Abstract
Background
Antipsychotic-induced subjective inner restlessness is one of the common and distressing adverse effects associated with antipsychotics; however, its underlying neurobiological basis is not well understood. We examined the relationship between antipsychotic-induced subjective inner restlessness and autonomic neurocardiac function.
Methods
Twenty-two schizophrenia patients with antipsychotic-induced subjective restlessness, 28 schizophrenia patients without antipsychotic-induced subjective restlessness, and 28 matched healthy control subjects were evaluated. Assessments of the linear and nonlinear complexity measures of heart rate dynamics were performed. Multivariate analysis of variance and correlation analysis were conducted.
Results
The mean interbeat (RR) interval value was significantly higher in control subjects than in patients with and without antipsychotic-induced subjective restlessness (P < 0.05). The low frequency/high frequency ratio was significantly higher in patients with antipsychotic-induced subjective restlessness than in control subjects and in patients without antipsychotic-induced subjective restlessness (P < 0.05), while the approximate entropy value was significantly lower in patients with antipsychotic-induced subjective restlessness than in control subjects and in patients without antipsychotic-induced subjective restlessness (P < 0.05). Correlation analyses controlling for psychotic symptom severity showed that the degree of antipsychotic-induced restlessness had a significant negative correlation with the value of approximate entropy (P < 0.05).
Conclusion
The results indicate that antipsychotic-induced subjective restlessness is associated with altered heart rate dynamics parameters, particularly the nonlinear complexity measure, suggesting that it might adversely affect autonomic neurocardiac integrity. Further prospective research is necessary to elucidate the precise interrelationships and causality.
Keywords: antipsychotics, subjective restlessness, heart rate dynamics
Introduction
Several psychotropic drugs produce subjective inner restlessness in therapeutic or nontoxic doses.1 The most important clinical syndrome in this context is that caused by dopamine antagonists.1,2 Antipsychotic-induced subjective restlessness has been receiving increased attention because of its significant impact on drug compliance.3 In addition, it causes considerable distress in an already vulnerable group of patients and may also represent a risk factor for suicidal behavior.3
The neurobiological basis for antipsychotic-induced subjective restlessness is not well understood, although the associated strong affective component suggests that it is of central origin. One of the crucial elements may be dysfunction of cortical-subcortical circuits involving dopamine and other neurotransmitter systems.1,3 The basal ganglia, the common site of action for antipsychotics, also interact strongly with the limbic system, which controls the affective domain.1,3 Considering that the limbic system is substantially involved in the pathophysiology of autonomic nervous system (ANS) reactivity,4 it is possible that the parameters measuring autonomic neurocardiac regulation may reflect the functional state of the cortical and mesolimbic system, which could be linked to the pathogenesis of antipsychotic-induced restlessness. Nevertheless, few studies have examined its relationship with autonomic neurocardiac dysregulation. The investigation of autonomic correlates of antipsychotic-induced subjective restlessness may provide valuable insight and understanding into its pathophysiology.
In the present study, we examined the relationship between antipsychotic-induced subjective inner restlessness and autonomic neurocardiac function using analysis of heart rate variability (HRV) in patients with schizophrenia treated with risperidone.
Materials and methods
Subjects
The study protocol was approved by the local ethical committee, and all procedures used in the study were conducted in accordance with international ethical standards, Declaration of Helsinki. The criteria for patient recruitment were (1) a diagnosis of schizophrenia by Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV),5 which was established using the Structured Clinical Interview for DSM-IV6; (2) age 20–50 years; (3) inpatients on monotherapy with risperidone without adjunctive medications; and (4) no treatment with psychotropic drugs known to cause subjective inner restlessness other than antipsychotics within the 2-week period prior to the beginning of the study. The drugs included lithium carbonate, tricyclic antidepressants, selective serotonin reuptake inhibitors, and calcium channel blockers. None of the patients were taking drugs known to treat akathisia, such as beta blockers, mianserin, or mirtazapine, at the time of enrollment.
