Abstract
Female cynomolgus monkeys are excellent models for understanding cardiovascular disease and the relationships between inflammatory processes and conditions such as atherogenesis. This review summarizes published research findings obtained through comprehensive, multidisciplinary, multi-investigator studies in nonhuman primates over the past two decades. These studies examined the effects of exogenous estrogens and dietary soy protein/isoflavones (IFs) on atherosclerosis, circulating biomarkers, and tissue inflammation in pre- and postmenopausal female cynomolgus monkeys. Inflammation may play a role in the initiation and progression of disease, be a consequence of the disease, or both. Circulating and tissue biomarkers with inflammatory and anti-inflammatory characteristics (including adhesion molecules such as e-selectin, VCAM-1, and ICAM-1, chemokines such as MCP-1, cytokines such as interleukins, and acute phase reactants such as CRP, and others) may be useful indicators of disease status. Treatment of postmenopausal subjects with estrogen resulted in significant reductions in several key inflammatory mediators as well as atherosclerosis, while dietary IF had a more limited effect on inflammation and atherogenesis. Circulating concentrations of key inflammatory proteins, including monocyte-chemoattractant protein-1 (MCP-1) and interleukin-6 (IL-6), were associated with atherosclerosis and lesion characteristics in these animals. In premenopausal female monkeys, a diet enriched in soy protein reduced arterial inflammation as well as atherogenesis in comparison to a diet enriched in casein-lactalbumin. Expression levels of arterial inflammation associated genes (MCP-1, ICAM-1) and markers for inflammatory cell types (macrophages and T cells) correlated with plaque size, were differentially influenced by treatments, and represent potential targets for interventions. Arterial expression of estrogen receptor α, the key mediator of estrogenic effects, was inversely correlated with plaque size and indices of inflammation, suggestive of an atheroprotective role. The findings provide additional evidence that circulating inflammatory markers (particularly MCP-1) may be useful indicators of atherosclerotic disease progression and responses to treatment in female primates, and that estrogens and dietary soy may inhibit atherogenesis in part through anti-inflammatory mechanisms.
Keywords: inflammation, atherogenesis, estrogen receptor, macrophage, T cell
INTRODUCTION
This review provides a summary of peer-reviewed published research findings related to inflammation and atherogenesis obtained from comprehensive, multidisciplinary, multi-investigator studies in nonhuman primates over the past two decades. These studies have been designed to explore the effects of diet, menopause, and hormone therapies on the cardiovascular, skeletal, reproductive, endocrine, and other systems imparting morbidity and mortality in women, and have provided important insights into mechanisms of disease and potential biomarkers useful in basic science and clinical studies. Considerable attention has been paid to the health and well-being of these primates during the course of these studies. Detailed methods and procedures used can be found in the referenced publications.
Inflammatory Processes Promote Initiation and Progression of Atherosclerosis
The initiation and progression of atherosclerosis occurs through a complex interrelated series of events in which inflammation plays a key role [for review see Hansson et al., 2006; Naghavi et al., 2003; Ross, 1999]. The very earliest events include activation of the endothelium in response to dyslipidemia alone or in combination with other stimuli. This leads to the endothelial cell (EC) expression of cell adhesion molecules, which facilitate adhesion of circulating leukocytes (primarily monocyte/macrophages and T-cells) to activated endothelium. P- and E-selectin facilitate the slowing and rolling of leukocytes while VCAM-1 and ICAM-1 promote firm cell attachment to the endothelium. E-selectin is produced primarily by ECs, P-selectin by platelets and ECs, while VCAM-1 and ICAM-1 are produced by endothelial and smooth muscle cells as well as macrophages. Figure 1 contains examples of immunohistochemical staining for ICAM-1 and VCAM-1 in selected frozen sections of aorta from an ovariectomized female cynomolgus macaque, which had been consuming an atherogenic diet for 8 months. At this stage of early atherogenesis demonstration of adhesion molecule expression by immunohistochemistry is sporadic and limited. Adhesion molecule expression is followed by adherence of leukocytes, which are enticed to migrate into the intima by monocyte chemoattractant protein-1 (MCP-1), a potent mononuclear cell chemoattractant produced by endothelium and smooth muscle cells. Once monocyte–macrophages are present in the arterial intima and in the face of continued insult, these cells can produce inflammation associated proteins and other chemicals which promote (1) continued influx and accumulation of inflammatory cells and (2) migration and proliferation of medial vascular smooth muscle cells into the intimal space, leading to the formation of an atherosclerotic lesion. As lesions progress they become more complicated, with the formation of a fibrous cap overlying the lesion and central areas of necrosis and calcification. Clinical events occur when plaques rupture and expose thrombogenic plaque contents to the circulation, leading to clot formation, impedance of blood flow, and ischemia, which can lead to myocardial infarction. Plaque rupture has been associated with proteolytic enzymes such as matrix metalloproteases (MMPs) and cathepsins, which degrade the extracellular matrix leading to unstable plaques prone to rupture [Hansson et al., 2006]. Macaques and humans consuming diets relatively high in fat and similar to the typical North American diet experience comparable rates of myocardial infarction (approximately 1/300 at risk per year) [Clarkson and Klumpp, 1990]. The relative rarity of such an event precludes its use as an experimental outcome in primate or other animal models.
Fig. 1.

