Abstract
Background
Native American children have higher rates of morbidity associated with acute respiratory infection than children in the general United States population, yet detailed information is lacking regarding their principal clinical presentations and infectious etiologies.
Methods
We pursued a comprehensive molecular survey of bacteria and viruses in nasal wash specimens from children with acute respiratory disease collected prospectively over one year (January 1 through December 31, 2009) from 915 Navajo and White Mountain Apache children in their second or third year of life who had been enrolled in an efficacy study of an RSV monoclonal antibody in the first year of life.
Results
During the surveillance period, 1476 episodes of disease were detected in 669 children. Rates of outpatient and inpatient lower respiratory tract illness were 391 and 79 per 1000 child-years, respectively, and were most commonly diagnosed as pneumonia. Potential pathogens were detected in 88% of specimens. Viruses most commonly detected were respiratory syncytial virus (RSV) and human rhinovirus (HRV); 2009 pandemic influenza A (H1N1) illnesses primarily occurred in the fall. Streptococcus pneumoniae was detected in 60% of subjects; only HRV was significantly associated with S. pneumoniae carriage. The presence of influenza virus, HRV, or S. pneumoniae was not associated with increased risk for lower respiratory tract involvement or hospitalization.
Conclusions
Acute lower respiratory illnesses occur at disproportionately high rates among young American Indian children, and are associated with a range of common pathogens. This study provides critical evidence to support reducing the disproportionate burden of acute respiratory disease among young Native Americans.
Keywords: American Indian, respiratory infections, child, diagnosis, influenza
Introduction
Native American (NA) children, particularly Alaska Natives and American Indians in the southwestern United States, have high rates of morbidity and mortality related to acute lower respiratory infections (ALRI) (1;2). Prospective, population-based studies in these communities thus far have examined only specific clinical presentations associated with single pathogens, such as invasive disease due to Streptococcus pneumoniae or Haemophilus influenzae type b (Hib), or bronchiolitis due to respiratory syncytial virus (RSV) (3–10). Data regarding the impact of influenza in NA communities, even during the 2009 pandemic, have been limited to retrospective observational studies (11–15). Although these investigations all demonstrate disproportionate rates of acute disease, the restricted range of their clinical and microbiologic evaluations prevents a more complete characterization of this elevated burden, which could direct where prevention efforts should be focused. Here, we describe the major clinical presentations and respiratory pathogens associated with ALRI events requiring medical attention in a cohort of young American Indian children followed prospectively through the 2009 calendar year.
Methods
Study population
Subjects for this analysis were drawn from a prospective clinical trial evaluating the effectiveness of motavizumab, an investigational monoclonal antibody directed against the RSV F protein, in preventing severe RSV-related disease in American Indian infants of the American Southwest (NCT00121108, www.clinicaltrials.gov; (16)). Cohorts of children 6 months of age or younger belonging to the Navajo and White Mountain Apache or San Carlos Apache tribes were recruited each autumn during 2004–2007 and randomized in a 2:1 ratio to receive study drug or placebo in five monthly doses during their first RSV season. Subjects were monitored for all medically-attended acute respiratory illnesses (MAARI) until their third birthday. Following reports of elevated morbidity related to H1N1pdm within Native American communities (11;12), we sought to investigate the impact of influenza in relation to other respiratory pathogens in this group of children during that time period. Therefore, all children from the third and fourth enrollment seasons (2006 and 2007) who were still participating in the trial follow-up as of January 1, 2009 were included in this report.
Clinical surveillance
Active surveillance was conducted six days a week at Indian Health Service clinical facilities where the study subjects primarily sought care. A nasal wash specimen was collected in the clinic or at home by a study nurse within 72 hours of any visit with a respiratory illness diagnosis, or if presenting signs/symptoms suggested a respiratory illness. Because specimen collection occurred prior to formal assessment of the respiratory category of the illness, nursing staff was instructed to collect specimens in children with diagnoses suggesting a lower respiratory illness (pneumonia, bronchiolitis, asthma, croup, etc.) or common presenting signs/symptoms from a pre-specified list (cough, wheeze, crackles, shortness of breath, etc.). Therefore, samples were obtained from a subset of upper respiratory illnesses when the determination of lower vs upper respiratory involvement had not been made within the specimen collection window. Nasal washes were obtained by instilling 15–20 mL of lactated Ringers solution into the nasopharynx with a bulb syringe and passively collecting the fluid into a sterile specimen cup. Specimens were combined with viral transport medium (VTM), snap frozen in liquid nitrogen, and stored at −70C.
A study physician categorized each clinical visit; a MAARI episode was defined as an event in a study child with any lower or upper respiratory symptoms; the presence of an abnormality on chest examination (e.g. wheeze, crackles, rhonchi); or where an acute respiratory illness diagnosis was made by the primary care provider based on clinical judgment. Study physicians then categorized the episode as an upper versus lower respiratory illness and assigned a final study diagnosis using a standardized protocol based on clinical exam findings, imaging reports, laboratory testing, clinical diagnoses, and where necessary, discussion with the provider. For study purposes, the protocol diagnosis took precedence when the assessments of the study and treating physicians were discordant. Study physicians communicated regularly with each other to discuss cases in which the final diagnosis was not straightforward, in order to maximize consistency and standardization across the study.
Diagnostic testing
All specimens obtained during January 1-December 31, 2009 were tested for this study. Total nucleic acid (TNA) was extracted from 250 µL aliquots of the nasal wash samples using the easyMAG extraction platform (bioMérieux) and eluted in 35 µL. cDNA was generated from 10 µL aliquots of TNA using the Superscript II reverse transcription kit (Invitrogen) and analyzed by the MassTag PCR respiratory panel (17–21). Each sample was screened using a panel of viral and bacterial respiratory pathogens, which included influenza A, influenza B, human rhinoviruses, human enteroviruses, human metapneumovirus, human parainfluenza viruses 1–4, human coronaviruses 229E and OC43, respiratory syncytial virus A and B, adenovirus, Chlamydophila pneumoniae, Haemophilus influenzae, Mycobacterium tuberculosis, Mycoplasma pneumoniae, Legionella pneumophila, and Streptococcus pneumoniae. Samples positive by MassTag PCR were re-amplified by singleplex PCR and confirmed by sequencing.
Statistical analysis
Data were analyzed using Stata 11.0 (StataCorp, College Station, TX). Health care provider records were reviewed by a study physician. MAARI episodes were categorized as an outpatient or inpatient ALRI as appropriate, and diagnoses were classified by major clinical syndrome (Table 1). All clinical data were recorded on paper forms and submitted to the trial sponsor (MedImmune, Gaithersburg, MD), after which a cleaned, locked dataset was returned to the study investigators for further analysis. Clinical episodes from which multiple nasal washes were collected were reviewed and molecular testing results were consolidated if clinically compatible. Illness incidence rates were calculated based on the subjects under active surveillance, and were compared using rate ratios and exact binomial 95% confidence intervals. Person-time at risk for each participant was calculated from January 1, 2009 through the date of study withdrawal/completion or December 31, 2009, whichever occurred first. Pathogen-specific incidence rates were adjusted for the proportion of episodes sampled. Associations between diagnostic categories or clinical syndromes and individual pathogens or pathogen combinations were examined using the two-sample test of proportions, Chi-square analysis, or Fisher’s exact test, as appropriate. Baseline characteristics (Table 2) were evaluated as risk factors for lower respiratory tract disease or hospitalization using multiple logistic regression. The median ages between groups were compared using the Mann-Whitney test. A p-value of <0.05 was considered statistically significant.
Table 1.