Patients were excluded if they (1) met diagnostic criteria for a psychiatric diagnosis other than schizophrenia; (2) had a concurrent diagnosis of substance abuse or dependence (including nicotine dependence); or (3) had concurrent cardiovascular, neurological, or endocrinological diseases. Healthy control subjects were also recruited and screened by a complete medical and psychiatric examination, and none had a history of any disease or medication that might affect the ANS. Informed consent was obtained after a full explanation of the study procedure.
Assessments
Antipsychotic-induced subjective inner restlessness was assessed using the Liverpool University Neuroleptic Side Effect Rating Scale (LUNSERS).7 Antipsychotic-induced subjective restlessness was distinguished from psychotic agitation by a sense of being driven and the awareness of inner tension, as proposed previously.2,3,7 The diagnosis of antipsychotic-induced subjective restlessness was made if the score on the item of LUNSERS was more than 2.7 The severity of schizophrenic symptoms was assessed using the Positive and Negative Syndrome Scale (PANSS).8
HRV measurements were performed after assessing subjective inner restlessness and schizophrenic symptoms on the same day. Smoking was strictly prohibited by hospital policy. In addition, vigorous exercise and caffeinated beverages including coffee and tea were not allowed on the day prior to HRV measurement. After each subject had been allowed to adapt to the experimental conditions for approximately 10 minutes, a 10-minute single channel (three-lead) electrocardiogram (ECG) recording was performed in the seated position at complete rest. The ECG signal was amplified and digitized at a sampling rate of 400 Hz (width path, 0.05–35 Hz). The interbeat (RR) interval time series was generated using the automatic scheme in order to detect the R peak in the ECG, using methods proposed previously.9
In the conventional time domain analysis of HRV, the mean length of all RR intervals was computed according to standardized procedures.10 For the frequency domain analysis, a spectral analysis was carried out using fast Fourier transformation and the low frequency ([LF]: 0.04–0.15 Hz)/high frequency ([HF]: 0.15–0.4 Hz) ratio was calculated to assess the sympathovagal balance, as proposed previously.10 For the nonlinear complexity measure, the approximate entropy (ApEn), which is a parameter developed to quantify the degree of regularity versus the unpredictability in a higher dimensional attractor reconstructed from a time series, such as the instantaneous heart rate time series, was calculated using the method proposed by Pincus.11 The ApEn measures the difference between the logarithmic frequencies of similar runs of length m and runs with the length m + 1, calculating the logarithmic likelihood that runs of patterns that are close to each other will remain close in the next incremental comparisons.12–16 The ApEn is strongly correlated with HF power, which mainly reflects respiratory sinus arrhythmia.14–16 A lower value of ApEn reflects a higher degree of regularity, and the higher the entropy value, the more unpredictable the time series.
Statistical analysis
Demographic and clinical variables were compared between the groups using one-way analysis of variance, t-test, and Chi-square test, as appropriate. The values of the HRV measures of the groups were compared using multivariate analysis of variance with post hoc Scheffé tests. The relationship between antipsychotic-induced subjective restlessness and HRV measures was also evaluated using Pearson’s partial correlation analyses controlling for PANSS total score. The level of statistical significance was defined as P < 0.05 (two-tailed).
Results
Fifty inpatients (35 men, 15 women) with schizophrenia and 28 healthy control subjects were enrolled. The patients had a mean age of 32.0 years ± 9.2 years and a mean duration of illness of 7.3 years ± 5.0 years. All patients were receiving risperidone monotherapy with a mean dosage of 2.9 mg/day ± 1.5 mg/day at the time of enrollment. The mean total PANSS score of the patients was 93.7 ± 15.8 at enrollment.
