Abstract
OBJECTIVES
To evaluate the relationship between platelet reactivity and atherosclerotic burden in patients undergoing percutaneous coronary intervention (PCI) with pre-intervention volumetric intravascular ultrasound (IVUS) imaging.
BACKGROUND
Atherosclerosis progresses by the pathologic sequence of sub-clinical plaque rupture, thrombosis and healing. In this setting, increased platelet reactivity may lead to more extensive arterial thrombosis at the time of plaque rupture, leading to a more rapid progression of the disease. Alternatively, abnormal vessel wall biology with advanced atherosclerosis is known to enhance platelet reactivity. Therefore, it is possible that by either mechanism, increased platelet reactivity may be associated with greater atherosclerotic burden.
METHODS
We analyzed patients who underwent PCI with pre-intervention IVUS imaging and platelet reactivity functional assay (P2Y12 reaction-units [PRU]) performed >16 hours post-PCI after stabilization of clopidogrel therapy (administered pre-PCI). A PRU value of >230 defined high on-treatment platelet reactivity (HPR).
RESULTS
Among 335 patients (mean age 65.0; 71% male), there were 109 patients with HPR (32.5%) and 226 without HPR (67.5%), with HPR being associated with diabetes and chronic renal insufficiency. By IVUS analysis, HPR patients had significantly greater target lesion calcium length, calcium arc, and calcium index. Furthermore, HPR patients tended to have longer lesions and greater volumetric dimensions, indicating higher plaque volume, larger total vessel volume and also greater lumen volume, despite similar plaque burden. By multivariable analysis controlling for baseline clinical variables, HPR was the single consistent predictor of all IVUS parameters examined, including plaque volume, calcium length and calcium arc.
CONCLUSIONS
Increased platelet reactivity on clopidogrel treatment, as defined by a PRU value of >230, is associated with greater coronary artery atherosclerotic disease burden and plaque calcification.
Keywords: atherosclerosis, clopidogrel, plaque progression, platelets, platelet reactivity
Introduction
Enhanced platelet reactivity plays a pivotal role in arterial ischemic events, acute coronary syndromes (ACS) and complications of percutaneous coronary interventions (PCIs) (1). In addition, the complex interactions between platelets, inflammatory cells, vascular cells and chemokines, also play a major role in atherosclerotic plaque and neointimal formation (2–3). Putative mechanisms whereby platelets may promote atherosclerosis include: 1) Releasing chemokines and their precursors that trigger the atherogenic recruitment of vascular cells or modulate processes such as angiogenesis or lipoprotein metabolism; 2) Inducing chemokine secretion by endothelial and other vascular cells; 3) Binding and presenting vascular cell-derived chemokines to trigger arrest of circulating mononuclear cells (2, 4–8). Huo at el have shown that the injection of activated platelets exacerbated atherosclerotic lesion formation, a process involving platelet surface receptors that facilitate mononuclear cell recruitment (9). The deposition of the most abundant platelet chemokine, platelet factor 4 (PF4; CXCL4), has been correlated with lesion severity and symptomatic atherosclerosis, suggesting that persistent platelet activation may contribute to the evolution of vascular lesions and supporting the rationale for chronic antiplatelet therapy in patients at risk for atherosclerosis (10). These observations extend the current view of platelets as not only being responsible for adhesion to the endothelium and propagation of endovascular thrombosis, but also suggest that activated platelets play an important role in promoting the atherosclerotic process itself, in particular the stages relating to ACS. Furthermore, it is also well described that alterations in the vascular wall or situations inducing high shear stress may lead to secondary platelet activation (11–12). Therefore, either as a primary cause or a secondary consequence there is strong rationale to expect that platelet activation may be associated with atherosclerotic plaque burden.
Antiplatelet therapy is a cornerstone of cardiovascular disease management and secondary prevention (13). Significant reductions in ischemic complications in a wide range of coronary artery disease patients have been demonstrated in major randomized controlled trials by the use of dual antiplatelet therapy with a thienopyridine plus aspirin (14–15). On the other hand, clopidogrel non-responsiveness (characterized as high on-treatment platelet reactivity - HPR) (16) has been recognized to correlate with adverse events after ACS and PCI (17–24). Since most patients with known coronary artery disease are receiving chronic anti-platelet therapy, assessment of platelet reactivity and ascertainment of HPR status is relevant to clinical practice and patient outcomes. Importantly, a recent meta-analysis demonstrated that HPR is associated with long-term cardiovascular events after PCI, including death, myocardial infarction, and stent thrombosis (24). While it is widely assumed that this is due to increased thrombotic events, a preliminary study recently reported that HPR may be associated with increased coronary artery atherosclerotic burden as assessed by cine-angiography (25). In the present study, we sought to extend these findings and determine if HPR, as an indicator of high residual platelet reactivity in patients receiving clopidogrel, correlates with more extensive atherosclerotic disease as determined by volumetric intravascular ultrasound (IVUS) imaging, the gold-standard imaging modality for the assessment of atherosclerotic burden and calcification.
Methods
Patient population, PCI and IVUS image acquisition
We analyzed 335 consecutive patients who underwent PCI with pre-intervention IVUS imaging and who had platelet function testing performed on the day post-PCI. Only a single culprit target lesion and associated target vessel were included in this study per patient. IVUS of other vessels was not clinically indicated and was not performed. Key enrolment criteria were as follows: (1) Guideline appropriate requirement for PCI, typically based on severe disease, positive stress test or presentation with unstable coronary syndrome; (2) Age >18 years; (3) Signed informed consent. Exclusion criteria were as follows: (1) Presentation with ST-segment elevation myocardial infarction; (2) Serum Creatinine ≥2.0 mg/dL; (3) Prior heart transplantation; (4) Active autoimmune disease; (5) Illicit drug use; (6) HIV positive; (7) Prior malignancy with mediastinal irradiation, bone marrow transplantation or high-dose chemotherapy; (8) Adult congenital heart disease.
PCI procedures were performed according to current standard guidelines, and the type of stent implanted and the use of pharmacological agents were at the discretion of the operator. The decision to perform IVUS was also at the discretion of the operator and was made entirely independently of this study, with > 80% of all IVUS images acquired before this study was conceived. If the patient had no prior exposure to clopidogrel (132 patients), a dose of 600 mg was administered no later than 2 hours before PCI; patients already treated with clopidogrel before hospitalization received a loading dose of 300 mg before PCI (203 patients). Following PCI, all patients received aspirin, clopidogrel and a statin. For the purposes of this analysis, patients with anemia (hemoglobin < 10.5 g/dl), thrombocytopenia (platelet count < 125,000/ml) and those receiving a glycoprotein IIb/IIIa inhibitor during or after PCI were excluded due to the possibility of interference with platelet assay measurements.
