Abstract
Acute rejection (AR) is a common complication following lung transplantation and is an established risk factor for bronchiolitis obliterans syndrome (BOS). AR clinical presentation varies considerably and is sometimes associated with an acute decrease in forced expiratory volume in one second (FEV1). We hypothesized that lung transplant recipients who experience such spirometrically significant AR (SSAR), as defined by a ≥10% decline in FEV1 relative to the prior pulmonary function test, are subsequently at increased risk for BOS and worse overall survival. In a large single center cohort (n = 339) SSAR occurred in 79 subjects (23%) and significantly increased the risk for BOS (p < 0.0001, HR = 3.2, 95% CI 2.3 – 4.6) and death (p = 0.0001, HR = 2.3, 95% CI 1.5 – 3.5). These effects persisted after multivariate adjustment for pre-BOS AR and lymphocytic bronchiolitis burden. An analysis of the subset of patients who experienced severe SSAR (≥20% decline in FEV1) resulted in even greater hazards for BOS and death. Thus we demonstrate a novel physiological measure that allows discrimination of patients at increased risk for worse posttransplant outcomes. Further studies are needed to determine mechanisms of airflow impairment and whether aggressive clinical interventions could improve post-SSAR outcomes.
Keywords: bronchiolitis obliterans syndrome, acute rejection, lung transplantation, survival
Introduction
Lung transplantation has emerged as a viable therapy for patients with advanced lung disease with nearly 3,000 lung transplant procedures performed annually (1). However, despite the current scale and steady growth over the last twenty years, long-term outcomes remain disappointing with posttransplant five-year survival of approximately fifty percent (1). Bronchiolitis obliterans syndrome (BOS), a condition of progressive airflow obstruction associated with small airway fibrosis, is the chief limitation to successful long-term outcomes and represents the leading cause of death after the first year posttransplant (2, 3).
Although numerous risk factors for BOS have been identified, prior acute rejection (AR) is most consistently associated with BOS development (2–6). A definitive diagnosis of AR is based upon the presence of mononuclear perivascular cell infiltrates on histological examination of lung tissue, also referred to as A grade rejection. The severity of A grade rejection is assigned depending upon the extent of inflammation (7). Clinical presentation is extremely variable with many AR episodes diagnosed in clinically stable patients undergoing surveillance transbronchial biopsies (8–12). When clinically manifest, AR can present with a range of nonspecific signs and symptoms including dyspnea, cough, wheezing, fever, radiographic changes, pleural effusion, and/or a drop in lung function (12).
Despite the variable clinical presentation, previous studies of AR have focused on the histopathologic grade, frequency, or timing as a risk factors for BOS (2, 4–6, 13–18). As a result, the impact of clinically manifest AR, as opposed to clinically silent AR, has not been characterized with regard to BOS and other long-term outcomes. We hypothesized that clinically manifest AR associated with an acute decline in lung function represents a more aggressive formof AR that may portend a worse long-term prognosis. Therefore, in our current analysis, we defined spirometrically significant AR (SSAR) as an AR event concurrent with a ≥10% drop in forced expiratory volume in one second (FEV1) from baseline. We then applied this objective definition to a large single center cohort of lung transplant recipients to determine if SSAR at any time prior to BOS or SSAR during the first year posttransplant predicts an increased risk for BOS or a decrease in survival.
Materials and Methods
Patient population and study design
The eligible cohort is drawn from all consecutive adult, first, single or bilateral lung transplants performed at Duke University Medical Center between January 1, 1998, and October 1, 2008 (n = 560). As SSAR evaluation is dependent upon AR episodes with both concurrent and prior baseline pulmonary function test (PFT) measurements, 129 subjects were excluded for a lack of biopsy or PFT data (generally patients who died early posttransplant or were never discharged from the transplant hospitalization). An additional 92 subjects never experienced AR prior to BOS and thus were not eligible for SSAR. The remaining 339 subjects comprised the final study cohort and were distributed between the SSAR and non-SSAR analysis groups. Patient flow through the study is summarized in Figure 1. This study was approved under Duke IRB protocol number 00029129.
Figure 1.
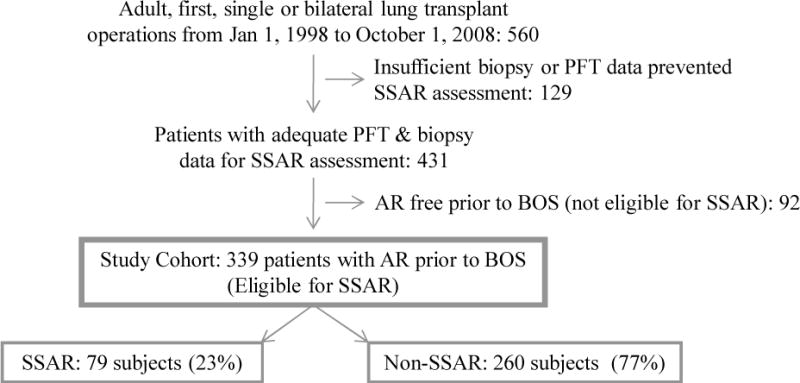
Patient flow through the study. The cohort is comprised of 339 (79 SSAR and 260 non-SSAR) subjects who underwent sufficient biopsy and PFT procedures and experienced at least one AR event prior to BOS; AR, acute rejection; BOS, bronchiolitis obliterans syndrome; PFT, pulmonary function test; SSAR, spirometrically significant acute rejection.
