Abstract
We explored whether maternal exercise during pregnancy moderates the effect of fetal breathing movements on fetal cardiac autonomic control assessed by metrics of heart rate (HR) and heart rate variability (HRV). Thirty women were assigned to Exercise or Control group (n=15/group) based on the modifiable physical activity questionnaire (MPAQ). Magnetocardiograms (MCG) were recorded using a dedicated fetal biomagnetometer. Periods of fetal breathing activity and apnea were identified using the fetal diaphragmatic magnetomyogram (dMMG) as a marker. MCG R-waves were marked. Metrics of fetal HR and HRV were compared using 1 breathing and1 apneic epoch/fetus. The main effects of group (Exercise vs. Control) and condition (Apnea vs. Breathing) and their interactions were explored. Fetal breathing resulted in significantly lower fetal HR and higher vagally-mediated HRV. Maternal exercise resulted in significantly lower fetal HR, higher total HRV and vagally-mediated HRV with no difference in frequency band ratios. Significant interactions between maternal exercise and fetal breathing were found for metrics summarizing total HRV and a parasympathetic metric. Post hoc comparison showed no group difference during fetal apnea. Fetal breathing was associated with a loss of Total HRV in the Control group and no difference in the Exercise group. Both groups show enhanced vagal function during fetal breathing; greater in the Exercise group. During in utero breathing movements, the fetus of the exercising mother has enhanced cardiac autonomic function that may give the offspring an adaptive advantage.
Keywords: Exercise, Autonomic Nervous System, Magnetocardiogram, Fetal Biomagnetometry, Diaphragm Activation
1. Introduction
Fetal breathing movements have been characterized using biomagnetometry by our group (1) and others (2). Ulusar, et al., used simultaneous ultrasound to identify and quantify fetal breathing movements and identified a sinusoidal waveform linked to the breathing movements seen on ultrasound. Recently, an automatic algorithm was developed that identifies spectral peaks in the fetal magnetocardiogram (MCG) interbeat interval (IBI) time-series consistent with fetal respiratory sinus arrhythmia (RSA)(3). These recent developments in the field of fetal biomagnetometry allow for the investigation of neural integration of central circuits that govern fetal cardiac autonomic control.
We independently observed what we termed the fetal diaphragmatic magnetomyogram (dMMG) in maternal-fetal MCG recordings (1). We then used the dMMG as marker of fetal breathing activity in order to compare fetal cardiac autonomic control during breathing and non-breathing epochs (apnea) in the active fetal states. We found that fetal breathing activity resulted in significantly lower heart rate (HR) and higher heart rate variability (HRV), particularly in those metrics influenced by parasympathetic input. Our next goal was to use this method to compare fetal HR and HRV in two groups of women; those who performed moderate intensity exercise during pregnancy and those who did not.
In the non-pregnant population, exercise has many documented effects including improved glucose tolerance, increased insulin sensitivity, improved endothelial function, increased parasympathetic tone and reduced inflammation. Exercise also has been found to have many positive maternal effects related to pregnancy and delivery; for example, shorter labor and delivery times, faster recovery after delivery, decreased discomforts of pregnancy and fewer pregnancy complications (4). Currently, the American Congress of Obstetrics and Gynecology (ACOG) guidelines suggest that pregnant women participate in aerobic activity at moderate to vigorous intensities most days of the week for 30 or more minutes (5). Given the grave health care concerns surrounding maternal obesity, fetal over-nutrition, and the potential heritability of obesity, insulin resistance and cardiovascular disease in the offspring (6), maternal exercise during pregnancy may be an important intervention strategy that could improve offspring cardiovascular health and reduce the prevalence of inherited obesity. While the benefits of exercise for pregnant women are well documented, the effects on the developing cardiovascular system of the fetus are less well understood.
Previously, we reported that regular aerobic maternal exercise throughout pregnancy was associated with improved fetal cardiac autonomic control (7). In that prospective, longitudinal pilot study, we found significant differences in fetal HR and HRV at 36 weeks GA. During active fetal states, fetal HR was lower by approximately 10 beats per minute (bpm) and HRV was higher in all time and frequency domain metrics if women exercised during pregnancy. During quiet fetal states, power in the very low frequency (VLF, 0.02-0.08Hz) and high frequency (HF, 0.4-1.7 Hz) bands was significantly higher in the Exercise group. No group difference was observed for any metric of fetal HR or HRV at the 28 or 32 week GA time points.
Following these studies, our next aim was to determine whether maternal exercise moderates the effect of fetal breathing on cardiac autonomic control as assessed by metrics of fetal HR and HRV. In this retrospective analysis we used the fetal dMMG as a marker of in utero breathing activity and explored the effects of maternal group (Exercise, Control), fetal condition (Breathing, Apnea) and their interactions.
2. Methods
2.1 Study Population
Sixty-six pregnant women were part of a longitudinal, non-blinded study designed to determine the effects of self-reported exercise on fetal cardiac autonomic nervous system development. Fifty-one of the 66 women enrolled in the pilot study remained enrolled at 36 weeks GA; (24 Exercise, 27 Control). Because group differences in fetal HR and HRV were previously found only at 36 weeks, this retrospective analysis was limited to the 51 maternal-fetal data sets. All protocols were approved by the Kansas City University of Medicine and Biosciences and the University of Kansas Medical Center Institutional Review Boards and Human Subjects Committees. Informed consent was obtained from each participant. Women were tested between 10:00 and 17:00 hours. Women were assigned to the Exercise group if they performed moderate to vigorous aerobic exercise throughout their pregnancy for a minimum of 30 minutes, three times per week. This criterion was based on the ACOG minimum recommendation for women previously sedentary prior to pregnancy. Women in the non-exercising group were below the minimum ACOG requirements throughout their pregnancy. Reported pre-pregnancy BMI of the women whose data were used in this retrospective analysis was [Mean (+/− 1 SD)]: Exercise group [23.4 (2.6)], Control group [25.4 (5.9)]. All women carried singleton pregnancies and delivered healthy, term infants.
2.2 Data Acquisition
An ultrasound was obtained immediately prior to the MCG recording in order to document the position of the fetal body and note the presence or absence of fetal movements (breathing, mouthing or body movements). Biomagnetic signals were recorded using an investigational 83 channel dedicated fetal biomagnetometer (CTF Systems, Inc.), housed in a magnetically shielded room. The axial gradiometer sensors are spatially distributed to cover the gravid maternal abdomen. Pregnant subjects were comfortably seated, slightly reclined and in contact with the surface of the biomagnetometer interface without applying pressure to the abdomen. The data were acquired in a continuous 18 minute recording using a 300 Hz sampling rate and recording filter of 0-75 Hz.