Guidelines for clinical syndrome categorization of acute respiratory illness episodes
| Diagnostic category | Categorization Rule |
|---|---|
| Pneumonia | Takes precedence over any co-diagnoses of a wheezing disorder or an etiology (e.g., RSV, influenza) |
| Bronchiolitis | Includes bronchitis |
| Wheezing illnesses | Includes asthma, reactive airways disease (RAD), wheeze, small airways disease |
| ALRI not otherwise specified |
Takes precedence over any co-diagnosis of URI |
| Croup | Includes laryngotracheobronchitis |
| Allergic rhinitis | Includes seasonal allergies |
| Viral syndromes | Includes flu-like illness, viral syndrome, and stand-alone diagnoses of RSV, influenza, etc. |
| Pharyngitis, tonsillitis | Includes sore throat |
| Sinusitis, otitis media | Takes precedence over any co-diagnosis of URI |
| URI, cough | Takes precedence over any co-diagnosis of an etiology |
Table 2.
Demographic, clinical, and environmental factors among study subjects.a
| 2006 Enrollment Cohort |
2007 Enrollment Cohort |
Total | |
|---|---|---|---|
| N=260 | N=655 | N=915 | |
| Gender (n, % male) | 136 (52) | 326 (50) | 462 (50) |
| Assigned to study drug (motavizumab) |
176 (68) | 435 (66) | 611 (67) |
| Age on January 1, 2009 (in months; median, range) |
26.3 (24.1–31.2) | 15.4 (12.2–20.6) | 16.9 (12.2–31.2) |
| Breastfed as an infant (n, %) | 224 (86) | 561 (86) | 785 (86) |
| Hospitalized prior to enrollment in parent trial (n, %) |
30 (13) | 62 (11) | 92 (12) |
| Attending daycare(n, %) | 5 (2) | 19 (3) | 24 (3) |
| Siblings <6 years old at home(n, %) | 168 (65) | 436 (67) | 604 (66) |
| Smoker in the household | 53 (20) | 123 (19) | 176 (19) |
| Mother smoked during pregnancy | 11 (4) | 27 (4)b | 38 (4) |
| Wood/coal stove in home | 155 (60) | 409 (62) | 564 (62) |
| Family member with asthma* | 92 (35) | 180 (27) | 272 (30) |
| Family member with wheeze | 61 (23) | 114 (17)b | 175 (19) |
| Family member with hay fever* | 36 (14) | 60 (9)b | 196 (10) |
| Family member with eczema | 31 (12) | 73 (11) | 104 (11) |
| Assigned to motavizumab treatment | 176 (68) | 435 (66) | 611 (67) |
Baseline demographic and exposure data were collected by parent or guardian interview at the time of enrollment.
Data not available for 1 subject.
p<0.05 comparing 2006 cohort 2007 cohort; p≥0.05 for all other variables.
Human subject research issues
The study was approved by the Navajo and White Mountain Apache tribes, as well as the Institutional Review Boards of the Johns Hopkins Bloomberg School of Public Health, the Navajo Nation, and the Phoenix Area Indian Health Service.
Results
Incidence of Medically-Attended Acute Respiratory Illness
At the start of the analysis period, 915 children (median age at start on January 1, 2009: 16.9 months) were under active follow-up; 260 (28%) enrolled in 2006, and 655 (72%) enrolled in 2007 (Table 2). The two cohorts were well balanced at enrollment in the parent trial, with the exception of having a family history of asthma or hay fever. A high proportion of subjects was breastfed in infancy (86%), had young siblings at home (66%), was exposed to secondhand smoke (19%) or wood- or coal-burning stoves (62%) in the home, or had been hospitalized prior to enrollment in the parent trial (12%). Although two-thirds of subjects lived with other young children, formal daycare attendance (3%) was low.
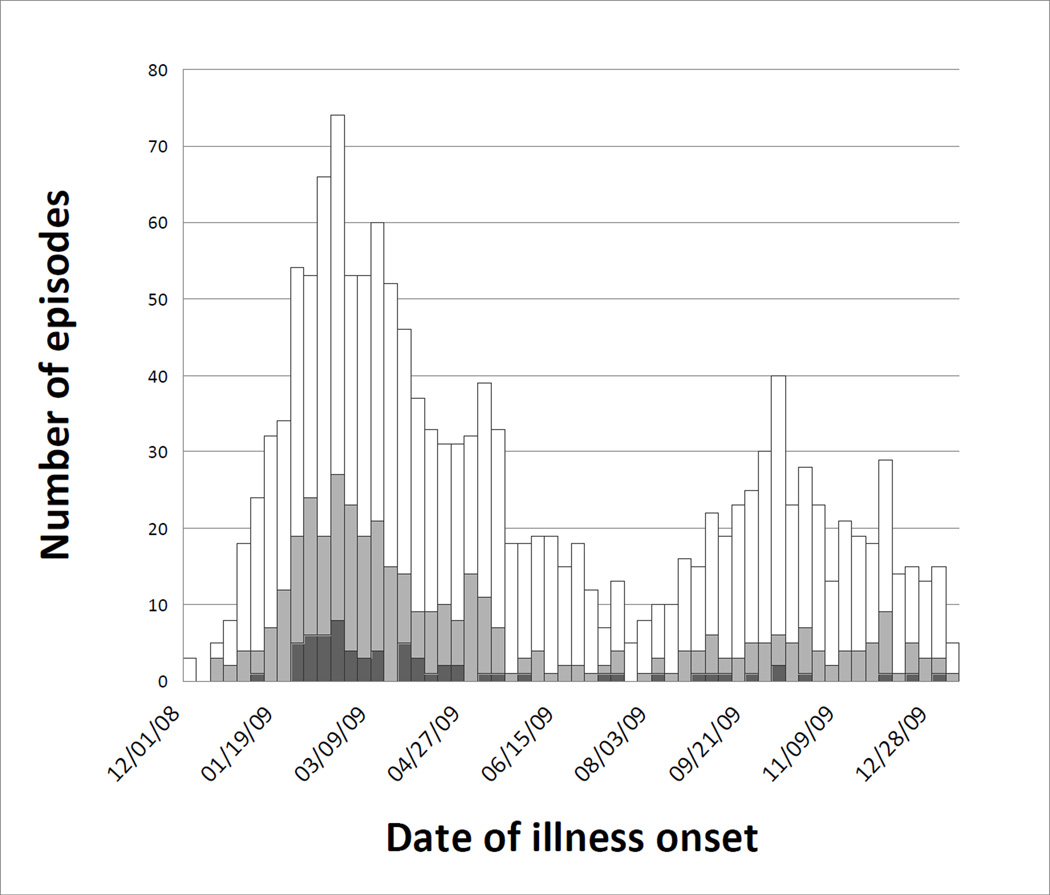
Of the 915 subjects under surveillance, 669 (73%) experienced a total of 1476 MAARI episodes, resulting in an incidence rate of 1756 episodes per 1000 child-years (Table 3). Of these episodes, 329 were ALRI managed on an outpatient basis, while 66 were ALRI requiring hospitalization, resulting in incidence rates of 391 and 79 episodes per 1000 child-years, respectively. Rates of respiratory illness were higher in the younger cohort compared to the older cohort for all categories (p<0.05 for all). Subjects experienced 2.2 MAARI episodes on average (range 0–11), while 246 (27%) experienced none. Repeat visits accounted for 27% and 12% of inpatient and outpatient ALRI episodes, respectively (Table 4). Of the 240 children who experienced an outpatient ALRI, 25 (10%) also experienced an inpatient ALRI episode. Episodes occurred in two waves during 2009 (Figure 1). The first wave, peaking in mid- to late winter, represented the second or third winter respiratory season experienced by the two cohorts, while the second wave occurred throughout the autumn, after a proportion of the older cohort had completed follow up.
Table 3.
