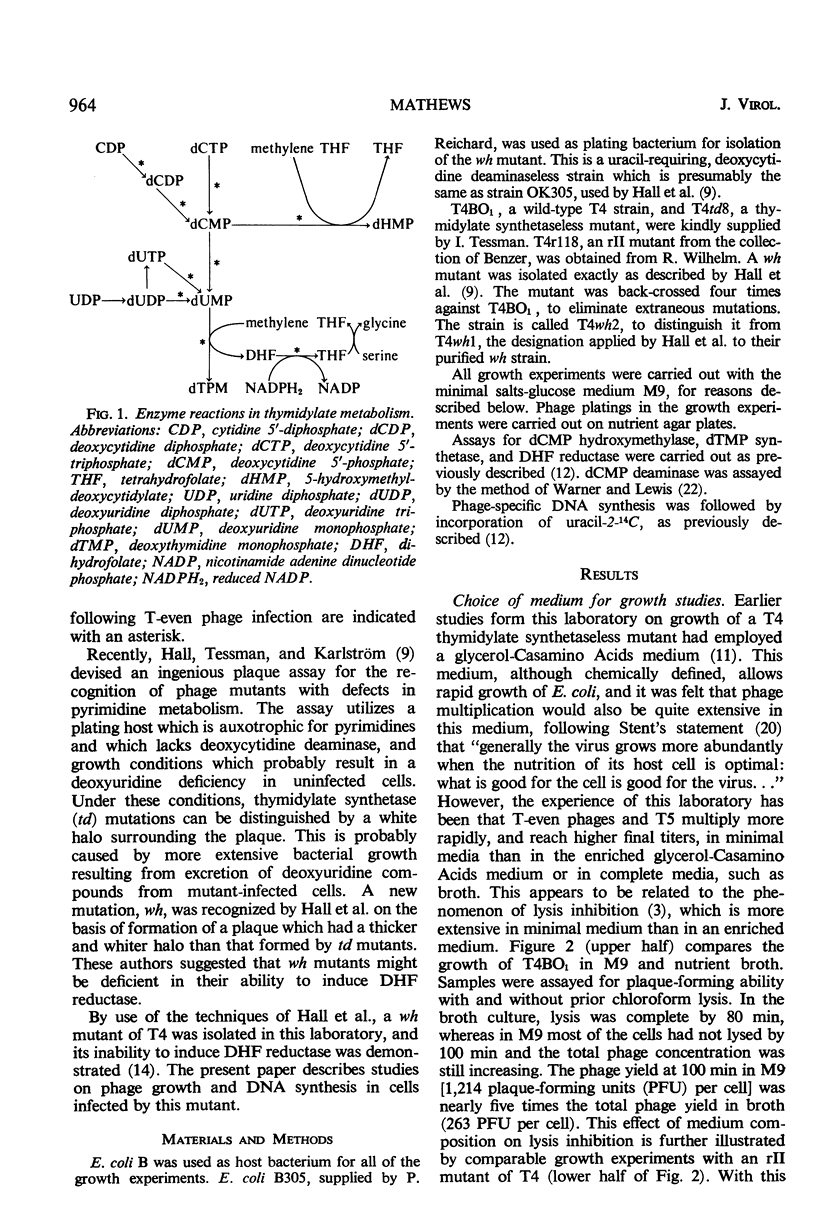
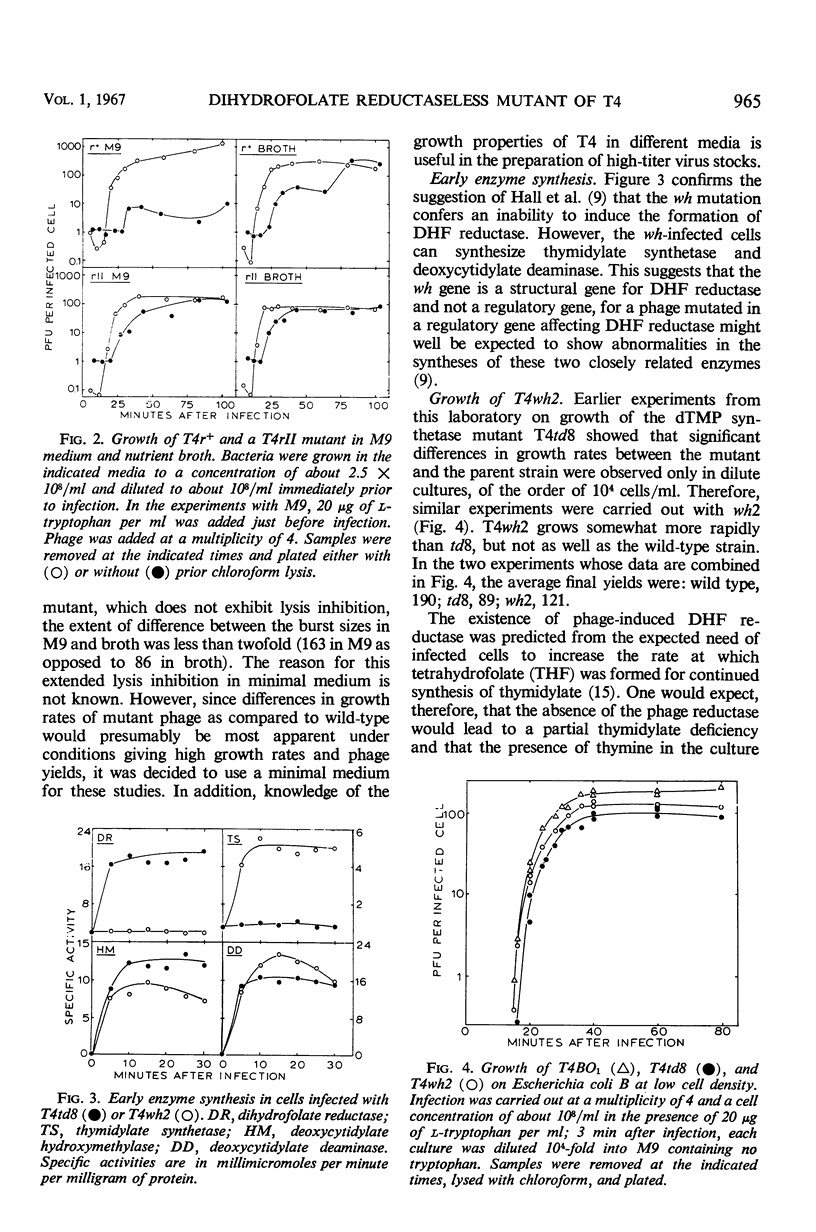
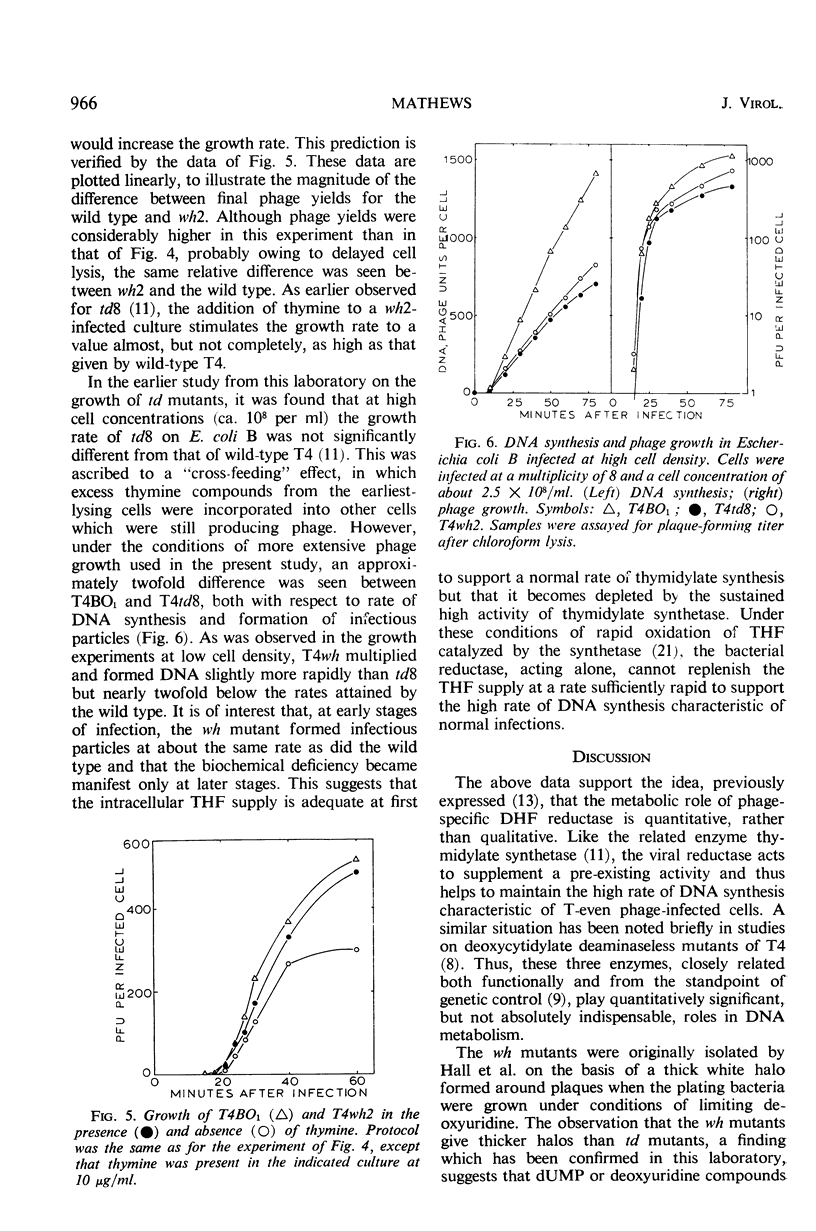
Abstract
A mutant of bacteriophage T4 was isolated which was unable to induce virus-specific dihydrofolate reductase in infected cells. The mutant was able to form several other early enzymes of pyrimidine metabolism. Growth of the mutant in a wild-type host, Escherichia coli B, was compared with that of the parent strain, T4BO1, and T4td8, a mutant which lacks the ability to induce thymidylate synthetase. Growth studies were carried out in minimal medium, which gave higher growth rates and phage yields than the supplemented media used in previous studies. The reductase mutant formed deoxyribonucleic acid and plaque-forming particles at a rate slightly higher than the synthetase mutant but 1.5-to 2-fold lower than that of the wild-type phage under all conditions studied. The addition of thymine to a culture infected by the mutant increased the growth rate significantly, suggesting that the genetic lesion leads to a partial thymidylate deficiency. Like other viral genes controlling steps in thymidylate metabolism, the dihydrofolate reductase gene appears to be useful but not completely essential for growth.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERTANI L. E., HAGGMARK A., REICHARD P. Synthesis of pyrimidine deoxyribonucleoside diphosphates with enzymes from Escherichia coli. J Biol Chem. 1961 Oct;236:PC67–PC68. [PubMed] [Google Scholar]