2.3 Data Processing
Data were digitally filtered between 0.5 and 40 Hz offline (bidirectional fourth-order Butterworth filter) and then divided into 3 consecutive 6 minute sections for independent component analysis (ICA). ICA is a blind-source separation technique used to segregate the contributions from multivariate and spatially distinct electrophysiological sources into individual components (e.g., maternal, fetal, heart, diaphragm)(8, 9) and was implemented in EEGLAB toolbox (version 4.311) (10). In order to construct the inter-beat interval (IBI) series for frequency domain analysis, the fetal MCG was identified and fiducial R-peaks were automatically detected using a template-matching algorithm described in detail in May, et. al (7).
Fetal activity state has a significant influence on fetal HR and HRV, therefore; all records undergo state classification by visual inspection of the fetal HR pattern (11) by two independent investigators (KMG/LEM). States 1F (calm, non-REM) and 3F (calm wakefulness) associated with absent, sporadic or short-lasting HR accelerations were classified as quiet states. States 2F (active REM) and 4F (active wakefulness) with frequent, long-lasting accelerations that return to the baseline were classified as active states (12). While fetal breathing occurs in both active (2F, 4F) and quiet (1F, 3F) states, the chance of observing these movements is significantly higher in the active states (13). If state determination differed between investigators, a third opinion was sought and consensus was reached.
The ICA data were visually inspected for the fetal dMMG, a clearly identifiable sinusoidal component (Fig. 1) described in detail in Gustafson, et.al (1). Eleven records at 36 weeks GA were classified as quiet states (n=6 Exercise, n=5 Control) and none of these showed evidence of fetal dMMG activity. This left 40 active state records available for visual inspection of fetal dMMG activity and further HR and HRV analysis (n=18 Exercise, n=22 Control). When observed, the duration (time onset and offset) of the dMMG was marked and only epochs greater than or equal to 1 minute in duration were used in the analysis of fetal HR and HRV. Non-breathing epochs (apnea) were determined by selecting a silent region in the same component that immediately preceded or followed the dMMG that was equal to the duration of the breathing epoch. Epoch length ranged from 60.2 to 144.2 seconds; mean and standard deviation were 80.2 +/− 22.4 seconds. Fetal HR and HRV were determined from the R-R intervals observed in one breathing and one non-breathing epoch per fetus. Thirty (15 Exercise,15 Control) of the 40 records contained fetal dMMG activity using the criteria we established. This represents 75% of the active state datasets and 59% of the total sample at 36 weeks GA.
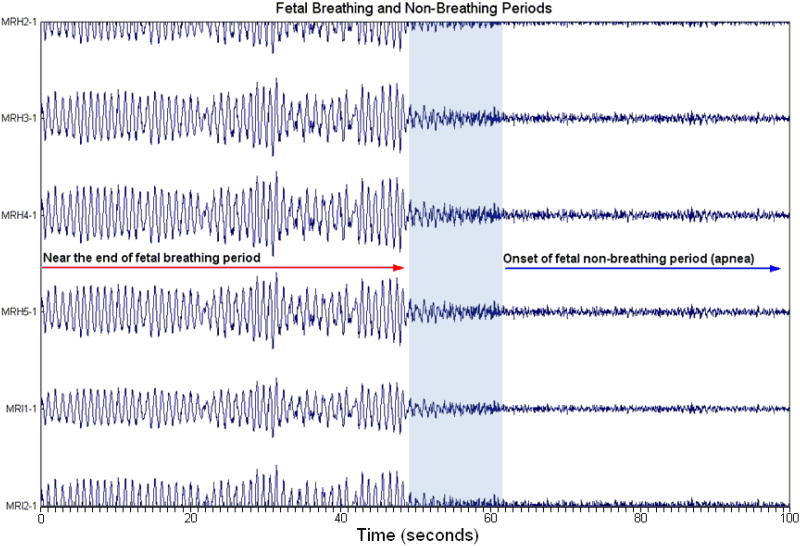
Fig. 1.
An example of a reconstructed fetal diaphragmatic magnetomyogram (dMMG) in channel space. After independent components analysis, the components linked to fetal breathing activity are reconstructed in channel space. The designations on the Y-axis are channel numbers assigned to the 83 channel system based on their location. The X-axis represents time in seconds. At zero seconds, fetal dMMG at a frequency of approximately 1.1 Hz is observed, lasting for about 48 seconds. At 60 seconds, a period of fetal apnea begins. In this particular case, fetal dMMG was observed at the end of the previous 6 minute dataset so this segment of breathing activity represents the last 48 seconds of that entire segment. This shorter segment is shown only to illustrate the morphology of the waveform and to demonstrate the distinctive difference between breathing and non-breathing activity (apnea). Areas of disorganized activity that likely represent the transition from breathing activity to apnea are in the shaded area and are not included in the analysis.
2.4 Analysis of Fetal HR and HRV
To compare time-domain metrics during breathing and non-breathing periods, the fetal IBI series was imported into QRSTool and CMetX, a suite of freely available tools for transforming ECG/MCG data to metrics of HRV (14) (http://www.psychofizz.org). QRSTool provides a graphical user interface that allows extraction of the interbeat interval (IBI) series from the MCG data. CMetX calculates several metrics of cardiac chronotropy based on the imported IBI series (IBI values in milliseconds). The IBI series is first converted to an IBI time-series by linear interpolation at 10 Hz, resulting in a time-series that is appropriate for frequency analyses. Epochs comprising fetal breathing were analyzed separately from those comprising non-breathing (Apnea). We used the following metrics from the CMetX output:
A putative parasympathetic metric, the natural log of the variance in the band-limited IBI time-series (Log RSA). The band-limited signal was selected to capture the breathing frequency of the fetus and neonate (0.4-1.7 Hz); (14), and following filtering of the IBI time-series in this band, the natural log of the variance in this filtered time series yields the metric Log RSA
-
Metrics of summarizing total HRV influenced by both parasympathetic and sympathetic activity which include:
The root mean square of successive differences between consecutive IBIs (RMSSD)
The natural Log of the variance in the IBI time-series (Log HRV)
Metrics of rate (mean IBI, mean HR) which are influenced by both parasympathetic and sympathetic activity
We also used a template-matching algorithm developed by our team (15) in EEGLab 4.311 (10) to automatically detect R-peaks for frequency domain analysis. False positive and false negative detections and abnormal beats were manually corrected. After generating the IBI time-series using the 10 Hz linear interpolation described above, we implemented a time-frequency analysis that was modified to provide a specified frequency resolution (0.0–1.7 Hz). The output from time-frequency analysis (power integral of defined frequency bands expressed in msec2) is based on the work of David, et. al (16), using the following frequency bands for fetal HRV analysis: very low frequency (VLF) [0.02-0.08 Hz], low frequency (LF) [0.08-0.2 Hz], intermediate (INT) [0.2-0.4 Hz], high frequency (HF) [0.4-1.7 Hz] and Total Power, [0.02-1.7 Hz]. Total Power is a measure of total HRV as the band encompasses all frequencies. The VLF, LF and INT bands reflect contributions from both sympathetic and parasympathetic activity. The HF band, similar to Log RSA above, captures the breathing frequency and thus is a parasympathetic metric.