Immunohistochemical staining for the cell adhesion molecules ICAM-1 (top) and VCAM-1 (bottom) in sections of aortas from ovariectomized female monkeys fed an atherogenic diet for 8 months. Aortic segments preserved frozen in OCT were cryosectioned and incubated with mouse-anti-human ICAM-1 mAb or mouse anti-human VCAM-1 mAb from R&D Systems (MN), which were detected with HRP-conjugated goat anti-mouse secondary antibodies. Sections were counterstained with light green. Positive immunoreactivity is depicted by brown stained areas. Control sections not incubated with primary antibody were negative for brown reaction product. Staining was heterogeneous, as the majority of sections from animals consuming atherogenic diets were negative, consistent with the localized nature of early atherogenesis. Color figures can be viewed in the online issue, which is available at www.interscience.Wiley.com
Circulating Inflammatory Markers and CHD
Circulating levels of inflammatory mediators have been associated with atherosclerosis and/or clinical events. In the Atherosclerosis Risk in Communities (ARIC) Study, plasma levels of soluble (s) sE-selectin and sICAM-1 [Hwang et al., 1997] as well as the chemokine MCP-1 [Hoogeveen et al., 2005] were associated with coronary artery atherosclerosis and CHD. TNF-α, IL1β, and IL-6 are other pro-inflammatory cytokines more important in inducing endothelial dysfunction than factors such as oxidized lipoproteins [Bolton et al., 2001]. Circulating MCP-1, IL-6, and other mediators have also been associated with atherosclerosis in nonhuman primates [Register et al., 2002, 2005]. Expression of these proteins is regulated by nuclear factor kappa-B (NF-κB), a transcription factor linked to inflammation and atherogenesis. NF-κB is activated in response to many stimuli associated with atherogenesis and CHD, including oxidative stress, dyslipidemia, hypertension, diabetes, etc. Activated NF-κB (p65) has been detected immunohistochemically in athero-sclerotic lesions but not in normal arteries [Brand et al., 1996].
Atherogenesis, Estrogen Receptors, and Anti-inflammatory Effects of Estrogen and IF
Estrogens, either 17β-estradiol or conjugated equine estrogens (CEE), are potent inhibitors of atherogenesis (intimal atherosclerotic plaque size) when administered soon after ovariectomy in female nonhuman primates [Adams et al., 1990, 1997; Clarkson et al., 2001; Register et al., 2002, 2005]. This inhibition is largely independent of circulating plasma lipids and lipoproteins, suggesting other mechanisms may be involved, such as direct effects on cells in the artery wall. Estrogen receptors (ERs) mediate the effects of estrogen, and both ERα and ERβ have been shown to be expressed in both female and male primate arteries [Register and Adams, 1998; Walker et al., 2008a,b]. Importantly, estrogen (E) has been shown to inhibit NF-κB mediated gene expression through the multiple ER-dependent mechanisms [for review see Kalaitzidis and Gilmore, 2005; also Biswas et al., 2005; also Spier et al., 2000; Chadwick et al., 2005; Ghisletti et al., 2005] and to reduce circulating levels of NF-κB regulated mediators in human [Caulin-Glaser et al., 1998; Cushman et al., 1999; Hu et al., 2006] and in nonhuman primate studies [Register et al., 2002, 2005]. Thus, anti-inflammatory effects of estrogens may occur in the artery as well as in other tissues and organs. IFs have also been postulated to have anti-inflammatory characteristics mediated through ER/NF-kB interactions, through anti-oxidant activity, and through other mechanisms [see Register et al., 2005 and references cited within].
C-Reactive Protein
Current evidence suggests that human and non-human primates may differ in the effect of oral estrogens on circulating C-reactive protein (CRP), an acute phase reactant produced primarily by the liver, which is associated with inflammation. In women, oral E has been shown to simultaneously reduce circulating levels of NF-κB regulated mediators such as MCP-1 and adhesion molecules while increasing plasma CRP levels [Cushman et al., 1999; Hu et al., 2006]. This apparently divergent effect of E may be explained in part by differences in regulatory regions of these genes, as CRP is regulated through complex mechanisms [Agrawal et al., 2003] distinct from the classic NF-κB driven gene expression observed in MCP-1 and many other inflammatory genes. The elevation in CRP in response to oral E appears to be due to a first pass effect, as transdermal E does not cause CRP elevation in women [Hemelaar et al., 2008]. Interestingly, administration of oral E in the diet to ovariectomized female cynomolgus monkeys has not been associated with increased circulating CRP. This apparent lack of parallelism might be due to the fact that women generally take estrogen orally in a bolus while administration in primate studies usually involves incorporation of E into the diet, although subcutaneous E2 has been used in some studies and shown to be atheroprotective [Adams et al., 1997]. Circulating CRP concentrations have been proposed as a potentially important (albeit controversial) risk factor for future clinical CHD events in people [Ridker et al., 1999, 2005]. While the primate studies are under-powered to detect an effect of serum CRP levels on MI or other parallel-clinical events, they are adequately powered to assess relationships between circulating biomarkers and disease endpoints and have demonstrated that there is not a significant relationship of CRP levels with atherosclerosis extent or plaque characteristics in the coronary or other arteries examined to date [Register et al., 2005].
Serum Markers of Inflammation: Effects of CEE and Soy IF and Relationships with Atherosclerosis
IFs, compounds present in soy and other plants with the potential to bind and activate ERs, have been investigated as potential alternatives to mammalian estrogens, which are associated with increases in the risk of endometrial cancer unless administered with a progestogen. Unfortunately, co-administered estrogens and progestins may increase the risk of breast cancer, so an alternative is desirable. We investigated the effects of dietary soy IF and CEEs on circulating inflammatory markers and atherosclerotic lesion characteristics at the end of a 3-yr study of ovariecto-mized monkeys consuming a moderately atherogenic diet [Register et al., 2005]. Treatments were as follows: (1) Control, receiving alcohol-extracted soy-protein-based diet with low IF content (comparable to 5 mg/day for a woman); (2) CEE, added to the control diet at a dose comparable to 0.625 mg/day; and (3) IF, consumed as a part of unextracted soy protein isolate (SPI) at a dose comparable to 129 mg/day. Coronary, iliac, and carotid artery atherosclerosis were signifi-cantly reduced by CEE in relation to Control [Clarkson et al., 2001]. IF reduced carotid artery atherosclerosis and tended to reduce atherosclerosis in iliac and coronary arteries. Serum monocyte chemoat-tractant protein (MCP)-1 was reduced by CEE but not by IF [Register et al., 2005]. On the contrary, serum soluble vascular cell adhesion molecule-1 (sVCAM-1) was reduced by both IF and CEE relative to Controls. Neither treatment affected serum IL-6, soluble E-selectin, or CRP levels. Thus, soy IF provided an anti-inflammatory effect, which appeared to be selective for sVCAM-1, whereas CEE reduced serum levels of both sVCAM-1 and MCP1. Reductions in serum sVCAM-1 levels in response to dietary soy have also been observed in naturally postmenopausal women [Colacurci et al., 2005], in hypertensive postmenopausal women [Nasca et al., 2008], and in women with a specific ERβ genotype [Vafeiadou et al., 2006], although some studies have found no effect of dietary IF on sVCAM-1 [Greany et al., 2008].
