Abstract
Although circadian clocks normally run with a 24 hr period, Brancaccio et al. (2013) report in this issue of Neuron that transiently activating G protein signaling can lengthen period even after the stimulus is removed, revealing an unexpected plasticity in the central brain clock.
Most organisms have internal circadian clocks that anticipate the daily environmental changes at dawn and dusk and drive 24 hr rhythms in behavior and physiology. We are usually only aware of our internal clocks when they become desynchronized from the environment during jetlag, but these clocks are impressively accurate and run with periods close to 24 hr even in constant darkness.
Genetic screens in flies and mammals identified numerous molecular components that help us understand how circadian clocks work at the single-cell level. Point mutants can change the normal ~24 hr period of animal clocks to periods ranging from 16 hr to 33 hr (Konopka et al., 1994; Rothenfluh et al., 2000), and the dramatic effects of these mutants promoted the idea that clock genes determine time for an organism. These clock genes form a transcriptional-translational feedback loop (TTFL) that is largely conserved across the metazoans. In mammals, the transcription factors Clk and Bmal1 activate expression of the Period (mPer1–3) and Cryptochrome genes (mCry1–2), whose protein products inhibit Clk/Bmal1 activity. This feedback loop generates 24 hr rhythms in expression of several of its components (including the Per and Cry genes) as well as hundreds of downstream genes that link the core clock to the rhythmic physiology and metabolism of clock-containing cells.
However, there is mounting evidence that communication between neurons containing these molecular clocks is as important for accurate time keeping as the clock genes themselves. Communication synchronizes the periods of individual clock neurons in the mammalian master clock located in the suprachiasmatic nucleus (SCN) of the hypothalamus (Liu et al., 1997) and can even compensate for deletion of the core clock genes mPer1 and mCry1 (Liu et al., 2007). Similarly, SCN explants from Bmal1−/− mutant mice still show dampened oscillations of an mPer2 reporter gene—these quasi-circadian rhythms are driven by communication since they are abolished by dissociating SCN neurons, by preventing them from firing or by blocking either Adenylate cyclase or Protein Kinase A activity (Ko et al., 2010). Cytosolic signaling therefore works alongside the TTFL in neurons (see also O’Neill et al. [2008]). Now, in this issue of Neuron, Brancaccio et al. (2013) focused on G protein signaling as a likely regulator of the SCN cytosolic oscillator.
First, Brancaccio et al. (2013) temporally ordered molecular rhythms in wild-type SCN neurons in constant darkness using a set of reporter genes. The first change they observed each day was a sharp rise and fall in intracellular Ca2+ at about noon, measured via a virally transduced GCaMP3 sensor. Approximately 100 min later, they observed peak expression of a CRE-luciferase (CRE-luc) reporter gene. CREs (cAMP-responsive elements) bind the CREB family of transcription factors and respond to both Ca2+and cAMP. Since cAMP levels are also rhythmic in the SCN and peak in the morning (O’Neill et al., 2008), CRE-luc probably reflects the transcriptional responses to 24 hr oscillations in both cAMP and Ca2+ levels. The number of neurons showing CRE activity oscillations was greatly reduced when communication between SCN neurons was blocked and the remaining rhythmic neurons had much more broadly distributed phases of oscillation than in control explants. Thus, Brancaccio et al. (2013) concluded that these CRE rhythms depend on intercellular communication in the SCN. The principal neuropeptide in the SCN is vaso-intestinal peptide (VIP) and SCN explants taken from VIP mutant mice showed similar phenotypes to blocking signaling with tetrodotoxin. Since VIP signals via the G protein coupled receptor VPAC2, the authors concluded that communication between SCN neurons depends on G protein signaling.
To understand how Ca2+/cAMP signals relate to the TTFL, Brancaccio et al. (2013) also measured three different luciferase-based reporters of the molecular clock. The Per1 promoter contains E boxes (that respond to Clk/Bmal1) as well as CREs and peak Per1-luc reporter expression trailed the Ca2+ rhythm by 2.6 hr. A PER2:Luciferase fusion protein peaked ~4.8 hr after the Ca2+ peak and finally a Cry1-luc reporter displayed a 24 hr oscillation peaking 5.5 hr after Ca2+. Since the Cry1 promoter contains E boxes but no CREs, cAMP and/or Ca2+ seem to initiate clock gene transcription ahead of Clk/Bmal1. Brancaccio et al. (2013) noted that the narrow-peaked Ca2+ rhythm feeds into a “saw tooth” CRE response followed by more sinusoidal clock gene oscillations, presumably modulated by gradual changes in Clk/Bmal1 activity.