2.5 Statistical Approach
We first examined the distribution of each fetal cardiac metric of rate and variability defined in section 2.4. The measures with skewed distributions were log transformed to make their distribution approximately Gaussian (RMSSD, VLF, LF, INT, HF and Total Power). Two metrics from CMetX were already natural-log transformed (Log RSA, Log HRV). Fetal cardiac metrics (either in original or natural log-transformed scales) were summarized by mean and standard deviation (SD). Because each subject had correlated, repeated fetal HRV measures during apnea and breathing conditions, we evaluated the effects of maternal exercise and fetal breathing conditions by the mixed-effects models using a random intercept. Predictor variables included group (Control and Exercise), breathing condition (Breathing and Apnea), and their interaction. If the interaction was significant, post hoc comparisons were then performed to compare Exercise vs. Control during each breathing condition and to compare apnea vs. breathing within Exercise and Control groups separately.
Because some measures showed a significant group-by-condition interaction while other highly correlated measures did not, we explored the difference in interactions between measures using a bivariate version of mixed-effects models in order to assess the group-by-condition interactions. The bivariate mixed-models include factors of measure, group, condition, group-by-condition interaction, and a 3-way interaction of measure-by-group-by-condition. A significant 3-way interaction would suggest the group-by condition interaction effects differed between measures.
3. Results
3.1 Summary statistics for metrics of fetal cardiac autonomic control for main effects and interactions
The mean and standard deviation for each metric by group and condition are listed in Table 1. Results of the mixed-models and group-by-condition interactions are listed in Table 2. The results of the post-hoc comparison are shown in Table 3. Means and standard errors for all metrics in each group under each condition are shown in Fig. 2. In Table 3, (Control vs. Exercise – Apnea) we show that the groups are similar during fetal apnea for all HRV metrics with significant or marginal interactions.
Table 1.
Fetal cardiac metrics during apnea and breathing.
| Exercise n = 15 | Control n = 15 | |||
|---|---|---|---|---|
|
|
|
|||
| [mean ± SD] | [mean ± SD] | |||
|
|
|
|||
| Apnea | Breathing | Apnea | Breathing | |
| Epoch Duration (sec) | 87.4 ±27.7 | 88.3 ±27.1 | 72.5 ±13.2 | 72.5 ±13.3 |
| Parasympathic Metrics | ||||
| Log HF | 1.62 ± 0.52 | 3.02 ± 0.98 | 1.47 ± 0.60 | 2.21 ± 0.58 |
| Log RSA | 1.34 ± 0.67 | 2.51 ± 0.98 | 1.23 ± 0.69 | 1.87 ± 0.58 |
| Metrics Summarizing Total HRV | ||||
| Log RMSSD | 1.70 ± 0.35 | 2.19 ± 0.51 | 1.58 ± 0.37 | 1.85 ± 0.29 |
| Log HRV | 5.67 ± 1.13 | 5.61 ± 1.01 | 5.45 ± 1.08 | 4.33 ± 0.73 |
| Log Total Power | 4.76 ± 1.06 | 5.10 ± 1.05 | 4.66 ± 1.06 | 4.04 ± 0.72 |
| Other Frequency Domain Metrics | ||||
| Log VLF | 3.93 ± 1.18 | 3.93 ± 1.36 | 3.86 ± 1.17 | 2.68 ± 1.24 |
| Log LF | 2.95 ± 1.14 | 3.33 ± 1.22 | 2.52 ± 1.58 | 2.31 ± 0.98 |
| Log INT | 1.25 ± 1.12 | 2.30 ± 1.51 | 0.86 ± 1.04 | 1.08 ± 0.87 |
| Metrics of Rate | ||||
| IBI (msec) | 428.9 ± 29.6 | 456.6 ± 29.4 | 405.9 ± 28.7 | 423 ± 27.8 |
| HR(bpm) | 140.9 ± 9.6 | 132.2 ± 8.8 | 148.8 ± 10.5 | 142.5 ± 9.6 |
| Ratios Attributed to Sympatho-Vagal Balance | ||||
| Log VLF/LF | 0.98 ± 0.94 | 0.61 ±1.07 | 1.25 ± 1.23 | 0.37 ± 1.20 |
| Log VLF/HF | 2.31 ± 1.03 | 0.90 ± 1.26 | 2.42 ± 1.04 | 0.32 ± 1.63 |
| Log LF/HF | 1.33 ± 0.88 | 0.31 ± 0.88 | 1.17 ± 1.15 | 0.10 ± 1.12 |
Note: Measures of parasympathetically-controlled heart rate variability include Log RSA = natural Log of variance of filtered [0.4-1.7 Hz] IBI time-series and high frequency power (Log HF) [0.4-1.7 Hz]. Time and frequency domain measures of total heart rate variability include RMSSD = root mean square of differences between IBIs; Log HRV = natural log of variance of IBI time-series, and Log Total Power [0.02-1.7 Hz]. Other frequency domain measures of band specific power (integrals in msec2) include very low frequency (Log VLF) [0.02-0.08 Hz], low frequency (Log LF) [0.08-0.2 Hz], and intermediate frequency (Log INT) [0.2-0.4 Hz] and their ratios. Assessments of rate include IBI=mean interbeat interval; HR=mean heart rate. Time domain HRV metrics are in msec.
