Abstract
Reversible protein phosphorylation plays a pivotal role in intercellular communication. Together with protein tyrosine kinases, protein tyrosine phosphatases (PTPs) are involved in the regulation of key cellular processes by controlling the phosphorylation levels of diverse effectors. Among PTPs, receptor-like protein tyrosine phosphatases (RPTPs) are involved in important developmental processes, particularly in the formation of the nervous system. Until recently, few ligands had been identified for RPTPs, making it difficult to grasp the effects these receptors have on cellular processes as well as the mechanisms through which their functions are mediated. However, several potential RPTP ligands have now been identified to provide us with unparalleled insights into RPTP function. In this review, we will focus on the nature and biological outcomes of these extracellular interactions between RPTPs and their associated ligands.
Keywords: Phosphatases, Ligands, Receptor-ligand interactions, RPTP
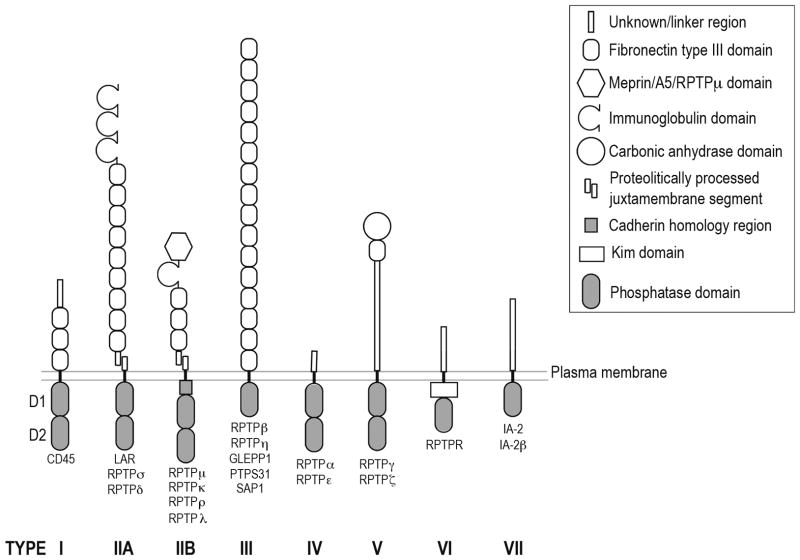
Protein tyrosine phosphatases (PTPs) are enzymes, which, alongside protein tyrosine kinases (PTK), play a crucial role in the regulation of cellular protein tyrosine phosphorylation levels. Receptor-like protein tyrosine phosphatases (RPTPs) were first discovered in the late 1980s [1]; there are now 39 human PTPs grouped into 17 subtypes, of which 22 are RPTPs grouped into 8 subtypes [2] (Figure 1). Structurally, (RPTPs) consist of one or two highly conserved intracellular phosphatase domains and an extracellular portion, which is variable across different subtypes. The extracellular domains of most RPTPs have a modular architecture reminiscent of that found for cell-adhesion molecules (CAMs) and are involved in cell-cell and cell-matrix interactions (Figure 1) [3]. Driven by the impetus to understand the biological functions and signaling properties of these receptors, many groups worked to identify putative ligands for RPTPs to identify the extracellular cues to which they respond. Historically, these investigations have proven challenging, in contrast to the relative facility with which ligands of PTKs were identified, and to this day most RPTPs remain orphan receptors. An additional challenge presents itself when we consider the possibility that not all RPTPs interact exclusively with ligands, but rather initiation of downstream signaling events might be mediated through homophilic interactions, as a number of RPTPs are capable of homophilic binding.
Figure 1. Domain organization of human receptor protein tyrosine phosphatases.
The eight families of RPTPs are shown here, many of which participate in various neural processes such as neuronal survival, synapse formation, axon guidance and neurite outgrowth (types IIa, IIb, III and V) [3].
A second major obstacle to understanding RPTP function is that it is difficult to discern a single model for the regulation of their phosphatase activity. Initially, a mechanism of RPTP regulation was proposed based on the crystal structure of the membrane-proximal domain of RPTPα (encoded by PTPRA). In this structure, two PTP domains are organized in symmetrical dimers so that an inhibitory motif of one domain, called a wedge, occludes the active site of another domain [4]. This finding came on the heels of experiments that demonstrated that the phosphatase activity of a chimera of the EGF receptor ectodomain fused with the intracellular region of CD45 (PTPRC) could be inhibited upon EGF binding [5] and led to the hypothesis that RPTPs could be regulated by a dimerization/inhibition mechanism. Subsequently, however, the structure of the catalytic domain of RPTPμ (PTPRM) did not reveal dimers similar to the one formed by RPTPα [6] and the structures of the tandem phosphatase domains of LAR (PTPRF) and CD45 indicated that the placement of the D2 domain is incompatible with the arrangement of the D1 domains in the RPTPα dimer [7, 8]. The final twist in the regulation of phosphatase activity by dimerization came from the crystal structure of the D1D2 region of RPTPγ (PTPRG) in which the active site is occluded by formation of the dimer interface [9] so that the phosphatase activity is regulated by modulating the availability of the active site [10].
There still are gaps in our understanding of RPTPs and their ligands, but recent work has provided us with novel insights into this mysterious group of receptors. In this review, we will describe some of these recent advances and introduce the various ligands of RPTPs described thus far and the effects they have on downstream signaling pathways.
I. Heterophilic interactions
RPTPζ and Pleiotrophin
RPTPζ (PTPRZ1) occupies a special place in the phosphatase field. Indeed, it is the first RPTP for which a heterophilic ligand was discovered (contactin, see below). Yet perhaps more importantly, RPTPζ is an RPTP for which a link between ligand-dependent dimerization and inhibition of the intracellular tyrosine phosphatase activity has been clearly established. A decade ago, Meng and colleagues reported that the heparin-binding growth factor pleiotrophin (PTN) interacted with the extracellular region of RPTPζ and that this interaction led to a decrease in the intrinsic tyrosine phosphatase activity of RPTPζ [11]. Furthermore, it was later shown that PTN induced the formation of RPTPζ oligomers, which was again accompanied by a loss of phosphatase activity in cells [12]. What are the physiological consequences of interactions between PTN and RPTPζ? In cells transfected with RPTPζ, phosphorylation of its endogenous substrate G protein-coupled receptor kinase-interactor 1 (Git1) was increased following treatment with PTN establishing a direct link between PTN binding, RPTPζ oligomerization and decreased phosphatase activity [12]. Moreover, addition of PTN to U373 cells, a glioblastoma cell line that expresses RPTPζ, leads to increased levels of phosphorylated β-catenin, thereby impairing the association between β-catenin and N-cadherin and disrupting adherens junction complexes [11, 13]. Importantly, these PTN/RPTPζ-induced changes culminate with an epithelial-mesenchymal transition in U373 cells, which is consistent with the finding that knockdown of RPTPζ expression in glioblastoma cells prevents their migration after treatment by PTN [14].
Interestingly, the effect of the PTN/RPTPζ complex on cell migration has been linked to integrin αvβ3 [15]. Integrins are heterodimers consisting of both an α and β subunits that mediate various processes such as cell adhesion, migration and differentiation [16]. Although PTN was shown to induce migration of endothelial cells via interaction with RPTPζ [17], further experiments demonstrated that RPTPζ-expressing cells, when stimulated with PTN, did not migrate as readily and that loss of RPTPζ led to pleiotrophin-mediated adhesion and migration [18]. However, cell migration could be observed when αvβ3 was expressed with RPTPζ, in line with the finding that integrin αvβ3 directly interacts with PTN and RPTPζ, and is required for PTN-induced phosphorylation of the β3 subunit [15]. A possible explanation for these observations is that the phosphatase activity of RPTPζ is attenuated via interaction with integrin αvβ3, thereby allowing for PTN-induced migration to occur.
RPTPζ/RPTPγ and the contactins
The type V subgroup of RPTPs includes the two homologues RPTPγ and RPTPζ, which are implicated in axon guidance and neurite outgrowth [3]. RPTPγ is involved in chick spinal cord neurogenesis, where it is important for the proliferation of neuronal precursors and motor neuron precursor migration [19]. It is also expressed in mouse sensory neurons and organs, but its deficiency is not associated with any significant defects of development [20]. Both RPTPs are expressed in the developing and adult brains of vertebrates [21]. Structurally, the extracellular portion of RPTPγ and RPTPζ is comprised of a carbonic anhydrase-like (CA) domain and a single fibronectin type III (FNIII) domain; the intracellular portion consists of two phosphatase domains [22, 23]. Though similar in structure, RPTPζ and RPTPγ are expressed by different cell types of the CNS and have different effects. RPTPζ is expressed by astrocytes and oligodendrocytes as well as a subset of neurons, while RPTPγ is predominantly found on neurons [21].
The first binding partner reported for RPTPζ is the cell-adhesion molecule contactin 1 (CNTN1) [24], a member of the contactin protein family of neural recognition molecules. Contactins are extracellular neural immunoglobulin (Ig) domain-containing CAMs, which are composed of six N-terminal Ig domains, four FNIII domains, and a C-terminal glycophosphatidylinositol anchor. Contactins are expressed during the various stages of neural development and in the adult brain [25]. The identification of CNTN1 as the binding partner of RPTPζ along with the high degree of homology within the contactin family suggested that other contactins could also bind RPTPζ or RPTPγ. Affinity binding assays have shown that RPTPγ binds CNTN3, 4, 5 and 6, while RPTPζ binds only CNTN1 [21].
