Abstract
Purpose
To retrospectively evaluate the pattern of recurrence and outcome of node-negative breast cancer (BC) according to major subtypes.
Patients and Methods
In all, 1,951 patients with node-negative, early-stage BC randomly assigned in International Breast Cancer Study Group Trials VIII and IX with centrally reviewed pathology data were included. BC subtypes were defined as triple negative (TN; n = 310), human epidermal growth factor receptor 2 (HER2) positive (n = 369), and hormone receptor positive with high (luminal B–like [LB-like]; n = 763) or low (luminal A–like [LA-like]; n = 509) proliferative activity by Ki-67 labeling index. BC-free interval (BCFI) events were invasive BC recurrence in local, contralateral breast, nodal, bone, or visceral sites. Time to first site–specific recurrence was evaluated by using cumulative incidence and competing risks regression analysis.
Results
Median follow-up was 12.5 years. The 10-year BCFI was higher for patients with LA-like (86%) BC compared with LB-like (76%), HER2 (73%), and TN (71%; P < .001) BC. TN and HER2 cohorts had higher hazard of BCFI event in the first 4 years after diagnosis (pre-trastuzumab). LB-like cohorts had a continuously higher hazard of BCFI event over time compared with LA-like cohorts. Ten-year overall survival was higher for LA-like (89%) compared with LB-like (83%), HER2 (77%), and TN (75%; P < .001) BC. LB-like subtypes had higher rates of bone as first recurrence site than other subtypes (P = .005). Visceral recurrence as first site was lower for the LA-like subgroup, with similar incidence among the other subgroups when treated with chemotherapy (P = .003).
Conclusion
BC subtypes have different distant recurrence patterns over time. Defining different patterns of BC recurrence can improve BC care through surveillance guidelines and can guide the design of clinical studies.
INTRODUCTION
Approximately one-third of patients with early-stage breast cancer (BC) experience disease recurrence after initial diagnosis.1 BC site-specific recurrence patterns are influenced by classic prognostic factors, such as nodal status, histologic grade, and the status of hormone receptors and human epidermal growth factor receptor 2 (HER2) expression.2–5 In addition, BC recurrence varies considerably over time and is influenced by adjuvant therapeutic modalities. Despite recognized variability, most recurrences occur within the first 5 years of diagnosis.3 However, in the subset of hormone receptor–positive BC, recurrences continue to occur even after 10 years from diagnosis, whereas in patients with hormone receptor–negative disease, recurrences occur earlier.1,6–8
The classification of BC has been evolving and now encompasses a group of heterogeneous genomically defined disease subsets.9,10 Genomic studies have defined four-major intrinsic BC subtypes: basal-like, which is mainly represented by triple-negative BC (estrogen receptor (ER) negative, progesterone receptor (PgR) negative, and HER2 negative); luminal A, represented by hormone receptor–positive tumors with low proliferative activity; luminal B, also mainly represented by ER-positive tumors but with high proliferative activity; and HER2-positive, represented by tumors with high expression of the ERBB2 gene.
In light of the evolving classification of BC, limited information is available for each BC subtype with regard to patterns of disease recurrence with long-term clinical follow-up. This analysis describes the BC recurrence pattern according to subtypes defined by immunohistochemical (IHC) surrogates. A total of 1,951 patients with BC carefully followed since 1988 were included.
PATIENTS AND METHODS
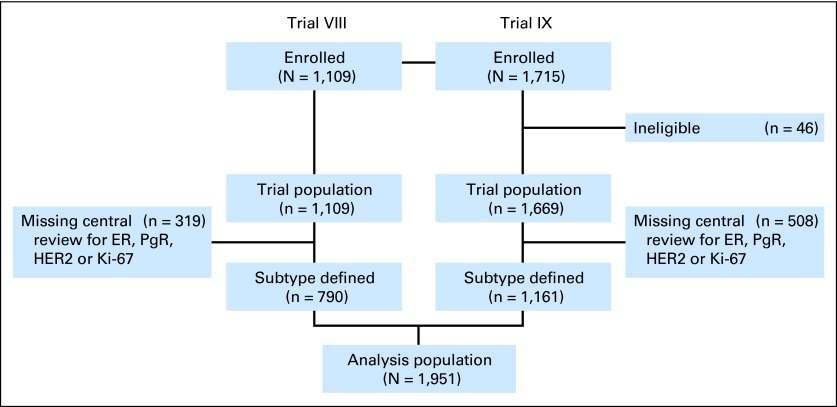
We analyzed data from 1,951 patients with early-stage BC enrolled onto International Breast Cancer Study Group (IBCSG) trials VIII and IX between 1988 and 199911–13 (Fig 1). IBCSG trial VIII randomly assigned 1,109 assessable pre- or perimenopausal women with lymph node–negative BC. There were 1,063 patients randomly assigned to sequential treatment with six cycles of classical (oral) cyclophosphamide, methotrexate, and fluorouracil (CMF) followed by 18 months of goserelin, six courses of CMF alone, or 24 months of goserelin alone.12 A no-treatment arm was discontinued (n = 46). IBCSG trial IX randomly assigned 1,669 eligible and assessable postmenopausal women with lymph node–negative BC to sequential treatment with three cycles of classical (oral) CMF followed by tamoxifen for 57 months or tamoxifen alone for 5 years.11 No patients in these trials received adjuvant trastuzumab, because it had not yet been approved.
Fig 1.
CONSORT diagram showing the numbers of patients enrolled onto International Breast Cancer Study Group Trials VIII and IX, and the derivations of the 1,951 patients in the analysis population. ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; PgR, progesterone receptor.
The trial patients were followed every 3 months during years 1 to 2, every 6 months during years 3 to 5, and annually thereafter for survival and disease status, including sites of first recurrence and postrecurrence survival. Informed consent was required according to the criteria established within participating countries. The trial protocols were reviewed and approved by local institutional review boards, also in accordance with local requirements.