Episodes and incidence ratesa of medically-attended acute respiratory infections, outpatient acute lower respiratory illnesses, and inpatient acute lower respiratory illnesses, by seasonal cohort.
| 2006 Cohort N=260 |
2007 Cohort N=655 |
Total N=915 |
p-valueb | |
|---|---|---|---|---|
| MAARI | ||||
| No. of Children (%) | 163 (63) | 506 (77) | 669 (73) | <0.0001 |
| No. of Episodes | 283 | 1193 | 1476 | |
| Incidence | 1428 (1267, 1605) | 1858 (1754, 1966) | 1756 (1668, 1848) | <0.0001 |
| Outpatient ALRI | ||||
| No. of Children (%) | 44 (17) | 196 (30) | 240 (26) | 0.0001 |
| No. of Episodes | 56 | 273 | 329 | |
| Incidence | 283 (214, 367) | 425 (376, 479) | 391 (350, 436) | 0.0020 |
| Inpatient ALRI | ||||
| No. of Children (%) | 6 (2) | 52 (8) | 58 (6) | 0.0016 |
| No. of Episodes | 6 | 60 | 66 | |
| Incidence | 30 (11, 66) | 93 (71, 120) | 79 (61, 100) | 0.0014 |
episodes per 1000 child-years (95% confidence interval).
Comparing 2006 cohort to 2007 cohort.
Table 4.
Number of times a child was seen, according to visit type.
| Number of children | ||||
|---|---|---|---|---|
| All MAARI |
All ALRI |
Outpt ALRI |
Inpt ALRI |
|
| Number of visits |
||||
| No visits of this type |
246 | 642 | 675 | 857 |
| Once | 267 | 197 | 179 | 53 |
| Twice | 198 | 49 | 43 | 3 |
| Three times | 101 | 16 | 11 | 1 |
| Four times | 51 | 5 | 5 | 1 |
| Five times | 23 | 4 | 1 | 0 |
| Six times | 19 | 2 | 1 | 0 |
| Seven times | 8 | |||
| Ten times | 1 | |||
| Eleven times | 1 | |||
Figure 1.
Medically-attended acute respiratory illnesses by date of onset among participants (N=915), January 1 – December 31, 2009, including inpatient acute lower respiratory illnesses (black), outpatient acute lower respiratory illnesses (gray), and all other medically-attended acute respiratory illnesses (white).
Of the 1476 episodes, 989 (67%) were diagnosed as upper respiratory infection (URI) or cough, whereas pneumonia, bronchiolitis, and other wheezing illnesses accounted for 23% (Table 5). Among outpatient ALRI, bronchiolitis was seen in one-fifth (21%), while pneumonia accounted for more than one-quarter (28%) and other wheezing illnesses accounted for one-third (32%). Pneumonia was diagnosed in a majority (59%) of ALRI hospitalizations, followed by bronchiolitis and other wheezing illnesses.
Table 5.
Number and incidence ratesa of clinical syndrome categories by visit type.
| Diagnostic category |
All MAARI | All ALRI | Outpatient ALRI | Inpatient ALRI | |||
|---|---|---|---|---|---|---|---|
| N (%) | N (%) | Incidence | N (%) | Incidence | N (%) | Incidence | |
| Total | 1476 | 395 | 329 | 66 | |||
| Pneumonia | 132 (9) | 132 (33) | 157 (131, 186) | 93 (28) | 111 (89, 136) | 39 (59) | 46 (33, 63) |
| Bronchiolitis | 83 (6) | 83 (21) | 99 (79, 122) | 68 (21) | 81 (63, 103) | 15 (23) | 18 (10, 29) |
| Wheezing illnessesb |
114 (8) | 114 (29) | 136 (112, 163) | 104 (32) | 124 (101, 150) | 10 (15) | 12 (6, 22) |
| ALRI not otherwise specified |
47 (3) | 47 (12) | 46 (14) | 1 (2) | |||
| Croup | 65 (4) | 11 (3) | 10 (3) | 1 (2) | |||
| Allergic rhinitis | 11 (1) | - | - | - | |||
| Viral syndromes | 23 (2) | 2 (1) | 2 (1) | - | |||
| Pharyngitis, tonsillitis |
2 (<1) | - | - | - | |||
| Sinusitis, otitis media |
10 (1) | 1 (<1) | 1 (<1) | - | |||
| URI or cough | 989 (67) | 5 (2) | 5 (2) | - | |||
episodes per 1000 child-years (95% confidence interval).
asthma, RAD, wheeze, small airway disease (other than bronchiolitis).
Detection of Viruses and Bacteria
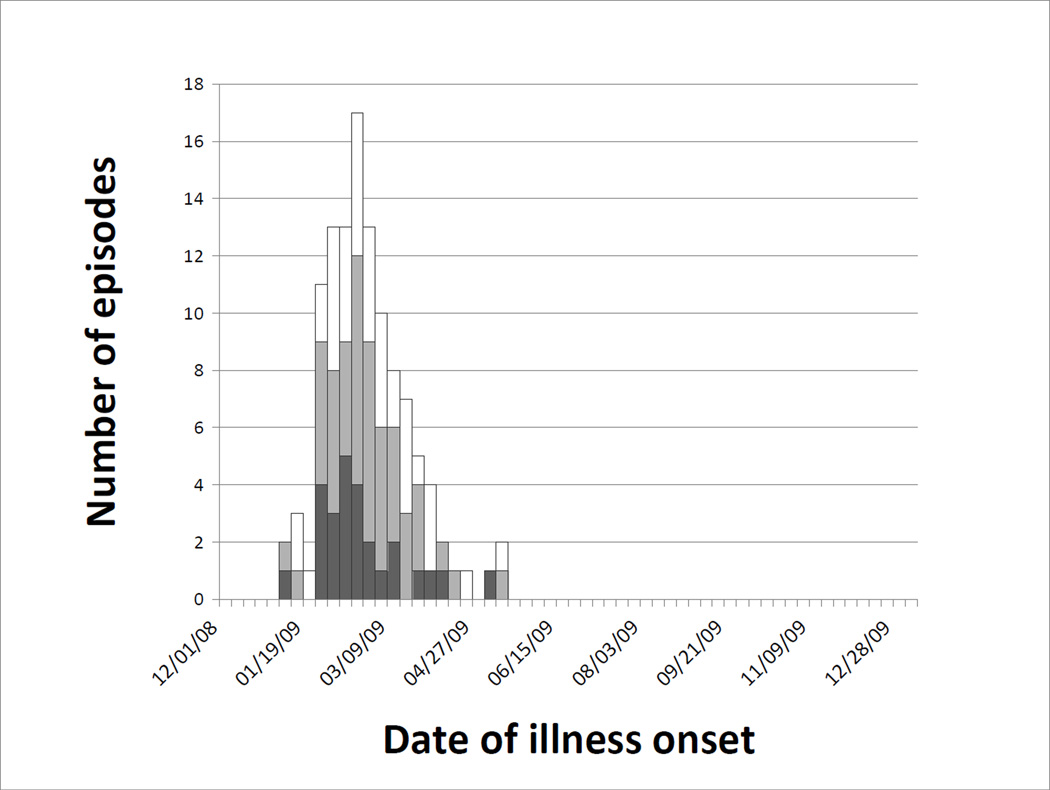
A total of 699 respiratory specimens were collected from 639 (43%) of the 1476 MAARI events, including 295 (30%) of the 984 URI episodes, 236 (72%) of the 329 outpatient ALRI episodes and 59 (89%) of the 66 ALRI hospitalizations. Samples were collected a median of 4 days (IQR 2–6 days) after illness onset. Molecular diagnostic testing detected one or more potential pathogens in 88% of episodes overall, including 91% of outpatient ALRI and 93% of inpatient ALRI (Table 6). Virus detection decreased in frequency as the number of days between illness onset and sample collection increased (p=0.001). Among the respiratory viruses, human rhinovirus (HRV) and respiratory syncytial virus (RSV) were most commonly detected across all categories, with RSV predominant among the more severe episodes (e.g., 44% of ALRI hospitalizations). RSV episodes were seasonal, peaking in early February, and ending by early May (Figure 2). HRV was detected in roughly one-quarter of episodes, with winter and fall waves similar to those observed for MAARI overall. HRV was detected among outpatient and inpatient ALRI cases in similar proportions; of these, less than one-fifth were associated with HRV alone. Influenza, parainfluenza (PIV) (particularly type 3), and human metapneumovirus (hMPV) were detected in roughly similar proportions (8–13%). S. pneumoniae was detected in 60% of episodes, with a smaller proportion positive for H. influenzae (all types) (19%) and C. pneumoniae (3%).
