to treat patients with newly diagnosed primary CNS lymphoma with immunotherapy plus high-dose chemotherapy in the multicenter, cooperative group setting and achieve efficacy comparable to that of regimens using brain irradiation. We implemented a two-step, dose-intensive immunochemotherapy regimen designed to be tolerated by patients with PCNSL, particularly in the first few weeks after diagnosis, when performance status and neurologic function are most severely impaired. Induction therapy involves three components: HD-MTX, administered every 2 weeks; weekly rituximab; and temozolomide, prescribed monthly. Rituximab is administered during the first 6 weeks of therapy, an interval during which the blood-brain barrier may be most compromised.7 Temozolomide reliably penetrates the blood-brain barrier, has established activity in CNS lymphomas as monotherapy and in combination with rituximab,8–10 and has been demonstrated to provide superior health-related quality of life and toxicity profile compared with procarbazine in patients with brain tumors.11,12
To consolidate response and potentiate PFS, patients who obtained a complete response (CR) after induction chemotherapy received the second step of this regimen: infusional etoposide plus high-dose cytarabine as intensive consolidation with non–cross-resistant agents. Notably, the combination of etoposide plus cytarabine (EA) was previously demonstrated to be highly active as first-line salvage in recurrent/refractory CNS lymphoma.13 The importance of high-dose cytarabine in the treatment of PCNSL is established.14 Etoposide is effective in the treatment of CNS complications of lymphoid leukemia and other brain tumors and, when administered in combination with CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) in aggressive lymphoma, may decrease the risk of secondary CNS lymphoma.15,16
PATIENTS AND METHODS
Eligibility
Patients were eligible provided they had histologic confirmation of CNS non-Hodgkin lymphoma (NHL), with central review of diagnostic specimens. Measurable disease based on gadolinium enhancement of brain or spine magnetic resonance imaging (MRI) and/or positive CSF cytology was also required. Patients were excluded for positive HIV serology, if pregnant or nursing, or for evidence of systemic NHL by computed tomography (CT) of chest, abdomen, and pelvis and bone marrow biopsy. Other exclusion criteria included baseline pleural effusions or ascites, Eastern Cooperative Oncology Group performance status (ECOG PS) > 2, absolute neutrophil count (ANC) < 1,500/mL, ALT/AST > 2× upper limit of normal, total bilirubin > 2 g/dL, and creatinine clearance < 50 mL/min. Each participant signed an institutional review board–approved informed consent document in accordance with federal and institutional guidelines.
On-Study Procedures
At enrollment, physical and neurologic examinations were performed in addition to laboratory studies, including complete blood count, differential and platelet count, and serum electrolyte and chemistries. MRI of brain and total spine, ocular slit lamp and CSF examination, and CT or MRI of chest/abdomen/pelvis as well as bone marrow aspirate and biopsy were performed.
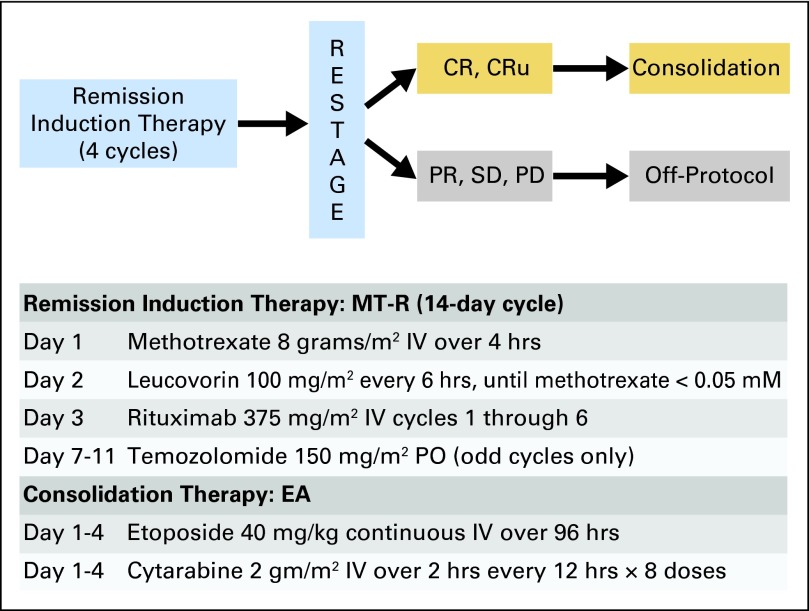
Protocol Treatment
CALGB 50202 had two treatment modules (Fig 1). Remission induction chemotherapy consisted of HD-MTX, temozolomide, and rituximab (MT-R). HD-MTX was administered intravenously (IV) once every 2 weeks (for the first seven doses) at 8 g/m2 over 4 hours, with leucovorin rescue every 6 hours, and adjusted for creatinine clearance, as described previously.2 Rituximab 375 mg/m2 was administered once per week for six doses, beginning on day +3 (patients with T-cell PCNSL did not receive rituximab). Temozolomide (150 mg/m2) was administered once per day on days 7 to 11 each of the first 5 months. No intrathecal chemotherapy was administered. Consolidation chemotherapy consisted of etoposide 5 mg/kg administered by continuous IV infusion every 12 hours for eight doses (total dose, 40 mg/kg), with cytarabine 2 g/m2 IV over 2 hours every 12 hours for eight doses (total dose, 16 g/m2), as described previously.17
Fig 1.
Protocol schema. Patients were restaged after 4 months of high-dose methotrexate-based therapy (seven doses of high-dose methotrexate, every 2 weeks; six doses of weekly rituximab; and 4 months of temozolomide over 5 days [MT-R]). Patients who achieved a complete response (CR) or CR/unconfirmed (CRu) received an additional course of high-dose methotrexate plus one of temozolomide. Three to 5 weeks later, patients received intensive consolidation with etoposide plus cytarabine (EA). High-dose EA chemotherapy doses were based on corrected body weight (kg), defined as ideal weight plus 0.25 (actual weight − ideal weight), as described previously.17 IV, intravenous; PD, progressive disease; PO, orally; PR, partial response; SD, stable disease.
Supportive Care
Hydration and urine alkalinization during methotrexate administration were achieved by administration of NaHCO3 (100 to 150 mEq/L) at 150 mL/h IV until urine output of ≥ 100 mL/h and urine pH > 7 for 4 hours before methotrexate and continued until completion of leucovorin rescue. During EA consolidation, patients showered twice daily, and corticosteroid eye drops, two drops per eye, were administered four times per day on days 1 to 6 to prevent cytarabine keratoconjunctivitis. Granulocyte CSF (5 mcg/kg/d) or granulocyte macrophage CSF 250 (mcg/m2/d) were administered subcutaneously starting day 14 of therapy and continued until ANC reached ≥ 500/μL for 2 days or ≥ 1,500/μL for 1 day. Bacterial prophylaxis with fluoroquinolone antibiotics was initiated at ANC < 500/μL and continued until ANC reached ≥ 500/μL. Fungal prophylaxis (azole) was started day 6 of therapy and continued until ANC reached ≥ 500/μL. Herpes simplex virus and Varicella zoster virus prophylaxis consisted of acyclovir or valacyclovir. Pneumocystis pneumonia prophylaxis was provided with trimethoprim/sulfamethoxazole or dapsone. Febrile neutropenia and transfusion support were managed according to institutional guidelines.
Documentation of Response
At 4 months of remission induction therapy (after the seventh course of HD-MTX), patients were restaged by MRI of brain (plus spine and lumbar puncture if previously positive). Patients who achieved a CR or CR/unconfirmed received an additional (eighth) cycle of HD-MTX followed by a fifth course of temozolomide followed by remission consolidation therapy with EA. After consolidation therapy, patients were restaged with repeat brain MRI every 2 months for the first year, then every 4 months for years 2 and 3. Beginning at 3.5 years, patients were evaluated every 6 months until 6.5 years after induction. The International Primary CNS Lymphoma Collaborative Group response criteria were used, as described previously.18
Statistical Considerations
The study used a two-stage design to address the primary end point—CR rate—with exact binomial 95% CI. An interim analysis was conducted when response data were available from the first 27 patients, with a planned early stopping rule for a CR rate < 44%. With a target accrual of 45 patients, a successful trial was prospectively defined as a CR rate of at least 53% for the therapeutic approach to be acceptable for further investigation. Efficacy end points PFS, time to progression (TTP), and overall survival (OS) were defined as per the Revised Response Criteria for Malignant Lymphoma.19
Neurologic and other toxicities were closely monitored with the first 6, 10, 20, 30, and 45 patients. Rates ≥ 5% grade 4 neurotoxicity and ≥ 10% grade 5 other toxicities were prospectively defined as unacceptable, and if observed, the trial would be stopped. Toxicities were scored using the National Cancer Institute Common Toxicity Criteria, version 3.0.
