Abstract
Objective
We have previously shown that transient coronary artery occlusion stimulated coronary collateral growth (CCG) in healthy (SD) but not in metabolic syndrome (JCR) rats. Here, we sought to determine whether matrix metalloproteinases (MMPs) negatively regulate CCG in the metabolic syndrome via release of endostatin and angiostatin.
Approach/Results
Rats underwent transient, repetitive LAD occlusion (RI) for 0-10 days. CCG was measured in the collateral-dependent (CZ) and normal (NZ) zones using microspheres, MMP activation by Western blot, and endostatin and angiostatin by ELISA on days 0, 3, 6, 9 or 10 of RI. Endostatin and angiostatin were increased in JCR but not in SD rats on days 6 and 9 of RI. Increased endostatin and angiostatin correlated with increased MMP12 (∼4 fold) activation in JCR but not in SD rats on days 6 and 9 of RI. Inhibition of MMP12 in JCR rats nearly completely blocked endostatin (∼85%) and angiostatin (∼90%) generation and significantly improved CCG (CZ flow was ∼66% of NZ flow vs. ∼12% for JCR RI).
Conclusions
Compromised CCG in the metabolic syndrome is in large part due to increased MMP12 activation and consequent increased generation of endostatin and angiostatin, which inhibits late-stage collateral remodeling.
Keywords: coronary collateral growth, MMPs, angiostatin, endostatin
Introduction
Transient repetitive coronary artery occlusion and resultant myocardial ischemia (RI) stimulate adaptive enlargement of pre-existing high resistance arterioles with minimal to no blood flow to conduit arteries in a process termed coronary collateral growth (CCG).1 Well-developed coronary collaterals provide an alternative source of blood supply and can help preserve myocardial function in the setting of total coronary occlusion.1 Clinically, patients with stable angina have decreased incidence of fatal myocardial infarction, which is associated with better developed collateral networks.2 However, development of collateral networks is severely impaired in patients with metabolic syndrome.3-7 Similarly, CCG is impaired in our metabolic syndrome rat model (JCR:LA-cp or JCR).8 JCR rats mimic the complex pathology of human metabolic syndrome. By 8 weeks of age, the rats develop obesity with fatty liver, insulin resistance with glucose intolerance, complex dyslipidemia (low HDL, high LDL and vLDL), and vasculopathy characterized by decreased endothelium-dependent and -independent vasorelaxation and intimal lesions morphologically identical to early atherosclerotic lesions in humans. By 12 weeks of age, JCR rats exhibit wide-spread atherosclerosis and evidence of myocardial and cerebral (micro)infarctions. In addition, like the development of metabolic syndrome and cardiovascular disease in humans, the apparent complexity of the cardio-metabolic phenotype exhibited by JCR rats is suspected to be multifactorial and polygenetic in etiology, arising from a combination of environmental and genetic factors.9 Thus, the JCR rat acts as an unparalleled rodent model for the study of CCG in the metabolic syndrome.
Temporally regulated reorganization of the extracellular matrix (ECM) is an integral part of collateral remodeling. First, the ECM acts as a mechanical barrier to the migration of endothelial cells (ECs) and vascular smooth muscle cells (VSMCs), which must be removed to allow for effective cell proliferation and migration in the early stages of CCG then reformed to allow for collateral vessel maturation in the late stages of CCG. In addition, the ECM also acts as a repository for pro-angiogenic growth factors such as the vascular endothelial growth factor (VEGF) and the fibroblast growth factor (FGF) as well as for anti-angiogenic peptides such as angiostatin and endostatin. The extent of CCG depends on the balance between the pro-angiogenic growth factors and the anti-angiogenic peptides. Therefore, ECM degradation, through the release of the above mentioned angiogenic factors can either promote or impede collateral development.
Angiostatin and endostatin have been negatively associated with coronary collateral development in patients with coronary artery disease10 and type II diabetes11. Chronic hyperglycemia was also shown to inhibit CCG via an increase in angiostatin in a dog model.12 Angiostatin is a 38 kDa cleavage product of plasminogen13, which has been shown to exert its anti-angiogenic effects via induction of endothelial cell (EC) apoptosis14-17 and impairment of EC proliferation18, migration, and tube formation19. Endostatin is a 20 kDa cleavage product of collagen XVIII.20 It also functions to induce EC apoptosis thorough multiple signaling pathways21, 22 and diminish endothelial cell migration, adhesion, and proliferation23, 24. Targets of angiostatin and endostatin in collateral growth have not been investigated but are presumed to also be ECs.
Several proteases, including matrix metalloproteinases (MMPs), have been shown to be able to generate angiostatin and endostatin by cleavage of plasminogen and collagen XVIII, respectively, in vitro and in cell culture studies. MMP225 and MMP1226 have been shown to release angiostatin in an in vitro model of lung metastasis. MMP12 has also been shown to inhibit microvascular EC proliferation in vitro by generating angiostatin.26, 27 MMP328, MMP729, and MMP929 have been shown to be able to release angiostatin in in vitro studies in which human plasminogen was incubated with various MMPs. In an in vitro assay, MMPs 1, 2, 3, 9, 12, 13, 14 and 20 were able to release endostatin from a fragment of human collagen XVIII.30 One study showed that MMP12 releases endostatin to inhibit VEGF-induced chemotaxis of osteoclasts.31 Very little is known about MMP8 and its ECM substrates; however, the other two collagenase, MMP1 and MMP13, have been shown to generate endostatin in in vitro assays.30 The ability of these MMPs to generate these anti-angiogenic peptides in vivo, especially during collateral development, has not been evaluated. MMP2 and MMP9 are the only MMPs that have been studied in collateral growth. One study correlated their increased expression with actively remodeling collaterals.32 However their decreased activation has been correlated with decreased CCG in humans with type II diabetes.11 Furthermore, we have recently definitively shown that they are required for CCG and that they are not activated in metabolic syndrome33 and several studies demonstrated lack of correlation between increased angiostatin and endostatin levels and increased MMP2 or MMP9 activation in humans with impaired CCG10, 11, suggesting that MMP2 and MMP9 are not the MMPs responsible for the generation of angiostatin and endostatin associated with decreased coronary collateral development. The role of the other members of the MMP family has not been studied in association with collateral development. Moreover, it is not known whether their activation is altered in metabolic syndrome.
Our results in this study demonstrated that elevated angiostatin and endostatin levels in the metabolic syndrome rat model (JCR) correlated with enhanced MMP12 and MMP8 activation. Therefore, the goal of this study was to determine if MMP12 and/or MMP8 were involved in the corruption of CCG in the metabolic syndrome via upregulation of angiostatin and endostatin in the later stages of CCG.
Materials and Methods
See online Supplement.