Table 6.
Number (%) and incidence ratesa of pathogens detected, by visit type.
| All MAARI episodes | Outpatient LRI | Inpatient LRI | ||||
|---|---|---|---|---|---|---|
| N (%) | Incidence | N (%) | Incidence | N (%) | Incidence | |
| Total episodes tested |
639 (100) | 236 (100) | 59 (100) | |||
| Influenza | ||||||
| All Type A | 59 (9.2) | 19 (8.1) | 3 (5.1) | |||
| H1N1pdm | 33 (5.2) | 91 (63, 128) | 11 (4.7) | 18 (9, 33) | 3 (5.1) | 4 (1, 12) |
| Type B | 4 (1) | 1 (0.4) | 0 (0) | |||
| Total | 63 (9.9) | 174 (134, 223) | 20 (8.5) | 33 (20, 51) | 3 (5.1) | 4 (1, 12) |
| Enteroviruses | 29 (4.5) | 80 (54, 115) | 9 (3.8) | 15 (7, 28) | 6 (10.2) | 8 (3, 17) |
| Rhinoviruses | 171 (26.8) | 473 (405, 550) | 61 (25.9) | 101 (77, 129) | 14 (23.7) | 19 (10, 31) |
| Coronaviruses 229E and OC43 |
11 (1.7) | 30 (15, 54) | 4 (1.7) | 7 (2, 17) | 0 (0) | 0 (0, 5) |
| Human metapneumovirus |
51 (8.0) | 141 (105, 186) | 18 (7.6) | 30 (18, 47) | 5 (8.5) | 7 (2, 16) |
| Parainfluenza | ||||||
| Type 1 | 18 (2.8) | 5 (2.1) | 0 (0) | |||
| Type 2 | 7 (1.1) | 1 (0.4) | 0 (0) | |||
| Type 3 | 57 (8.9) | 20 (8.5) | 6 (10.2) | |||
| Type 4 | 5 (1) | 3 (1.3) | 1 (1.7) | |||
| Total | 87 (13.6) | 241 (193, 297) | 29 (12.3) | 48 (32, 69) | 7 (11.9) | 9 (4, 19) |
| RSV | ||||||
| RSV A | 32 (5.0) | 12 (5.1) | 10 (17.0) | |||
| RSV B | 82 (12.8) | 37 (15.7) | 16 (27.1) | |||
| Total | 114 (17.8) | 315 (260, 379) | 49 (20.1) | 81 (60, 107) | 26 (44.1) | 35 (23, 51) |
| Adenovirus | 14 (2.2) | 39 (21, 65) | 3 (1.3) | 5 (1, 14) | 5 (8.5) | 7 (2, 16) |
| S. pneumoniae | 381 (59.6) | 148 (62.7) | 34 (57.6) | |||
| H. influenzae | 120 (18.8) | 47 (19.9) | 13 (22.0) | |||
| C. pneumoniae | 20 (3.1) | 55 (34, 85) | 9 (3.8) | 15 (7, 28) | 1 (1.7) | 1 (0, 7) |
episodes per 1000 child-years (95% confidence interval), adjusted by the proportion sampled.
Figure 2.
Medically-attended acute respiratory illnesses by date of onset and visit type associated with RSV including inpatient acute lower respiratory illnesses (black), outpatient acute lower respiratory illnesses (gray), and all other medically-attended acute respiratory illnesses (white).
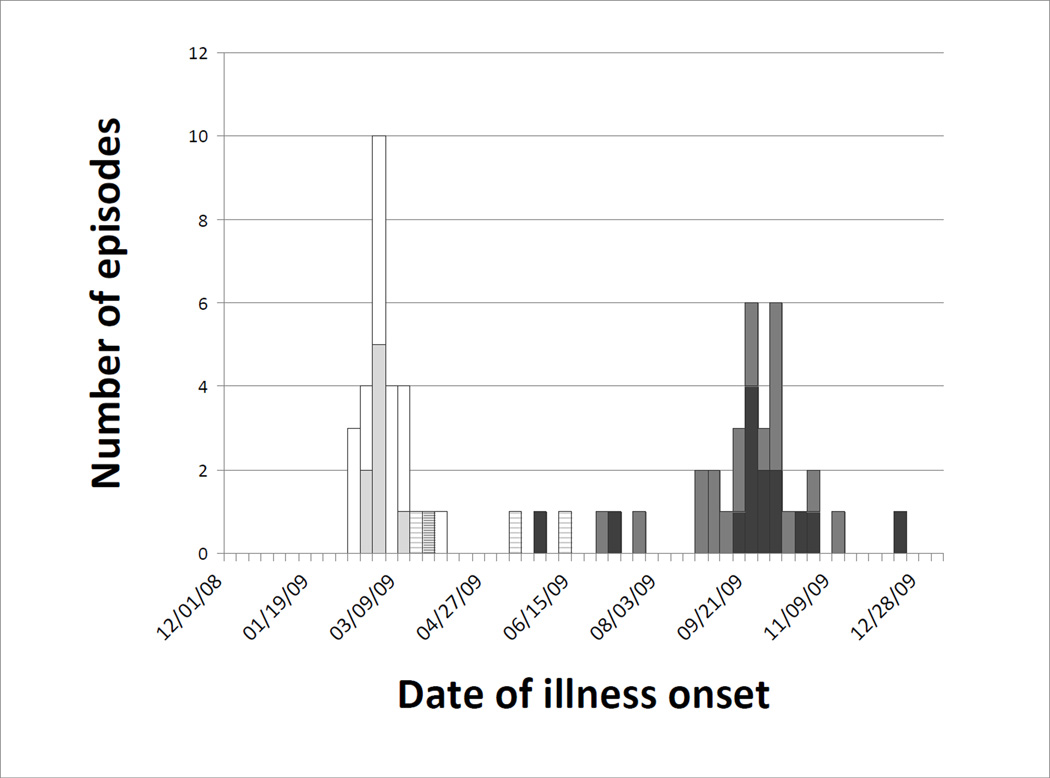
Among influenza detections, episodes during the first three months of the year were associated with seasonal influenza A(H1N1) and influenza B viruses, while pandemic influenza A(H1N1) (H1N1pdm) was first identified in late May (Figure 3). Although the first spring wave was largely absent in this population, H1N1pdm ultimately accounted for more than half of all influenza detections, including 11 (64%) of the 22 ALRI cases and all three illnesses requiring hospitalization.
Figure 3.
Medically-attended acute respiratory illnesses associated with influenza by subtype and illness category. Seasonal influenza A acute lower respiratory illnesses (light gray) and other medically-attended acute respiratory illnesses (white), influenza B acute lower respiratory illnesses (heavy stripe) and other medically-attended acute respiratory illnesses (light stripe), and H1N1pdm acute lower respiratory illnesses (dark gray) and other medically-attended acute respiratory illnesses (medium gray).
