Summary
Few data exist on risk factors for candidemia in pediatric intensive care unit (PICU) patients who are at high risk of mortality from infection. We conducted a population-based case-control study to determine risk factors and predictors for candidemia in the PICU.
Background
Candida species are the leading cause of invasive fungal infections in hospitalized children and are the third most common isolates recovered from pediatric healthcare-associated bloodstream infection in the US [1]. Few data exist on risk factors for candidemia in pediatric intensive care unit (PICU) patients.
Methods
We conducted a population-based case-control study of PICU patients at Children's Hospital of Philadelphia (CHOP) from 1997-2004. Cases were identified using laboratory records, controls were selected from PICU rosters. Controls were matched to cases by incidence density sampling, adjusting for time at risk. Following conditional multivariate analysis, we performed weighted multivariate analysis to determine predicted probabilities for candidemia given certain risk factor combinations.
Results
We identified 101 cases of candidemia(incidence,3.5/1,000 PICU admissions). Factors independently associated with candidemia included presence of a central venous catheter(OR 30.4;CI,7.7,119.5), malignancy(OR 4.0;CI,1.23,13.1), use of vancomycin for >3 days in the prior two weeks(OR 6.2;CI,2.4,16), and receipt of agents with activity against anaerobic organisms for >3 days in the prior two weeks(OR 3.5;CI, 1.5,8.4). Predicted probability of various combinations of the factors above ranged from 10.7%-46%. The 30-day mortality rate was 44% in cases compared to 14% in controls (OR 4.22;CI,2.35,7.60).
Conclusions
To our knowledge, this is the first study to evaluate independent risk factors and to determine a population of children in PICUs at high risk for developing candidemia. Future efforts should focus on validation of these risk factors identified in a different PICU population and development of interventions for prevention of candidemia in critically ill children.
Keywords: Candidemia, Pediatrics, Risk factors, Intensive Care
Candida species are the leading cause of invasive fungal infections in hospitalized children and are the third most common isolates recovered from pediatric cases of healthcare-associated bloodstream infection in the United States [1]. Candidemia is frequently associated with signs and symptoms of sepsis syndrome [2]. The annual number of cases of sepsis caused by fungal organisms increased by 207% between 1979 and 2000 [3]. Fungal infections possess the second highest case fatality rate (13%) among all causes of sepsis in children [4].
The attributable mortality of candidemia in children has been reported to be 10%. In children, candidemia is associated with prolonged hospital length of stay (median=21 days) and hospital charges (median=$39,331.00) [5]. Pediatric intensive care unit (PICU) patients are at highest risk for death to due candidemia [6,7], however few data exist on the risk factors for candidemia in PICU patients. Understanding these risk factors may provide an epidemiologically based rationale for development of preventative strategies. Antifungal prophylaxis has been an effective preventative strategy in other pediatric populations at high risk for candidemia including neonates and oncology patients [8,9]. If the rate of candidemia is sufficiently high, demonstrating the value of a preventative strategy is straightforward. However, the potential benefit of instituting preventative or prophylactic strategies in a large group of patients with a lower event (candidemia) rate is also weighed against the potential risks (e.g., antifungal drug resistance, toxicity). Previous investigators have suggested that preventative strategies in intensive care should be targeted to populations with a baseline rate of candidemia of 10% or more [10]. Further refining the risk factors helps to identify the subpopulations that may benefit most from antifungal prophylaxis and other preventive measures.
Therefore, we conducted a population-based case-control study to determine the risk factors and predictors for candidemia in the PICU.
Methods
Study Population
We conducted a population-based case-control study of all patients 18 years of age and younger admitted to the PICU at the Children's Hospital of Philadelphia (CHOP) between 1997 and 2004. CHOP is an academic tertiary care center with 418 beds and approximately 24,000 hospital admissions per year. The PICU at CHOP consists of a 45 bed critical care unit that has approximately 3,000 admissions per year and a cardiac intensive care unit with 24 beds and approximately 3,600 admissions per year. All patients admitted to the PICU were identified using both hospital and unit-specific databases.
Definition of Cases
Candidemia was defined as a blood culture that yielded Candida spp. in a patient hospitalized in the CHOP PICU. Cases were identified through the records of the Clinical Microbiology Laboratory at CHOP. If multiple episodes of candidemia occurred in the same patient during the study period, the patient was included as a study participant using only the first episode of candidemia.
Selection of Controls: Incidence Density Sampling
Study controls were selected from unit-specific patient admission databases. To increase statistical efficiency, incidence density sampling was used to match controls to cases with respect to time at risk for developing infection. Time at risk is an important confounding variable because it represents the opportunity for both exposures (e.g., antibiotics) and development of candidemia. For example, a patient who develops candidemia on day 10 of his ICU stay becomes a case and the potential controls are patients who have been in the ICU for at least 10 days and have not developed candidemia by day 10 of their stay. As such, a patient who ultimately develops candidemia is eligible to be selected as a control for the exposure period prior to his infection. In addition, time at risk is likely to be associated with severity of illness, which is another important confounding variable.