Platelet reactivity testing
Verify Now point-of-care assay (Accumetrics, San Diego, CA) was used to measure platelet reactivity. This test has been previously described in detail (26); it is a turbidimetry-based optical detection device that measures platelet induced aggregation in a system containing fibrinogen-coated beads. The instrument measures changes in light transmission and thus the rate of aggregation in whole blood. In the cartridge used for this assay, there is a channel in which inhibition of the P2Y12 receptor is measured; this channel contains ADP as platelet agonist and prostaglandin E1 as a suppressor of intracellular free calcium levels, to reduce the nonspecific contribution of ADP binding to P2Y1 receptors. Venous blood samples anticoagulated with sodium citrate 0.109 mol/L (ratio 9:1) were tested from each patient 16 – 24 hours after PCI (as part of the clinically determined morning blood tests) but before the next dose of clopidogrel. Results are expressed as P2Y12 reaction units (PRU). A PRU value of > 230 was used to define patients with HPR clopidogrel resistance (27).
IVUS image analysis
Target lesion IVUS imaging studies were performed after intracoronary administration of 200 µg nitroglycerin using a commercially available IVUS system (Atlantis SR Pro, 40 MHz catheter, Boston Scientific Corp., Natick, MA; Eagle Eye, 20MHz catheter or Revolution 45 MHz catheter, Volcano Corp., Rancho Cordova, CA) and prior to any coronary intervention. The IVUS catheter was advanced distal to the stenosis, and imaging was performed with retrograde pull-back to the aorto-ostial junction at an automatic pullback speed of 0.5 mm/sec. All analyses were performed off-line without knowledge of the platelet assay results using planimetry software (INDEC Systems Inc., MountainView, CA). The minimum lumen cross sectional area (MLA) site was the image slice with the smallest lumen cross sectional area (CSA). The reference sites were the most normal-appearing cross-sections within 5 mm proximal and distal to the stenosis, but before any side branch, and were used to calculate a mean reference. For each patient the lesion with the smallest lumen CSA was chosen for analysis. The lesion itself was defined as the segment between the proximal and distal reference sites whose length (mm) was calculated using the pullback duration and pullback speed. Quantitative analysis included measurement of the external elastic membrane (EEM) and lumen CSA every 1 mm within the length of the lesion. Plaque & media CSA were calculated as EEM minus lumen CSA. Once a complete set of CSA measurements were obtained, EEM, plaque+media, and lumen volumes were calculated using Simpson’s rule. Plaque burden was calculated as plaque & media divided by EEM volume or CSA. A remodeling index was calculated as the lesion divided by the mean reference EEM CSA. Calcium was identified as an echo signal brighter than the adventitia with acoustic shadowing. The maximum arc of calcium (°) within the lesion was measured with the electronic protractor centered on the lumen. Calcium length (mm) within the lesion was measured as the length of the lesion in which there was IVUS-detectable calcium. Calcium Index was calculated as total calcium length/lesion length × maximum calcium arc/360 degrees.
Clinical patient follow-up
All patients had follow-up completed out to 12 months or to fatal event. Follow-up data was collected from patients using scripted telephone interview and simultaneous electronic medical records review. If telephone contact was not established then a mailed questionnaire was used. Major adverse cardiovascular events (MACE = death, myocardial infarction or target lesion revascularization [TLR]) were adjudicated by an independent committee. Social security death index was utilized to obtain vital status of the patient. These methods are the standard operation of the Columbia University Medical Center PCI outcomes database registry, which includes data collection and follow-up under an institutional review board approval.
Statistical analyses
Continuous variables are expressed as mean and SD or median [interquartile range] as indicated and compared using Student’s t test or Wilcoxon rank sum test if applicable. Discrete variables and clinical outcomes are presented as frequencies and percentages and compared with the χ2 test, unless the observation in any cell was < 5, in which case Fisher’s exact test was used. All statistical tests are two-sided with a significance level of < 0.05. Multivariate linear regression analysis is used to assess the relationship between High On-Treatment Platelet Reactivity and IVUS measurements (Table 5) while controlling for the following baseline factors: age, gender, diabetes and chronic renal failure. A stepwise selection algorithm with entry/stay criteria of 0.1/0.1 is used to identify multivariate predictors. Adjusted mean values for IVUS measurements (Table 6) are calculated using least square means estimation from a linear regression model. All statistical analyses are performed using SAS software, version 9.2. (Cary, NC).
Table 5.
Multivariable analysis results for the respective IVUS variables derived among the following candidate predictors: High On-Treatment Platelet Reactivity, age, gender, diabetes and chronic renal failure.
| IVUS variables and respective independent predictors |
Coefficient | Standard Error |
P-value |
|---|---|---|---|
| Lesion length/mm | |||
| HPR | 1.58 | 0.83 | 0.0585 |
| Max Calcium/° | |||
| HPR | 32.4 | 12.5 | 0.0101 |
| Calcium length/mm | |||
| HPR | 1.76 | 0.70 | 0.0118 |
| EEM volume/mm3 | |||
| HPR | 23.2 | 10.5 | 0.0284 |
| Male gender | 31.6 | 10.9 | 0.0039 |
| Lumen volume/mm3 | |||
| HOT PR | 7.9 | 3.3 | 0.0161 |
| Male gender | 7.4 | 3.4 | 0.0281 |
| Plaque&media volume/mm3 | |||
| HPR | 15.3 | 7.5 | 0.0432 |
| Male gender | 24.2 | 7.8 | 0.0021 |
IVUS=intravascular ultrasound, EEM=external elastic membrane
Table 6.
Mean values for IVUS variables in relation to platelet reactivity following adjustment for age, gender, diabetes and chronic renal failure.
| HPR n = 109 |
No HPR n = 226 |
P value | |
|---|---|---|---|
| Lesion length, mm | 11.0±0.7 | 9.4±0.5 | 0.0585 |
| Max calcium arc, ° | 152.8±10.3 | 120.4±7.2 | 0.0101 |
| Calcium length, mm | 7.2±0.6 | 5.4±0.4 | 0.0118 |
| EEM volume, mm3 | 133.6±8.7 | 110.4±6.6 | 0.0284 |
| Lumen volume, mm3 | 49.2±2.7 | 41.3±−2.0 | 0.0161 |
| Plaque&media volume, mm3 | 84.4±6.2 | 69.0±4.7 | 0.0432 |
IVUS=intravascular ultrasound, HOTPREEM=external elastic membrane
Results
Among the 335 patients analyzed, there were 109 patients with HPR (32.5%) and 226 without HPR (67.5%). Patient baseline characteristics are presented in Table 1; HPR was associated with diabetes and chronic renal insufficiency.