Pathology Assessment
Central pathology retrospective evaluation included ER, PgR, HER2, and Ki-67 labeling index (LI). A total of 827 patients (319 [29%] from trial VIII and 508 [30%] from trial IX) were missing central pathology evaluation of one or more of the four tumor features and hence were excluded (Fig 1). Baseline characteristics of excluded patients were comparable to those included except that more of the patients who were included had tumors larger than 2 cm and of a higher grade compared with patients who were excluded (data not shown).
Expression of ER, PgR, and Ki-67 LI in the primary tumors was determined by IHC.14,15 ER- or PgR-negative status was defined as less than 1% immunoreactive cells, in accordance with recent guidelines.16 Ki-67 LI was reported as the percentage of cells that showed definite nuclear immunoreactivity with the antibody MIB-1 among 2,000 invasive neoplastic cells in randomly selected high-power fields (×400) at the periphery of the tumor.15 HER2-positive tumors were defined as IHC3+ or IHC2+ and fluorescent in situ hybridization (FISH) positive, and HER2-negative tumors were defined as IHC0 or IHC1+ or IHC 2+ and FISH negative. Additional information related to pathology assessment is provided in the Data Supplement.
Subtype Definitions
Tumors were classified into four subtypes by using IHC surrogates. A patient's tumor was considered triple negative (TN) if ER and PgR were both absent and HER2 status was negative; HER2-positive if HER2 was positive, subdivided into hormone receptor–positive (ER- and/or PgR-positive) and hormone receptor–negative (ER- and PgR-negative); luminal A–like (LA-like) if ER- and/or PgR-positive, HER2-negative, and Ki-67 less than 14%; or luminal B–like (LB-like) if ER- and/or PgR-positive, HER2-negative, and Ki-67 ≥ 14%. The Ki-67 LI cutoff of 14% was selected to best recapitulate LA and LB intrinsic subtypes.17
BC-Free Interval and Sites of Recurrence
BC-free interval (BCFI) was defined as the length of time from the date of random assignment to any invasive BC recurrence (including ipsilateral or contralateral breast recurrence) and was censored at date of last follow-up or at date of death without recurrence. The first site of BC recurrence was defined hierarchically according to the worst site as follows: local (confined to ipsilateral breast or chest wall and including mastectomy scars), contralateral, nodal (including ipsilateral axillary, supraclavicular, and internal mammary lymph node metastases and distant soft tissue nodes), bone, or visceral (including bone marrow, lung, liver, and other organs). Overall survival (OS) was defined as time from random assignment to death as a result of any cause.
Statistical Methods
The χ2 test and Fisher's exact test18 were used to evaluate associations between BC subtypes and menopausal status, tumor size, tumor grade, peritumoral vascular invasion, primary local treatment (surgery and radiotherapy), and random assignment to a chemotherapy-containing regimen. The Kruskal-Wallis test was used to evaluate associations with age.19 Kaplan-Meier estimates were used to estimate the event-time distributions, and log-rank test was used to compare the differences among the BC subtypes in terms of BCFI and OS. The kernel-smoothed hazard functions of BCFI were estimated on the basis of the method described by Allison.20
Time to the first BC recurrence in a specific site was evaluated by using cumulative incidence analysis (Gray's test) and competing risks regression analysis to estimate subdistribution hazard ratios (HRs) and 95% CIs,21 treating recurrences in other sites and death without recurrence as competing events. In the competing risks regression analyses, the differences among the BC subtypes were examined adjusting for potential risk factors, and the P values were calculated on the basis of a Wald test. Wald tests were also used to determine the interaction effect of treatment group and BC subtypes. If no significant interaction of treatment and subtype was observed for a specific site, the difference of time to first recurrence in that site was examined among BC subtypes for all patients. If significant interactions of treatment and subtype were observed for a specific site, the difference of time to first recurrence in that site was examined among BC subtypes by each treatment group separately.
RESULTS
A total of 1,951 patients with node-negative early-stage BC at a median follow-up of 12.5 years were analyzed and were classified as having LA-like (26%), LB-like (39%), HER2 (19%), or TN (16%) BC. Clinical and pathological characteristics according to BC subtypes are shown in Table 1. Tumors larger than 2 cm and grade 3 tumors were more commonly observed among TN patients, followed by those with HER2, LB-like, or LA-like disease. Peritumoral vascular invasion occurred more often in HER2 tumors followed by TN, LB-like, or LA-like tumors. Breast-conserving surgery with radiotherapy was more common in LA-like patients, followed by LB-like, HER2, and TN patients. In total, 1,096 patients were assigned to receive chemotherapy and 855 were not.
Table 1.
Characteristics of the Patients, Tumors, and Primary Treatments of 1,951 Patients With Lymph Node–Negative BC According to BC Subtypes Defined by Using IHC Surrogates
| Characteristic | LA-Like (n = 509) |
LB-Like (n = 763) |
HER2 (n = 369) |
TN (n = 310) |
P | ||||
|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | ||
| Age at diagnosis, years | .38 | ||||||||
| Mean | 55.3 | 54.2 | 53.5 | 52.6 | |||||
| SD | 9.5 | 1.02 | 10.4 | 10.2 | |||||
| Menopausal status | .05 | ||||||||
| Premenopausal | 180 | 35.4 | 312 | 40.9 | 154 | 41.7 | 138 | 44.5 | |
| Postmenopausal | 329 | 64.6 | 451 | 59.1 | 215 | 58.3 | 172 | 55.5 | |
| Tumor size, cm | < .001 | ||||||||
| 0-2 | 368 | 72.3 | 435 | 57.0 | 217 | 58.8 | 130 | 41.9 | |
| > 2 | 128 | 25.1 | 326 | 42.7 | 148 | 40.1 | 176 | 56.8 | |
| Missing/unknown | 13 | 2.6 | 2 | 0.3 | 4 | 1.1 | 4 | 1.3 | |
| Histologic grade | < .001 | ||||||||
| 1 | 191 | 37.5 | 80 | 10.5 | 21 | 5.7 | 6 | 1.9 | |
| 2 | 252 | 49.5 | 393 | 51.5 | 135 | 36.6 | 65 | 21.0 | |
| 3 | 64 | 12.6 | 288 | 37.7 | 207 | 56.1 | 239 | 77.1 | |
| Missing | 2 | 0.4 | 2 | 0.3 | 6 | 1.6 | 0 | 0 | |
| Peritumoral vascular invasion | .006 | ||||||||
| No | 422 | 82.9 | 605 | 79.3 | 277 | 75.1 | 237 | 76.5 | |
| Yes | 55 | 10.8 | 114 | 14.9 | 71 | 19.2 | 47 | 15.2 | |
| Missing/unknown | 32 | 6.3 | 44 | 5.8 | 21 | 5.7 | 26 | 8.4 | |
| Surgery | .06 | ||||||||
| Mastectomy | 225 | 44.2 | 349 | 45.7 | 186 | 50.4 | 163 | 52.6 | |
| Breast conservation | 284 | 55.8 | 414 | 54.3 | 183 | 49.6 | 147 | 47.4 | .02 |
| With RT planned | 262 | 51.5 | 367 | 48.1 | 157 | 42.5 | 122 | 39.4 | |
| With no RT planned | 22 | 4.3 | 47 | 6.2 | 26 | 7.0 | 25 | 8.1 | |
| Randomly assigned to CT | .90 | ||||||||
| No | 223 | 43.8 | 337 | 44.2 | 165 | 44.7 | 130 | 41.9 | |
| Yes | 286 | 56.2 | 426 | 55.8 | 204 | 55.3 | 180 | 58.1 | |
Abbreviations: BC, breast cancer; CT, chemotherapy; HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; LA, luminal A; LB, luminal B; RT, radiotherapy; SD, standard deviation; TN, triple negative.
