Abstract
Purpose
To analyze outcomes of hematopoietic cell transplantation (HCT) in T-cell non-Hodgkin lymphoma.
Patients and Methods
Outcomes of 241 patients (112 anaplastic large-cell lymphoma, 102 peripheral T-cell lymphoma not otherwise specified, 27 angioimmunoblastic T-cell lymphoma) undergoing autologous HCT (autoHCT; n = 115; median age, 43 years) or allogeneic HCT (alloHCT; n = 126; median age, 38 years) were analyzed. Primary outcomes were nonrelapse mortality (NRM), relapse/progression, progression-free survival (PFS), and overall survival (OS). Patient, disease, and HCT-related variables were analyzed in multivariate Cox proportional hazard models to determine association with outcomes.
Results
AutoHCT recipients were more likely in first complete remission (CR1; 35% v 14%; P = .001) and with chemotherapy-sensitive disease (86% v 60%; P < .001), anaplastic large-cell histology (53% v 40%; P = .04), and two or fewer lines of prior therapy (65% v 44%; P < .001) compared with alloHCT recipients. Three-year PFS and OS of autoHCT recipients beyond CR1 were 42% and 53%, respectively. Among alloHCT recipients who received transplantations beyond CR1, 31% remained progression-free at 3 years, despite being more heavily pretreated and with more refractory disease. NRM was 3.5-fold higher (95% CI, 1.80 to 6.99; P < .001) for alloHCT. In multivariate analysis, chemotherapy sensitivity (hazard ratio [HR], 1.8; 95% CI, 1.16 to 2.87) and two or fewer lines of pretransplantation therapy (HR, 5.02; 95% CI, 2.15 to 11.72) were prognostic of survival.
Conclusion
These data describe the roles of autoHCT and alloHCT in T-cell non-Hodgkin lymphoma and suggest greater effectiveness earlier in the disease course, and limited utility in multiply relapsed disease. Notably, autoHCT at relapse may be a potential option for select patients, particularly those with anaplastic large-cell lymphoma histology.
INTRODUCTION
Peripheral T-cell lymphomas such as T-cell non-Hodgkin lymphoma (T-NHL) are heterogeneous malignancies sharing common elements of chemotherapy resistance and poor outcome with standard treatments. The International T-Cell Lymphoma Project highlights that fewer than one third of patients with T-cell lymphomas survive 5 years, although histology strongly influences survival.1 Patients with anaplastic lymphoma kinase (ALK) –positive variants of anaplastic large-cell lymphoma (ALCL) have better outcomes, with 5-year survival rates of 70%. In contrast, survival sequentially declines for ALK-negative ALCL (49%), peripheral T-cell lymphoma not otherwise specified (PTCL-NOS; 32%), and angioimmunoblastic T-cell lymphoma (AITL; 14%). The German High Grade Non-Hodgkin's Lymphoma Study Group (DSHNHL) similarly reported on more than 200 patients with T-NHL enrolled onto prospective trials; 3-year event-free survival was best for ALK-positive ALCL (75%) and suboptimal for all other histologies.2
Attempts to improve outcomes have included autologous or allogeneic hematopoietic cell transplantation (autoHCT or alloHCT). Single-institution studies and retrospective analyses suggest that both modalities lead to durable remissions in recurrent disease settings and might be important in consolidating first remission.3–6 However, key questions remain, including identification of optimal populations, relative efficacy of autologous versus allogeneic approaches, and HCT timing (first-line consolidation v relapse). Herein, we analyzed outcomes of a large cohort of autoHCT or alloHCT recipients with the three most common T-NHL histologies reported to the Center for International Blood and Marrow Transplant Research (CIBMTR).
PATIENTS AND METHODS
Data Sources
CIBMTR is a voluntary working group of more than 500 transplantation centers worldwide. Participating centers register basic information on consecutive transplantations to a statistical center at the Medical College of Wisconsin with two levels of data collection. Comprehensive patient- and disease-related data were collected by using a weighted randomization scheme in a subset of patients. A larger registration data set consisted of consecutive data on all transplantations from all centers reporting to the CIBMTR and was used to estimate transplantation activity. This registration showed 946 autologous transplantations and 346 allogeneic transplantations from US centers during the period specified. These numbers represent 55% and 95% of all US auto and allo transplantation activity for T-NHL, corresponding to an estimated 1,048 autoHCTs and 629 alloHCTs performed in the United States. We compared outcome data for our selected representative cohort with higher-level data (Case Report Forms) versus the registration data set that included all patients. Outcomes were similar, confirming that our data set was representative of HCT outcomes for T-NHL.
Patients Were Followed Longitudinally, With Annual Follow-Up
Patients with T-NHL age ≤ 60 years who received first autoHCT or alloHCT between 1996 and 2006 were included. Two hundred forty-one patients who underwent autoHCT (n = 115) or alloHCT (n = 126) and restricted to ALCL (n = 112), PTCL-NOS (n = 102), and AITL (n = 27) histologies were identified. Exclusion criteria were precursor T-cell neoplasms, primary cutaneous T-cell lymphomas, or second transplantations as well as identical twin (n = 4), mismatched related donor (n = 13), or cord blood (n = 8) transplantations. When available, primary pathology reports were reviewed (n = 143). Patients older than age 60 years were excluded because of the small number who underwent alloHCT (n = 6).
Definitions of alloHCT Conditioning Regimens
Lower-intensity conditioning regimens were categorized as nonmyeloablative stem-cell transplantation (NST) or reduced-intensity conditioning (RIC) by using established criteria.7 Previously validated criteria defined donor-recipient HLA matching quality on the basis of the number of HLA loci examined and resolution of HLA typing at each locus.8
End Points
Primary outcomes were nonrelapse mortality (NRM), relapse/progression, progression-free survival (PFS), and overall survival (OS). NRM was defined as death as a result of any cause in the first 28 days or death without evidence of lymphoma relapse/progression; progression, increase of ≥ 25% in lymphoma sites or development of new sites; relapse, recurrence of lymphoma after complete remission (CR); primary induction failure (PIF) sensitive, never in CR but with partial remission to treatment; PIF other, never in CR but with stable or progressive disease on treatment; relapse sensitive, relapsing from prior remission but with a partial remission to treatment for relapse; and relapse other, relapsing from prior remission with stable disease or progression thereafter.
Treatment failure was defined as time of relapse, progression, or death as a result of any cause. Patients alive without evidence of disease relapse/progression were censored at last follow-up, and PFS events were summarized by survival curves. The OS interval variable was time from date of transplantation to date of death or last contact and was summarized by a survival curve. Other outcomes included acute and chronic graft-versus-host disease (AGVHD and CGVHD) and cause of death (COD). AGVHD was defined and graded on the basis of patterns and severity of organ involvement by using established criteria. CGVHD was defined as the development of any chronic GVHD on the basis of clinical criteria. Both events were summarized by corresponding cumulative incidence estimates with death without development of GVHD as the competing risk.