Table 2. Fetal HR and HRV metrics fitted by the mixed models.
| Exercise vs. Control | Apnea vs. Breathing | Group-by-condition | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Estimate | SE | p† | Estimate | SE | P† | Estimate | SE | p† | |
| Epoch Duration (sec) | 15.8 | 7.86 | 0.062 | −0.94 | 0.41 | 0.13 | 0.97 | 0.59 | 0.11 |
| Log HF | 0.81 | 0.25 | 0.028 | −1.40 | 0.21 | <0001 | 0.65 | 0.29 | 0.033 |
| Log RSA | 0.64 | 0.27 | 0.11 | −1.17 | 0.21 | <.0001 | 0.52 | 0.30 | 0.09 |
| Log RMSSD | 0.34 | 0.14 | 0.077 | −0.49 | 0.10 | <.0001 | 0.22 | 0.14 | 0.11 |
| Log HRV | 1.28 | 0.37 | 0.013 | 0.06 | 0.33 | 0.016 | 1.06 | 0.46 | 0.029 |
| Log Total Power | 1.06 | 0.36 | 0.057 | −0.34 | 0.30 | 0.62 | 0.96 | 0.42 | 0.029 |
| Log VLF | 1.25 | 0.45 | 0.064 | −0.003 | 0.42 | 0.057 | 1.18 | 0.59 | 0.056 |
| LogLF | 1.01 | 0.46 | 0.069 | −0.37 | 0.35 | 0.74 | 0.58 | 0.49 | 0.25 |
| Log INT | 1.22 | 0.42 | 0.032 | −1.05 | 0.32 | 0.009 | 0.83 | 0.45 | 0.078 |
| IBI (msec) | 33.6 | 10.5 | 0.008 | −27.8 | 5.22 | <.0001 | 10.7 | 7.40 | 0.16 |
| HR(bpm) | −10.3 | 3.50 | 0.01 | 8.68 | 1.65 | <0001 | 2.36 | 2.33 | 0.32 |
| Log VLF/LF | 0.24 | 0.41 | 0.96 | 0.37 | 0.41 | 0.038 | 0,51 | 0.58 | 0.38 |
| Log VLF/HF | 0.59 | 0.46 | 0.48 | 1.40 | 0.46 | <0001 | 0.70 | 0.65 | 0.29 |
| Log LF/HF | 0.20 | 0.37 | 0.55 | 1.02 | 0.31 | <0001 | 0.05 | 0.44 | 0.92 |
based on Type 3 tests for the fixed effects
mixed models include random intercepts only
Table 3. Post hoc comparison of those variables with marginal.
| Apnea vs. Breathing Control | Apnea vs. Breathing Exercise | Control vs. Exercise Apnea | Control vs. Exercise Breathing | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||||||
| Est. | SE | t | P | Est. | SE | t | P | Est. | SE | t | P | Est. | SE | t | P | |
| Log HF‡ | −0.74 | 0.21 | −3.62 | 0.001 | −1.40 | 0.21 | −6.80 | <.0001 | −0.16 | 0.25 | −0.61 | 054 | −0.81 | 0.25 | −3.19 | 0.004 |
| Log RSA‡ | −0.65 | 0.21 | −3.04 | 0.005 | −1.17 | 0.2 I | −5.50 | <.0001 | −0.12 | 0.27 | −0 43 | 067 | −0.64 | 0.27 | −2.36 | 0.026 |
| Log HRV‡ | 1.12 | 033 | 3.45 | 0.002 | 0.06 | 0.33 | 0.1? | 0.85 | −0.22 | 0.37 | −0.61 | 055 | −1.28 | 0.37 | −3.51 | 0.002 |
| Log Total Power‡ | 0.62 | 0.30 | 2.09 | 0.046 | −0.34 | 0.30 | −1.16 | 0.26 | −0.10 | 0.36 | −0.27 | 0.7S | −1.06 | 0.36 | −2.95 | 0.006 |
| Log VLF† | 1.18 | 042 | 2.81 | 0.009 | −0.003 | 0.42 | −0.C1 | 0.995 | −0.07 | 0.45 | −0.15 | 088 | −1.25 | 0.45 | −2.77 | 0.010 |
| Log INT† | −0 22 | 032 | −0.69 | 0.50 | −1.05 | 0.32 | −3.28 | 0.003 | −0.39 | 0.42 | −0.92 | 0.36 | −1.22 | 0.42 | −−2.89 | 0.007 |
(p between 0.05 and 0.10) and significant
(p≤0.05) group-by-condition interaction
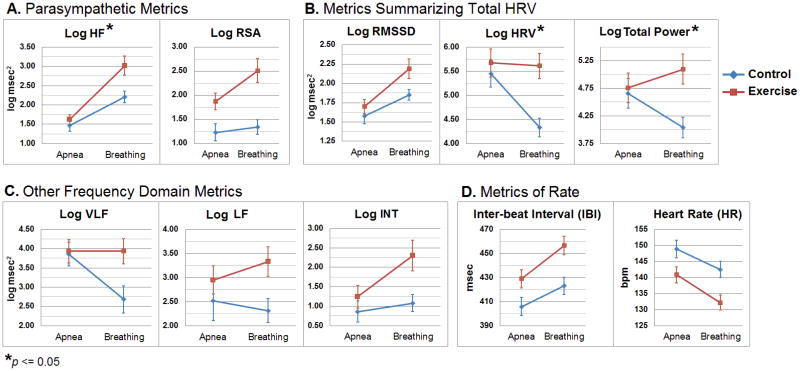
Fig. 2.
Means and standard errors of the group-by-condition interactions for fetal heart rate variability (HRV) and heart rate (HR) metrics used in the analysis. Asterisks denote significant interactions between maternal exercise and fetal breathing (p ≤ 0.05). Abbreviations: RMSSD = root mean square of successive difference, RSA = respiratory sinus arrhythmia. Frequency domain HRV measures: Total Power (log Total Power), [0.02-1.7 Hz].very low frequency (log VLF) [0.02-0.08 Hz], low frequency (log LF) [0.08-0.2 Hz], intermediate (log INT) [0.2-0.4 Hz], high frequency (log HF) [0.4-1.7 Hz].
3.1.1 Parasympathetic Metrics
Maternal exercise does not significantly increase Log RSA after adjusting for fetal breathing (p = 0.11). During fetal breathing, Log RSA is significantly higher than apnea (p <.0001) after adjusting for maternal exercise status. Importantly, the group-by-condition interaction for Log RSA is marginal (p=0.09) but is significant for Log HF power (p = 0.033). The post-hoc comparison reveals that fetal breathing, when compared to apnea, results in significantly greater Log HF power (p = 0.001) and higher Log RSA (p = 0.005) in the Control group and in the Exercise group (both p <.0001), and maternal exercise increases these two measures during fetal breathing (Log HF, p = 0.004, Log RSA, p = 0.026).