BC Subtypes and Outcome
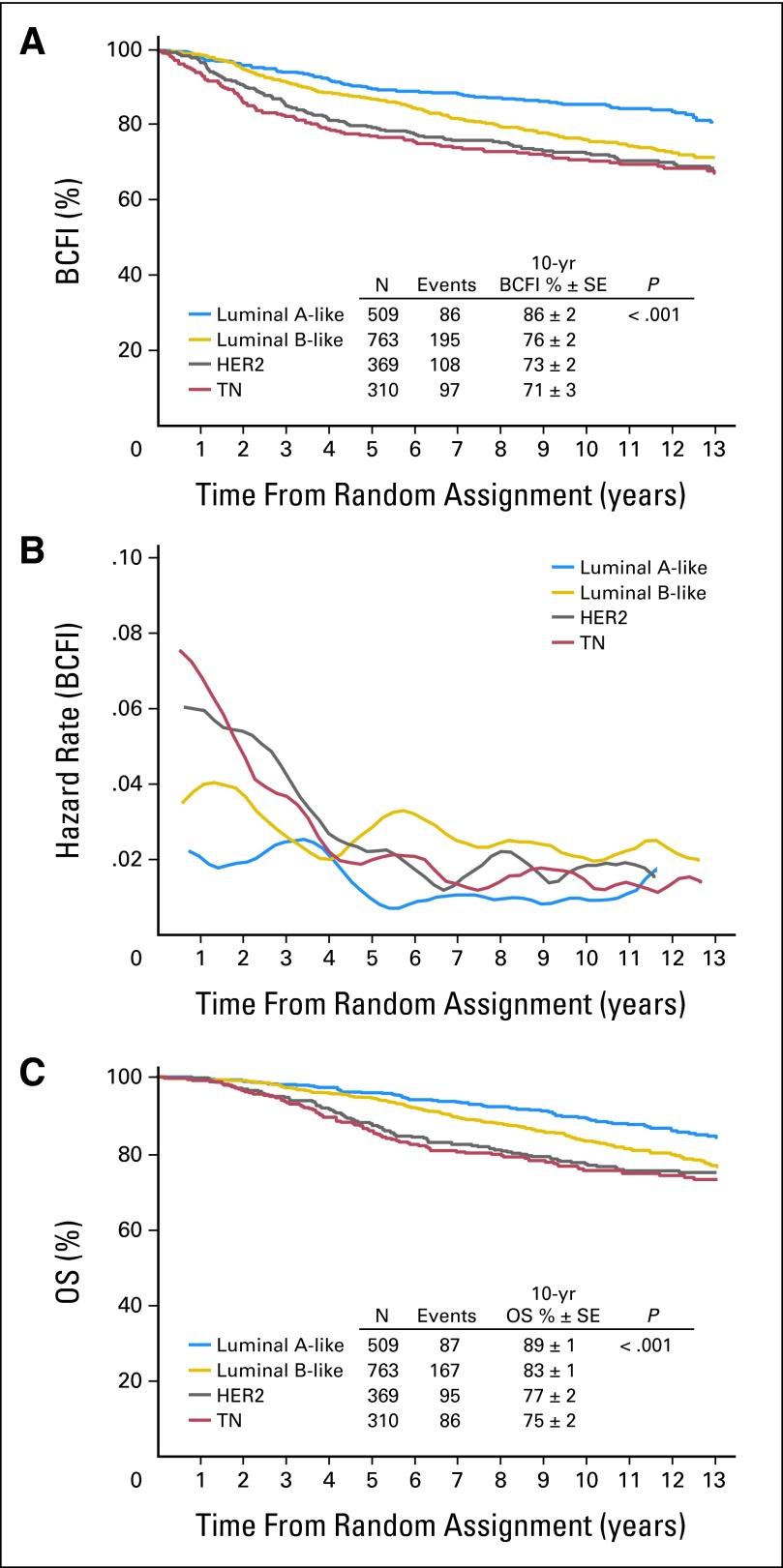
A total of 486 patients (25%) had a BCFI event (ie, BC recurrence). BCFI and OS were significantly different among the different subtypes. The LA-like subtype had a higher 10-year BCFI (86%) compared with LB-like (76%), HER2 (73%), and TN (71%) subtypes (P < .001; Fig 2A). In the HER2 subset, hormone receptor–negative disease had worse BCFI outcome than hormone receptor–positive disease, but the difference was not statistically significant and was attenuated with long-term follow-up (P = .40). HER2 and TN subtypes had a higher hazard rate in the first 4 years, and LA-like and LB-like subtypes had constant BCFI hazard rates over time. The LB-like subtype generally had a higher risk or hazard of BCFI event than the LA-like subtype (Fig 2B).The estimated 10-year OS was 89% in the LA-like subtype compared with 83% in LB-like, 77% in HER2, and 75% in TN subtypes (P < .001; Fig 2C). Median duration of survival from time of first disease recurrence was 3.4, 2.7, 1.6, and 1.9 years for LA-like, LB-like, HER2, and TN subtypes, respectively.