Results
MMP12 and MMP8 activation is increased in the later stages of CCG in the metabolic syndrome but not in the normal rat model
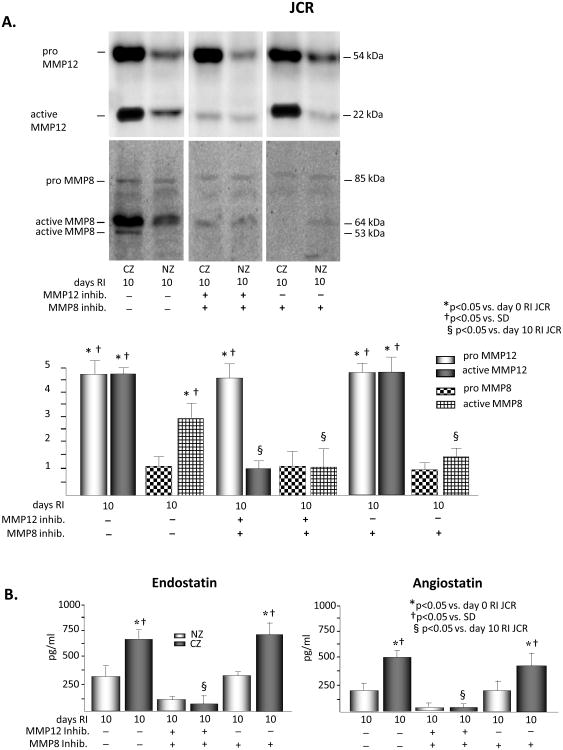
Of the MMPs which have been shown to be able to generate angiostatin and endostatin in in vitro or cell culture studies, MMPs 1, 2, 3, 9, 12, 13, and 14, we have found MMPs 2, 9 and 14 (MT1-MMP) to be activated in response to RI and required for CCG in the normal rat phenotype (SD rat)33 (Figure I, Supplement). Importantly, of these MMPs, we have found only MMP12 to be activated in the metabolic syndrome (JCR) rats in response to RI. Moreover, in our screen of MMPs, which are known to be expressed in the cardiovascular system34, by expression of the pro and active forms of the MMP proteins, we have found only one additional MMP to be activated in response to RI, MMP8 (Figure 1 and Figure I, Supplement).
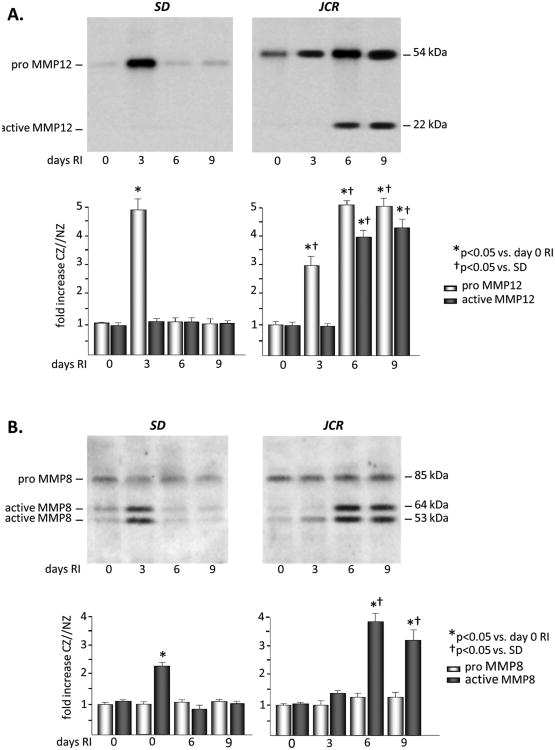
Figure 1.
SD or JCR rats were sacrificed on day 0, 3, 6 or 9 of RI. Tissue samples were collected from the NZ or the CZ. Pro and active MMP12 (A) and MMP8 (B) expression was determined by Western Blot in SD (left) and JCR (right) rats. Top: Representative Western blot using the anti-MMP12 and anti-MMP8 antibodies. Bottom: Cumulative data, *p<0.05 vs. day 0 RI, †p<0.05 vs. SD, n=5.
Basal MMP12 expression was ∼2 fold higher in the JCR phenotype; however, there was no difference in its basal activation between the two phenotypes. RI resulted in a significant increase in pro-MMP12 on day 3 of RI in the SD rats (∼5 fold), but this did not translate into an increase in its activation on any day of the RI protocol in the SD animals. Pro-MMP12 was also increased in the JCR rats on days 3 (∼3 fold), 6 (∼5 fold), and 9 (∼5 fold) of RI. However, in contrast to the SD rats, MMP12 was also highly activated beginning at day 6 of RI (∼4 fold vs. baseline) in the JCR animals. This activation remained sustained for the duration of the RI protocol (Figure 1A). Increased RI-induced MMP12 expression and activation in the heart did not translate into increased RI-induced MMP12 expression or activation in the plasma of JCR rats (Figure III, Supplement). Basal expression of MMP8 was ∼2 fold higher in the JCR animals; however, RI did not significantly alter MMP8 expression in either phenotype. MMP8 activity was increased ∼2 fold on day 3 of the RI protocol in the SD rat. In the JCR rat, MMP8 activation was greater in magnitude and the timing of activation was shifted from the early to the later staged of CCG. MMP8 was maximally activated on days 6 (∼4 fold) and 9 (∼3 fold) of RI (Figure 1B).
All changes were observed in the CZ only. RI did not induce any changes in MMP12 or MMP8 expression or activation in the NZ (Figure 3 and data not shown)
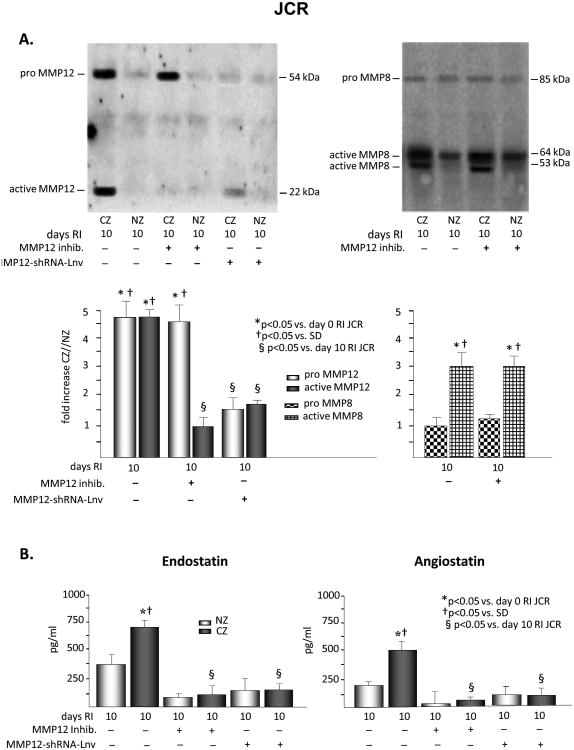
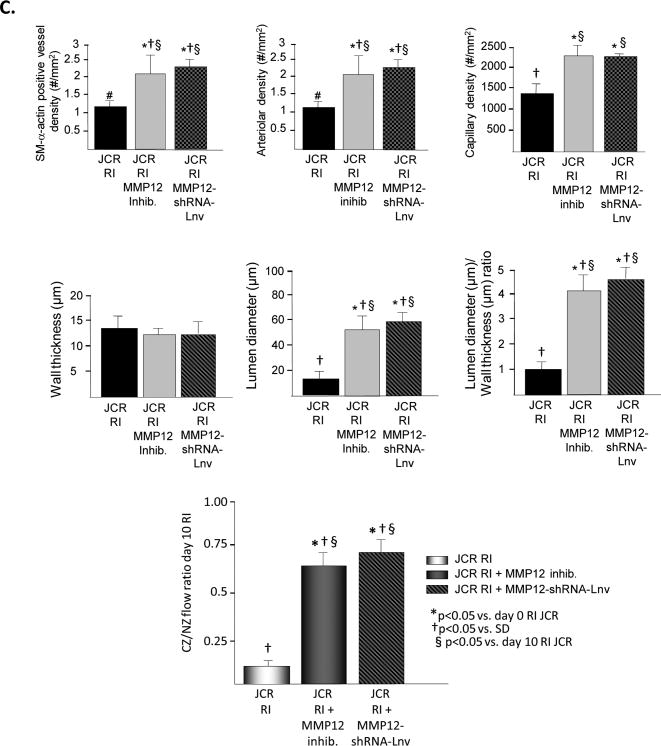
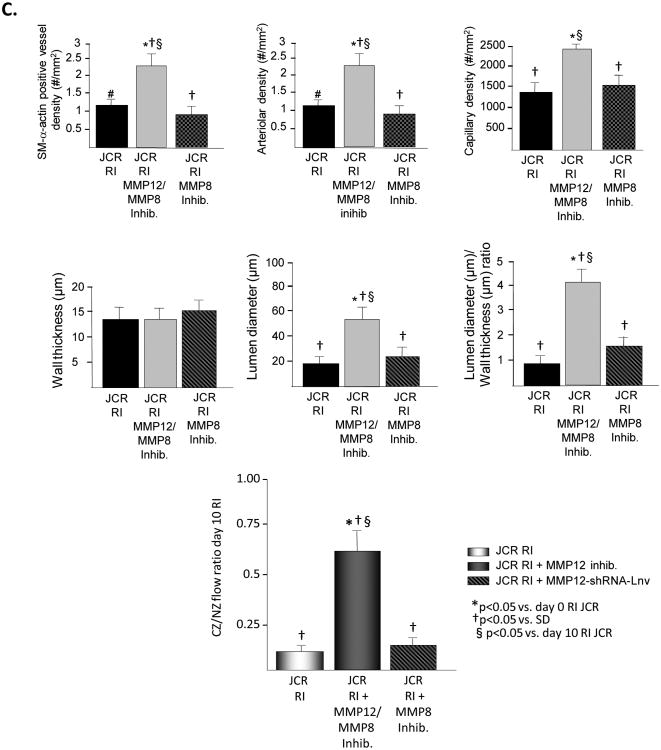
Figure 3.