Assessment of clinical prognostic factors was based on International Extranodal Lymphoma Study Group (IELSG) score20 using the log-rank test. Assessment of candidate molecular prognostic markers BCL6 and MYC was performed by immunohistochemistry in archival formalin-fixed paraffin-embedded tissue. The percent tumor cell nuclei staining for each marker was independently scored (nearest 10% increment) by two pathologists blinded to clinical outcome (E.D.H., M.O.N.) with near-100% reproducibility (R2 > 0.9; P < .001). Cox proportional hazards models were fitted for outcomes using candidate molecular markers as continuous variables. If the model was statistically significant, the best cut point in the data was determined using an iterative procedure.
Patient registration and data collection were managed by the CALGB (Alliance) Statistics and Data Center, with data as of May 24, 2012, analyzed by CALGB statisticians. Data quality was ensured through review by CALGB (Alliance) statistical center staff and by the study chairperson. As part of the CALGB quality assurance program, members of the audit committee visit all participating institutions at least once every 3 years to verify compliance with federal regulations and protocol requirements. Review of medical records was performed for 13 (28%) of the 47 patients registered to this study.
RESULTS
Patients and Disease Characteristics and Study
Forty-seven patients enrolled between October 2004 and November 2009 at 12 CALGB sites. Three patients were excluded from analysis because of failure to meet eligibility criteria or to receive protocol therapy (Table 1). Large B-cell lymphoma was diagnosed in 43 (98%) of 44 patients. The median age was 61 years, and 48% of patients were male. Among the 40 patients for whom complete IELSG PCNSL prognostic parameters were available, 27 (68%) had IELSG risk scores ≥ 2. Ten patients (24%) had positive CSF cytology, and one had intraocular lymphoma.
Table 1.
Patient Demographic and Clinical Characteristics (N = 44)
| Characteristic | No. | % |
|---|---|---|
| Age, years | ||
| Median | 61 | |
| Range | 12-76 | |
| Male sex | 21 | 48 |
| ECOG PS | ||
| 0 | 8 | 18 |
| 1 | 28 | 64 |
| 2 | 8 | 18 |
| Elevated LDH* | 12 | 29 |
| Elevated CSF protein* | 20 | 48 |
| Deep brain lesions† | 20 | 47 |
| IELSG risk group‡ | ||
| 0-1 | 13 | 33 |
| 2-3 | 23 | 58 |
| 4-5 | 4 | 10 |
| Positive CSF cytology | 10 | 24 |
| Intraocular lymphoma | 1 | 2 |
| Large B-cell lymphoma§ | 43 | 98 |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; IELSG, International Extranodal Lymphoma Study Group; LDH, lactate dehydrogenase.
Data regarding serum LDH and CSF protein concentration were available in 42 patients.
Documentation of deep tumor location was made in 43 patients.
Complete IELSG prognostic characteristics were available in 40 of 44 patients.
Large B-cell histology was diagnosed in 98% of patients; one patient had B-cell lymphoma, unspecified.
Response and Survival
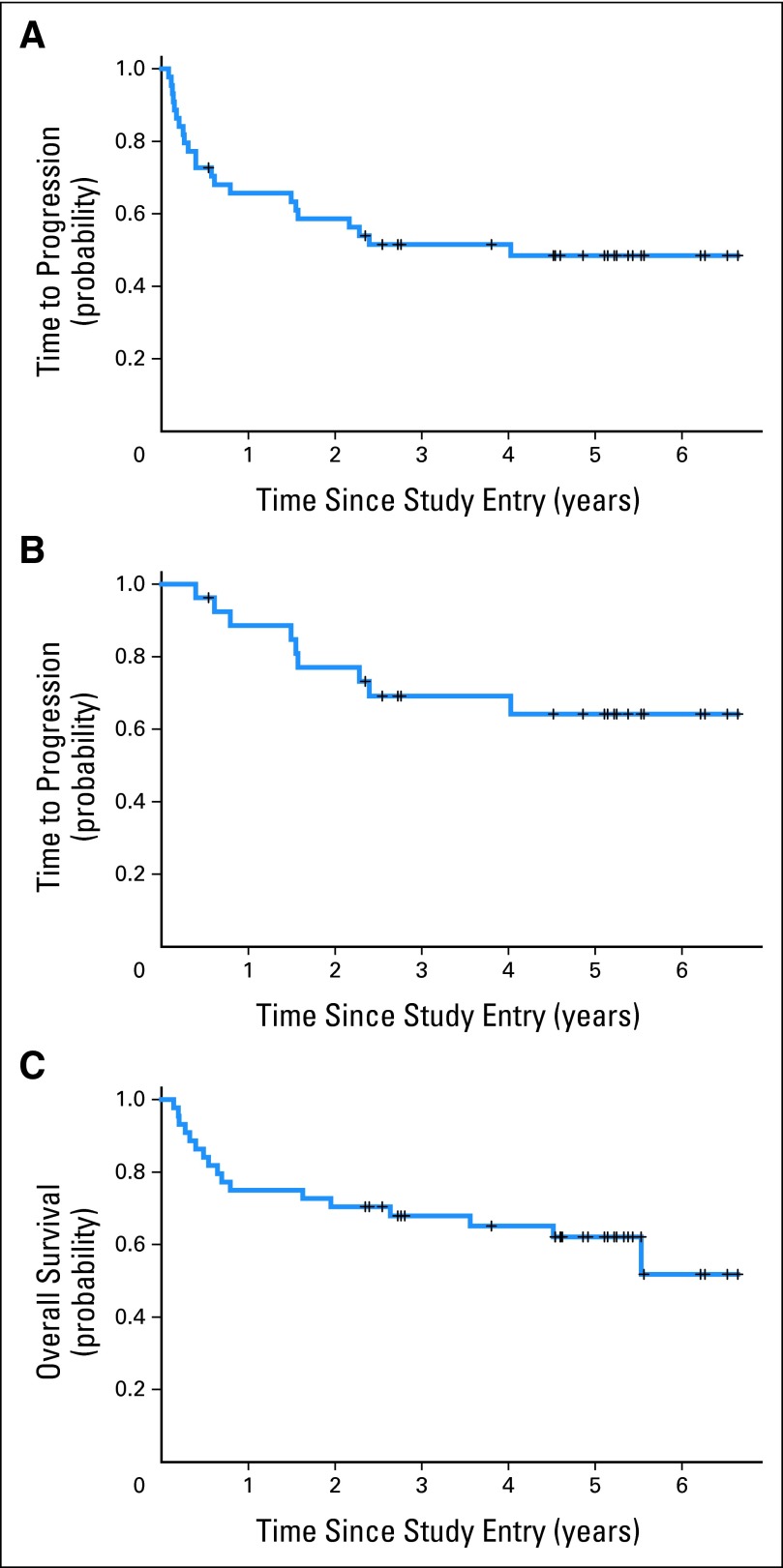
After MT-R induction, nine patients (20%) experienced disease progression, one had stable disease, five (11%) achieved a partial response, and 29 (66%) achieved a CR, yielding a final CR rate of 66% (95% CI, 50% to 80%). The 2-year rate of PFS was 0.57; 2-year TTP was 0.59, and median TTP was 4.0 years. The 2-year TTP for those patients who completed the entire regimen was 0.77 (range, 0.56 to 0.89). The median PFS was 2.4 years, with one treatment-related death and one death resulting from lung cancer at 4.5 years. Median OS for the study population has not been reached, with estimated probability of OS at 4 years of 0.65 (range, 0.49 to 0.77; Figs 2A to 2C). To date, 17 patients have died, with median survival time among the 27 surviving patients of 4.9 years (range, 2.3 to 6.6 years).