Our detailed knowledge of how the molecular clock keeps time makes the circadian system ideal for studying how extracellular signals are integrated by neurons to regulate both gene expression and neuronal plasticity. To test the role of different G protein subunits in the SCN, Brancaccio et al. (2013) used specific designer receptors exclusively activated by designer drugs (DREADDs) to activate Gs, Gi, or Gq in SCN pacemaker neurons. DREADDs are modified GPCRs that respond only to C clozapine-N-oxide (CNO), an otherwise inert small molecule (Rogan and Roth, 2011) and couple to endogenous G proteins.
Each DREADD was virally transduced along with CRE-luc into SCN explants and CNO added to activate a specific G protein. Activating Gs or Gi increased or decreased overall CRE-luc activity respectively, as expected given the well-known effects of Gs and Gi on Adenylate cyclase activity. CRE-luc oscillations maintained 24 hr periods during and after CNO was removed in both cases.
DREADD activation of Gq produced some surprising results. Activation of Gq increased CRE activity and lengthened the period of both CRE-luc and Per1-luc oscillations by over an hour. Strikingly, these long period oscillations remained even after removing CNO, indicating that Gq activation had reprogrammed the SCN. This result is even more surprising because <20% of SCN neurons were transduced by Gq DREADD and yet the whole SCN was affected. The SCN is therefore far more plastic than previously imagined and can be engineered to run with a long period even with genetically wild-type clock components. Along with the period changes, the topography of reporter gene oscillations across the SCN was also disrupted by Gq activation. Normal circadian oscillations peak first in the dorsomedial SCN (the “shell”) and then move ventrolaterally (to the “core”). After Gq activation, these waves of Ca2+ and clock gene expression largely collapsed and were mainly visible only in the ventrolateral SCN. Not surprisingly, the overall amplitudes of these oscillations were also reduced after Gq activation. Oscillations were only monitored for 6 days after CNO washout, so the period may eventually return to 24 hr. Even so, this unexpected and intriguing result further emphasizes how important cytosolic inputs are to the core clock. It will be very interesting to find out if these altered oscillations are translated into long-period behavioral rhythms.
These long-lasting changes in the SCN indicate that the SCN has a memory of Gq activity and that the reductions in the amplitude of circadian Ca2+ oscillations induced by Gq activation are maintained and feed back into the molecular clock. The Gq effect on Ca2+ seems to be very important because Gs activation (to increase cAMP) did not stably alter rhythms. It has already been shown that SCN neurons go through quite dramatic daily changes in neuronal excitability (Belle et al., 2009). Now the period change and altered pattern of oscillations in response to Gq are additional forms of plasticity in the SCN.
But why would the SCN “want” to reorganize its oscillations in response to Gq? The 25 hr period is probably an artifact of constitutively activating Gq for several days. Gq is likely normally activated in a temporally restricted manner in response to a specific stimulus and would therefore have a smaller effect on intracellular Ca2+ than in these studies, allowing modulation of molecular clock oscillations by extracellular signals. But this intriguing study shows the importance of the Gq signal and raises the questions: What normally activates Gq in the SCN and which cells respond to this signal?
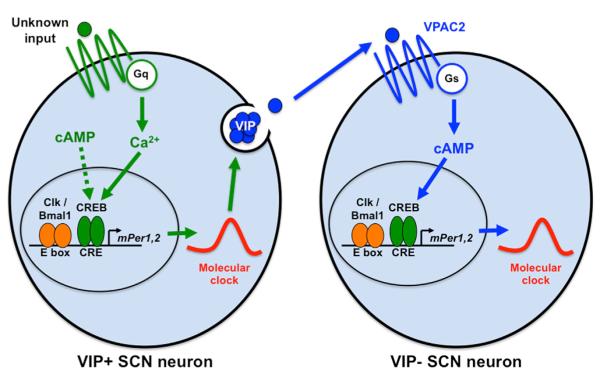
Addressing the second question, Brancaccio et al. (2013) showed that the VIP-expressing subset of SCN neurons reprogram the entire SCN circuit after Gq activation (see Figure 1). First, using grafts between VIP−/− and wild-type SCNs, they found that activating Gq in neurons lacking VIP did not lengthen period. Second, they used an intersectional Cre recombinase approach to target DREADD expression specifically to VIP-expressing neurons. They found that directly activating Gq only in VIP-expressing neurons was sufficient to increase the period of molecular clock oscillations to 25 hr across the SCN. The spatiotemporal dynamics of PER2:Luc oscillations were also displaced and compressed, identical to the phenotypes of untargeted Gq activation. Since synchronization of the SCN depends on VIP (Aton et al., 2005), these data fit with the idea that Gq activation in VIP-expressing neurons sets the period of other clock neurons in the SCN via VIP (see Figure 1).
Figure 1. A Model for G Protein Signaling in the SCN.
In this simplified model, VIP-expressing clock neurons in the SCN receive inputs via Gq, which increases intracellular Ca2+ levels and modifies clock gene oscillations that, in turn, determine the timing of VIP release. VPAC2, the VIP receptor, activates Gs signaling in SCN neurons that do not express VIP and this alters clock gene oscillations in these downstream clock neurons.