Statistical Analyses
Probabilities of PFS and OS were calculated by using Kaplan-Meier product limit estimates. Probabilities of NRM, lymphoma relapse/progression, and AGVHD and CGVHD were calculated by using cumulative incidence curves to accommodate competing risks. Associations between patient-, disease-, and transplantation-related factors and primary outcomes of interest were assessed by using multivariate Cox proportional hazards regression. Variables in multivariate analyses included the following: (1) main effect was autoHCT versus alloHCT; (2) patient-related variables included age at transplantation (≤ 20 v 21 to 40 v 41 to 60 years), sex, Karnofsky performance score (≥ 90 v ≤ 90); (3) disease-related variables included histology (ALCL v PTCL v AITL), “B” symptoms, number of lines of therapy before transplantation (1 v 2 v > 2), disease stage at diagnosis (stage I to II v III to IV), extranodal involvement at diagnosis, time from diagnosis to transplantation (≤ 12 v > 12 months), disease status before transplantation (CR1 v CR2+ v primary induction failure v relapsed), and chemotherapy sensitivity (sensitive v resistant); and (4) treatment-related variables include conditioning regimen (total-body irradiation–containing v no total-body irradiation), graft type (bone marrow v peripheral blood stem cells, and year of transplantation (1996 to 1998 v 1999 to 2001 v 2002 to 2004 v 2005 to 2006). AlloHCT recipients had additional comparisons: impact of conditioning regimen (myeloablative v NST/RIC), donor-recipient sex match, donor-recipient cytomegalovirus status, donor type (HLA-identical sibling v matched unrelated v mismatched unrelated), and GVHD prophylaxis (T-cell depletion v other). A stepwise forward selection multivariate model was built to identify covariates that influenced outcomes. Covariates with a P value less than .05 were considered significant. The main effect studied (ie, autoHCT v alloHCT) was included in all models. The proportionality assumption for Cox regression was tested by adding time-dependent covariates for each risk factor and each outcome. All variables met the proportional hazards assumption. Results were expressed as relative risks or relative rate of occurrence of the event.
RESULTS
Patient-, Disease-, and Treatment-Related Characteristics
Table 1 lists patient characteristics, and Table 2 lists treatment- or transplantation-related features. Most patients in both groups had B symptoms at diagnosis, had more than one line of therapy before transplantation, lacked bone marrow involvement at transplantation, and had advanced disease or extranodal disease at time of diagnosis. There were no differences in median age, age distribution, sex distribution, disease stage, or median time from diagnosis to transplantation by HCT type. AutoHCT patients had more ALCL histology. AlloHCT recipients had more bone marrow involvement, more lines of chemotherapy pretransplantation, extranodal disease at diagnosis, and higher second-line prognostic index of PTCLs9 at transplantation (P = .02).
Table 1.
Patient Characteristics
| Characteristic | Autologous HCT |
Allogeneic HCT |
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| No. of patients* | 115 | 126 | |||
| No. of centers | 67 | 72 | |||
| Age at transplantation, years | .10 | ||||
| Median | 43 | 38 | |||
| Range | 4-60 | 5-60 | |||
| ≤ 20 | 11 | 9 | 22 | 17 | |
| 21-30 | 18 | 16 | 20 | 16 | |
| 31-40 | 24 | 21 | 27 | 21 | |
| 41-50 | 21 | 18 | 30 | 24 | |
| 51-60 | 41 | 36 | 27 | 21 | |
| Male | 70 | 61 | 91 | 72 | .06 |
| Karnofsky score pretransplantation | .62 | ||||
| < 90 | 31 | 27 | 41 | 33 | |
| Histology† | .04 | ||||
| Anaplastic large-cell lymphoma‡ | 61 | 53 | 51 | 40 | |
| Peripheral T-cell lymphoma, unspecified | 39 | 34 | 63 | 50 | |
| Angioimmunoblastic T-cell lymphoma | 15 | 13 | 12 | 10 | |
| B symptoms at diagnosis | 67 | 58 | 69 | 55 | .13 |
| LDH at diagnosis | .15 | ||||
| Normal | 19 | 17 | 12 | 10 | |
| Increased | 26 | 23 | 39 | 31 | |
| Unknown | 70 | 61 | 75 | 60 | |
| No. of lines of therapy prior to transplantation | |||||
| Median | 2 | 3 | .002 | ||
| 1 | 19 | 17 | 18 | 14 | < .001 |
| 2 | 55 | 48 | 38 | 30 | |
| ≥ 3 | 39 | 34 | 55 | 43 | |
| Unknown | 2 | 2 | 7 | 6 | |
| CNS involvement§ | 2 | 2 | 6 | 5 | .28 |
| BM involvement at diagnosis | 21 | 18 | 44 | 35 | .0142 |
| BM involvement at time of transplantation | 1 | 1 | 19 | 15 | < .001 |
| Extranodal involvement at diagnosis | 64 | 56 | 86 | 68 | .05 |
| PIT at transplantation | .02 | ||||
| 0 | 49 | 43 | 42 | 33 | |
| 1 | 41 | 36 | 42 | 33 | |
| 2 | 3 | 2 | 16 | 13 | |
| Unknown | 22 | 19 | 26 | 21 | |
| Disease stage at diagnosis | .28 | ||||
| I | 10 | 9 | 5 | 4 | |
| II | 21 | 18 | 15 | 12 | |
| III | 32 | 28 | 36 | 28 | |
| IV | 47 | 41 | 64 | 51 | |
| Unknown | 5 | 4 | 6 | 5 | |
| Time from diagnosis to transplantation, months | |||||
| Median | 10 | 11 | |||
| Range | 2-229 | 3-69 | |||
| ≤ 6 | 14 | 12 | 22 | 17 | .32 |
| 6-12 | 57 | 50 | 53 | 42 | |
| 12-18 | 15 | 13 | 26 | 21 | |
| 18-24 | 11 | 10 | 9 | 7 | |
| > 24 | 18 | 16 | 16 | 13 | |
| Follow-up of survivors, months | |||||
| Median | 71 | 49 | |||
| Range | 3-167 | 3-157 | |||
NOTE. Completeness index follow-up: 96% at 1 year; 88% at 3 years.
Abbreviations: BM, bone marrow; HCT, hematopoietic cell transplantation; LDH, lactate dehydrogenase; PIT, prognostic index of T-cell non-Hodgkin lymphoma.
Patients who had autologous transplantation followed by allogeneic transplantation (four twins, eight cord blood, 13 other-related) were not included in allogeneic population.
Pathology reports were reviewed for 143 patients.
Anaplastic lymphoma kinase status: positive, n = 14; negative, n = 8; unknown, n = 90.
CNS involvement was at any time prior to transplantation.
Table 2.