A diagnosis of antipsychotic-induced subjective restlessness based on the LUNSERS was made in 22 (44%) of the 50 patients. Table 1 presents the demographic characteristics of the groups. There were no significant differences in age, sex, or smoking status among the groups (P > 0.1). No significant differences in illness duration (8.5 years ± 5.2 years versus 6.4 years ± 4.7 years, P = 0.15) or antipsychotic dosage (risperidone: 3.1 mg/day ± 1.3 mg/day versus 2.6 mg/day ± 1.6 mg/day, P = 0.24) were observed between patients with and without antipsychotic-induced subjective restlessness (Table 1).
Table 1.
Comparison of demographic variables among groups
| Variables | Group 1a | Group 2b | Group 3c | F value | P value |
|---|---|---|---|---|---|
| Aged | 32.3 ± 7.9 | 31.7 ± 10.3 | 29.6 ± 8.0 | 0.69 | 0.51 |
| Sex (male/female) | 17/5 | 18/10 | 19/9 | 1.01e | 0.60 |
| Smokers/nonsmokers | 12/10 | 11/17 | 10/18 | 1.95e | 0.38 |
| Duration of illness (years)d | 8.5 ± 5.2 | 6.4 ± 4.7 | Na | 1.48f | 0.15 |
| Antipsychotic dosage (mg/day)d | 3.1 ± 1.3 | 2.6 ± 1.6 | Na | 1.19f | 0.24 |
Notes:
Patients with antipsychotic-induced subjective restlessness
patients without antipsychotic-induced subjective restlessness
healthy control subjects
values are presented as mean ± standard deviation
Chi-square value
t value.
Abbreviations: Na, not applicable.
A comparison of the HRV parameters among groups is shown in Table 2. The analysis using multivariate analysis of variance (Wilks’ λ = 0.53, F = 9.20, P < 0.001) and follow-up F tests showed significant group differences in all parameters (P < 0.01). Post hoc analyses with Scheffé tests indicated that the mean RR interval value was significantly higher in the healthy control group than in patients with and without antipsychotic-induced subjective restlessness (P < 0.01) (Table 2). The LF/HF ratio was significantly higher in patients with antipsychotic-induced subjective restlessness than in control subjects and in patients without antipsychotic-induced subjective restlessness (P < 0.05) (Table 2). Regarding the nonlinear complexity measure, the ApEn value was significantly lower in patients with antipsychotic-induced subjective restlessness than in control subjects and in patients without antipsychotic-induced subjective restlessness (P < 0.01) (Table 2).
Table 2.
Comparison of heart rate variability parameters among groups
| Variables | Group 1a | Group 2b | Group 3c | F value | P value |
|---|---|---|---|---|---|
| Mean RR intervald | 637.28 ± 106.96 | 681.91 ± 97.75 | 799.83 ± 80.50 | 20.28 | <0.01e |
| LF/HFd | 9.67 ± 7.53 | 6.13 ± 4.34 | 3.35 ± 2.19 | 10.08 | <0.01f |
| ApEnd | 1.13 ± 0.26 | 1.34 ± 0.19 | 1.47 ± 0.17 | 17.02 | <0.01g |
Notes:
Patients with antipsychotic-induced subjective restlessness
patients without antipsychotic-induced subjective restlessness
healthy control subjects
values are presented as mean ± standard deviation
differences between groups 1 and 3 (P < 0.01) as well as between groups 2 and 3 (P < 0.01) on scheffé tests
differences between groups 1 and 2 (P < 0.05) as well as between groups 1 and 3 (P < 0.01) on scheffé tests;
differences between groups 1 and 2 (P < 0.01) as well as between groups 1 and 3 (P < 0.01) on scheffé tests.
Abbreviations: ApEn, approximate entropy; HF, high frequency; LF, low frequency; RR, interbeat.