- BESSMAN M. J., BELLO L. J. The enzymology of virus-infected bacteria. III. Evidence for the formation of a trifunctional deoxynucleotide kinase. J Biol Chem. 1961 Oct;236:PC72–PC73. [PubMed] [Google Scholar]
- Doermann A. H. Lysis and Lysis Inhibition with Escherichia coli Bacteriophage. J Bacteriol. 1948 Feb;55(2):257–276. doi: 10.1128/jb.55.2.257-276.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLAKS J. G., COHEN S. S. Virus-induced acquisition of metabolic function. I. Enzymatic formation of 5-hydroxymethyldeoxycytidylate. J Biol Chem. 1959 Jun;234(6):1501–1506. [PubMed] [Google Scholar]
- FLAKS J. G., COHEN S. S. Virus-induced acquisition of metabolic function. III. Formation and some properties of thymidylate synthetase of bacteriophage-infected Escherichia coli. J Biol Chem. 1959 Nov;234:2981–2986. [PubMed] [Google Scholar]
- FRASER D., JERREL E. A. The amino acid composition of T3 bacteriophage. J Biol Chem. 1953 Nov;205(1):291–295. [PubMed] [Google Scholar]
- GREENBERG G. R., SOMERVILLE R. L., DEWOLF S. Resolution of phage-initiated and normal host thymidylate synthetases of Escherichia coli. Proc Natl Acad Sci U S A. 1962 Feb;48:242–247. doi: 10.1073/pnas.48.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall D. H., Tessman I., Karlström O. Linkage of T4 genes controlling a series of steps in pyrimidine biosynthesis. Virology. 1967 Mar;31(3):442–448. doi: 10.1016/0042-6822(67)90224-3. [DOI] [PubMed] [Google Scholar]
- Hall D. H., Tessman I. T4 mutants unable to induce deoxycytidylate deaminase activity. Virology. 1966 Jun;29(2):339–345. doi: 10.1016/0042-6822(66)90041-9. [DOI] [PubMed] [Google Scholar]
- KECK K., MAHLER H. R., FRASER D. Synthesis of deoxycytidine-5'-phosphate deaminase in Escherichia coli infected by T2 bacteriophage. Arch Biochem Biophys. 1960 Jan;86:85–88. doi: 10.1016/0003-9861(60)90373-8. [DOI] [PubMed] [Google Scholar]
- MATHEWS C. K., SUTHERLAND K. E. COMPARATIVE BIOCHEMISTRY OF BACTERIAL AND PHAGE-INDUCED DIHYDROFOLATE REDUCTASES. J Biol Chem. 1965 May;240:2142–2147. [PubMed] [Google Scholar]
- Mathews C. K. On the metabolic role of T6 phage-induced dihydrofolate reductase. Intracellular reduced pyridine nucleotides. J Biol Chem. 1966 Nov 10;241(21):5008–5012. [PubMed] [Google Scholar]