Table 1.
Baseline patient characteristcs in the overall study population and in the groups with or without High On-Treatment Platelet Reactivity (HPR).
| Overall group | HPR | No HPR | P-value | |
|---|---|---|---|---|
| n=335 | n=109 | n=226 | ||
| Age | 65.0 [58.0, 73.0] | 66.0 [58.4, 74.0] | 65.0 [57.8, 72.0] | 0.28 |
| Male | 71.0% (238/335) | 61.5% (67/109) | 75.7% (171/226) | 0.01 |
| Body surface area (m2) | 1.93 [1.79, 2.13] | 1.95 [1.80, 2.16] | 1.93 [1.79, 2.12] | 0.70 |
| Platelet Count | 196 [164, 232] | 189 [158, 220] | 198 [167, 236] | 0.037 |
| Prior MI | 21.5% (72/335) | 18.3% (20/109) | 23.0% (52/226) | 0.40 |
| Stroke/TIA | 6.6% (22/335) | 3.7% (4/109) | 8.0% (18/226) | 0.16 |
| Congestive Heart Failure | 7.5% (25/335) | 8.3% (9/109) | 7.1% (16/226) | 0.67 |
| Hypertension | 77.9% (261/335) | 79.8% (87/109) | 77.0% (174/226) | 0.67 |
| Hyperlipidemia | 80.5% (269/334) | 81.5% (88/108) | 80.1% (181/226) | 0.88 |
| Diabetes Mellitus | 31.6% (106/335) | 45.9% (50/109) | 24.8% (56/226) | 0.0002 |
| Chronic Renal Insufficiency | 9.3% (31/335) | 14.7% (16/109) | 6.6% (15/226) | 0.026 |
| Current Smoking | 9.9% (33/335) | 8.3% (9/109) | 10.6% (24/226) | 0.56 |
| Additional Medication usage | ||||
| β-blocker | 66.9% (220/329) | 67.3% (72/107) | 66.7% (148/222) | 1.00 |
| Statins | 70.9% (234/330) | 66.7% (72/108) | 73.0% (162/222) | 0.25 |
| ACE I | 34.8% (115/330) | 32.4% (35/108) | 36.0% (80/222) | 0.54 |
| Anatomical Location of Target Lesion | ||||
| Left Main | 2.1% (7/333) | 3.7% (4/108) | 1.3% (3/225) | 0.22 |
| Left Anterior Descending (LAD) | 44.4% (148/333) | 47.2% (51/108) | 43.1% (97/225) | 0.48 |
| LAD branch (Diagonal) | 3.6% (12/333) | 3.7% (4/108) | 3.6% (8/225) | 1.00 |
| Left Circumflex (LCx) | 14.1% (47/333) | 9.3% (10/108) | 16.4% (37/225) | 0.093 |
| LCx branch (Ramus, OM, LPL, LPDA) | 8.1% (27/333) | 9.3% (10/108) | 7.6% (17/225) | 0.67 |
| Right Coronary Artery (RCA) | 24.6% (82/333) | 23.1% (25/108) | 25.3% (57/225) | 0.69 |
| RCA branch (AVcon, RPDA, RPL) | 3.0% (10/333) | 3.7% (4/108) | 2.7% (6/225) | 0.73 |
Diabetes = diabetes requiring medical therapy, Chronic renal insufficiency = Glomerular filtration rate < 60 ml/min/1.72m2, CHF = NYHA class III or IV, MI = myocardial infarction, TIA = transient ischemic attack. Coronary anatomy: LAD = left anterior descending, LCx = left circumflex, Ramus = ramus intermedius, OM = obtuse marginal, LPL = left postero-lateral, LPDA = left posterior descending, RCA = right coronary artery, AVcon = A–V continuation, RPDA = right posterior descending, RPL = right posterolateral. Continuous variables are summarized as median [interquartile range] (count/n). Categorical variables are summarized as % (count/n).
Volumetric IVUS analysis was performed in all 335 patients (Figure 1 and Table 2). There was no difference in proximal or distal reference segments in terms of plaque area or plaque burden. Plaque burden at the minimum lumen site was similar between the 2 groups. The HPR group had significantly greater calcification length, calcification arc and calcium index. In addition, HPR patients tended to have longer lesions and greater volumetric dimensions despite a similar plaque burden. Similar results were obtained when only patients receiving clopidogrel at the time of enrolment were evaluated (Table 3).
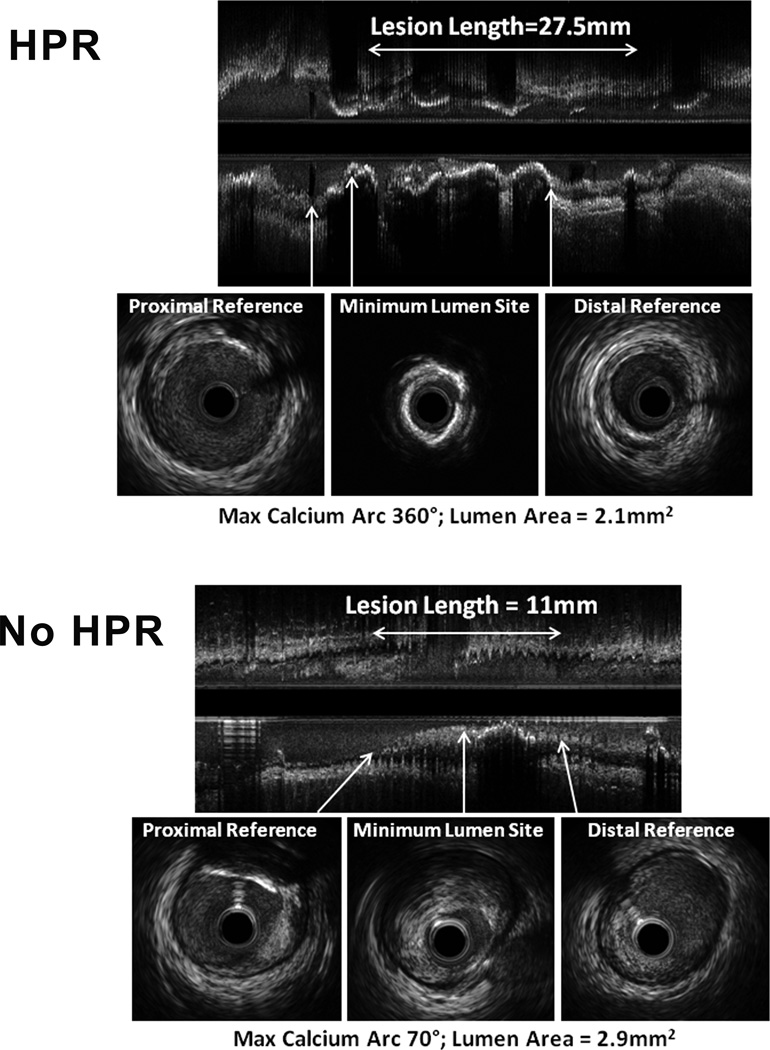
Figure 1. Illustrative volumetric IVUS images from ‘HPR’ and ‘No HPR’ patients.