Clinical management
Patients were consistently managed according to our previously published clinical protocols (19). Briefly, all patients received induction immunosuppression including a CD25 antagonist followed by triple maintenance immunosuppression with cyclosporine (prior to 4/1/2002) or tacrolimus (after 4/1/2002), azathioprine, and corticosteroids. Ganciclovir cytomegalovirus (CMV) prophylaxis was given based on donor and recipient CMV antibody serology status, and CMV immunoglobulin preparations were not used. Gastroesophageal refluxwas evaluated prior to transplant using 24 hour pH probe and was treated with posttransplant fundoplication when indicated. Transbronchial biopsies were performed at 1, 3, 6, 9 and 12 months posttransplant, annually thereafter, or at any point as clinically indicated. Posttransplant PFTs were performed weekly within the first six weeks posttransplant and then quarterly thereafter. All A (≥ A1) and B (≥ B1) grade rejection was first treated with methylprednisone followed by a prednisone taper. Recurrent or refractory rejection was treated with anti-thymocyte globulin or alemtuzumab.
AR, LB, and BOS Criteria
All transbronchial biopsies were prospectively graded for AR and LB by a single experienced lung transplant pathologist. AR and LB were defined respectively as perivascular or peribronchial mononuclear inflammation according to the established International Society for Heart and Lung Transplantation (ISHLT) criteria (20). Histologic appraisal of AR and LB was conducted in accordance with accepted ISHLT standards in terms of the minimum number of biopsy samples and exclusion of opportunistic infection. Specimens failing to meet minimum standards were given grades of Ax and Bx as appropriate (20). The percent of graded biopsies relative to the total number of biopsies performed was calculated separately for A and B grade rejection. All AR ≥ A1 and LB ≥ B1 was considered in the analysis. In accordance with previously published studies, AR and LB scores were calculated through summation of the numerical histopathology grades (e.g. A2+A1=AR score of 3) (13, 16, 21, 22). BOS was calculated according to ISHLT guidelines as a persistent drop in FEV1 relative to the average of the two highest previous FEV1 values measured at least three weeks apart (4). For analysis purposes, BOS was defined as grades ≥ 1.
Definition of SSAR vs. non-SSAR episodes
As shown in supplemental figure S1, each episode of pre-BOS biopsy proven AR was categorized as either SSAR, non-SSAR, or unclassified. LB wasnot used to define SSAR. The presence of SSAR was assessed as follows: first, each biopsy proven AR episode was paired with an FEV1 measurement taken between 0 and 7 days prior to the biopsy, thus creating an AR-FEV1 pair. If more than one FEV1 was measured within this window for a given AR episode, the closest preceding FEV1 was used. Second, a prior baseline FEV1 was established for each AR-FEV1 pair such that the baseline FEV1 occurred ≥7 days prior to the paired FEV1 and was not itself paired with an AR episode. Third, AR-FEV1 pairs were compared to prior baseline FEV1 values (AR-FEV1/baseline FEV1), and all AR-FEV1 pairs exhibiting a ≥ 10% decline from baseline were tentatively categorized as SSAR episodes. Finally, each SSAR episode was evaluated for concurrent infection, clinically significant airway stenosis, and pleural effusion by medical chart review. All SSAR episodes with concurrent infection or clinically significant airway stenosis were excluded and labeled as unclassified. Episodes with concurrent pleural effusion were still classified as SSAR, but as described in the results, analyses were performed with and without these episodes to ensure that the presence of effusion did not confound our findings. All other AR-FEV1 pairs exhibiting a <10% decline from baseline were classified as non-SSAR episodes. Remaining AR episodes for which concurrent and baseline FEV1s could not be established were excluded and labeled as unclassified. In some cases, these unclassified episodes occurred in subjects who developed clinical signs or symptoms and proceeded directly to biopsy without undergoing formal PFTs in the clinic. In other cases they were diagnosed very early posttransplant in an inpatient setting, and outpatient PFTs were simply not performed. Subjects who experienced ≥ 1 SSAR episode were placed into the SSAR analysis group; those who experienced no SSAR episodes and ≥ 1 non-SSAR episodes were placed into the non-SSAR analysis group, and remaining subjects who exclusively experienced unclassified AR episodes were not included in the analysis.