Fig 2.
Outcome for all 50202 study patients; y-axis refers to cumulative probability of event. (A) Time to progression (TTP) for all patients; median TTP was 4.0 years (22 patients experienced disease progression). Estimated TTPs at 1, 2, 3, and 4 years were 0.66 (95% CI, 0.50 to 0.78), 0.59 (95% CI, 0.43 to 0.72), 0.52 (95% CI, 0.36 to 0.65), and 0.48 (95% CI, 0.33 to 0.63), respectively. (B) TTP for those patients (n = 27) who completed entire treatment protocol (induction plus consolidation). One- and 2-year probabilities of TTP from start of etoposide plus cytarabine consolidation were 0.85 (95% CI, 0.64 to 0.94) and 0.69 (95% CI, 0.47 to 0.83), respectively. (C) Overall survival (OS) for all patients; median OS has not been reached. Estimated OS at 1, 2, 3, and 4 years were 0.75 (95% CI, 0.59 to 0.85), 0.70 (95% CI, 0.52 to 0.80), 0.70 (95% CI, 0.52 to 0.80), and 0.65 (95% CI, 0.49 to 0.77), respectively. TTP is defined as time from date of study entry until progression or date of last follow-up while in remission, with censoring of deaths not resulting from progressive lymphoma. OS is defined as time from date of study entry until death resulting from any cause or date of last follow-up while in remission.
Toxicity
As expected, 55% of patients experienced grade 4 neutropenia, and 50% of patients experienced grade 4 thrombocytopenia; 81% of these episodes occurred after remission consolidation chemotherapy with EA (Table 2). There were four cases of febrile neutropenia, (grade 3, three; grade 4, one); there was one grade 5 infectious complication (sepsis), which also occurred after EA. There was only one case of grade 3 renal failure (reversible) and no episodes of grade 3 or 4 cytarabine or other neurotoxicity. Summary of adverse events is provided in Appendix Table A1 (online only).
Table 2.
Common Toxicities by Grade Occurring in Each Arm*
| AE | Grade 3 |
Grade 4 |
Grade 5 |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Hemoglobin | ||||||
| MT-R | 5 | 11 | 1 | 2 | 0 | 0 |
| EA | 3 | 12 | 0 | 0 | 0 | 0 |
| Lymphopenia | ||||||
| MT-R | 3 | 7 | 1 | 2 | 0 | 0 |
| EA | 0 | 0 | 1 | 4 | 0 | 0 |
| Neutropenia | ||||||
| MT-R | 7 | 16 | 4 | 9 | 0 | 0 |
| EA | 1 | 4 | 21 | 81 | 0 | 0 |
| Thrombocytopenia | ||||||
| MT-R | 4 | 9 | 1 | 2 | 0 | 0 |
| EA | 1 | 4 | 21 | 81 | 0 | 0 |
| Diarrhea | ||||||
| MT-R | 2 | 5 | 0 | 0 | 0 | 0 |
| EA | 2 | 8 | 0 | 0 | 0 | 0 |
| Mucositis/stomatitis | ||||||
| MT-R | 0 | 0 | 0 | 0 | 0 | 0 |
| EA | 2 | 8 | 0 | 0 | 0 | 0 |
| Nausea | ||||||
| MT-R | 2 | 5 | 0 | 0 | 0 | 0 |
| EA | 1 | 4 | 0 | 0 | 0 | 0 |
| Febrile neutropenia | ||||||
| MT-R | 0 | 0 | 0 | 0 | 0 | 0 |
| EA | 3 | 12 | 1 | 4 | 0 | 0 |
| Infection | ||||||
| MT-R | 3 | 7 | 0 | 0 | 0 | 0 |
| EA | 6 | 14 | 0 | 0 | 1 | 4 |
| ALT | ||||||
| MT-R | 7 | 16 | 3 | 7 | 0 | 0 |
| EA | 0 | 0 | 0 | 0 | 0 | 0 |
| AST | ||||||
| MT-R | 8 | 18 | 2 | 5 | 0 | 0 |
| EA | 0 | 0 | 0 | 0 | 0 | 0 |
| High serum glucose | ||||||
| MT-R | 6 | 14 | 1 | 2 | 0 | 0 |
| EA | 0 | 0 | 1 | 4 | 0 | 0 |
| Low serum potassium | ||||||
| MT-R | 11 | 25 | 1 | 2 | 0 | 0 |
| EA | 3 | 12 | 0 | 0 | 0 | 0 |
| High serum potassium | ||||||
| MT-R | 3 | 7 | 0 | 0 | 0 | 0 |
| EA | 1 | 4 | 0 | 0 | 0 | 0 |
| Low serum sodium | ||||||
| MT-R | 3 | 7 | 0 | 0 | 0 | 0 |
| EA | 0 | 0 | 0 | 0 | 0 | 0 |
| Muscle weakness | ||||||
| MT-R | 3 | 7 | 0 | 0 | 0 | 0 |
| EA | 0 | 0 | 0 | 0 | 0 | 0 |
| Pain | ||||||
| MT-R | 3 | 7 | 0 | 0 | 0 | 0 |
| EA | 1 | 4 | 0 | 0 | 0 | 0 |
| Pneumonitis | ||||||
| MT-R | 3 | 7 | 0 | 0 | 0 | 0 |
| EA | 0 | 0 | 0 | 0 | 0 | 0 |
| Maximum overall AE | ||||||
| MT-R | 24 | 55 | 12 | 27 | 0 | 0 |
| EA | 1 | 4 | 21 | 81 | 1 | 4 |
Abbreviations: AE, adverse event; EA, etoposide plus cytarabine; MT-R, methotrexate, temozolomide, and rituximab.
Toxicities occurring in ≥ 5% of patients. No EA toxicity data were available for one patient who experienced disease progression and died within 10 days of intensive consolidation.
Clinical Prognostic Variables
We evaluated the relationship between established clinical prognostic variables and outcome in patients with PCNSL treated with the 50202 regimen (Figs 3A to 3D). ECOG PS > 1 and high IELSG score (4 to 5) were associated with shorter PFS. Unlike previous studies in PCNSL,21,22 patients age > 60 years experienced outcomes similar to those of younger patients. The most significant clinical variable identified in this series was treatment delay. Although the median interval between diagnosis and initiation of protocol treatment for the entire 50202 cohort was 15 days, review of study throughput data revealed that 10 patients started MT-R therapy > 30 days after diagnosis (median, 39 days; range, 31 to 83 days). PFS for these patients was significantly shorter than for patients who started therapy soon after diagnosis (two-sided t-test P = .05). Among the cohort of patients for whom remission induction therapy was delayed > 30 days after diagnosis, two experienced disease progression during induction MT-R, three achieved only a partial response, and one had stable disease and went off study. Of the four patients with treatment delay who did achieve a CR, two subsequently experienced early disease progression, and only two remain in remission. Half of the patients with treatment delay succumbed to CNS lymphoma progression, whereas only 29% of the patients (12 of 34) who were treated within 1 month of diagnosis have died. Notably, there was no association between ECOG PS or IELSG risk score and delay in remission induction therapy, supporting treatment delay as a previously unrecognized, independent clinical prognostic variable in PCNSL.
Fig 3.