Controls were selected for cases using the following mechanism: we determined the length of ICU stay prior to infection for a given case, restricted the roster of ICU patients to those who had lengths of stay at least as long as the case's time to infection, and then randomly selected two controls per case. For purposes of the conditional regression analysis, each subject was assigned an index date, which was the day of infection for the cases and the corresponding day in the ICU for the matched controls.
Data Collection
Research assistants used a structured data collection instrument to retrieve clinical and laboratory data from the inpatient medical record. Data obtained included age, sex, race, type of ICU, duration of hospitalization prior to infection, date of infection, and reason for admission.
All antimicrobial therapy in the 2 weeks prior to the index date was recorded. Data regarding the specific antimicrobial agent, duration of use, as well as the class of antibiotics to which it belongs (e.g., cephalosporin) were also collected. Antimicrobial therapy was further classified as to whether it had activity against anaerobic bacteria in the gastrointestinal tract. Antimicrobials with anti-anaerobic activity (metronidazole, clindamycin, piperacillin-tazobactam, ticarcillin-clavulanic acid, ceftriaxone, ampicillin-sulbactam, amoxicillin-clavulanic acid, oral vancomycin, and the carbapenems) were of particular interest because they eliminate normal flora and thereby promote the growth of Candida[11,12]. Vancomycin was included because it has potent in vitro activity against Gram-positive anaerobes and because oral administration results in broader activity against Gram-negative species such as Bacteroides[13]. Similarly, ceftriaxone was included because it markedly decreases the levels of anaerobic GI flora in humans [14,15].
Comorbid conditions at the time of study entry were considered as potential confounders. These conditions included malignancy (specifying the type of malignancy), renal insufficiency (including requirement of hemodialysis or peritoneal dialysis), human immunodeficiency virus infection (HIV), primary immunodeficiency, neutropenia (absolute neutrophil count of <500mm3) and duration of neutropenia, prior organ transplant (specifying the date and type of transplant), use of immunosuppressive agents (specifying which agents) in the preceding 2 weeks, and surgical procedure or trauma in the 2 weeks preceding the index date.
Information was collected regarding devices that were in place prior to the index date. The presence of central venous catheter (CVC) (including type and anatomic location), urinary catheter, arterial catheter, mechanical ventilation, and administration of enteral nutrition and/or hyperalimentation (TPN) were also recorded.
Statistical Analysis
Following data collection, continuous variables were summarized using the median and interquartile range (IQR) while categorical variables were summarized using frequencies and percents. Univariate p-values were obtained, adjusting for the matched analysis. All analysis was performed using Stata 10.1 (Stata Corp., College Station, TX 2008). After univariate statistics were generated, we analyzed the data using two paradigms, conditional logistic regression and weighted logistic regression.
Conditional logistic regression
All factors with a univariate p-value of <0.20 were considered for inclusion in the multivariate model. Conditional logistic regression was used to identify independent risk factors for development of candidemia as we matched cases to controls based on time at risk.
Weighted multivariate logistic regression
Because the case-control design with incidence density sampling was matched on time, we could not estimate the association between candidemia and time. To this end, we reconstructed through the use of weights the entire cohort by estimating the probability of the selection of each case and each control under incidence density sampling. The inverse of this selection probability was the sampling weight, and the sum of these sampling weights over the sampled subjects equaled the number of children in the cohort. The cases plus the weighted controls represent the entire group of children. Then using a weighted logistic regression, we examined the association between time and other risk factors with candidemia, just as a logistic regression could be applied to the entire cohort. In addition, we compared the results of these analyses with those of conditional logistic regression that are detailed above. Each variable included in the regression model was first cross classified with each other variable to identify zero cells that would prohibit their simultaneous inclusion in a regression model. Factors that were pre-specified as clinically important were forced into a multivariable model. Owing to the small number of cases, we took care to avoid both overfitting (inclusion of too many variables) and confounding (omission of a factor related to both outcome and exposure).
Predicted probabilities
Using the weighted multivariate model, we derived predicted probabilities and confidence intervals (CI) for combinations of factors that were independently associated with the acquisition of candidemia. All combinations with predicted probability point estimates of .10 or greater were considered for our predictive model.
Results
During the study period, we identified a total of 101 cases of candidemia. The incidence of candidemia was 3.5 per 1000 PICU admissions. The most commonly isolated Candida spp. was C. albicans (46%), followed by C. parapsilosis (30%). Other Candida spp. accounted for 15% of the isolates (6% C. tropicalis, 3% C. glabrata, 3% C. krusei, 3% C. lusitaniae). The remaining 9% of organisms isolated were multiple or unknown Candida spp. A total of 184 controls were selected; 18 cases were matched with only one control.