Statistical analysis
Mean, standard deviation, median, and interquartile range were used to describe cohort demographics and clinical characteristics as appropriate. Clinical data was included from time of transplant until the general study censor date of October 11, 2010. Continuous variables were analyzed using the Wilcoxon rank sum test and binary variables were analyzed with chi-square tests. In the primary analysis of SSAR at any time prior to BOS, estimation of the impact of SSAR (time to first SSAR episode prior to BOS considered as a continuous, time-dependent covariate) on time to BOS and time to death among all eligible subjects was performed with Cox regression models. Predictor variables including AR count, AR score, LB count, and LB score were treated as time varying covariates within the primary analysis period (any time prior to BOS). In the sub-analysis of SSAR in the first year posttransplant, SSAR was considered as a binary covariate in the first year of transplant, and Kaplan-Meier models in addition to Cox regression models were used to estimate the impact of SSAR on time to BOS and death. Predictor variables in this one year sub-analysis were limited to the first posttransplant year, and the impact of each predictor was only assessed on outcomes occurring after one year posttransplant. Predictor variables were thus fixed in time for the purposes of the one year sub-analysis. Time to BOS was defined as the time in days from transplant until BOS onset (grade ≥1), and BOS free subjects were censored on the date of their last PFT measurement at our center. The survival analysis was conducted using time from transplant to either retransplant or death, and surviving subjects were censored on the date of their last PFT measurement at our center. Lost to follow-up subjects who subsequently died were also censored on the date of their last PFT measurement at our center to avoid confounding events that may have occurred in the time after their last follow-up at our center. Known predictors of BOS and death that differed significantly between the SSAR and non-SSAR groupswere included in bivariate and multivariate Cox models. All analyses were performed with SAS statistical software version 9.2 (Cary, NC).
Results
Characteristics of the study cohort
As shown in Figure 1, the primary analysis study cohort consisted of 339 subjects, including 79 that experienced ≥ 1 episode of pre-BOS SSAR (SSAR group) and 260 that experienced ≥ 1 pre-BOS non-SSAR episodes and no pre-BOS SSAR episodes (non-SSAR group). Among the 79 subjects in the SSAR analysis group, 68 experienced one SSAR episode, 10 experienced two SSAR episodes, and 1 experienced three SSAR episodes. The 260 subjects in the non-SSAR analysis group experienced a total of 545 non-SSAR episodes.
The 339 subjects in the study cohort had a median posttransplant follow-up of 4.5 years. Clinical characteristics of the SSAR and non-SSAR groups (Table 1) were generally similar, including gender, age at transplant, race, native disease, transplant type, transplant era, CMV pneumonitis incidence, and PFT frequency. The SSAR group experienced a higher number of pre-BOS AR episodes (median of 4 vs. 2) and had higher cumulative pre-BOS AR scores (median of 6 vs. 4) as compared to the non-SSAR group. Differences between SSAR and nonSSAR pre-BOS LB scores (mean of 1.55 and 0.82 for SSAR and non-SSAR groups respectively) and episode counts (mean of 0.96 and 0.50 for SSAR and non-SSAR groups respectively) were nearly significant although the group medians did not differ substantially. The SSAR group also underwent a higher number of pre-BOS biopsies (median of 13 vs. 10). Rates of graded vascular tissue (A grade) biopsies were similar between the SSAR (median: 100%, IQR: 95 – 100) and non-SSAR (median: 100%, IQR: 93 – 100) analysis groups (p = 0.53). Likewise rates of graded bronchial tissue (B grade) biopsies were similar between the SSAR (median: 62% graded, IQR: 50 – 73) and non-SSAR (median: 64% graded, IQR: 47 – 76) analysis groups (p = 0.82).
Table 1.
Clinical characteristics of the non-SSAR and SSAR analysis groups
| Non-SSAR Group, N=260 | SSAR Group, N=79 | p-value | |
|---|---|---|---|
|
| |||
| Female Gender† | 42% (109) | 47% (37) | 0.44 |
|
| |||
| Age at Transplant‡ | 56 (43 – 62) | 55 (39 – 62) | 0.62 |
|
| |||
| Race† | |||
| Caucasian | 91% (236) | 91% (72) | 0.92 |
| African American | 9% (22) | 8% (6) | 0.81 |
|
| |||
| Native Disease† | |||
| Obstructive | 43% (112) | 43% (34) | 0.99 |
| Restrictive | 34% (89) | 35% (28) | 0.84 |
| Cystic | 21% (54) | 19% (15) | 0.73 |
| Vascular | 2% (5) | 3% (2) | 0.74 |
|
| |||
| Bilateral Transplant† | 92% (239) | 91% (72) | 0.82 |
|
| |||
| Modern Transplant Era† ¥ | 70% (182) | 71% (56) | 0.88 |
|
| |||
| Pre-BOS CMVP Count‡ | 0 (0 – 1) | 0 (0 – 1) | 0.70 |
|
| |||
| Pre-BOS AR Episode Count‡ | 2 (1 – 4) | 4 (2 – 6) | <0.0001 |
|
| |||
| Pre-BOS AR Score‡ | 4 (2 – 6) | 6 (3 – 9) | 0.0001 |
|
| |||
| Pre-BOS LB Episode Count‡ | 0 (0 – 1) | 0 (0 – 1) | 0.053 |
|
| |||
| Pre-BOS LB Score‡ | 0 (0 – 1) | 0 (0 – 2) | 0.092 |
|
| |||
| Pre-BOS PFTs‡ | 25 (18 – 33) | 26 (20 – 36) | 0.24 |
|
| |||
| Pre-BOS Biopsies‡ | 10 (7 – 14) | 13 (8 – 17) | 0.0054 |
Percent (Number),
Median (IQ Range),
Transplanted after March 2002; AR, acute rejection (A grade); CMVP, cytomegalovirus pneumonitis; LB, lymphocytic bronchiolitis (B grade); PFT, pulmonary function test.
