Abstract
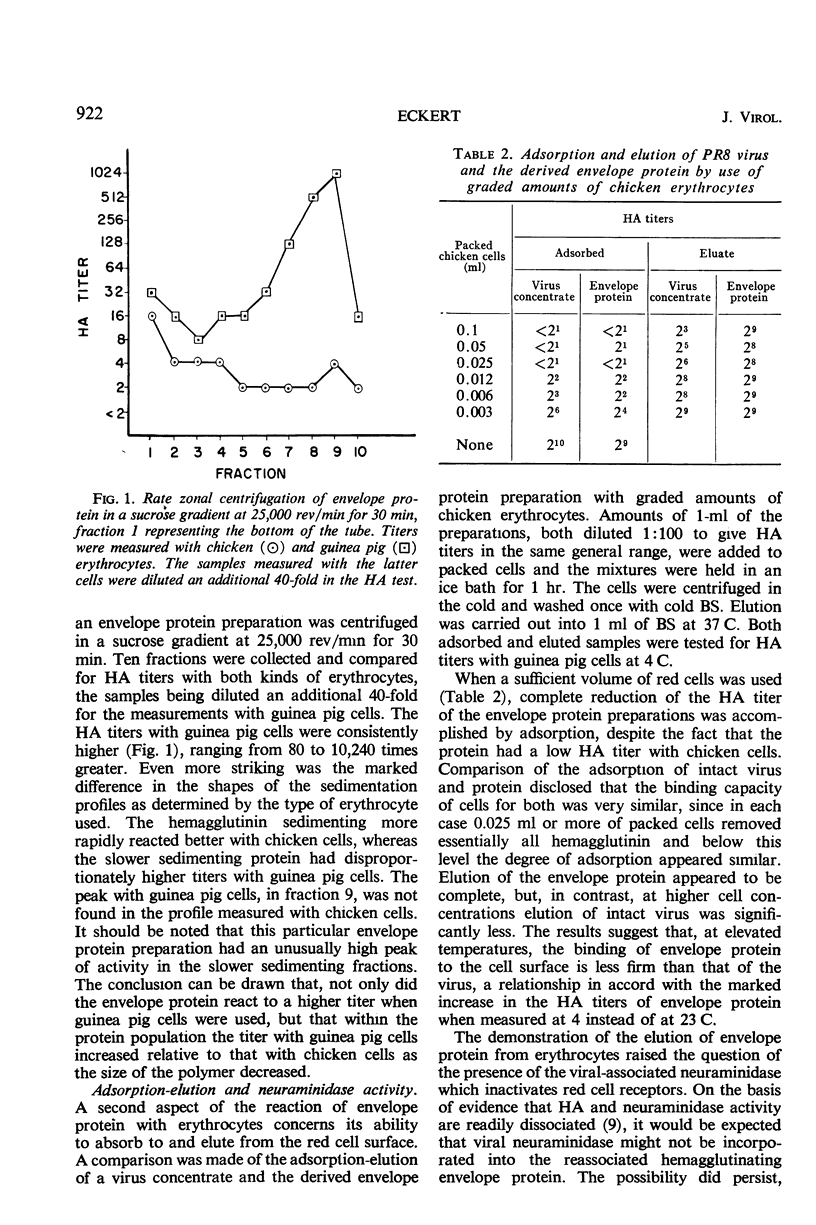
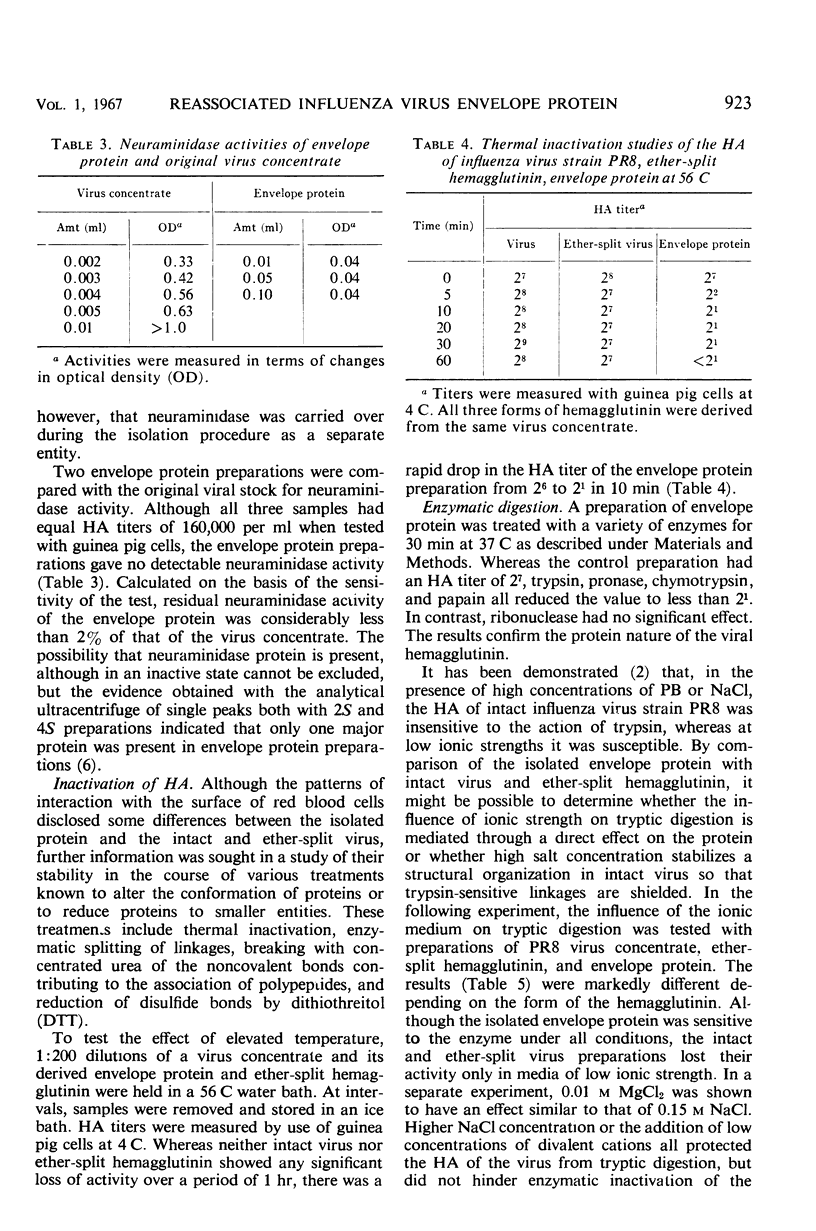
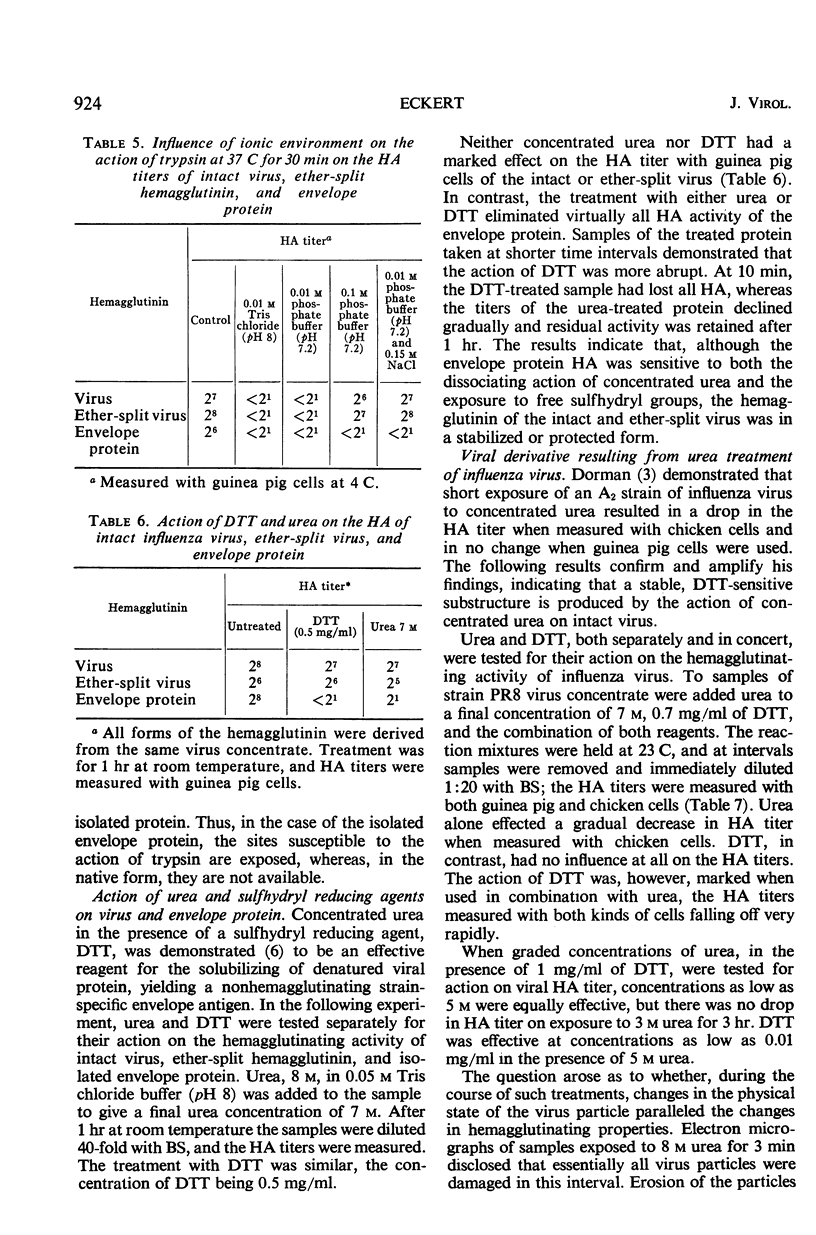
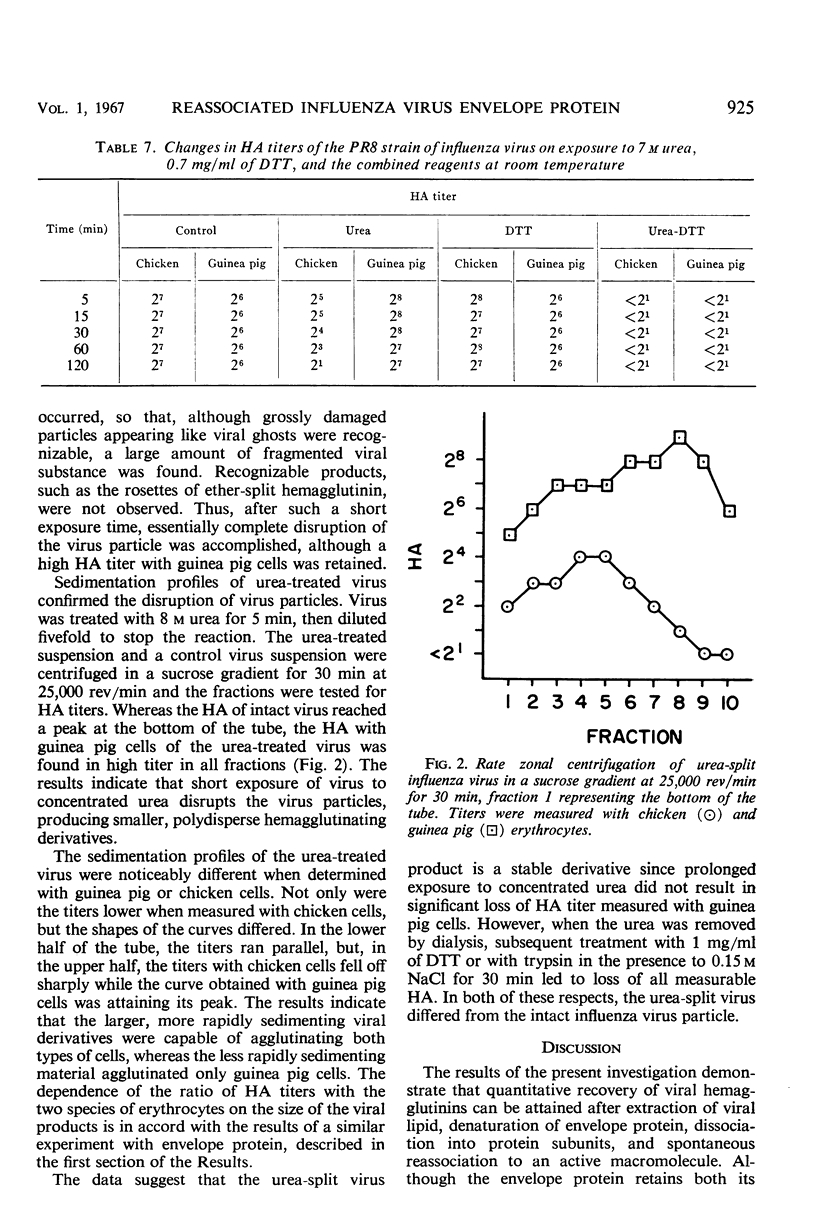
The hemagglutinating properties of influenza virus envelope protein, prepared by reassociation of polypeptide subunits, have been defined and compared with those of virus and ether-split hemagglutinin. In general, the characteristics of the intact and ether-split virus were found to be similar, whereas those of the envelope protein were distinctly different. The use of chicken, pigeon, and guinea pig erythrocytes both at 23 and 4 C disclosed that the hemagglutinating titers of envelope protein preparations were particularly dependent on the system employed. Under optimal conditions, with