Upper panel shows an example of a ‘HPR’ patient. This long lesion (27.5 mm from proximal to distal reference site) contains diffuse calcification (note the acoustic shadows beyond regions of calcification) with a maximum calcium arc of 360° at the minimum lumen area (MLA) site. Lower panel shows a ‘No HPR’ patient. While not intended to be representative, the lesion length was shorter (11 mm from proximal to distal reference site) with non-calcified plaque at the MLA site; the maximum arc of calcium (70°) was located at the proximal reference. Both lesions had lumen areas at the lesion site of < 3.0 mm2, considered to be highly hemodynamically significant.
Table 2.
Intravascular ultrasound measurements in the overall study population by groups according to platelet reactivity status (HPR versus no HPR).
| Overall group | HPR | No HPR | P-value | |
|---|---|---|---|---|
| n=335 | n=109 | n=226 | ||
| Reference site* | ||||
| EEM CSA, mm2 | 12.0 [9.3, 15.2] | 12.2 [9.8, 15.0] | 11.6 [9.1, 15.4] | 0.355 |
| Lumen CSA, mm2 | 6.5 [5.1, 7.9] | 6.7 [5.1, 8.0] | 6.3 [5.1, 7.9] | 0.535 |
| Plaque burden | 0.45 [0.38, 0.52] | 0.45 [0.39, 0.53] | 0.45 [0.37, 0.52] | 0.751 |
| Minimum Lumen area site | ||||
| EEM CSA, mm2 | 10.7 [8.5, 13.6] | 11.0 [8.5, 14.0] | 10.6 [8.4, 13.5] | 0.310 |
| Lumen CSA, mm2 | 3.0 [2.3, 3.8] | 3.1 [2.4, 4.0] | 3.0 [2.2, 3.7] | 0.229 |
| Plaque&media CSA, mm2 | 7.5 [5.5, 10.0] | 7.9 [5.6, 10.0] | 7.2 [5.4, 10.0] | 0.434 |
| Plaque burden | 0.71 [0.64, 0.77] | 0.70 [0.64, 0.77] | 0.71 [0.65, 0.77] | 0.720 |
| Volumetric Data | ||||
| EEM volume, mm3 | 100.9 [58.6, 174.4] | 111.6 [66.6, 177.5] | 89.4 [57.4, 169.0] | 0.0526 |
| Lumen volume, mm3 | 38.5 [25.0, 61.7] | 44.4 [26.3, 69.5] | 36.3 [22.6, 58.8] | 0.0244 |
| Plaque&media volume, mm3 | 58.6 [33.1, 108.7] | 71.2 [36.7, 111.2] | 53.9 [32.9, 103.6] | 0.0726 |
| Plaque burden | 0.61 [0.55, 0.66] | 0.61 [0.56, 0.66] | 0.61 [0.55, 0.66] | 0.869 |
| Lesion length, mm | 8.1 [5.5, 13.1] | 8.8 [6.3, 14.0] | 7.6 [5.2, 12.8] | 0.0710 |
| Calcium length, mm | 4.7 [1.7, 8.8] | 5.9 [2.6, 10.5] | 4.3 [1.4, 7.4] | 0.0096 |
| Maximum calcium arc, ° | 110 [50, 190] | 130 [70, 260] | 90 [40, 170] | 0.0149 |
| Calcium index | 0.17 [0.04, 0.40] | 0.25 [0.07, 0.45] | 0.15 [0.03, 0.38] | 0.0250 |
| Remodeling index | 0.90 [0.79, 1.02] | 0.92 [0.79, 1.03] | 0.90 [0.79, 1.02] | 0.834 |
Calculated as the average of proximal and distal reference sites. EEM = external elastic membrane, CSA = cross sectional area
Table 3.
Intravascular ultrasound measurements in patients already receiving clopidogrel at enrolment by groups according to platelet reactivity status (HPR versus no HPR).
| Overall group | HPR | No HPR | P-value | |
|---|---|---|---|---|
| n=203 | n=58 | n=145 | ||
| Reference site* | ||||
| EEM CSA, mm2 | 11.6 [9.3, 15.0] | 11.9 [10.2, 14.5] | 11.4 [9.1, 15.4] | 0.461 |
| Lumen CSA, mm2 | 6.3 [5.0, 7.6] | 6.4 [5.3, 7.6] | 6.3 [5.0, 7.6] | 0.659 |
| Plaque burden | 0.46 [0.38, 0.53] | 0.47 [0.39, 0.53] | 0.46 [0.38, 0.52] | 0.592 |
| Minimum Lumen area site | ||||
| EEM CSA, mm2 | 10.7 [8.7, 13.9] | 10.6 [8.5, 13.9] | 10.7 [8.8, 13.9] | 0.829 |
| Lumen CSA, mm2 | 3.0 [2.3, 3.7] | 3.0 [2.5, 3.8] | 3.0 [2.2, 3.7] | 0.485 |
| Plaque&media CSA, mm2 | 7.5 [5.5, 10.3] | 7.7 [5.5, 9.9] | 7.5 [5.5, 10.3] | 0.714 |
| Plaque burden | 0.71 [0.64, 0.77] | 0.72 [0.63, 0.77] | 0.71 [0.65, 0.78] | 0.867 |
| Volumetric Data | ||||
| EEM volume, mm3 | 92.3 [55.8, 174.4] | 106.4 [67.8, 200.3] | 86.8 [51.8, 161.2] | 0.0962 |
| Lumen volume, mm3 | 37.4 [22.6, 59.5] | 42.2 [26.3, 69.5] | 34.7 [20.5, 53.8] | 0.0526 |
| Plaque&media volume, mm3 | 56.0 [32.9, 110.6] | 67.4 [37.4, 127.5] | 51.3 [30.9, 102.9] | 0.111 |
| Plaque burden | 0.62 [0.55, 0.66] | 0.61 [0.56, 0.67] | 0.62 [0.55, 0.66] | 0.822 |
| Lesion length, mm | 7.6 [5.3, 13.0] | 8.6 [6.4, 14.5] | 7.3 [4.9, 12.5] | 0.0652 |
| Calcium length, mm | 4.4 [1.3, 8.7] | 7.0 [2.9, 11.0] | 3.8 [1.1, 6.9] | 0.0022 |
| Maximum calcium arc, ° | 100 [40, 180] | 125 [70, 270] | 90 [33, 165] | 0.0055 |
| Calcium index | 0.15 [0.03, 0.42] | 0.28 [0.07, 0.53] | 0.11 [0.02, 0.40] | 0.0116 |
| Remodeling index | 0.91 [0.79, 1.03] | 0.90 [0.78, 1.04] | 0.91 [0.80, 1.02] | 0.954 |
Calculated as the average of proximal and distal reference sites. EEM = external elastic membrane, CSA = cross sectional area
Clinical events were rare (Table 4), and there was no significant difference between HPR and non-HPR patients. However, the present study was not designed or powered to detect differences in clinical outcomes.