Timing of SSAR and non-SSAR episodes
As shown in Figure 2, SSAR episodes generally occurred substantially later posttransplant (median: 639 days, IQR: 71 – 1275) in comparison to the non-SSAR episodes (median: 361 days, IQR: 90 – 828). However, both SSAR and nonSSAR episode counts peaked in the first year posttransplant (33 and 356 episodes respectively) and in general followed an exponential distribution with increasingly fewer episodes occurring in successive years.
Figure 2.
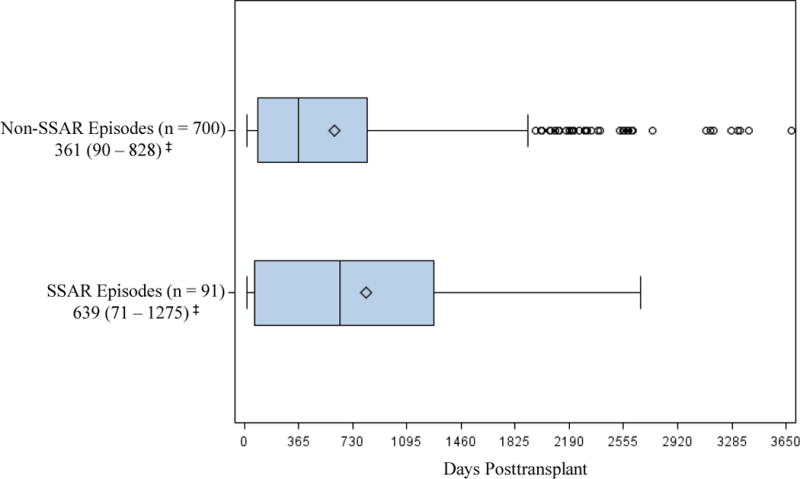
Timing of SSAR and non-SSAR episodes after transplantation (box-and-whisker plot). SSAR episodes occurred an average of 818 (median: 639, IQR: 71 – 1275) days posttransplant while non-SSAR episodes occurred an average of 606 (median: 361, IQR: 90 –828) days posttransplant; max upper whisker = upper quartile + (1.5 * IQR); ‡Median (IQR); ◊ Mean; ○ value > upper whisker; SSAR, spirometrically significant acute rejection.
Impact of SSAR on time to BOS
Increasing time to first pre-BOS SSAR episode significantly increased the risk for BOS (p < 0.0001, HR = 3.2, 95% CI = 2.3 – 4.6). The effect of SSAR on increased risk for BOS persisted after bivariate adjustment in independent models for pre-BOS AR score, pre-BOS AR episode count, pre-BOS LB score, and pre-BOS LB episode count (data not shown). Likewise in multivariate analyses of SSAR adjusted for AR count and LB count as well as SSAR adjusted for AR score and LB score, the effect of SSAR on increasing risk for BOS remained significant (Table 2).
Table 2.
Impact on time to BOS, univariate and multivariate analyses.
| Parameter | HR | 95% CI | p-value | |
|---|---|---|---|---|
| SSAR | 3.2 | 2.3 | 4.6 | <0.0001 |
| SSAR adjusted for AR Count† and LB Count† | 2.7 | 1.8 | 4.0 | <0.0001 |
| SSAR adjusted for AR Score† and LB Score† | 2.7 | 1.8 | 4.0 | <0.0001 |
Pre-BOS time varying covariate; AR, acute rejection (A grade); BOS, bronchiolitis obliterans syndrome; LB, lymphocytic bronchiolitis (B grade); SSAR, spirometrically significant acute rejection (above parameter refers to days from transplant to first pre-BOS SSAR episode)
Impact of SSAR on posttransplant survival
Increasing time to first pre-BOS SSAR episode significantly increased the risk for death (p = 0.0001, HR = 2.3, 95% CI 1.5 – 3.5). The effect of SSAR on increased risk for death persisted after bivariate adjustment in independent models for pre-BOS AR score, pre-BOS AR episode count, pre-BOS LB score, and pre-BOS LB episode count (data not shown). Likewise in multivariate analyses of SSAR adjusted for AR count and LB count as well as SSAR adjusted for AR score and LB score, the effect of SSAR on increasing risk for death remained significant (Table 3).
Table 3.
Impact on time to death, univariate and multivariate analyses.
| Parameter | HR | 95% CI | p-value | |
|---|---|---|---|---|
| SSAR | 2.3 | 1.5 | 3.5 | 0.0001 |
| SSAR adjusted for AR Count† and LB Count† | 2.3 | 1.5 | 3.7 | 0.0003 |
| SSAR adjusted for AR Score† and LB Score† | 2.3 | 1.5 | 3.6 | 0.0003 |
Pre-BOS time varying covariate; AR, acute rejection (A grade); BOS, bronchiolitis obliterans syndrome; LB, lymphocytic bronchiolitis (B grade); SSAR, spirometrically significant acute rejection (above parameter refers to days from transplant to first pre-BOS SSAR episode)
Post-SSAR FEV1 Trajectory
The majority (78%, 71/91) of SSAR episodes were followed by either an increasing or stable FEV1 trajectory prior to the final FEV1 decline representing BOS onset. In the remaining episodes (n = 20), progression to BOS generally occurred over the next several months with a continued decline in FEV1. When the subjects (n=17) who experienced these 20 SSAR episodes were removed from the analysis, SSAR remained a significant predictor of BOS (p = 0.0021, HR = 1.9, 95% CI 1.3 – 3.0) and death (p = 0.0033, HR = 2.0, 95% CI 1.3 – 3.2). The median time from SSAR occurrence to BOS onset was 187 days (IQR: 50 – 624).