In spite of this distinct binding specificity, both RPTPζ and RPTPγ use their homologous CA domains to form a complex with contactins. The crystal structure of the mouse CNTN4Ig14/RPTPγCA complex has revealed that the interaction between these two molecules is mediated mostly by the β-hairpin loop of the RPTPγ CA domain and the Ig domains 2 and 3 of CNTN4, which adopt a horseshoe–like conformation. The two strands of RPTPγCA β-hairpin combine with three antiparallel strands of CNTN4Ig14 to form a 5-strand antiparallel β-sheet and amino acid residues in the hairpin region make key contacts with CNTN4 residues [21]. All the CNTN4 residues which mediate the binding interactions within the binding site are conserved in CNTN3, 5, and 6 [21], so it is likely that RPTPγ uses a similar binding mode to interact with these receptors. Given the similarities between CNTN1 and CNTN4 on one hand and RPTPγ and RPTPζ on the other hand, it was predicted that the structure of the CNTN1/RPTPζ complex would be similar to that of the CNTN4/RPTPγ complex. The crystal structure of CNTN1Ig23/RPTPζCA indeed shows this to be the case: Ig domains 2 and 3 of CNTN1 adopt a horseshoe-like conformation which bind to the β-hairpin loop of RPTPζ [26]. The binding sites in the CNTN1/RPTPζ and the CNTN4/RPTPγ complexes overlap completely and the strict specificity observed between CNTN1 and RPTPζ can be solely explained by amino acid substitutions at the complex interface in both RPTPζ and CNTN1.
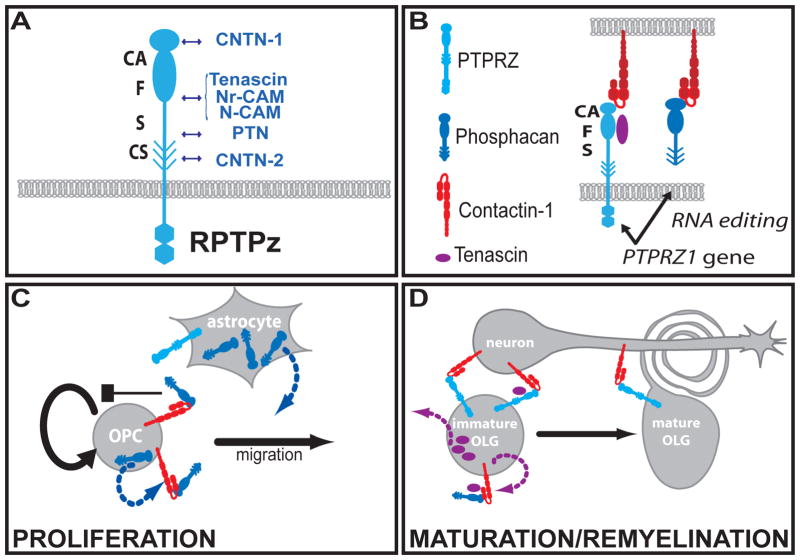
The physiological roles of the interactions between RPTPs and CNTNs are still not fully understood. It has been shown that RPTPζ, which is expressed on the surface of glial cells, is able to bind CNTN1 expressed on the surface of axons. This interaction appears to be important in the promotion of neurite outgrowth and glial adhesion [19]. Recent investigations also suggest that the formation of the complex between the soluble form of RPTPζ (phosphacan) and CNTN1 expressed on the surface oligodendrocyte precursor cells (OPCs) regulates the proliferation of these cells. The existing model suggests that at first, phosphacan binds CNTN1 expressed on the surface of OPCs, thereby inhibiting OPC proliferation. As the expression of phosphacan decreases, OPCs differentiate into immature oligodendrocytes that express less CNTN1. Finally, the phosphacan/CNTN1 complex is replaced by another complex, which consists of transmembrane RPTPζ on the surface of glial cells and CNTN1 on the surface of axons. This complex is believed to promote the differentiation of oligodendrocytes and the ensheathment of axons [26]. In this model, soluble RPTPζ is at first a ligand for CNTN1, later trading roles with CNTN1 acting as a ligand of transmembrane RPTPζ (Fig. 2). In addition, using cultures lacking RPTPζ, the CA domain of PTPRZ engagement of OPC CNTN1 is sufficient to repress OPC proliferation, while engagement with the domains CAFS (carbonic anhydrase-like (CA), fibronectin type III (F), and the spacer (S) domains) is needed for a differentiation-inducing signal that partially restores the morphological maturation of oligodendrocytes [26]. These results indicate that the FS (fibronectin type III and spacer) domain of RPTPζ is required to recruit differentiation-inducing molecules. For example, tenascins bind to phosphacan and CNTN1 [27] and play a role in oligodendrocyte development [28, 29] and thus could be implicated in the function of the RPTPζ-CNTN1 complex in oligodendrocyte differentiation (Figure 2).
Figure 2. Schematic model of PTPRz and its ligands in OPC development.
A. Ligands of PTPRZ. PTPRZ contains a carbonic anhydrase (CA) domain followed by a fibronectin type III domain (F), a spacer (S) then a stretch of chondroitine sulfate proteoglycan (CS) followed by a transmembrane domain and 2 intracellular phosphatase domains. B. Structure of PTPRZ and CNTN-1: CNTN-1 is composed of 6 immunoglobulin (Ig) repeats and 4 FNIII domains. The Ig2-Ig3 tandem repeats of CNTN1 adopt a horseshoe-like conformation and bind to the CA domain of PTPRZ. C–D. Model of PTPRZ and CNTN-1 function. Phosphacan binds to CNTN1 expressed by OPCs and inhibits proliferation. As OPCs differentiate into immature OLG, CNTN1 and phosphacan levels decrease at the surface of OLG (C). In a second step, the transmembrane isoform of PTPRZ on OLG binds to the axonal CNTN1 (D), a complex probably needed for complete myelination [26].
Our understanding of interactions between CNTN1 and RPTPζ and the various CNTNs and RPTPγ continues to expand. However, there is still much work that can be done towards furthering our understanding of the expression profile of these molecules, their interactions, and the resulting effects of such interactions on cell growth and development.
Type IIa RPTPs, Heparan sulfate and chondroitin sulfate
Type IIa RPTPs were the first family members shown to be involved in the development of the nervous system and probably are the best characterized family of RPTPs. Pioneering work showed that the sole Drosophila type IIa RPTP called leukocyte-antigen related receptor (Dlar) is involved in the guidance of motor axons [30] and later work demonstrated that Dlar mediates the formation of synapses at the neuromuscular junction [31]. Dlar and its vertebrate homologues LAR, RPTPδ (PTPRD) and RPTPσ (PTPRS) all include a cell adhesion molecule-like extracellular domain that includes three N-terminal Ig domains and eight to nine FNIII repeats as well as tandem intracellular tyrosine phosphatase domains. Mice deficient for type IIa RPTPs exhibited developmental abnormalities, decreased brain size, impaired learning ability, tremors and spastic movements, as well as decreased conduction velocity of peripheral nerves [32–36].
The first report of potential binding partners for a type IIa RPTP identified the heparan sulfate proteoglycans (HSPGs) agrin and collagen XVIII as ligands for chicken RPTPσ [37]. Interestingly, binding was entirely mediated by the glycosaminoglycan chains and affinities measured were in the nanomolar range. Subsequently, it was reported that fly syndecan and dally-like protein function as ligands for Dlar in vivo during the formation of neuromuscular junctions with dissociation constants in the nanomolar range [31, 38]. Syndecan and dally-like protein bind competitively to the N-terminal Ig region of Dlar, yet mediate distinct physiological outputs: syndecan expressed on the same cell as Dlar promoted normal synapse growth whereas dally-like protein found on an opposing cell antagonized the activity of LAR and led to the formation of stable synapses, which is mediated by the dephosphorylation of Enabled (Ena), an actin regulatory protein, by Dlar [31].
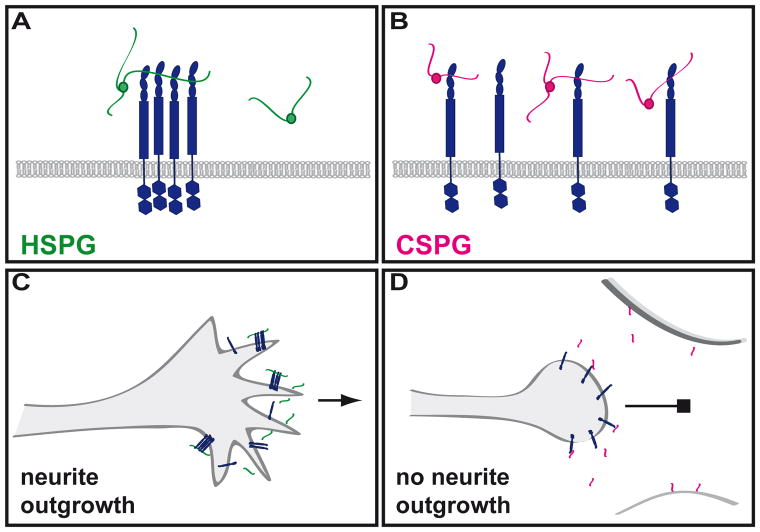
Similarly, further analysis of the function of RPTPσ in vivo demonstrated that interactions with proteoglycans mediate drastically distinct biological outcomes. In addition to HSPGs, RPTPσ binds to chondroitin sulfate proteoglycans (CSPGs) with dissociation constants comparable to those measured for HSPGs [39]. Importantly, however, interactions between HSPGs and RPTPσ mediate axonal growth whereas interactions with CSPGs inhibit it [40]. Both glycosaminoglycan chains bind to a basic region in the first Ig domain of RPTPσ that is conserved in Dlar and vertebrate type IIa RPTPs [37, 39–41], yet they exert opposite control on the oligomeric state of RPTPσ, as binding to heparan sulfate (HS) chains, but not to chondroitin sulfate (CS) chains, induces the clustering of the extracellular region of RPTPσ [40]. Even though the composition of CS and HS chains differ significantly, structural analyses of Ig domains 1 and 2 of RPTPσ indicate that differences in carbohydrate composition and in sulfation could be accommodated by a flexible loop in the glycosaminoglycan-binding site of RPTPσ [40].