Treatment- and Transplantation-Related Characteristics
| Characteristic | Autologous HCT |
Allogeneic HCT |
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Chemotherapy sensitivity | < .001 | ||||
| Sensitive | 99 | 86 | 75 | 60 | |
| Resistant | 9 | 8 | 37 | 29 | |
| Untreated | 0 | 2 | 2 | ||
| Unknown | 7 | 6 | 12 | 10 | |
| Disease status at transplantation | .001 | ||||
| CR1 | 40 | 35 | 18 | 14 | |
| CR2+ | 24 | 21 | 20 | 16 | |
| PIF, sensitive | 16 | 14 | 23 | 18 | |
| PIF, other | 6 | 5 | 23 | 18 | |
| Relapse, sensitive | 17 | 15 | 21 | 17 | |
| Relapse, other | 10 | 9 | 18 | 14 | |
| Missing | 2 | 2 | 3 | 3 | |
| Conditioning regimen (allogeneic) | N/A | ||||
| Myeloablative | 74 | 59 | |||
| NST/RIC | 45 | 36 | |||
| Unknown | 7 | 5 | |||
| Conditioning regimen (autologous) | N/A | ||||
| TBI containing | 26 | 23 | |||
| BEAM and similar | 65 | 57 | |||
| Cyclophosphamide or similar | 14 | 12 | |||
| Busulfan + melphalan/busulfan + cyclophosphamide | 4 | 3 | |||
| Other | 6 | 5 | |||
| Conditioning regimen (allogeneic) | N/A | ||||
| TBI containing | 60 | 48 | |||
| Donor HLA match | N/A | ||||
| HLA-identical sibling | 76 | 60 | |||
| Matched unrelated | 30 | 24 | |||
| Mismatched unrelated | 20 | 16 | |||
| Donor/recipient cytomegalovirus status | N/A | ||||
| −/− | 39 | 31 | |||
| +/– | 23 | 18 | |||
| −/+ | 21 | 17 | |||
| +/+ | 38 | 30 | |||
| Unknown | 5 | 4 | |||
| Donor-recipient sex match | |||||
| Male-male | N/A | 52 | 41 | ||
| Male-female | 20 | 16 | |||
| Female-male | 39 | 31 | |||
| Female-female | 15 | 12 | |||
| Graft type | < .001 | ||||
| Bone marrow | 10 | 9 | 36 | 29 | |
| Peripheral blood | 105 | 91 | 90 | 71 | |
| Year of transplantation | < .001 | ||||
| 1996-1998 | 43 | 37 | 16 | 13 | |
| 1999-2001 | 39 | 34 | 23 | 18 | |
| 2002-2004 | 22 | 19 | 39 | 31 | |
| 2005-2006 | 11 | 10 | 48 | 38 | |
| GVHD prophylaxis | N/A | ||||
| T-cell depletion ± other | 14 | 11 | |||
| Methotrexate + cyclosporine ± other | 46 | 37 | |||
| Cyclosporine ± other | 28 | 22 | |||
| Methotrexate + tacrolimus ± other | 23 | 18 | |||
| Tacrolimus ± other | 12 | 10 | |||
| Other | 3 | 2 | |||
Abbreviations: BEAM, carmustine, etoposide, cytarabine, melphalan; CR1, first complete remission; CR2, second complete remission; GVHD, graft-versus-host disease; HCT, hematopoietic cell transplantation; PIF, primary induction failure; N/A, not applicable; NST, nonmyeloablative stem-cell transplantation; RIC, reduced-intensity conditioning; TBI, total-body irradiation.
AutoHCT recipients were more likely in first complete remission (CR1; 35% v 14%; P = .001) and with chemotherapy-sensitive disease (86% v 60%; P < .001). Myeloablative (MA) conditioning was more common than NST/RIC for alloHCT, although increased use of alloHCT was seen in later years (69% of alloHCTs were performed after 2002). Forty percent of alloHCT recipients had unrelated donors. Peripheral blood was the most common graft source in both groups.
Univariate Analysis of Outcomes
There were no outcome differences between MA and NST/RIC conditioning (Table 3; Figs 1B and 1C). Both NRM and overall mortality were higher in alloHCT patients (Fig 1). There was no difference in relapse/progression between autoHCT and alloHCT patients. For autoHCT patients, the 1- and 3-year PFS rates were 58% and 47%, and the 1- and 3-year OS rates were 68% and 59%, respectively. For alloHCT, the 1- and 3-year PFS rates were 42% and 37%, and the 1- and 3-year OS rates were 55% and 46%, respectively (Fig 1B; Table 3). Patients in CR1 undergoing autoHCT (n = 40) had 1- and 3-year PFS rates of 75% and 58%, respectively, whereas OS at 1 year and 3 years was 80% and 70%, respectively (data not shown). Few patients (n = 18) underwent alloHCT in CR1.
Table 3.
Univariate Outcomes of Patients With HCT
| Variable | All Patients |
Patients Beyond CR1 |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AutoHCT |
Myeloablative |
NST/RIC |
P | AutoHCT |
Myeloablative |
NST/RIC |
P | |||||||
| % | 95% CI (%) | % | 95% CI (%) | % | 95% CI (%) | % | 95% CI (%) | % | 95% CI (%) | % | 95% CI (%) | |||
| Nonrelapse mortality | ||||||||||||||
| At 100 days | 2 | 0 to 6 | 19 | 11 to 29 | 18 | 8 to 30 | < .001 | 3 | 1 to 8 | 21 | 12 to 32 | 20 | 9 to 33 | < .001 |
| At 1 year | 7 | 3 to 13 | 29 | 19 to 40 | 27 | 15 to 40 | < .001 | 4 | 1 to 10 | 30 | 19 to 42 | 27 | 14 to 41 | < .001 |
| At 3 years | 11 | 6 to 17 | 32 | 22 to 43 | 27 | 15 to 40 | < .001 | 6 | 2 to 13 | 34 | 22 to 46 | 27 | 14 to 41 | < .001 |
| Relapse/progression | ||||||||||||||
| At 1 year | 34 | 26 to 43 | 29 | 19 to 40 | 38 | 24 to 52 | .5694 | 46 | 35 to 57 | 33 | 22 to 45 | 39 | 24 to 53 | .2978 |
| At 3 years | 43 | 33 to 52 | 32 | 21 to 43 | 40 | 26 to 54 | .3282 | 53 | 41 to 64 | 37 | 25 to 49 | 42 | 27 to 56 | .1627 |
| Progression-free survival | ||||||||||||||
| At 1 year | 58 | 49 to 67 | 42 | 31 to 53 | 36 | 22 to 49 | .0113 | 50 | 38 to 60 | 37 | 25 to 49 | 34 | 20 to 49 | .1813 |
| At 3 years | 47 | 37 to 56 | 36 | 25 to 47 | 33 | 20 to 47 | .1834 | 41 | 29 to 52 | 29 | 18 to 41 | 32 | 18 to 46 | .3449 |
| Overall survival | ||||||||||||||
| At 1 year | 68 | 59 to 76 | 49 | 37 to 60 | 59 | 44 to 72 | .0266 | 62 | 50 to 72 | 43 | 30 to 55 | 58 | 41 to 71 | .0701 |
| At 3 years | 59 | 49 to 68 | 39 | 28 to 51 | 52 | 36 to 66 | .0356 | 53 | 40 to 64 | 31 | 20 to 44 | 50 | 33 to 64 | .0349 |
Abbreviations: autoHCT, autologous hematopoietic cell transplantation; CR1, first complete remission; NST, nonmyeloablative stem-cell transplantation; RIC, reduced-intensity conditioning.