- Mathews C. K. Phage Growth and Deoxyribonucleic Acid Synthesis in Escherichia coli Infected by a Thymine-Requiring Bacteriophage. J Bacteriol. 1965 Sep;90(3):648–652. doi: 10.1128/jb.90.3.648-652.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews C. K. Deoxyribonucleic acid metabolism and virus-induced enzyme synthesis in a thymine-requiring bacterium infected by a thymine-requiring bacteriophage. Biochemistry. 1966 Jun;5(6):2092–2100. doi: 10.1021/bi00870a042. [DOI] [PubMed] [Google Scholar]
- SHAPIRO D. M., EIGNER J., GREENBERG G. R. INABILITY OF THYMINE-DEPENDENT MUTANTS OF BACTERIOPHAGE T4 TO INDUCE THYMIDYLATE SYNTHETASE. Proc Natl Acad Sci U S A. 1965 Apr;53:874–881. doi: 10.1073/pnas.53.4.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMON E. H., TESSMAN I. THYMIDINE-REQUIRING MUTANTS OF PHAGE T4. Proc Natl Acad Sci U S A. 1963 Sep;50:526–532. doi: 10.1073/pnas.50.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WYATT G. R., COHEN S. S. The bases of the nucleic acids of some bacterial and animal viruses: the occurrence of 5-hydroxymethylcytosine. Biochem J. 1953 Dec;55(5):774–782. doi: 10.1042/bj0550774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner H. R., Lewis N. The synthesis of deoxycytidylate deaminase and dihydrofolate reductase and its control in Escherichia coli infected with bacteriophage T4 and T-4 amber mutants. Virology. 1966 May;29(1):172–175. doi: 10.1016/0042-6822(66)90208-x. [DOI] [PubMed] [Google Scholar]