Lower respiratory illnesses were more likely to be associated with RSV compared to upper respiratory illness (detected in 25% vs. 11%, p<0.001) while inpatient status was associated with the detection of RSV (p=0.029), enterovirus (p<0.001), and adenovirus (p<0.001) when compared with all outpatient MAARI. By contrast, the detection of influenza virus (including H1N1pdm), rhinovirus, or pneumococcus did not increase the risk of either lower respiratory tract involvement or hospitalization. Notably, all five inpatient episodes associated with adenovirus involved the detection of an additional virus (RSV in four, HRV in one), and only one of the six enterovirus-associated hospitalizations involved its detection as a single pathogen.
Approximately one-third of episodes were associated with only one pathogen across all visit types; the largest proportion demonstrated two pathogens (Table 7). Detection of three or more pathogens was more frequently seen among hospitalized episodes (29%) than among MAARI episodes overall (17%) (p=0.030). S. pneumoniae was identified in combination with a virus in 48–58% of episodes, yet HRV was the only virus specifically associated with its presence (p=0.028 for all MAARI; p=0.034 for all ALRI; p=0.145 for outpatient ALRI; p=0.069 for inpatient ALRI). Influenza virus, RSV, and rhinovirus were negatively associated with each other (p<0.05 for all by pair-wise combination).
Table 7.
Multiple pathogen detections, by visit type.
| Number of specimens in category (%) | ||||
|---|---|---|---|---|
| Number Pathogens detected in specimen |
All MAARI | All ALRI | Outpatient ALRI |
Inpatient ALRI |
| 0a | 75 (12) | 26 (9) | 22 (9) | 4 (7) |
| 1 | 192 (30) | 96 (33) | 78 (33) | 18 (31) |
| 2 | 261 (41) | 114 (39) | 94 (40) | 20 (34) |
| 3 | 98 (15) | 50 (17) | 38 (16) | 12 (20) |
| 4 | 12 (2) | 8 (3) | 3 (1) | 5 (8) |
| 5 | 1 (<1) | 1 (<1) | 1 (<1) | - |
| Virus + S. pneumoniae |
307 (48) | 148 (50) | 114 (48) | 34 (58) |
| Total | 639 | 295 | 236 | 59 |
specimen negative.
The frequencies of pathogens according to diagnostic category among ALRI were unevenly distributed (Table 8). As expected, RSV was prominently seen among pneumonia and bronchiolitis cases, while rhinovirus was more frequent in other wheezing illnesses and pneumonia. Influenza and parainfluenza cases were notable for pneumonia and nonspecific ALRI, but were seen in a considerable proportion of wheezing illnesses as well. In contrast with previous reports (22;23), and similar to more recent data (24), hMPV was most frequently detected with pneumonia and nonspecific ALRI as opposed to bronchiolitis and other wheezing illnesses.
Table 8.
Pathogen detection (number, % positive among those tested) by diagnostic category among outpatient and inpatient ALRI episodes.
| Outpatient ALRI | Inpatient ALRI | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diagnostic Category |
All Diagnoses |
Pneumonia | Bronchiolitis | Wheezing Illnessesa |
LRI NOSb |
Croup | Viral Syndromes |
URI or Cough |
All Diagnoses |
Pneumonia | Bronchiolitis | Wheezing Illnessesa |
LRI NOSb |
Croup |
| Total episodes | 329 | 93 | 68 | 104 | 46 | 10 | 2 | 5 | 66 | 39 | 15 | 10 | 1 | 1 |
| Episodes tested (N, %) |
236 (72) | 72 (77) | 47 (69) | 72 (69) | 32 (70) | 9 (90) | 1 (50) | 3 (60) | 59 (89) | 35 (90) | 15 (100) | 7 (70) | 1 (100) | 1 (100) |
| Pathogen: | ||||||||||||||
| Influenza | ||||||||||||||
| All Type A | 19 (8) | 5 (7) | 3 (6) | 8 (11) | 3 (9) | 0 (0) | 0 (0) | 0 (0) | 3 (5) | 2 (6) | 0 (0) | 0 (0) | 1 (100) | 0 (0) |
| H1N1pdm | 11 (5) | 2 (3) | 2 (4) | 5 (7) | 2 (6) | 0 (0) | 0 (0) | 0 (0) | 3 (5) | 2 (6) | 0 (0) | 0 (0) | 1 (100) | 0 (0) |
| Type B | 1 (0) | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Total | 20 (8) | 6 (8) | 3 (6) | 8 (11) | 3 (9) | 0 (0) | 0 (0) | 0 (0) | 3 (5) | 2 (6) | 0 (0) | 0 (0) | 1 (100) | 0 (0) |
| Enteroviruses | 9 (4) | 1 (1) | 2 (4) | 5 (7) | 1 (3) | 0 (0) | 0 (0) | 0 (0) | 6 (10) | 3 (9) | 1 (7) | 1 (14) | 1 (100) | 0 (0) |
| Rhinoviruses | 61 (26) | 16 (22) | 7 (15) | 25 (35) | 9 (28) | 3 (33) | 0 (0) | 1 (33) | 14 (24) | 5 (14) | 4 (27) | 4 (57) | 1 (100) | 0 (0) |
| Coronaviruses | 4 (2) | 2 (3) | 0 (0) | 2 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| (229E and OC43) | ||||||||||||||
| Human metapneumovirus |
18 (8) | 4 (6) | 3 (6) | 5 (7) | 6 (19) | 0 (0) | 0 (0) | 0 (0) | 5 (8) | 3 (9) | 2 (13) | 0 (0) | 0 (0) | 0 (0) |
| Parainfluenza | 0 (0) | |||||||||||||
| Type 1 | 5 (2) | 2 (3) | 0 (0) | 1 (1) | 2 (6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Type 2 | 1 (0) | 0 (0) | 0 (0) | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Type 3 | 20 (8) | 6 (8) | 3 (6) | 8 (11) | 2 (6) | 0 (0) | 0 (0) | 1 (33) | 6 (10) | 4 (11) | 2 (13) | 0 (0) | 0 (0) | 0 (0) |
| Type 4 | 3 (1) | 0 (0) | 1 (2) | 2 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2) | 1 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Total | 29 (12) | 8 (11) | 4 (9) | 12 (17) | 4 (13) | 0 (0) | 0 (0) | 1 (33) | 7 (12) | 5 (14) | 2 (13) | 0 (0) | 0 (0) | 0 (0) |
| RSV | ||||||||||||||
| RSV A | 12 (5) | 2 (3) | 3 (6) | 1 (1) | 6 (19) | 0 (0) | 0 (0) | 0 (0) | 10 (17) | 4 (11) | 3 (20) | 2 (29) | 0 (0) | 1 (100) |
| RSV B | 37 (16) | 11 (15) | 12 (26) | 7 (10) | 4 (13) | 2 (22) | 1 (100) | 0 (0) | 16 (27) | 12 (34) | 3 (20) | 1 (14) | 0 (0) | 0 (0) |
| Total | 49 (21) | 13 (18) | 15 (32) | 8 (11) | 10 (31) | 2 (22) | 1 (100) | 0 (0) | 26 (44) | 16 (46) | 6 (40) | 3 (43) | 0 (0) | 1 (100) |
| Adenovirus | 3 (1) | 0 (0) | 2 (4) | 0 (0) | 1 (3) | 0 (0) | 0 (0) | 0 (0) | 5 (8) | 2 (6) | 3 (20) | 0 (0) | 0 (0) | 0 (0) |
| S. pneumoniae | 148 (63) | 37 (51) | 33 (70) | 41 (57) | 28 (88) | 7 (78) | 0 (0) | 2 (67) | 34 (58) | 19 (54) | 10 (67) | 4 (57) | 1 (100) | 0 (0) |
| H. influenzae | 47 (20) | 13 (18) | 10 (21) | 16 (22) | 4 (13) | 3 (33) | 1 (100) | 0 (0) | 13 (22) | 5 (14) | 5 (33) | 3 (43) | 0 (0) | 0 (0) |
| C. pneumoniae | 9 (4) | 5 (7) | 0 (0) | 3 (4) | 0 (0) | 0 (0) | 0 (0) | 1 (33) | 4 (7) | 3 (9) | 1 (7) | 0 (0) | 0 (0) | 0 (0) |
| None detected | 22 (9) | 9 (13) | 3 (6) | 7 (10) | 1 (3) | 2 (22) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
asthma, RAD, wheeze, small airway disease (other than bronchiolitis).