3.1.2 Maternal Exercise may Augment the Effects on Parasympathetic Metrics
Log RSA and Log HF power are two metrics that are highly correlated (r=.935) and reflect respiratory linked variability, but maternal exercise significantly enhances the breathing effects for Log HF power and is only marginally significant for Log RSA. Therefore, we used a bivariate mixed-effects model to assess whether the group-by-condition interactions between measures of Log RSA and Log HF were significantly different. The results showed a non-significant 3-way interaction (F(3,84) = 0.46, p = 0.71) that indicates the 2-way group-by-condition interactions were not significantly different between Log RSA and Log HF power. This matched the patterns of between-condition and between-group differences (Apnea < Breathing in both Control and Exercise groups; Control < Exercise during fetal breathing). In other words, the trend-level group-by-condition interaction for Log RSA is in fact similar to that seen for Log HF. In support of this claim are the findings that Log RSA and Log HF Power functioned similarly (same signs in estimates and same conclusions of significance) but with Log RSA having slightly smaller magnitude of effect size, and hence the p-value for Log RSA by group-by-condition interaction on Log RSA was marginal (0.09). Nonetheless, in the key comparison of Control vs. Exercise groups during breathing episodes, both measures perform similarly and significantly differentiate Control and Exercise groups (Table 3, far right column).
3.1.3 Metrics Summarizing Total HRV
After adjusting for the fetal breathing condition, fetuses in the Exercise group have marginally higher Log RMSSD (p = 0.077). During fetal breathing, Log RMSSD is significantly higher than during the apneic condition (p<0.0001).
A significant group-by-condition interaction was seen for Log HRV and Log Total Power (both p = 0.029) but not for Log RMSSD (p = 0.11). Because of the interactions, the exercise and breathing effects were interpreted by post hoc comparison. In the Control group, fetal breathing reduced both Log HRV and Log Total Power when compared to apnea (p = 0.002 and 0.046, respectively), but not in the Exercise group. During fetal breathing, the Exercise group showed greater variability in both measures when compared to the Control group (Log HRV, p = 0.002, Log Total Power, p = 0.006).
3.1.4 Other Frequency Domain Metrics
Maternal exercise results in marginally higher Log LF power after adjusting for fetal breathing (p = 0.069). Maternal exercise does not result in a group difference for the frequency band ratios thought to reflect fetal sympatho-vagal balance (Log VLF/LF p = 0.96, Log VLF/HF p = 0.48, Log LF/HF, p = 0.55).
There is no significant difference in Log LF power during fetal breathing after adjusting for maternal exercise (p = 0.74). The ratios thought to reflect sympatho-vagal balance are all significantly lower during fetal breathing when compared to apnea (Log VLF/LF p = 0.038, Log VLF/HF p = <.0001, Log LF/HF, p = <.0001). No significant group-by-condition interactions are seen for Log LF power or the frequency band ratios.
The group-by-condition interaction is marginally significant for Log VLF (p = 0.056) and Log INT power (p = 0.078), indicating that maternal exercise might modify the fetal breathing effects. Results of the post-hoc comparison show that fetal breathing results in lower Log VLF in the Control group (p = 0.009) but had no effect on the Exercise group (p = 0.995); consequently, there is a significant group difference during fetal breathing (p = 0.010). There is no significant difference in Log INT during fetal breathing in the Control group (p = .50) however; Log INT is significantly increased in the Exercise group during breathing (p = 0.003). During fetal breathing, the Exercise group has greater power in the INT band when compared to the Control group (p = 0.007).
3.1.5 Metrics of Rate
Both maternal exercise and fetal breathing result in significantly lower fetal HR. In the Exercise group, the mean IBI is longer (p = 0.008) and, consequently, fetal HR is lower (p = 0.01) when compared to the Control group after adjusting for the fetal breathing condition. Fetal breathing activity results in a longer IBI and lower HR compared to the apneic condition (both p<.0001) after adjusting for maternal exercise status. No group-by-condition interaction was seen for either metric of fetal HR (IBI, p = 0.16; HR p = 0.32)
4. Discussion
We compared metrics of fetal HR and HRV during in utero breathing and apneic periods in women who, based on the MPAQ, did or did not participate in regular aerobic exercise throughout their pregnancy. Using the fetal dMMG as a marker for breathing activity, we compared metrics of fetal HR and HRV in both time and frequency domains. We examined the main effects of fetal breathing condition (apnea vs. breathing) and maternal exercise group (Exercise vs. Control) on fetal HR and HRV and the group-by-condition interaction.
When we examined the main effect of maternal exercise, we found that maternal exercise was associated with significantly lower fetal HR (long IBI), higher Total HRV (Log HRV) and higher vagal input (Log HF power). We did not observe a group difference in the frequency band ratios, similar to our previous report on the effects of maternal exercise on fetal cardiac autonomic control (7) suggesting that maternal exercise does not alter fetal sympatho-vagal balance.
When we examined the main effect of fetal breathing, we found that fetal breathing movements were associated with significantly lower fetal HR (longer IBI), higher Total HRV (Log RMSSD) and higher vagal input as indexed by the parasympathetic metrics Log HF power and Log RSA. Fetal breathing had a greater effect on the parasympathetic metrics than maternal exercise. Consequently, the frequency band ratios showed significantly lower values during fetal breathing, comparable to the results in our previous report describing the effects of fetal breathing on cardiac autonomic control (1).
The novel finding in this report is the significant interaction between maternal exercise and fetal breathing for Log HRV, Log Total Power, Log VLF, Log INT and Log HF power (Fig. 2). The exercise effects are only seen during fetal breathing activity, a time when vagal function is enhanced. However, exposure to maternal exercise may also prevent sympathetic withdrawal during fetal breathing, if we assume that there are significant sympathetic contributions to VLF and LF power. Other investigators have concluded that the fetal VLF band reflects primarily sympathetic activity and the LF band has both sympathetic and parasympathetic components (16). Attributing power in the lower frequency bands to specific contributions from one or both limbs of the developing autonomic nervous system warrants caution. However, there does seem to be general agreement that fetal breathing activity contributes primarily to HF power (17-19) although, where the lower cutoff for the HF band is not clear. David, et al., described a “power gap” in the INT frequency band (0.2-0.4 Hz) where it was nearly impossible to observe any power. We agree that this band has the least power, but it is not without significance. Our results show an increase in Log INT power during fetal breathing movements for the Exercise group, and for both groups in Log HF power and Log RSA. Despite not being able to unequivocally assign specific contributions of one or both limbs of the autonomic nervous system to the lower frequency bands, based on the results of the group-by-condition interactions, we conclude that the fetus of the exercising mother is more able to initiate greater vagal activation with less sympathetic withdrawal during breathing activity. This suggests that maternal exercise results in a more flexible, responsive and adaptive autonomic nervous system in the fetus. Our results are important for two reasons. First, they show that that fetal breathing activity strongly influences HRV and needs to be accounted for when comparing group differences. Second, while the actions of the sympathetic and parasympathetic nervous systems are often regarded as opposing each other, they can function in synergy. It has been shown that simultaneous activation of the vagal and sympathetic cardiac nerves results in greater cardiac output and lower HR (20). While power in defined frequency bands cannot be used as a proxy for cardiac output, the finding is intriguing and warrants further investigation.