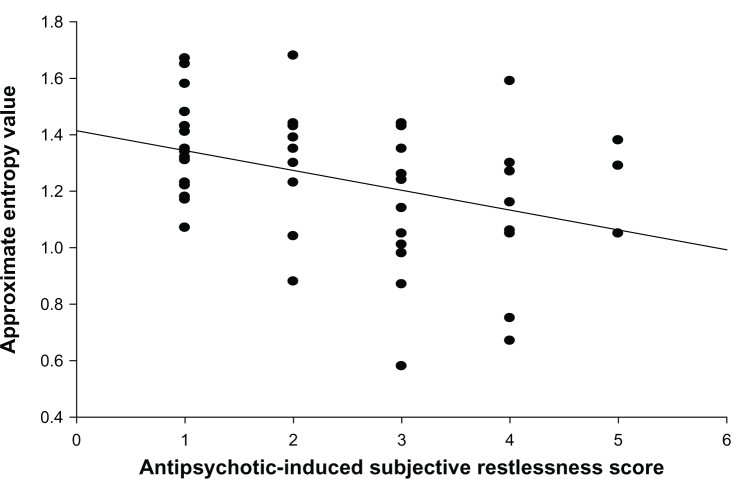
In the patient group, partial correlation analyses controlling for PANSS total score showed that the severity of antipsychotic-induced subjective restlessness had a significant negative correlation with the ApEn value (r = −0.29, P = 0.04) (Figure 1). No significant correlations were observed between the other HRV measures and the antipsychotic-induced subjective restlessness score (mean RR interval: r = −0.04, P = 0.81; LF/HF: r = 0.14, P = 0.33).
Figure 1.
Scatter plots showing the relationship between the severity of antipsychotic-induced subjective restlessness as measured by the liverpool University Neuroleptic Side Effect Rating Scale and the approximate entropy value.
Discussion
In the present study, we examined the relationship between antipsychotic-induced subjective restlessness and autonomic neurocardiac function using analysis of HRV in patients with schizophrenia. We observed that the ApEn value was significantly lower in patients with antipsychotic-induced subjective restlessness than in those without it as well as in healthy control subjects, while the LF/HF ratio was significantly higher in patients with antipsychotic-induced subjective restlessness than in control subjects and in patients without antipsychotic-induced subjective restlessness, suggesting a shift in sympathetic–parasympathetic balance in favor of sympathetic tone. Notably, a significant negative correlation between antipsychotic-induced subjective restlessness and the nonlinear complexity measure; ie, ApEn, was observed while controlling for the influence of psychotic symptom severity, suggesting its association with reduced neurocardiac dynamics.
The results are partly in line with previous reports showing that patients with Parkinson’s disease exhibit lower HRV measures than those of healthy controls and that the severity of extrapyramidal features is negatively associated with HRV measures;9 however, previous studies did not include symptoms of restlessness nor apply the nonlinear complexity measures of HRV.
Although the pathogenesis of antipsychotic-induced subjective restlessness is unclear, the neuronal circuits involved in its pathophysiology are thought to have complex afferent and efferent connections with large parts of the cortical and subcortical areas.1,17 Considering the primary role of the limbic system in both central ANS reactivity and emotional behavior, it may be that certain HRV parameters reflect the functional state of mesolimbic activity, which could be associated with the pathophysiology of antipsychotic-induced subjective restlessness. The altered neurocardiac dynamics associated with antipsychotic-induced restlessness may also be attributed, in part, to dysphoric affective symptoms closely related to it such as depressive and anxiety symptoms.1
Considering the accumulating evidence demonstrating that autonomic dysfunction seen in patients with schizophrenia may indicate underlying disease-inherent vulnerability,4,18 which was supported by the low HRV findings in the patient groups in our study, the reduced dynamics of HRV associated with the adverse effects of antipsychotics might exacerbate compromised autonomic cardiac modulation and increase cardiovascular morbidity in vulnerable patients.4,18–23
It is interesting to note that the nonlinear complexity measure was significantly correlated with the severity of antipsychotic-induced subjective restlessness and that a significant difference between the patient groups was also found in this parameter. Central autonomic regulation causes nonlinear phenomena in sinus rhythm generation. An analysis of HRV by methods based on the nonlinear theory has been shown to better depict the multiple regulatory systems influencing the heart rate time series modulation in complex biological systems.12 The decrease in entropy value means a decrease in the number of the variables and their levels of interactions involved in neuroautonomic regulation.15 Therefore, decreased entropy measures in patients with antipsychotic-induced subjective restlessness suggest that the degree of distribution of stochastic process gets lower11 and that the neuroautonomic control system governing the heart rate loses complexity in those patients. These changes may be substantially associated with the diminished adaptability of the biosystem.15 However, the effect size of the correlation between the antipsychotic-induced subjective restlessness and the ApEn was 0.29, indicating a less than moderate effect size. Therefore, the mechanisms causing antipsychotic-induced subjective restlessness may differ from those leading to impairment of neuroautonomic regulation and other mechanisms may also be involved.