Fig 1.
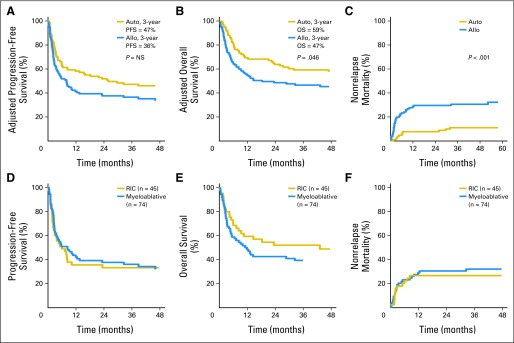
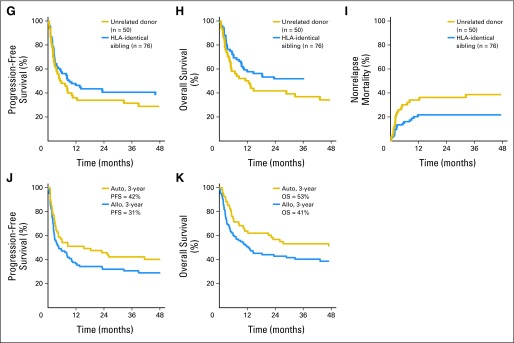
(A) Adjusted progression-free survival (PFS), (B) adjusted overall survival (OS), and (C) nonrelapse mortality (NRM) for all patients (n = 241). (D) PFS, (E) OS, and (F) NRM for patients who underwent nonmyeloablative stem-cell transplantation/reduced-intensity conditioning (RIC) versus myeloablative allogeneic hematopoietic cell transplantation (allo). NS, not significant. (G) PFS, (H) OS, and (I) NRM for patients who underwent HLA-identical sibling versus unrelated donor allo. (J) PFS and (K) OS for patients who underwent autologous HCT (auto) and allo when excluding patients in first complete remission.
Univariate outcomes after excluding CR1 patients are listed in Table 3. NRM at all time points was higher for alloHCT recipients. Overall NRM for autoHCT recipients was 6% at 3 years compared with 34% for alloHCT recipients. Unadjusted OS and PFS were similar for both cohorts. When excluding CR1 patients, relapse was lower for the alloHCT cohort (53% v 38%; P = .0437), but PFS and OS were similar (Fig 1D).
Among 241 patients, 33 were younger than 21 years of age, including 11 patients (eight ALCL, three PTCL-NOS) undergoing autoHCT and 22 (20 ALCL, two PTCL-NOS) undergoing alloHCT. When excluding pediatric patients from the overall NRM, PFS and OS at 1 year and 3 years were similar to those for the entire group (data not shown).
Outcomes by Histology
Subanalyses by histology (ALCL, PTCL-NOS, AITL) were performed (Appendix Table A1, online only). Patients with ALCL undergoing autoHCT (n = 61) had superior PFS (55% v 35%; P = .0319) and OS (68% v 41%; P = .0034), with significantly reduced NRM and overall mortality compared with alloHCT recipients (n = 51). Even when excluding CR1 patients, autoHCT recipients had higher 3-year OS (62% v 33%; P = .0088) and lower transplantation-related mortality (5% v 32%; P < .001), with no difference in PFS or relapse/progression.
When specifically examining autoHCT recipients beyond CR1 by histology, patients with ALCL (n = 39) had 1-year and 3-year PFS rates of 53% (95% CI, 37% to 69%) and 50% (95% CI, 34% to 66%) and 1-year and 3-year OS rates of 74% (95% CI, 59% to 86%) and 65% (95% CI, 49% to 80%), respectively. Patients with PTCL-NOS (n = 28) had 1-year and 3-year PFS rates of 52% (95% CI, 33% to 71%) and 29% (95% CI, 12% to 50%) and 1-year and 3-year OS rates of 57% (95% CI, 38% to 75%) and 42% (95% CI, 22% to 62%), respectively. Only six patients with AITL underwent autoSCT beyond CR1; they had 1-year PFS and OS rates of 33% (95% CI, 5% to 72%). Histology was included in multivariate analyses but did not have an impact on relapse/progression for patients beyond CR1 (Table 4).
Table 4.
Multivariate Analysis of Prognostic Factors for AutoHCT and AlloHCT Recipients, Excluding CR1 Patients (significant covariates are shown)
| Variables | No. of Patients | Nonrelapse Mortality* |
Relapse/Progression† |
Treatment Failure‡ |
Overall Mortality§ |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RR | 95% CI | P | RR | 95% CI | P | RR | 95% CI | P | RR | 95% CI | P | ||
| Main effect | |||||||||||||
| AutoHCT | 72 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | ||||||||
| AlloHCT | 99 | 6.836 | 2.399 to 19.483 | < .001 | 0.696 | 0.428 to 1.133 | .1451 | 1.24 | 0.818 to 1.880 | .3108 | 1.515 | 1.001 to 2.294 | .0494 |
| Time from diagnosis to HCT, months | |||||||||||||
| ≤ 12 | 87 | 1.00 (ref) | |||||||||||
| > 12 | 84 | 2.394 | 1.164 to 4.922 | .0176 | |||||||||
| No. of chemotherapy lines prior to transplantation | .0308‖ | .0022‖ | |||||||||||
| 1 | 17 | 1.00 (ref) | 1.00 (ref) | ||||||||||
| 2 | 68 | 2.312 | 0.978 to 5.468 | .0563 | 3.31 | 1.176 to 9.316 | .0234 | ||||||
| > 2 | 86 | 3.012 | 1.286 to 7.058 | .0111 | 5.139 | 1.858 to 14.210 | .0016 | ||||||
| Chemotherapy-sensitive disease | |||||||||||||
| Sensitive | 110 | 1.00 (ref) | 1.00 (ref) | ||||||||||
| Resistant | 44 | 1.97 | 1.114 to 3.487 | .0198 | 1.744 | 1.116 to 2.725 | .0146 | ||||||
| Disease status prior to transplantation | .0221‖ | .0226‖ | |||||||||||
| CR2+ | 42 | 1.00 (ref) | 1.00 (ref) | ||||||||||
| PIF | 67 | 2.447 | 1.004 to 5.965 | .0489 | 1.377 | 0.690 to 2.749 | .3648 | ||||||
| Relapse | 62 | 0.815 | 0.304 to 2.189 | .6853 | 2.259 | 1.180 to 4.324 | .0139 | ||||||
Abbreviations: alloHCT, allogeneic hematopoietic cell transplantation; autoHCT, autologous HCT; CR1, first complete remission; CR2, second complete remission; PIF, primary induction failure; ref, reference group; RR, relative risk.