Fetal breathing has been shown in both humans (1) and animals (21-23) to result in lower HR and higher parasympathetic-dominated HRV. How maternal exercise influences fetal cardiac autonomic function is less clear.
4.1. Potential mechanisms of the effect of maternal exercise on fetal cardiac control
Aerobic exercise can increase activity of the parasympathetic nervous system and decrease sympathetic activity (24). At rest, the conditioned individual has lower HR and increased parasympathetic input compared with a sedentary individual; therefore, one would assume greater parasympathetic input at rest to represent the healthy state of autonomic balance. Autonomic imbalance with greater sympathetic input is associated with obesity (25), insulin resistance (26, 27), diabetes (28) and cardiovascular disease (29). Exercise is anti-inflammatory, anti-oxidant and anti-atherogenic (30). These effects may be ascribed to some extent to an increase in cholinergic anti-inflammatory pathway activity. Acetylcholine, the primary vagal neurotransmitter, is considered an anti-inflammatory molecule due to its ability to attenuate the release of pro-inflammatory cytokines (31). Interestingly, there is evidence of interaction between acetylcholine and long-chain polyunsaturated fatty acids (32, 33). During the third trimester, there is increased maternal transfer of docosahexaenoic acid (DHA) to the fetus. DHA has also been shown to lower HR and enhance parasympathetic control (34, 35). We did not see an effect of maternal exercise on fetal cardiac autonomic control in our previous longitudinal study until 36 weeks GA. It is conceivable that exercise may influence DHA incorporation into fetal cell membranes based on the work of Chytrova, et al, who showed that exercise can complement the action of DHA and may play a role in membrane stability and fluidity (36). Since DHA is abundant in all cell membranes and dietary supplementation has been shown to lower HR in the infant and fetus, (37, 38) it is not inconceivable that there may be a synergistic effect of maternal exercise and DHA that results in lower fetal HR and higher HRV. Therefore, maternal DHA status will be an important consideration in future studies.
4.2 Limitations of the Present Sample
This was a non-randomized, observational pilot study with a relatively small sample of pregnant women who, based on the MPAQ, did or did not exercise during pregnancy. While there is inherent bias associated with questionnaires, the tool used in this study has been shown to be a reliable and valid instrument for assessing the intensity and duration of physical activities performed in the preceding 12 months in various populations, including pregnant women (39, 40). Because of the non-randomized nature of the sample, it is possible that a variable other than exercise might account for the findings (i.e., a third-variable problem), whereby an individual difference in the mothers impacts both the likelihood of exercise and factors related to fetal autonomic function. Nonetheless, the present findings support the promise of a larger randomized controlled trial of maternal exercise on fetal cardiac autonomic control during pregnancy.
Due to the sensitivity and precision of the biomagnetometer and our ability to observe simultaneous fetal dMMG and MCG activity we were able to capture data with no loss of signal. To our knowledge, we are the only group to use the dMMG as a marker for human fetal breathing activity with the purpose of comparing fetal HR and HRV during breathing and apneic periods. Due to the periodic nature of fetal breathing activity, these recordings are unavoidably short. Nonetheless, frequency domain measures, especially HF components of HRV, can be assessed in as little as 1 minute (41). This is a novel area of HRV research that allows us to investigate the longitudinal development of fetal cardiac autonomic function during different fetal behaviors.
4.3 Implications
In a healthy pregnancy, there are myriad maternal physiologic and cardiovascular adaptations to prevent rejection of the fetus and support fetal growth. These include modifications to the innate immune system(42), alterations in cytokine production (43), increased insulin resistance (44) and changes in autonomic circulatory function that result in an increasingly hyper-sympathetic maternal state as gestation progresses (45). When these normal adaptations are complicated by excess maternal weight and inflammation, then pregnancy can be complicated by gestational diabetes, pre-eclampsia, abnormal placental development and pre-term delivery (46).
It is now clear that a sedentary lifestyle increases metabolic disease risk. The consequences of maternal over-nutrition, obesity, gestational diabetes leading to increased risk for metabolic disorders in the offspring are already evident. Furthermore, there is now convincing evidence that these maternal complications can have long-term health consequences for the next generation (47). Maternal exercise is associated with lower fetal HR and higher HRV in both time and frequency domains. Furthermore, during in utero breathing movements, the fetus of the exercising mother is able to exert greater cardiac autonomic input, suggesting a programming effect that may give the offspring an adaptive advantage with respect to cardiac autonomic function.
The question remains as to whether these effects of maternal exercise offer a transitory or long-lasting benefit to the offspring. This is an area of continuing and future investigation. Exercise is a natural, holistic and often overlooked therapy that has the potential to counter-balance the natural physiologic changes in pregnancy. We suggest that moderate maternal exercise during pregnancy may help reduce the risks associated with excess maternal weight gain and inflammatory conditions of pregnancy.
Acknowledgments
The authors acknowledge the contributions of JoAnn Liermann, RNC, PhD and Lori Blanck, R. EEG/EP T. for their assistance with ultrasonography, data acquisition and processing. This study was supported in part by pilot funding from the Hoglund Brain Imaging Center, through a generous gift from Forrest and Sally Hoglund, an intramural grant from the Kansas City University of Medicine and Biosciences (May), and the Kansas Intellectual Development and Disabilities Research Center (P30 NICHD HD 002528). K.M. Gustafson is supported in part by NICHD R21 HD059019 and R01 HD047315.