Demographic and clinical characteristics
The demographic and clinical characteristics of patients with candidemia and controls are shown in Table 1. Cases were more likely than controls to have a malignancy (17% v. 7%, p = 0.008) and neutropenia (6% v. 1%, p = 0.036) prior to study entry. In addition, cases were more likely than controls to have a CVC in place (92% v. 57%, p < 0.0001) and receive total parenteral nutrition (68% v. 33%, p < 0.0001).
Table 1. Unadjusted Risk Factors for Candidemia in the Pediatric ICU*.
| Characteristic | Control (n = 184) | Case (n = 101) | Unadjusted OR/CI | |
|---|---|---|---|---|
| Median age, interquartile range | 1.94 (0.3, 11.2) | 3.4 (0.7, 11.1) | 1.02 (0.98, 1.06) | |
| Male | 85 (46%) | 43 (43%) | 0.86 (.52, 1.42) | |
| Transferred from another healthcare institution | 95 (52%) | 58 (57%) | 1.31 (.79, 2.17) | |
| Comorbidities and Clinical Procedures | ||||
| Receipt of a prior transplantation | 13 (7%) | 13 (13%) | 1.91 (.82, 4.46) | |
| Malignancy | 12 (7%) | 17 (17%) | 3.22 (1.36, 7.60) | |
| Dialysis (peritoneal or hemodialysis) | 8 (4%) | 9 (9%) | 2.0 (0.72, 5.57) | |
| Graft Versus Host Disease | 1 (1%) | 2 (2%) | 3.24 (.29, 36.63) | |
| Mechanical ventilation | 110 (60%) | 71 (70%) | 1.68 (.99, 2.86) | |
| Clinical Features within 1 Week of Study Entry | ||||
| Presence of a central venous catheter | 104 (57%) | 93 (92%) | 13.4 (4.80, 37.42) | |
| Presence of an arterial catheter | 81 (44%) | 56 (55%) | 1.77 (1.02, 3.06) | |
| Presence of a urinary catheter | 77 (42%) | 49 (49%) | 1.30 (.78, 2.17) | |
| Receipt of total parenteral nutrition | 61 (33%) | 69 (68%) | 5.30 (2.80, 10.05) | |
| Receipt of enteral nutrition | 124 (68%) | 62 (61%) | 0.75 (.44, 1.27) | |
| Clinical Features within 15 Days of Study Entry | ||||
| Receipt of a surgical procedure | 61 (33%) | 41 (41%) | 1.05 (.95, 1.17) | |
| Neutropenia | 2 (1%) | 6 (6%) | 5.58 (1.12, 27.79) | |
| Non-candidal blood stream infection | 26 (14%) | 29 (29%) | 2.47 (1.35, 4.52) | |
| Medication Use within 15 Days of Study Entry | ||||
| Receipt of immunosuppressive agents | 74 (41%) | 35 (35%) | 0.78 (.47, 1.32) | |
| Receipt of antifungal agents | 23 (13%) | 29 (29%) | 2.86 (1.44, 5.66) | |
| Receipt of antibiotics | 146 (80%) | 93 (92%) | 5.44 (1.87, 15.77) | |
| Receipt of antibiotics by class | ||||
| Receipt of parenteral or oral vancomycin | ||||
| 0 days | 123 (67%) | 42 (42%) | REF | |
| 1 to 3 days | 24 (13%) | 21 (21%) | 2.56 (1.27, 5.16) | |
| 4 or more days | 37 (20%) | 38 (38%) | 3.17 (1.73, 5.82) | |
| Receipt of extended spectrum cephalosporins | ||||
| 0 days | 128 (70%) | 54 (53%) | REF | |
| 1 to 3 days | 23 (13%) | 16 (16%) | 1.64 (.79, 3.39) | |
| 4 or more days | 33 (18%) | 31 (31%) | 2.31 (1.26, 4.22) | |
| Receipt of carbapenems | ||||
| 0 days | 176 (96%) | 90 (89%) | REF | |
| 1 to 3 days | 3 (2%) | 2 (2%) | 1.44 (.19, 11.12) | |
| 4 or more days | 5 (3%) | 9 (9%) | 3.29 (1.10, 9.89) | |
| Receipt of aminoglycosides | ||||
| 0 days | 104 (57%) | 43 (43%) | REF | |
| 1 to 3 days | 24 (13%) | 15 (15%) | 1.73 (.80, 3.76) | |
| 4 or more days | 56 (30%) | 43 (43%) | 2.09 (1.17, 3.74) | |
| Receipt of antibiotics by spectrum of action | ||||
| Receipt of agents covering anaerobic organismsˆ | ||||
| 0 days | 122 (66%) | 52 (52%) | REF | |
| 1 to 3 days | 12 (7%) | 6 (6%) | 1.34 (.47, 3.81) | |
| 4 or more days | 50 (27%) | 43 (43%) | 2.30 (1.29, 4.11) | |
All data in Table 1 are derived from the conditional matched analysis.