JCR rats were treated the MMP12 inhibitor or MMP12-shRNA-Lnv as indicated and underwent 10 days of RI. A. Tissue samples were collected from the NZ or the CZ. Pro and active MMP12 and MMP8 expression was determined by Western Blot. Top: Representative Western blot detecting pro and active forms MMP12 (left) and MMP8 (right). Bottom: Cumulative data. B. Tissue samples were collected from the NZ or the CZ. Myocardial endostatin (left) and angiostatin (right) levels were determined by ELISA. C. Top: Arteriolar (SM-a-actin positive vessels >20μM) and capillary (vessels <20μM) densities, wall thickness, lumen diameter and lumen diameter/wall thickness ratio of collateral arteries (anti-SM-a-actin and PCNA positive arteries) were determined in cardiac (CZ) cross-sections on day 10 RI. Bottom: Coronary blood flow was measured in the CZ and the NZ using microspheres during LAD occlusion and is expressed as the ratio between CZ and NZ flows at day 10 of RI. *p<0.05 vs. SD sham, †p<0.05 vs. SD RI, §p<0.05 vs. JCR RI, n=3 for MMP12 inhibitor, n=5 for MMP12-shRNA-Lnv.
Elevated myocardial angiostatin and endostatin levels in the later stages of CCG in the metabolic syndrome correlate with increased MMP12 and MMP8 activation
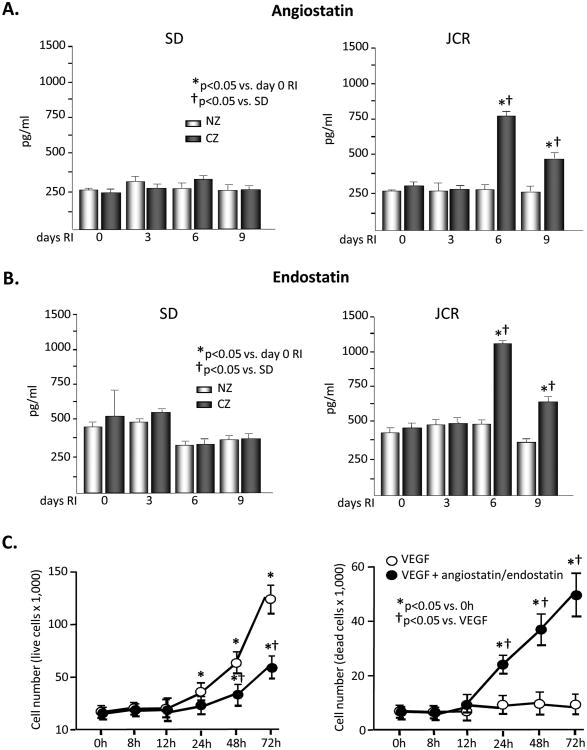
Basal angiostatin and endostatin levels were similar between the two phenotypes. RI did not alter either angiostatin or endostatin in the CZ of the SD rats. In contrast, both angiostatin and endostatin levels were markedly upregulated on days 6 (∼3 fold and 2 fold, respectively) and 9 (∼2 fold and 2 fold, respectively) of RI in the CZ of the JCR rats. Because the angiostatin and endostatin fragments are inaccessible to the respective antibodies within the tertiary structures of intact plasminogen and collagen XVIII in the ELIZA assays, the observed increases specifically represent cleaved angiostatin and endostatin. Neither endostatin nor angiostatin were altered by RI in the NZ of either rat phenotype (Figure 2A and 2B). Thus, increased angiostatin and endostatin levels correlate with the increased activation of MMP8 and MMP12 in the later stages of CCG in the metabolic syndrome animals.
Figure 2.
SD or JCR rats were sacrificed on day 0, 3, 6 or 9 of RI. Tissue samples were collected from the NZ or the CZ. Myocardial angiostatin (A) and endostatin (B) levels were determined by ELISA in SD (left) and JCR (right) rats. *p<0.05 vs. day 0 RI, †p<0.05 vs. SD, n=5. C. HCAEC were treated with VEGF (50 ng/ml) or VEGF (50 ng/ml) + angiostatin (750 pg/ml) + endostatin (1000 pg/ml) for the times indicated. Live and dead cells were counted. *p<0.05 vs. 0 hours, †p<0.05 vs. VEGF, n=3.
To determine whether the concentrations of angiostatin and endostatin generated by RI in JCR rats induced the classical endothelium-specific anti-angiogenic effects of these peptides, we examined whether they decreased VEGF-induced HCAEC proliferation and survival. While neither angiostatin (750 pg/ml) nor endostatin (1000 pg/ml) alone were able to significantly decrease the VEGF-induced increase in HCAEC number or survival (data not shown), results in Figure 2C demonstrate that their combined effect on both parameters was significant.
Inhibition of MMP12 activation decreased myocardial endostatin and angiostatin levels and partially restored coronary collateral growth in the metabolic syndrome
To determine whether MMP12 activation alone was responsible for the increased level of angiostatin and/or endostatin in response to RI in the metabolic syndrome, JCR rats were treated with the specific MMP12 inhibitor (3.5 mg/kg/day, 1/day, i.p.) on days 6-9 of the RI protocol. Complete inhibition of MMP12 activation was confirmed by Western blot analysis of the active (MW=22 kDa) MMP12 band (Figure 3A). MMP12 expression was not affected. MMP8 activation was not affected (Figure 3A) confirming the specificity of the inhibitor. MMP12 inhibition resulted in a significant reduction in both endostatin and angiostatin levels (85% and 90%, respectively) at day 10 of RI (Figure 3B). Moreover, inhibition of MMP12 partially restored CCG in JCR rat as evident by increased artery/arteriolar density (∼2 50μM arteries/mm2 vs. ∼1 20 μM arteriole in JCR RI vs. ∼3 60 μM arteries in SD RI) and increased collateral-dependent flow (CZ/NZ flow ratio was 0.66±0.05 vs. 0.12±0.02 in JCR RI vs. 0.87±0.04 in SD RI33) and completely restored RI-dependent angiogenesis as indicated by an increase in capillary density equivalent to that observed in normal animals (Figures 3C and Figure 5). Nearly identical results were obtained when MMP12 expression was decreased (∼80%) using shRNA, delivered to JCR rats via a lentiviral vector (Figure 3). Control-Lnv, carrying a non-targeting shRNA, had no effect (data not shown).