Footnotes
Conflict of Interest Statement: The authors have no financial or personal relationships with other people or organizations that could inappropriately influence their work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gustafson KM, Allen JJ, Yeh HW, May LE. Characterization of the fetal diaphragmatic magnetomyogram and the effect of breathing movements on cardiac metrics of rate and variability. Early Hum Dev. 2011 Apr 13; doi: 10.1016/j.earlhumdev.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ulusar UD, Wilson JD, Murphy P, Govindan RB, Preissl H, Lowery CL, et al. Bio-magnetic signatures of fetal breathing movement. Physiol Meas. 2011 Feb;32(2):263–73. doi: 10.1088/0967-3334/32/2/009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Leeuwen P, Voss A, Cysarz D, Edelhauser F, Gronemeyer D. Automatic identification of fetal breathing movements in fetal RR interval time series. Comput Biol Med. 2011 May 27; doi: 10.1016/j.compbiomed.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 4.Clapp JF., 3rd The course of labor after endurance exercise during pregnancy. Am J Obstet Gynecol. 1990 Dec;163(6 Pt 1):1799–805. doi: 10.1016/0002-9378(90)90753-t. [DOI] [PubMed] [Google Scholar]
- 5.ACOG committee opinion. Exercise during pregnancy and the postpartum period. Number 267, January 2002. American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet. 2002 Apr;77(1):79–81. doi: 10.1016/s0020-7292(02)80004-2. [DOI] [PubMed] [Google Scholar]
- 6.Catalano PM, Hauguel-De Mouzon S. Is it time to revisit the Pedersen hypothesis in the face of the obesity epidemic? Am J Obstet Gynecol. 2011 Jun;204(6):479–87. doi: 10.1016/j.ajog.2010.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.May LE, Glaros A, Yeh HW, Clapp JF, 3rd, Gustafson KM. Aerobic exercise during pregnancy influences fetal cardiac autonomic control of heart rate and heart rate variability. Early Hum Dev. 2010 Apr;86(4):213–7. doi: 10.1016/j.earlhumdev.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Delorme A, Sejnowski T, Makeig S. Enhanced detection of artifacts in EEG data using higher-order statistics and independent component analysis. Neuroimage. 2007 Feb 15;34(4):1443–9. doi: 10.1016/j.neuroimage.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bell AJ, Sejnowski TJ. An information-maximization approach to blind separation and blind deconvolution. Neural Comput. 1995 Nov;7(6):1129–59. doi: 10.1162/neco.1995.7.6.1129. [DOI] [PubMed] [Google Scholar]
- 10.Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004 Mar 15;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Nijhuis IJ, ten Hof J. Development of fetal heart rate and behavior: indirect measures to assess the fetal nervous system. Eur J Obstet Gynecol Reprod Biol. 1999 Nov;87(1):1–2. doi: 10.1016/s0301-2115(99)00143-8. [DOI] [PubMed] [Google Scholar]
- 12.Ten Hof J, Nijhuis IJ, Mulder EJ, Nijhuis JG, Narayan H, Taylor DJ, et al. Longitudinal study of fetal body movements: nomograms, intrafetal consistency, and relationship with episodes of heart rate patterns a and B. Pediatr Res. 2002 Oct;52(4):568–75. doi: 10.1203/00006450-200210000-00017. [DOI] [PubMed] [Google Scholar]
- 13.Pillai M, James D. Hiccups and breathing in human fetuses. Arch Dis Child. 1990 Oct;65(10 Spec No):1072–5. doi: 10.1136/adc.65.10_spec_no.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allen JJB, Chambers AS, Towers DN. The many metrics of cardiac chronotropy: a pragmatic primer and a brief comparison of metrics. Biol Psychol. 2007 Feb;74(2):243–62. doi: 10.1016/j.biopsycho.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Popescu EA, Popescu M, Bennett TL, Lewine JD, Drake WB, Gustafson KM. Magnetographic assessment of fetal hiccups and their effect on fetal heart rhythm. Physiol Meas. 2007 Jun;28(6):665–76. doi: 10.1088/0967-3334/28/6/005. [DOI] [PubMed] [Google Scholar]
- 16.David M, Hirsch M, Karin J, Toledo E, Akselrod S. An estimate of fetal autonomic state by time-frequency analysis of fetal heart rate variability. J Appl Physiol. 2007 Mar;102(3):1057–64. doi: 10.1152/japplphysiol.00114.2006. [DOI] [PubMed] [Google Scholar]
- 17.Groome LJ, Mooney DM, Bentz LS, Singh KP. Spectral analysis of heart rate variability during quiet sleep in normal human fetuses between 36 and 40 weeks of gestation. Early Hum Dev. 1994 Jul;38(1):1–9. doi: 10.1016/0378-3782(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 18.Van Leeuwen P, Geue D, Lange S, Hatzmann W, Gronemeyer D. Changes in the frequency power spectrum of fetal heart rate in the course of pregnancy. Prenat Diagn. 2003 Nov;23(11):909–16. doi: 10.1002/pd.723. [DOI] [PubMed] [Google Scholar]
- 19.Wakai RT, Wang M, Leuthold AC, Martin CB. Foetal magnetocardiogram amplitude oscillations associated with respiratory sinus arrhythmia. Physiol Meas. 1995 Feb;16(1):49–54. doi: 10.1088/0967-3334/16/1/006. [DOI] [PubMed] [Google Scholar]
- 20.Koizumi K, Terui N, Kollai M, Brooks CM. Functional significance of coactivation of vagal and sympathetic cardiac nerves. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2116–20. doi: 10.1073/pnas.79.6.2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jansen AH, Chernick V. Fetal breathing and development of control of breathing. J Appl Physiol. 1991 Apr;70(4):1431–46. doi: 10.1152/jappl.1991.70.4.1431. [DOI] [PubMed] [Google Scholar]
- 22.Metsala TH, Siimes AS, Antila KJ, Tuominen J, Valimaki IA. Computer analysis of heart rate variation and breathing movements in fetal lambs. Med Biol Eng Comput. 1993 May;31(3):221–8. doi: 10.1007/BF02458040. [DOI] [PubMed] [Google Scholar]
- 23.Myers MM, Fifer W, Haiken J, Stark RI. Relationships between breathing activity and heart rate in fetal baboons. Am J Physiol. 1990 Jun;258(6 Pt 2):R1479–85. doi: 10.1152/ajpregu.1990.258.6.R1479. [DOI] [PubMed] [Google Scholar]