guinea pig cells at 4 C, the titers of envelope protein preparations were equivalent to those of the original virus concentrates. The hemagglutinating activity of envelope protein was particularly sensitive to elevated temperature, concentrated urea, sulfhydryl-reducing reagents, and tryptic digestion at high salt concentrations. In all these respects, the intact virus was more resistant than the envelope protein. Interpretation of the data indicates that the hemagglutinin is stabilized when associated with the lipid micelle at the surface of the virus.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHOPPIN P. W., STOECKENIUS W. INTERACTIONS OF ETHER-DISRUPTED INFLUENZA A2 VIRUS WITH ERYTHROCYTES, INHIBITORS, AND ANTIBODIES. Virology. 1964 Apr;22:482–492. doi: 10.1016/0042-6822(64)90069-8. [DOI] [PubMed] [Google Scholar]
- CLEELAND R., SUGG J. Y. SEROLOGIC AND ANTIGENIC PROPERTIES OF TYPE A INFLUENZA VIRUS AFTER TRYPSIN TREATMENT. J Immunol. 1964 Sep;93:414–419. [PubMed] [Google Scholar]
- DORMAN D. C. EFFECT OF UREA ON A2 INFLUENZA VIRUS. Nature. 1964 Aug 15;203:789–791. doi: 10.1038/203789b0. [DOI] [PubMed] [Google Scholar]
- Eckert E. A. Characterization of a low molecular weight antigenic protein from the envelope of influenza virus. J Bacteriol. 1966 Nov;92(5):1430–1434. doi: 10.1128/jb.92.5.1430-1434.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert E. A. Envelope protein(s) derived from influenza virus. J Bacteriol. 1966 May;91(5):1907–1910. doi: 10.1128/jb.91.5.1907-1910.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOYLE L., HORNE R. W., WATERSON A. P. The structure and composition of the myxoviruses. II. Components released from the influenza virus particle by ether. Virology. 1961 Apr;13:448–459. doi: 10.1016/0042-6822(61)90276-8. [DOI] [PubMed] [Google Scholar]
- NOLL H., AOYAGI T., ORLANDO J. The structural relationship of sialidase to the influenza virus surface. Virology. 1962 Sep;18:154–157. doi: 10.1016/0042-6822(62)90193-9. [DOI] [PubMed] [Google Scholar]
- Seto JT BRZENIEK R., Rott R. Isolation of a low molecular weight sialidase (neuraminidase) from influenza virus. Biochim Biophys Acta. 1966 Feb 14;113(2):402–404. doi: 10.1016/s0926-6593(66)80081-4. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]