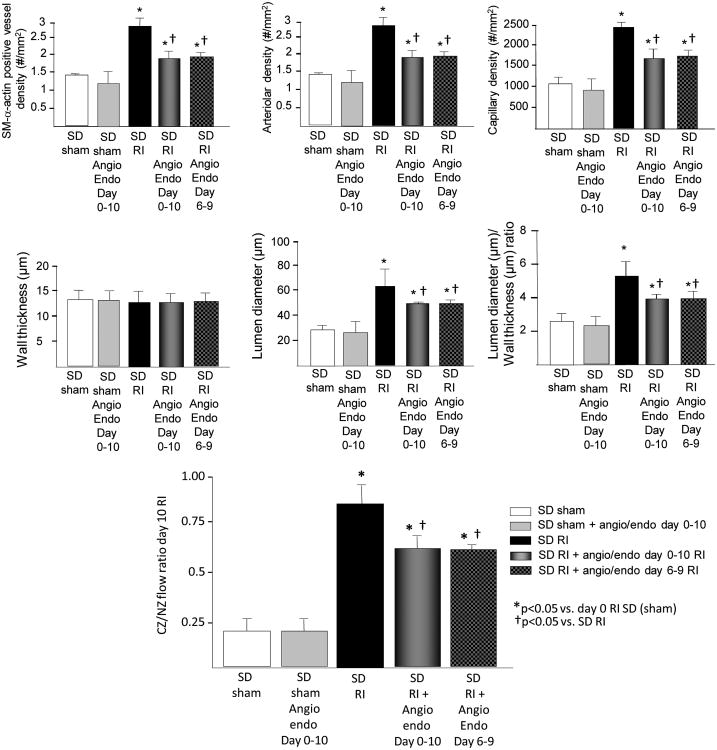
Figure 5.
SD rats were treated angiostatin (750 pg/kg/day) and endostatin (1000 mg/kg/day) on days 0-10 RI or 6-9 RI as indicated and underwent 10 days of RI. Top: Arteriolar (SM-a-actin positive vessels >20μM) and capillary (vessels <20μM) densities, wall thickness, lumen diameter and lumen diameter/wall thickness ratio of collateral arteries (anti-SM-a-actin and PCNA positive arteries) were determined in cardiac (CZ) cross-sections on day 10 RI. Bottom: Coronary blood flow was measured in the CZ and the NZ using microspheres during LAD occlusion and is expressed as the ratio between CZ and NZ flows at day 10 of RI. **p<0.05 vs. SD sham, †p<0.05 vs. SD RI, §p<0.05 vs. JCR RI, n=5.
To determine whether additional inhibition of MMP8 would have an additional beneficial effect on CCG in the metabolic syndrome, in addition to the specific MMP12 inhibitor to inhibit MMP12 activity, we used the MMP8 inhibitor (0.25 mg/kg/day, 1/day, i.v.) to inhibit MMP8 activity at days 6-9 of RI in JCR rats. The MMP8 inhibitor may inhibit the other collagenases, MMP1 and MMP13 at similar doses; however, since neither MMP1 nor MMP13 are activated at these time points in the JCR rat (Figure I, Supplement), the non-specific effects on the other collagenases were not a concern. The collagenase inhibitor successfully and completely inhibited MMP8 activation without effecting MMP8 expression, and did not alter the effectiveness of the MMP12 inhibitor on MMP12 activity (Figure 4A). Concomitant inhibition of MMP12 and MMP8 resulted in a slightly greater but statistically insignificant reduction in endostatin levels (90% vs. 85% with MMP12 inhibition alone), while the extent of reduction in angiostatin levels remained unchanged (Figure 4B). The additional inhibition of MMP8 likewise did not have any greater of an effect on CCG recovery or angiogenesis then MMP12 inhibition alone (CZ/NZ flow ratio was 0.62±0.08) (Figure 4C).
Figure 4.
JCR rats were treated the MMP12 and the MMP8 inhibitor as indicated and underwent 10 days of RI. A. Tissue samples were collected from the NZ or the CZ. Pro and active MMP12 and MMP8 expression was determined by Western Blot. Top: Representative Western blot detecting pro and active forms MMP12 and MMP8. Bottom: Cumulative data, **p<0.05 vs. day 0, †p<0.05 vs. SD, §p<0.05 vs. day 10 RI, n=3. B. Tissue samples were collected from the NZ or the CZ. Myocardial endostatin (left) and angiostatin (right) levels were determined by ELISA. **p<0.05 vs. day 0, †p<0.05 vs. SD, §p<0.05 vs. day 10 RI, n=3. C. Top: Arteriolar (SM-a-actin positive vessels >20μM) and capillary (vessels <20μM) densities, wall thickness, lumen diameter and lumen diameter/wall thickness ratio of collateral arteries (anti-SM-a-actin and PCNA positive arteries) were determined in cardiac (CZ) cross-sections on day 10 RI. Bottom: Coronary blood flow was measured in the CZ and the NZ using microspheres during LAD occlusion and is expressed as the ratio between CZ and NZ flows at day 10 of RI. **p<0.05 vs. SD sham, †p<0.05 vs. SD RI, §p<0.05 vs. JCR RI, n=3.
To confirm that MMP12 but not MMP8 plays the major role in the regulation of angiostatin and endostatin production and CCG in metabolic syndrome, we also inhibited MMP8 alone. Complete inhibition of MMP8 activation had no effect on RI-induced angiostatin or endostatin production (Figure 4B), CCG or angiogenesis in JCR rats (Figure 4C).
Angiostatin and endostatin infusion decreased coronary collateral growth in normal animals
Finally we tested the effect of the amounts of angiostatin and endostatin produced in the JCR rats on CCG in the normal animals to provide proof-of-concept as well as examine the physiological relevance of these amounts. Infusion of 750 pg/ml/day angiostatin and 1000 pg/ml/day endostatin (days 0-10 or 6-9 of RI) moderately but significantly decreased RI-induced angiogenesis and CCG in SD rats while having predictably no effect on pre-existing collaterals and capillary networks (Figure 5). These results suggest that angiostatin and endostatin amounts produced in JCR rats are physiologically significant. The caveat to these conclusions is that ECs of SD rats are normal while JCR rats display marked endothelial dysfunction9; thus, these anti-angiogenic peptides would be predicted to more severely impact the already apoptosis-prone JCR ECs.
Discussion
The most important novel findings of this study are that: 1) MMP12 and MMP8 are differentially regulated in the normal vs. the metabolic syndrome phenotype during the process of CCG, 2) the temporal pattern of MMP12 and MMP8 activation in the later stages of CCG correlates with elevated levels of angiostatin and endostatin in the metabolic syndrome, and 3) inhibition of MMP12 but not MMP8 activation blocks RI-induced generation of angiostatin and endostatin and significantly improves CCG in the metabolic syndrome.
Proteolysis of ECM and plasma proteins is a common theme in inhibition of angiogenesis. Many inhibitors of tumor angiogenesis, such as angiostatin, endostatin, serpin antithrombin, tumstatin, canstatin, vasostatin, restin, and arrestin are proteolytic fragments.20, 35-43 Of these, angiostatin and endostatin have been investigated in collateral growth. Results in our study demonstrating elevated levels of angiostatin and endostatin during the later stages of repetitive ischemia in the metabolic syndrome are in agreement with previous studies which showed that these anti-angiogenic peptides were increased in hyperglycemic dogs with impaired CCG during the later stages of repetitive ischemia12 and in humans with type II diabetes and impaired coronary collateral development10, 11.