LRI NOS: Lower respiratory illness, not otherwise specified.
Risk Factor Analyses
Subjects who experienced one or more MAARI (n=669) were more likely to have been younger at the start of the study period compared those who had not (n=246) (median age 16.5 vs. 18.4 months, p=0.0002). These two groups were otherwise similar in terms of baseline characteristics. Among subjects who had a MAARI, those who experienced lower respiratory involvement (n=273) were younger (p=0.0001), were more likely to be male (p=0.017), to have had an ALRI as an infant prior to enrollment in the trial (p=0.034), and to have a family history of an atopic condition (p=0.001) compared to those who had not had ALRI (n= 396), by multiple logistic regression. Among the children with ALRI, those who required hospitalization (n=58) were more likely to have been younger (p=0.017), or to have a history of eczema (p=0.014), compared to subjects who were managed as outpatients (n=215), by multiple logistic regression. The effects of motavizumab on bronchiolitis and RSV will be reported separately.
Discussion
Here we provide, for the first time, prospective data regarding the major presentations and pathogens associated with acute respiratory illness from a cohort of young American Indian children. Collected through a full calendar year, these findings document a wide spectrum of respiratory pathogens, including the emergence of pandemic influenza, in this population known to be at high risk for respiratory illness. These data indicate high rates of medical attendance beyond infancy for respiratory disease, particularly involving the lower respiratory tract. Our rates of ALRI hospitalizations exceed estimates of 17–20 per 1000/year derived from retrospective database analyses (2;25). This difference may be due to our use of active surveillance in a well-defined prospective cohort. Similarly, the incidence of pneumonia observed here exceeds published rates of community-acquired pneumonia for children in the general US population (16.9–22.4 outpatient visits per 1000 children aged 1–5 years (26) and 8.1–9.1 and 3.9–4.8 hospitalizations per 1000 children aged <2 years and 2–4 years, respectively (27)). Although limited by differences in age ranges, case definitions, and laboratory methods, such comparisons provide strong indication of the higher burden of acute respiratory disease among young American Indian children relative to the general U.S. pediatric population. These elevated rates of pneumonia, despite the presence of high vaccine coverage among Native Americans against important serotypes of S. pneumoniae and H. influenzae type b (28), demonstrate the relevance of other respiratory pathogens. Our data also confirm the continuing high burden of bronchiolitis among young American Indian children, even into their second and third respiratory seasons, underscoring the need for additional strategies in the prevention and control of this condition.
We demonstrate the continued dominance of RSV in the years following infancy, responsible for a large proportion of pneumonia, bronchiolitis, and other acute wheezing illnesses. We also confirm the high prevalence of HRV among these children, although it is unclear if HRV was the causative pathogen in all cases, as multiple detections were common. An additional virus was detected in approximately one-third of HRV ALRI episodes, compared with 17% of ALRI cases positive for hMPV, 18% of those with influenza, and 24% of those with RSV. Nevertheless, HRV may play at least a contributing role, as co-detection of HRV with bacteria was common, and among ALRI cases and hospitalizations, was statistically associated with S. pneumoniae.
Consistent with prior reports (14;15), we found substantially higher rates of influenza-related respiratory illness in our population compared to U.S. children in general (29;30). On a national level, the 2008–09 influenza season was mild in terms of severity and extent, while pandemic influenza affected children disproportionately worldwide (31–33). Therefore, it is not surprising that H1N1pdm accounted for all the influenza-related hospitalizations in this cohort. Nevertheless, seasonal H1N1 and B viruses contributed almost as much to the total influenza burden in terms of outpatient ALRI episodes (42%) and overall MAARI visits (44%), and within a shorter span of time. These unexpectedly comparable proportions of seasonal and pandemic influenza cases may be because young age is an independent risk factor for influenza-related complications, and the study cohort was younger at the start of 2009, when seasonal viruses were in circulation. Moreover, the spring wave of H1N1pdm was comparatively mild in this population, and our surveillance did not capture the fall wave experiences of the full 2006 cohort, as up to 30% of this group (representing 8% of the total cohort) had completed follow-up by September 1.
Similar to prior studies conducted in Native American communities, pneumococcal colonization within this cohort exceeded rates found among similar age groups in the general U.S. population (34–40). While these high rates have been implicated in the increased burden of invasive pneumococcal disease observed among Native Americans, the mechanisms underlying this relationship remain unclear. The potential for a synergistic effect when viruses and bacteria co-infect the respiratory tract has received increased attention in recent years, particularly between influenza and S. pneumoniae (41;42). One study that employed a similar diagnostic approach to ours demonstrated that co-detection of S. pneumoniae in nasopharyngeal samples was associated with more severe H1N1pdm illness (20). We did not observe such correlations, other than a potential interaction with rhinovirus, which has been reported previously (43–46). The significance of this combination is unclear, as both pathogens can be detected in respiratory specimens in the absence of symptoms. The high overall burden of disease and elevated background rates of bacterial colonization present in this population may have obscured such statistical relationships in our study. In addition, we only established the presence or absence of S. pneumoniae and did not measure colonization density, which may be more closely associated with disease (47;48). More generally, multiple pathogen detection appeared to be related to hospitalization in our study, supporting previous reports suggesting that co-infection increases severity (49;50). This statistical association does not prove a causal relationship, however, and several studies dispute this effect (51–53).
Our study was subject to limitations. First, our study did not include microbial testing in asymptomatic subjects, thus limiting our ability to draw causal inferences between the pathogens detected in the nasal wash and the clinical illness observed in the child. Second, clinical syndromes were assigned according to diagnoses made by the treating provider, and could only be standardized and confirmed at the level of medical record review by our study physicians. Third, we report data only from a single year, focusing on children in a restricted age range. Finally, the detection of bacteria or other pathogens in upper respiratory samples may not always be correlated with lower tract disease, thus limiting our ability to identify potential associations with co-infections or clinical outcomes.
In summary, we present the first comprehensive assessment of the clinical diagnoses and pathogens associated with acute respiratory illness among young American Indian children. These findings demonstrate the prevalence of numerous respiratory pathogens, including RSV, rhinovirus, and pneumococcus in this community, and indicate that seasonal and pandemic influenza imposed a considerably higher burden compared to the general U.S. population. Our observations offer valuable insights into the complex picture of respiratory infections and provide critical evidence to support prevention and control efforts that can be applied to epidemiologically similar communities throughout the world.
Acknowledgements
The authors express their appreciation to the children and adults from the Navajo, White Mountain Apache and San Carlos Apache communities who participated actively in this study and the Indian Health Service (IHS) physicians who cared for them. We also gratefully acknowledge the dedicated efforts of the Center for American Indian Health field staff who collected these data over many years. We also gratefully acknowledge the work by MedImmune staff on the parent clinical trial.