Fig 2.
(A) Kaplan-Meier estimates of breast cancer–free interval (BCFI) according to breast cancer subtype; (B) hazard rate of BCFI over time; (C) Kaplan-Meier estimates of overall survival (OS) according to breast cancer subtype. HER2, human epidermal growth factor receptor 2; TN, triple negative.
Site-Specific Recurrence Pattern According to BC Subtype
Table 2 lists the numbers of events and the 10-year cumulative incidence rates of site-specific events over time according to BC subtype. Table 3 shows the subdistribution HRs and their 95% CIs from a competing risks regression model, adjusted for age, menopausal status, tumor size, tumor grade, peritumoral vascular invasion, primary local treatment, and chemotherapy received.
Table 2.
Patterns of 10-Year Cumulative Incidence of First Site of BCFI Event According to BC Subtypes
| Site of First BC Occurrence | LA-Like (n = 509) |
LB-Like (n = 763) |
HER2 (n = 369) |
TN (n = 310) |
P† | ||||
|---|---|---|---|---|---|---|---|---|---|
| No. | 10-year % ± SE* | No. | 10-year % ± SE* | No. | 10-year % ± SE* | No. | 10-year % ± SE* | ||
| Local | 29 | 5.00 ± 0.01 | 54 | 6.01 ± 0.01 | 28 | 6.93 ± 0.01 | 22 | 6.60 ± 0.01 | .66 |
| Contralateral breast | 18 | 2.60 ± 0.01 | 31 | 3.82 ± 0.01 | 10 | 2.64 ± 0.01 | 16 | 3.95 ± 0.01 | .43 |
| Nodal (no CT) | 0 | 0 ± 0.00 | 11 | 3.39 ± 0.01 | 2 | 1.23 ± 0.01 | 11 | 7.71 ± 0.02 | < .001 |
| Nodal (CT) | 5 | 1.40 ± 0.01 | 4 | 0.80 ± 0.00 | 7 | 2.99 ± 0.01 | 5 | 2.79 ± 0.01 | .17 |
| Bone | 14 | 2.06 ± 0.01 | 43 | 5.64 ± 0.01 | 12 | 2.49 ± 0.01 | 6 | 2.01 ± 0.01 | .005 |
| Visceral (no CT)‡ | 11 | 4.63 ± 0.01 | 14 | 3.67 ± 0.01 | 25 | 14.91 ± 0.03 | 21 | 16.26 ± 0.03 | < .001 |
| Bone marrow | 0 | 0 | 0 | 0 | |||||
| Lung | 1 | 7 | 12 | 13 | |||||
| Liver | 5 | 9 | 12 | 2 | |||||
| CNS | 1 | 1 | 5 | 4 | |||||
| Other | 5 | 1 | 2 | 3 | |||||
| Visceral (CT)‡ | 9 | 2.88 ± 0.01 | 38 | 7.98 ± 0.01 | 24 | 11.36 ± 0.02 | 16 | 7.82 ± 0.02 | .003 |
| Bone marrow | 1 | 0 | 0 | 0 | |||||
| Lung | 1 | 18 | 12 | 6 | |||||
| Liver | 6 | 12 | 11 | 6 | |||||
| CNS | 0 | 2 | 2 | 4 | |||||
| Other | 1 | 11 | 3 | 2 | |||||
NOTE. Nodal and visceral recurrence were reported according to adjuvant treatment due to the interaction between treatment and recurrence at these sites (nodal interaction P < .001; visceral interaction P = .007).
Abbreviations: BC, breast cancer; BCFI, BC-free interval; CT, chemotherapy; HER2, human epidermal growth factor receptor 2; LA, luminal A; LB, luminal B; TN, triple negative.
Ten-year cumulative incidence % ± SE.
P values are based on Gray's test.
Visceral recurrence may occur in one or more of the sites listed. Individual patients may be counted more than once.
Table 3.
Subdistribution HRs and 95% CIs From Competing Risks Regression Models Adjusted for Age, Menopausal Status, Tumor Size, Tumor Grade, Peritumoral Vascular Invasion, Primary Local Treatment, and Assigned CT According to BC Subtypes
| Site of First BC Recurrence | LA-Like |
LB-Like |
HER2 |
TN HR | P* | |||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | |||
| Local | 1.17 | 0.57 to 2.42 | 1.11 | 0.62 to 1.98 | 1.20 | 0.66 to 2.18 | 1 | .94 |
| Contralateral breast | 0.96 | 0.41 to 2.27 | 0.96 | 0.47 to 1.88 | 0.49 | 0.21 to 1.14 | 1 | .34 |
| Nodal (no CT)† | — | — | 0.25 | 0.08 to 0.80 | 0.14 | 0.02 to 0.77 | 1 | .04 |
| Nodal (CT) | 1.95 | 0.34 to 11.3 | 0.59 | 0.13 to 2.77 | 1.90 | 0.52 to 7.01 | 1 | .30 |
| Bone | 2.57 | 0.82 to 8.04 | 4.54 | 1.73 to 12.0 | 2.31 | 0.80 to 6.68 | 1 | .005 |
| Visceral (no CT) | 0.29 | 0.11 to 0.76 | 0.28 | 0.13 to 0.61 | 1.03 | 0.54 to 1.96 | 1 | < .001 |
| Visceral (CT) | 0.39 | 0.13 to 1.19 | 1.30 | 0.68 to 2.48 | 1.57 | 0.78 to 3.15 | 1 | .03 |
NOTE. Nodal and visceral recurrence were reported according to adjuvant treatment because of the interaction between treatment and recurrence at these sites (nodal interaction P < .001; visceral interaction P = .007). Triple negative (TN) is the reference group in the model.