Antibiotic agents covering anaerobic organisms included ampicillin/sulbactam, clindamycin, imipenem, meropenem, metronidazole, and ticarcillin/clavulanate.
Multivariate analysis
Table 2 displays the results from the weighted multivariate regression. The following factors remained independently associated with candidemia: the presence of a CVC (OR, 30.4; 95% CI, 7.7, 119.5), malignancy (OR, 4.0; 95% CI, 1.23, 13.1), the use of vancomycin for > 3 days in the two weeks preceding study entry (OR, 6.2; 95% CI, 2.4,16), and the receipt of antimicrobial agents with activity against anaerobic organisms for > 3 days in the two weeks preceding study entry (OR 3.5; 95% CI, 1.5, 8.4).
Table 2. Multivariate Model for Candidemia in the Pediatric ICU.
| Characteristic | Unadjusted OR/CI | Adjusted OR/CI |
|---|---|---|
| Receipt of vancomycin | ||
| 0 days | REF | REF |
| 1 to 3 days | 2.56 (1.27, 5.16) | 3.04 (.98, 9.46) |
| 4 or more days | 3.17 (1.73, 5.82) | 6.19 (2.40, 15.99) |
| Receipt of agents covering anaerobic organisms | ||
| 0 days | REF | REF |
| 1 to 3 days | 1.34 (.47, 3.81) | .86 (.17, 4.37) |
| 4 or more days | 2.30 (1.29, 4.11) | 3.51 (1.47, 8.38) |
| Receipt of total parenteral nutrition | 5.30 (2.80, 10.05) | 2.03 (.80, 5.12) |
| Presence of a central venous catheter | 13.4 (4.80, 37.42) | 30.45 (7.76, 119.49) |
| Malignancy | 3.22 (1.36, 7.60) | 4.02 (1.23, 13.11) |
Adjusted ORs and CIs are derived from the weighted regression analysis (see Methods for details). Other factors were considered and ruled out as potential confounders.
Predictive Model for Candidemia
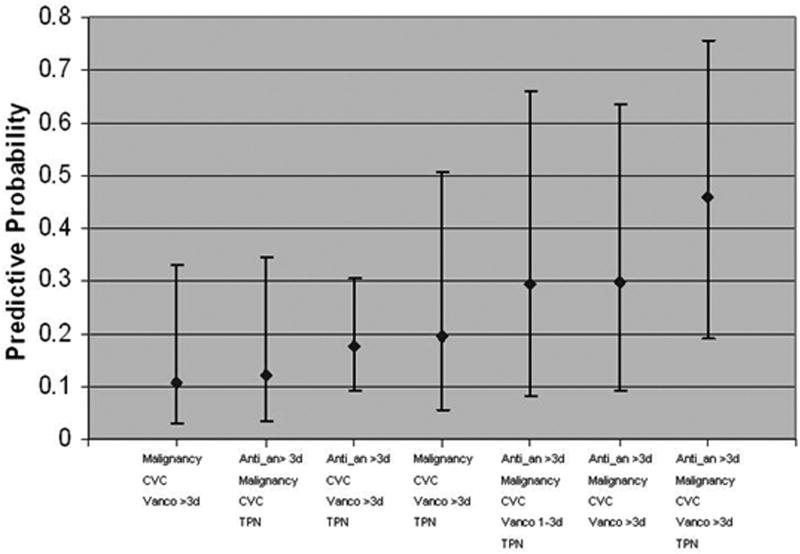
Figure 1 displays the predicted probability of candidemia in children who have various combinations of the risk factors significantly associated (>10% risk) with candidemia in weighted multivariate logistic regression analysis. Based on our data, the predicted probability of candidemia varied from 10.7% to 46%. Children with malignancy, a CVC in place, and who receive vancomycin for >3 days are at 10.7% risk of candidemia (CI= 2.8%, 32.9%). Children with malignancy who receive >3 days of vancomycin and >3 days of antimicrobial agents covering anaerobic organisms, who have a CVC and received total parenteral nutrition are at 46% risk of candidemia (CI= 19.0%, 75.5%). Table 3 includes point estimates of predicted probabilities in children who have various combinations of risk factors significantly associated with candidemia.
Figure 1. Predicted Probabilities and 95% Confidence Intervals for Candidemia in Children in the ICU by Risk Factor Combinations.