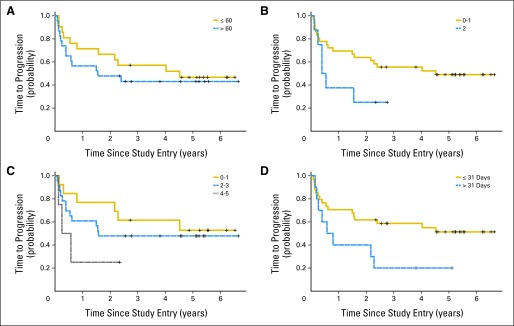
Clinical prognostic variables and their relationship to progression-free survival (PFS); median PFS survival was 2.4 years (22 patients who experienced disease progression plus two patients achieving complete response who succumbed to sepsis and lung cancer, respectively). Estimated PFS at 1, 2, 3, and 4 years were 0.64 (95% CI, 0.48 to 0.76), 0.57 (95% CI, 0.41 to 0.70), 0.50 (95% CI, 0.34 to 0.64), and 0.47 (95% CI, 0.32 to 0.61; not shown). (A) PFS was similar for patients age > 60 years (n = 23) and for younger patients (n = 21; P = .48). (B) PFS was shorter for patients with Eastern Cooperative Oncology Group performance status of 2 (n = 8; P < .06). (C) There was a trend between shorter PFS and highest International Extranodal Lymphoma Study Group risk score of 4 to 5 (P = .16). (D) Treatment delay was associated with shorter PFS. Patients with delayed initiation of remission induction therapy, beyond 30 days after diagnosis, experienced significantly shorter PFS compared with patients whose therapy began within 1 month of diagnosis (P = .050). Three-year PFS was 0.59 (95% CI, 0.40 to 0.73) for those without treatment delay and 0.2 (95% CI, 0.03 to 0.47) for those with treatment delay. PFS is defined as time from date of study entry until progression, death resulting from any cause, or date of last follow-up while in remission. There was no association between malignant CSF cytology at pretreatment staging and response rate or outcome.
Molecular Prognostic Variables
Previous immunohistochemical analyses of prognostic molecules in PCNSL have been retrospective in nature and identified BCL6 and MYC as candidate biomarkers.23–26 Importantly, to date, there have been no prospective studies of molecular biomarkers in patients with PCNSL treated uniformly in the setting of a multicenter clinical trial. Furthermore, candidate prognostic biomarkers in PCNSL have not been examined in the context of rituximab and high-dose chemotherapy.
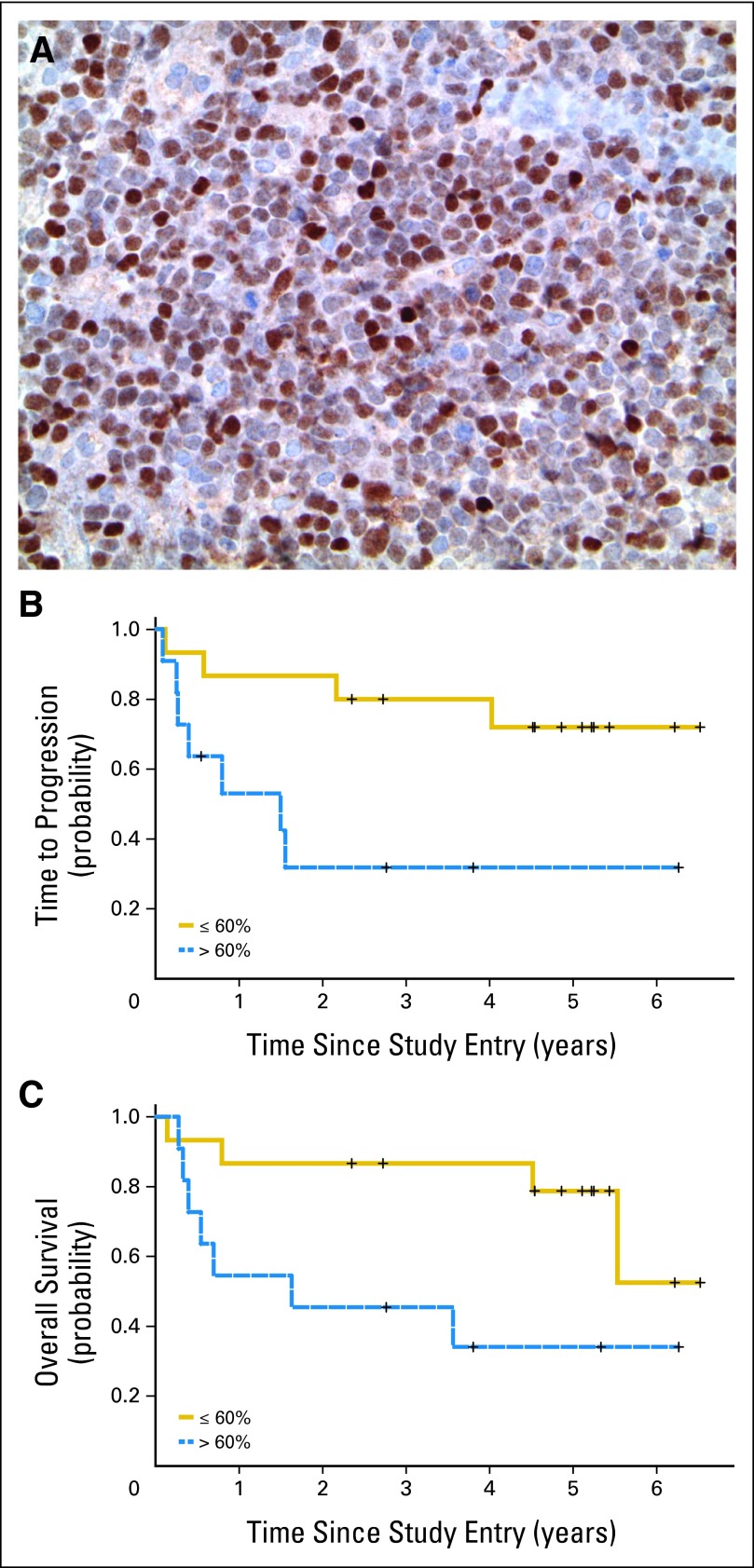
In CALGB 50202, diagnostic specimens were requested from all participating patients, and sufficient biopsy material was available for immunohistochemical staining from 26 patient cases (59%). High MYC expression (> 50% of lymphoma nuclei) was detected in 54% of patient cases, an increased proportion of patient cases compared with systemic diffuse large B-cell lymphoma,27 but MYC expression in this series did not correlate with outcome. By contrast, high BCL6 expression (≥ 30% of lymphoma nuclei) was detected in 19 patient cases (59%), consistent with previous reports.28 High BCL6 expression by lymphoma nuclei, correlated as a continuous variable with inferior TTP, PFS, and OS. The two-sided P values for these models were P = .045, P = .019, and P = .045, respectively (log-rank test). Because the global test was significant in all three cases, the most significant cut point for dichotomizing BCL6 expression was evaluated using an iterative method and determined to be 60% (Figs 4A to 4C).
Fig 4.
BCL6 expression is associated with short time to progression (TTP) and overall survival (OS) in patients with primary CNS lymphoma (PCNSL) treated in the 50202 study. (A) Example of strong nuclear BCL6 expression in a PCNSL case from patient treated in study (40× magnification). (B) High BCL6 expression (ie, > 60% of lymphoma nuclei) is associated with short TTP (two-sided P < .016). (C) High BCL6 is also associated with shorter OS (two-sided P < .009). For BCL6, monoclonal antibody Pg-B6p (Dako, Carpenteria, CA) was used. For MYC (not shown), monoclonal antibody Y69 (Epitomics, Burlingame, CA) was used. An automated immunostainer with iView diaminobenzidine detection (Ventana Medical Systems, Oro Valley, AZ) was used with CC1 heat-induced epitope retrieval for both assays.
DISCUSSION
CALGB 50202 demonstrates for the first time to our knowledge the feasibility of high-dose chemotherapy consolidation administered in the multicenter, cooperative group setting for newly diagnosed PCNSL. The 0.57 rate of 2-year PFS exceeds those of other chemotherapy-alone studies2,29,30 and is at least comparable to combined-modality approaches with reduced-dose WBRT.31 The median TTP of all 50202 patients—4 years—is 2× longer than that achieved with combined-modality therapy in multicenter trials using standard-dose WBRT.4,22 Survival for the cohort of patients who completed EA dose-intensive consolidation is particularly promising, confirming prior single-institution data.32 The survival curves show encouraging evidence of a stable plateau, and the median OS for 50202 patients has not yet been reached, with a median follow-up of 4.9 years.