Abbreviations: BC, breast cancer; CT, chemotherapy; HER2, human epidermal growth factor receptor 2; HR, hazard ratio; LA, luminal A; LB, luminal B; TN, triple negative.
P values are based on Wald test.
In patients randomly assigned to receive no CT, there was no nodal as first recurrence site in patients with LA-like subtype. For this group of patients, competing risks regression was performed on the LB-like, HER2, and TN subgroups using TN as the reference group.
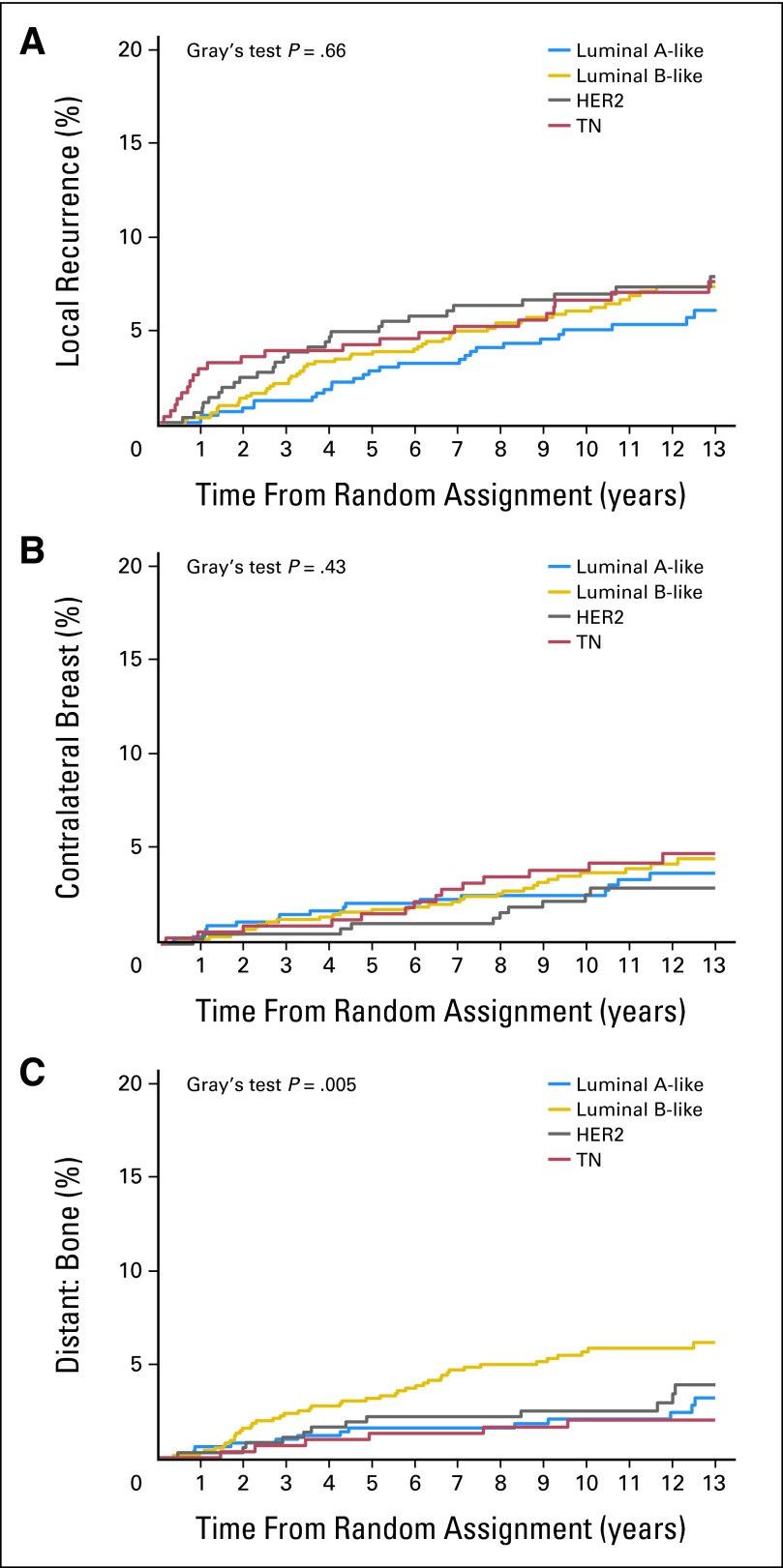
For time to first local recurrence, contralateral breast recurrence, and bone recurrence, no significant interaction of treatment group (chemotherapy v no chemotherapy) and BC subtype was observed (Fig 3). The 10-year cumulative incidence rates and their subdistribution of HRs and 95% CIs are listed for the overall group (Tables 2 and 3).
Fig 3.
Estimated cumulative incidence of (A) local recurrence, (B) contralateral breast recurrence, and (C) bone recurrence over time according to breast cancer subtype. For time to first local recurrence, contralateral breast recurrence, and bone recurrence, no significant interaction of treatment group and breast cancer subtype was observed. The P values are based on Gray's test for comparing the distribution for each site-specific recurrence across subtypes. HER2, human epidermal growth factor receptor 2; TN, triple negative.
A significant interaction of treatment group and BC subtype was observed for nodal (P < .001) and visceral events as the first site of recurrence (P = .007; Fig 4). The 10-year cumulative incidence rates and their subdistribution of HRs and 95% CIs at these sites are presented according to treatment (Tables 2 and 3).
Fig 4.
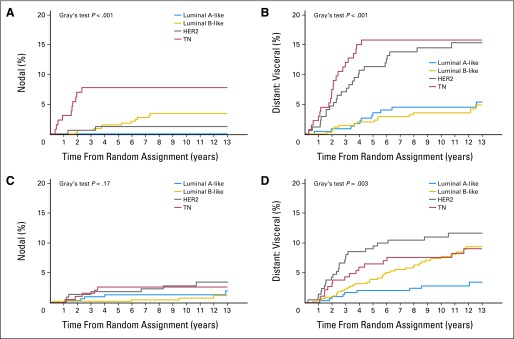
Estimated cumulative incidence of nodal recurrence and visceral recurrence over time according to subtype for patients who were randomly assigned to receive no chemotherapy (A, B) or chemotherapy (C, D). The P values were based on Gray's test for comparing the distribution for each site-specific recurrence across subtype within treatment group. HER2, human epidermal growth factor receptor 2; TN, triple negative.