Although Brancaccio et al. did not identify the source of the signal that activates Gq signaling in VIP neurons, one possibility is that Gq is activated by signals from other clock neurons, allowing time of day information to be shared across the SCN. Alternatively, since many VIP-expressing neurons respond to light inputs arriving via the retinohypothalamic tract (Cassone et al., 1988), Gq may transform light inputs into molecular clock changes. This is when an SCN memory trace could be important. There are similar day lengths at spring and fall equinoxes, and yet what follows is very different for animals in the wild: spring heralds abundant food and the mating season, while fall heralds cold and extreme physiological changes including hibernation for some. So could Gq signaling act as a memory of the previous day length and measure photoperiod? Certainly the SCN has a major role in controlling the length of nocturnal melatonin production which seems to encode day length in animals (Goldman, 2001). The functional significance of Gq as a measure of photoperiodic device for humans is less clear, but we too show seasonal changes, including pathologies such as seasonal affective disorder—for which, intriguingly, bright light therapy is the recommended treatment.
Finally, the ability to specifically manipulate subsets of SCN neurons through intersectional techniques described here should allow mammalian clock researchers to determine how mammalian clock neurons function together to generate rhythmic behavior. In Drosophila, the s-LNv master pacemaker neurons release the neuropeptide PDF. Since the PDF receptor is the homolog of the VIP receptor, VPAC2, could VIP-expressing neurons and s-LNvs be functionally equivalent? If so, then recent experiments from Drosophila on the roles of different clock neurons (e.g., Collins et al., 2012; Stoleru et al., 2007; Stoleru et al., 2005) suggest some immediate avenues for mammalian researchers. The tools are now available to test if circadian neural circuits are as well conserved across evolution as their molecular clocks.
ACKNOWLEDGMENTS
We thank the NIH (R01 GM063911 and R03 NS077156) for support.
REFERENCES
- Aton SJ, Colwell CS, Harmar AJ, Waschek J, Herzog ED. Nat. Neurosci. 2005;8:476–483. doi: 10.1038/nn1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belle MD, Diekman CO, Forger DB, Piggins HD. Science. 2009;326:281–284. doi: 10.1126/science.1169657. [DOI] [PubMed] [Google Scholar]
- Brancaccio M, Maywood ES, Chesham JE, Loudon ASI, Hastings MH. Neuron. 2013;78(this issue):714–728. doi: 10.1016/j.neuron.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassone VM, Speh JC, Card JP, Moore RY. J. Biol. Rhythms. 1988;3:71–91. doi: 10.1177/074873048800300106. [DOI] [PubMed] [Google Scholar]
- Collins B, Kane EA, Reeves DC, Akabas MH, Blau J. Neuron. 2012;74:706–718. doi: 10.1016/j.neuron.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman BD. J. Biol. Rhythms. 2001;16:283–301. doi: 10.1177/074873001129001980. [DOI] [PubMed] [Google Scholar]
- Ko CH, Yamada YR, Welsh DK, Buhr ED, Liu AC, Zhang EE, Ralph MR, Kay SA, Forger DB, Takahashi JS. PLoS Biol. 2010;8:e1000513. doi: 10.1371/journal.pbio.1000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka RJ, Hamblen-Coyle MJ, Jamison CF, Hall JC. J. Biol. Rhythms. 1994;9:189–216. doi: 10.1177/074873049400900303. [DOI] [PubMed] [Google Scholar]
- Liu C, Weaver DR, Strogatz SH, Reppert SM. Cell. 1997;91:855–860. doi: 10.1016/s0092-8674(00)80473-0. [DOI] [PubMed] [Google Scholar]
- Liu AC, Welsh DK, Ko CH, Tran HG, Zhang EE, Priest AA, Buhr ED, Singer O, Meeker K, Verma IM, et al. Cell. 2007;129:605–616. doi: 10.1016/j.cell.2007.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill JS, Maywood ES, Chesham JE, Takahashi JS, Hastings MH. Science. 2008;320:949–953. doi: 10.1126/science.1152506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogan SC, Roth BL. Pharmacol. Rev. 2011;63:291–315. doi: 10.1124/pr.110.003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenfluh A, Young MW, Saez L. Neuron. 2000;26:505–514. doi: 10.1016/s0896-6273(00)81182-4. [DOI] [PubMed] [Google Scholar]
- Stoleru D, Peng Y, Nawathean P, Rosbash M. Nature. 2005;438:238–242. doi: 10.1038/nature04192. [DOI] [PubMed] [Google Scholar]
- Stoleru D, Nawathean P, Fernández MP, Menet JS, Ceriani MF, Rosbash M. Cell. 2007;129:207–219. doi: 10.1016/j.cell.2007.02.038. [DOI] [PubMed] [Google Scholar]