Potential confounding of SSAR by BOS 0-p
Because our primary analysis considered SSAR episodes that occurred prior to the onset of BOS (grade 1 or higher), the possibility exists that SSAR could simply represent BOS 0-p with concurrent AR. In order to address this issue we first determined that 79% (72/91) of SSAR episodes occurred in patients that were free of BOS 0-p both before and after the acute SSAR event. In order to ensure that the presence of those infrequent SSAR episodes that overlap with BOS 0-p did not drive the observed relationship between SSAR and BOS or death, we performed a sub-analysis in which only SSAR episodes occurring prior to BOS 0-p onset were considered. This analysis produced results similar to the primary analysis, and in both univariate and multivariate analysis SSAR remained a predictor of BOS and death independent of AR and LB count or AR and LB score (Tables S1 and S2).
Potential confounding effects of undiagnosed LB
In our study, as in all lung transplant studies that consider bronchial grade rejection, an inherent limitation is the large percentage of biopsy samples that included Bx results (ungraded due to absent bronchial tissue). In order to address the possibility that undiagnosed LB was driving the drop in lung function and the subsequent poor outcomes that we were associating with SSAR, we performed an additional sub-analysis in which we considered only biopsy results that included a defined B grade (B0 – 4). In this analysis, all Bx biopsies were excluded. This sub-analysis produced 53 SSAR patients (60 SSAR episodes) and 197 non-SSAR patients (423 non-SSAR episodes). Importantly, in univariate and multivariate analysis using this subset, SSAR remained a significant predictor of BOS and death independent of A and B grade rejection (Tables S3 and S4).
Confounding effects of pleural effusion
Although in most cases SSAR was not associated with radiographic changes, 14% (13/91) of SSAR episodes were accompanied by a new or increasing pleural effusion that could have contributed to the decrease in FEV1. We therefore performed an analysis in which we excluded all 13 SSAR episodes associated with pleural effusions. Consistent with our previous results, even after exclusion of these episodes, SSAR significantly increased the risk for BOS (p < 0.0001, HR = 3.4, 95% CI = 2.3 – 4.8) and death (p = 0.0011, HR = 2.1, 95% CI 1.4 – 3.4).
Dose response relationship between severe SSAR and worse clinical outcomes
In order to better understand the relationship between SSAR and both BOS and death, we sought to determine if a dose response relationship might exist, such that more severe SSAR further increased the risk for BOS and death. To test this hypothesis, we increased the threshold to identify SSAR and defined a severe SSAR episode as having an FEV1 decline of ≥ 20%. In this analysis, subjects who experienced at least one pre-BOS severe SSAR episode (28/339, 8%) had an increased hazard for BOS (p < 0.0001, HR = 5.2, 95% CI = 3.1 – 8.7) and death (p < 0.0001, HR = 3.3, 95% CI = 1.9 – 5.6) in comparison to the non-SSAR subjects (311/339, 92%). Consistent with a dose response relationship, the hazard imposed by severe SSAR (FEV1 decline of ≥ 20%) was greater in magnitude across all outcomes than the effect observed under the original SSAR definition (FEV1 decline of ≥ 10%).
Impact of SSAR in the first year posttransplant
Given the high rate of AR during the first year posttransplant, we sought to both confirm our primary findings and determine if SSAR during the first year posttransplant offered a method of early identification for subjects at increased risk for BOS and death. A sub-analysis was thus performed to examine the effects of SSAR in the first year alone. By nature of the reduced time frame, this sub-analysis was limited to a study cohort of 254 subjects including 32 that experienced ≥ 1 episode of SSAR (SSAR group) and 222 that experienced ≥ 1 non-SSAR episodes but no SSAR episodes (non-SSAR group). Consistent with our primary analysis, this sub-analysis demonstrated that SSAR within the first year posttransplant significantly increased the risk for BOS after the first year (p = 0.020, HR = 1.9, 95% CI = 1.1 – 3.2). Two, four, and six year Kaplan Meier BOS incidence estimates among the SSAR and non-SSAR analysis groups were 32%, 50%, and 63% vs. 11%, 33%, and 51% respectively. Likewise SSAR occurring within the first year posttransplant significantly increased the risk for death after the first year (p < 0.0001, HR = 3.4, 95% CI 2.0 – 5.6). Two, four, and six year Kaplan Meier death incidence estimates among the SSAR and non-SSAR analysis groups were 22%, 51%, and 77% vs. 6%, 19%, and 35% respectively. All effects persisted after bivariate adjustment for known BOS risk factors including year one AR count, year one AR score, year one LB count, and year one LB score. The effects of pleural effusion and dose response upon SSAR in the first year posttransplant were consistent with the results of our primary analysis (data not shown).
