Abstract
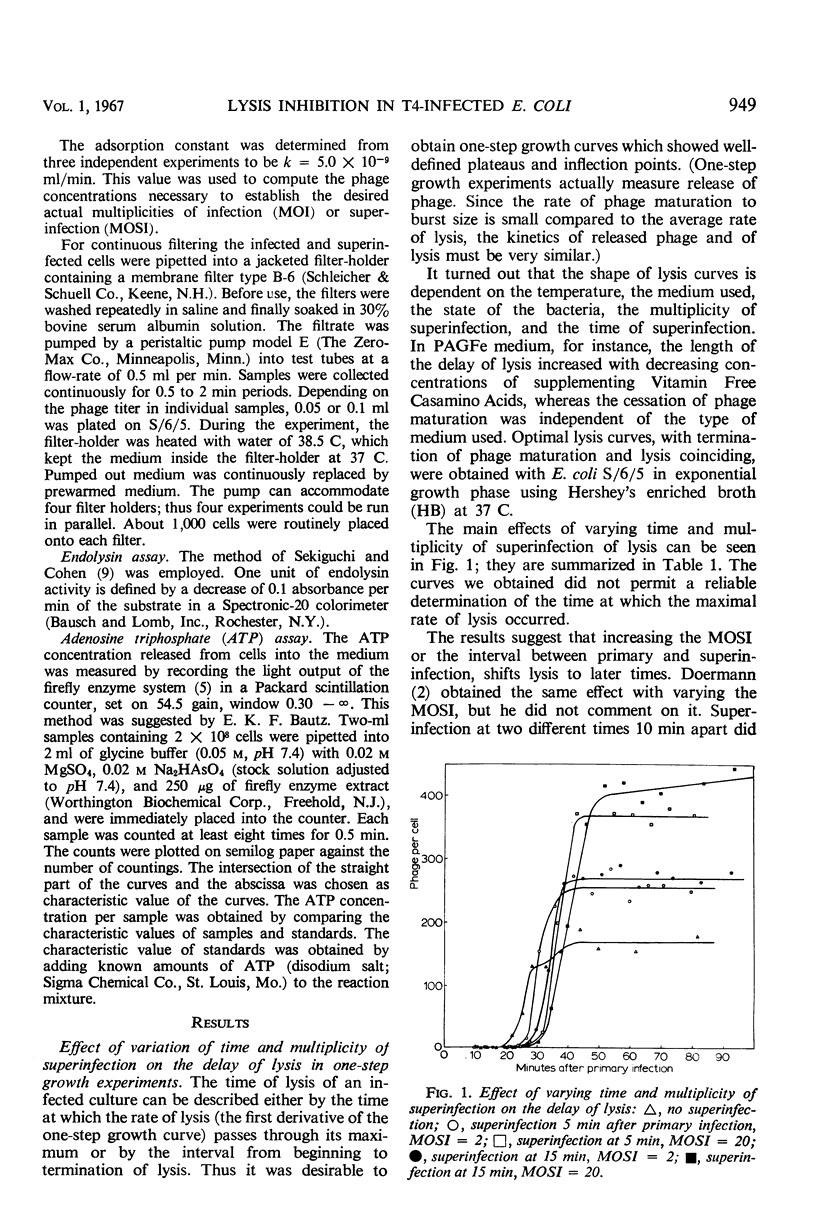
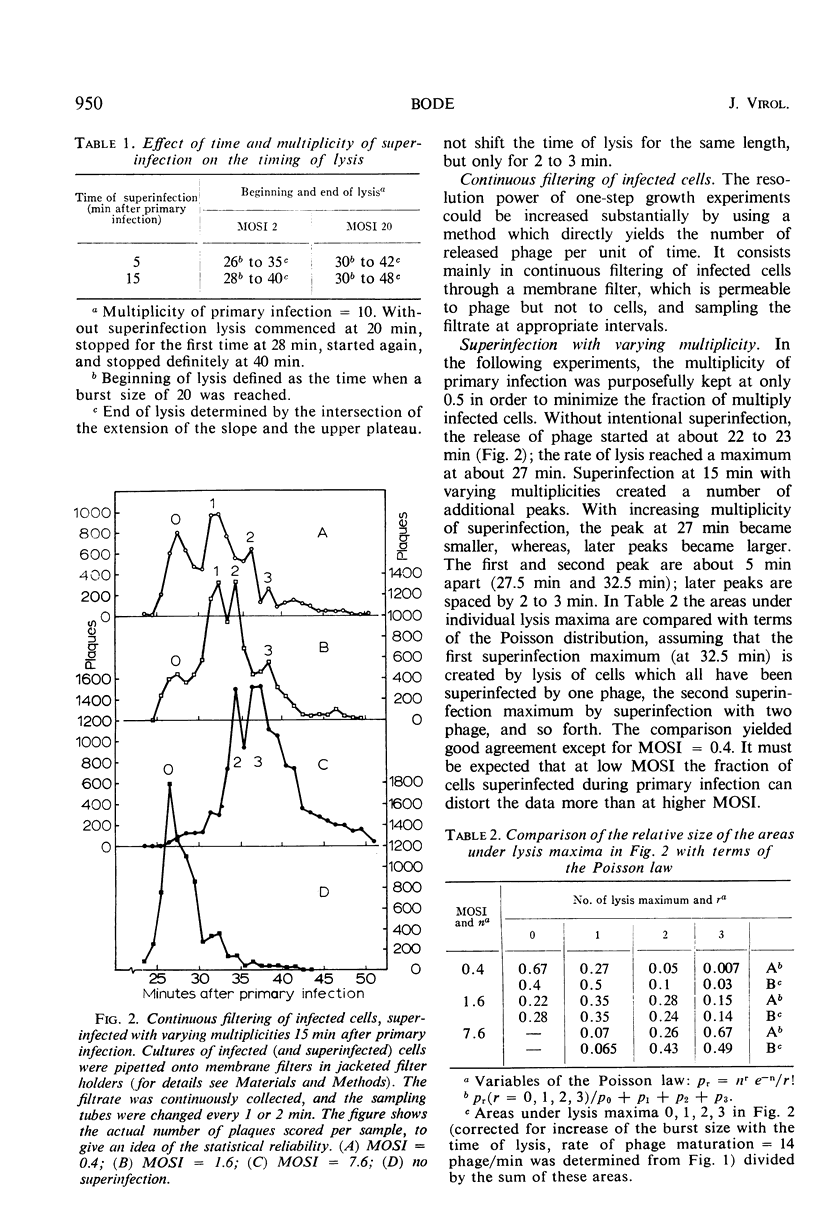
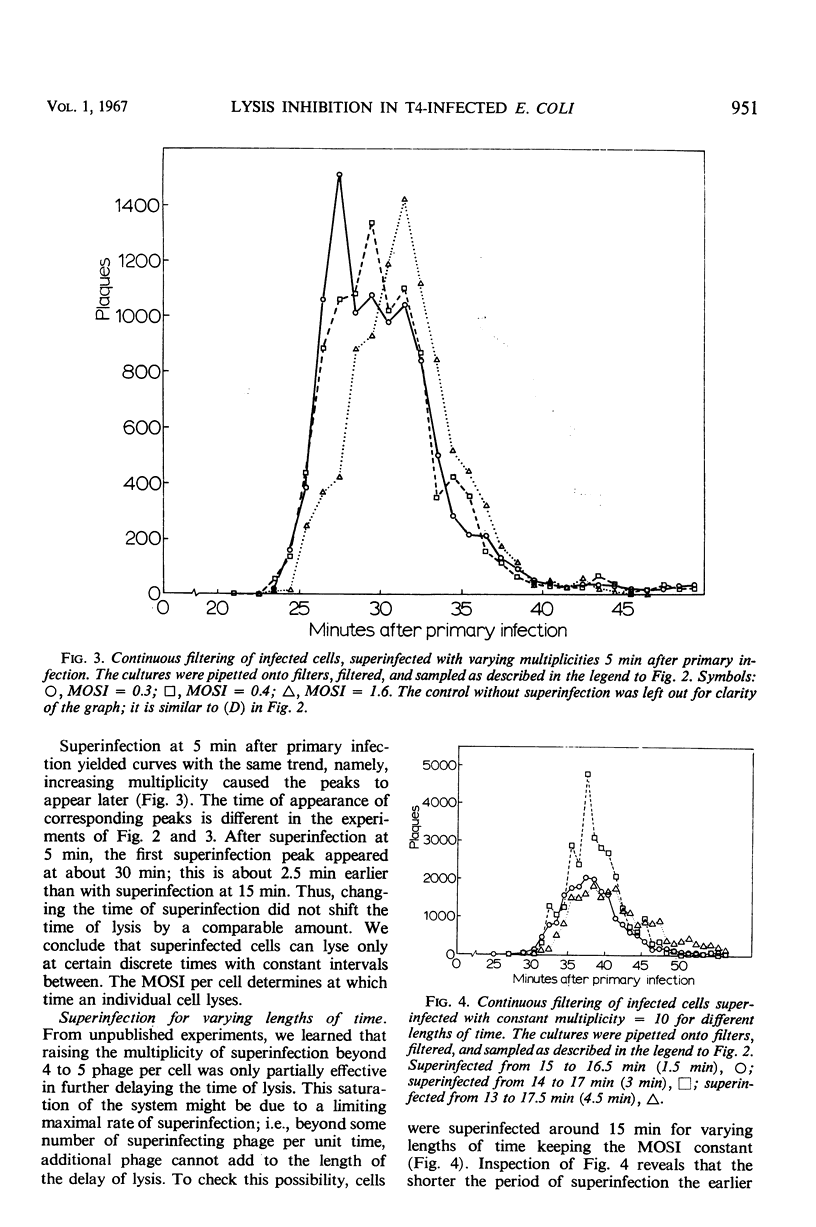
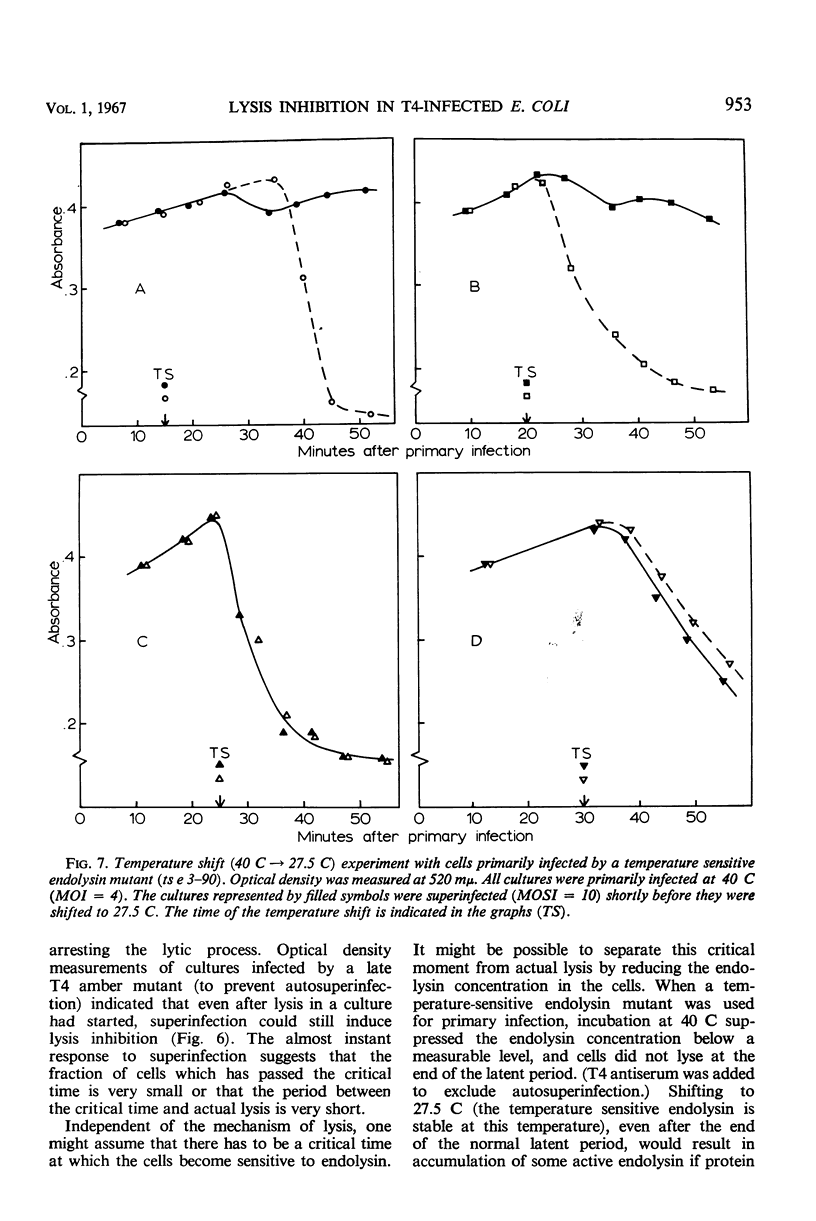
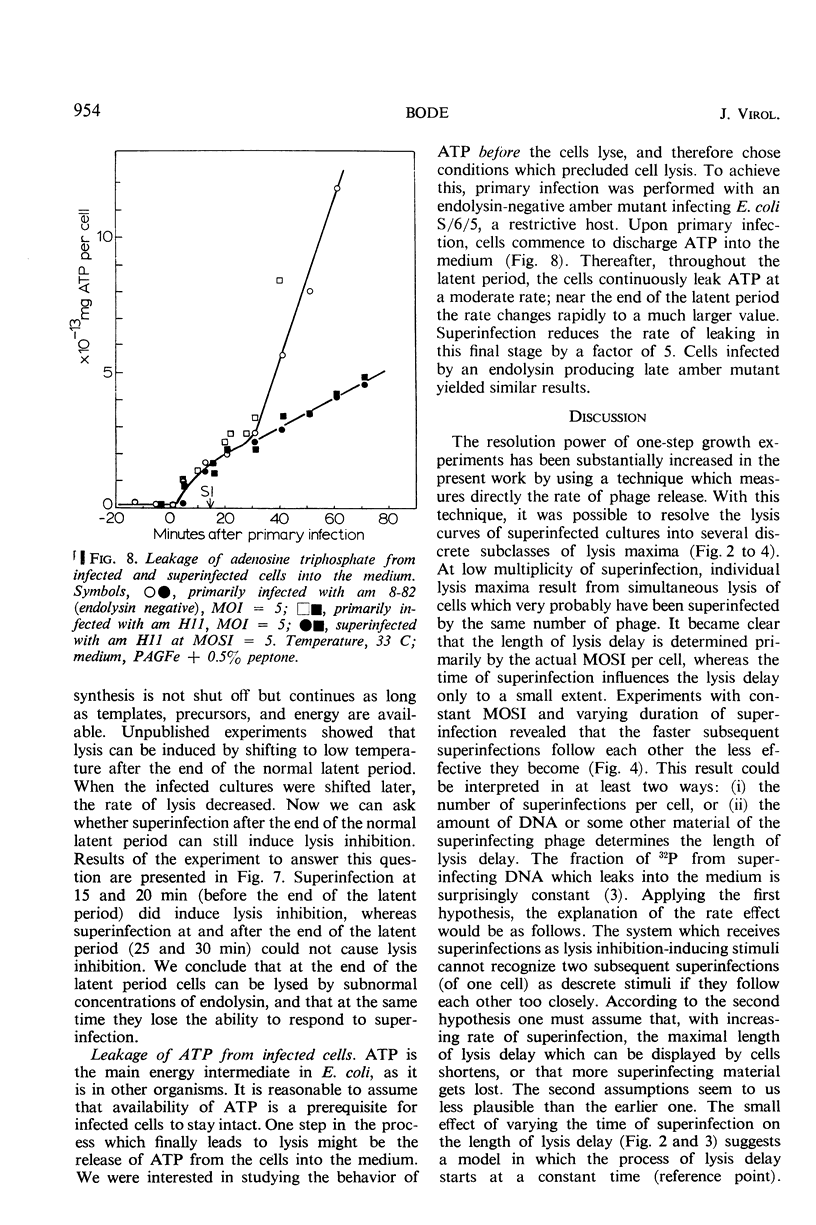
A technique of continuous filtration of T4-infected Escherichia coli has been devised to study the phenomenon of lysis inhibition. Studies using this technique revealed that the length of the lysis delay caused by superinfection can attain only certain discrete values, which for low average multiplicity of superinfection is thought to be a reflection of the actual number of superinfecting particles per cell. The time interval between primary and superinfection had little effect