Table 4.
Clinical outcomes in the overall study population and in the groups with or without High On-Treatment Platelet Reactivity (HPR).
| Overall group | HPR | No HPR | P-value | |
|---|---|---|---|---|
| n=335 | n=109 | n=226 | ||
| MACE | 18.2% (61/335) | 22.9% (25/109) | 15.9% (36/226) | 0.132 |
| Myocardial Infarction | 0.9% (3/335) | 0.9% (1/109) | 0.9% (2/226) | 1.000 |
| TLR | 17.9% (60/335) | 22.9% (25/109) | 15.5% (35/226) | 0.128 |
| Death | 0.6% (2/335) | 0.0% (0/109) | 0.9% (2/226) | 1.000 |
MACE = Major adverse cardiovascular events (death, myocardial infarction or TLR). No patient underwent coronary artery bypass graft surgery.
After exploratory analyses to identify univariable predictors, multivariable analysis was performed against each of the IVUS-derived variables using the following candidate predictors: HPR, age, gender, diabetes and chronic renal insufficiency. Table 5 indicates that HPR was the single consistent predictor of all IVUS parameters examined. Male gender also correlated with external elastic membrane, lumen and volumetric plaque burden.
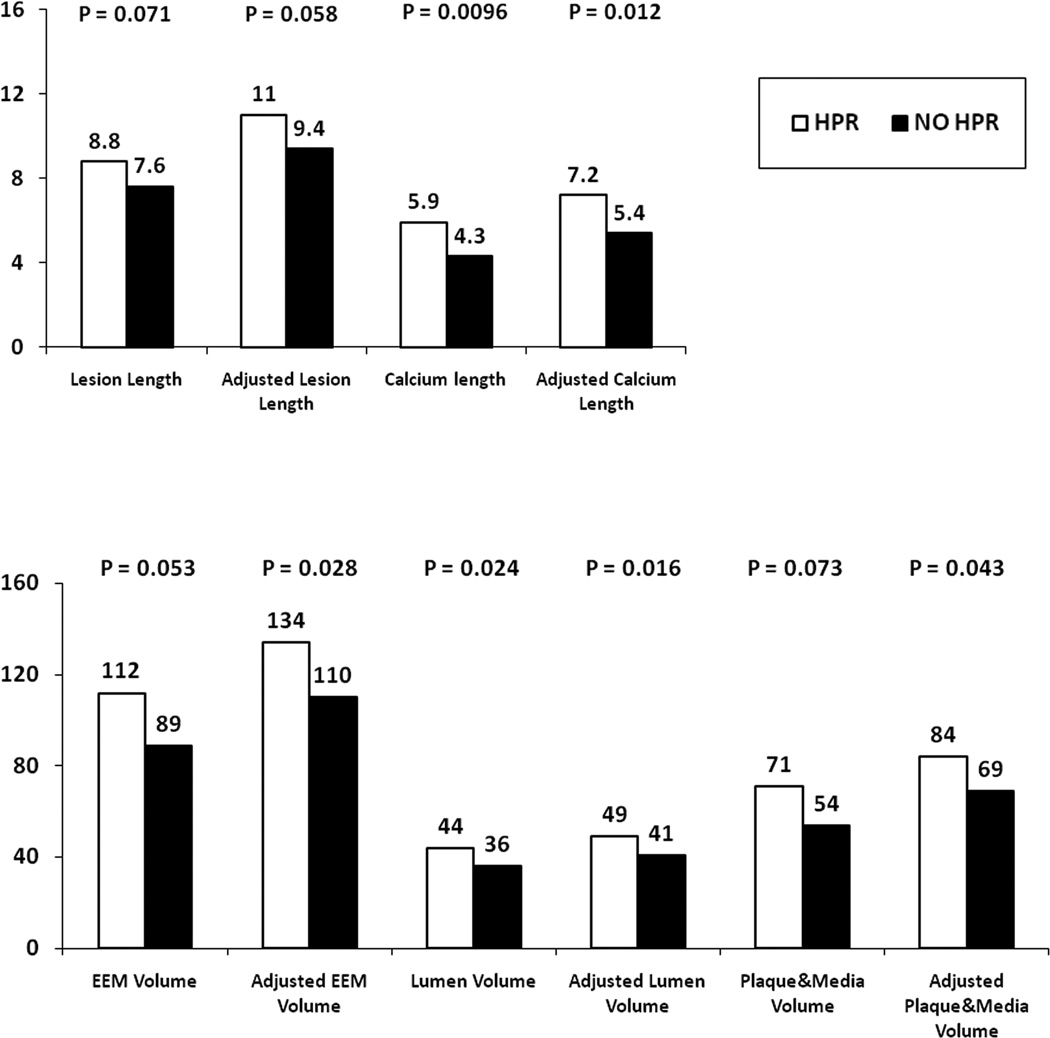
Table 6 depicts the IVUS parameters in the 2 groups after adjustment for age, gender, diabetes and chronic renal failure. Figure 2 indicates that the trends observed in the univariable analyses between the 2 study groups with respect to the IVUS parameters are significantly strengthened after adjustment for clinical variables.
Figure 2. Relationship between IVUS-derived arterial wall measurements and platelet reactivity.
Data represents mean values pergroup. Adjustment performed for age, gender, diabetes and chronic renal failure.