Inflammation, Inflammatory Markers, and Vulnerable Plaque Characteristics
The onset of clinical cardiovascular events depends on the presence of a vulnerable atherosclerotic plaque with prothombotic characteristics, often as a result of rupture [Hansson et al., 2006; Naghavi et al., 2003; Ross, 1999]. In addition to the central role of inflammation, plaque remodeling as well as other characteristics are involved in the development of vulnerable plaques. Criteria for defining vulnerable plaques include the presence of active inflammation with monocyte/macrophage or T cell infiltration, a thin fibrous cap juxtaposed to a large lipid core, and endothelial denudation accompanied by platelet aggregation, fissure, and/or severe stenosis (>90%) [Naghavi et al., 2003]. Minor criteria include the presence of a superficial calcified nodule, a “glistening yellow” appearance, intraplaque hemorrhage, endothelial dysfunction, and outward remodeling. Clinically, the characteristics of atherosclerotic lesions may be as important as the size and extent of these lesions. Acute coronary events are not necessarily associated with large, stenotic coronary artery atherosclerotic plaques, but more often are a result of the disruption of small to medium size plaques that are heavily infiltrated by macrophages [Falk, 1983; Lendon et al., 1991]. These macrophages in the shoulder region of fibrous caps can produce MMPs, which can weaken the fibrous cap and lead to plaque rupture, mural thrombosis, and arterial occlusion [Moreno et al., 1994; van der Wal et al., 1994]. Pro-inflammatory cytokines are also important in the lesion inflammatory process. Interleukins (IL-1, IL-6) have a broad range of humoral and cellular immune effects related to inflammation [Papanicolaou et al., 1998].
To explore potential relationships between serum biomarkers and atherosclerotic lesion characteristics in animals treated with CEE and IFs, histologic sections representative of the length of the left circumflex (LCX) coronary artery were stained with hematoxylin and eosin or with Verhoeff-van Gieson’s and characterized [Register et al., 2005]. The degree of inflammation associated with the monkey’s atherosclerotic plaque was determined based on the density of leukocytes accumulated both within the adventitia and within the intimal plaque. A subjective score of 4 was used for the most extensively involved of those cases within the data set. The intermediate grades of 1, 2, and 3 were used relative to reference slides available in the laboratory. The amount of atheronecrosis was estimated as a percent of the cross-sectional area of the plaque. The fibro-muscular caps overlying the plaques were measured histomorphometrically at points of maximum and minimum thickness. Data were then averaged across all the sections for an individual animal before statistical analysis. Partial correlations (corrected for treatment effects) between serum markers and indices of atherosclerosis, inflammation, and matrix remodeling were calculated. Serum MCP-1 levels were significantly correlated with numerous indices of atherosclerosis, including plaque area in the iliac artery obtained at necropsy, and in the coronary and carotid (common and internal) arteries (Table I) [Register et al., 2005]. Serum IL-6 was correlated with atherosclerosis extent in the iliac and carotid arteries. Serum MCP-1 also correlated with estimates of plaque inflammation, necrosis, and matrix remodeling, the latter indicated by immunohistochemical analyses of MMP-9 (Table II). Serum CRP levels were not associated with size or characteristics of atherosclerotic plaques.
TABLE I.
Partial Correlations and P-values of Circulating Markers with Plaque Area in Different Vascular Sites across All Animals in this Study of Ovariectomized Female Monkeys
| MCP-1 | IL-6 | CRP | |
|---|---|---|---|
| Coronary | 0.220 | 0.089 | −0.050 |
| P = 0.009 | P = 0.302 | P = 0.559 | |
| Iliac Artery | 0.219 | 0.197 | −0.035 |
| (Common) | P = 0.010 | P = 0.021 | P = 0.684 |
| Carotid Artery | 0.219 | 0.228 | 0.007 |
| (Common) | P = 0.003 | P = 0.003 | P = 0.923 |
| Carotid Artery | 0.314 | 0.250 | 0.108 |
| (Internal) | P = 0.001 | P = 0.003 | P = 0.208 |
Iliac artery (final) refers to the common iliac artery obtained at necropsy. Correlations were adjusted for effect of treatment. Significant correlations are represented by bold italicized correlation coefficients. Adapted from Register et al. [2005]. Reprinted with permission from The Endocrine Society (Copyright 2005, The Endocrine Society).
TABLE II.
Partial Correlations and P-values of Circulating Markers with Plaque Characteristics Determined in the Left Circumflex Coronary Artery Across All Animals in the Study
| Plaque characteristics | MCP-1 | IL-6 | CRP |
|---|---|---|---|
| Plaque area | 0.272 | 0.019 | −0.082 |
| P = 0.001 | P = 0.828 | P = 0.337 | |
| Inflammation index | 0.256 | 0.100 | 0.008 |
| P = 0.002 | P = 0.246 | P = 0.925 | |
| Necrosis area | 0.362 | 0.107 | −0.046 |
| P = 0.001 | P = 0.213 | P = 0.596 | |
| Percent necrosis | 0.304 | 0.066 | 0.076 |
| P = 0.001 | P = 0.446 | P = 0.375 | |
| Fibrous cap thickness | 0.352 | 0.070 | −0.045 |
| P = 0.001 | P = 0.415 | P = 0.601 | |
| Fibrous cap thinness | 0.296 | 0.032 | −0.080 |
| P = 0.001 | P = 0.707 | P = 0.351 | |
| Intimal MMP-9 | 0.361 | 0.120 | −0.025 |
| P = 0.001 | P = 0.240 | P = 0.813 |
Significant correlations are represented by bold italicized correlation coefficients. Correlations were corrected for effects of treatment. Adapted from Register et al. [2005]. Reprinted with permission from The Endocrine Society (Copyright 2005, The Endocrine Society).