PIF v relapse, P = .0119.
Chemotherapy-sensitive disease: resistant v unknown, P = .6435; disease status prior to transplantation: CR2+ v PIF, P = .3799; disease status prior to transplantation: PIF v relapse, P = .0544.
No. of chemotherapy treatments prior to transplantation: 2 v > 2, P = .2086; chemotherapy-sensitive disease: resistant v unknown, P = .1808.
No. of chemotherapy treatments prior to transplantation: 2 v > 2, P = .0429.
Overall P value.
Allogeneic Transplantation
Among alloHCT recipients, there was no difference in AGVHD or CGVHD relapse/progression, PFS, OS, or overall mortality when comparing HLA-identical sibling donors (n = 76) and unrelated donors (n = 50; data not shown). Regimen intensity did not have an impact on PFS, OS, or NRM between MA and NST/RIC HCT recipients. Neither AGVHD nor CGVHD affected relapse or survival. Only 14 patients had T-cell depletion as part of their transplantation, and impact on relapse and survival could not be determined.
COD
The most common COD was lymphoma (Appendix Table A2, online only). Progressive lymphoma leading to death in the three groups (auto v myeloablative v NST/RIC) was significantly higher in the autologous cohort (P = .0036).
Multivariate Analysis
In multivariate models (Table 5), alloHCT (hazard ratio [HR], 3.543) or two or more pretransplantation chemotherapy regimens (HR, 4.059 and HR, 7.035, respectively) were strongly predictive of worse NRM, with no improvement in relapse/progression. Chemotherapy-resistant disease doubled the risk of relapse/progression. Among alloHCT recipients, risk of overall mortality and treatment failure was higher in those not in CR or after more than two lines of chemotherapy (Table 4).
Table 5.
Multivariate Analysis of Prognostic Factors for AutoHCT and AlloHCT Recipients (significant covariates are shown)
| Variable | Nonrelapse Mortality* |
Relapse/Progression |
Treatment Failure† |
Overall Mortality‡ |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RR | 95% CI | P | RR | 95% CI | P | RR | 95% CI | P | RR | 95% CI | P | |
| Main effect | ||||||||||||
| AutoHCT | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | ||||||||
| AlloHCT | 3.543 | 1.795 to 6.993 | < .001 | 0.728 | 0.460 to 1.150 | .1738 | 1.274 | 0.876 to 1.852 | .2047 | 1.425 | 0.948 to 2.141 | .0883 |
| No. of chemotherapy lines prior to transplantation | .0118§ | .0024§ | < .001§ | |||||||||
| 1 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | |||||||||
| 2 | 4.059 | 0.932 to 17.665 | .0619 | 2.069 | 1.072 to 3.990 | .0301 | 3.385 | 1.432 to 7.997 | .0054 | |||
| > 2 | 7.035 | 1.671 to 29.612 | .0078 | 2.988 | 1.564 to 5.708 | < .001 | 5.016 | 2.147 to 11.720 | < .001 | |||
| Chemotherapy-sensitive disease | .0323§ | .0076§ | .0332§ | |||||||||
| Sensitive | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | |||||||||
| Resistant | 2.006 | 1.137 to 3.539 | .0163 | 1.981 | 1.285 to 3.052 | .0019 | 1.821 | 1.155 to 2.872 | .0099 | |||
| Disease status prior to transplantation | .0012§ | |||||||||||
| CR1 | 1.00 (ref) | |||||||||||
| CR2+ | 1.626 | 0.736 to 3.591 | .2293 | |||||||||
| PIF | 2.215 | 1.066 to 4.606 | .0331 | |||||||||
| Relapse | 3.661 | 1.834 to 7.309 | < .001 | |||||||||
Abbreviations: alloHCT, allogeneic hematopoietic cell transplantation; autoHCT, autologous HCT; CR1, first complete remission; CR2, second complete remission; PIF, primary induction failure; ref, reference group; RR, relative risk.
No. of chemotherapy treatments prior to transplantation: 2 v > 2, P = .0802 (nonrelapse mortality).
No. of chemotherapy treatments prior to transplantation: 2 v > 2, P = .0569; chemotherapy-sensitive disease: resistant v unknown, P = .3014.
No. of chemotherapy treatments prior to transplantation: 2 v > 2, P = .0583; chemotherapy-sensitive disease: resistant v unknown, P = .1430.
Overall P value.
When excluding CR1 patients (Table 4), NRM risk was higher in alloHCT recipients, in those who experienced PIF, and in those receiving HCT more than 12 months from diagnosis. Relapse risk was higher for chemotherapy-resistant disease or relapsed disease at time of transplantation. Higher number of chemotherapy lines before HCT correlated with higher risk of mortality and treatment failure.
In multivariate analysis restricted to patients with PTCL-NOS, receipt of alloHCT remained a significant risk factor for NRM (HR, 3.031; 95% CI, 1.025 to 8.961). Similar to the overall group, chemotherapy resistance predicted for increased relapse, increased treatment failure, and worse overall mortality. However, the relative risk of relapse/progression was halved by use of alloHCT (HR, 0.504; P = .04). Transplantation type (autoHCT v alloHCT) did not have an impact on OS.
DISCUSSION
To the best of our knowledge, this is the largest report on the outcomes and analyses of patient-, disease-, and treatment-related factors for patients with systemic T-NHL undergoing HCT. HCT, whether autologous or allogeneic, can benefit a considerable subset of patients with T-NHL, with few patients relapsing beyond 3 years. Despite baseline differences in autoHCT and alloHCT recipients, a unifying finding was that transplantation was often offered late in the disease course, with 50% of alloHCT and 30% of autoHCT patients receiving more than two prior treatment regimens. Such late referral was detrimental; the use of either modality for patients receiving more than two lines of therapy was associated with a three-fold increased risk of relapse, five-fold increased risk of overall mortality, and a seven-fold increased risk of NRM. A corollary of the number of regimens is chemotherapy sensitivity; patients with chemotherapy-resistant disease had essentially a two-fold increased risk of relapse, treatment failure, and overall mortality. Considering that chemotherapy-resistant patients more often receive more than two lines of pretransplantation therapy, these findings are likely related. The main implication is that if transplantation is to be applied, both toxicity and efficacy are optimized by fewer prior chemotherapy regimens, and transplantation is most beneficial when offered as part of first- or second-line therapy.