Funding Sources: This work was supported by the National Center for Research Resources (NCRR) at the National Institutes of Health (NIH) (grant 1KL2RR025006-01 to N.B.); the National Institutes of Health (grant AI57158 to the Northeast Biodefense Center - W.I.L.); the Bill and Melinda Gates Foundation and the Defense Threats Reduction Agency (to the Center for Infection and Immunity, Columbia University). The parent clinical trial was funded by MedImmune, who played no role in the design, conduct, analysis or funding of the molecular diagnostic pathogen sub-study. N.B is currently an employee of the United States Food and Drug Administration.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: K.L.O., A.C., R.W., R.R., and M.S. received funding for the parent trial from MedImmune.
Conflict of Interest: The authors have no conflicts of interest to disclose.
Disclaimer: The findings and conclusions in this report are solely the responsibility of the authors and do not necessarily represent the official view of the IHS, NCRR, or NIH.
References
- 1.Holman RC, Curns AT, Cheek JE, Singleton RJ, Anderson LJ, Pinner RW. Infectious disease hospitalizations among American Indian and Alaska native infants. Pediatrics. 2003;111(2):E176–E182. doi: 10.1542/peds.111.2.e176. [DOI] [PubMed] [Google Scholar]
- 2.Peck AJ, Holman RC, Curns AT, et al. Lower respiratory tract infections among american Indian and Alaska Native children and the general population of U. S. Children. Pediatr Infect Dis J. 2005;24(4):342–351. doi: 10.1097/01.inf.0000157250.95880.91. [DOI] [PubMed] [Google Scholar]
- 3.O’Brien KL, Shaw J, Weatherholtz R, et al. Epidemiology of invasive Streptococcus pneumoniae among Navajo children in the era before use of conjugate pneumococcal vaccines, 1989–1996. Am J Epidemiol. 2004;160(3):270–278. doi: 10.1093/aje/kwh191. [DOI] [PubMed] [Google Scholar]
- 4.Bockova J, O’Brien KL, Oski J, et al. Respiratory syncytial virus infection in Navajo and White Mountain Apache children. Pediatrics. 2002;110(2 Pt 1):e20. doi: 10.1542/peds.110.2.e20. [DOI] [PubMed] [Google Scholar]
- 5.Millar EV, O’Brien KL, Levine OS, Kvamme S, Reid R, Santosham M. Toward elimination of Haemophilus influenzae type B carriage and disease among high-risk American Indian children. Am J Public Health. 2000;90(10):1550–1554. doi: 10.2105/ajph.90.10.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weatherholtz R, Millar EV, Moulton LH, et al. Invasive pneumococcal disease a decade after pneumococcal conjugate vaccine use in an American Indian population at high risk for disease. Clin Infect Dis. 2010;50(9):1238–1246. doi: 10.1086/651680. [DOI] [PubMed] [Google Scholar]
- 7.Davidson M, Parkinson AJ, Bulkow LR, Fitzgerald MA, Peters HV, Parks DJ. The epidemiology of invasive pneumococcal disease in Alaska, 1986–1990--ethnic differences and opportunities for prevention. J Infect Dis. 1994;170(2):368–376. doi: 10.1093/infdis/170.2.368. [DOI] [PubMed] [Google Scholar]
- 8.Singleton R, Hammitt L, Hennessy T, et al. The Alaska Haemophilus influenzae type b experience: lessons in controlling a vaccine-preventable disease. Pediatrics. 2006;118(2):e421–e429. doi: 10.1542/peds.2006-0287. [DOI] [PubMed] [Google Scholar]
- 9.Singleton RJ, Bruden D, Bulkow LR. Respiratory syncytial virus season and hospitalizations in the Alaskan Yukon-Kuskokwim Delta. Pediatr Infect Dis J. 2007;26(11 Suppl):S46–S50. doi: 10.1097/INF.0b013e318157da9b. [DOI] [PubMed] [Google Scholar]
- 10.Singleton RJ, Butler JC, Bulkow LR, et al. Invasive pneumococcal disease epidemiology and effectiveness of 23-valent pneumococcal polysaccharide vaccine in Alaska native adults. Vaccine. 2007;25(12):2288–2295. doi: 10.1016/j.vaccine.2006.11.065. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention (CDC) Deaths related to 2009 pandemic influenza A (H1N1) among American Indian/Alaska Natives - 12 states, 2009. MMWR Morb Mortal Wkly Rep. 2009;58(48):1341–1344. [PubMed] [Google Scholar]
- 12.La Ruche G, Tarantola A, Barboza P, et al. The 2009 pandemic H1N1 influenza and indigenous populations of the Americas and the Pacific. Euro Surveill. 2009;14(42):19366. doi: 10.2807/ese.14.42.19366-en. [DOI] [PubMed] [Google Scholar]
- 13.Groom AV, Jim C, Laroque M, et al. Pandemic influenza preparedness and vulnerable populations in tribal communities. Am J Public Health. 2009;99(Suppl 2):S271–S278. doi: 10.2105/AJPH.2008.157453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nair H, Brooks WA, Katz M, et al. Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta-analysis. Lancet. 2011;378(9807):1917–1930. doi: 10.1016/S0140-6736(11)61051-9. [DOI] [PubMed] [Google Scholar]
- 15.Dee DL, Bensyl DM, Gindler J, et al. Racial and ethnic disparities in hospitalizations and deaths associated with 2009 pandemic Influenza A (H1N1) virus infections in the United States. Ann Epidemiol. 2011;21(8):623–630. doi: 10.1016/j.annepidem.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Chandran A, Millar E, Weatherholtz R, et al. Safety and efficacy of motavizumab in the prevention of RSV disease in healthy infants. 2008 May;3 2008. [Google Scholar]
- 17.Briese T, Palacios G, Kokoris M, et al. Diagnostic system for rapid and sensitive differential detection of pathogens. Emerg Infect Dis. 2005;11(2):310–313. doi: 10.3201/eid1102.040492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lamson D, Renwick N, Kapoor V, et al. MassTag polymerase-chain-reaction detection of respiratory pathogens, including a new rhinovirus genotype, that caused influenza-like illness in New York State during 2004–2005. J Infect Dis. 2006;194(10):1398–1402. doi: 10.1086/508551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dominguez SR, Briese T, Palacios G, et al. Multiplex MassTag-PCR for respiratory pathogens in pediatric nasopharyngeal washes negative by conventional diagnostic testing shows a high prevalence of viruses belonging to a newly recognized rhinovirus clade. J Clin Virol. 2008;43(2):219–222. doi: 10.1016/j.jcv.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palacios G, Hornig M, Cisterna D, et al. Streptococcus pneumoniae coinfection is correlated with the severity of H1N1 pandemic influenza. PloS one. 2009;4(12):e8540. doi: 10.1371/journal.pone.0008540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tokarz R, Kapoor V, Wu W, et al. Longitudinal molecular microbial analysis of influenza-like illness in New York City, May 2009 through May 2010. Virol J. 2011;8:288. doi: 10.1186/1743-422X-8-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams JV, Harris PA, Tollefson SJ, et al. Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N Engl J Med. 2004;350(5):443–450. doi: 10.1056/NEJMoa025472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schildgen V, van den Hoogen B, Fouchier R, et al. Human Metapneumovirus: lessons learned over the first decade. Clin Microbiol Rev. 2011;24(4):734–754. doi: 10.1128/CMR.00015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edwards KM, Zhu Y, Griffin MR, et al. Burden of human metapneumovirus infection in young children. N Engl J Med. 2013;368(7):633–643. doi: 10.1056/NEJMoa1204630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singleton RJ, Holman RC, Folkema AM, Wenger JD, Steiner CA, Redd JT. Trends in lower respiratory tract infection hospitalizations among American Indian/Alaska Native children and the general US child population. J Pediatr. 2012;161(2):296–302. doi: 10.1016/j.jpeds.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 26.Kronman MP, Hersh AL, Feng R, Huang YS, Lee GE, Shah SS. Ambulatory visit rates and antibiotic prescribing for children with pneumonia, 1994–2007. Pediatrics. 2011;127(3):411–418. doi: 10.1542/peds.2010-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention (CDC) Pneumonia hospitalizations among young children before and after introduction of pneumococcal conjugate vaccine--United States, 1997–2006. MMWR Morb Mortal Wkly Rep. 2009;58(1):1–4. [PubMed] [Google Scholar]