Dietary Soy Reduces Atherosclerosis and Arterial Inflammation in Premenopausal Female Monkeys
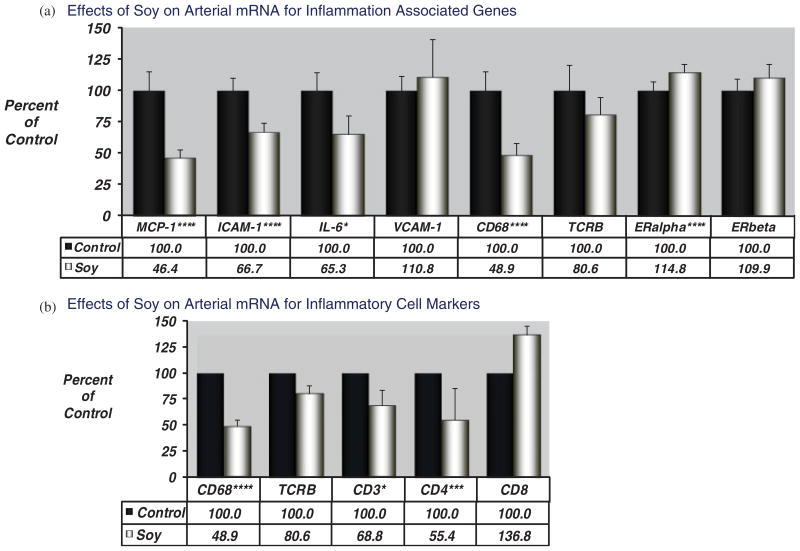
The iliac artery is a reliable surrogate for plaque development in the coronary artery [Clarkson et al., 2001], and provides a method for assessment of atherosclerosis and arterial biology without sacrificing the animal. We used the iliac artery biopsy to determine atherosclerosis development and plaque characteristics in a project designed to assess effects of dietary soy protein containing IF relative to dietary casein-lactalbumin protein [Walker et al., 2008a]. Premenopausal cynomolgus macaques (n = 84) were fed an atherogenic diet deriving protein from casein/lactalbumin or SPI (containing 1.88 mg IF/g). Animals consumed atherogenic diets for 3 years, at which time a biopsy of the left iliac artery was removed for the determination of atherosclerosis and expression of mRNA transcripts related to inflammation, macrophage and T-cell content, and ERs. Lesion size was determined in the middle iliac artery section (preserved in paraformaldehyde), RNA was obtained from adjacent sections of biopsies (preserved in RNAlater), and quantitative real time RT-PCR was then used with monkey specific oligonucleotide primer-probe reagents to determine the arterial expression of target genes associated with inflammation and atherogenesis (MCP-1, VCAM-1, ICAM-1, IL6), and estrogen action (ERα and ERβ). We also used qRT-PCR to quantitate expression of genes indicative of inflammatory cell populations expected to be present in these lesions. For these studies we used cell-type specific markers for macrophages (CD68, a scavenger receptor expressed almost exclusively by macrophages) and T cells (CD3, a classic T cell marker) and T cell subsets (CD4, a marker of T helper cells; CD8, a marker of cytotoxic or killer T cells as well as natural killer cells [NK]). All data were normalized to the geometric mean of the expression of 3 housekeeping genes (GAPDH, β-actin, and RPLP). The group receiving dietary soy had reduced atherosclerotic plaque size and reduced levels of arterial mRNA transcripts for inflammation associated targets MCP-1, intercellular adhesion molecule-1 (ICAM-1), and interleukin-6 (Fig. 2a). In addition, transcripts indicative of macrophage (CD68) and T cell (CD3, CD4) populations were significantly reduced in the group receiving soy protein (Fig. 2b). Arterial mRNA expression levels of inflammatory mediators and markers of inflammatory cell types were significantly correlated with plaque size, with the exception of arterial VCAM-1 (Table III).
Fig. 2.
(a) Effects of dietary soy protein on molecular indices of inflammation in an iliac artery biopsy obtained from female cynomolgus macaques (without euthanasia of the animals). Data represent mean±standard error of the data expressed as percent of control group. All data were corrected to the geometric mean of the control genes GAPDH, β-actin, and RPLP. Data were analyzed by ANOVA, significance levels are denoted by asterisks: ****P < 0.001; ***P < 0.005; **P < 0.01; *P < 0.05. (b) Effects of dietary soy protein on molecular indices of cell type markers in an iliac artery biopsy obtained from female cynomolgus macaques (without euthanasia of the animals). Data represent mean±standard error of the data expressed as percent of control group. All data were corrected to the geometric mean of the control genes GAPDH, β-actin, and RPLP. Data were analyzed by ANOVA, significance levels are denoted by asterisks: ***P < 0.001; ***P < 0.005; **P < 0.01; *P < 0.05. Adapted from Walker et al. [2008b].
TABLE III.
Spearman Partial Correlations of Iliac Artery Plaque Area and mRNA Expression of Inflammation Related Genes, Inflammatory Cell Markers, and Estrogen Receptors
| MCP-1 | VCAM-1 | ICAM-1 | IL6 | CD68 | CD3 | CD4 | TCRβ | ERα | ERβ | |
|---|---|---|---|---|---|---|---|---|---|---|
| Plaque size | 0.5936 | 0.0458 | 0.696 | 0.3179 | 0.7255 | 0.7839 | 0.8139 | 0.5111 | −0.5088 | −0.1092 |
| P = 0.000 | P = 0.696 | P = 0.000 | P = 0.005 | P = 0.000 | P = 0.000 | P = 0.000 | P = 0.000 | P = 0.000 | P = 0.351 | |
| MCP-1 | 0.0362 | 0.782 | 0.5205 | 0.7566 | 0.5852 | 0.6456 | 0.2926 | −0.524 | −0.2468 | |
| P = 0.758 | P = 0.000 | P = 0.000 | P = 0.000 | P = 0.000 | P = 0.000 | P = 0.011 | P = 0.000 | P = 0.033 | ||
| VCAM-1 | 0.0467 | −0.0184 | 0.0671 | 0.0406 | −0.0207 | 0.0376 | −0.0467 | −0.0894 | ||
| P = 0.691 | P = 0.876 | P = 0.567 | P = 0.729 | P = 0.860 | P = 0.749 | P = 0.691 | P = 0.445 | |||
| ICAM-1 | 0.5237 | 0.8819 | 0.7225 | 0.6987 | 0.5048 | −0.4125 | −0.083 | |||
| P = 0.000 | P = 0.00 | P = 0.000 | P = 0.000 | P = 0.000 | P = 0.000 | P = 0.479 | ||||
| IL6 | 0.4045 | 0.3462 | 0.2846 | 0.1595 | −0.309 | −0.1657 | ||||
| P = 0.000 | P = 0.002 | P = 0.013 | P = 0.172 | P = 0.007 | P = 0.155 | |||||
| CD68 | 0.7837 | 0.7791 | 0.5066 | −0.4661 | −0.1781 | |||||
| P = 0.000 | P = 0.000 | P = 0.000 | P = 0.000 | P = 0.126 | ||||||
| CD3 | 0.8277 | 0.6123 | −0.3723 | −0.0168 | ||||||
| P = 0.00 | P = 0.000 | P = 0.001 | P = 0.886 | |||||||
| CD4 | 0.5709 | −0.478 | −0.1661 | |||||||
| P = 0.000 | P = 0.000 | P = 0.154 | ||||||||
| TCRβ | −0.1923 | −0.0117 | ||||||||
| P = 0.098 | P = 0.921 | |||||||||
| ERα | 0.4172 | |||||||||
| P = 0.000 |
Correlations were adjusted for effect of diet group. Significant correlations are represented by bold italicized correlation coefficients and P values.