The mechanism by which angiostatin and endostatin inhibit collateral growth has not been investigated, but is inferred from a related process of tumor angiogenesis. It has been shown that angiostatin directly targets only EC proliferation and survival and does not affect either tumor cells or other cells of non-endothelial origin in vitro.44 In agreement with these findings, results in this study show that the concentrations of angiostatin and endostatin generated in the metabolic syndrome animals can suppress EC proliferation and survival. It was shown that angiostatin directly induces apoptosis of ECs without a compensatory increase in proliferation, resulting in a net reduction of viable ECs via altering focal adhesion kinase signaling and disrupting normal focal adhesion turnover.45, 46 Secondly, angiostatin directly reduces EC migration, proliferation and tube formation by inhibiting FGF and VEGF-dependent ERK1/2 activation.47 Lastly, angiostatin may mediate its anti-angiogenic effects by binding to the cell surface ATP synthase and depriving ECs of ATP.15 Like angiostatin, endostatin does not affect the in vitro proliferation of tumor cells nor other non-endothelial cell lines; thus, its actions appear to be EC-specific.20, 21 Endostatin increases EC apoptosis by reducing the expression of anti-apoptotic proteins Bcl-2 and Bcl-XL21 and inducing Caspase 3 activation48. Furthermore, endostatin inhibited ERK1/2 phosphorylation in VEGF- and FGF-stimulated primary arterial EC cultures without interfering with growth factor receptor binding.49 Our unpublished observations suggest that ERK1/2 activation is also impaired in the metabolic syndrome during CCG (Rocic, unpublished data, [2008]).
MMPs, non-MMP elastases and plasmin are capable of generating angiostatin from plasminogen, while a wide variety of MMPs are able to generate endostatin from collagen XVIII. Of the possible candidates relevant to the cardiovascular system, our results show that only MMP12 and MMP8 activation correlate with increased levels of angiostatin and endostatin in the later stages of CCG in metabolic syndrome. Our findings further indicate that MMP12 is the primary regulator of angiostatin and endostatin in the metabolic syndrome since its inhibition alone was sufficient to completely block their generation, additional inhibition of MMP8 had no further effect and MMP8 inhibition alone likewise had no effect. MMP12 has extensive activity against elastin but is also capable of degrading proteoglycans, fibronectin, laminin, non-fibrilar collagen including collagen XVIII, heparin sulfate, plasminogen and vitronectin. Importantly, it has been shown to be anti-angiogenic in several studies in cancer angiogenesis. MMP12 was decreased in breast carcinoma compared to normal mammary cells and these breast carcinoma lines were more efficient in capillary tube formation than the normal mammary cells.50 Decreased MMP12 expression correlated with decreased expression of angiostatin.51 MMP12 was also able to generate angiostatin from purified plasminogen in vitro27 and in an in vitro model of lung metastasis26. MMP12 has also been shown to inhibit microvascular endothelial cell proliferation in vitro by generating angiostatin.26, 27 One study showed that MMP12 can release endostatin, which inhibited VEGF-induced chemotaxis of osteoclasts.31
Not much is known about MMP8. Its role in collateral growth or angiogenesis is completely unknown, as are its substrates. However, MMP8 belongs to the family of interstitial collagenases, which in the cardiovascular system, includes MMP1 and MMP13, and its active site is structurally identical to that of MMP1 and MMP13. Neither MMP1 nor MMP13 are activated during coronary collateral remodeling in either the normal or the metabolic syndrome rat phenotype. Thus, it is reasonable to propose that MMP8 may be the representative active interstitial collagenase under these circumstances. Interstitial collagenases cleave collagen types I and III, which are components of the myocardial interstitium and vascular adventitia. MMP1 has been shown to suppress angiogenesis in sarcoma; its silencing significantly increased mean vascular volume per unit volume of tumor.52 MMP 13 has been found to have a direct role in the formation of endostatin. Because both MMP1 and MMP13 share the collagen XVIII cleavage site, MMP1 was also thought to mediate its anti-angiogenic effects via endostatin production.52
Paradoxically, many of the same proteases that release anti-angiogenic peptides are also capable of releasing pro-angiogenic growth factors and stimulate angiogenesis. For example, plasmin mediates cell invasion into neighboring tissues, which requires angiogenesis.53, 54 MMP1 effectively releases pro-angiogenic growth factors, VEGF and FGF, from the ECM.55, 56 This is interesting since in our study, in addition to being activated late in CCG in the metabolic syndrome animals, which fail to grow collaterals, MMP8 was also activated early during CCG in the normal animals. Thus, our data suggest that MMP8 may play a differential role in CCG in normal vs. the metabolic syndrome phenotype. Depending on the timing of its activation, it may release either pro-angiogenic growth factors from their binding proteins in the ECM (early in CCG) or anti-angiogenic peptides (late in CCG). The extent of collateral growth is determined by the balance of the pro-angiogenic growth factors and anti-angiogenic peptides. Therefore, our results in this study further support the concept that ECM proteolysis within the context of collateral growth is a temporally tightly regulated process, which can be profoundly affected by the temporal regulation of MMPs.
Regarding the possible cause of the later and sustained increase in MMP8 activation in the metabolic syndrome, MMP8 is a neutrophil collagenase (collagenase-2). Although transient accumulation of a low number of neutrophils and other inflammatory cells early in collateral growth has been associated with beneficial effects on collateral remodeling57, prolonged or excessive neutrophil accumulation and inflammation which characterizes the vasculature of type II diabetes and metabolic syndrome is strongly negatively correlated with CCG.58, 59 Also, increases in the expression, activation, and collagenase activity of MMP8 and MMP13 have been shown in response to Angiotensin II (Ang II) administration.60 We have previously shown that infusion of a hypertensive dose of Ang II negatively affected collateral growth. Moreover and explicitly relevant to the scenario in the current study, Ang II type I receptor (AT1R) levels are increased in JCR rats and AT1R blockade partially but significantly restored CCG in the JCR rat.61 Another possibility is that MMP8 upregulation in the late stages of CCG in metabolic syndrome may serve some other as of yet unknown function or be coincidental.
Our results suggest that increased angiostatin and endostatin production provide a very substantial impediment to coronary collateral development in the metabolic syndrome since their downregulation results in ∼65% recovery of coronary blood flow to the collateral-dependent zone. Upstream regulators of MMP12 activation in collateral growth are not known. The mechanisms responsible for impaired collateral remodeling in the metabolic syndrome have not been completely elucidated, but involve increased oxidative stress and altered redox-dependent signaling8, 62, which might provide an upstream activation mechanism. All MMPs have a disulfide bond in their hinge region63, which makes them directly susceptible to oxidation by reactive oxygen species (ROS), which results in their activation63, 64. We have previously shown that oxidative stress is highly elevated in response to RI in the metabolic syndrome and that reduction in oxidative stress significantly improved CCG in the metabolic syndrome.8, 62 Results in the present study are compatible with the idea that observed recovery of CCG in response to oxidative stress reduction was in part due to the downstream inhibition of MMP12 activation and the consequent decrease in angiostatin and endostatin generation.