Discussion
BOS remains the leading cause of death beyond one year posttransplant, affecting nearly half of recipients at five years posttransplant and 75% at ten years posttransplant (1). Previous studies have suggested that histological factors such as the cumulative frequency, severity, or timing of biopsy proven A or B grade rejection are the strongest risk factors for subsequent BOS onset (2, 4–6, 13–18). However to our knowledge, no previous studies have systematically considered the prognostic importance of any clinical signs or symptoms accompanying AR episodes on long-term outcomes such as BOS or survival. In this analysis, we sought to formally evaluate the prognostic importance of a decline in FEV1 associated with an AR episode. We demonstrate for the first time that the presence of one or more SSAR episodes prior to BOS is associated with a significantly increased risk for BOS as well as a reduction in overall survival.
We chose to define SSAR based upon an acute drop in lung function from baseline in order to apply an objective, highly reproducible definition. Ten percent FEV1 decline was a logical benchmark for SSAR as previous studies have used a 10% decline in FEV1 to describe clinical indication for bronchoscopy (8–11). Furthermore, we chose to apply this benchmark using the immediate prior PFT as a baseline measurement in order to provide the most meaningful estimate of an acute FEV1 decline at the time of AR. Although we considered using the best ever prior FEV1 as a baseline (similar to the approach used to assign BOS grade), we noted that in contrast to our analysis, BOS seeks to measure a sustained drop from a stable FEV1 trajectory. Based on the variation in lung function, especially over the first year posttransplant, we reasoned that a recent prior PFT provided a more accurate representation of the acute FEV1 changes on which our study is focused.
When critically evaluating our results, we first considered the possibility that SSAR was simply a marker for patients with both AR and BOS 0-p; however we found that concurrent BOS 0-p and SSAR was actually infrequent among SSAR patients. When we performed a subanalysis in which SSAR events occurring after BOS 0-p onset were excluded, the results were similar to those of our primary analysis, suggesting that the relationship between SSAR and BOS or death is not mediated by BOS 0-p. We next considered the relationship between SSAR and LB; namely because transbronchial biopsy poorly samples bronchial tissue, we understood that ungraded B grade rejection may have confounded our results. In order to address this concern, we performed a sub-analysis that included only biopsy results with evaluable B grade tissue. Reassuringly this sub-analysis, being limited to biopsies with known B grades (B0 – 4), produced results similar to our primary findings and demonstrated that the effect of SSAR on BOS or death is not occurring as a result of undiagnosed LB.
Given our retrospective definition of SSAR, we recognize that there are several limitations to our approach. First, we did not consider any prognostic significance of other clinical signs (e.g. radiographic changes) or symptoms (e.g. increased dyspnea) because such definitions were likely to be biased when applied retrospectively. Second, because our method strictly limited SSAR to AR episodes with both a concurrent and prior baseline FEV1, we were not able to classify all AR episodes, and as such, some subjects who potentially experienced SSAR could have been misclassified. Finally, our study was drawn from a cohort that underwen both clinically indicated and regular surveillance bronchoscopies; thus treatment for clinically silent AR following surveillance bronchoscopy may have preempted SSAR development. Presumably SSAR rates would differ at a center where patients only undergo clinically indicate bronchoscopy. However, the practice of surveillance bronchoscopy is quite common and is performed at nearly 70% of transplant centers, making our results broadly relevant to most clinical transplant programs (23).
Despite these limitations, our results are unique in demonstrating that a physiological parameter concurrent with AR offers important prognostic value. In our primary analysis, we demonstrate that SSAR, objectively defined as an AR episode with concurrent drop in FEV1 from prior baseline, at any time prior to BOS poses an increased risk for BOS and death independent of other major BOS risk factors including the cumulative frequency or severity of histological AR or LB. Our sub-analysis of SSAR in the first year posttransplant supports our primary findings and offers a method of early identification for at risk patients. Our results are not surprising given that clinical outcomes are quite variable among patients with AR, and that not all patients with AR develop BOS nor do all BOS patients have a significant history of prior AR (2). Although to our knowledge no previous studies have considered the significance of SSAR as performed in this analysis, at least one prior study suggested that early physiological responses to methacholine challenge after transplant could predict the onset of BOS, consistent with the idea that physiological differences in response to AR, as shown in this study, could impact long-term prognosis (24).
The mechanism underlying our results remains uncertain. The relationship between AR and BOS, while not fully understood, is generally thought to involve a process of epithelial injury leading to airway fibrosis (2). Importantly our results suggest that, independent of the cumulative frequency or severity of AR and LB, clinical changes in FEV1 reflect important prognostic information about the underlying biology of the rejection process. We therefore postulate that SSAR is a marker for those patients with greater upregulation of inflammatory signals or profibrotic growth factors in response to AR (rather than simply a marker of the presence of LB), an idea that warrants further testing in future prospective studies.
In summary, we developed and applied a novel, reproducible definition of SSAR and demonstrated that the occurrence of even a single episode of SSAR is associated with a significantly increased risk for BOS and worse posttransplant survival independent of other clinical risk factors such as AR and LB frequency or cumulative severity. We also conducted aseries of sub-analyses that demonstrate that the effects of SSAR are not simply due to concurrent BOS 0-p, undiagnosed B grade rejection, or pleural effusion. Furthermore, our results became even more striking when a more stringent FEV1 definition of SSAR was applied, demonstrating that more severe SSAR is associated with an even higher risk for BOS and early death. Given its poor prognosis, SSAR represents an important opportunity to identify patients for more aggressive clinical interventions and to potentially alter the natural history of BOS. In particular, our analysis of SSAR within the first year posttransplant offers a method of early identification for at risk patients and provides a greater opportunity to improve the associated poor prognosis with aggressive treatment. Given the lack of mechanistic understanding of BOS, SSAR also represents a key opportunity for additional translational research to better understand the biological and immunological processes by which AR leads to BOS.