Dietary Soy Protein Reduces Arterial Inflammation Independently of Plasma Lipid Effects in Primates
Comprehensive meta analyses of human studies have demonstrated beneficial effects of dietary soy on plasma lipoprotein profiles, although the magnitude of the beneficial effect and its contribution to atheroprotection is still debated [Reynolds et al., 2006; Taku et al., 2007; Zhan and Ho, 2005]. Diet studies in the cynomolgus monkey model enable a more detailed examination of mechanisms of atheroprotection, and allow a glimpse into biologic effects occurring in the artery which may be independent of circulating plasma lipoprotein concentrations. Statistical analyses of the iliac artery data above suggested that most of the effects of dietary soy on plaque size could be accounted for by effects of dietary SPI on plasma lipids. On the contrary, reductions in arterial inflammatory gene and macrophage and T cell marker expression were largely independent of the plasma lipid effects of dietary soy protein [Walker et al., 2008a]. Briefly, plasma lipid concentration-mediated effects on gene expression were evaluated by a bootstrapping analysis that treated the TPC/HDLc ratio as a mediator and plaque size and social status as covariates. These analyses suggested that the beneficial effects of dietary soy protein on arterial expression of ICAM-1, IL-6, CD68, CD3, and CD4 were largely unexplained by effects on plasma lipid concentrations. This question was further examined by analyses of subsets of animals having similar TPC/HDLc ratios in each dietary treatment condition. Consistently across all subset groups generated, MCP-1, ICAM-1, and IL-6 expression was significantly lower in the SPI treatment group. Similar results were obtained when subsets of animals were matched for both TPC/HDLc and plaque size, supporting the suggestion that dietary soy inhibited arterial inflammatory expression independently of plasma lipids and lesion size, thereby illuminating a potential benefit of dietary soy not detectable by assessment of plasma lipoproteins alone.
Differential Effects of Dietary Soy Protein on Inflammatory Gene Expression in Male vs. Female Monkeys
The results in the premenopausal female monkeys contrasted with the effects of dietary soy protein in male monkeys, where soy protein consumption was associated with reduced atherosclerotic plaque size but did not significantly influence arterial expression of inflammatory mediators. Nevertheless, arterial inflammatory gene expression was very highly correlated with atherosclerotic plaque size in the males [Walker et al., 2008b]. The mechanisms underlying this sex difference are unknown, but could be related to differences in endogenous sex hormone concentrations, degree of atherosclerosis, or some other factor.
Inflammation, Atherogenesis, and Arterial ER Expression
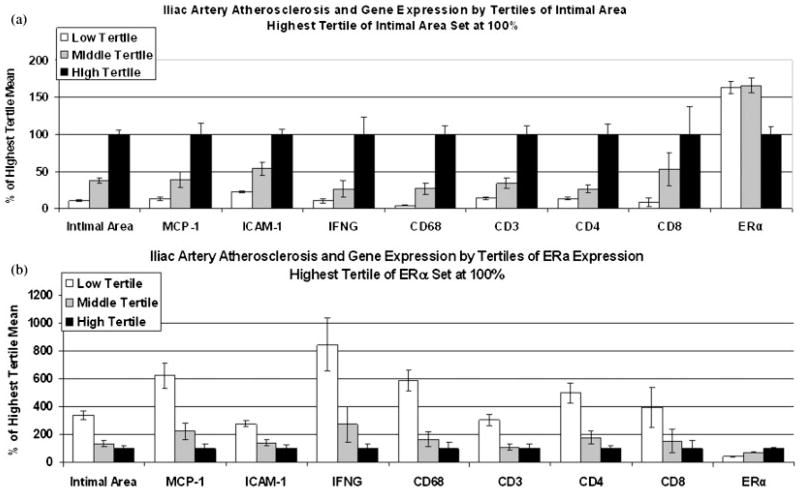
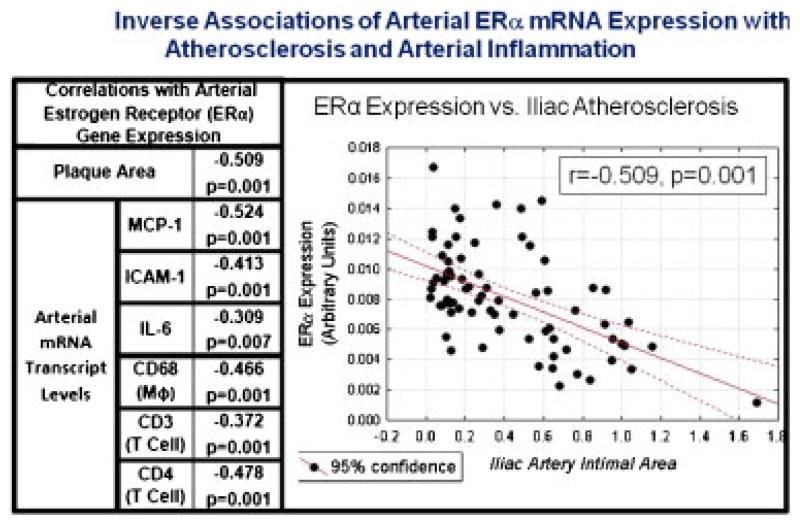
ERα and ERβ are expressed in the coronary arteries and vascular smooth muscle cells of cynomolgus monkeys [Register and Adams, 1998]. We assessed the potential relationships of arterial ER expression with plaque characteristics and found that arterial ERα and ERβ mRNA expression was inversely correlated with atherosclerotic plaque size and inflammatory gene expression in both male [Walker et al., 2008b] and female [Walker et al., 2008a] macaques. Figure 3 shows the relationships of arterial gene expression as a function of plaque size (Fig. 3a) or ERα expression (Fig. 3b) in premenopausal female monkeys. Expression of the proinflammatory genes MCP-1, ICAM-1, IL-6, IL-2, and IFN-γ as well as markers for macrophages (CD 68), T cells (CD3), and T helper cells (CD4) were found to be closely associated with intimal area. The dataset was first examined with respect to intimal lesion area: data were subdivided into tertiles based upon intimal lesion area (Fig. 3a). Subjects in the highest tertile of intimal area (black bars, set at 100%) had the highest level of inflammatory gene expression and the lowest level of ERα expression (Fig. 3a). Conversely, partitioning of the data by tertiles of ERα