on the length of lysis delay. With increasing rate of superinfection, the length of lysis delay decreased. In superinfected cells, the concentration of endolysin exceeded the final concentration in nonsuperinfected cells. Superinfection of a lysing culture induced lysis inhibition immediately. Temperature-shift experiments, with cells primarily infected by a temperature-sensitive endolysin mutant, revealed that after the normal latent period superinfection was unable to induce lysis inhibition. Amber-restrictive cells, which were primarily infected by an endolysin negative amber mutant, released adenosine triphosphate (ATP) at the end of the normal latent period although lysis did not occur. Superinfection reduced the loss of ATP markedly. The hypothetical role of the cytoplasmic membrane in lysis inhibition is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chase M, Doermann A H. High Negative Interference over Short Segments of the Genetic Structure of Bacteriophage T4. Genetics. 1958 May;43(3):332–353. doi: 10.1093/genetics/43.3.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doermann A. H. Lysis and Lysis Inhibition with Escherichia coli Bacteriophage. J Bacteriol. 1948 Feb;55(2):257–276. doi: 10.1128/jb.55.2.257-276.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRENCH R. C., LESLEY S. M., GRAHAM A. F., van ROOYEN C. E. Studies on the relationship between virus and host cell. III. The breakdown of P32 labelled T2r+ bacteriophage adsorbed to E. coli previously infected by other coliphages of the T group. Can J Med Sci. 1951 Jun;29(3):144–148. [PubMed] [Google Scholar]
- Rutberg B., Rutberg L. Role of superinfecting phage in lysis inhibition with phage T4 in Escherichia coli. J Bacteriol. 1965 Oct;90(4):891–894. doi: 10.1128/jb.90.4.891-894.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEKIGUCHI M., COHEN S. S. THE SYNTHESIS OF MESSENGER RNA WITHOUT PROTEIN SYNTHESIS. II. SYNTHESIS OF PHAGE-INDUCED RNA AND SEQUENTIAL ENZYME PRODUCTION. J Mol Biol. 1964 May;8:638–659. doi: 10.1016/s0022-2836(64)80114-5. [DOI] [PubMed] [Google Scholar]