CVC = central venous catheter; Vanco >3d = receipt of vancomycin for >3 days in the two weeks prior to study entry; Anti_an >3d = receipt of antimicrobials with anti-anaerobic activity for > 3 days in the two weeks prior to study entry; Malignancy = malignancy as a comorbid condition; TPN = total parenteral nutrition in the week prior to study entry; Vanco 1-3d = receipt of vancomycin for 1-3 days in the two weeks prior to study entry
Table 3. Predicted probabilities of all risk factor combinations with >10% risk of candidemia.
| Anti-an | Malignancy | CVC | Vanco | TPN | Predicted probability |
|---|---|---|---|---|---|
| None | YES | YES | > 3 days | NO | 0.106903 (0.028384, 0.329068) |
| > 3 days | YES | YES | None | YES | 0.120818 (0.034794, 0.343777) |
| > 3 days | NO | YES | > 3 days | YES | 0.174846 (0.09297, 0.304614) |
| None | YES | YES | > 3 days | YES | 0.195158 (0.054084, 0.506984) |
| > 3 days | YES | YES | 1-3 days | YES | 0.29465 (0.082548, 0.659801) |
| > 3 days | YES | YES | > 3 days | NO | 0.295844 (0.09158, 0.63649) |
| > 3 days | YES | YES | > 3 days | YES | 0.459778 (0.190498, 0.754787) |
Anti_an = receipt of antimicrobials with anti-anaerobic activity in the two weeks prior to study entry; Malignancy = malignancy as a comorbid condition; CVC = central venous catheter; Vanco = receipt of vancomycin in the two weeks prior to study entry; TPN = total parenteral nutrition in the one week prior to study entry
Outcomes
The 30-day mortality rate was 44% in children with candidemia compared to 14% in controls (OR: 4.22; CI: 2.35, 7.60). The median length of PICU stay for children with candidemia was 35 days (IQR: 17, 69 days) versus a median of 27 days (IQR: 14,67 days) for controls (OR: 1.0; 95% CI: 0.99, 1.01). The hospital length of stay for children with candidemia was 46 days (IQR: 24, 79 days) versus 36 days (IQR: 17, 77 days) for controls (OR 1.0; 95% CI: 0.99, 1.01). There was no statistical difference between cases and controls with respect to time between study entry and ICU discharge (p = 0.509).
Discussion
To our knowledge, the present study is the first to evaluate independent risk factors and to determine a population of children in the PICU at high risk for developing candidemia. We found that the presence of a CVC, a diagnosis of malignancy, and receipt of either vancomycin or antimicrobials with activity against anaerobic organisms for greater than 3 days were independently associated with the development of candidemia in the PICU. Children in the PICU with 3 or more of these risk factors in different combinations had between 10% and 46% predicted probability of candidemia.
Previous studies have described risk factors for candidemia in neonatal intensive care unit (NICU) patients with an emphasis on premature neonates, a population of children with unique characteristics that may not be relevant to other pediatric patients [16,17]. There is a paucity of data on risk factors outside the NICU. Several studies have reported the general characteristics of children outside the neonatal period who have developed candidemia [18-22]; however data from studies using multivariate analysis to adjust for confounding are limited. In one of the previously conducted controlled pediatric studies of 24 cases of candidemia, investigators identified hyperalimentation as an independent risk factor for candidemia in children [20]. Although, hyperalimentation was not independently associated with candidemia in our study, it did contribute significantly to predicting candidemia in addition to the other variables.
Both the presence of a CVC and malignancy have been previously identified as risk factors for candidemia [23,24]; however, we were surprised by the magnitude of the effect CVCs had in PICU patients, suggesting that CVCs may be a significant source of candidemia in this patient population. Patients with malignancy are clearly at increased risk for candidemia due to their underlying immunocompromised state. That Candida parapsilosis comprised 30% of all isolates causing candidemia is consistent with the role of vascular catheters as a potential portal of entry. However, the source of Candida in patients with neoplastic diseases more likely derives from the gastrointestinal tract [24]. Mucosal disruption caused by cytotoxic chemotherapy and abrogation of normal gastrointestinal flora by antimicrobial therapy create a permissive environment that allows Candida to invade the mesenteric circulation.
There have been several studies that have investigated the relationship between antibiotic use and candidemia [25-32], but little is known about the relationships between the spectrum of antimicrobial activity or the duration of antibiotic use and candidemia. Numerous studies in animals have shown that the normal anaerobic gastrointestinal flora provide an important defense mechanism against infection by inhibiting the growth of potentially pathogenic organisms, a concept known as colonization resistance [33-35]. Colonization resistance is the limiting action of the normal flora that prevents overcolonization by endogenous organisms such as Candida spp [34,36,37]. It has been clearly established that the presence of anaerobic bacteria in the gut inhibits the overgrowth of Candida[34,36,37]. Therefore, we hypothesized that the use of antimicrobials with activity against the anaerobic GI flora would be associated with the development of candidemia, an independent association that was found in our analysis. Although we did not include parenteral vancomycin in the group of antimicrobials with activity against anaerobic organisms, it is not surprising that parenteral vancomycin use was an independent risk factor for candidemia because it does have significant activity against many anaerobic bacteria found in the GI tract.