Since CSPGs and HSPGs are both present at the cell surface, the mechanisms underpinning their opposite biological functions are particularly interesting. Changing ratios of HSPGs versus CSPGs could mediate the differential clustering of RPTPσ, which would in turn alter the distribution of tyrosine phosphatase activity at the cell surface [40]. Indeed, clustering of RPTPσ in response to HSPGs would presumably lead to a reduction of phosphatase activity and the resulting increase in local tyrosine phosphorylation would favor the growth of axons. Conversely, binding of RPTPσ to CSPGs prevents RPTPσ clustering and thus also prevents inhibition of phosphatase activity and increased local tyrosine phosphorylation, leading to prevention of axonal growth (Figure 3). Furthermore, binding of HS chains displayed on the same cell as RPTPσ would be responsible for growth activation whereas the CSPG ligands would be found on an apposing cell [40], a situation which echoes the findings obtained for Dlar with syndecan and dally-like protein [31].
Figure 3. Interactions of HSPG and CSPGs with the Type IIa RPTP, RPTPσ.
A, C. Interaction of HSPG with RPTPσ. Interaction with HSPGs induces tetrameric clustering of RPTPσ. Such interaction results in the promotion of neurite outgrowth. RPTPσ is found expressed at the growth cone in a punctate fashion. RPTPσ expression is similar but not identical to HSPG expression pattern. B, C. Interaction of CSPG with RPTPσ. Interaction of CSPGs with RPTPσ does not induce clustering of the RPTP. Neurite outgrowth is inhibited. CSPGs are expressed by surrounding, non-neuronal cells.
The association of RPTPσ with both CSPGs and HSPGs illustrates an interesting property of RPTPs compared to receptor tyrosine kinases (RTKs). Whereas RTKs tend to form discrete dimers in the presence of ligands, RPTPs could cluster in response to their cognate partners. This reorganization of the RPTP population could lead to, at the very least, a redistribution of phosphatase activity on the cell or even a complete inhibition of phosphatase activity. Because the first two Ig domains of LAR and RPTPδ are close structural homologues of those of RPTPσ and contain an essentially identical glycosaminoglycan-binding site [40, 41], it is tempting to speculate that they too bind to both HSPGs and CSPGs during neurogenesis. However, the specific roles that proteoglycans play in the physiological function of LAR and RPTPδ remain unclear.
Although critical, HSPGs and CSPGs are not the sole physiological binding partners of type IIa RPTPs, and recent work has identified non-proteoglycan ligands that would mediate adhesive interactions important for the organization of the synapse. The leucine-rich repeat molecule netrin-G ligand-3 (NGL3) binds to all three vertebrate type IIa RPTPs during the formation of excitatory synapses [42, 43]. In contrast to proteoglycans, NGL-3 binds to FNIII repeats 1 and 2 of LAR, RPTPδ and RPTPσ, thus illustrating that physiological functions of type IIa RPTPs can be mediated by distinct modules found in the extracellular regions of these receptors. Although they involve an identical ligand, the biological outputs of the synaptic adhesion complexes formed by NGL-3 and individual type IIa RPTPs are distinct. Interaction of LAR and RPTPσ with NGL-3 induces postsynaptic clustering of PSD-95 and promotes bidirectional formation of synapses in contrast to the NGL-3/RPTPδ complex, which favors only pre-synaptic differentiation in one direction [42]. It is noteworthy that binding of both LAR and RPTPσ resulted in clustering of PSD-95, which might indicate that LAR and RPTPσ themselves form clusters upon binding to NGL-3 and could thus lead to changes in dephosphorylation reminiscent of the effect of HSPG binding to RPTPσ. In such a case, differential clustering of receptors could once again account for the full spectrum of physiological processes mediated by type IIa RPTPs. On the other hand, bidirectional synaptic organization can also be mediated by the formation of a complex between the non-catalytic form of the neurotrophin receptor tyrosine kinase TrkC and RPTPσ [44]. The two molecules bind with nanomolar affinities and formation of the complex leads to a clustering of RPTPσ, as was observed with NGL-3. In contrast to NGL-3, TrkC does not interact with either LAR or RPTPδ and the interaction with RPTPσ is mediated by the N-terminal Ig regions and not by FNIII domains 1 and 2 so that, in theory, this interaction could compete with that of CSPGs and HSPGs. These recent studies are a significant development because they demonstrate that RPTPσ controls axonal growth and synapse formation by binding to distinct ligands using several modules in its ectodomain.
CD45 (RPTPc), RPTPκ and Galectin -1 and -3
CD45 is the prototypical receptor protein tyrosine phosphatase and the first to have been identified as such [45, 46]. It is highly expressed on all nucleated hematopoietic cells [47], plays a crucial role in thymocyte development [47–49], and modulates signal transduction in both T and B cells [50, 51] thereby influencing their survival and activation state. Various isoforms of the extracellular domain of CD45 exist due to alternative splicing of consecutive exons 4, 5 and 6 (termed A, B and C in the protein) [47]; these regions contain multiple sites for O-linked glycosylation with CD45RABC being the most O-glycosylated isoform and CD45RO, which lacks all three exons, the least [52]. Other isoforms have been detected at the protein level as well, including: CD45RB, RAB, and RBC [53]. The variable glycosylation of the CD45 isoforms influences the size and shape, as well as the charge, of the molecule, thus influencing ligand binding and, as a result, intracellular signaling [47, 54, 55]. The extracellular domain of CD45, following the variable regions, further consists of a cysteine-rich domain followed by three FNIII domains [53, 56] that are heavily N-glycosylated. The presence of these N-glycans are not only necessary for stability and proper transport of the phosphatase to the cell surface [57], but also serve as binding sites for galectin-1 [58, 59], macrophage mannose receptor, and CD22 [56]. Various molecules have been described to interact with and bind to CD45; among these galectin-1 has been described as a ligand for CD45.
The galectins are a family of β-galactoside-binding proteins [60] that contain conserved carbohydrate-recognition sites. Galectins have distinct specificities and recognize various galactose-containing glycans [61]. Some galectins are secreted and found in the extracellular space, in spite of the absence of a signal sequence normally required for protein secretion [62]. Out of the 15 known galectins, galectin-1 and -3 interact with two RPTPs: CD45 (RPTPc) and RPTPκ (PTPRK), respectively.
Galectin-1 has been described as a ligand for CD45 expressed on T cells [63], playing a role in regulating T cell death at various stages of thymocyte development and maturation [59]. Several binding sites are present on CD45 for galectin-1 [64] and, upon binding of galectin-1 to the extracellular domain of CD45, clustering is induced [65] and the intrinsic phosphatase activity is inhibited [56, 63, 66]. However, the outcome of the interaction between CD45 and galectin-1 is dependent on the glycosylation state of CD45 as galectin-1 binds to lactosamine sequences on N- and O-glycans [58]. The specific glycan influences the ability of galectin-1 to bind to and modulate CD45 phosphatase activity. For instance, in cells expressing low molecular weight CD45, the presence of core 2 O-glycans are required for galectin-1 binding and subsequent decrease in CD45 activity; however, the presence of α2,6-linked sialic acids on N-glycans blocks galectin-1 binding [58].
The ectodomain of RPTPκ, when cleaved from the cell surface, has been shown to mediate cancer cell migration during cancer progression [67]. It was demonstrated that RPTPκ is cleaved by a secreted form of proprotein convertase 5 (PC5A) [68]. It was also shown that RPTPκ cleavage is modulated by galectin-3 binding protein, likely mediated through its binding to galectin-3 [68]. Galectin-3 is a secreted glycoprotein, which recognizes the N-glycans of RPTPκ. It is structurally unique among the galectins and has two distinct domains: the carbohydrate-recognition domain, and an atypical N-terminal domain required for full biological activity and which may mediate formation of oligomers [69]. In its interaction with RPTPκ, whereas galectin-3 binding protein facilitates cleavage of RPTPκ, the presence of galectin-3 decreases the likelihood of RPTPκ cleavage. Accordingly, suppression of galectin-3 gene expression increases shedding of RPTPκ [68].
II. Homophilic interactions
RPTPμ is a type II RPTP that undergoes homophilic interactions, mediating cell-cell aggregation [70–72]. Other RPTPs demonstrated to mediate homophilic binding include RPTPδ [73], RPTPκ [72] RPTPλ/PCP-2 (PTPRU) [74] and RPTPρ (PTPRT) [75]. Structurally, the latter two tyrosine phosphatases are closely related and display similarity to cell-adhesion molecules [70, 72, 74]. However, in spite of their structural similarity, these RPTPs do not interact with each other heterophilically [70, 76].
Using transfected insect Sf9 cells expressing full-length RPTPμ, this molecule was demonstrated to promote cell aggregation in a homophilic manner [71, 77] (Figure 4B), independently of its phosphatase activity [71, 77] and of Ca2+ [77]. Similarly, other members of the same RPTP family (RPTPρ, RPTPκ) mediate cell aggregation [72, 75, 78] although RPTPλ does not [76]. Homophilic interactions were shown to be mediated by the Ig domain of the extracellular domain [71, 79]. In contrast, Zondag et al. showed the MAM domain to be essential, although not sufficient, for homophilic interactions [70] in cis rather than in trans [80]. However, more recent studies now show that the MAM, Ig and FN domains are all required for dimerization interactions, with the MAM and Ig domains on one molecule of RPTPμ interacting with the FN1 and 2 domains of the other [81, 82] (Figure 4A).