The CALGB 50202 regimen was generally well tolerated, with the exception of one treatment-related death caused by sepsis in a patient managed as an outpatient after EA chemotherapy; this event highlights the recommendation of detailed inpatient monitoring during the neutropenic and thrombocytopenic nadirs expected after the intensive consolidation phase of treatment. On the other hand, myelosuppression during the remission induction phase was mild; few patients required growth factor support; and there was only one case of grade 3 renal toxicity, despite high doses of methotrexate administered. Although there were no reported episodes of severe acute neurotoxicity, detailed post-treatment neurocognitive testing was not performed.
Remarkably, the PFS of patients age > 60 years treated in 50202 was similar compared with that of younger patients, a result that contrasts previous studies in PCNSL, which demonstrated that patients age > 60 years fare significantly worse.21 Although preliminary, this observation suggests that many of the established prognostic features of PCNSL may be dependent on treatment-related variables, including radiotherapy.5 It should also be noted that there may be subgroups of patients with PCNSL for whom radiotherapy may be necessary and who may potentially be identified in randomized studies.
In addition, our finding that the late initiation of remission induction therapy correlates with a population at higher risk of early disease progression, although novel, is supported by prior assertions that delayed diagnosis of PCNSL correlates with adverse outcome.33,34 Among the factors that may contribute to delayed initiation of therapy after the diagnosis of PCNSL are the relative rarity of the diagnosis, a lack of familiarity with therapeutic options in community practice, the fact that many patients may choose to delay treatment to obtain a second opinion, and the assumption that PCNSL is an incurable disease. Whatever the cause of treatment delay, its association with adverse outcome has important implications for the management of these patients and provides evidence that the prompt initiation of therapy after diagnosis may translate to improved outcomes in PCNSL. This result also suggests that interventions that facilitate early diagnosis of PCNSL and intraocular lymphoma may also translate into improved outcomes for patients.
Finally, CALGB 50202 is the first clinical trial in PCNSL to our knowledge to prospectively evaluate molecular prognostic biomarkers expressed within diagnostic lymphoma specimens. As a lymphoma subtype, PCNSL tumors exhibited high MYC expression relative to systemic large-cell lymphoma, consistent with previous transcriptional evidence35; however, MYC was not prognostic. By contrast, our prospective data demonstrate that BCL6 expression was predictive of shorter survival in patients with PCNSL treated with the 50202 regimen. This observation is in agreement with previous reports that expression of the BCL6 oncoprotein correlates with adverse outcome in PCNSL.25,26 Because the vast majority of B-cell PCNSL patient cases express MUM1, and prior studies have shown ongoing immunoglobulin gene somatic hypermutation, the concept of BCL6-positive PCNSL having an activated germinal center B-cell origin seems reasonable and may explain the adverse outcome of this phenotype.28,36,37 Nevertheless, it should be pointed out that at least two retrospective, single-institution studies have suggested that BCL6 expression correlates with improved outcome in PCNSL.23,24 Possible explanations for these disparate findings are the retrospective nature of previous studies, the possibility that previous studies may not have considered the prognostic impact of high expression of the BCL6 oncogene, and the possibility that the prognostic relevance of individual biomarkers may be dictated by treatment-related variables including brain radiotherapy and rituximab. In support of this explanation are recent prospective data demonstrating that the addition of rituximab to CHOP chemotherapy selectively improved survival in BCL6-negative systemic diffuse large B-cell lymphoma.38 Although our study is the first to our knowledge to evaluate the significance of BCL6 expression in PCNSL in the setting of rituximab, assessment of clinical and prognostic variables in this trial will require validation given the small size and number of biopsy specimens available, with consequent limited power. In any case, the observation that BCL6 expression is predictive of adverse outcome in newly diagnosed PCNSL, if confirmed, suggests that this biomarker could prospectively be used in risk-adapted therapy and supports the rational application of BCL6 antagonists in the treatment of this disease.39
Given the encouraging results of CALGB 50202 in terms of toxicity, response, and survival achieved in the multicenter setting, the MT-R regimen is being evaluated in a successor intergroup, randomized phase II trial—CALGB 51101 (Alliance)—which compares dose-intensive EA chemotherapy with myeloablative chemotherapy using carmustine plus thiotepa followed by autologous stem-cell transplantation.40 Validation of BCL6 and other molecular prognostic biomarkers and detailed neurocognitive testing are key correlative goals of this first randomized trial in PCNSL in which neither arm involves WBRT.
Supplementary Material
Appendix
The following institutions participated in this study:
Christiana Care Health Services, Wilmington, DE, Stephen Grubbs, MD, supported by Grant No. CA45418; Dana-Farber Cancer Institute, Boston, MA, Harold J. Burstein, MD, PhD, supported by Grant No. CA32291; Dartmouth Medical School–Norris Cotton Cancer Center, Lebanon, NH, Konstantin Dragnev, MD, supported by Grant No. CA04326; Rhode Island Hospital, Providence, RI, William Sikov, MD, supported by Grant No. CA08025; Roswell Park Cancer Institute, Buffalo, NY, Ellis Levine, MD, supported by Grant No. CA59518; Southeast Cancer Control Consortium, Goldsboro, NC, James N. Atkins, MD, supported by Grant No. CA45808; Southern Nevada Cancer Research Foundation, Las Vegas, NV, John Ellerton, MD, supported by Grant No. CA35421; State University of New York Upstate Medical University, Syracuse, NY, Stephen L. Graziano, MD, supported by Grant No. CA21060; Ohio State University Medical Center, Columbus, OH, Clara D. Bloomfield, MD, supported by Grant No. CA77658; University of California at San Francisco, San Francisco, CA, Charles J. Ryan, MD, supported by Grant No. CA60138; University of Chicago, Chicago, IL, Hedy L. Kindler, MD, supported by Grant No. CA41287; University of Minnesota, Minneapolis, MN, Bruce A. Peterson, MD, supported by Grant No. CA16450; and University of Vermont, Burlington, VT, Steven M. Grunberg, MD, supported by Grant No. CA77406 from the National Cancer Institute.
Table A1.