In the absence of a clinical prediction rule that could be used to identify patients who will benefit most from antifungal prophylaxis, other strategies to decrease rates of candidemia could be considered. Anecdotally, NICUs have observed decreasing rates of candidemia by improving infection control practices and antimicrobial stewardship strategies. Additionally, given the significant risk CVCs posed for developing candidemia in our study, re-education of healthcare providers that are involved in the day-to-day care of critically ill patients on best practices surrounding central catheter maintenance may yield decreased rates of the illness.
There were several potential limitations to our study. Selection bias is normally of concern in a case-control study, but the nested case-control study design applied to our analysis allows selection of cases and controls from the same distinct source cohort (ICU admission), thus minimizing the likelihood of selection bias. Misclassification bias is likewise of concern in case-control studies. Cases and control subjects were drawn from the same hospitalized patient population and were identified solely on whether Candida was isolated from blood culture. Because these cultures were conducted for clinical care without previous knowledge of the patient's status regarding possible exposures of interest, there was unlikely to be any differential misclassification. Missing data can be an issue in studies involving retrospective review of medical records; however, past studies utilizing the same database of inpatient medical records used in this study found 97% of records complete and available for review. Any missing data would result in a non-differential bias and bias toward the null; yet, our results show strong associations between the hypothesized risk factors and candidemia.
Although the population from which the cases and controls was drawn was large, the number of observations is necessarily limited by the incidence of candidemia. Consequently, the confidence bounds around the ORs (Table 2) and predicted probabilities (Figure 2) are wide. Despite this limitation, our results suggest that combinations of risk factors are strongly associated with a higher probability of candidemia. Our institution is one of the largest children's hospitals in the United States but our findings may not be generalizable to other institutions; therefore, further work to validate our results will require multicenter collaboration.
We identified several combinations of predictors that identified a group of children in the PICU with a greater than 10% risk of candidemia that may potentially benefit from prophylaxis. Clinical prediction rules for candidemia are currently being evaluated in adult ICU patients [38,39]. Currently, no such scores exist for PICU patients. A predictive model could be of great clinical value to intensivists who care for children in the PICU, patients who are at high risk for candidemia due to their underlying severity of illness. A predictive model would also facilitate the evaluation of preventative strategies. Future efforts should focus on validation of the risk factors identified in our study in a different PICU population and development of interventions for prevention of candidemia in critically ill children.
Acknowledgments
The authors acknowledge Lesli Davis for her assistance with data abstraction.
Financial Support: National Institutes of Health (1K23 AI0629753-01 to T.E.Z) and Merck Research Funding. This study was supported in part by the intramural research program of the National Cancer Institute.
Footnotes
Potential Conflicts of Interest: T.E.Z has received research funding from Merck, Enzon, Schering-Plough, AstraZeneca, and Wyeth-Ayerst Laboratories and has received speaking honoraria from Cephalon. All other authors: no conflicts.
References
- 1.Wisplinghoff H, Seifert H, Tallent SM, Bischoff T, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in pediatric patients in United States hospitals: epidemiology, clinical features and susceptibilities. Pediatr Infect Dis J. 2003 Sep;22(8):686–691. doi: 10.1097/01.inf.0000078159.53132.40. [DOI] [PubMed] [Google Scholar]
- 2.Rex JH, Walsh TJ, Sobel JD, et al. Practice guidelines for the treatment of candidiasis. Infectious Diseases Society of America. Clin Infect Dis. 2000 Apr;30(4):662–678. doi: 10.1086/313749. [DOI] [PubMed] [Google Scholar]