Figure 4. RPTPμ interactions and effects.
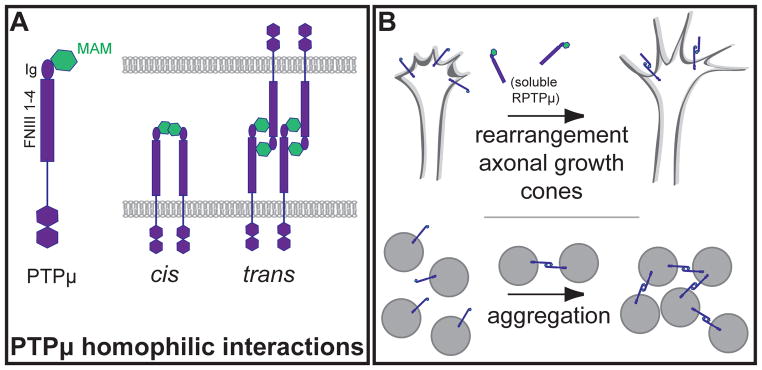
A. RPTPμ and homophilic binding. Structure of RPTPμ: the ectodomain consists of four FNIII repeats, an Ig domain, and the MAM domain. RPTPμ can interact in cis and in trans. B. Effects of RPTPμ homophilic interactions. (top) RPTPμ-mediated homophilic interactions have been shown to trigger rearrangement of growth cones [83]; with addition of soluble RPTPμ and subsequent homophilic binding, growth cones transition from lamellipodia-dominant to fillipodia-dominant morphology. (bottom) Cells aggregate in clusters, mediated by RPTPμ interactions.
The effect of homophilic interactions beyond cell-cell adhesion and whether such interactions also trigger phosphatase activity requires further investigation. Such an effect does seem likely: RPTPμ-mediated homophilic interactions have been shown to trigger the rearrangement of axonal growth cones [83] (Figure 4B). Homophilic binding of RPTPμ leads to changes in the cytoskeleton thus affecting axon outgrowth, or repulsion, via tyrosine phosphatase-dependent signaling [84]. On the other hand, the aggregation of Sf9 cells expressing RPTPμ did not result in significant changes in cellular phosphotyrosine levels [71]. Another study, by Aricescu et al., describes RPTPμ as forming homophilic dimers in trans, responsible for maintaining the spacing between cells. Such interactions and the resulting clustering of various molecules and substrates at cell-cell contacts may act to ‘lock’ and concentrate phosphatase activity to a specific region [85].
In general, the homophilic interactions leading to dimerization of protein tyrosine phosphatases may be a way to regulate the activity of the phosphatases. However, whether this regulation is inhibitory or activating may depend on the RPTP under consideration. For instance, the crystal structure of RPTPα indicates the presence of an inhibitory wedge that, in the dimerized structure, reciprocally occludes the catalytic sites of the D1 domains [4, 86]. It seems CD45 may also be regulated in a similar fashion, undergoing cis dimerization thereby inhibiting enzymatic activity [87]. However, in the case of RPTPσ, while it has been shown to exist in a dimeric state, this interaction is not inhibitory. It is the dimeric form of this RPTP rather than the monomers which bind to extracellular ligands [88]. In this case, dimerization still does regulate phosphatase activity, albeit an activating rather than inhibitory regulation.
Whether the other RPTPs capable of homophilic interactions trigger, or inhibit, downstream signaling is yet to be determined and further studies are warranted.
III. Protein tyrosine kinases, substrates or ligands of RPTPs?
Protein tyrosine kinases (PTKs) transmit extracellular signals and regulate various cellular process which are initiated through dimerization, induced by ligand-binding, leading to autophosphorylation [89, 90]. RPTPs can act as negative regulators of PTK signaling: for example, RPTPO (RPTPO) can diminish NT-3-induced TrkC phosphorylation [91], RPTPζ exerts an influence on TrkA [92, 93], and RPTPβ (PTPRB) dephosphorylates its substrate Met, specifically at the binding site for several downstream effector molecules [94].
PTPRO is a neuronal type III RPTP which forms dimers in living cells, the formation of which is most likely regulated by disulfide linkages [91]. This RPTP is co-expressed with tropomyosin-related kinase (Trk) C in neurons of the sensory and cranial ganglia, the spinal cord, and cortex [95] thus making TrkC a potential substrate for PTPRO. PTPRO is regulated via ligand-induced dimerization; it was found that nerve growth factor (NGF)-induced dimerization of the transmembrane and intracellular domains of a TrkC-RPTPO fusion protein resulted in decreased PTP activity [91]. Neurotrophin-3 (NT-3) is a ligand for TrkC and induces phosphorylation of the receptor; co-expression of TrkC with RPTPO leads to TrkC dephosphorylation, reducing the degree of activation induced by NT-3 [91]. TrkC also co-precipitates with PTPRO [91] indicating a physical interaction between these two proteins exists and thereby supporting TrkC as a possible substrate for RPTPO. In mice lacking full-length PTPRO, axon guidance from TrkC-expressing sensory neurons is perturbed [96] indicating the importance of TrkC regulation by this phosphatase. Further studies on the regulation and effect of PTPRO phosphatase activity are required.
Another Trk, TrkA, which is normally activated by NGF, also interacts with RPTPζ. As with all tyrosine kinases, TrkA becomes phosphorylated at specific tyrosine residues upon ligand binding [97] thus recruiting and activating downstream signaling molecules. Interacting with RPTPζ leads to dephosphorylation of TrkA at two sites (Y674 and Y675) [93], which are responsible for the regulation of Trk tyrosine kinase activity, thereby modulating the sensitivity of cells to NGF [93]. However, while interaction with RPTPζ is responsible for the dephosphorylation of TrkA, this interaction itself is regulated by PTN, which inactivates RPTPζ.
We have already considered the interactions between PTN and RPTPζ here. To complete the picture, we will also examine the interaction of PTN and RPTPζ with anaplastic lymphoma kinase (ALK), another RTK. ALK has been proposed as a receptor for PTN [98–100] and there are various reports to support this. One study demonstrates indirect activation of ALK by PTN via RPTPζ. They suggest that RPTPζ dephosphorylates ALK, but upon stimulation by PTN, RPTPζ is inactivated, allowing ALK to retain an activated, phosphorylated state [92]. Supporting this notion, PTN had opposing effects depending on whether RPTPζ or ALK was expressed. A loss of RPTPζ led to pleiotrophin-mediated adhesion and migration whereas loss of ALK resulted in an inhibition of migration [18]. Whether PTN directly interacts with ALK or indirectly via RPTPζ, is still undetermined.
RPTPs regulate the phosphorylation state of various PTKs, either directly or indirectly via downstream signaling pathways. They themselves are regulated, via ligand-induced dimerization, which attenuates phosphatase activity thus influencing the phosphorylation state of the PTKs with which they interact. These interactions are complex and do involve a plethora of interactions between various PTKs and RPTPs, in addition to their respective ligands, all of which contribute to the fine-tuning of the phosphorylation, and thus activation, state of the proteins involved.
IV. Shedding of PTPs: generation of ligands?
Various RPTPs undergo proteolytic processing to generate different forms of the receptors; the products of such processing may have unique roles and functions. Cleavage and/or shedding of RPTP ectodomains may either modify phosphatase activity or may also be implicated in the generation of ligands, as in the case of RPTPζ and phosphacan. Among other effects, processing of PTPs may influence the subcellular localization, which has implications in the regulation of their physiological roles [101, 102]. For instance, the transmembrane form of RPTPε is involved with the down-regulation of insulin receptor signaling [103, 104] among other functions, whereas the cytoplasmic form dephosphorylates and down-regulates activity of delayed-rectifier potassium channels [103]. There are a number of PTPs that undergo processing post-translationally. RPTPζ, Type IIa RPTPs LAR and RPTPσ, PTPRR (PTPRR) isoform RPTP-BR7, and RPTPα and RPTPε all have been shown to undergo proteolytic processing, thereby modulating their roles [105–110].
LAR is composed of two subunits, one containing the extracellular domain and the other the intracellular, phosphatase domains [110]. The extracellular portion is cleaved and shed during cell growth, implicating shedding as a mechanism to regulate phosphatase function [109, 110]. RPTPσ also undergoes cleavage, resulting in shedding of the extracellular domain. One study shows that in addition and subsequent to proteolytic processing and cleavage, the intracellular portions of both LAR and RPTPσ are internalized and relocated in the cell, away from cell-cell contact zones, thereby further regulating phosphatase activity [108].
There already are various isoforms under the PTPRR umbrella, of which PTPBR7 is one. This RPTP is located at the cell membrane, at vesicles and the Golgi apparatus [105]. It is cleaved at the cell surface, undergoing proteolytic processing at its N-terminal [105]. Proteolytic processing of RPTP-BR7 may be triggered by extracellular factors and may possibly depend on the MAPK cascade signaling [105]. It was noted that serum-starved cells contained much greater numbers of uncleaved (GFP-tagged) RPTP-BR7 than their counterparts grown in the presence of serum. Further experiments demonstrated only a minor role for serum proteases; rather, a role for growth-factors is indicated in inducing proteolytic cleavage, thereby potentially influencing the impact of RPTP-BR7 on MAPK signaling pathways [105].