AEs by Grade
| Hematologic AE | Grade 3 (severe) |
Grade 4 (life threatening) |
Grade 5 (lethal) |
Total No. | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| Hemoglobin | |||||||
| C2 | 3 | 12 | 0 | 0 | 0 | 0 | 26 |
| MTX | 5 | 11 | 1 | 2 | 0 | 0 | 44 |
| Leukocytes (total WBC) | |||||||
| C2 | 0 | 0 | 8 | 31 | 0 | 0 | 26 |
| MTX | 3 | 7 | 3 | 7 | 0 | 0 | 44 |
| Lymphopenia | |||||||
| C2 | 0 | 0 | 1 | 4 | 0 | 0 | 26 |
| MTX | 3 | 7 | 1 | 2 | 0 | 0 | 44 |
| Neutrophils/granulocytes (ANC/AGC) | |||||||
| C2 | 1 | 4 | 21 | 81 | 0 | 0 | 26 |
| MTX | 7 | 16 | 4 | 9 | 0 | 0 | 44 |
| Platelets | |||||||
| C2 | 1 | 4 | 21 | 81 | 0 | 0 | 26 |
| MTX | 4 | 9 | 1 | 2 | 0 | 0 | 44 |
| Maximum hematologic AE | |||||||
| C2 | 1 | 4 | 21 | 81 | 0 | 0 | 26 |
| MTX | 11 | 25 | 6 | 14 | 0 | 0 | 44 |
| Nonhematologic AE | Grade 3 (severe) |
Grade 4 (life threatening) |
Grade 5 (lethal) |
Total No. | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| Cardiac arrhythmia, other | |||||||
| C2 | 0 | 0 | 0 | 0 | 0 | 0 | 26 |
| MTX | 1 | 2 | 0 | 0 | 0 | 0 | 44 |
| Cardiac general | |||||||
| Cardiopulmonary arrest, cause unknown | |||||||
| C2 | 0 | 0 | 1 | 4 | 0 | 0 | 26 |
| MTX | 0 | 0 | 0 | 0 | 0 | 0 | 44 |
| Hypertension | |||||||
| C2 | 0 | 0 | 0 | 0 | 0 | 0 | 26 |
| MTX | 1 | 2 | 0 | 0 | 0 | 0 | 44 |
| Hypotension | |||||||
| C2 | 1 | 4 | 0 | 0 | 0 | 0 | 26 |
| MTX | 0 | 0 | 0 | 0 | 0 | 0 | 44 |
| Constitutional symptoms | |||||||
| Fatigue (asthenia, lethargy, malaise) | |||||||
| C2 | 0 | 0 | 0 | 0 | 0 | 0 | 26 |
| MTX | 1 | 2 | 1 | 2 | 0 | 0 | 44 |
| Insomnia | |||||||
| C2 | 0 | 0 | 0 | 0 | 0 | 0 | 26 |
| MTX | 1 | 2 | 0 | 0 | 0 | 0 | 44 |
| Dermatology/skin | |||||||
| Rash/desquamation | |||||||
| C2 | 1 | 4 | 0 | 0 | 0 | 0 | 26 |
| MTX | 0 | 0 | 0 | 0 | 0 | 0 | 44 |
| GI | |||||||
| Dehydration | |||||||
| C2 | 1 | 4 | 0 | 0 | 0 | 0 | 26 |
| MTX | 1 | 2 | 0 | 0 | 0 | 0 | 44 |
| Diarrhea | |||||||
| C2 | 2 | 8 | 0 | 0 | 0 | 0 | 26 |
| MTX | 2 | 5 | 0 | 0 | 0 | 0 | 44 |
| Heartburn/dyspepsia | |||||||
| C2 | 0 | 0 | 0 | 0 | 0 | 0 | 26 |
| MTX | 1 | 2 | 0 | 0 | 0 | 0 | 44 |
| Mucositis/stomatitis (functional) | |||||||
| C2 | 2 | 8 | 0 | 0 | 0 | 0 | 26 |
| MTX | 0 | 0 | 0 | 0 | 0 | 0 | 44 |
| Nausea | |||||||
| C2 | 1 | 4 | 0 | 0 | 0 | 0 | 26 |
| MTX | 2 | 5 | 0 | 0 | 0 | 0 | 44 |
| Vomiting | |||||||
| C2 | 0 | 0 | 0 | 0 | 0 | 0 | 26 |
| MTX | 1 | 2 | 0 | 0 | 0 | 0 | 44 |
| Hemorrhage/bleeding | |||||||
| Hemorrhage CNS | |||||||
| C2 | 0 | 0 | 1 | 4 | 0 | 0 | 26 |
| MTX | 0 | 0 | 0 | 0 | 0 | 0 | 44 |
| Hemorrhage, GI | |||||||
| C2 | 1 | 4 | 0 | 0 | 0 | 0 | 26 |
| MTX | 0 | 0 | 0 | 0 | 0 | 0 | 44 |
| Petechiae/purpura | |||||||
| C2 | 1 | 4 | 0 | 0 | 0 | 0 | 26 |
| MTX | 0 | 0 | 0 | 0 | 0 | 0 | 44 |
| Infection | |||||||
| Febrile neutropenia | |||||||
| C2 | 3 | 12 | 1 | 4 | 0 | 0 | 26 |
| MTX | 0 | 0 | 0 | 0 | 0 | 0 | 44 |
| Infection (documented clinically) | |||||||
| C2 | 5 | 19 | 0 | 0 | 0 | 0 | 26 |
| MTX | 3 | 7 | 0 | 0 | 0 | 0 | 44 |
| Infection, other | |||||||
| C2 | 1 | 4 | 0 | 0 | 1 | 4 | 26 |
| MTX | 0 | 0 | 0 | 0 | 0 | 0 | 44 |
| Infection, normal ANC | |||||||
| C2 | 0 | 0 | 0 | 0 | 0 | 0 | 26 |
| MTX | 1 | 2 | 0 | 0 | 0 | 0 | 44 |
| Infection, unknown ANC | |||||||
| C2 | 0 | 0 | 0 | 0 | 0 | 0 | 26 |
| MTX | 1 | 2 | 0 | 0 | 0 | 0 | 44 |
| Lymphatics | |||||||
| Edema, limb | |||||||
| C2 | 0 | 0 | 0 | 0 | 0 | 0 | 26 |
| MTX | 1 | 2 | 0 | 0 | 0 | 0 | 44 |
| Metabolic/laboratory | |||||||
| ALT, SGPT | |||||||
| C2 | 0 | 0 | 0 | 0 | 0 | 0 | 26 |
| MTX | 7 | 16 | 3 | 7 | 0 | 0 | 44 |
| AST, SGOT | |||||||
| C2 | 0 | 0 | 0 | 0 | 0 | 0 | 26 |
| MTX | 8 | 18 | 2 | 5 | 0 | 0 | 44 |
| Low serum albumin | |||||||
| C2 | 0 | 0 | 0 | 0 | 0 | 0 | 26 |
| MTX | 2 | 5 | 0 | 0 | 0 | 0 | 44 |
| Alkalosis (metabolic or respiratory) | |||||||
| C2 | 0 | 0 | 0 | 0 | 0 | 0 | 26 |
| MTX | 1 | 2 | 0 | 0 | 0 | 0 | 44 |
| Bilirubin | |||||||
| C2 | 1 | 4 | 0 | 0 | 0 | 0 | 26 |
| MTX | 1 | 2 | 0 | 0 | 0 | 0 | 44 |
| Low serum calcium | |||||||
| C2 | 0 | 0 | 0 | 0 | 0 | 0 | 26 |
| MTX | 5 | 11 | 1 | 2 | 0 | 0 | 44 |
| GGT | |||||||
| C2 | 0 | 0 | 0 | 0 | 0 | 0 | 26 |
| MTX | 2 | 5 | 0 | 0 | 0 | 0 | 44 |
| High serum glucose | |||||||
| C2 | 0 | 0 | 1 | 4 | 0 | 0 | 26 |
| MTX | 6 | 14 | 1 | 2 | 0 | 0 | 44 |
| Metabolic/laboratory, other | |||||||
| C2 | 0 | 0 | 0 | 0 | 0 | 0 | 26 |
| MTX | 0 | 0 | 1 | 2 | 0 | 0 | 44 |
| Low serum phosphate | |||||||
| C2 | 0 | 0 | 0 | 0 | 0 | 0 | 26 |
| MTX | 3 | 7 | 0 | 0 | 0 | 0 | 44 |
| High serum potassium | |||||||
| C2 | 1 | 4 | 0 | 0 | 0 | 0 | 26 |
| MTX | 3 | 7 | 0 | 0 | 0 | 0 | 44 |
| Low serum potassium | |||||||
| C2 | 3 | 12 | 0 | 0 | 0 | 0 | 26 |
| MTX | 11 | 25 | 1 | 2 | 0 | 0 | 44 |
| High serum sodium | |||||||
| C2 | 1 | 4 | 0 | 0 | 0 | 0 | 26 |
| MTX | 0 | 0 | 0 | 0 | 0 | 0 | 44 |
| Low serum sodium | |||||||
| C2 | 0 | 0 | 0 | 0 | 0 | 0 | 26 |
| MTX | 3 | 7 | 0 | 0 | 0 | 0 | 44 |
| High serum uric acid | |||||||
| C2 | 0 | 0 | 0 | 0 | 0 | 0 | 26 |
| MTX | 1 | 2 | 0 | 0 | 0 | 0 | 44 |
| Musculoskeletal/soft tissue | |||||||
| Muscle weakness | |||||||
| C2 | 0 | 0 | 0 | 0 | 0 | 0 | 26 |
| MTX | 3 | 7 | 0 | 0 | 0 | 0 | 44 |
| Neurology | |||||||
| Ataxia (incoordination) | |||||||
| C2 | 0 | 0 | 0 | 0 | 0 | 0 | 26 |
| MTX | 2 | 5 | 0 | 0 | 0 | 0 | 44 |
| Confusion | |||||||
| C2 | 0 | 0 | 0 | 0 | 0 | 0 | 26 |
| MTX | 1 | 2 | 1 | 2 | 0 | 0 | 44 |
| Dizziness | |||||||
| C2 | 0 | 0 | 0 | 0 | 0 | 0 | 26 |
| MTX | 1 | 2 | 0 | 0 | 0 | 0 | 44 |
| Mood alteration | |||||||
| C2 | 0 | 0 | 0 | 0 | 0 | 0 | 26 |
| MTX | 2 | 5 | 0 | 0 | 0 | 0 | 44 |
| Neurology, other | |||||||
| C2 | 0 | 0 | 1 | 4 | 0 | 0 | 26 |
| MTX | 0 | 0 | 0 | 0 | 0 | 0 | 44 |
| Seizure | |||||||
| C2 | 1 | 4 | 0 | 0 | 0 | 0 | 26 |
| MTX | 0 | 0 | 0 | 0 | 0 | 0 | 44 |
| Syncope (fainting) | |||||||
| C2 | 0 | 0 | 0 | 0 | 0 | 0 | 26 |
| MTX | 1 | 2 | 0 | 0 | 0 | 0 | 44 |
| Pain | |||||||
| C2 | 1 | 4 | 0 | 0 | 0 | 0 | 26 |
| MTX | 3 | 7 | 0 | 0 | 0 | 0 | 44 |
| Pulmonary/upper respiratory | |||||||
| Hypoxia | |||||||
| C2 | 0 | 0 | 0 | 0 | 0 | 0 | 26 |
| MTX | 1 | 2 | 0 | 0 | 0 | 0 | 44 |
| Pneumonitis/pulmonary infiltrates | |||||||