A limitation of our study inherent to the rat model of coronary occlusion, where it is not possible to isolate collateral vessels from the heart tissue, is that we did not determine the cellular source of the active MMPs in this study. The most likely source of both MMP12 and MMP8 are inflammatory cells, specifically macrophages and/or neutrophils. Metabolic syndrome is a chronic pro-inflammatory state, where the vasculature is characterized by increased infiltration of neutrophils and monocytes, and increased adhesion and extravasation of inflammatory cells into the vascular wall.65, 66 The infiltration of inflamatory cells, especially neutrophils and monocytes, is a normal and required component of collateral remodeling57 which further increases the total number of these cells in and around the forming collaterals in the metabolic syndrome. Our findings are consistent with the idea that these highly elevated numbers of neutrophils, adherent monocytes and/or extravasated tissue macrophages in the metabolic syndrome result in the release of high levels of MMP12 and MMP8, which lead to generation of high levels of angiostatin and endostatin, which are sufficient to significantly contribute to collateral growth impairement in the metabolic syndrome. However, endothelial cells50, 67 and VSMC68 have also been shown to be able to secrete MMP12; therefore, other cell types cannot be excluded as possible contributors to the production of these MMPs. Our results showing ∼2 fold higher basal MMP12 activation in the plasma of JCR rats is also intriguing. This may be largely a consequence of adipose tissue macrophage-mediated MMP12 production.69 While clearly not a consequence of cardiac repetitive ischemia and probably of little predictive value within the context of coronary collateral growth, the contributing value of this factor to impaired CCG in metabolic syndrome is difficult to predict.
Supplementary Material
Significance.
This study identifies MMP12 as a novel positive regulator of angiostatin and endostatin production, which inhibit coronary collateral development in a rat model of metabolic syndrome. Inhibition of MMP12 activation significantly improved coronary collateral development in the metabolic syndrome animals thus characterizing MMP12 as a potentially important target for revascularization therapy through collateral growth. Furthermore, this study catalogues MMPs which are activated during coronary collateral remodeling in metabolic syndrome vs. the normal phenotype thus identifying targets for future investigation.
Acknowledgments
None
Sources of Funding: NIH R01 HL093052
Footnotes
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chen W, Gabel S, Steenbergen C, Murphy E. A redox-based mechanism for cardioprotection induced by ischemic preconditioning in perfused rat heart. Circ Res. 1995;77:424–429. doi: 10.1161/01.res.77.2.424. [DOI] [PubMed] [Google Scholar]
- 2.Seiler C. The human coronary collateral circulation. Heart. 2003;89:1352–1357. doi: 10.1136/heart.89.11.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turhan H, Yasar AS, Erbay AR, Yetkin E, Sasmaz H, Sabah I. Impaired coronary collateral vessel development in patients with metabolic syndrome. Pathophysi Nat Hist. 2005;16:281–285. doi: 10.1097/00019501-200508000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Yilmaz MB, Biyikoglu BF, Akin Y, Guray U, Kisacik HL, Korkmaz S. Obesity is associated with impaired coronary collateral vessel development. Int J Obes Relat Metab Disord. 2003;27:1541–1545. doi: 10.1038/sj.ijo.0802474. [DOI] [PubMed] [Google Scholar]
- 5.Hutheson R, Terry R, Rocic P. Why is coronary collateral growth impaired in type ii diabetes and the metabolic syndrome? Vascul Pharmacol. 2012;57:179–186. doi: 10.1016/j.vph.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teunissen P, Horrevoets A, van Royen N. The coronary collateral circulation: Genetic and environmental determinants in experimental models and humans. J Mol Cell Cardiol. 2012;52:897–904. doi: 10.1016/j.yjmcc.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 7.van Weel V, de Vries M, Voshol PJ, Verloop RE, Eilers P, van Hinsbergh V, van Brockel Hajo, Quax P. Hypercholesterolemia reduces collateral artery growth more dominantly than hyperglycemia or insulin resistance in mice. Arterioscler Thromb Vasc Biol. 2006;26:1383–1390. doi: 10.1161/01.ATV.0000219234.78165.85. [DOI] [PubMed] [Google Scholar]
- 8.Reed R, Kolz C, Potter B, Rocic P. The mechanistic basis for the disparate effects of angiotension ii on coronary collateral growth. Arterioscler Thromb Vasc Biol. 2008;28:61–67. doi: 10.1161/ATVBAHA.107.154294. [DOI] [PubMed] [Google Scholar]
- 9.Russell JC, Graham SE, Richardson M. Cardiovascular disease in the jcr:La-cp rat. Mol Cell. 1998;188:113–126. [PubMed] [Google Scholar]
- 10.Matsunaga T, Chilian WM, March K. Angiostatin is negatively associated with coronary collateral growth in patients with coronary artery disease. Am J Physiol Heart Circ Physiol. 2005;288:H2042–H2046. doi: 10.1152/ajpheart.00669.2004. [DOI] [PubMed] [Google Scholar]
- 11.Sodha NR, Clements RT, Boodhwani M, Xu SH, Laham RJ, Bianchi C, Selke FW. Endostatin and angiostatin are increased in diabetic patients with coronary artery disease and associated with impaired coronary collateral formation. Am J Physiol Heart Circ Physiol. 2009;296:H428–434. doi: 10.1152/ajpheart.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weihrauch D, Lohr NL, Mraovic B, Ludwig LM, Chilian WM, Pagel PS, Warltier DC, Kersten JR. Chronic hyperglycemia attenuates coronary collateral development and impairs proliferation properties of myocardial interstitial fluid by production of angiostatin. Circulation. 2004;109:2343–2348. doi: 10.1161/01.CIR.0000129225.67353.1F. [DOI] [PubMed] [Google Scholar]
- 13.Wahl ML, Kenan DJ, Gonzalez-Gronow M, Pizzo SV. Angiostatin's molecular mechanism: Aspects of specificity and regulation elucidated. J Cell Biochem. 2005;96:242–261. doi: 10.1002/jcb.20480. [DOI] [PubMed] [Google Scholar]
- 14.Chen YH, Wu HL, Li C, Huang YH, Chiang CW, Wu MP, Wu LW. Anti-angiogenesis mediated by angiostatin k1-3, k1-4 and k1-4.5 involvement of p53, fasl, akt and mrna deregulation. Thromb Haemost. 2006;95:668–677. [PubMed] [Google Scholar]
- 15.Moser TL, Stack SM, Asplin I, Enghild JJ, Hojrup P, Eeveritt L, Hubchak S, Schnaper HW, Pizzo SV. Angiostatin binds atp synthase on the surface of human endothelial cells. Proc Natl Acad Sci USA. 1999;96:2811–2816. doi: 10.1073/pnas.96.6.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moser TL, Kenan DJ, Ashley TA, Roy JA, Goodman MD, Misra UK, Cheek DJ, Pizzo SV. Endothelial cell surface f1-f0 atp synthase is active in atp synthesis and is inhibited by angiostatin. Proc Natl Acad Sci USA. 2001;98:6656–6661. doi: 10.1073/pnas.131067798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tarui T, Miles LA, Takada Y. Specific interaction of angiostatin with integrin alpha (v) beta (3) in endothelial cells. J Biol Chem. 2001;152:39562–39568. doi: 10.1074/jbc.M101815200. [DOI] [PubMed] [Google Scholar]