The initial intent was to compare autoHCT v alloHCT outcomes, but there were substantially differing baseline characteristics by treatment modality. Patients undergoing autoHCT were more likely to be in CR1, have chemotherapy-sensitive disease, have ALCL subtype, and have two or fewer lines of prior therapy. To account for these differences, an adjusted model was used in multivariate analysis to assess the relative risk of autoHCT v alloHCT and evaluate prognostic factors. This model did not find a difference in relapse/progression between autoHCT and alloHCT, although the latter significantly increased NRM (HR, 3.543).
An intriguing finding is that high-dose chemotherapy as part of autoHCT can be beneficial at relapse, which conflicts with several prior reports.10–13 When excluding patients in first remission, the aggregate group of autoHCT recipients had 3-year PFS and OS rates of 41% and 53%, respectively, with a robust median follow-up time of 73 months. In particular, patients with ALCL undergoing autoHCT at relapse had superior overall survival compared with alloHCT recipients; similar conclusions for other histologies is precluded by small numbers. NRM, although higher than that reported in many B-cell lymphoma series, was 4% at 1 year and 6% at 3 years. These findings along with the results of the multivariate analysis (Table 4) suggest that, despite discouraging retrospective reports previously discussed, a subset of relapsed patients with T-NHL can benefit from effective salvage treatment with autoHCT, particularly if performed as part of second-line therapy and if chemotherapy sensitivity is demonstrated. Given that half the pool of patients undergoing autoSCT beyond CR1 had ALCL histology, this likely influences the results of the multivariate analysis; however, histology did not specifically emerge as a significant factor.
AlloHCT has been proposed as an alternative to autoHCT, given the potential for graft-versus-leukemia effects and reports of durable remissions and few late relapses.14–17 Among 126 patients undergoing alloHCT in our series, we did not find differences in outcome on the basis of either donor type (related v unrelated) or regimen intensity (myeloablative v NST/RIC). When considering regimen intensity, there are few comparative reports, but La Société Française de Greffe de Moëlle et de Thérapie Cellulaire (SFGM-TC) found no difference in either toxicity or survival between MA and NST regimens; the impact of regimen intensity on relapse was not reported.18 Of note, a single-center review of 52 patients found that RIC regimens conferred a seven-fold increased risk of relapse but no difference in either PFS or OS.19 Our series found no difference in 3-year transplantation-related mortality, PFS, OS, or risk of relapse/progression by alloHCT regimen intensity. In addition, neither AGVHD nor CGVHD correlated with outcome, although patients not in remission at time of transplantation had a four-fold increased risk of CGVHD.
A limitation of this analysis was lack of central pathology review for all patients. For example, the WHO defines two distinct subsets of ALCL on the basis of ALK status that differ by age. ALK-positive ALCL occurs primarily in males younger than age 30 years, and many consider this a pediatric disease. In contrast, ALK-negative ALCL is more common beyond age 40. Despite the impact of ALK on response to initial treatment, we were unable to include ALK status in our analysis because of unavailable data. Several lines of evidence suggest that higher International Prognostic Index and increased age are powerful surrogates for ALK, with high-risk patients faring poorly, independent of ALK status.20 A subanalysis of the International T-Cell Lymphoma Project observed that patients older than age 40 years had no differential outcome (PFS or OS) on the basis of ALK positivity.21 In our analysis, the median age of patients was 43 years for autoHCT and 38 years for alloHCT recipients, making a high proportion of ALK-positive patients unlikely. Furthermore, the significance of ALK status at relapse is unknown, suggesting an attenuated impact of ALK status in this setting that hopefully limits the impact of this deficiency.
Another issue inherent to transplantation registries is that only patients undergoing transplantation are included, with no data regarding patients unable to receive HCT because of refractory disease, age, comorbidities, or other factors. In light of the advanced median age of patients with T-NHL, clearly only a portion of them undergo transplantation. Furthermore, there is keen awareness of inadequate first-line regimens, with primary refractory disease often precluding transplantation. A Spanish intent-to-treat analysis found that only 40% of patients undergoing induction underwent autoHCT because of primary treatment failure,22 although other prospective trials show that higher proportions of patients (66% to 80%) of patients can proceed.
Despite these caveats, this large series shows that HCT can benefit patients with T-NHL in both relapsed and first-line settings, and with both autoHCT and alloHCT approaches. Importantly, approximately 40% of patients undergoing autoHCT at the time of relapse attain long-term benefit and disease control, particularly for ALCL histology. One-third of alloHCT recipients remain progression-free at 3 years, despite being more heavily pretreated and having more refractory disease. Our results suggest that if HCT is considered, outcomes are best in chemotherapy-sensitive patients at the time of first- or second-line therapy, perhaps supporting evolving treatment paradigms in T-NHL in which HCT is considered earlier in overall management.
Acknowledgment
We thank Laren C. Pinter-Brown, Maria L. Delioukina, Christopher Dvorak, Mark B. Juckett, Mary Jo Lechowicz, Miguel A. Pavlovsky, and Julie M. Vose for their helpful comments and insights as members of the study committee.
Appendix
Table A1.