Finally, RPTPζ, a strong CNS RPTP, undergoes proteolytic processing by plasmin (in the mouse brain) [107]. It is suggested that cleavage of RPTPζ is involved in functional remodeling of synapses during learning and memory. Three isoforms exist due to alternative splicing [111, 112]: RPTPζ A, B and a secreted from, phosphacan. Plasmin has been demonstrated to cleave the extracellular regions of the various isoforms of RPTPζ at multiple sites [107]. Since phosphacan expression peaks at P8 in the CNS, this mechanism might generate another source of soluble RPTPζ, phosphacan, later during development and adulthood. Indeed, the authors of this latter study suggest that the extracellular processing of RPTPζ might result in a ligand with a role in reverse signaling, at the synapse [107].
IV. Concluding remarks
We can see that while much work has been done to deepen our understanding of the biology behind RPTPs, there still remains much to be discovered. The ligands of the various RPTPs are yet to be described, the interactions they have with other molecules detailed, and their overall role and effects in cellular processes to be clarified. Adding to the complexity, it is not only protein-protein interactions that might have to be considered. The presence of sugars, whether on the ligand or on the RPTPs themselves, seems to be implicated in some way in the ligand-receptor interaction. Type IIa RPTPs interact with HSPGs and CSPGs, glycans are found on CD45 and RPTPκ, and phosphacan contains CSPG, implicated in PTN binding. Collaborative works with glycobiologists and using mice deficient in synthesis of some glycans would be needed to further pursue this search.
We have considered here a number of molecules that interact with RPTPs and act as ligands or as substrates. RPTP-ligand interactions result in modulation of downstream signaling, affecting cellular processes, or may result in deactivation of the RPTP itself, in a manner similar to the regulation of RTKs. Again, the proteins potentially responsible have yet to be definitively determined. However, while there still is much to be done, we do have the tools and techniques available to be able to further elucidate and build our base of knowledge concerning RPTP function and role. It is only a matter of time before the seemingly complex and fairly unknown interactions of RPTP with ligand or substrate are no longer as mysterious.
Acknowledgments
We apologize to all colleagues whose original work could not be referred to due to space constraints. We thank A. Elson for the critical reading of the manuscript and B.L.B. Sharma for assistance with the illustration. This work was supported in part by European Research Community Funds to PTPNET (MRTN-CT-2006-035830) and by award number R01GM088806 from the National Institute of General Medical Sciences (SB). ANM is the recipient of the ENP Graduate Program fellowship from Ecole des Neurosciences de Paris Ile-de-France.
Bibliography
- 1.Tonks NK, Diltz CD, Fischer EH. Purification of the major protein-tyrosine-phosphatases of human placenta. Journal of Biological Chemistry. 1988;263:6722–6730. [PubMed] [Google Scholar]
- 2.Andersen JN, Mortensen OH, Peters GH, Drake PG, Iversen LF, Olsen OH, Jansen PG, Andersen HS, Tonks NK, Møller NPH. Sructural and Evolutionary Relationships among Protein Tyrosine Phosphatase Domains. Mol Cell Biol. 2001;21:7117–7136. doi: 10.1128/MCB.21.21.7117-7136.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson KG, Van Vactor D. Receptor protein tyrosine phosphatases in nervous system development. Physiol Rev. 2003;83:1–24. doi: 10.1152/physrev.00016.2002. [DOI] [PubMed] [Google Scholar]
- 4.Bilwes AM, den Hertog J, Hunter T, Noel JP. Structural basis for inhibition of receptor protein-tyrosine phosphatase-[alpha] by dimerization. Nature. 1996;382:555–559. doi: 10.1038/382555a0. [DOI] [PubMed] [Google Scholar]
- 5.Desai DM, Sap J, Schlessinger J, Weiss A. Ligand-mediated negative regulation of a chimeric transmembrane receptor tyrosine phosphatase. Cell. 1993;73:541–554. doi: 10.1016/0092-8674(93)90141-c. [DOI] [PubMed] [Google Scholar]
- 6.Hoffmann KMV, Tonks NK, Barford D. The Crystal Structure of Domain 1 of Receptor Protein-tyrosine Phosphatase μ. Journal of Biological Chemistry. 1997;272:27505–27508. doi: 10.1074/jbc.272.44.27505. [DOI] [PubMed] [Google Scholar]
- 7.Nam H-J, Poy F, Krueger NX, Saito H, Frederick CA. Crystal Structure of the Tandem Phosphatase Domains of RPTP LAR. Cell. 1999;97:449–457. doi: 10.1016/s0092-8674(00)80755-2. [DOI] [PubMed] [Google Scholar]
- 8.Nam H-J, Poy F, Saito H, Frederick CA. Structural basis for the function and regulation of the receptor protein tyrosine phosphatase CD45. The Journal of Experimental Medicine. 2005;201:441–452. doi: 10.1084/jem.20041890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barr AJ, Ugochukwu E, Lee WH, King ON, Filippakopoulos P, Alfano I, Savitsky P, Burgess-Brown NA, Muller S, Knapp S. Large-scale structural analysis of the classical human protein tyrosine phosphatome. Cell. 2009;136:352–63. doi: 10.1016/j.cell.2008.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tonks NK. Protein tyrosine phosphatases: from genes, to function, to disease. Nat Rev Mol Cell Biol. 2006;7:833–46. doi: 10.1038/nrm2039. [DOI] [PubMed] [Google Scholar]
- 11.Meng K, Rodriguez-Pena A, Dimitrov T, Chen W, Yamin M, Noda M, Deuel TF. Pleiotrophin signals increased tyrosine phosphorylation of beta beta-catenin through inactivation of the intrinsic catalytic activity of the receptor-type protein tyrosine phosphatase beta/zeta. Proc Natl Acad Sci U S A. 2000;97:2603–8. doi: 10.1073/pnas.020487997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukada M, Fujikawa A, Chow JP, Ikematsu S, Sakuma S, Noda M. Protein tyrosine phosphatase receptor type Z is inactivated by ligand-induced oligomerization. FEBS Lett. 2006;580:4051–6. doi: 10.1016/j.febslet.2006.06.041. [DOI] [PubMed] [Google Scholar]
- 13.Perez-Pinera P, Alcantara S, Dimitrov T, Vega JA, Deuel TF. Pleiotrophin disrupts calcium-dependent homophilic cell-cell adhesion and initiates an epithelial-mesenchymal transition. Proc Natl Acad Sci U S A. 2006;103:17795–800. doi: 10.1073/pnas.0607299103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muller S, Kunkel P, Lamszus K, Ulbricht U, Lorente GA, Nelson AM, von Schack D, Chin DJ, Lohr SC, Westphal M, Melcher T. A role for receptor tyrosine phosphatase zeta in glioma cell migration. Oncogene. 2003;22:6661–8. doi: 10.1038/sj.onc.1206763. [DOI] [PubMed] [Google Scholar]
- 15.Mikelis C, Sfaelou E, Koutsioumpa M, Kieffer N, Papdimitriou E. Integrin αvβ3 is a pleiotrophin receptor required for pleiotrophin-induced endothelial cell migration through receptor protein tyrosine phosphatase β/ζ. FASEB J. 2009;23:1459–1469. doi: 10.1096/fj.08-117564. [DOI] [PubMed] [Google Scholar]
- 16.Hodivala-Dilke K, Reynolds A, Reynolds L. Integrins in angiogenesis: multitalented molecules in a balancing act. Cell and Tissue Research. 2003;314:131–144. doi: 10.1007/s00441-003-0774-5. [DOI] [PubMed] [Google Scholar]
- 17.Polytarchou C, Hatziapostolou M, Poimenidi E, Mikelis C, Papadopoulou A, Parthymou A, Papadimitriou E. Nitric oxide stimulates migration of human endothelial and prostate cancer cells through up-regulation of pleiotrophin expression and its receptor protein tyrosine phosphatase β/ζ. International Journal of Cancer. 2009;124:1785–1793. doi: 10.1002/ijc.24084. [DOI] [PubMed] [Google Scholar]
- 18.Diamantopoulou Z, Courty J, Katsoris P. Europhosphatases 2011, Protein Phosphatases: From Molecules to Networks. Baden, Austria: 2011. Pleiotrophin biological activity results from the opposing effects of PTPRZ1 and ALK [abstract] p. 119.p. Abstract P18. [Google Scholar]