- 3.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003 Apr 17;348(16):1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 4.Watson RS, Carcillo JA, Linde-Zwirble WT, Clermont G, Lidicker J, Angus DC. The epidemiology of severe sepsis in children in the United States. Am J Respir Crit Care Med. 2003 Mar 1;167(5):695–701. doi: 10.1164/rccm.200207-682OC. [DOI] [PubMed] [Google Scholar]
- 5.Zaoutis TE, Argon J, Chu J, Berlin JA, Walsh TJ, Feudtner C. The epidemiology and attributable outcomes of candidemia in adults and children hospitalized in the United States: a propensity analysis. Clin Infect Dis. 2005 Nov 1;41(9):1232–1239. doi: 10.1086/496922. [DOI] [PubMed] [Google Scholar]
- 6.Singhi SC, Reddy TC, Chakrabarti A. Candidemia in a pediatric intensive care unit. Pediatr Crit Care Med. 2004 Jul;5(4):369–374. doi: 10.1097/01.pcc.0000123550.68708.20. [DOI] [PubMed] [Google Scholar]
- 7.Zaoutis TE, Coffin SE, Chu JH, et al. Risk factors for mortality in children with candidemia. Pediatr Infect Dis J. 2005 Aug;24(8):736–739. doi: 10.1097/01.inf.0000172938.76561.8e. [DOI] [PubMed] [Google Scholar]
- 8.Kaufman D, Boyle R, Hazen KC, Patrie JT, Robinson M, Donowitz LG. Fluconazole prophylaxis against fungal colonization and infection in preterm infants. N Engl J Med. 2001 Dec 6;345(23):1660–1666. doi: 10.1056/NEJMoa010494. [DOI] [PubMed] [Google Scholar]
- 9.Robenshtok E, Gafter-Gvili A, Goldberg E, et al. Antifungal prophylaxis in cancer patients after chemotherapy or hematopoietic stem-cell transplantation: systematic review and meta-analysis. J Clin Oncol. 2007 Dec 1;25(34):5471–5489. doi: 10.1200/JCO.2007.12.3851. [DOI] [PubMed] [Google Scholar]
- 10.Rex JH, Sobel JD. Prophylactic antifungal therapy in the intensive care unit. Clin Infect Dis. 2001 Apr 15;32(8):1191–1200. doi: 10.1086/319763. [DOI] [PubMed] [Google Scholar]
- 11.Donskey CJ, Chowdhry TK, Hecker MT, et al. Effect of antibiotic therapy on the density of vancomycin-resistant enterococci in the stool of colonized patients. N Engl J Med. 2000 Dec 28;343(26):1925–1932. doi: 10.1056/NEJM200012283432604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hecker MT, Aron DC, Patel NP, Lehmann MK, Donskey CJ. Unnecessary use of antimicrobials in hospitalized patients: current patterns of misuse with an emphasis on the antianaerobic spectrum of activity. Arch Intern Med. 2003 Apr 28;163(8):972–978. doi: 10.1001/archinte.163.8.972. [DOI] [PubMed] [Google Scholar]
- 13.Edlund C, Barkholt L, Olsson-Liljequist B, Nord CE. Effect of vancomycin on intestinal flora of patients who previously received antimicrobial therapy. Clin Infect Dis. 1997 Sep;25(3):729–732. doi: 10.1086/513755. [DOI] [PubMed] [Google Scholar]
- 14.Arvidsson A, Leijd B, Nord CE, Angelin B. Interindividual variability in biliary excretion of ceftriaxone: effects on biliary lipid metabolism and on intestinal microflora. Eur J Clin Invest. 1988 Jun;18(3):261–266. doi: 10.1111/j.1365-2362.1988.tb01256.x. [DOI] [PubMed] [Google Scholar]
- 15.Bodey GP, Fainstein V, Garcia I, Rosenbaum B, Wong Y. Effect of broad-spectrum cephalosporins on the microbial flora of recipients. J Infect Dis. 1983 Nov;148(5):892–897. doi: 10.1093/infdis/148.5.892. [DOI] [PubMed] [Google Scholar]
- 16.Saiman L, Ludington E, Pfaller M, et al. Risk factors for candidemia in Neonatal Intensive Care Unit patients. The National Epidemiology of Mycosis Survey study group. Pediatr Infect Dis J. 2000 Apr;19(4):319–324. doi: 10.1097/00006454-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Weese-Mayer DE, Fondriest DW, Brouillette RT, Shulman ST. Risk factors associated with candidemia in the neonatal intensive care unit: a case-control study. Pediatr Infect Dis J. 1987 Feb;6(2):190–196. doi: 10.1097/00006454-198702000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Stamos JK, Rowley AH. Candidemia in a pediatric population. Clin Infect Dis. 1995 Mar;20(3):571–575. doi: 10.1093/clinids/20.3.571. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez-Nunez A. Incidence and mortality of proven invasive Candida infections in pediatric intensive care patients. Infect Control Hosp Epidemiol. 2001 Aug;22(8):477–478. doi: 10.1086/503410. [DOI] [PubMed] [Google Scholar]
- 20.MacDonald L, Baker C, Chenoweth C. Risk factors for candidemia in a children's hospital. Clin Infect Dis. 1998 Mar;26(3):642–645. doi: 10.1086/514580. [DOI] [PubMed] [Google Scholar]
- 21.Turner RB, Donowitz LG, Hendley JO. Consequences of candidemia for pediatric patients. Am J Dis Child. 1985 Feb;139(2):178–180. doi: 10.1001/archpedi.1985.02140040080032. [DOI] [PubMed] [Google Scholar]