Subset Analysis by Histology: Univariate Probabilities of Outcome for ALCL, PTCL, and AITL
| Outcome Event | ALCL |
PTCL |
AITL |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AutoHCT (n = 61) |
AlloHCT (n = 51) |
P | AutoHCT (n = 39) |
AlloHCT (n = 63) |
P | AutoHCT (n = 15) |
AlloHCT (n = 12) |
P | |||||||
| % | 95% CI (%) | % | 95% CI (%) | % | 95% CI (%) | % | 95% CI (%) | % | 95% CI (%) | % | 95% CI (%) | ||||
| Acute GVHD, grades 2 to 4 | |||||||||||||||
| At 100 days | 18 | 9 to 30 | 10 | 4 to 19 | 8 | 0 to 30 | |||||||||
| Chronic GVHD | |||||||||||||||
| At 1 year | 20 | 10 to 32 | 41 | 28 to 53 | 27 | 6 to 56 | |||||||||
| At 3 years | 22 | 12 to 34 | 43 | 30 to 55 | 27 | 6 to 56 | |||||||||
| Nonrelapse mortality | |||||||||||||||
| At 100 days | 2 | 0 to 8 | 22 | 12 to 34 | < .001 | 3 | 0 to 12 | 16 | 8 to 26 | .0115 | 0 | 8 | 1 to 31 | ||
| At 1 year | 10 | 4 to 19 | 29 | 18 to 42 | .0093 | 3 | 0 to 12 | 28 | 17 to 39 | < .001 | 7 | 0 to 26 | 8 | 1 to 31 | .8709 |
| At 3 years | 10 | 4 to 19 | 31 | 19 to 44 | .0046 | 15 | 5 to 31 | 29 | 19 to 41 | .1164 | 7 | 0 to 26 | 8 | 1 to 31 | .8709 |
| Relapse/progression | |||||||||||||||
| At 1 year | 31 | 20 to 43 | 31 | 19 to 44 | .9989 | 37 | 22 to 52 | 32 | 21 to 44 | .6399 | 40 | 16 to 63 | 25 | 6 to 50 | .399 |
| At 3 years | 35 | 23 to 47 | 33 | 21 to 46 | .8679 | 56 | 37 to 71 | 38 | 26 to 50 | .098 | 47 | 21 to 69 | 25 | 6 to 50 | .2274 |
| Progression-free survival | |||||||||||||||
| At 1 year | 59 | 45 to 70 | 39 | 26 to 52 | .0376 | 60 | 43 to 74 | 40 | 28 to 52 | .045 | 53 | 26 to 74 | 67 | 34 to 86 | .4767 |
| At 3 years | 55 | 42 to 67 | 35 | 22 to 48 | .0319 | 29 | 14 to 47 | 33 | 22 to 45 | .7188 | 47 | 21 to 69 | 67 | 34 to 86 | .2858 |
| Overall survival | |||||||||||||||
| At 1 year | 73 | 60 to 83 | 49 | 35 to 62 | .0072 | 64 | 46 to 77 | 52 | 38 to 64 | .25 | 60 | 35 to 82 | 92 | 70 to 100 | .034 |
| At 3 years | 68 | 54 to 78 | 41 | 27 to 54 | .0034 | 45 | 27 to 62 | 42 | 30 to 55 | .7979 | 51 | 26 to 76 | 83 | 56 to 98 | .077 |
| Overall mortality | |||||||||||||||
| At 30 days | 0 | 8 | 3 to 20 | 0 | 2 | 0 to 11 | 7 | 1 to 39 | 8 | 1 to 46 | .87 | ||||
| At 100 days | 7 | 3 to 17 | 33 | 22 to 48 | < .001 | 8 | 3 to 22 | 22 | 14 to 35 | .0315 | 13 | 4 to 44 | 8 | 1 to 46 | .67 |
Abbreviations: AITL, angioimmunoblastic T-cell lymphoma; ALCL, anaplastic large-cell lymphoma; alloHCT, allogeneic hematopoietic cell transplantation; autoHCT, autologous HCT; GVHD, graft-versus-host disease; PTCL, peripheral T-cell lymphoma.
Table A2.
Cause of Death by Transplantation Modality
| Cause of Death | AutoHCT |
Myeloablative |
NST/RIC |
||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of Patients | No. | % | No. of Patients | No. | % | No. of Patients | No. | % | |
| No. of patients | 115 | 74 | 45 | ||||||
| No. of deaths | 51 | 45 | 24 | ||||||
| Primary disease | 37 | 73 | 18 | 40 | 11 | 46 | |||
| Infection | 4 | 8 | 2 | 4 | 3 | 13 | |||
| IPn | 0 | 4 | 9 | 2 | 8 | ||||
| ARDS | 2 | 4 | 2 | 4 | 0 | ||||
| Organ failure | 4 | 8 | 10 | 22 | 3 | 13 | |||
| Accidental death | 1 | 2 | 0 | 0 | |||||
| Graft failure | 1 | 2 | 0 | 0 | |||||
| Hemorrhage | 0 | 3 | 7 | 1 | 4 | ||||
| GVHD | 0 | 3 | 7 | 2 | 8 | ||||
| Pulmonary toxicity | 0 | 2 | 4 | 0 | |||||
| Vascular (cardiac or cerebral) | 0 | 1 | 2 | 1 | 4 | ||||
| Other, not specified | 2 | 4 | 0 | 1 | 4 | ||||
Abbreviations: ARDS, acute respiratory disease syndrome; autoHCT, autologous hematopoietic cell transplantation; GVHD, graft-versus-host disease; IPn, interstitial pneumonitis; NST, nonmyeloablative stem-cell transplantation; RIC, reduced-intensity conditioning.
Footnotes
Support information appears at the end of this article.
The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, or any other agency of the US Government.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Koen van Besien, Center for International Blood and Marrow Transplant Research (U); Ritsuro Suzuki, EUSA Pharma (C); Leona A. Holmberg, Seattle Genetics (C), Genzyme (C); Thomas G. Gross, Roche (C), Eli Lilly (C); Peter H. Wiernik, Celgene (C) Stock Ownership: None Honoraria: Ritsuro Suzuki, Kyowa Hakko Kirin; Jack W. Hsu, MedTrend; Peter H. Wiernik, Novartis; Silvia Montoto, Roche, Celgene Research Funding: Leona A. Holmberg, Merck, Millennium Pharmaceuticals, Otsuka Pharmaceutical, Sanofi/Genzyme, Seattle Genetics; Silvia Montoto, Genentech Expert Testimony: None Patents: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Sonali M. Smith, Linda J. Burns, Jennifer LeRademacher, Harry C. Schouten, Robert Peter Gale, Luciano J. Costa, Ginna G. Laport, Silvia Montoto, Parameswaran N. Hari
Collection and assembly of data: Linda J. Burns, Wensheng He, Parameswaran N. Hari
Data analysis and interpretation: Sonali M. Smith, Linda J. Burns, Koen van Besien, Jennifer LeRademacher, Wensheng He, Timothy S. Fenske, Ritsuro Suzuki, Jack W. Hsu, Harry C. Schouten, Gregory A. Hale, Leona A. Holmberg, Anna Sureda, Cesar O. Freytes, Richard Thomas Maziarz, David J. Inwards, Thomas G. Gross, Mitchell S. Cairo, Hillard M. Lazarus, Peter H. Wiernik, Dipnarine Maharaj, Ginna G. Laport, Silvia Montoto, Parameswaran N. Hari
Manuscript writing: All authors
Final approval of manuscript: All authors
Support
Supported by Public Health Service Grant No. U24-CA76518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI), and the National Institute of Allergy and Infectious Diseases; Grant No. 5U01HL069294 from NHLBI and NCI; Contract No. HHSH234200637015C with Health Resources and Services Administration, Department of Health and Human Services; Grants No. N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research; and grants from Allos Therapeutics; Amgen; Angioblast Systems; anonymous donation to the Medical College of Wisconsin; Ariad Pharmaceuticals; Be the Match Foundation; Blue Cross and Blue Shield Association; Buchanan Family Foundation; CaridianBCT; Celgene; CellGenix; Children's Leukemia Research Association; Fresenius-Biotech North America; Gamida Cell-Teva Joint Venture; Genentech; Genzyme; GlaxoSmithKline; HistoGenetics; Kiadis Pharma; The Leukemia & Lymphoma Society; The Medical College of Wisconsin; Merck; Millennium Pharmaceuticals; The Takeda Oncology Company; Milliman USA; Miltenyi Biotec; National Marrow Donor Program; OptumHealth Care Solutions; Osiris Therapeutics; Otsuka America Pharmaceutical; RemedyMD; Sanofi; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix; StemCyte; STEMSOFT Software; Swedish Orphan Biovitrum; Tarix Pharmaceuticals; Teva Neuroscience; Therakos; and WellPoint.