- 19.Sakurai T, Lustig M, Nativ M, Hemperly JJ, Schlessinger J, Peles E, Grumet M. Induction of neurite outgrowth through contactin and Nr-CAM by extracellular regions of glial receptor tyrosine phosphatase beta. Journal of Cell Biology. 1997;136:907–18. doi: 10.1083/jcb.136.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hashemi H, Hurley M, Gibson A, Panova V, Tchetchelnitski V, Barr A, Stoker AW. Receptor tyrosine phosphatase PTPgamma is a regulator of spinal cord neurogenesis. Mol Cell Neurosci. 2011;46:469–82. doi: 10.1016/j.mcn.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bouyain S, Watkins DJ. The protein tyrosine phosphatases PTPRZ and PTPRG bind to distinct members of the contactin family of neural recognition molecules. Proc Natl Acad Sci U S A. 2010;107:2443–8. doi: 10.1073/pnas.0911235107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barnea G, Silvennoinen O, Shaanan B, Honegger AM, Canoll PD, D’Eustachio P, Morse B, Levy JB, Laforgia S, Huebner K, et al. Identification of a carbonic anhydrase-like domain in the extracellular region of RPTP gamma defines a new subfamily of receptor tyrosine phosphatases. Mol Cell Biol. 1993;13:1497–506. doi: 10.1128/mcb.13.3.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krueger NX, Saito H. A human transmembrane protein-tyrosine-phosphatase, PTP zeta, is expressed in brain and has an N-terminal receptor domain homologous to carbonic anhydrases. Proc Natl Acad Sci U S A. 1992;89:7417–21. doi: 10.1073/pnas.89.16.7417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peles E, Nativ M, Campbell PL, Sakurai T, Martinez R, Lev S, Clary DO, Schilling J, Barnea G, Plowman GD, Grumet M, Schlessinger J. The carbonic anhydrase domain of receptor tyrosine phosphatase beta is a functional ligand for the axonal cell recognition molecule contactin. Cell. 1995;82:251–60. doi: 10.1016/0092-8674(95)90312-7. [DOI] [PubMed] [Google Scholar]
- 25.Zuko A, Bouyain S, van der Zwaag B, Burbach JP. Contactins: structural aspects in relation to developmental functions in brain disease. Adv Protein Chem Struct Biol. 2011;84:143–80. doi: 10.1016/B978-0-12-386483-3.00001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lamprianou S, Chatzopoulou E, Thomas JL, Bouyain S, Harroch S. A complex between contactin-1 and the protein tyrosine phosphatase PTPRZ controls the development of oligodendrocyte precursor cells. Proc Natl Acad Sci U S A. 2011;108:17498–503. doi: 10.1073/pnas.1108774108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peles E, Schlessinger J, Grumet M. Multi-ligand interactions with receptor-like protein tyrosine phosphatase β: implications for intercellular signaling. Trends in Biochemical Sciences. 1998;23:121–124. doi: 10.1016/s0968-0004(98)01195-5. [DOI] [PubMed] [Google Scholar]
- 28.Czopka T, von Holst A, ffrench-Constant C, Faissner A. Regulatory Mechanisms that Mediate Tenascin C-Dependent Inhibition of Oligodendrocyte Precursor Differentiation. The Journal of Neuroscience. 2010;30:12310–12322. doi: 10.1523/JNEUROSCI.4957-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Czopka T, Von Holst A, Schmidt G, Ffrench-Constant C, Faissner A. Tenascin C and tenascin R similarly prevent the formation of myelin membranes in a RhoA-dependent manner, but antagonistically regulate the expression of myelin basic protein via a separate pathway. Glia. 2009;57:1790–1801. doi: 10.1002/glia.20891. [DOI] [PubMed] [Google Scholar]
- 30.Desai CJ, Popova E, Zinn K. A Drosophila receptor tyrosine phosphatase expressed in the embryonic CNS and larval optic lobes is a member of the set of proteins bearing the “HRP” carbohydrate epitope. J Neurosci. 1994;14:7272–83. doi: 10.1523/JNEUROSCI.14-12-07272.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson KG, Tenney AP, Ghose A, Duckworth AM, Higashi ME, Parfitt K, Marcu O, Heslip TR, Marsh JL, Schwarz TL, Flanagan JG, Van Vactor D. The HSPGs Syndecan and Dallylike bind the receptor phosphatase LAR and exert distinct effects on synaptic development. Neuron. 2006;49:517–31. doi: 10.1016/j.neuron.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 32.Elchebly M, Wagner J, Kennedy TE, Lanctot C, Michaliszyn E, Itie A, Drouin J, Tremblay ML. Neuroendocrine dysplasia in mice lacking protein tyrosine phosphatase sigma. Nat Genet. 1999;21:330–3. doi: 10.1038/6859. [DOI] [PubMed] [Google Scholar]
- 33.Kolkman MJ, Streijger F, Linkels M, Bloemen M, Heeren DJ, Hendriks WJ, Van der Zee CE. Mice lacking leukocyte common antigen-related (LAR) protein tyrosine phosphatase domains demonstrate spatial learning impairment in the two-trial water maze and hyperactivity in multiple behavioural tests. Behav Brain Res. 2004;154:171–82. doi: 10.1016/j.bbr.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 34.Uetani N, Chagnon MJ, Kennedy TE, Iwakura Y, Tremblay ML. Mammalian motoneuron axon targeting requires receptor protein tyrosine phosphatases sigma and delta. J Neurosci. 2006;26:5872–80. doi: 10.1523/JNEUROSCI.0386-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uetani N, Kato K, Ogura H, Mizuno K, Kawano K, Mikoshiba K, Yakura H, Asano M, Iwakura Y. Impaired learning with enhanced hippocampal long-term potentiation in PTPdelta-deficient mice. EMBO J. 2000;19:2775–85. doi: 10.1093/emboj/19.12.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wallace MJ, Batt J, Fladd CA, Henderson JT, Skarnes W, Rotin D. Neuronal defects and posterior pituitary hypoplasia in mice lacking the receptor tyrosine phosphatase PTPsigma. Nat Genet. 1999;21:334–8. doi: 10.1038/6866. [DOI] [PubMed] [Google Scholar]
- 37.Aricescu AR, McKinnell IW, Halfter W, Stoker AW. Heparan sulfate proteoglycans are ligands for receptor protein tyrosine phosphatase sigma. Mol Cell Biol. 2002;22:1881–92. doi: 10.1128/MCB.22.6.1881-1892.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fox AN, Zinn K. The heparan sulfate proteoglycan syndecan is an in vivo ligand for the Drosophila LAR receptor tyrosine phosphatase. Curr Biol. 2005;15:1701–11. doi: 10.1016/j.cub.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 39.Shen Y, Tenney AP, Busch SA, Horn KP, Cuascut FX, Liu K, He Z, Silver J, Flanagan JG. PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science. 2009;326:592–6. doi: 10.1126/science.1178310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coles CH, Shen Y, Tenney AP, Siebold C, Sutton GC, Lu W, Gallagher JT, Jones EY, Flanagan JG, Aricescu AR. Proteoglycan-specific molecular switch for RPTPsigma clustering and neuronal extension. Science. 2011;332:484–8. doi: 10.1126/science.1200840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Biersmith BH, Hammel M, Geisbrecht ER, Bouyain S. The immunoglobulin-like domains 1 and 2 of the protein tyrosine phosphatase LAR adopt an unusual horseshoe-like conformation. J Mol Biol. 2011;408:616–27. doi: 10.1016/j.jmb.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kwon SK, Woo J, Kim SY, Kim H, Kim E. Trans-synaptic adhesions between netrin-G ligand-3 (NGL-3) and receptor tyrosine phosphatases LAR, protein-tyrosine phosphatase delta (PTPdelta), and PTPsigma via specific domains regulate excitatory synapse formation. J Biol Chem. 2010;285:13966–78. doi: 10.1074/jbc.M109.061127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woo J, Kwon SK, Choi S, Kim S, Lee JR, Dunah AW, Sheng M, Kim E. Trans-synaptic adhesion between NGL-3 and LAR regulates the formation of excitatory synapses. Nat Neurosci. 2009;12:428–37. doi: 10.1038/nn.2279. [DOI] [PubMed] [Google Scholar]
- 44.Takahashi H, Arstikaitis P, Prasad T, Bartlett TE, Wang YT, Murphy TH, Craig AM. Postsynaptic TrkC and presynaptic PTPsigma function as a bidirectional excitatory synaptic organizing complex. Neuron. 2011;69:287–303. doi: 10.1016/j.neuron.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tonks N, Charbonneau H, Diltz C, Fischer E, Walsh K. Demonstration that the leukocyte common antigen CD45 is a protein tyrosine phosphatase. Biochemistry. 1988;27:8695–8701. doi: 10.1021/bi00424a001. [DOI] [PubMed] [Google Scholar]
- 46.Charbonneau H, Tonks N, Walsh K, Fischer E. The leukocyte common antigen (CD45): a putative receptor-linked protein tyrosine phosphatase. Proc Natl Acad Sci USA. 1988;85:7182–7186. doi: 10.1073/pnas.85.19.7182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trowbridge I, Thomas M. CD45: an emerging role as a protein tyrosine phosphatase required for lymphocyte activation and development. Annu Rev Immunol. 1994;12:85–116. doi: 10.1146/annurev.iy.12.040194.000505. [DOI] [PubMed] [Google Scholar]
- 48.Kung C, Pingel J, Heikinheio M, Klemola T, Varkila K, Yoo L, Vuopala K, Poyhonen M, Uhari M, Rogers M, Speck S, Chatila T, Thomas M. Mutations in the tyrosine phosphatase CD45 gene in a child with severe combined immunodeficiency disease. Nat Med. 2000;6:343–345. doi: 10.1038/73208. [DOI] [PubMed] [Google Scholar]