| C2 | 0 | 0 | 0 | 0 | 0 | 0 | 26 |
| MTX | 3 | 7 | 0 | 0 | 0 | 0 | 44 |
| Renal/genitourinary | |||||||
| Renal failure | |||||||
| C2 | 0 | 0 | 0 | 0 | 0 | 0 | 26 |
| MTX | 1 | 2 | 0 | 0 | 0 | 0 | 44 |
| Vascular | |||||||
| Thrombosis/thrombus/embolism | |||||||
| C2 | 0 | 0 | 0 | 0 | 0 | 0 | 26 |
| MTX | 2 | 5 | 0 | 0 | 0 | 0 | 44 |
| Maximum nonhematologic AE | |||||||
| C2 | 10 | 38 | 1 | 4 | 1 | 4 | 26 |
| MTX | 26 | 59 | 8 | 18 | 0 | 0 | 44 |
| Maximum Overall AE | Grade 3 (severe) |
Grade 4 (life threatening) |
Grade 5 (lethal) |
Total No. | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| C2 | 1 | 4 | 20 | 77 | 1 | 4 | 26 |
| MTX | 24 | 55 | 12 | 27 | 0 | 0 | 44 |
Abbreviations: AE, adverse event; AGC, absolute granulocyte count; ANC, absolute neutrophil count; C2, etoposide plus cytarabine consolidation; GGT, γ-glutamyltransferase; MTX, methotrexate; SGOT, serum glutamic oxaloacetic transaminase; SGPT, serum glutamic pyruvate transaminase.
Footnotes
Processed as a Rapid Communication manuscript. See accompanying editorial on page 3051; listen to the podcast by Dr Batchelor at www.jco.org/podcasts
Written on behalf of the Alliance for Clinical Trials in Oncology.
Supported by National Cancer Institute Grants No. RO1CA1398301 and CA60138 (J.L.R., L.D.K.), CA21115 (E.D.H., M.O.N.), CA33601 (J.L.J., S.-H.J.), CA77406 (B.G.), CA77597 (B.D.C.), CA31946 to the Alliance for Clinical Trials in Oncology, and CA33601 to the Alliance Statistics and Data Center. J.L.R. is also supported by the Leukemia and Lymphoma Society.
The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT00098774.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: James L. Rubenstein, Eric D. Hsi
Administrative support: Sin-Ho Jung, Bruce D. Cheson
Provision of study materials or patients: James L. Rubenstein, Barbara Grant
Collection and assembly of data: James L. Rubenstein, Eric D. Hsi, Sin-Ho Jung, Barbara Grant, Bruce D. Cheson
Data analysis and interpretation: James L. Rubenstein, Eric D. Hsi, Jeffrey L. Johnson, Sin-Ho Jung, Megan O. Nakashima, Bruce D. Cheson, Lawrence D. Kaplan
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Batchelor T, Loeffler JS. Primary CNS lymphoma. J Clin Oncol. 2006;24:1281–1288. doi: 10.1200/JCO.2005.04.8819. [DOI] [PubMed] [Google Scholar]
- 2.Batchelor T, Carson K, O'Neill A, et al. Treatment of primary CNS lymphoma with methotrexate and deferred radiotherapy: A report of NABTT 96-07. J Clin Oncol. 2003;21:1044–1049. doi: 10.1200/JCO.2003.03.036. [DOI] [PubMed] [Google Scholar]
- 3.Herrlinger U, Schabet M, Brugger W, et al. German Cancer Society Neuro-Oncology Working Group NOA-03 multicenter trial of single-agent high-dose methotrexate for primary central nervous system lymphoma. Ann Neurol. 2002;51:247–252. doi: 10.1002/ana.10102. [DOI] [PubMed] [Google Scholar]
- 4.DeAngelis LM, Seiferheld W, Schold SC, et al. Combination chemotherapy and radiotherapy for primary central nervous system lymphoma: Radiation Therapy Oncology Group Study 93-10. J Clin Oncol. 2002;20:4643–4648. doi: 10.1200/JCO.2002.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Abrey LE, DeAngelis LM, Yahalom J. Long-term survival in primary CNS lymphoma. J Clin Oncol. 1998;16:859–863. doi: 10.1200/JCO.1998.16.3.859. [DOI] [PubMed] [Google Scholar]
- 6.Sun A, Bae K, Gore EM, et al. Phase III trial of prophylactic cranial irradiation compared with observation in patients with locally advanced non-small-cell lung cancer: Neurocognitive and quality-of-life analysis. J Clin Oncol. 2011;29:279–286. doi: 10.1200/JCO.2010.29.6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ott RJ, Brada M, Flower MA, et al. Measurements of blood-brain barrier permeability in patients undergoing radiotherapy and chemotherapy for primary cerebral lymphoma. Eur J Cancer. 1991;27:1356–1361. doi: 10.1016/0277-5379(91)90009-3. [DOI] [PubMed] [Google Scholar]
- 8.Wong ET, Tishler R, Barron L, et al. Immunochemotherapy with rituximab and temozolomide for central nervous system lymphomas. Cancer. 2004;101:139–145. doi: 10.1002/cncr.20339. [DOI] [PubMed] [Google Scholar]
- 9.Reni M, Ferreri AJ. Therapeutic management of refractory or relapsed primary central nervous system lymphomas. Ann Hematol. 2001;80(suppl 3):B113–B117. doi: 10.1007/pl00022772. [DOI] [PubMed] [Google Scholar]
- 10.Reni M, Zaja F, Mason W, et al. Temozolomide as salvage treatment in primary brain lymphomas. Br J Cancer. 2007;96:864–867. doi: 10.1038/sj.bjc.6603660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osoba D, Brada M, Yung WK, et al. Health-related quality of life in patients with anaplastic astrocytoma during treatment with temozolomide. Eur J Cancer. 2000;36:1788–1795. doi: 10.1016/s0959-8049(00)00165-9. [DOI] [PubMed] [Google Scholar]
- 12.Osoba D, Brada M, Yung WK, et al. Health-related quality of life in patients treated with temozolomide versus procarbazine for recurrent glioblastoma multiforme. J Clin Oncol. 2000;18:1481–1491. doi: 10.1200/JCO.2000.18.7.1481. [DOI] [PubMed] [Google Scholar]
- 13.Soussain C, Suzan F, Hoang-Xuan K, et al. Results of intensive chemotherapy followed by hematopoietic stem-cell rescue in 22 patients with refractory or recurrent primary CNS lymphoma or intraocular lymphoma. J Clin Oncol. 2001;19:742–749. doi: 10.1200/JCO.2001.19.3.742. [DOI] [PubMed] [Google Scholar]
- 14.Ferreri AJ, Reni M, Foppoli M, et al. High-dose cytarabine plus high-dose methotrexate versus high-dose methotrexate alone in patients with primary CNS lymphoma: A randomised phase 2 trial. Lancet. 2009;374:1512–1520. doi: 10.1016/S0140-6736(09)61416-1. [DOI] [PubMed] [Google Scholar]
- 15.Relling MV, Mahmoud HH, Pui CH, et al. Etoposide achieves potentially cytotoxic concentrations in CSF of children with acute lymphoblastic leukemia. J Clin Oncol. 1996;14:399–404. doi: 10.1200/JCO.1996.14.2.399. [DOI] [PubMed] [Google Scholar]
- 16.Boehme V, Zeynalova S, Kloess M, et al. Incidence and risk factors of central nervous system recurrence in aggressive lymphoma: A survey of 1693 patients treated in protocols of the German High-Grade Non-Hodgkin's Lymphoma Study Group (DSHNHL) Ann Oncol. 2007;18:149–157. doi: 10.1093/annonc/mdl327. [DOI] [PubMed] [Google Scholar]
