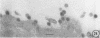Abstract
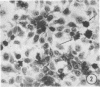
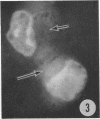
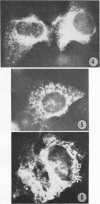
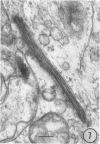
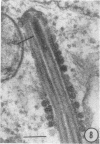
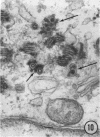
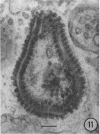
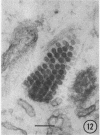
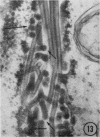
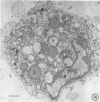
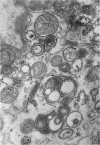
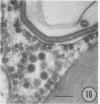
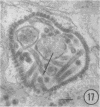
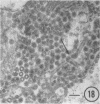
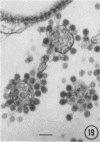
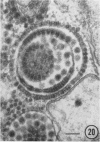
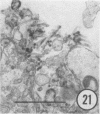
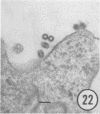
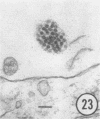
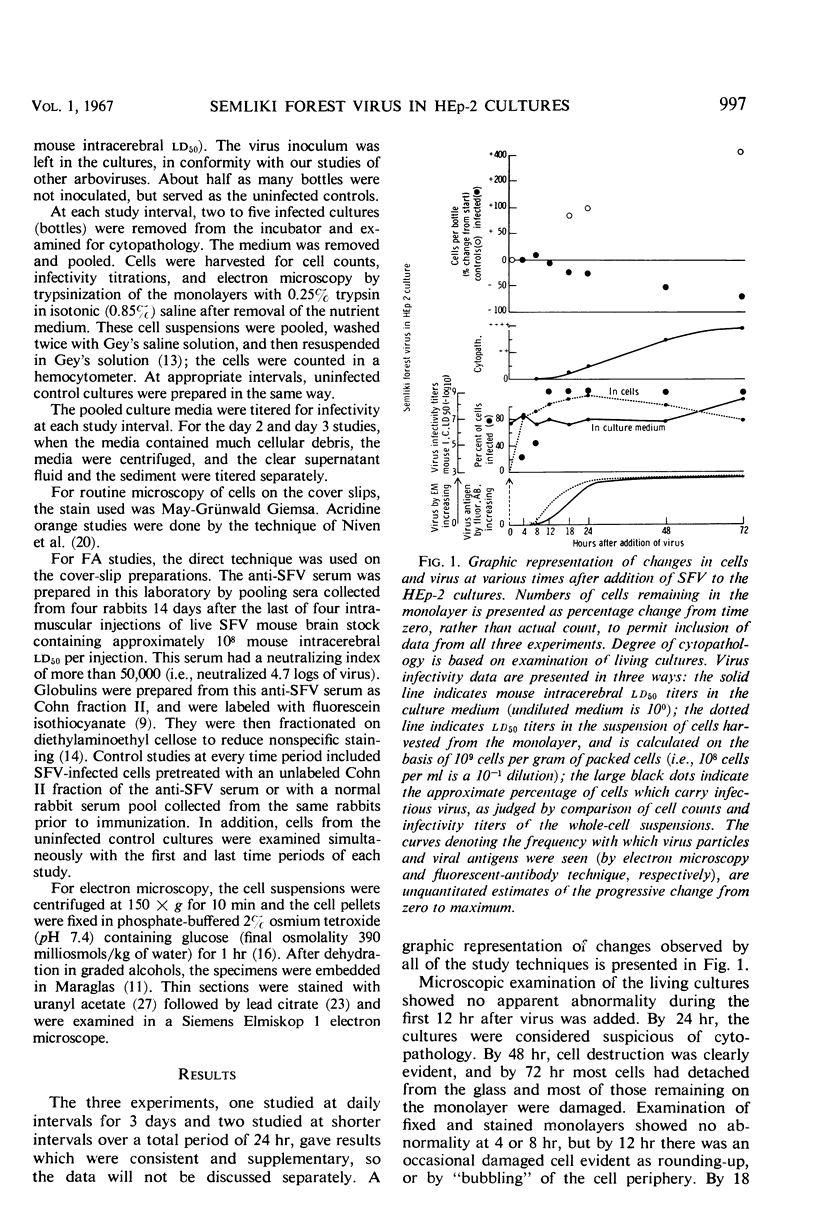
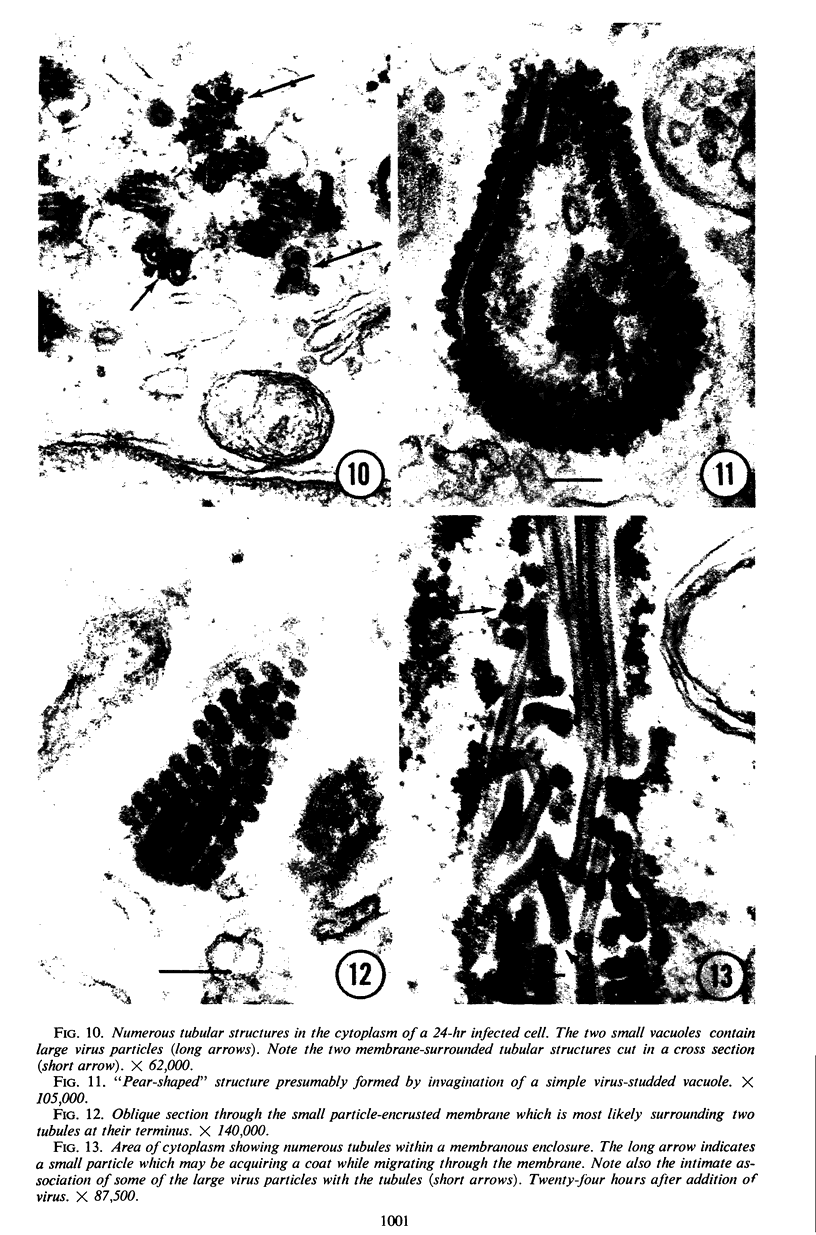
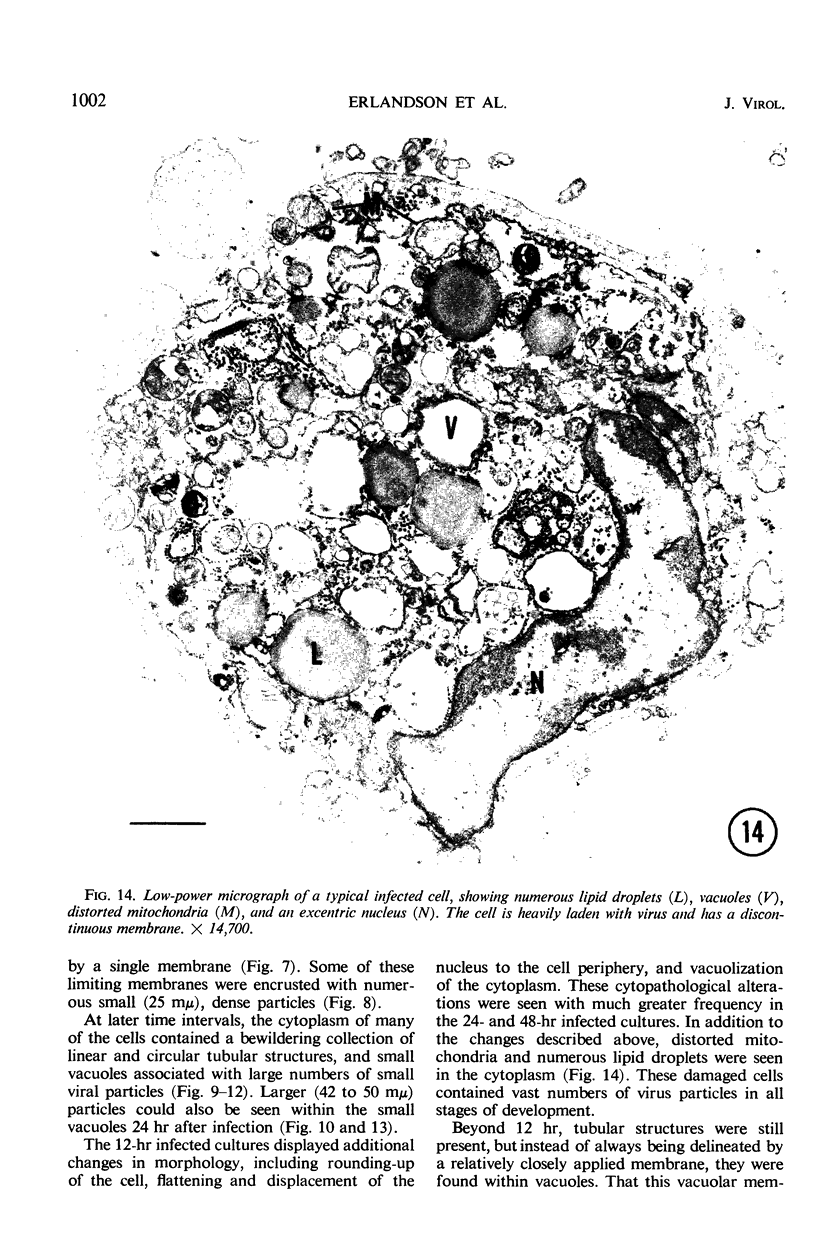
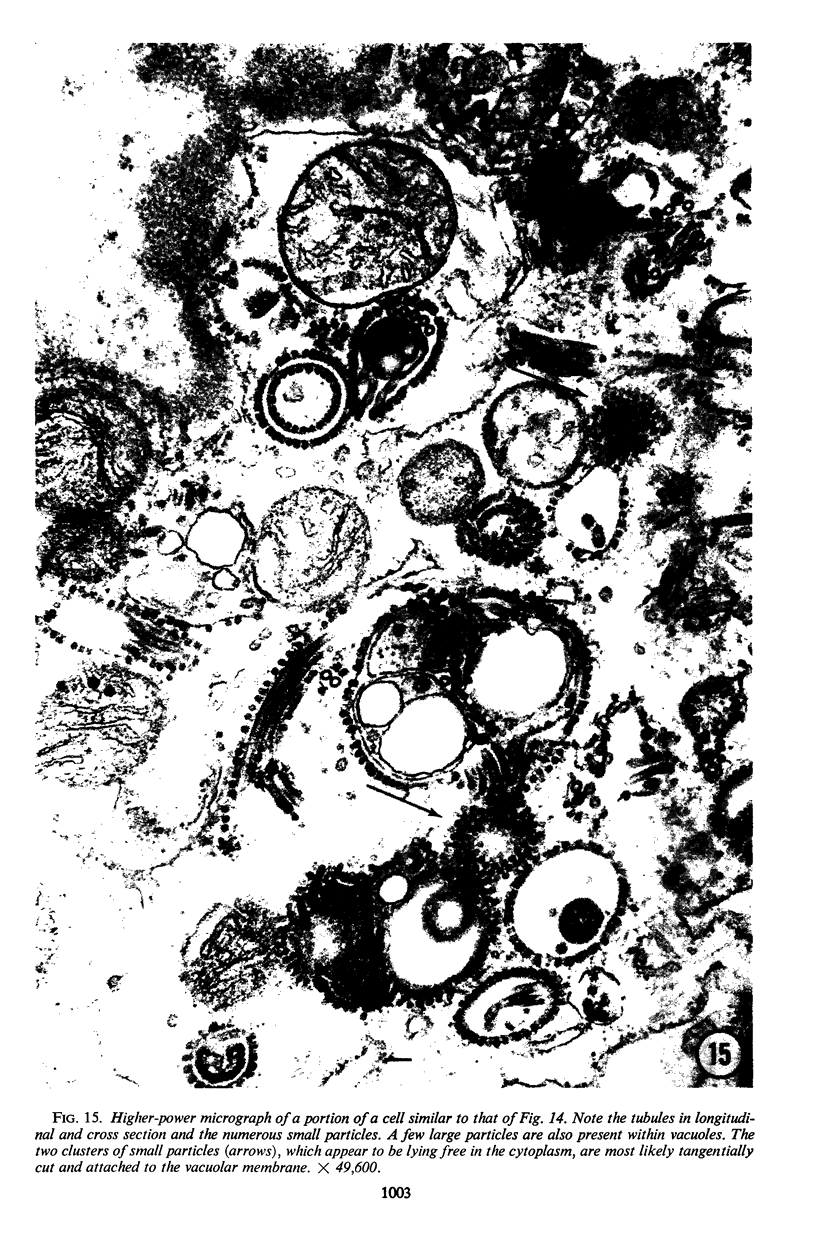
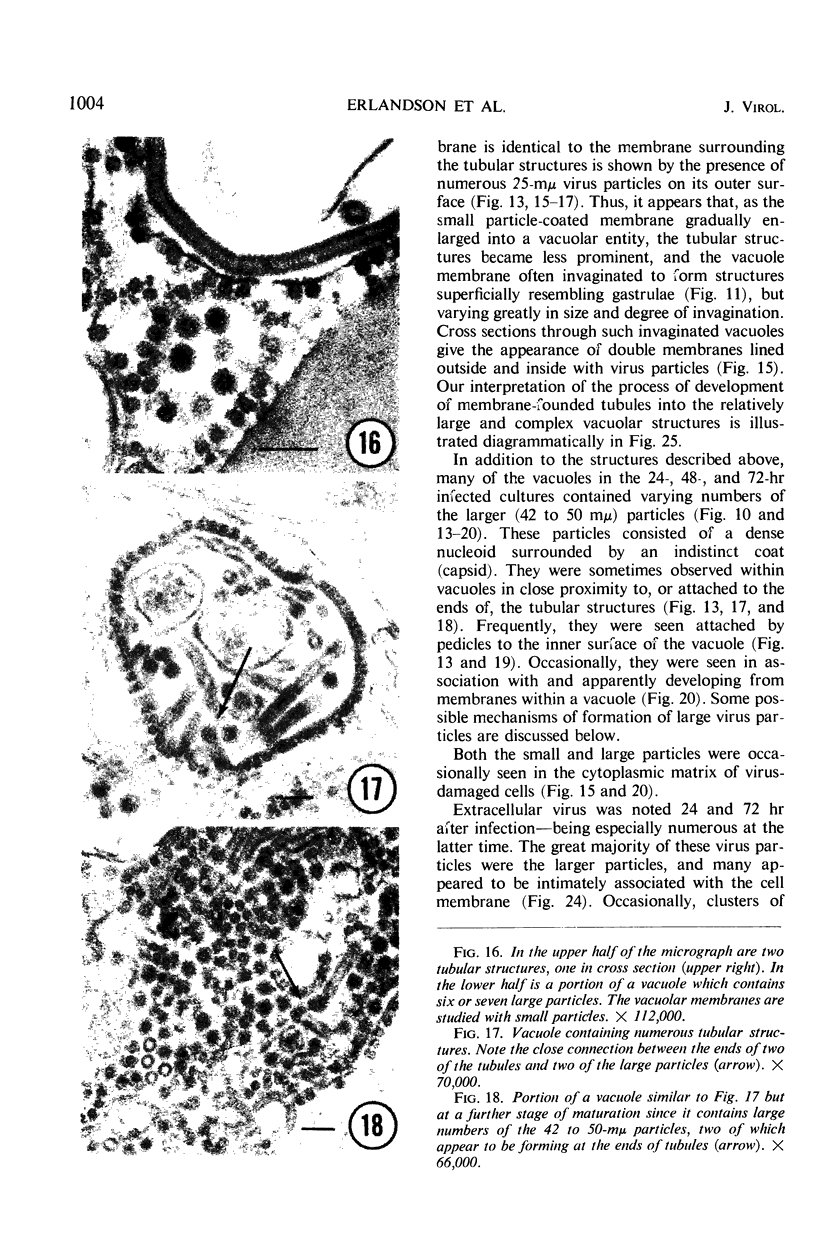
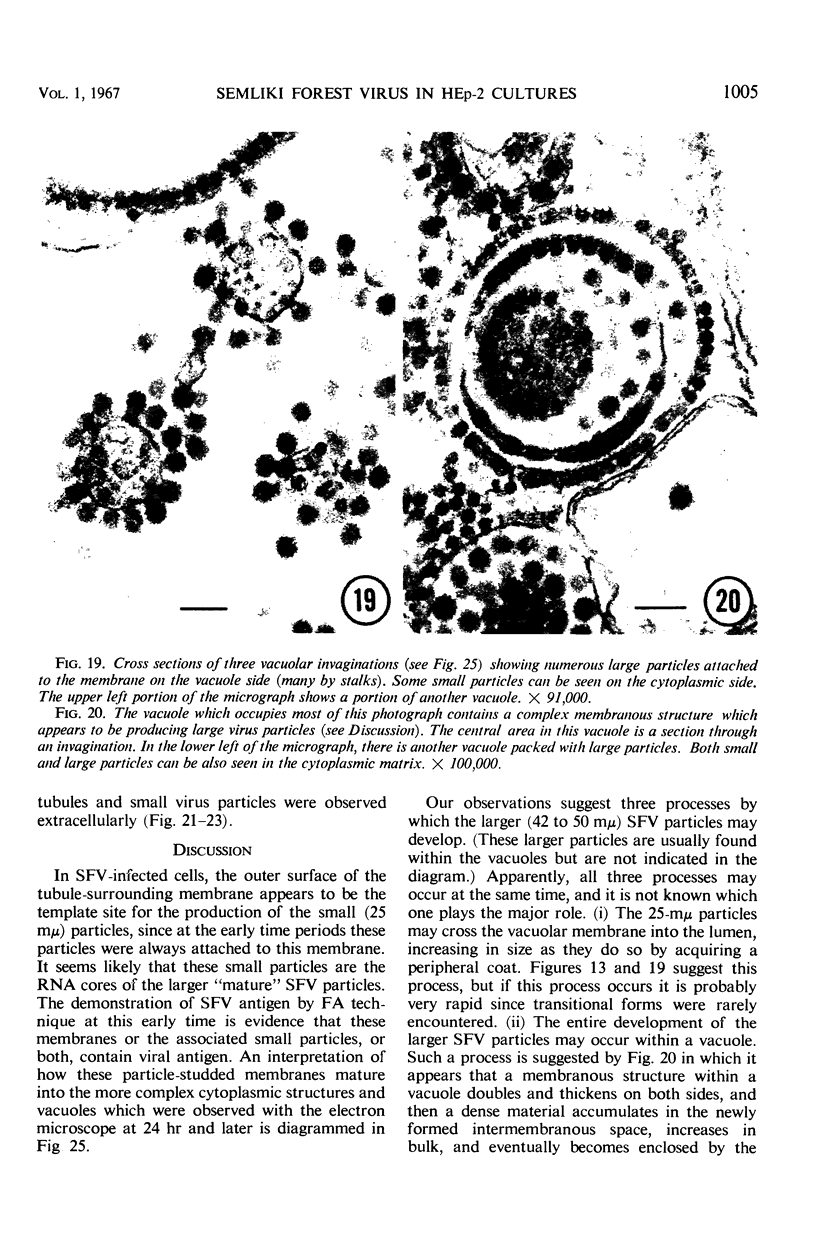
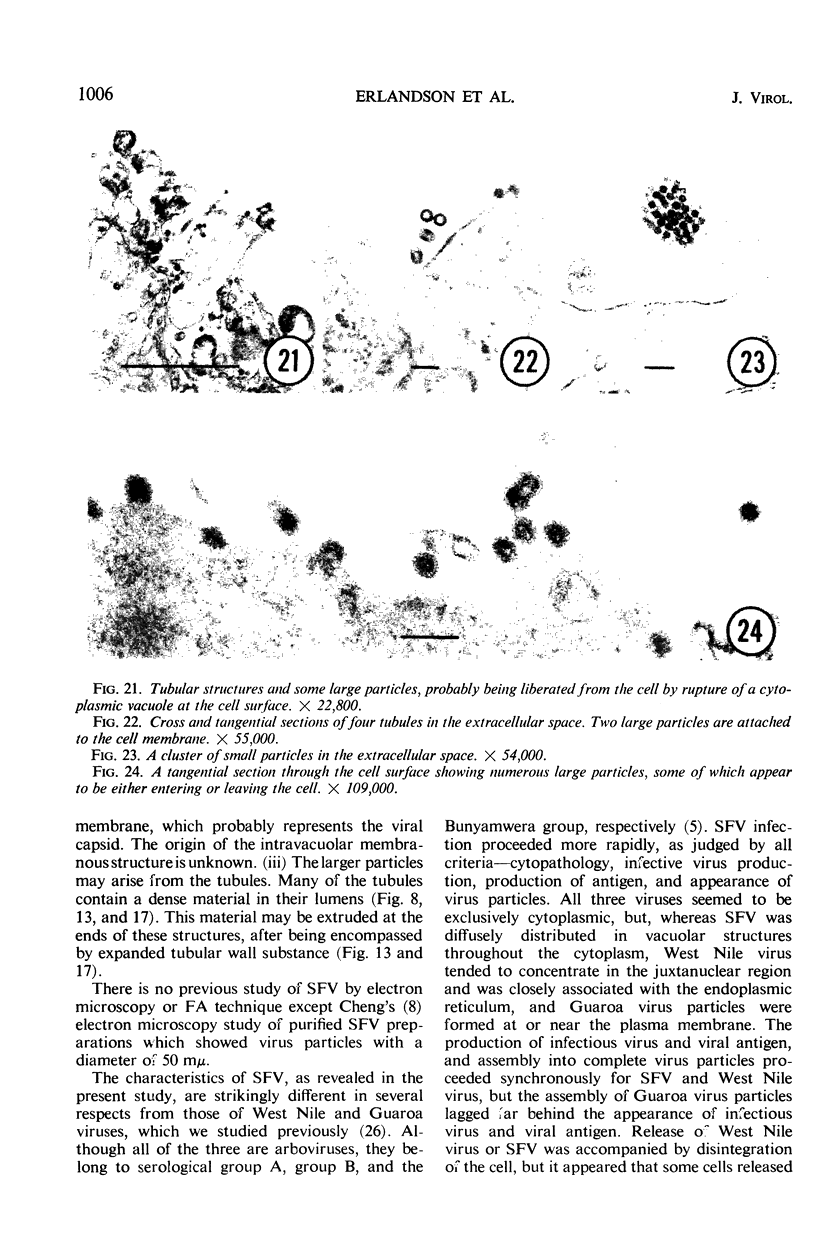
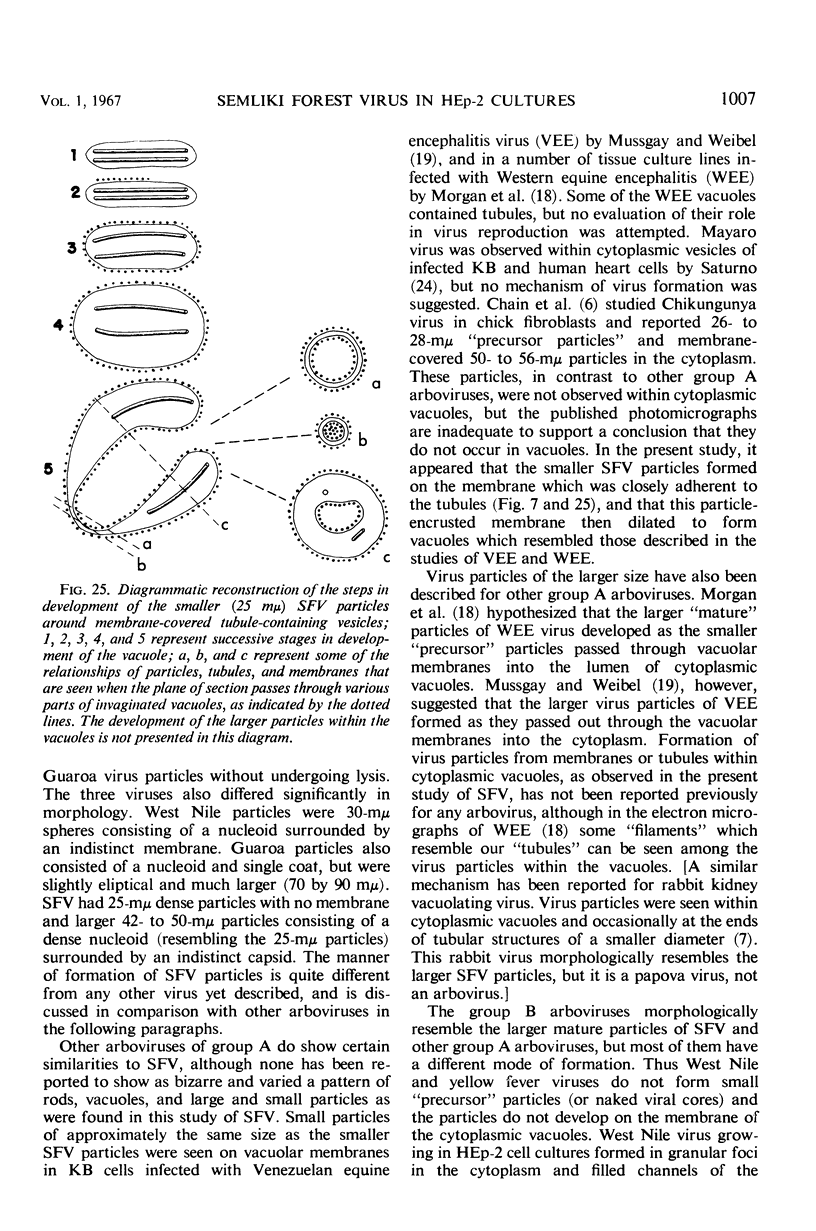