The HRV values of the patients included in our study were higher than those observed in patients receiving clozapine22–24 and similar to or slightly lower than those observed in patients receiving haloperidol.23 These findings indicate a relatively favorable profile of risperidone in terms of the effects on HRV,20,25–27 which reflects the fact that risperidone shows no anticholinergic activity in the in vitro receptor binding profile. However, the effects of antipsychotics on HRV in patients with schizophrenia cannot be attributed simply to their receptor binding profile, as multiple other factors, such as disease processes, symptom severity, and adverse effects, also play a significant role in the complex regulation of HRV.28 Therefore, further prospective investigations of the neurocardiac effects of various antipsychotics in patients with different clinical characteristics are required to better understand the complex interaction between antipsychotics and HRV.19
In the present study, we did not perform actometry recordings to evaluate objective restlessness, since we focused on the subjective aspect of restlessness. However, since psychomotor activity and autonomic cardiac functioning are closely interrelated,29 it would have been helpful to measure objective restlessness using an accelerometer, which has been reported as a valuable objective tool for assessing restlessness.30–33 Further studies using actometry are required to better understand the physiological basis of multiple aspects of restlessness and to investigate whether subjective restlessness and objective motor manifestations are differentially associated with HRV parameters.
The interpretation of the results should be considered in light of some limitations. A cross-sectional design limits the firm interpretation of the results observed. We evaluated the relationships in patients receiving risperidone monotherapy without adjunctive medications. Therefore, the results may not be generalized to other groups receiving different antipsychotics. We did not measure body mass index, which was reported to be one of the factors influencing baroreflex sensitivity.34 In our study, the consumption of coffee or tea was not allowed on the day prior to HRV measurement. However, we did not measure the daily average amount of coffee or tea intake, which might have affected autonomic regulation.35
In conclusion, the present study indicates that antipsychotic-induced subjective restlessness is associated with altered HRV parameters, particularly the nonlinear complexity measure, suggesting that it might adversely affect autonomic neurocardiac integrity. Further prospective studies are necessary to elucidate the precise interrelationships and causality. The nonlinear complexity measures of HRV may be useful in evaluating the subtle subjective forms of adverse effects of antipsychotics, serving as a practical noninvasive method.