No significant differences were observed among the BC subtypes for time to first local recurrence and contralateral breast recurrence (Tables 2 and 3; Fig 3). In contrast, patients with LB-like disease had a higher 10-year cumulative incidence of bone recurrence (5.64%) than all other BC subtypes (LA-like, 2.06%; HER2, 2.49%; and TN, 2.01%; Gray's test P = .005; Table 2; Fig 3). After adjusting for other potential risk factors, the LA-like HR was 2.57 (95% CI, 0.82 to 8.04), the LB-like HR was 4.54 (95% CI, 1.73 to 12.0), and the HER2 HR was 2.31 (95% CI, 0.80 to 6.68) relative to the TN subtype (overall P = .005; Table 3).
For patients not treated with chemotherapy, TN patients had significantly more nodal recurrence (10-year cumulative incidence rate, 7.71%) than other subtypes (LA-like, 0%; LB-like, 3.39%; HER2, 1.23%; Gray's test P < .001; Table 2; Fig 4). After adjusting for other potential risk factors, the LB-like HR was 0.25 (95% CI, 0.08 to 0.80) and the HER2 HR was 0.14 (95% CI, 0.02 to 0.77) relative to TN (overall P = .04; Table 3). However, for patients receiving chemotherapy, no significant differences were observed (Gray's test P = .17; Wald test P = .30; Tables 2 and 3; Fig 4).
Among patients not treated with chemotherapy, HER2 and TN patients had significantly more visceral recurrence (10-year cumulative incidence rate, 14.91% and 16.26%, respectively) than ER-positive patients (LA-like, 4.63%; LB-like, 3.67%; Gray's test P < .001; Table 2; Fig 4). After adjusting for other potential risk factors, the LA-like HR was 0.29 (95% CI, 0.11 to 0.76), the LB-like HR was 0.28 (95% CI, 0.13 to 0.61), and the HER2 HR was 1.03 (95% CI, 0.54 to 1.96) relative to TN (overall P < .001; Table 3). Among patients treated with chemotherapy, HER2 patients had more and earlier visceral recurrence (10-year cumulative incidence rate, 11.36%), followed by TN (7.82%), LB-like (7.98%), and LA-like patients (2.88%; Gray's test P = .003; Table 2; Fig 4). After adjusting for other potential risk factors, the LA-like HR was 0.39 (95% CI, 0.13 to 1.19), the LB-like HR was 1.30 (95% CI, 0.68 to 2.48), and the HER HR was 1.57 (95% CI, 0.78 to 3.15) relative to TN (overall P = .03).
The numbers of events at each visceral site (bone marrow, lung, liver, CNS, or other) by BC subtype are listed in Table 2. Liver and lung were the most common visceral sites of recurrence for LB-like (3.0% and 2.6%) and HER2 (6.7% and 6.4%) subtypes. Liver was the most common visceral site for LA-like (2.1%) and lung was the most common visceral site for TN (5.8%) subtypes. The 10-year cumulative incidence of site-specific events according to hormone receptor status in the HER2 subset is provided in the Data Supplement. When compared with patients with HER2-positive hormone receptor–negative BC, HER2-positive hormone receptor–positive patients had a higher incidence rate of bone recurrence. For patients treated with chemotherapy, HER2-positive hormone receptor–positive patients had a relatively lower incidence rate of nodal recurrence compared with HER2-positive hormone receptor–negative patients. However, the overall numbers of those events were small.
DISCUSSION
This study demonstrates that patients with major subtypes of node-negative BC have specific patterns of first recurrence over time. A major strength of this study is that the population comes from two large phase III clinical trials, and patients were carefully followed over a long period of time. In addition, determination of biomarkers (ER, PgR, HER2, and Ki-67) was performed in central laboratories with extensive BC pathology experience. A limitation of our study is that some of the adjuvant therapies administered in these trials do not represent current clinical practice (eg, chemotherapy was CMF-based, some patients with ER-negative disease received endocrine therapy, and no patients with HER2-positive disease received trastuzumab). In addition, defining molecular BC subtypes by using IHC-based surrogates has inherent limitations. In this analysis, 94 patients classified as HER2 IHC2+ (3.4% of IBCSG VIII and IX population) were excluded because of missing FISH results. IHC-based surrogates use a reduced set of biomarkers compared with genomic studies and were not expected to replace or classify patients with the same accuracy. Consequently, only an incomplete approximation to genomically defined BC subtypes can be achieved when biomarkers using IHC-based surrogates are assessed according to existing recommendations,17 which is a limitation of our retrospective exercise.
In general, a nonselected node-negative BC population has a 20% to 30% recurrence rate at 10 years.22 In this study, we found an overall BC-related event rate of 25% at 12.5 years of median follow-up. Survival outcomes observed for the BC subtypes defined in this analysis are similar to those reported for the genomically defined subtypes.9,23–26 LA-like was statistically significantly associated with better BCFI and OS when compared with the other subtypes, and TN and HER2 subtypes had worse survival outcome over the initial years after diagnosis. Given that patients with the HER2-positive subtype did not receive adjuvant trastuzumab, its impact for this subtype needs further study in a more recent trial after adequate follow-up.
In a series dating from an era in which BC was solely classified as ER-positive or ER-negative, Saphner et al3 wisely stated that “perhaps the long-term recurrence rate for ER-positive and ER-negative patients will be the same but with the ER-negative recurrences occurring more frequently in early follow-up and the ER-positive recurrences occurring in late follow-up.” This series provides data in support of this statement and further demonstrates that the long-term recurrence rates for LB-like and ER-negative tumors are similar, with ER-negative recurrences occurring more frequently in early follow-up and LB-like recurrences occurring both in early and late follow-up. Interestingly, the continuous recurrence pattern previously reported for ER-positive BC is observed for both LA-like and LB-like subtypes, but with a continuously higher HR of recurrence over time for the LB-like subtype.