Supplementary Material
Table S1. Impact on time to BOS, univariate and multivariate analyses (limited to pre-BOS 0-p SSAR).
Table S2. Impact on time to death, univariate and multivariate analyses (limited to pre-BOS 0-p SSAR).
Table S3. Impact on time to BOS, univariate and multivariate analyses (all Bx biopsies excluded).
Table S4. Impact on time to death, univariate and multivariate analyses (all Bx biopsies excluded).
Figure S1. Individual SSAR and non-SSAR episode classification. All 1181 pre-BOS AR episodes were evaluated and classified as shown to define the SSAR vs. non-SSAR patient analysis groups; AR, acute rejection; BOS, bronchiolitis obliterans syndrome; FEV1, forced expiratory volume in one second; SSAR, spirometrically significant acute rejection.
Acknowledgments
Funding:
National Institutes of Health KL2RR024127 and American Society of Transplantation Clinical Faculty Development Award (LD Snyder), National Heart Lung and Blood Institute SCCOR 1P50-HL084917-01 and K24-091140-01 (SM Palmer)
List of Non-standardized Abbreviations
- AR
acute rejection (A grade rejection)
- BOS
bronchiolitis obliterans syndrome
- CMV
cytomegalovirus
- CMVP
cytomegalovirus pneumonitis
- FEV1
forced expiratory volume in one second
- ISHLT
International Society for Heart and Lung Transplantation
- LB
lymphocytic bronchiolitis (B grade rejection)
- PFT
pulmonary function test
- SSAR
spirometrically significant acute rejection
Footnotes
Disclosure
This manuscript was neither prepared nor funded in any part by a commercial organization. The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting Information
Additional information may be found in the online version of this article:
References
- 1.Christie JD, Edwards LB, Kucheryavaya AY, Aurora P, Dobbels F, Kirk R, et al. The Registry of the International Society for Heart and Lung Transplantation: twenty-seventh official adult lung and heart-lung transplant report--2010. J Heart Lung Transplant. 2010 Oct;29(10):1104–18. doi: 10.1016/j.healun.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Belperio JA, Weigt SS, Fishbein MC, Lynch JP., 3rd Chronic lung allograft rejection: mechanisms and therapy. Proc Am Thorac Soc. 2009 Jan 15;6(1):108–21. doi: 10.1513/pats.200807-073GO. [DOI] [PubMed] [Google Scholar]
- 3.Bowdish ME, Arcasoy SM, Wilt JS, Conte JV, Davis RD, Garrity ER, et al. Surrogate markers and risk factors for chronic lung allograft dysfunction. Am J Transplant. 2004 Jul;4(7):1171–8. doi: 10.1111/j.1600-6143.2004.00483.x. [DOI] [PubMed] [Google Scholar]
- 4.Estenne M, Maurer JR, Boehler A, Egan JJ, Frost A, Hertz M, et al. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant. 2002 Mar;21(3):297–310. doi: 10.1016/s1053-2498(02)00398-4. [DOI] [PubMed] [Google Scholar]
- 5.Scott AI, Sharples LD, Stewart S. Bronchiolitis obliterans syndrome: risk factors and therapeutic strategies. Drugs. 2005;65(6):761–71. doi: 10.2165/00003495-200565060-00004. [DOI] [PubMed] [Google Scholar]
- 6.Sharples LD, McNeil K, Stewart S, Wallwork J. Risk factors for bronchiolitis obliterans: a systematic review of recent publications. J Heart Lung Transplant. 2002 Feb;21(2):271–81. doi: 10.1016/s1053-2498(01)00360-6. [DOI] [PubMed] [Google Scholar]
- 7.Stewart S, Fishbein MC, Snell GI, Berry GJ, Boehler A, Burke MM, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007 Dec;26(12):1229–42. doi: 10.1016/j.healun.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 8.Chakinala MM, Ritter J, Gage BF, Lynch JP, Aloush A, Patterson GA, et al. Yield of surveillance bronchoscopy for acute rejection and lymphocytic bronchitis/bronchiolitis after lung transplantation. J Heart Lung Transplant. 2004 Dec;23(12):1396–404. doi: 10.1016/j.healun.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 9.Hopkins PM, Aboyoun CL, Chhajed PN, Malouf MA, Plit ML, Rainer SP, et al. Prospective analysis of 1,235 transbronchial lung biopsies in lung transplant recipients. J Heart Lung Transplant. 2002 Oct;21(10):1062–7. doi: 10.1016/s1053-2498(02)00442-4. [DOI] [PubMed] [Google Scholar]
- 10.McWilliams TJ, Williams TJ, Whitford HM, Snell GI. Surveillance bronchoscopy in lung transplant recipients: risk versus benefit. J Heart Lung Transplant. 2008 Nov;27(11):1203–9. doi: 10.1016/j.healun.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Valentine VG, Gupta MR, Weill D, Lombard GA, LaPlace SG, Seoane L, et al. Singleinstitution study evaluating the utility of surveillance bronchoscopy after lung transplantation. J Heart Lung Transplant. 2009 Jan;28(1):14–20. doi: 10.1016/j.healun.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 12.Martinu T, Howell DN, Palmer SM. Acute cellular rejection and humoral sensitization in lung transplant recipients. Semin Respir Crit Care Med. 2009 Apr;31(2):179–88. doi: 10.1055/s-0030-1249113. [DOI] [PubMed] [Google Scholar]