- 28.Groom AV, Santibanez TA, Bryan RT. Vaccination coverage among American Indian and Alaska native children, 2006–2010. Pediatrics. 2012;130(6):e1592–e1599. doi: 10.1542/peds.2012-1001. [DOI] [PubMed] [Google Scholar]
- 29.Dawood FS, Fiore A, Kamimoto L, et al. Burden of seasonal influenza hospitalization in children, United States, 2003 to 2008. J Pediatr. 2010;157(5):808–814. doi: 10.1016/j.jpeds.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 30.Poehling KA, Edwards KM, Griffin MR, et al. The burden of influenza in young children, 2004–2009. Pediatrics. 2013;131(2):207–216. doi: 10.1542/peds.2012-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Louie JK, Acosta M, Winter K, et al. Factors associated with death or hospitalization due to pandemic 2009 influenza A(H1N1) infection in California. JAMA. 2009;302(17):1896–1902. doi: 10.1001/jama.2009.1583. [DOI] [PubMed] [Google Scholar]
- 32.Writing Committee of the WHO Consultation on Clinical Aspects of Pandemic. Bautista E, Chotpitayasunondh T, et al. Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. N Engl J Med. 2010;362(18):1708–1719. doi: 10.1056/NEJMra1000449. [DOI] [PubMed] [Google Scholar]
- 33.Dawood FS, Iuliano AD, Reed C, et al. Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: a modelling study. Lancet Infect Dis. 2012 doi: 10.1016/S1473-3099(12)70121-4. [DOI] [PubMed] [Google Scholar]
- 34.Hennessy TW, Singleton RJ, Bulkow LR, et al. Impact of heptavalent pneumococcal conjugate vaccine on invasive disease, antimicrobial resistance and colonization in Alaska Natives: progress towards elimination of a health disparity. Vaccine. 2005;23(48–49):5464–5473. doi: 10.1016/j.vaccine.2005.08.100. [DOI] [PubMed] [Google Scholar]
- 35.Millar EV, O’Brien KL, Watt JP, et al. Effect of community-wide conjugate pneumococcal vaccine use in infancy on nasopharyngeal carriage through 3 years of age: a cross-sectional study in a high-risk population. Clin Infect Dis. 2006;43(1):8–15. doi: 10.1086/504802. [DOI] [PubMed] [Google Scholar]
- 36.O’Brien KL, Millar EV, Zell ER, et al. Effect of pneumococcal conjugate vaccine on nasopharyngeal colonization among immunized and unimmunized children in a community-randomized trial. J Infect Dis. 2007;196(8):1211–1220. doi: 10.1086/521833. [DOI] [PubMed] [Google Scholar]
- 37.Millar EV, O’Brien KL, Zell ER, Bronsdon MA, Reid R, Santosham M. Nasopharyngeal carriage of Streptococcus pneumoniae in Navajo and White Mountain Apache children before the introduction of pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2009;28(8):711–716. doi: 10.1097/INF.0b013e3181a06303. [DOI] [PubMed] [Google Scholar]
- 38.Millar EV, Pimenta FC, Roundtree A, et al. Pre- and post-conjugate vaccine epidemiology of pneumococcal serotype 6C invasive disease and carriage within Navajo and White Mountain Apache communities. Clin Infect Dis. 2010;51(11):1258–1265. doi: 10.1086/657070. [DOI] [PubMed] [Google Scholar]
- 39.Scott JR, Hinds J, Gould KA, et al. Nontypeable pneumococcal isolates among navajo and white mountain apache communities: are these really a cause of invasive disease? J Infect Dis. 2012;206(1):73–80. doi: 10.1093/infdis/jis307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wroe PC, Lee GM, Finkelstein JA, et al. Pneumococcal carriage and antibiotic resistance in young children before 13-valent conjugate vaccine. Pediatr Infect Dis J. 2012;31(3):249–254. doi: 10.1097/INF.0b013e31824214ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Madhi SA, Klugman KP. Vaccine Trialist Group. A role for Streptococcus pneumoniae in virus-associated pneumonia. Nat Med. 2004;10(8):811–813. doi: 10.1038/nm1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCullers JA. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev. 2006;19(3):571–582. doi: 10.1128/CMR.00058-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Templeton KE, Scheltinga SA, van den Eeden WC, Graffelman AW, van den Broek PJ, Claas EC. Improved diagnosis of the etiology of community-acquired pneumonia with real-time polymerase chain reaction. Clin Infect Dis. 2005;41(3):345–351. doi: 10.1086/431588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jennings LC, Anderson TP, Beynon KA, et al. Incidence and characteristics of viral community-acquired pneumonia in adults. Thorax. 2008;63(1):42–48. doi: 10.1136/thx.2006.075077. [DOI] [PubMed] [Google Scholar]
- 45.Peltola V, Heikkinen T, Ruuskanen O, et al. Temporal association between rhinovirus circulation in the community and invasive pneumococcal disease in children. Pediatr Infect Dis J. 2011;30(6):456–461. doi: 10.1097/INF.0b013e318208ee82. [DOI] [PubMed] [Google Scholar]
- 46.Launes C, de-Sevilla MF, Selva L, Garcia-Garcia JJ, Pallares R, Munoz-Almagro C. Viral coinfection in children less than five years old with invasive pneumococcal disease. Pediatr Infect Dis J. 2012;31(6):650–653. doi: 10.1097/INF.0b013e31824f25b0. [DOI] [PubMed] [Google Scholar]
- 47.Vu HT, Yoshida LM, Suzuki M, et al. Association between nasopharyngeal load of Streptococcus pneumoniae, viral coinfection, and radiologically confirmed pneumonia in Vietnamese children. Pediatr Infect Dis J. 2011;30(1):11–18. doi: 10.1097/INF.0b013e3181f111a2. [DOI] [PubMed] [Google Scholar]
- 48.Albrich WC, Madhi SA, Adrian PV, et al. Use of a rapid test of pneumococcal colonization density to diagnose pneumococcal pneumonia. Clin Infect Dis. 2012;54(5):601–609. doi: 10.1093/cid/cir859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Drews AL, Atmar RL, Glezen WP, Baxter BD, Piedra PA, Greenberg SB. Dual respiratory virus infections. Clin Infect Dis. 1997;25(6):1421–1429. doi: 10.1086/516137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cilla G, Onate E, Perez-Yarza EG, Montes M, Vicente D, Perez-Trallero E. Viruses in community-acquired pneumonia in children aged less than 3 years old: High rate of viral coinfection. J Med Virol. 2008;80(10):1843–1849. doi: 10.1002/jmv.21271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Canducci F, Debiaggi M, Sampaolo M, et al. Two-year prospective study of single infections and co-infections by respiratory syncytial virus and viruses identified recently in infants with acute respiratory disease. J Med Virol. 2008;80(4):716–723. doi: 10.1002/jmv.21108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Singleton RJ, Bulkow LR, Miernyk K, et al. Viral respiratory infections in hospitalized and community control children in Alaska. J Med Virol. 2010;82(7):1282–1290. doi: 10.1002/jmv.21790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martin ET, Kuypers J, Wald A, Englund JA. Multiple versus single virus respiratory infections: viral load and clinical disease severity in hospitalized children. Influenza Other Respi Viruses. 2012;6(1):71–77. doi: 10.1111/j.1750-2659.2011.00265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]