Discussion
Our data demonstrate that platelet reactivity, as assessed by HPR measurement, correlates with diabetes and chronic renal insufficiency as well as with increased calcification and atheroma burden as assessed by IVUS in patients with coronary artery disease requiring PCI. Compared to non-HPR patients, those with HPR exhibited increased lesion length and plaque volume despite also having a larger lumen volume. Our analysis also confirmed other previously reported observations, including the fact that men have more plaque than women (28). Importantly, ours is not the first study to suggest HPR is related to the extent of coronary artery atherosclerotic disease. However, the single prior study that we are aware of which suggested this relationship did not incorporate a sensitive and objective imaging modality to determine atherosclerotic burden, but rather, relied entirely on cine-angiography with visual estimate of disease severity (25). Therefore, our IVUS-based findings add significantly to this former study, extending our understanding of this relationship and demonstrating that HPR is independently associated with sensitive measures of coronary atheroma burden and calcification.
Altered vessel wall characteristics may lead to HPR
Increased calcification and plaque burden in patients with HPR raises important biologic questions. Foremost, as a potential mechanistic explanation for our findings, intrinsic changes in the biology of the vessel wall may arise due to diabetes, chronic renal insufficiency or plaque development (Tables 1, 5, 6) which lead to functional platelet abnormalities and increased platelet reactivity (29). In support of this, both diabetes and chronic renal insufficiency play a significant role in arterial calcification and were preliminarily reported to be associated with HPR in another study (30). Furthermore, alterations in arterial shear forces due to developing atherosclerotic plaque may activate platelets (11), with additional human in vivo data corroborating the notion that primary vascular disease processes lead to platelet activation (12). Therefore, there is biologic plausibility to suggest that our findings may be due to atherosclerotic disease secondarily activating platelets and leading to HPR.
Increased platelet reactivity may promote atherosclerosis
As an alternative explanation, HPR may be a marker of intrinsic platelet characteristics or functionality that promote plaque formation and calcification. Importantly, silent plaque rupture with arterial thrombosis is a form of vascular wound healing that leads to atherosclerotic progression (31). Therefore, it is possible that increased platelet reactivity may potentiate arterial thrombosis at the time of plaque rupture, thereby driving inflammation and atherosclerotic progression. Moreover, in addition to facilitating arterial thrombosis, several studies have shown a key role for platelets in mediating various non-thrombotic, inflammatory pathways of plaque progression (32–36). On the endothelial layer, platelet-derived chemokines, in conjunction with increased expression of adhesion molecules, promote the recruitment of circulating monocytes that may migrate across the endothelial lining and augment plaque progression. Further, activated circulating platelets may induce monocyte differentiation (32) or form platelet-monocyte complexes that increase the adhesive and migratory capacities of monocytes and which promote the additional recruitment of inflammatory cells (37–38). Collectively, there are multiple possible mechanisms whereby patients with high platelet reactivity may develop more advanced atherosclerotic and calcific arterial disease. Importantly, although we measured platelet reactivity and ascertained HPR in patients receiving clopidogrel, it is critical to emphasize that high baseline platelet reactivity (in persons receiving no anti-platelet therapy) is very strongly correlated with subsequent platelet reactivity after the initiation of clopidogrel therapy (39–40). Therefore, HPR is a reliable measure for determining those patients with high intrinsic platelet reactivity during the preceding years of plaque development when not receiving clopidogrel.
Vascular calcification and platelet reactivity
It has been established that calcification of the coronary arteries accurately identifies coronary atherosclerosis (41). However, the relationship between vascular calcification and atherosclerosis is complex; Virmani and colleagues identified that vascular calcification is least in eroded plaques, greatest in acute and healed plaque ruptures, and intermediate in stable plaques (31, 42). Furthermore, at the cellular level, multiple cell types are potentially implicated in the process of vascular ossification (43–44). Again, increased vascular calcification may be either a cause or effect of HPR. Thus, more extensive, calcific plaque may lead to platelet activation, or, increased platelet reactivity may augment the progression of atherosclerosis and arterial calcification.
Limitations
This was a single center study. Only patients with available pre-intervention IVUS images were included in the study. Platelet reactivity was only assessed at a single time-point, at a relatively late stage in the development of atherosclerotic disease. Further, it was recently shown that platelet reactivity changes during the first weeks after initiation of clopidogrel therapy (27, 45). Therefore, in patients commencing this therapy, the measurement of platelet reactivity soon after PCI may not precisely reflect long-term HPR status. Only the culprit lesion and vessel were analyzed, and we did not assess more global measures of atherosclerotic disease. Our study demonstrates only correlative associations, and the possibility that platelet reactivity is causatively related to atherosclerotic burden and calcification, or vise-versa, remain speculative. While we attempted to account for potential confounding (Tables 5 and 6, and elsewhere), we are unable to completely exclude the possibility of residual confounding in our results.
Conclusions
In a large group of patients undergoing PCI with volumetric IVUS, we have identified that relative resistance to the anti-platelet effects of clopidogrel as defined by HPR status is associated with an increase in both atherosclerotic burden and plaque calcification. Further studies are now required to precisely define the mechanisms whereby platelets that are relatively resistant to the effects of clopidogrel are associated with this increase in atherosclerotic disease, and if this may be a contributing factor in adverse clinical events. The higher resolution provided by optical coherence tomography compared to IVUS may enable the further identification of atheromatous characteristics that are associated with high platelet reactivity. These findings have important implications for our understanding of the patho-biology and treatment of atherosclerotic disease.
Acknowledgments
Funding Sources
No specific funding or grant support was used to conduct this study (internal funds only were used).
Akiko Maehara has received a research grant from Boston Scientific and speaker’s honoraria Volcano-Japan. Gary S. Mintz is a consultant and has received grant support from Boston Scientific and Volcano. Roxana Mehran has received research grant support from Sanofi-Aventis/BMS, serves on advisory boards for Abbott Vascular, Cardiva, and Regado Bioscience, and has received honoraria from Cordis and The Medicines Company. George Dangas has received speaker’s honoraria from Cordis/Johnson & Johnson and Abbott Vascular.
Abbreviations and Acronyms
- ACS
acute coronary syndrome
- CSA
cross sectional area
- EEM
external elastic membrane
- HPR
high on-treatment platelet reactivity
- IVUS
intravascular ultrasound
- MACE
major adverse cardiovascular events
- MLA
minimum lumen cross sectional area
- PCI
percutaneous coronary intervention
- TLR
target lesion revascularization
Footnotes
Relationships to industry
Relevant to this submission, the following authors have no conflicts of interest to declare: Amala P. Chirumamilla, Sunil Kanwal, Giora Weisz, Ahmed Hassanein, Diaa Hakim, Ning Guo, Usman Baber, Robert Pyo, Jeffrey W. Moses, Martin Fahy, Jason C. Kovacic.