expression (highest ERα tertile mean at 100%, black bars) revealed a slightly different pattern (Fig. 3b). Arteries representing the lowest tertile of ERα expression (open bars) showed the highest level of expression of inflammatory targets (MCP-1, ICAM-1, IFN-γ) and inflammatory cell markers (macrophages (CD68), T cells (CD3), T helper cells (CD4), and cytotoxic T cells and/or NK cells (CD8)) compared with the two higher tertiles of ER expression (shaded, black bars). These relationships are also illustrated in Table III, which shows significant inverse correlations of arterial ERα expression with several indices of atherosclerosis and vascular inflammation, including expression levels of MCP-1, ICAM-1, IL-6, as well as the markers for macrophages (CD68) and T cell subsets (TCRβ, CD3, CD4). A plot and table illustrating the significant inverse relationship between ERα and iliac artery atherosclerotic plaque area and the correlations to inflammatory gene expression are shown in Figure 4. Similar inverse relationships between arterial ERα gene expression and plaque size and characteristics were also observed in male arteries [Walker et al., 2008a]. These inverse relationships between ER and plaque size and inflammatory gene expression could be causal: i.e. pre-existing receptor expression may have influenced the susceptibility of the artery to atherogenesis. It is also possible that the relationship is consequential: atherosclerosis and/or hypercholesterolemia dependent alterations in vascular biology of specific cell types may have modulated receptor expression. Alternatively, decreased expression accompanying increased plaque complications could simply be reflective of alterations in overall cellular composition of the atherosclerotic lesions (i.e. macrophages and T cells), which may have distinct receptor expression profiles. These data are retrospective, cross-sectional associations between plaque size, ERα expression and the various markers evaluated after 3 years of atherogenic diet, and thus, we cannot determine whether ERα expression was causally related to plaque progression or the result of plaque progression. Efforts are underway to more fully characterize the temporal nature underlying these relationships.
Fig. 3.
Relationships of arterial gene expression as a function of plaque size (intimal area) (a) or ERα expression (b) in premenopausal female monkeys. (a) Data was subdivided into tertiles based upon measures of atherosclerotic plaque size (intimal lesion area). (b) Data was subdivided into tertiles based upon levels of ERα expression. Adapted from Walker et al. [2008b].
Fig. 4.
Inverse association of arterial expression of ERα with expression of inflammation associated markers (index left) and plaque size (scatterplot right) in premenopausal monkeys. Partial correlations were determined after correction for treatment. Adapted from Walker et al. [2008b].
In conclusion, atherogenesis and arterial inflammation are linked in nonhuman primates, may be assessed to varying degrees by measuring key circulating inflammatory markers, and may be influenced by plasma lipid dependent and independent mechanisms. Targeting both plasma lipids and inflammatory mechanisms may be beneficial in reducing atherosclerosis progression and the evolution of an atherosclerotic plaque to a vulnerable “unstable” plaque likely to rupture and produce a clinical event.
Acknowledgments
Contract grant sponsor: NIH; Contract grant numbers: HL45666; HL79421; RR020890; AG18170; AG28641; and AA11204.
All research practices in these studies adhered to the American Journal of Primatology policies for the ethical treatment of nonhuman primates and were conducted humanely and in compliance with all institutional, state and federal laws for the usage of primates in laboratory settings. The author thanks the many collaborators and colleagues involved in these studies, including Drs. Michael R Adams, Mary Anthony, Susan Appt, Jennifer Cann, Haiying Chen, Thomas B. Clarkson, J. Mark Cline, Jay R. Kaplan, Tim Morgan, Carol A. Shively, Sara Walker, Janice Wagner, J. Koudy Williams, and Charles Wood.