Acknowledgments
This work was supported by a grant of the Korean Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (A070001).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Sachdev P. Akathisia and Restless Legs. New York: Cambridge University Press; 1995. [Google Scholar]
- 2.Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672–676. doi: 10.1192/bjp.154.5.672. [DOI] [PubMed] [Google Scholar]
- 3.Owens DGC. A guide to the extrapyramidal side-effects of antipsychotic drugs. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- 4.Bär KJ, Boettger MK, Koschke M, et al. Non-linear complexity measures of heart rate variability in acute schizophrenia. Clin Neurophysiol. 2007;118:2009–2015. doi: 10.1016/j.clinph.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 5.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders fourth edition. Washington DC: American Psychiatric Press; 1994. [Google Scholar]
- 6.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders Research Version (SCID-I) New York State Psychiatric Institute: Biometrics Research: 1996. [Google Scholar]
- 7.Day JC, Wood G, Dewey M, Bentall RP. A self-rating scale for measuring neuroleptic side-effects. Validation in a group of schizophrenic patients. Br J Psychiatry. 1995;166:650–653. doi: 10.1192/bjp.166.5.650. [DOI] [PubMed] [Google Scholar]
- 8.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 9.Haapaniemi TH, Pursiainen V, Korpelainen JT, Huikuri HV, Sotaniemi KA, Myllylä VV. Ambulatory ECG and analysis of heart rate variability in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2001;70:305–310. doi: 10.1136/jnnp.70.3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 11.Pincus SM. Approximate entropy as a measure of system complexity. Proc Natl Acad Sci USA. 1991;88:2297–2301. doi: 10.1073/pnas.88.6.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seely AJ, Macklem PT. Complex systems and the technology of variability analysis. Crit Care. 2004;8:R367–R384. doi: 10.1186/cc2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeragani VK, Pohl R, Mallavarapu M, et al. Approximate entropy of symptoms of mood: an effective technique to quantify regularity of mood. Bipolar Disord. 2003;5:279–286. doi: 10.1034/j.1399-5618.2003.00012.x. [DOI] [PubMed] [Google Scholar]
- 14.Yeragani VK, Sobolewski E, Kay J, et al. Effect of age on long-term heart rate variability. Cardiovasc Res. 1997;35:35–42. doi: 10.1016/s0008-6363(97)00107-7. [DOI] [PubMed] [Google Scholar]
- 15.Yeragani VK, Sobolewski E, Jampala VC, et al. Fractal dimension and approximate entropy of heart period and heart rate: awake versus sleep differences and methodological issues. Clin Sci (Lond) 1998;95:295–301. [PubMed] [Google Scholar]
- 16.Richman JS, Moorman JR. Physiological time-series analysis using approximate entropy and sample entropy. Am J Physiol Heart Circ Physiol. 2000;278:H2039–H2049. doi: 10.1152/ajpheart.2000.278.6.H2039. [DOI] [PubMed] [Google Scholar]
- 17.Kim JH, Byun HJ. Prevalence and characteristics of subjective akathisia, objective akathisia, and mixed akathisia in chronic schizophrenic subjects. Clin Neuropharmacol. 2003;26:312–316. doi: 10.1097/00002826-200311000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Bär KJ, Koschke M, Berger S, et al. Influence of olanzapine on QT variability and complexity measures of heart rate in patients with schizophrenia. J Clin Psychopharmacol. 2008;28:694–698. doi: 10.1097/JCP.0b013e31818a6d25. [DOI] [PubMed] [Google Scholar]
- 19.Agelink MW, Majewski T, Wurthmann C, et al. Effects of newer atypical antipsychotics on autonomic neurocardiac function: a comparison between amisulpride, olanzapine, sertindole, and clozapine. J Clin Psychopharmacol. 2001;21:8–13. doi: 10.1097/00004714-200102000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Hempel RJ, Tulen JH, van Beveren NJ, Röder CH, Hengeveld MW. Cardiovascular variability during treatment with haloperidol, olanzapine or risperidone in recent-onset schizophrenia. J Psychopharmacol. 2009;23:697–707. doi: 10.1177/0269881108091254. [DOI] [PubMed] [Google Scholar]