- 49.Kishihara K, Penninger J, Wallace V, Kündig T, Kawai K, Wakeham A, Timms E, Ffeffer PK, Ohashi P, Thomas M. Normal B lymphocyte development but impaired T cell maturation in CD45-exon6 protein tyrosine phosphatase-deficient mice. Cell. 1993;74:143–156. doi: 10.1016/0092-8674(93)90302-7. [DOI] [PubMed] [Google Scholar]
- 50.Ledbetter J, Tonks N, Fischer E, Clark E. CD45 regulates signal transduction and lymphocyte activation by specific association with receptor molecules on T or B cells. Proc Natl Acad Sci USA. 1988;85:8628–8632. doi: 10.1073/pnas.85.22.8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Janeway CJ. The T cell receptor as a multicomponent signalling machine: CD4/CD8 coreceptors and CD45 in T cell activation. Annu Rev Immunol. 1992;10:645–674. doi: 10.1146/annurev.iy.10.040192.003241. [DOI] [PubMed] [Google Scholar]
- 52.Hermiston M, Xu Z, Weiss A. CD45: a critical regulator of signaling thresholds in immune cells. Annu Rev Immunol. 2003;21:107–137. doi: 10.1146/annurev.immunol.21.120601.140946. [DOI] [PubMed] [Google Scholar]
- 53.Hermiston M, Zikherman J, Zhu J. CD45, CD148, and Lyp/Pep: critical phosphatases regulating Src family kinase signaling networks in immune cells. Immunol Rev. 2009;228:288–311. doi: 10.1111/j.1600-065X.2008.00752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Irles C, Symons A, Michel F, Bakker T, van der Merwe P, Acuto O. CD45 ectodomain controls interaction with GEMs and Lck activity for optimal TCR signaling. Nat Immunol. 2003;4:189–197. doi: 10.1038/ni877. [DOI] [PubMed] [Google Scholar]
- 55.Novak T, Farber D, Leitenberg D, Hong S, Johnson P, Bottomly K. Isoforms of the transmembrane tyrosine phosphatase CD45 differentially affect T cell recognition. Immunity. 1994;1:109–119. doi: 10.1016/1074-7613(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 56.Earl L, Baum L. CD45 Glycosylation controls T-cell life and death. Immunol Cell Biol. 2008;86:608–615. doi: 10.1038/icb.2008.46. [DOI] [PubMed] [Google Scholar]
- 57.Pulido R, Sánchez-Madrid F. Glycosylation of CD45: carbohydrate processing through Golgi appartaus is required for cell surface expression and protein stability. Eur J Immunol. 1992;22:463–468. doi: 10.1002/eji.1830220226. [DOI] [PubMed] [Google Scholar]
- 58.Earl L, Bi S, Baum L. N- and O-glycans modulate galectin-1 binding, CD45 signaling, and T cell death. J Biol Chem. 2010;285:2232–2244. doi: 10.1074/jbc.M109.066191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Perillo N, Pace K, Seilhamer J, Baum L. Apoptosis of T cells mediated by galectin-1. Nature. 1995;378:736–739. doi: 10.1038/378736a0. [DOI] [PubMed] [Google Scholar]
- 60.Barondes SH, Castronovo V, Cooper DNW, Cummings RD, Drickamer K, Feizi T, Gitt MA, Hirabayashi J, Hughes C, Kasai K-i, Leffler H, Liu F-T, Lotan R, Mercurio AM, Monsigny M, Pillai S, Poirer F, Raz A, Rigby PWJ, Rini JM, Wang JL. Galectins: A Family of Animal β-Galactoside-Binding Lectins. Cell. 1994;76:597–598. doi: 10.1016/0092-8674(94)90498-7. [DOI] [PubMed] [Google Scholar]
- 61.Hirabayashi J, Hashidate T, Arata Y, Nishi N, Nakamura T, Hirashima M, Urashima T, Oka T, Futai M, Muller WE, Yagi F, Kasai K-i. Oligosaccharide specificity of galectins: a search by frontal affinity chromatography. Biochim Biophys Acta. 2002;1572:232–254. doi: 10.1016/s0304-4165(02)00311-2. [DOI] [PubMed] [Google Scholar]
- 62.Hughes C. Secretion of the galectin family of mammalian carbohydrate-binding proteins. Biochim Biophys Acta. 1999;1473:172–185. doi: 10.1016/s0304-4165(99)00177-4. [DOI] [PubMed] [Google Scholar]
- 63.Walzel H, Schulz U, Neels P, Brock J. Galectin-1, a natural ligand for the receptor-type protein tyrosine phosphatase CD45. Immunol Lett. 1999;67:193–202. doi: 10.1016/s0165-2478(99)00012-7. [DOI] [PubMed] [Google Scholar]
- 64.Symons A, Cooper D, Barclay A. Characterization of the interaction between galectin-1 and lymphocyte glycoproteins CD45 and Thy-1. Glycobiology. 2000;10:559–563. doi: 10.1093/glycob/10.6.559. [DOI] [PubMed] [Google Scholar]
- 65.Pace K, Lee C, Steward P, Baum L. Restricted receptor segregation into membrane microdomains occurs on human T cells during apoptosis induced by galectin-1. J Immunol. 1999;163:3801–3811. [PubMed] [Google Scholar]
- 66.Nguyen J, Evans D, Galvan M, Pace K, Leitenberg D, Bui T, Baum L. CD45 modulates galectin-1-induced T cell death: regulation by expression of core 2 O-glycans. J Immunol. 2001;167:5697–5707. doi: 10.4049/jimmunol.167.10.5697. [DOI] [PubMed] [Google Scholar]
- 67.Kim Y-S, Kang H-Y, Kim J-Y, Oh S, Kim C-H, Ryo CJ, Miyoshi E, Taniguchi N, Ko JH. Identification of target proteins of N-acetylgulcosaminyl transferase V in huan colon cancer and implications of protein tyrosine phosphatase kappa in enhanced cancer cell migration. Proteomics. 2006;6:1187–1191. doi: 10.1002/pmic.200500400. [DOI] [PubMed] [Google Scholar]
- 68.Kim Y-S, Jung J-A, Kim H-J, Ahn YH, Yoo JS, Oh S, Cho C, Yoo H-S, Ko J-K. Galectin-3 binding protein promotes cell motility in colon cancer by stimulating the shedding of protein tyrosine prhosphatase kappa by proprotein convertase 5. Biochm Biophys Res Commun. 2011;404:96–102. doi: 10.1016/j.bbrc.2010.11.071. [DOI] [PubMed] [Google Scholar]
- 69.Seetharaman J, Kanigsberg A, Slaaby R, Leffler H, Barondes SH, Rini JM. X-ray crystal structure of the human galectin-3 carbohydrate recognition domain at 2.1-A resolution. J Biol Chem. 1998;273:13047–13052. doi: 10.1074/jbc.273.21.13047. [DOI] [PubMed] [Google Scholar]
- 70.Zondag GCM, Koningstein GM, Jiang YP, Sap J, Moolenaar WH, Gebbink MFBG. Homophilic Interactions Mediated by Receptor Tyrosine Phosphatases μ and κ. A critical role for the novel extracellular MAM domain. J Biol Chem. 1995;270:14247–14250. doi: 10.1074/jbc.270.24.14247. [DOI] [PubMed] [Google Scholar]
- 71.Brady-Kalnay S, Flint A, Tonks N. Homophilic Binding of PTPu, a Receptor-Type Protein Tyrosine Phosphatase, Can Mediate Cell-Cell Aggregation. J Cell Biol. 1993;122:961–972. doi: 10.1083/jcb.122.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sap J, Jiany Y, Friedlander D, Grumet M, Schlessinger J. Receptor tyrosine phosphatase R-PTP-kappa mediates homophilic binding. Mol Cell Biol. 1994;14:1–9. doi: 10.1128/mcb.14.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang J, Bixby JL. Receptor Tyrosine Phosphatase-δ Is a Homophilic, Neurite-Promoting Cell Adhesion Molecule for CNS Neurons. Molecular and Cellular Neuroscience. 1999;14:370–384. doi: 10.1006/mcne.1999.0789. [DOI] [PubMed] [Google Scholar]
- 74.Cheng J, Wu K, Armanini M, O’Rourke N, Dowbenko D, Lasky L. A Novel Protein-Tyrosine Phosphatase Related to the Homotypically Adhering k and u Receptors. J Biol Chem. 1997;272:7264–7277. doi: 10.1074/jbc.272.11.7264. [DOI] [PubMed] [Google Scholar]
- 75.Yu J, Becka S, Zhang P, Zhang X, Brady-Kalnay SM, Wang Z. Tumor-Derived Extracellular Mutations of PTPRT/PTPρ Are Defective in Cell Adhesion. Molecular Cancer Research. 2008;6:1106–1113. doi: 10.1158/1541-7786.MCR-07-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Becka S, Zhang P, Craig SE, Lodowski DT, Wang Z, Brady-Kalnay S. Characterization of the adhesive properties of the type iib subfamily of RPTPs. Cell Commun Adhes. 2010;17:34–47. doi: 10.3109/15419061.2010.487957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gebbink M, Zondag G, Wubbolts R, Beijersbergen R, van Etten I, Moolenaar W. Cell-Cell Adhesion Mediated by a Receptor-like Protein Tyrosine Phosphatase. J Biol Chem. 1993;268:16101–16104. [PubMed] [Google Scholar]
- 78.Zhang P, Becka S, Craig SE, Lodowski DT, Brady-Kalnay S, Wang Z. Cancer-derived mutations in the fibronectin III repeats of PTPRT/PTPrho inhibit cell-cell aggregation. Cell Commun Adhes. 2009;16:146–153. doi: 10.3109/15419061003653771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brady-Kalnay S, Tonks N. identification of the homophilic binding site of the receptor protein tyrosine phosphatase PTPM. J Biol Chem. 1994;269:28472–28477. [PubMed] [Google Scholar]
- 80.Cismasiu V, Denes S, Reiländer H, Michel H, Szedlacsek S. The MAM (Meprin/A5-protein/PTPmu) Domain Is a Homophilic Binding Site Promoting the Lateral Dimerization of Receptor-like Protein-tyrosine Phosphatase mu. J Biol Chem. 2004;279:26922–26931. doi: 10.1074/jbc.M313115200. [DOI] [PubMed] [Google Scholar]