- 18.Sharma MR, Tuszynski GP, Sharma MC. Angiostatin-induced inhibition of endothelial cell proliferation/apoptosis is associated with the down-regulation of cell cycle regulatory protein cdk5. J Cell Biochem. 2004:398–409. doi: 10.1002/jcb.10762. [DOI] [PubMed] [Google Scholar]
- 19.Troyanovsky B, Levchenko T, Mansson G, Matvijenko O, Holmgren L. Angiomotin: A angiostatin binding protein that regulates endothelial cell migration and tube formation. J Cell Biochem. 2001;152:1247–1254. doi: 10.1083/jcb.152.6.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O' Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: An endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 21.Dhanabal M, Ramchandran R, Waterman MJ, Lu H, Knebelmann B, Segal M, Sukhatme VP. Endostatin induces endothelial cell apoptosis. J Biol Chem. 1999;274:11726–11726. doi: 10.1074/jbc.274.17.11721. [DOI] [PubMed] [Google Scholar]
- 22.Kang HY, Shim D, Kang SS, Chang SI, Kim HY. Protein kinase b inhibits endostatin-induce apoptosis in huvecs. J Biochem Mol Bio. 2006;39:97–104. doi: 10.5483/bmbrep.2006.39.1.097. [DOI] [PubMed] [Google Scholar]
- 23.Kim YM, Hwang S, Pyun BJ, Kim TY, Lee ST, Gho YS, Kwon YG. Endostatin blocks vascular endothelial growth factor-mediated signaling via direct interation with kdr/flk-1. J Biol Chem. 2002;277:27872–27879. doi: 10.1074/jbc.M202771200. [DOI] [PubMed] [Google Scholar]
- 24.Rehn M, Veikkola T, Kukk-Valdre E, Nakamura H, Ilmonen M, Lombardo C, Pihlajaniemi T, Alitalo K, Vuori K. Interaction of endostatin with intergrins implicated in angiogenesis. Proc Natl Acad Sci USA. 2001;98:1024–1029. doi: 10.1073/pnas.031564998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Reilly MS, Wiederschain D, Stetler-Stevenson WG, Folkman J, Moses MA. Regulation of angiostatin production by matrix metalloproteinase-2 in a model of concomitant resistance. J Biol Chem. 1999;274:29568–29571. doi: 10.1074/jbc.274.41.29568. [DOI] [PubMed] [Google Scholar]
- 26.Dong Z, Kumar R, Yang X, Fidler IJ. Macrophage-derived metalloelastase is responsible for the generation of angiostatin in lewis lung carcinoma. Cell. 1997;88:801–810. doi: 10.1016/s0092-8674(00)81926-1. [DOI] [PubMed] [Google Scholar]
- 27.Cornelius LA, Nehring LC, Harding E, Bolanowski M, Welgus HG, Kobayashi DK, Pierce RA, Shapiro SD. Matrix metalloproteinase generate angiostatin: Effects on neovasculariation. J Immunol. 1998;161:6845–6852. [PubMed] [Google Scholar]
- 28.Lijnen HR, Ugwu F, Bini A, Collen D. Generation of an angiostatin-like fragment from plasminogen by stromelysin-1 (mmp-3) Biochemistry. 1998;37:4699–4702. doi: 10.1021/bi9731798. [DOI] [PubMed] [Google Scholar]
- 29.Patterson BC, Sang QA. Angiostatin-converting enzyme activities of human matrilysin (mmp-7) and gelatinase b/type iv collagenase (mmp 9) J Biol Chem. 1997;272:28823–28825. doi: 10.1074/jbc.272.46.28823. [DOI] [PubMed] [Google Scholar]
- 30.Ferreras M, Felbor U, Lenhard T, Olsen B, Delaisse JM. Generation and degradation of human endostatin protein by various proteinase. FEBS Letters. 2000;486:247–251. doi: 10.1016/s0014-5793(00)02249-3. [DOI] [PubMed] [Google Scholar]
- 31.Hou P, Troen T, Ovejero M, Kirkegaard T, Andersen T, Byrjalsen I, Ferreras M, Sato T, Shapiro S, Foged N, Delaisse JM. Matrix metalloproteinase-12 (mmp 12) in osteoclasts: New lesson on the involvement of mmps in bone resorption. Bone. 2004;34:37–47. doi: 10.1016/j.bone.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 32.Cai WJ, Kocsis E, Wu X, Rodriguez M, Luo X, Schaper W, Schaper J. Remodeling of the vascular tunica media is essential for development of collateral vessels in the canine heart. Mol Cell Bioch. 2004;264:201–210. doi: 10.1023/b:mcbi.0000044389.65590.57. [DOI] [PubMed] [Google Scholar]
- 33.Dodd T, Jadhav R, Wiggins L, Stewart J, Smith E, Russell J, Rocic P. Mmps 2 and 9 are essential for coronary collateral growth and are prominently regulated by p38 mapk. J Mol Cell Cardiol. 2011;6:1015–1025. doi: 10.1016/j.yjmcc.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siefert SA, Sarkar R. Matrix metalloproteinases in vascular physiology and disease. Vascular. 2012;20:210–216. doi: 10.1258/vasc.2011.201202. [DOI] [PubMed] [Google Scholar]
- 35.O'Reilly MS, Holmgren L, Cehn C, Folkman J. Angiostatin induces and sustains dormancy of human primary tumors in mice. Nat Me. 1996;2:689–692. doi: 10.1038/nm0696-689. [DOI] [PubMed] [Google Scholar]
- 36.Cao Y. Endogenous angiogenesis inhibitors and their therapeutic implications. Int J Biochem Cell Biol. 2001:357–369. doi: 10.1016/s1357-2725(01)00023-1. [DOI] [PubMed] [Google Scholar]
- 37.O'Reilly S, Pirie-Shepherd S, Lane WS, Folkman J. Antiangiogenic activity of the cleaved conformation of the serpin antithrombin. Science. 1999;285:1926–1928. doi: 10.1126/science.285.5435.1926. [DOI] [PubMed] [Google Scholar]
- 38.Maeshima Y, Colorado PC, Torre A, Holthaus KA, Grunkemeyer JA, Ericksen MB, Hopfer H, Xiao Y, Stillman IE, Kalluri R. Distinct antitumor properties of a type iv collagen domain derived from basement membranes. J Biol Chem. 2000;275:21340–21348. doi: 10.1074/jbc.M001956200. [DOI] [PubMed] [Google Scholar]
- 39.Kamphaus GD, Colorado PC, Panka DJ, Hopfer H, Ramchandran R, Torre A, Maeshima Y, Mier JW, Sukhatme VP, Kalluri R. Canstatin, a novel matrix-derived inhibitor of angiogenesis and tumor growth. J Biol Chem. 2000;275:1209–1215. doi: 10.1074/jbc.275.2.1209. [DOI] [PubMed] [Google Scholar]
- 40.Pike SE, Yao L, Jones KD, Cherney B, Appella E, Sakaquchi K, Nakhasi H, Teruya-Feldstein J, Wirth P, Gupta G, Tosato G. Vasostatin, a calreticulin fragment, inhibits angiogenesis and suppresses tumor growth. J Exp Med. 1998;188:2349–2356. doi: 10.1084/jem.188.12.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clapp C, Martial JA, Guzman RC, Rentier-Delura F, Weiner RI. The 16-kilodalton n-terminal fragment of human prolactin is a potent inhibitor of angiogenesis. Endocrinology. 1993;133:1292–1299. doi: 10.1210/endo.133.3.7689950. [DOI] [PubMed] [Google Scholar]
- 42.Ramchandran R, Dhanabal M, Volk R, Waterman MJ, Segal M, Lu H, Knebelmann B, Sukhatme VP. Antiangiogenic activity of restin, nc10 domiain of human collagen xv: Comparison to endostatin. Biochem Biophys Res Commun. 1999;255:735–739. doi: 10.1006/bbrc.1999.0248. [DOI] [PubMed] [Google Scholar]
- 43.Colorado PC, Torre A, Kamphaus G. Anti-angiogenic cues from vascular basement membrane collagen. Cancer Res. 2000;60:2520–2526. [PubMed] [Google Scholar]