The growth and development of Semliki Forest virus (SFV), an arbovirus of serological group A, in HEp-2 cells in tissue culture was examined by various techniques at frequent intervals. Infectivity and fluorescent-antibody studies demonstrated the presence of infective virus and viral antigens within the cells at 8 hr after infection. The antigen was particulate and distributed throughout the cytoplasm. Thereafter, there was rapid progression of virus production and cell destruction. By electron microscopy, tubular structures bounded by a fine membrane were observed in cytoplasm at 12 hr. Rows of small (25 mμ) virus particles were often present on the outer surface of these membranes, and at later times they became progressively more encrusted with the small virus particles. These structures subsequently increased rapidly in number, size, and complexity, and the space between the membrane and the tubules increased, thus forming vacuoles which contained tubules and were covered with the small particles. At later times (24 hr and later) larger (42 to 50 mμ) particles were observed, usually inside of the vacuoles. These larger particles (and occasionally the smaller ones) were also seen at the cell periphery and in the extracellular space. The large SFV particles appear to form by three distinct processes: (i) from the smaller particles, (ii) by development on an intravacuolar membrane, and (iii) at the ends of the tubules. The mode of development of SFV is unique among viruses studied to date, but in some characteristics it resembles that of other group A arboviruses. Its development differs from that of most arboviruses of group B and other serological groups.