- 21.Cohen H, Loewenthal U, Matar MA, Kotler M. Reversal of pathologic cardiac parameters after transition from clozapine to olanzapine treatment: a case report. Clin Neuropharmacol. 2001;24:106–108. doi: 10.1097/00002826-200103000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Mueck-Weymann M, Rechlin T, Ehrengut F, et al. Effects of olanzapine and clozapine upon pulse rate variability. Depress Anxiety. 2002;16:93–99. doi: 10.1002/da.10037. [DOI] [PubMed] [Google Scholar]
- 23.Cohen H, Loewenthal U, Matar M, Kotler M. Association of autonomic dysfunction and clozapine. Heart rate variability and risk for sudden death in patients with schizophrenia on long-term psychotropic medication. Br J Psychiatry. 2001;179:167–171. doi: 10.1192/bjp.179.2.167. [DOI] [PubMed] [Google Scholar]
- 24.Kim JH, Yi SH, Yoo CS, et al. Heart rate dynamics and their relationship to psychotic symptom severity in clozapine-treated schizophrenic subjects. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:371–378. doi: 10.1016/j.pnpbp.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Silke B, Campbell C, King DJ. The potential cardiotoxicity of antipsychotic drugs as assessed by heart rate variability. J Psychopharmacol. 2002;16:355–360. doi: 10.1177/026988110201600410. [DOI] [PubMed] [Google Scholar]
- 26.Henry BL, Minassian A, Paulus MP, Geyer MA, Perry W. Heart rate variability in bipolar mania and schizophrenia. J Psychiatr Res. 2010;44:168–176. doi: 10.1016/j.jpsychires.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang JS, Yoo CS, Yi SH, et al. Changes in heart rate dynamics of patients with schizophrenia treated with risperidone. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:924–929. doi: 10.1016/j.pnpbp.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 28.Agelink MW, Sayar K, Klieser E. Usefulness of heart rate variability (HRV) for monitoring clozapine plasma levels. Pharmacopsychiatry. 2003;36:166–167. doi: 10.1055/s-2003-41203. [DOI] [PubMed] [Google Scholar]
- 29.Volkers AC, Tulen JH, van den Broek WW, Bruijn JA, Passchier J, Pepplinkhuizen L. Motor activity and autonomic cardiac functioning in major depressive disorder. J Affect Disord. 2003;76:23–30. doi: 10.1016/s0165-0327(02)00066-6. [DOI] [PubMed] [Google Scholar]
- 30.Tuisku K, Holi MM, Wahlbeck K, Ahlgren AJ, Lauerma H. Quantitative rest activity in ambulatory monitoring as a physiological marker of restless legs syndrome: a controlled study. Mov Disord. 2003;18:442–448. doi: 10.1002/mds.10381. [DOI] [PubMed] [Google Scholar]
- 31.Tuisku K, Holi MM, Wahlbeck K, Ahlgren AJ, Lauerma H. Actometry in measuring the symptom severity of restless legs syndrome. Eur J Neurol. 2005;12:385–387. doi: 10.1111/j.1468-1331.2004.00960.x. [DOI] [PubMed] [Google Scholar]
- 32.Janno S, Holi MM, Tuisku K, Wahlbeck K. Neuroleptic-induced movement disorders in a naturalistic schizophrenia population: diagnostic value of actometric movement patterns. BMC Neurol. 2008;8:10. doi: 10.1186/1471-2377-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caligiuri MP, Teulings HL, Dean CE, Niculescu AB, 3rd, Lohr JB. Handwriting movement kinematics for quantifying extrapyramidal side effects in patients treated with atypical antipsychotics. Psychiatry Res. 2010;177:77–83. doi: 10.1016/j.psychres.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skrapari I, Tentolouris N, Perrea D, et al. Baroreflex sensitivity in obesity: relationship with cardiac autonomic nervous system activity. Obesity (Silver Spring) 2007;15:1685–1693. doi: 10.1038/oby.2007.201. [DOI] [PubMed] [Google Scholar]
- 35.Yeragani VK, Krishnan S, Engels HJ, Gretebeck R. Effects of caffeine on linear and nonlinear measures of heart rate variability before and after exercise. Depress Anxiety. 2005;21:130–134. doi: 10.1002/da.20061. [DOI] [PubMed] [Google Scholar]