References
- Adams MR, Kaplan JR, Manuck SB, Koritnik DR, Parks JS, Wolfe MS, Clarkson TB. Inhibition of coronary artery atherosclerosis by 17-beta estradiol in ovariectomized monkeys. Lack of an effect of added progesterone. Arteriosclerosis. 1990;10:1051–1057. doi: 10.1161/01.atv.10.6.1051. [DOI] [PubMed] [Google Scholar]
- Adams MR, Register TC, Golden DL, Wagner JD, Williams JK. Medroxyprogesterone acetate antagonizes inhibitory effects of conjugated equine estrogens on coronary artery atherosclerosis. Arterioscler Thromb Vasc Biol. 1997;17:217–221. doi: 10.1161/01.atv.17.1.217. [DOI] [PubMed] [Google Scholar]
- Agrawal A, Cha-Molstad H, Samols D, Kushner I. Overexpressed nuclear factor-kappaB can participate in endogenous C-reactive protein induction, and enhances the effects of C/EBPbeta and signal transducer and activator of transcription-3. Immunology. 2003;108:539–547. doi: 10.1046/j.1365-2567.2003.01608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas DK, Singh S, Shi Q, Pardee AB, Iglehart JD. Crossroads of estrogen receptor and NF-kappaB signaling. Science STKE. 2005;288:pe27. doi: 10.1126/stke.2882005pe27. [DOI] [PubMed] [Google Scholar]
- Bolton CH, Downs LG, Victory JG, Dwight JF, Tomson CR, Mackness MI, Pinkney JH. Endothelial dysfunction in chronic renal failure: roles of lipoprotein oxidation and pro-inflammatory cytokines. Nephrol Dial Transplant. 2001;16:1189–1197. doi: 10.1093/ndt/16.6.1189. [DOI] [PubMed] [Google Scholar]
- Brand K, Page S, Rogler G, Bartsch A, Brandl R, Knuechel R, Page M, Kaltschmidt C, Baeuerle PA, Neumeier D. Activated transcription factor nuclear factor-kappa B is present in the atherosclerotic lesion. J Clin Invest. 1996;97:1715–1722. doi: 10.1172/JCI118598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulin-Glaser T, Farrell WJ, Pfau SE, Zaret B, Bunger K, Setaro JF, Brennan JJ, Bender JR, Cleman MW, Cabin HS, Remetz MS. Modulation of circulating cellular adhesion molecules in postmenopausal women with coronary artery disease. J Am Coll Cardiol. 1998;31:1555–1560. doi: 10.1016/s0735-1097(98)00145-4. [DOI] [PubMed] [Google Scholar]
- Chadwick CC, Chippari S, Matelan E, Borges-Marcucci L, Eckert AM, Keith JC, Jr, Albert LM, Leathurby Y, Harris HA, Bhat RA, Ashwell M, Trybulski E, Winneker RC, Adelman SJ, Steffan RJ, Harnish DC. Identification of pathway-selective estrogen receptor ligands that inhibit NF-kappa B transcriptional activity. Proc Natl Acad Sci USA. 2005;102:2543–2548. doi: 10.1073/pnas.0405841102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson TB, Klumpp S. The contribution of nonhuman primates to understanding coronary artery atherosclerosis. ILAR Journal V32 Animal Models in Biomedical Research. 1990 http://dels.nas.edu/ilar_n/ilarjournal/32_2/32_2Contribution.shtml.
- Clarkson TB, Anthony MS, Morgan TM. Inhibition of postmenopausal atherosclerosis progression a comparison of the effects of conjugated equine estrogens and soy phytoestrogens. J Clin Endocrinol Metab. 2001;186:41–47. doi: 10.1210/jcem.86.1.7151. [DOI] [PubMed] [Google Scholar]
- Colacurci N, Chiàntera A, Fornaro F, de Novellis V, Manzella D, Arciello A, Chiàntera V, Improta L, Paolisso G. Effects of soy isoflavones on endothelial function in healthy post-menopausal women. Menopause. 2005;12:299–307. doi: 10.1097/01.gme.0000147017.23173.5b. [DOI] [PubMed] [Google Scholar]
- Cushman M, Legault C, Barrett-Connor E, Stefanick ML, Kessler C, Judd HL, Sakkinen PA, Tracy RP. Effect of postmenopausal hormones on inflammation-sensitive proteins: the Postmenopausal Estrogen/Progestin Interventions (PEPI) Study. Circulation. 1999;100:717–722. doi: 10.1161/01.cir.100.7.717. [DOI] [PubMed] [Google Scholar]
- Falk KE. Plaque rupture with severe pre-existing stenosis precipitating coronary thrombosis. Characteristics of coronary atherosclerotic plaques underlying fatal occlusive thrombi. Br Heart J. 1983;50:127–134. doi: 10.1136/hrt.50.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghisletti S, Meda C, Maggi A, Vegeto E. 17beta-Estradiol inhibits inflammatory gene expression by controlling NF-kappaB intracellular localization. Mol Cell Biol. 2005;25:2957–2968. doi: 10.1128/MCB.25.8.2957-2968.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greany KA, Nettleton JA, Wangen KE, Thomas W, Kurzer MS. Consumption of isoflavone-rich soy protein does not alter homocysteine or markers of inflammation in postmenopausal women. Eur J Clin Nutr. 2008;62:1419–1425. doi: 10.1038/sj.ejcn.1602885. [DOI] [PubMed] [Google Scholar]
- Hansson GK, Robertson AK, Söderberg-Nauclér C. Inflammation and atherosclerosis. Annu Rev Pathol. 2006;1:297–329. doi: 10.1146/annurev.pathol.1.110304.100100. [DOI] [PubMed] [Google Scholar]
- Hemelaar M, van der Mooren MJ, Rad M, Kluft C, Kenemans P. Effects of non-oral postmenopausal hormone therapy on markers of cardiovascular risk: a systematic review. Fertil Steril. 2008;90:642–672. doi: 10.1016/j.fertnstert.2007.07.1298. [DOI] [PubMed] [Google Scholar]
- Hoogeveen RC, Morrison A, Boerwinkle E, Miles JS, Rhodes CE, Sharrett AR, Ballantyne CM. Plasma MCP-1 level and risk for peripheral arterial disease and incident coronary heart disease: atherosclerosis risk in communities study. Atherosclerosis. 2005;183:301–307. doi: 10.1016/j.atherosclerosis.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Hu P, Greendale GA, Palla SL, Reboussin BA, Herrington DM, Barrett-Connor E, Reuben DB. The effects of hormone therapy on the markers of inflammation and endothelial function and plasma matrix metalloproteinase-9 level in postmenopausal women: the postmenopausal estrogen progestin intervention (PEPI) trial. Atherosclerosis. 2006;185:347–352. doi: 10.1016/j.atherosclerosis.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Hwang SJ, Ballantyne CM, Sharrett AR, Smith LC, Davis CE, Gotto AM, Jr, Boerwinkle E. Circulating adhesion molecules VCAM 1, ICAM 1, and E selectin in carotid atherosclerosis and incident coronary heart disease cases: the Atherosclerosis Risk In Communities (ARIC) study. Circulation. 1997;96:4219–4225. doi: 10.1161/01.cir.96.12.4219. [DOI] [PubMed] [Google Scholar]