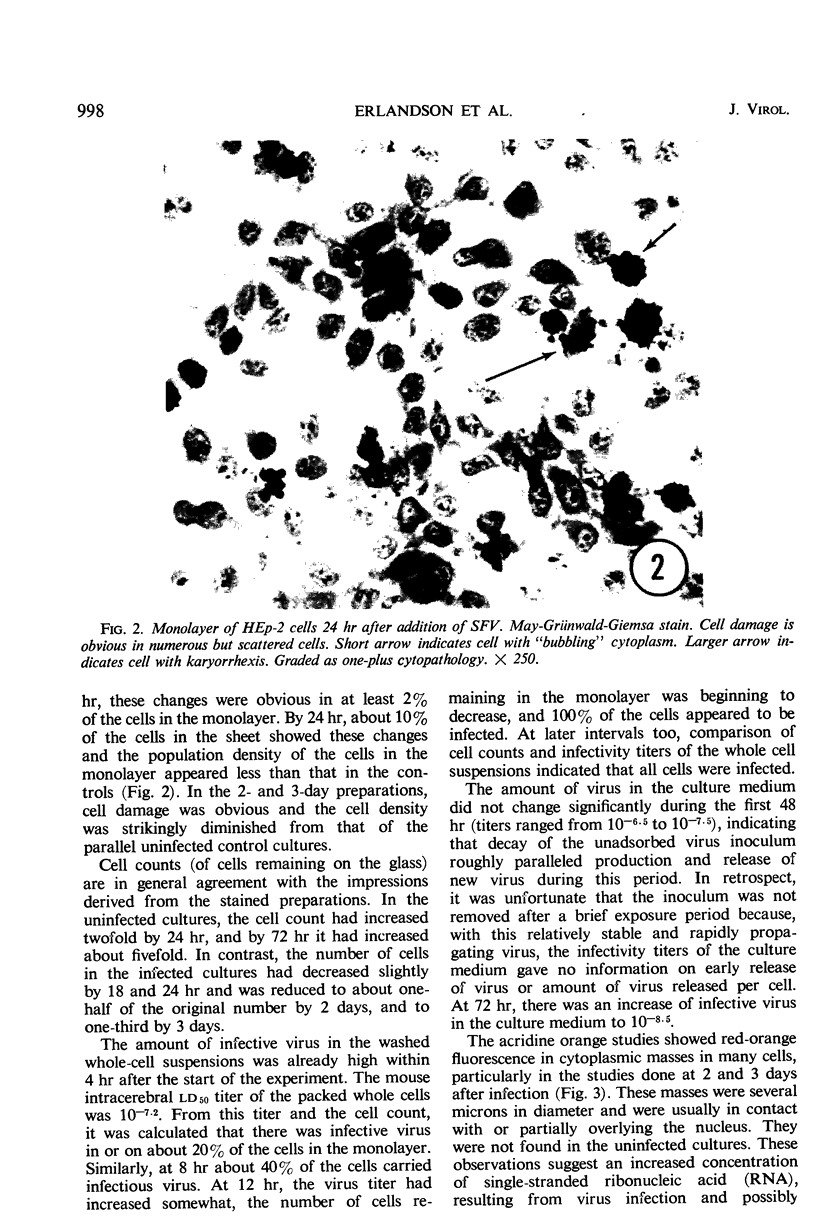
Full text
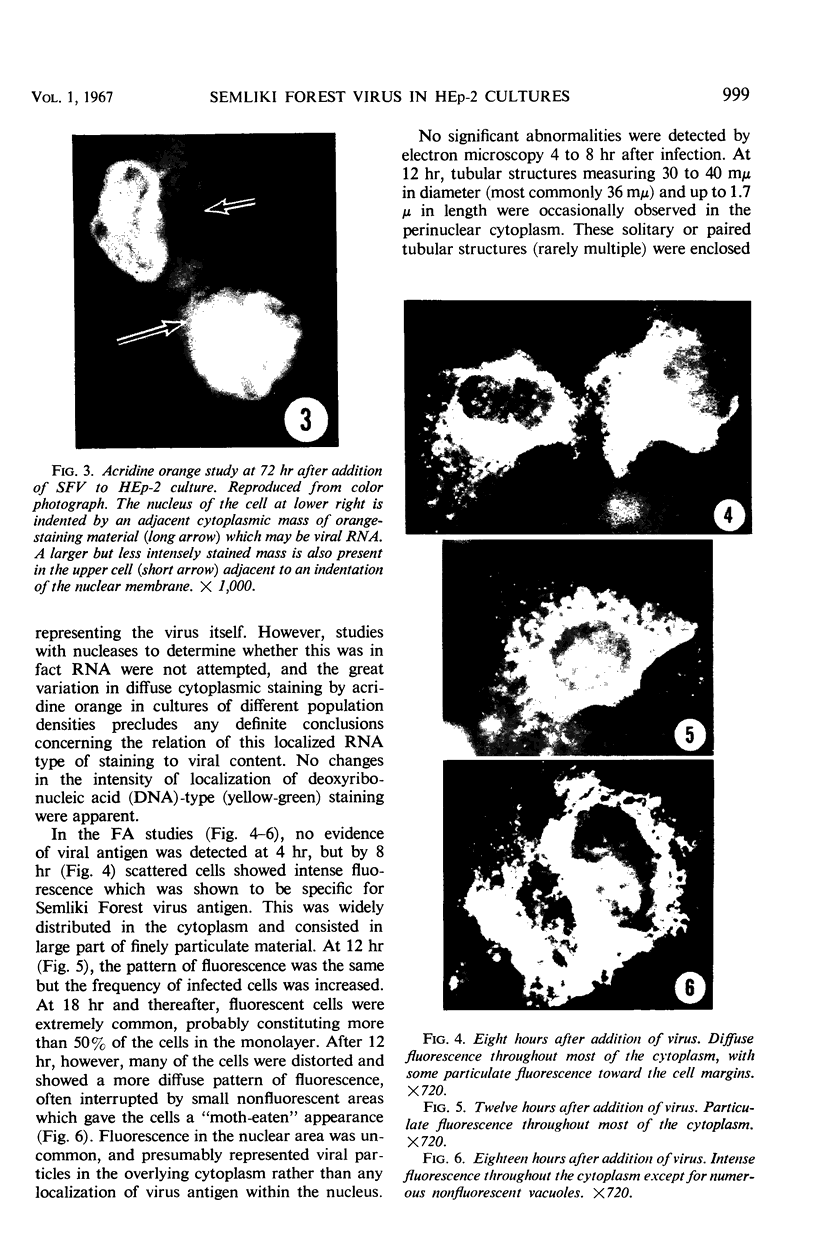
PDF
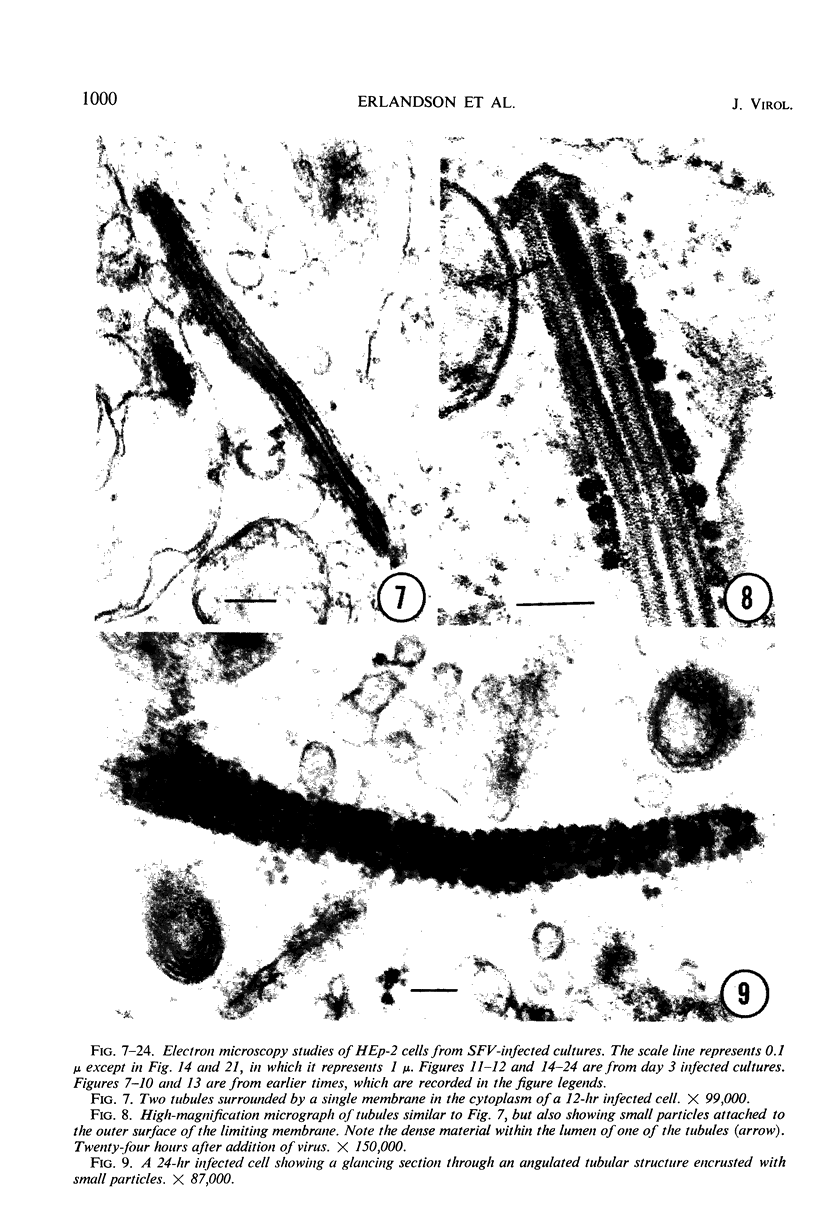


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABDELWAHAB K. S., ALMEIDA J. D., DOANE F. W., MCLEAN D. M. POWASSAN VIRUS: MORPHOLOGY AND CYTOPATHOLOGY. Can Med Assoc J. 1964 May 2;90:1068–1072. [PMC free article] [PubMed] [Google Scholar]
- BARUCH E. ELECTRON MICROSCOPIC STUDY OF SPINAL CORD OF MICE INFECTED WITH YELLOW FEVER VIRUS. J Ultrastruct Res. 1963 Oct;59:209–224. doi: 10.1016/s0022-5320(63)80003-9. [DOI] [PubMed] [Google Scholar]
- BEARCROFT W. G. Electron-microscope studies on the liver cells of yellow-fever-infected rhesus monkeys. J Pathol Bacteriol. 1960 Oct;80:421–426. [PubMed] [Google Scholar]
- BERGOLD G. H., WEIBEL J. Demonstration of yellow fever virus with the electron microscope. Virology. 1962 Aug;17:554–562. doi: 10.1016/0042-6822(62)90155-1. [DOI] [PubMed] [Google Scholar]
- CASALS J. Procedures for identification of arthropod-borne viruses. Bull World Health Organ. 1961;24:723–734. [PMC free article] [PubMed] [Google Scholar]
- CHENG P. Y. Purification, size, and morphology of a mosquitoborne animal virus, Semliki Forest virus. Virology. 1961 May;14:124–131. doi: 10.1016/0042-6822(61)90139-8. [DOI] [PubMed] [Google Scholar]
- COONS A. H. Fluorescent antibody methods. Gen Cytochem Methods. 1958;1:399–422. [PubMed] [Google Scholar]
- Chain M. M., Doane F. W., McLean D. M. Morphological development of Chikungunya virus. Can J Microbiol. 1966 Oct;12(5):895–900. doi: 10.1139/m66-122. [DOI] [PubMed] [Google Scholar]