REFERENCES
- 1.Vose J, Armitage J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: Pathology findings and clinical outcomes. J Clin Oncol. 2008;26:4124–4130. doi: 10.1200/JCO.2008.16.4558. [DOI] [PubMed] [Google Scholar]
- 2.Schmitz N, Trumper L, Ziepert M, et al. Treatment and prognosis of mature T-cell and NK-cell lymphoma: An analysis of patients with T-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood. 2010;116:3418–3425. doi: 10.1182/blood-2010-02-270785. [DOI] [PubMed] [Google Scholar]
- 3.Kyriakou C, Canals C, Goldstone A, et al. High-dose therapy and autologous stem-cell transplantation in angioimmunoblastic lymphoma: Complete remission at transplantation is the major determinant of Outcome-Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2008;26:218–224. doi: 10.1200/JCO.2008.12.6219. [DOI] [PubMed] [Google Scholar]
- 4.Nademanee A, Palmer JM, Popplewell L, et al. High-dose therapy and autologous hematopoietic cell transplantation in peripheral T cell lymphoma (PTCL): Analysis of prognostic factors. Biol Blood Marrow Transplant. 2011;17:1481–1489. doi: 10.1016/j.bbmt.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reimer P, Rüdiger T, Geissinger E, et al. Autologous stem-cell transplantation as first-line therapy in peripheral T-cell lymphomas: Results of a prospective multicenter study. J Clin Oncol. 2009;27:106–113. doi: 10.1200/JCO.2008.17.4870. [DOI] [PubMed] [Google Scholar]
- 6.Rodríguez J, Conde E, Gutiérrez A, et al. Frontline autologous stem cell transplantation in high-risk peripheral T-cell lymphoma: A prospective study from The Gel-Tamo Study Group. Eur J Haematol. 2007;79:32–38. doi: 10.1111/j.1600-0609.2007.00856.x. [DOI] [PubMed] [Google Scholar]
- 7.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: Working definitions. Biol Blood Marrow Transplant. 2009;15:1628–1633. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weisdorf D, Spellman S, Haagenson M, et al. Classification of HLA-matching for retrospective analysis of unrelated donor transplantation: Revised definitions to predict survival. Biol Blood Marrow Transplant. 2008;14:748–758. doi: 10.1016/j.bbmt.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallamini A, Stelitano C, Calvi R, et al. Peripheral T-cell lymphoma unspecified (PTCL-U): A new prognostic model from a retrospective multicentric clinical study. Blood. 2004;103:2474–2479. doi: 10.1182/blood-2003-09-3080. [DOI] [PubMed] [Google Scholar]
- 10.Chen AI, McMillan A, Negrin RS, et al. Long-term results of autologous hematopoietic cell transplantation for peripheral T cell lymphoma: The Stanford experience. Biol Blood Marrow Transplant. 2008;14:741–747. doi: 10.1016/j.bbmt.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feyler S, Prince HM, Pearce R, et al. The role of high-dose therapy and stem cell rescue in the management of T-cell malignant lymphomas: A BSBMT and ABMTRR study. Bone Marrow Transplant. 2007;40:443–450. doi: 10.1038/sj.bmt.1705752. [DOI] [PubMed] [Google Scholar]
- 12.Kewalramani T, Zelenetz AD, Teruya-Feldstein J, et al. Autologous transplantation for relapsed or primary refractory peripheral T-cell lymphoma. Br J Haematol. 2006;134:202–207. doi: 10.1111/j.1365-2141.2006.06164.x. [DOI] [PubMed] [Google Scholar]
- 13.Song KW, Mollee P, Keating A, et al. Autologous stem cell transplant for relapsed and refractory peripheral T-cell lymphoma: Variable outcome according to pathological subtype. Br J Haematol. 2003;120:978–985. doi: 10.1046/j.1365-2141.2003.04203.x. [DOI] [PubMed] [Google Scholar]
- 14.Corradini P, Dodero A, Zallio F, et al. Graft-versus-lymphoma effect in relapsed peripheral T-cell non-Hodgkin's lymphomas after reduced-intensity conditioning followed by allogeneic transplantation of hematopoietic cells. J Clin Oncol. 2004;22:2172–2176. doi: 10.1200/JCO.2004.12.050. [DOI] [PubMed] [Google Scholar]
- 15.Delioukina M, Zain J, Palmer JM, et al. Reduced-intensity allogeneic hematopoietic cell transplantation using fludarabine-melphalan conditioning for treatment of mature T-cell lymphomas. Bone Marrow Transplant. 2012;47:65–72. doi: 10.1038/bmt.2011.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dodero A, Spina F, Narni F, et al. Allogeneic transplantation following a reduced-intensity conditioning regimen in relapsed/refractory peripheral T-cell lymphomas: Long-term remissions and response to donor lymphocyte infusions support the role of a graft-versus-lymphoma effect. Leukemia. 2012;26:520–526. doi: 10.1038/leu.2011.240. [DOI] [PubMed] [Google Scholar]
- 17.Zain J, Palmer JM, Delioukina M, et al. Allogeneic hematopoietic cell transplant for peripheral T-cell non-Hodgkin lymphoma results in long-term disease control. Leuk Lymphoma. 2011;52:1463–1473. doi: 10.3109/10428194.2011.574754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Gouill S, Milpied N, Buzyn A, et al. Graft-versus-lymphoma effect for aggressive T-cell lymphomas in adults: A study by the Société Française de Greffe de Moëlle et de Thérapie Cellulaire. J Clin Oncol. 2008;26:2264–2271. doi: 10.1200/JCO.2007.14.1366. [DOI] [PubMed] [Google Scholar]
- 19.Jacobsen ED, Kim HT, Ho VT, et al. A large single-center experience with allogeneic stem-cell transplantation for peripheral T-cell non-Hodgkin lymphoma and advanced mycosis fungoides/Sezary syndrome. Ann Oncol. 2011;22:1608–1613. doi: 10.1093/annonc/mdq698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki R, Kagami Y, Takeuchi K, et al. Prognostic significance of CD56 expression for ALK-positive and ALK-negative anaplastic large-cell lymphoma of T/null cell phenotype. Blood. 2000;96:2993–3000. [PubMed] [Google Scholar]
- 21.Savage KJ, Harris NL, Vose JM, et al. ALK-anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: Report from the International Peripheral T-Cell Lymphoma Project. Blood. 2008;111:5496–5504. doi: 10.1182/blood-2008-01-134270. [DOI] [PubMed] [Google Scholar]
- 22.Mercadal S, Briones J, Xicoy B, et al. Intensive chemotherapy (high-dose CHOP/ESHAP regimen) followed by autologous stem-cell transplantation in previously untreated patients with peripheral T-cell lymphoma. Ann Oncol. 2008;19:958–963. doi: 10.1093/annonc/mdn022. [DOI] [PubMed] [Google Scholar]