In addition to time-dependent recurrence pattern, important site-specific recurrence patterns were observed across BC subtypes. However, these results should be considered as hypothesis generating, and they require independent validation. No significant differences were observed for local recurrence or for nodal recurrence when treated with chemotherapy across the four BC subtypes, whereas significant differences were observed for nodal recurrence in the absence of chemotherapy. Previous studies reported a statistically significant lower occurrence of axillary lymph node metastasis in patients with TN when compared with patients with other subtypes,27,28 but other research did not demonstrate such a difference.29–31 In the largest series describing locoregional recurrence patterns across BC subtypes (including node-positive patients), an increased incidence of nodal recurrence was observed for basal-like (defined as TN and epidermal growth factor receptor or CK5/6 positive).32 In contrast to studies including node-positive patients, our results showed similar local recurrence rates across the four major BC subtypes.
In a retrospective series using similar IHC-based surrogates such as the ones used in this study, bone was the most common metastatic site in all subtypes with the exception of basal-like (defined as TN and epidermal growth factor receptor or CK5/6 positive).5 Previous series suggested bone as the most common metastatic site for ER-positive BC.33–35 In our study, a higher incidence of bone metastasis was observed for patients with LB-like subtype.
As expected, higher rates of visceral recurrence occurred among TN and HER2 subtypes, evident over time in the absence of chemotherapy. However, with longer-term follow-up after chemotherapy, patients classified as LB-like eventually had a cumulative incidence of visceral recurrence similar to that of TN and HER2 patients. It is important to note that studies including node-positive BC have reported increased incidence of CNS metastasis among HER2 and TN subtypes.36–38 In a retrospective series evaluating CNS recurrence pattern, the 15-year cumulative incidence of brain metastasis among 4,000 node-negative patients was less than 1%.2 This study corroborates a low incidence of CNS metastasis among patients with node-negative BC irrespective of subtype.
To the best of our knowledge, this retrospective study represents the largest series reporting outcome and recurrence pattern of patients with node-negative BC subdivided into its major subtypes. Dent et al7 made the insightful observation that the time-dependence of an effect will be overlooked if the majority of patients have a short follow-up. Our retrospective results, based on a reanalysis of tumor phenotype, reinforce the need for long-term clinical follow-up in order to understand the pattern of recurrence of BC subtypes. However, results based on long-term follow-up can lead to the inclusion of patients treated in a noncontemporary fashion, which limits inferences on the effect of modern treatment approaches.
Defining the pattern of BC recurrence according to time after diagnosis and site of recurrence can provide useful information for guiding the development of surveillance recommendations and for designing future therapeutic approaches.
Supplementary Material
Acknowledgment
Presented as a poster at the 33rd Annual San Antonio Breast Cancer Symposium, San Antonio, TX, December 8-12, 2010.
We thank the patients, physicians, nurses, and data managers who participate in the International Breast Cancer Study Group trials VIII and IX. We thank Barry Gusterson for central review of histologic grade and peritumoral vessel invasion. Participating centers and investigators are listed in the Data Supplement.
Footnotes
Written on behalf of the International Breast Cancer Study Group.
Supported in part by the Swiss Group for Clinical Cancer Research, Frontier Science and Technology Research Foundation, The Cancer Council Australia, Australian New Zealand Breast Cancer Trials Group (National Health Medical Research Council), Grant No. CA-75362 from the National Institutes of Health, Swedish Cancer Society, Cancer Association of South Africa, and the Foundation for Clinical Cancer Research of Eastern Switzerland.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Otto Metzger-Filho, Aron Goldhirsch, Fatima Cardoso
Administrative support: Karen N. Price
Provision of study materials or patients: Diana Crivellari, Raymond D. Snyder, Monica Castiglione-Gertsch
Collection and assembly of data: Giuseppe Viale, Karen N. Price, Diana Crivellari, Raymond D. Snyder, Monica Castiglione-Gertsch, Aron Goldhirsch
Data analysis and interpretation: Otto Metzger-Filho, Zhuoxin Sun, Karen N. Price, Richard D. Gelber, Alan S. Coates, Aron Goldhirsch, Fatima Cardoso
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 2.Pestalozzi BC, Zahrieh D, Price KN, et al. Identifying breast cancer patients at risk for central nervous system (CNS) metastases in trials of the International Breast Cancer Study Group (IBCSG) Ann Oncol. 2006;17:935–944. doi: 10.1093/annonc/mdl064. [DOI] [PubMed] [Google Scholar]
- 3.Saphner T, Tormey DC, Gray R. Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol. 1996;14:2738–2746. doi: 10.1200/JCO.1996.14.10.2738. [DOI] [PubMed] [Google Scholar]
- 4.Grann VR, Troxel AB, Zojwalla NJ, et al. Hormone receptor status and survival in a population-based cohort of patients with breast carcinoma. Cancer. 2005;103:2241–2251. doi: 10.1002/cncr.21030. [DOI] [PubMed] [Google Scholar]
- 5.Kennecke H, Yerushalmi R, Woods R, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28:3271–3277. doi: 10.1200/JCO.2009.25.9820. [DOI] [PubMed] [Google Scholar]
- 6.Pagani O, Price KN, Gelber RD, et al. Patterns of recurrence of early breast cancer according to estrogen receptor status: A therapeutic target for a quarter of a century. Breast Cancer Res Treat. 2009;117:319–324. doi: 10.1007/s10549-008-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dent R, Hanna WM, Trudeau M, et al. Pattern of metastatic spread in triple-negative breast cancer. Breast Cancer Res Treat. 2009;115:423–428. doi: 10.1007/s10549-008-0086-2. [DOI] [PubMed] [Google Scholar]