- 44.O'Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Moses M, Lane WS, Cao Y, Sage EH, Folkman J. Angiostatin: A novel angiogenesis inhibitor that mediates the suppression of metastases by a lewis lung carcinoma. Cell. 1994;79:315–328. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 45.Claesson-Welsh L, Welsh M, Ito N, Anand-Apte B, Soker S, Zetter B, O'Reilly M, Folkman J. Angiostatin induces endothelial cell apoptosis and activation of focal adhesion kinase independently of the integrin-binding motif rgd. Proc Natl Acad Sci USA. 1998;95:5579–5583. doi: 10.1073/pnas.95.10.5579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lucas R, Holmgren L, Garcia I, Jimenez B, Mandriota SJ, Borlat F, Sim BKL, Wu Z, Grau GE, Shing Y, Soff GA, Bouck N, Pepper MS. Multiple forms of angiostatin induce apoptosis in endothelial cells. Blood. 1998;92:4730–4741. [PubMed] [Google Scholar]
- 47.Redlitz A, Daum G, Sage EH. Angiostatin diminishes activation of the mitogen-activated protein kinases erk-1 and erk-2 in human dermal microvascular endothelial cells. J Vasc Res. 1999;36:28–34. doi: 10.1159/000025623. [DOI] [PubMed] [Google Scholar]
- 48.Salvesen GS, Dixit VM. Caspases: Intracelluar signaling by proteolysis. Cell. 1997;91:443–446. doi: 10.1016/s0092-8674(00)80430-4. [DOI] [PubMed] [Google Scholar]
- 49.Knebelmann B, Dhanabal M, Ramchandran R, Waterman M, Lu H, Sukhatme VP. Endostatin inhibits vegf and bfgf induced mapk activation in endothelial cells. Proc Ameri Assoc Cancer Res Ann Meet. 1999;40:414–420. [Google Scholar]
- 50.Margheri F, Serrati S, Lapucci A, Anastasia C, Giusti B, Pucci M, Torre E, Bianchini F, Calorini L, Albini A, Ventura A, Fibbi G, Dell Rossa M. Systemic sclerosis-endothelial cell antiangiogenic pentraxin 3 and matrix metalloprotease 12 control human breast cancer tumor vascularization and development in mice. Neoplasia. 2009;11:1106–1115. doi: 10.1593/neo.09934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gorrin-Rivas MJ, Arii S, Mori A, Hanaki K, Maeda M, Furuyama H, Kondo Y, Imamura M. Mouse macrophage metalloelastase gene transfer into a murine melanoma suppresses primary tumor growth by halting angiogenesis. Clin Cancer Res. 2000;6:1647–1654. [PubMed] [Google Scholar]
- 52.Jawad MU, Garamszegi N, Garamszegi SP, Correa-Medina M, Diez JA, Wen R, Scully SP. Matrix metalloproteinase 1: Role in sarcoma biology. PLoS One. 2010;5:e14250. doi: 10.1371/journal.pone.0014250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kramer MD, Reinartz J, Brunner G, V S. Plasmin in pericellular proteolysis and cellular invasion. Invasion metastasis. 1994;14:210–222. [PubMed] [Google Scholar]
- 54.Andreasen PA, Egelund R, Petersen HH. The plasminogen activation system in tumor growth, invasion, and metastasis. Cell Mol Life Sci. 2000;57:25–40. doi: 10.1007/s000180050497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hashimoto G, Inoki I, Fujii Y, Aoki T, Ikeda E, Okada Y. Matrix metalloproteinases cleave connective tissue growth factor and reactivate angiogenic activity of vascular endothelial growth factor. J Biol Chem. 2002;277:36288–36295. doi: 10.1074/jbc.M201674200. [DOI] [PubMed] [Google Scholar]
- 56.Whitelock JM, Murdoch AD, Iozzo RV, Underwood PA. The degradation of human endothelial cell-derived perlecan and release of bound basic fibroblast growth factor by stromelysin, collagenase, plasmin, and heparanases. J Biol Chem. 1996;271:10079–10086. doi: 10.1074/jbc.271.17.10079. [DOI] [PubMed] [Google Scholar]
- 57.Meisner JK, Price RJ. Spatial and temporal coordination of bone marrow-derived cell activity during arteriogenesis: Regulation of the endogenous response and therapeutic implications. Microcirculation. 2010;17:583–599. doi: 10.1111/j.1549-8719.2010.00051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guerci B, Bohme P, Kearney-Schwartz A, Zannad F, Drouin P. Endothelial dysfunction and type 2 diabetes. Part 2: Altered endothelial function and the effects of treatment in type 2 diabetes mellitus. Diabetes Metab. 2001;27:436–447. [PubMed] [Google Scholar]
- 59.Hanzu FA, Paloma M, Kalko SG, Parrizas M, Garaulet M, Escolar G, Gomis R, Diaz-Ricart M. Translational evidence of endothelial damage in obese individuals: Inflammatory and prothrombotic responses. J Thromb Haemost. 2011;9:1236–1245. doi: 10.1111/j.1538-7836.2011.04285.x. [DOI] [PubMed] [Google Scholar]
- 60.Chen C, Tempel D, Van Haperen R. Activation of mmp 8 and mmp13 by angiotensin ii correlates to severe intraplaque hemorrhages and collagen breakdown in atherosclerotic lesions with a vulnerable phenotype. Atherosclerosis. 2009;204:26–33. doi: 10.1016/j.atherosclerosis.2009.01.025. [DOI] [PubMed] [Google Scholar]
- 61.Jadhav R, Dodd T, Smith E, Bailey E, Delucia AL, Russell JC, Madison R, Potter B, Walsh K, Jo H, Rocic P. Angiotensin type 1 receptor blockade in conjunction with enhanced akt activation restores coronary collateral growth in the metabolic syndrome. Am J Physiol Heart Circ Physiol. 2011;300:H1938–H1949. doi: 10.1152/ajpheart.00282.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reed R, Potter B, Smith E, Jadhav R, Villalta P, Jo H, Rocic P. Redox-sensitive akt and src regulate coronary collateral growth in metabolic syndrome. Am J Physiol Heart Circ Physiol. 2009;296:H1811–H1821. doi: 10.1152/ajpheart.00920.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and timps. Cardiovasc res. 2006;69:562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 64.Haorah J, Ramirez SH, Schall K, Smith D, Pandya R, Persidsky Y. Oxidative stress activates protein tyrosine kinase and matrix metalloproteinases leading to blood-brain barrier dysfunction. J Neurochem. 2007;101:566–576. doi: 10.1111/j.1471-4159.2006.04393.x. [DOI] [PubMed] [Google Scholar]
- 65.Zambon A, Pauletto P, Crepaldi G. Review article: The metabolic syndrome- a chronic cardiovascular inflammatory. Aliment Pharmacol Ther. 2005;22:20–23. doi: 10.1111/j.1365-2036.2005.02589.x. [DOI] [PubMed] [Google Scholar]
- 66.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome-a new world-wide definition. A consensus statement from the international diabetes federation. Diabet Med. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 67.Margheri F, Serrati S, Lapucci A, Chilli A, Bazzichi L, Bombardieri S, Kahaleh B, Calorini L, Bianchini F, Fibbi G, Del Rosso M. Modulation of the angiogenic phenotype of normal and systemic sclerosis endothelial cells by gain-loss of function of pentraxin 3 and matrix metalloproteinase 12. Arthritis Rheum. 2010;62:2488–2498. doi: 10.1002/art.27522. [DOI] [PubMed] [Google Scholar]
- 68.Harris LK, Smith SD, Keogh RJ, Jones RL, Baker PN, Knöfler M, Cartwright JE, Whitley GS, Aplin JD. Trophoblast- and vascular smooth muscle cell-derived mmp-12 mediates elastolysis during uterine spiral artery remodeling. Am J Pathol. 2010;177:2103–2115. doi: 10.2353/ajpath.2010.100182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.O'Hara A, Lim FL, Mazzatti DJ, Trayhurn P. Microarray analysis identifies matrix metalloproteinases (mmps) as key genes whose expression is up-regulated in human adipocytes by macrophage-conditioned medium. Pflugers Arch. 2009;458:1103–1114. doi: 10.1007/s00424-009-0693-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.