- 81.Aricescu AR, Hon W-C, Siebold C, Lu W, van der Merwe PA, Jones EY. Molecular analysis of receptor protein tyrosine phosphatase [mu]-mediated cell adhesion. EMBO J. 2006;25:701–712. doi: 10.1038/sj.emboj.7600974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aricescu AR, Jones EY. Immunoglobulin superfamily cell adhesion molecules: zippers and signals. Current opinion in cell biology. 2007;19:543–50. doi: 10.1016/j.ceb.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 83.Rosdahl J, Ensslen S, Niedenthal J, Brady-Kalnay S. PTPμ-dependent growth cone rearrangement is regulated by Cdc42. J Neurobiol. 2003;56:199–208. doi: 10.1002/neu.10231. [DOI] [PubMed] [Google Scholar]
- 84.Ensslen-Craig SE, Brady-Kalnay SM. PTPμ expression and catalytic activity are required for PTPμ-mediated neurite outgrowth and repulsion. Molecular and Cellular Neuroscience. 2005;28:177–188. doi: 10.1016/j.mcn.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 85.Aricescu AR, Siebold C, Choudhuri K, Chang VT, Lu W, Davis SJ, van der Merwe PA, Jones EY. Structure of a tyrosine phosphatase adhesive interaction reveals a spacer-clamp mechanism. Science. 2007;317:1217–20. doi: 10.1126/science.1144646. [DOI] [PubMed] [Google Scholar]
- 86.Majeti R, Bilwes AM, Noel JP, Hunter T, Weiss A. Dimerization-Induced Inhibition of Receptor Protein Tyrosine Phosphatase Function Through an Inhibitory Wedge. Science. 1998;279:88–91. doi: 10.1126/science.279.5347.88. [DOI] [PubMed] [Google Scholar]
- 87.Takeda A, Wu JJ, Maizel AL. Evidence for monomeric and dimeric forms of CD45 associated with a 30-kDa phosphorylated protein. Journal of Biological Chemistry. 1992;267:16651–9. [PubMed] [Google Scholar]
- 88.Lee S, Faux C, Nixon J, Alete D, Chilton J, Hawadle M, Stoker AW. Dimerization of Protein Tyrosine Phosphatase σ Governs both Ligand Binding and Isoform Specificity. Molecular and Cellular Biology. 2007;27:1795–1808. doi: 10.1128/MCB.00535-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Heldin C, Ostman A. Ligand-induced dimerization of growth factor receptors: variations on the theme. Cytokine Growth Factor Rev. 1996;7:3–10. doi: 10.1016/1359-6101(96)00002-0. [DOI] [PubMed] [Google Scholar]
- 90.Hubbard S, Mohammadi M, Schlessinger J. Autoregulatory mechanisms in protein-tyrosine kinases. J Biol Chem. 1998;15:11987–11990. doi: 10.1074/jbc.273.20.11987. [DOI] [PubMed] [Google Scholar]
- 91.Hower A, Beltran P, Bixby J. Dimerization of tyrosine phosphatase PTPRO decreases its activity and ability to inactivate TrkC. J Neurochem. 2009;110:1635–1647. doi: 10.1111/j.1471-4159.2009.06261.x. [DOI] [PubMed] [Google Scholar]
- 92.Perez-Pinera P, Zhang W, Chang Y, Vega JA, Deuel TF. Anaplastic Lymphoma Kinase is Activated Through the Pleiotrophin/Receptor Protein-tyrosine Phosphatase β/ζ Signaling Pathway. J Biol Chem. 2007;282:28683–28690. doi: 10.1074/jbc.M704505200. [DOI] [PubMed] [Google Scholar]
- 93.Shintani T, Noda M. Protein Tyrosine Phosphatase Receptor Type Z Dephosphorylates TrkA Receptors and Attenuates NGF-dependent Neurite Outgrowth of PC12 Cells. J Biochem. 2008;144:259–266. doi: 10.1093/jb/mvn064. [DOI] [PubMed] [Google Scholar]
- 94.Xu Y, Xia W, Baker D, Zhou J, Cha HC, Voorhees JJ, Fisher GJ. Receptor-type Protein Tyrosine Phsphatase β (RPTP-β) Directly Dephosphorylates and Regulates Hepatocyte Growth Factor Receptor (HGFR/Met) Function. J Biol Chem. 2011;286:15980–15988. doi: 10.1074/jbc.M110.212597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Beltran P, Bixby J, Masters B. Expression of PTPRO during mouse development suggests involvement in axonogenesis and differentiation of NT-3 and NGF-dependent neurons. J Comp Neurol. 2003;456:384–395. doi: 10.1002/cne.10532. [DOI] [PubMed] [Google Scholar]
- 96.Gonzalez-Brito M, Bixby J. Protein tyrosine phosphatase receptor type O regulates development and function of the sensory nervous system. Mol Cell Neurosci. 2009;42:458–465. doi: 10.1016/j.mcn.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Segal RA. Selectivity in Neurotrophin Signaling: Theme and Variations. Annu Rev Neurosci. 2003;26:299–330. doi: 10.1146/annurev.neuro.26.041002.131421. [DOI] [PubMed] [Google Scholar]
- 98.Bowden ET, Stoica GE, Wellstein A. Anti-apoptotic Signaling of Pleiotrophin through Its Receptor, Anaplastic Lymphoma Kinase. Journal of Biological Chemistry. 2002;277:35862–35868. doi: 10.1074/jbc.M203963200. [DOI] [PubMed] [Google Scholar]
- 99.Powers C, Aigner A, Stoica GE, McDonnell K, Wellstein A. Pleiotrophin Signaling through Anaplastic Lymphoma Kinase Is Rate-limiting for Glioblastoma Growth. Journal of Biological Chemistry. 2002;277:14153–14158. doi: 10.1074/jbc.M112354200. [DOI] [PubMed] [Google Scholar]
- 100.Stoica GE, Kuo A, Aigner A, Sunitha I, Souttou B, Malerczyk C, Caughey DJ, Wen D, Karavanov A, Riegel AT, Wellstein A. Identification of Anaplastic Lymphoma Kinase as a Receptor for the Growth Factor Pleiotrophin. Journal of Biological Chemistry. 2001;276:16772–16779. doi: 10.1074/jbc.M010660200. [DOI] [PubMed] [Google Scholar]
- 101.Fischer E. Cell signaling by protein tyrosine phosphorylation. Advances in Enzyme Regulation. 1999;39:359–369. doi: 10.1016/s0065-2571(98)00014-4. [DOI] [PubMed] [Google Scholar]
- 102.Mauro L, Dixon J. ‘Zip codes’ direct intracellular protein tyrosine phosphatases to the corect ellular ‘address’. Trends Biochem Sci. 1994;19:151–155. doi: 10.1016/0968-0004(94)90274-7. [DOI] [PubMed] [Google Scholar]
- 103.Andersen J, Elson A, Lammers R, Rømer J, Clausen J, Møller K, Møller N. Comparative study of protein tyrosine phopshatase-epsilon isoforms: membrane localization confers specificity in cellular signalling. Biochem J. 2001;354:581–590. doi: 10.1042/0264-6021:3540581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Møller N, Møller K, Lammers R, Kharitonenkov A, Hoppe E, Wiberg F, Sures I, Ullrich A. Selective down-regulation of the insulin receptor signal by protein-tyrosine phosphatase epsilon. J Biol Chem. 1995;270:126–131. doi: 10.1074/jbc.270.39.23126. [DOI] [PubMed] [Google Scholar]
- 105.Dilaver G, van de Vorstenbosch R, Tárrega C, Ríos P, Pulido R, van Aerde K, Fransen J, Hendriks W. Proteolytic processing of the receptor-type protein tyrosine phosphatase PTPBR7. FEBS J. 2007;274:96–108. doi: 10.1111/j.1742-4658.2006.05568.x. [DOI] [PubMed] [Google Scholar]
- 106.Gil-Henn H, Volohonsky G, Elson A. Regulation of Protein-tyrosine Phosphatases alpha and epsilon by Calpain-mediated Proteolytic Cleavage. J Biol Chem. 2001;276:31772–31779. doi: 10.1074/jbc.M103395200. [DOI] [PubMed] [Google Scholar]
- 107.Chow J, Fujikawa A, Shimizu H, Noda M. Plasmin-mediated processing of protein tyrosine phosphatase receptor type Z in the mouse brain. Neuro letters. 2008;442:208–212. doi: 10.1016/j.neulet.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 108.Aicher B, Lerch MM, Müller T, Schilling J, Ullrich A. Cellular Redistribution of Protein Tyrosine Phosphatases LAR and PTPσ by Inducible Proteolytic Processing. The Journal of Cell Biology. 1997;138:681–696. doi: 10.1083/jcb.138.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Serra-Pages C, Saito H, Streuli M. Mutational analysis of proprotein processing, subunit association, and shedding of the LAR transmembrane protein tyrosine phosphatase. Journal of Biological Chemistry. 1994;269:23632–23641. [PubMed] [Google Scholar]
- 110.Streuli M, Krueger NX, Ariniello PD, Tang M, Munro JM, Blattler WA, Adler DA, Disteche CM, Saito H. Expression of the receptor-linked protein tyrosine phosphatase LAR: proteolytic cleavage and shedding of the CAM-like extracellular region. EMBO J. 1992;11:897–907. doi: 10.1002/j.1460-2075.1992.tb05128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Maeda N, Hamanaka H, Shintani T, Nishiwaki T, Noda M. Multiple receptor-like protein tyrosine phosphatases in the form of chondroitin sulfate proteoglycan. FEBS Lett. 1994;354:67–70. doi: 10.1016/0014-5793(94)01093-5. [DOI] [PubMed] [Google Scholar]
- 112.Sakurai T, Friedlander D, Grumet M. Expression of polypeptide variants of receptor-type protein tyrosine phosphatase beta: the secreted form, phosphacan, increase dramatically during embryonic development and modulates glial cell behavior in vitro. J Neurosci Res. 1996;43:694–706. doi: 10.1002/(SICI)1097-4547(19960315)43:6<694::AID-JNR6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]