- 17.Damon LE, Johnson JL, Niedzwiecki D, et al. Immunochemotherapy and autologous stem-cell transplantation for untreated patients with mantle-cell lymphoma: CALGB 59909. J Clin Oncol. 2009;27:6101–6108. doi: 10.1200/JCO.2009.22.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abrey LE, Batchelor TT, Ferreri AJ, et al. Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol. 2005;23:5034–5043. doi: 10.1200/JCO.2005.13.524. [DOI] [PubMed] [Google Scholar]
- 19.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 20.Ferreri AJ, Blay JY, Reni M, et al. Prognostic scoring system for primary CNS lymphomas: The International Extranodal Lymphoma Study Group experience. J Clin Oncol. 2003;21:266–272. doi: 10.1200/JCO.2003.09.139. [DOI] [PubMed] [Google Scholar]
- 21.Abrey LE, Ben-Porat L, Panageas KS, et al. Primary central nervous system lymphoma: The Memorial Sloan-Kettering Cancer Center prognostic model. J Clin Oncol. 2006;24:5711–5715. doi: 10.1200/JCO.2006.08.2941. [DOI] [PubMed] [Google Scholar]
- 22.Thiel E, Korfel A, Martus P, et al. High-dose methotrexate with or without whole brain radiotherapy for primary CNS lymphoma (G-PCNSL-SG-1): A phase 3, randomised, non-inferiority trial. Lancet Oncol. 2010;11:1036–1047. doi: 10.1016/S1470-2045(10)70229-1. [DOI] [PubMed] [Google Scholar]
- 23.Braaten KM, Betensky RA, de Leval L, et al. BCL-6 expression predicts improved survival in patients with primary central nervous system lymphoma. Clin Cancer Res. 2003;9:1063–1069. [PubMed] [Google Scholar]
- 24.Levy O, Deangelis LM, Filippa DA, et al. Bcl-6 predicts improved prognosis in primary central nervous system lymphoma. Cancer. 2008;112:151–156. doi: 10.1002/cncr.23149. [DOI] [PubMed] [Google Scholar]
- 25.Chang CC, Kampalath B, Schultz C, et al. Expression of p53, c-Myc, or Bcl-6 suggests a poor prognosis in primary central nervous system diffuse large B-cell lymphoma among immunocompetent individuals. Arch Pathol Lab Med. 2003;127:208–212. doi: 10.5858/2003-127-208-EOPMOB. [DOI] [PubMed] [Google Scholar]
- 26.Momota H, Narita Y, Maeshima AM, et al. Prognostic value of immunohistochemical profile and response to high-dose methotrexate therapy in primary CNS lymphoma. J Neurooncol. 2010;98:341–348. doi: 10.1007/s11060-009-0078-z. [DOI] [PubMed] [Google Scholar]
- 27.Kluk MJ, Chapuy B, Sinha P, et al. Immunohistochemical detection of MYC-driven diffuse large B-cell lymphomas. PLoS One. 2012;7:e33813. doi: 10.1371/journal.pone.0033813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Camilleri-Broët S, Crinière E, Broët P, et al. A uniform activated B-cell-like immunophenotype might explain the poor prognosis of primary central nervous system lymphomas: Analysis of 83 cases. Blood. 2006;107:190–196. doi: 10.1182/blood-2005-03-1024. [DOI] [PubMed] [Google Scholar]
- 29.Hoang-Xuan K, Taillandier L, Chinot O, et al. Chemotherapy alone as initial treatment for primary CNS lymphoma in patients older than 60 years: A multicenter phase II study (26952) of the European Organisation for Research and Treatment of Cancer Brain Tumor Group. J Clin Oncol. 2003;21:2726–2731. doi: 10.1200/JCO.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 30.Angelov L, Doolittle ND, Kraemer DF, et al. Blood-brain barrier disruption and intra-arterial methotrexate-based therapy for newly diagnosed primary CNS lymphoma: A multi-institutional experience. J Clin Oncol. 2009;27:3503–3509. doi: 10.1200/JCO.2008.19.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shah GD, Yahalom J, Correa DD, et al. Combined immunochemotherapy with reduced whole-brain radiotherapy for newly diagnosed primary CNS lymphoma. J Clin Oncol. 2007;25:4730–4735. doi: 10.1200/JCO.2007.12.5062. [DOI] [PubMed] [Google Scholar]
- 32.Wieduwilt MJ, Valles F, Issa S, et al. Immunochemotherapy with intensive consolidation for primary CNS lymphoma: A pilot study and prognostic assessment by diffusion-weighted MRI. Clin Cancer Res. 2012;18:1146–1155. doi: 10.1158/1078-0432.CCR-11-0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haldorsen IS, Espeland A, Larsen JL, et al. Diagnostic delay in primary central nervous system lymphoma. Acta Oncol. 2005;44:728–734. doi: 10.1080/02841860500256272. [DOI] [PubMed] [Google Scholar]
- 34.Hormigo A, Abrey L, Heinemann MH, et al. Ocular presentation of primary central nervous system lymphoma: Diagnosis and treatment. Br J Haematol. 2004;126:202–208. doi: 10.1111/j.1365-2141.2004.05028.x. [DOI] [PubMed] [Google Scholar]
- 35.Rubenstein JL, Fridlyand J, Shen A, et al. Gene expression and angiotropism in primary CNS lymphoma. Blood. 2006;107:3716–3723. doi: 10.1182/blood-2005-03-0897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Montesinos-Rongen M, Küppers R, Schlüter D, et al. Primary central nervous system lymphomas are derived from germinal-center B cells and show a preferential usage of the V4-34 gene segment. Am J Pathol. 1999;155:2077–2086. doi: 10.1016/S0002-9440(10)65526-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thompsett AR, Ellison DW, Stevenson FK, et al. V(H) gene sequences from primary central nervous system lymphomas indicate derivation from highly mutated germinal center B cells with ongoing mutational activity. Blood. 1999;94:1738–1746. [PubMed] [Google Scholar]
- 38.Winter JN, Weller EA, Horning SJ, et al. Prognostic significance of Bcl-6 protein expression in DLBCL treated with CHOP or R-CHOP: A prospective correlative study. Blood. 2006;107:4207–4213. doi: 10.1182/blood-2005-10-4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cerchietti LC, Ghetu AF, Zhu X, et al. A small-molecule inhibitor of BCL6 kills DLBCL cells in vitro and in vivo. Cancer Cell. 2010;17:400–411. doi: 10.1016/j.ccr.2009.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Illerhaus G, Müller F, Feuerhake F, et al. High-dose chemotherapy and autologous stem-cell transplantation without consolidating radiotherapy as first-line treatment for primary lymphoma of the central nervous system. Haematologica. 2008;93:147–148. doi: 10.3324/haematol.11771. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.