- 24.Carter JB, Banister EW, Blaber AP. Effect of endurance exercise on autonomic control of heart rate. Sports Med. 2003;33(1):33–46. doi: 10.2165/00007256-200333010-00003. [DOI] [PubMed] [Google Scholar]
- 25.Hall JE, da Silva AA, do Carmo JM, Dubinion J, Hamza S, Munusamy S, et al. Obesity-induced hypertension: role of sympathetic nervous system, leptin, and melanocortins. J Biol Chem. 2010 Jun 4;285(23):17271–6. doi: 10.1074/jbc.R110.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaaja RJ, Poyhonen-Alho MK. Insulin resistance and sympathetic overactivity in women. J Hypertens. 2006 Jan;24(1):131–41. doi: 10.1097/01.hjh.0000194121.19851.e5. [DOI] [PubMed] [Google Scholar]
- 27.Egan BM. Insulin resistance and the sympathetic nervous system. Curr Hypertens Rep. 2003 Jun;5(3):247–54. doi: 10.1007/s11906-003-0028-7. [DOI] [PubMed] [Google Scholar]
- 28.Masuo K, Rakugi H, Ogihara T, Esler MD, Lambert GW. Cardiovascular and renal complications of type 2 diabetes in obesity: role of sympathetic nerve activity and insulin resistance. Curr Diabetes Rev. 2010 Mar;6(2):58–67. doi: 10.2174/157339910790909396. [DOI] [PubMed] [Google Scholar]
- 29.Malpas SC. Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol Rev. 2010 Apr;90(2):513–57. doi: 10.1152/physrev.00007.2009. [DOI] [PubMed] [Google Scholar]
- 30.Szostak J, Laurant P. The forgotten face of regular physical exercise: a [natural] anti-atherogenic activity. Clin Sci (Lond) 2011 Aug 1;121(3):91–106. doi: 10.1042/CS20100520. [DOI] [PubMed] [Google Scholar]
- 31.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000 May 25;405(6785):458–62. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 32.Aid S, Vancassel S, Linard A, Lavialle M, Guesnet P. Dietary docosahexaenoic acid [22: 6(n-3)] as a phospholipid or a triglyceride enhances the potassium chloride-evoked release of acetylcholine in rat hippocampus. J Nutr. 2005 May;135(5):1008–13. doi: 10.1093/jn/135.5.1008. [DOI] [PubMed] [Google Scholar]
- 33.Almeida T, Cunha RA, Ribeiro JA. Facilitation by arachidonic acid of acetylcholine release from the rat hippocampus. Brain Res. 1999 Apr 24;826(1):104–11. doi: 10.1016/s0006-8993(99)01267-6. [DOI] [PubMed] [Google Scholar]
- 34.Christensen JH, Schmidt EB. Autonomic nervous system, heart rate variability and n-3 fatty acids. J Cardiovasc Med (Hagerstown) 2007 Sep;8(1):S19–22. doi: 10.2459/01.JCM.0000289276.10675.a1. [DOI] [PubMed] [Google Scholar]
- 35.O'Keefe JH, Jr, Abuissa H, Sastre A, Steinhaus DM, Harris WS. Effects of omega-3 fatty acids on resting heart rate, heart rate recovery after exercise, and heart rate variability in men with healed myocardial infarctions and depressed ejection fractions. Am J Cardiol. 2006 Apr 15;97(8):1127–30. doi: 10.1016/j.amjcard.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 36.Chytrova G, Ying Z, Gomez-Pinilla F. Exercise contributes to the effects of DHA dietary supplementation by acting on membrane-related synaptic systems. Brain Res. 2010 Jun 23;1341:32–40. doi: 10.1016/j.brainres.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Colombo J, Carlson SE, Cheatham CL, Fitzgerald-Gustafson KM, Kepler A, Doty T. Long Chain Polyunsaturated Fatty Acid Supplementation in Infancy Reduces Heart Rate and Positively Affects Distribution of Attention. Pediatr Res. 2011 Oct;70(4):406–10. doi: 10.1203/PDR.0b013e31822a59f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gustafson KM, Colombo J, Carlson SE. Docosahexaenoic acid and cognitive function: Is the link mediated by the autonomic nervous system? Prostaglandins Leukot Essent Fatty Acids. 2008 Sep-Nov;79(3-5):135–40. doi: 10.1016/j.plefa.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cramp AG, Bray SR. Pre- and postnatal women's leisure time physical activity patterns: a multilevel longitudinal analysis. Res Q Exerc Sport. 2009 Sep;80(3):403–11. doi: 10.1080/02701367.2009.10599578. [DOI] [PubMed] [Google Scholar]
- 40.Bauer PW, Pivarnik JM, Feltz DL, Paneth N, Womack CJ. Validation of an historical physical activity recall tool in postpartum women. J Phys Act Health. 2010 Sep;7(5):658–61. doi: 10.1123/jpah.7.5.658. [DOI] [PubMed] [Google Scholar]
- 41.Camm AJ, Malik M, Bigger JT., Jr Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996 Mar 1;93(5):1043–65. [PubMed] [Google Scholar]
- 42.Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF, 3rd, Petraglia F. Inflammation and pregnancy. Reprod Sci. 2009 Feb;16(2):206–15. doi: 10.1177/1933719108329095. [DOI] [PubMed] [Google Scholar]
- 43.Orsi NM. Cytokine networks in the establishment and maintenance of pregnancy. Hum Fertil (Camb) 2008 Dec;11(4):222–30. doi: 10.1080/14647270802206879. [DOI] [PubMed] [Google Scholar]
- 44.Lain KY, Catalano PM. Factors that affect maternal insulin resistance and modify fetal growth and body composition. Metab Syndr Relat Disord. 2006 Summer;4(2):91–100. doi: 10.1089/met.2006.4.91. [DOI] [PubMed] [Google Scholar]
- 45.Fu Q, Levine BD. Autonomic circulatory control during pregnancy in humans. Semin Reprod Med. 2009 Jul;27(4):330–7. doi: 10.1055/s-0029-1225261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ornoy A. Prenatal origin of obesity and their complications: Gestational diabetes, maternal overweight and the paradoxical effects of fetal growth restriction and macrosomia. Reprod Toxicol. 2011 May 19; doi: 10.1016/j.reprotox.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 47.Jarvie E, Hauguel-de-Mouzon S, Nelson SM, Sattar N, Catalano PM, Freeman DJ. Lipotoxicity in obese pregnancy and its potential role in adverse pregnancy outcome and obesity in the offspring. Clin Sci (Lond) 2010 Aug;119(3):123–9. doi: 10.1042/CS20090640. [DOI] [PMC free article] [PubMed] [Google Scholar]