- Chambers V. C., Hsia S., Ito Y. Rabbit kidney vacuolating virus: ultrastructural studies. Virology. 1966 May;29(1):32–43. doi: 10.1016/0042-6822(66)90193-0. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- ERLANDSON R. A. A NEW MAGAGLAS, D.E.R.(R) 732, EMBEDMENT FOR ELECTRON MICROSCOPY. J Cell Biol. 1964 Sep;22:704–709. doi: 10.1083/jcb.22.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEDLAENDER M., MOORE D. H., KOPROWSKI H. Studies with the electron microscope of virus-host relationships in Ehrlich ascites tumor cells. II. The localization and possible development of anopheles A virus within the endoplasmic reticulum of the host cell. J Exp Med. 1955 Oct 1;102(4):371–378. doi: 10.1084/jem.102.4.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCDEVITT H. O., PETERS J. H., POLLARD L. W., HARTER J. G., COONS A. H. PURIFICATION AND ANALYSIS OF FLUORESCEIN-LABELED ANTISERA BY COLUMN CHROMATOGRAPHY. J Immunol. 1963 Apr;90:634–642. [PubMed] [Google Scholar]
- MOORE A. E., SABACHEWSKY L., TOOLAN H. W. Culture characteristics of four permanent lines of human cancer cells. Cancer Res. 1955 Oct;15(9):598–602. [PubMed] [Google Scholar]
- MORGAN C., HOWE C., ROSE H. M. Structure and development of viruses as observed in the electron microscope. V. Western equine encephalomyelitis virus. J Exp Med. 1961 Jan 1;113:219–234. doi: 10.1084/jem.113.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUSSGAY M., WEIBEL J. Electron microscopic and biological studies on the growth of Venezuelan equine encephalitis virus in KB cells. Virology. 1962 Jan;16:52–62. doi: 10.1016/0042-6822(62)90201-5. [DOI] [PubMed] [Google Scholar]