- Kalaitzidis D, Gilmore TD. Transcription factor crosstalk: the estrogen receptor and NF-kappaB. Trends Endocrinol Metab. 2005;16:46–52. doi: 10.1016/j.tem.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Lendon CL, Davis MJ, Born GVR, Richardson PD. Atherosclerotic plaque caps are locally weakened when macrophages density is increased. Atherosclerosis. 1991;87:87–90. doi: 10.1016/0021-9150(91)90235-u. [DOI] [PubMed] [Google Scholar]
- Moreno PR, Falk E, Palacios IF, Newell JB, Fuster V, Fallon JT. Macrophage infiltration in acute coronary syndromes: implications for plaque rupture. Circulation. 1994;90:775–778. doi: 10.1161/01.cir.90.2.775. [DOI] [PubMed] [Google Scholar]
- Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, Badimon JJ, Stefanadis C, Moreno P, Pasterkamp G, Fayad Z, Stone PH, Waxman S, Raggi P, Madjid M, Zarrabi A, Burke A, Yuan C, Fitzgerald PJ, Siscovick DS, de Korte CL, Aikawa M, Juhani Airaksinen KE, Assmann G, Becker CR, Chesebro JH, Farb A, Galis ZS, Jackson C, Jang IK, Koenig W, Lodder RA, March K, Demirovic J, Navab M, Priori SG, Rekhter MD, Bahr R, Grundy SM, Mehran R, Colombo A, Boerwinkle E, Ballantyne C, Insull W, Jr, Schwartz RS, Vogel R, Serruys PW, Hansson GK, Faxon DP, Kaul S, Drexler H, Greenland P, Muller JE, Virmani R, Ridker PM, Zipes DP, Shah PK, Willerson JT. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: parts I and II. Circulation. 2003;108108:1664–1668. 1772–1778. doi: 10.1161/01.CIR.0000087480.94275.97. [DOI] [PubMed] [Google Scholar]
- Nasca MM, Zhou JR, Welty FK. Effect of soy nuts on adhesion molecules and markers of inflammation in hypertensive and normotensive postmenopausal women. Am J Cardiol. 2008;102:84–86. doi: 10.1016/j.amjcard.2008.02.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanicolaou DA, Wilder RL, Manolagas SC, Chrousos GP. The pathophysiologic roles of interleukin-6 in human disease. Ann Intern Med. 1998;128:127–137. doi: 10.7326/0003-4819-128-2-199801150-00009. [DOI] [PubMed] [Google Scholar]
- Register TC, Adams MR. Coronary artery and cultured aortic smooth muscle cells express mRNA for both the classical estrogen receptor and the newly described estrogen receptor beta. J Steroid Biochem Mol Biol. 1998;64:187–191. doi: 10.1016/s0960-0760(97)00155-6. [DOI] [PubMed] [Google Scholar]
- Register TC, Wagner JD, Zhang L, Hall J, Clarkson TB. Effects of tibolone and conventional hormone replacement therapies on arterial and hepatic cholesterol accumulation and on circulating endothelin-1, vascular cell adhesion molecule-1, and E-selectin in surgically menopausal monkeys. Menopause. 2002;9:411–421. doi: 10.1097/00042192-200211000-00006. [DOI] [PubMed] [Google Scholar]
- Register TC, Cann JA, Kaplan JR, Williams JK, Adams MR, Morgan TM, Anthony MS, Blair RM, Wagner JD, Clarkson TB. Effects of soy isoflavones and conjugated equine estrogens on inflammatory markers in atherosclerotic, ovariectomized monkeys. J Clin Endocrinol Metab. 2005;90:1734–1740. doi: 10.1210/jc.2004-0939. [DOI] [PubMed] [Google Scholar]
- Reynolds K, Chin A, Lees KA, Nguyen A, Bujnowski D, He J. A meta-analysis of the effect of soy protein supplementation on serum lipids. Am J Cardiol. 2006;98:633–640. doi: 10.1016/j.amjcard.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Hennekens CH, Rifai N, Buring JE, Manson JE. Hormone replacement therapy and increased plasma concentration of C-reactive protein. Circulation. 1999;100:713–716. doi: 10.1161/01.cir.100.7.713. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Rifai N, Cook NR, Bradwin G, Buring JE. Non-HDL cholesterol, apolipoproteins A-I and B100, standard lipid measures, lipid ratios, and CRP as risk factors for cardiovascular disease in women. JAMA. 2005;294:326–333. doi: 10.1001/jama.294.3.326. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Taku K, Umegaki K, Sato Y, Taki Y, Endoh K, Watanabe S. Soy isoflavones lower serum total and LDL cholesterol in humans: a meta-analysis of 11 randomized controlled trials. Am J Clin Nutr. 2007;85:1148–1156. doi: 10.1093/ajcn/85.4.1148. Erratum in: Am J Clin Nutr 2007, 86, 809. [DOI] [PubMed] [Google Scholar]
- Vafeiadou K, Hall WL, Williams CM. Does genotype and equol-production status affect response to isoflavones? Data from a pan-European study on the effects of isoflavones on cardiovascular risk markers in post-menopausal women. Proc Nutr Soc. 2006;65:106–115. doi: 10.1079/pns2005483. [DOI] [PubMed] [Google Scholar]
- Van der Wal AC, Becker AE, van der Loos CM, Das PK. Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation. 1994;89:36–44. doi: 10.1161/01.cir.89.1.36. [DOI] [PubMed] [Google Scholar]
- Walker SE, Adams MR, Franke AA, Register TC. Effects of dietary soy protein on iliac and carotid artery atherosclerosis and gene expression in male monkeys. Atherosclerosis. 2008a;196:106–113. doi: 10.1016/j.atherosclerosis.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker SE, Register TC, Appt SE, Adams MR, Clarkson TB, Chen H, Isom S, Franke AA, Kaplan JR. Plasma lipid-dependent and -independent effects of dietary soy protein and social status on atherogenesis in premenopausal monkeys: implications for postmenopausal atherosclerosis burden. Menopause. 2008b;15:950–957. doi: 10.1097/gme.0b013e3181612cef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan S, Ho SC. Meta-analysis of the effects of soy protein containing isoflavones on the lipid profile. Am J Clin Nutr. 2005;81:397–408. doi: 10.1093/ajcn.81.2.397. [DOI] [PubMed] [Google Scholar]