- 8.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Peto R, Davies C, et al. Comparisons between different polychemotherapy regimens for early breast cancer: Meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2011;379:432–444. doi: 10.1016/S0140-6736(11)61625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 10.Prat A, Perou CM. Deconstructing the molecular portraits of breast cancer. Mol Oncol. 2011;5:5–23. doi: 10.1016/j.molonc.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.International Breast Cancer Study Group (IBCSG) Endocrine responsiveness and tailoring adjuvant therapy for postmenopausal lymph node-negative breast cancer: A randomized trial. J Natl Cancer Inst. 2002;94:1054–1065. doi: 10.1093/jnci/94.14.1054. [DOI] [PubMed] [Google Scholar]
- 12.Karlsson P, Sun Z, Braun D, et al. Long-term results of International Breast Cancer Study Group Trial VIII: Adjuvant chemotherapy plus goserelin compared with either therapy alone for premenopausal patients with node-negative breast cancer. Ann Oncol. 2011;22:2216–2226. doi: 10.1093/annonc/mdq735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colleoni M, Cole BF, Viale G, et al. Classical cyclophosphamide, methotrexate, and fluorouracil chemotherapy is more effective in triple-negative, node-negative breast cancer: Results from two randomized trials of adjuvant chemoendocrine therapy for node-negative breast cancer. J Clin Oncol. 2010;28:2966–2973. doi: 10.1200/JCO.2009.25.9549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Viale G, Giobbie-Hurder A, Regan MM, et al. Prognostic and predictive value of centrally reviewed Ki-67 labeling index in postmenopausal women with endocrine-responsive breast cancer: Results from Breast International Group Trial 1-98 comparing adjuvant tamoxifen with letrozole. J Clin Oncol. 2008;26:5569–5575. doi: 10.1200/JCO.2008.17.0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Viale G, Regan MM, Mastropasqua MG, et al. Predictive value of tumor Ki-67 expression in two randomized trials of adjuvant chemoendocrine therapy for node-negative breast cancer. J Natl Cancer Inst. 2008;100:207–212. doi: 10.1093/jnci/djm289. [DOI] [PubMed] [Google Scholar]
- 16.Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–2795. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes: Dealing with the diversity of breast cancer—Highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fisher RA. The logic of inductive inference. J R Stat Soc. 1935;98:39–82. [Google Scholar]
- 19.Kruskal WH, Wallis WA. Use of ranks in one-criterion variance analysis. J Am Stat Assoc. 1952;47:583–621. [Google Scholar]
- 20.Allison PD. Cary, NC: SAS Institute; 2005. Survival Analysis Using the SAS System: A Practical Guide. [Google Scholar]
- 21.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 22.Fitzgibbons PL, Page DL, Weaver D, et al. Prognostic factors in breast cancer: College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000;124:966–978. doi: 10.5858/2000-124-0966-PFIBC. [DOI] [PubMed] [Google Scholar]
- 23.Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sorlie T, Tibshirani R, Parker J, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003;100:8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sotiriou C, Neo SY, McShane LM, et al. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc Natl Acad Sci U S A. 2003;100:10393–10398. doi: 10.1073/pnas.1732912100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu Z, Fan C, Oh DS, et al. The molecular portraits of breast tumors are conserved across microarray platforms. BMC Genomics. 2006;7:96. doi: 10.1186/1471-2164-7-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim MJ, Ro JY, Ahn SH, et al. Clinicopathologic significance of the basal-like subtype of breast cancer: A comparison with hormone receptor and Her2/neu-overexpressing phenotypes. Hum Pathol. 2006;37:1217–1226. doi: 10.1016/j.humpath.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 28.Fulford LG, Reis-Filho JS, Ryder K, et al. Basal-like grade III invasive ductal carcinoma of the breast: Patterns of metastasis and long-term survival. Breast Cancer Res. 2007;9:R4. doi: 10.1186/bcr1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haffty BG, Yang Q, Reiss M, et al. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J Clin Oncol. 2006;24:5652–5657. doi: 10.1200/JCO.2006.06.5664. [DOI] [PubMed] [Google Scholar]
- 30.Calza S, Hall P, Auer G, et al. Intrinsic molecular signature of breast cancer in a population-based cohort of 412 patients. Breast Cancer Res. 2006;8:R34. doi: 10.1186/bcr1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jumppanen M, Gruvberger-Saal S, Kauraniemi P, et al. Basal-like phenotype is not associated with patient survival in estrogen-receptor-negative breast cancers. Breast Cancer Res. 2007;9:R16. doi: 10.1186/bcr1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Voduc KD, Cheang MC, Tyldesley S, et al. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol. 2010;28:1684–1691. doi: 10.1200/JCO.2009.24.9284. [DOI] [PubMed] [Google Scholar]
- 33.Hess KR, Pusztai L, Buzdar AU, et al. Estrogen receptors and distinct patterns of breast cancer relapse. Breast Cancer Res Treat. 2003;78:105–118. doi: 10.1023/a:1022166517963. [DOI] [PubMed] [Google Scholar]
- 34.Alanko A, Heinonen E, Scheinin T, et al. Significance of estrogen and progesterone receptors, disease-free interval, and site of first metastasis on survival of breast cancer patients. Cancer. 1985;56:1696–1700. doi: 10.1002/1097-0142(19851001)56:7<1696::aid-cncr2820560738>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 35.Smid M, Wang Y, Zhang Y, et al. Subtypes of breast cancer show preferential site of relapse. Cancer Res. 2008;68:3108–3114. doi: 10.1158/0008-5472.CAN-07-5644. [DOI] [PubMed] [Google Scholar]
- 36.Lin NU, Bellon JR, Winer EP. CNS metastases in breast cancer. J Clin Oncol. 2004;22:3608–3617. doi: 10.1200/JCO.2004.01.175. [DOI] [PubMed] [Google Scholar]
- 37.Miller KD, Weathers T, Haney LG, et al. Occult central nervous system involvement in patients with metastatic breast cancer: Prevalence, predictive factors and impact on overall survival. Ann Oncol. 2003;14:1072–1077. doi: 10.1093/annonc/mdg300. [DOI] [PubMed] [Google Scholar]
- 38.Dawood S, Broglio K, Esteva FJ, et al. Survival among women with triple receptor-negative breast cancer and brain metastases. Ann Oncol. 2009;20:621–627. doi: 10.1093/annonc/mdn682. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.