- McGavran M. H., Easterday B. C. Rift Valley Fever Virus Hepatitis: Light and Electron Microscopic Studies in the Mouse. Am J Pathol. 1963 May;42(5):587–607. doi: 10.21236/ad0410394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIVEN J. S., ARMSTRONG J. A., BALFOUR B. M., KLEMPERER H. G., TYRRELL D. A. Cellular changes accompanying the growth of influenza virus in bovine cell cultures. J Pathol Bacteriol. 1962 Jul;84:1–18. [PubMed] [Google Scholar]
- OTA Z. ELECTRON MICROSCOPE STUDY OF THE DEVELOPMENT OF JAPANESE B ENCEPHALITIS VIRUS IN PORCINE KIDNEY STABLE (PS) CELLS. Virology. 1965 Mar;25:372–378. doi: 10.1016/0042-6822(65)90057-7. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SATURNO A. THE MORPHOLOGY OF MAYARO VIRUS. Virology. 1963 Sep;21:131–133. doi: 10.1016/0042-6822(63)90313-1. [DOI] [PubMed] [Google Scholar]
- SOUTHAM C. M., SHIPKEY F. H., BABCOCK V. I., BAILEY R., ERLANDSON R. A. VIRUS BIOGRAPHIES. I. GROWTH OF WEST NILE AND GUAROA VIRUSES IN TISSUE CULTURE. J Bacteriol. 1964 Jul;88:187–199. doi: 10.1128/jb.88.1.187-199.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON M. L. Staining of tissue sections for electron microscopy with heavy metals. J Biophys Biochem Cytol. 1958 Jul 25;4(4):475–478. doi: 10.1083/jcb.4.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YASUZUMI G., TSUBO I. ANALYSIS OF THE DEVELOPMENT OF JAPANESE B ENCEPHALITIS (JBE) VIRUS. II. ELECTRON MICROSCOPE STUDIES OF NEURONS INFECTED WITH JBE VIRUS. J Ultrastruct Res. 1965 Apr;12:304–316. doi: 10.1016/s0022-5320(65)80101-0. [DOI] [PubMed] [Google Scholar]