- 22.Gray J, Gossain S, Morris K. Three-year survey of bacteremia and fungemia in a pediatric intensive care unit. Pediatr Infect Dis J. 2001 Apr;20(4):416–421. doi: 10.1097/00006454-200104000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Blyth CC, Chen SC, Slavin MA, et al. Not just little adults: candidemia epidemiology, molecular characterization, and antifungal susceptibility in neonatal and pediatric patients. Pediatrics. 2009 May;123(5):1360–1368. doi: 10.1542/peds.2008-2055. [DOI] [PubMed] [Google Scholar]
- 24.Nucci M, Anaissie E. Should vascular catheters be removed from all patients with candidemia? An evidence-based review. Clin Infect Dis. 2002 Mar 1;34(5):591–599. doi: 10.1086/338714. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz RS, Mackintosh FR, Schrier SL, Greenberg PL. Multivariate analysis of factors associated with invasive fungal disease during remission induction therapy for acute myelogenous leukemia. Cancer. 1984 Feb 1;53(3):411–419. doi: 10.1002/1097-0142(19840201)53:3<411::aid-cncr2820530308>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 26.Marr KA, Seidel K, White TC, Bowden RA. Candidemia in allogeneic blood and marrow transplant recipients: evolution of risk factors after the adoption of prophylactic fluconazole. J Infect Dis. 2000 Jan;181(1):309–316. doi: 10.1086/315193. [DOI] [PubMed] [Google Scholar]
- 27.Wey SB, Mori M, Pfaller MA, Woolson RF, Wenzel RP. Risk factors for hospital-acquired candidemia. A matched case-control study. Arch Intern Med. 1989 Oct;149(10):2349–2353. [PubMed] [Google Scholar]
- 28.Bross J, Talbot GH, Maislin G, Hurwitz S, Strom BL. Risk factors for nosocomial candidemia: a case-control study in adults without leukemia. Am J Med. 1989 Dec;87(6):614–620. doi: 10.1016/s0002-9343(89)80392-4. [DOI] [PubMed] [Google Scholar]
- 29.Richet HM, Andremont A, Tancrede C, Pico JL, Jarvis WR. Risk factors for candidemia in patients with acute lymphocytic leukemia. Rev Infect Dis. 1991 Mar-Apr;13(2):211–215. doi: 10.1093/clinids/13.2.211. [DOI] [PubMed] [Google Scholar]
- 30.Burchard KW, Minor LB, Slotman GJ, Gann DS. Fungal sepsis in surgical patients. Arch Surg. 1983 Feb;118(2):217–221. doi: 10.1001/archsurg.1983.01390020065011. [DOI] [PubMed] [Google Scholar]
- 31.Karabinis A, Hill C, Leclercq B, Tancrede C, Baume D, Andremont A. Risk factors for candidemia in cancer patients: a case-control study. J Clin Microbiol. 1988 Mar;26(3):429–432. doi: 10.1128/jcm.26.3.429-432.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pittet D, Monod M, Suter PM, Frenk E, Auckenthaler R. Candida colonization and subsequent infections in critically ill surgical patients. Ann Surg. 1994 Dec;220(6):751–758. doi: 10.1097/00000658-199412000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vollaard EJ, Clasener HA. Colonization resistance. Antimicrob Agents Chemother. 1994 Mar;38(3):409–414. doi: 10.1128/aac.38.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van der Waaij D. Colonization resistance of the digestive tract: clinical consequences and implications. J Antimicrob Chemother. 1982 Oct;10(4):263–270. doi: 10.1093/jac/10.4.263. [DOI] [PubMed] [Google Scholar]
- 35.Dubos R. The microbiota of the gastrointestinal tract. Gastroenterology. 1966 Nov;51(5):868–874. [PubMed] [Google Scholar]
- 36.Samonis G, Gikas A, Anaissie EJ, et al. Prospective evaluation of effects of broad-spectrum antibiotics on gastrointestinal yeast colonization of humans. Antimicrob Agents Chemother. 1993 Jan;37(1):51–53. doi: 10.1128/aac.37.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Louie TJ, Chubb H, Bow EJ, et al. Preservation of colonization resistance parameters during empiric therapy with aztreonam in the febrile neutropenic patient. Rev Infect Dis. 1985 Nov-Dec;7(4):S747–761. doi: 10.1093/clinids/7.supplement_4.s747. [DOI] [PubMed] [Google Scholar]
- 38.Ostrosky-Zeichner L, Sable C, Sobel J, et al. Multicenter retrospective development and validation of a clinical prediction rule for nosocomial invasive candidiasis in the intensive care setting. Eur J Clin Microbiol Infect Dis. 2007 Apr;26(4):271–276. doi: 10.1007/s10096-007-0270-z. [DOI] [PubMed] [Google Scholar]
- 39.Leon C, Ruiz-Santana S, Saavedra P, et al. A bedside scoring system (“Candida score”) for early antifungal treatment in nonneutropenic critically ill patients with Candida colonization. Crit Care Med. 2006 Mar;34(3):730–737. doi: 10.1097/01.CCM.0000202208.37364.7D. [DOI] [PubMed] [Google Scholar]