References
- 1.Lange RA, Hillis LD. Antiplatelet therapy for ischemic heart disease. N Engl J Med. 2004;350:277–280. doi: 10.1056/NEJMe038191. [DOI] [PubMed] [Google Scholar]
- 2.Weber C. Platelets and chemokines in atherosclerosis: partners in crime. Circulation Research. 2005;96:612–616. doi: 10.1161/01.RES.0000160077.17427.57. [DOI] [PubMed] [Google Scholar]
- 3.Kovacic JC, Gupta R, Lee AC, et al. Stat3-dependent acute Rantes production in vascular smooth muscle cells modulates inflammation following arterial injury in mice. J Clin Invest. 2010;120:303–314. doi: 10.1172/JCI40364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dickfeld T, Lengyel E, May AE, et al. Transient interaction of activated platelets with endothelial cells induces expression of monocyte-chemoattractant protein-1 via a p38 mitogen-activated protein kinase mediated pathway. Implications for atherogenesis. Cardiovasc Res. 2001;49:189–199. doi: 10.1016/s0008-6363(00)00220-0. [DOI] [PubMed] [Google Scholar]
- 5.Gawaz M. Role of platelets in coronary thrombosis and reperfusion of ischemic myocardium. Cardiovasc Res. 2004;61:498–511. doi: 10.1016/j.cardiores.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 6.Gawaz M, Brand K, Dickfeld T, et al. Platelets induce alterations of chemotactic and adhesive properties of endothelial cells mediated through an interleukin-1-dependent mechanism. Implications for atherogenesis. Atherosclerosis. 2000;148:75–85. doi: 10.1016/s0021-9150(99)00241-5. [DOI] [PubMed] [Google Scholar]
- 7.Gawaz M, Langer H, May AE. Platelets in inflammation and atherogenesis. J Clin Invest. 2005;115:3378–3384. doi: 10.1172/JCI27196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruggeri ZM. Platelets in atherothrombosis. Nat Med. 2002;8:1227–1234. doi: 10.1038/nm1102-1227. [DOI] [PubMed] [Google Scholar]
- 9.Huo Y, Schober A, Forlow SB, et al. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat Med. 2003;9:61–67. doi: 10.1038/nm810. [DOI] [PubMed] [Google Scholar]
- 10.Pitsilos S, Hunt J, Mohler ER, et al. Platelet factor 4 localization in carotid atherosclerotic plaques: correlation with clinical parameters. Thromb Haemost. 2003;90:1112–1120. doi: 10.1160/TH03-02-0069. [DOI] [PubMed] [Google Scholar]
- 11.Hathcock JJ. Flow effects on coagulation and thrombosis. Arterioscler Thromb Vasc Biol. 2006;26:1729–1737. doi: 10.1161/01.ATV.0000229658.76797.30. [DOI] [PubMed] [Google Scholar]
- 12.Touat Z, Lepage L, Ollivier V, et al. Dilation-dependent activation of platelets and prothrombin in human thoracic ascending aortic aneurysm. Arterioscler Thromb Vasc Biol. 2008;28:940–946. doi: 10.1161/ATVBAHA.107.158576. [DOI] [PubMed] [Google Scholar]
- 13.Gachet C. Regulation of platelet functions by P2 receptors. Annu Rev Pharmacol Toxicol. 2006;46:277–300. doi: 10.1146/annurev.pharmtox.46.120604.141207. [DOI] [PubMed] [Google Scholar]
- 14.Kushner FG, Hand M, Smith SC, Jr, et al. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2009;54:2205–2241. doi: 10.1016/j.jacc.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 15.Patrono C, Baigent C, Hirsh J, Roth G. Antiplatelet drugs: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133:199S–233S. doi: 10.1378/chest.08-0672. [DOI] [PubMed] [Google Scholar]
- 16.Gurbel PA, Tantry US. Drug insight: Clopidogrel nonresponsiveness. Nat Clin Pract Cardiovasc Med. 2006;3:387–395. doi: 10.1038/ncpcardio0602. [DOI] [PubMed] [Google Scholar]
- 17.Aleil B, Ravanat C, Cazenave JP, Rochoux G, Heitz A, Gachet C. Flow cytometric analysis of intraplatelet VASP phosphorylation for the detection of clopidogrel resistance in patients with ischemic cardiovascular diseases. J Thromb Haemost. 2005;3:85–92. doi: 10.1111/j.1538-7836.2004.01063.x. [DOI] [PubMed] [Google Scholar]
- 18.Barragan P, Bouvier JL, Roquebert PO, et al. Resistance to thienopyridines: clinical detection of coronary stent thrombosis by monitoring of vasodilator-stimulated phosphoprotein phosphorylation. Catheter Cardiovasc Interv. 2003;59:295–302. doi: 10.1002/ccd.10497. [DOI] [PubMed] [Google Scholar]
- 19.Bliden KP, DiChiara J, Tantry US, Bassi AK, Chaganti SK, Gurbel PA. Increased risk in patients with high platelet aggregation receiving chronic clopidogrel therapy undergoing percutaneous coronary intervention: is the current antiplatelet therapy adequate? J Am Coll Cardiol. 2007;49:657–666. doi: 10.1016/j.jacc.2006.10.050. [DOI] [PubMed] [Google Scholar]
- 20.Gurbel PA, Becker RC, Mann KG, Steinhubl SR, Michelson AD. Platelet function monitoring in patients with coronary artery disease. J Am Coll Cardiol. 2007;50:1822–1834. doi: 10.1016/j.jacc.2007.07.051. [DOI] [PubMed] [Google Scholar]
- 21.Gurbel PA, Bliden KP, Guyer K, et al. Platelet reactivity in patients and recurrent events post-stenting: results of the PREPARE POST-STENTING Study. J Am Coll Cardiol. 2005;46:1820–1826. doi: 10.1016/j.jacc.2005.07.041. [DOI] [PubMed] [Google Scholar]
- 22.Järemo P, Lindahl TL, Fransson SG, Richter A. Individual variations of platelet inhibition after loading doses of clopidogrel. J Intern Med. 2002;252:233–238. doi: 10.1046/j.1365-2796.2002.01027.x. [DOI] [PubMed] [Google Scholar]
- 23.Matetzky S, Shenkman B, Guetta V, et al. Clopidogrel resistance is associated with increased risk of recurrent atherothrombotic events in patients with acute myocardial infarction. Circulation. 2004;109:3171–3175. doi: 10.1161/01.CIR.0000130846.46168.03. [DOI] [PubMed] [Google Scholar]
- 24.Brar SS, ten Berg J, Marcucci R, et al. Impact of Platelet Reactivity on Clinical Outcomes after Percutaneous Coronary Intervention: Collaborative Meta-analysis of Individual Participant Data. J Am Coll Cardiol. doi: 10.1016/j.jacc.2011.06.059. In press. [DOI] [PubMed] [Google Scholar]