- 13.Husain AN, Siddiqui MT, Holmes EW, Chandrasekhar AJ, McCabe M, Radvany R, et al. Analysis of risk factors for the development of bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 1999 Mar;159(3):829–33. doi: 10.1164/ajrccm.159.3.9607099. [DOI] [PubMed] [Google Scholar]
- 14.Schulman LL, Weinberg AD, McGregor CC, Suciu-Foca NM, Itescu S. Influence of donor and recipient HLA locus mismatching on development of obliterative bronchiolitis after lung transplantation. Am J Respir Crit Care Med. 2001 Feb;163(2):437–42. doi: 10.1164/ajrccm.163.2.2005031. [DOI] [PubMed] [Google Scholar]
- 15.Bando K, Paradis IL, Similo S, Konishi H, Komatsu K, Zullo TG, et al. Obliterative bronchiolitis after lung and heart-lung transplantation. An analysis of risk factors and management. J Thorac Cardiovasc Surg. 1995 Jul;110(1):4–13. doi: 10.1016/S0022-5223(05)80003-0. discussion –4. [DOI] [PubMed] [Google Scholar]
- 16.El-Gamel A, Sim E, Hasleton P, Hutchinson J, Yonan N, Egan J, et al. Transforming growth factor beta (TGF-beta) and obliterative bronchiolitis following pulmonary transplantation. J Heart Lung Transplant. 1999 Sep;18(9):828–37. doi: 10.1016/s1053-2498(99)00047-9. [DOI] [PubMed] [Google Scholar]
- 17.Khalifah AP, Hachem RR, Chakinala MM, Yusen RD, Aloush A, Patterson GA, et al. Minimal acute rejection after lung transplantation: a risk for bronchiolitis obliterans syndrome. Am J Transplant. 2005 Aug;5(8):2022–30. doi: 10.1111/j.1600-6143.2005.00953.x. [DOI] [PubMed] [Google Scholar]
- 18.Hachem RR, Khalifah AP, Chakinala MM, Yusen RD, Aloush AA, Mohanakumar T, et al. The significance of a single episode of minimal acute rejection after lung transplantation. Transplantation. 2005 Nov 27;80(10):1406–13. doi: 10.1097/01.tp.0000181161.60638.fa. [DOI] [PubMed] [Google Scholar]
- 19.Hartwig MG, Snyder LD, Finlen-Copeland A, Lin SS, Zaas DW, Davis RD, et al. Lung transplantation at Duke University. Clin Transpl. 2009:197–210. [PubMed] [Google Scholar]
- 20.Yousem SA, Berry GJ, Cagle PT, Chamberlain D, Husain AN, Hruban RH, et al. Revision of the 1990 working formulation for the classification of pulmonary allograft rejection: Lung Rejection Study Group. J Heart Lung Transplant. 1996 Jan;15(1 Pt 1):1–15. [PubMed] [Google Scholar]
- 21.Glanville AR, Aboyoun CL, Havryk A, Plit M, Rainer S, Malouf MA. Severity of lymphocytic bronchiolitis predicts long-term outcome after lung transplantation. Am J Respir Crit Care Med. 2008 May 1;177(9):1033–40. doi: 10.1164/rccm.200706-951OC. [DOI] [PubMed] [Google Scholar]
- 22.Finlen Copeland CA, Snyder LD, Zaas DW, Turbyfill WJ, Davis WA, Palmer SM. Survival after bronchiolitis obliterans syndrome among bilateral lung transplant recipients. Am J Respir Crit Care Med. 2010 Sep 15;182(6):784–9. doi: 10.1164/rccm.201002-0211OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levine SM. A survey of clinical practice of lung transplantation in North America. Chest. 2004 Apr;125(4):1224–38. doi: 10.1378/chest.125.4.1224. [DOI] [PubMed] [Google Scholar]
- 24.Reid DW, Walters EH, Johns DP, Ward C, Burns GP, Liakakos P, et al. Bronchial hyperresponsiveness and the bronchiolitis obliterans syndrome after lung transplantation. J Heart Lung Transplant. 2005 Apr;24(4):489–92. doi: 10.1016/j.healun.2004.02.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Impact on time to BOS, univariate and multivariate analyses (limited to pre-BOS 0-p SSAR).
Table S2. Impact on time to death, univariate and multivariate analyses (limited to pre-BOS 0-p SSAR).
Table S3. Impact on time to BOS, univariate and multivariate analyses (all Bx biopsies excluded).
Table S4. Impact on time to death, univariate and multivariate analyses (all Bx biopsies excluded).
Figure S1. Individual SSAR and non-SSAR episode classification. All 1181 pre-BOS AR episodes were evaluated and classified as shown to define the SSAR vs. non-SSAR patient analysis groups; AR, acute rejection; BOS, bronchiolitis obliterans syndrome; FEV1, forced expiratory volume in one second; SSAR, spirometrically significant acute rejection.