- 25.Mangiacapra F, De Bruyne B, Muller O, et al. High residual platelet reactivity after clopidogrel: extent of coronary atherosclerosis and periprocedural myocardial infarction in patients with stable angina undergoing percutaneous coronary intervention. JACC Cardiovasc Interv. 2010;3:35–40. doi: 10.1016/j.jcin.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 26.Price MJ. Bedside evaluation of thienopyridine antiplatelet therapy. Circulation. 2009;119:2625–2632. doi: 10.1161/CIRCULATIONAHA.107.696732. [DOI] [PubMed] [Google Scholar]
- 27.Price MJ, Berger PB, Teirstein PS, et al. Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. JAMA. 2011;305:1097–1105. doi: 10.1001/jama.2011.290. [DOI] [PubMed] [Google Scholar]
- 28.Qian J, Maehara A, Mintz GS, et al. Impact of gender and age on in vivo virtual histology-intravascular ultrasound imaging plaque characterization (from the global Virtual Histology Intravascular Ultrasound [VH-IVUS] registry) Am J Cardiol. 2009;103:1210–1214. doi: 10.1016/j.amjcard.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 29.Angiolillo DJ. Antiplatelet therapy in diabetes: efficacy and limitations of current treatment strategies and future directions. Diabetes Care. 2009;32:531–540. doi: 10.2337/dc08-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baber U, Mehran R, Weisz G, et al. Differences In Coronary Plaque Composition And Morphology In Patients With And Without Chronic Kidney Disease Presenting With Acute Coronary Syndromes: Insights From The Prospect Study. J Am Coll Cardiol. 2011;57(Suppl 1):E1442. [Google Scholar]
- 31.Burke AP, Kolodgie FD, Farb A, et al. Healed plaque ruptures and sudden coronary death: evidence that subclinical rupture has a role in plaque progression. Circulation. 2001;103:934–940. doi: 10.1161/01.cir.103.7.934. [DOI] [PubMed] [Google Scholar]
- 32.Scheuerer B, Ernst M, Durrbaum-Landmann I, et al. The CXC-chemokine platelet factor 4 promotes monocyte survival and induces monocyte differentiation into macrophages. Blood. 2000;95:1158–1166. [PubMed] [Google Scholar]
- 33.Burger PC, Wagner DD. Platelet P-selectin facilitates atherosclerotic lesion development. Blood. 2003;101:2661–2666. doi: 10.1182/blood-2002-07-2209. [DOI] [PubMed] [Google Scholar]
- 34.Fateh-Moghadam S, Li Z, Ersel S, et al. Platelet degranulation is associated with progression of intima-media thickness of the common carotid artery in patients with diabetes mellitus type 2. Arterioscler Thromb Vasc Biol. 2005;25:1299–1303. doi: 10.1161/01.ATV.0000165699.41301.c5. [DOI] [PubMed] [Google Scholar]
- 35.Massberg S, Brand K, Gruner S, et al. A critical role of platelet adhesion in the initiation of atherosclerotic lesion formation. J Exp Med. 2002;196:887–896. doi: 10.1084/jem.20012044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Massberg S, Schurzinger K, Lorenz M, et al. Platelet adhesion via glycoprotein IIb integrin is critical for atheroprogression and focal cerebral ischemia: an in vivo study in mice lacking glycoprotein IIb. Circulation. 2005;112:1180–1188. doi: 10.1161/CIRCULATIONAHA.105.539221. [DOI] [PubMed] [Google Scholar]
- 37.Seizer P, Gawaz M, May AE. Platelet-monocyte interactions--a dangerous liaison linking thrombosis, inflammation and atherosclerosis. Curr Med Chem. 2008;15:1976–1980. doi: 10.2174/092986708785132852. [DOI] [PubMed] [Google Scholar]
- 38.van Gils JM, Zwaginga JJ, Hordijk PL. Molecular and functional interactions among monocytes, platelets, and endothelial cells and their relevance for cardiovascular diseases. J Leukoc Biol. 2009;85:195–204. doi: 10.1189/jlb.0708400. [DOI] [PubMed] [Google Scholar]
- 39.Gurbel PA, Bliden KP, Hiatt BL, O'Connor CM. Clopidogrel for coronary stenting: response variability, drug resistance, and the effect of pretreatment platelet reactivity. Circulation. 2003;107:2908–2913. doi: 10.1161/01.CIR.0000072771.11429.83. [DOI] [PubMed] [Google Scholar]
- 40.Michelson AD, Linden MD, Furman MI, et al. Evidence that pre-existent variability in platelet response to ADP accounts for 'clopidogrel resistance'. J Thromb Haemost. 2007;5:75–81. doi: 10.1111/j.1538-7836.2006.02234.x. [DOI] [PubMed] [Google Scholar]
- 41.Wexler L, Brundage B, Crouse J, et al. Coronary artery calcification: pathophysiology, epidemiology, imaging methods, and clinical implications. A statement for health professionals from the American Heart Association. Writing Group. Circulation. 1996;94:1175–1192. doi: 10.1161/01.cir.94.5.1175. [DOI] [PubMed] [Google Scholar]
- 42.Burke AP, Taylor A, Farb A, Malcom GT, Virmani R. Coronary calcification: insights from sudden coronary death victims. Z Kardiol. 2000;89:49–53. doi: 10.1007/s003920070099. [DOI] [PubMed] [Google Scholar]
- 43.Kovacic JC, Moreno P, Nabel EG, Hachinski V, Fuster V. Cellular senescence, vascular disease, and aging: part 2 of a 2-part review: clinical vascular disease in the elderly. Circulation. 2011;123:1900–1910. doi: 10.1161/CIRCULATIONAHA.110.009118. [DOI] [PubMed] [Google Scholar]
- 44.Kovacic JC, Randolph GJ. Vascular calcification: harder than it looks. Arterioscler Thromb Vasc Biol. 2011;31:1249–1250. doi: 10.1161/ATVBAHA.111.227868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Campo G, Parrinello G, Ferraresi P, et al. Prospective evaluation of on-clopidogrel platelet reactivity over time in patients treated with percutaneous coronary intervention relationship with gene polymorphisms and clinical outcome. J Am Coll Cardiol. 2011;57:2474–2483. doi: 10.1016/j.jacc.2010.12.047. [DOI] [PubMed] [Google Scholar]