Abstract
TNFs are major mediators of inflammation and inflammation-related diseases, hence, the United States Food and Drug Administration (FDA) has approved the use of blockers of the cytokine, TNF-α, for the treatment of osteoarthritis, inflammatory bowel disease, psoriasis and ankylosis. These drugs include the chimeric TNF antibody (infliximab), humanized TNF-α antibody (Humira) and soluble TNF receptor-II (Enbrel) and are associated with a total cumulative market value of more than $20 billion a year. As well as being expensive ($15 000–20 000 per person per year), these drugs have to be injected and have enough adverse effects to be given a black label warning by the FDA. In the current report, we describe an alternative, curcumin (diferuloylmethane), a component of turmeric (Curcuma longa) that is very inexpensive, orally bioavailable and highly safe in humans, yet can block TNF-α action and production in in vitro models, in animal models and in humans. In addition, we provide evidence for curcumin's activities against all of the diseases for which TNF blockers are currently being used. Mechanisms by which curcumin inhibits the production and the cell signalling pathways activated by this cytokine are also discussed. With health-care costs and safety being major issues today, this golden spice may help provide the solution.
Linked Articles
This article is part of a themed section on Emerging Therapeutic Aspects in Oncology. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2013.169.issue-8
Keywords: bioavailability, chronic diseases, curcumin, inflammation, TNF blockers, TNF
Introduction
Extensive research during the past century has revealed that inflammation plays a major role in most chronic diseases. It was Cornelius Celsus, a physician in first century Rome, who first attempted to describe inflammation as heat (calor), pain (dolor), redness (rubor) and swelling (tumour). Rudolf Virchow, a German scientist from Wurzburg, in 1850, was the first to observe a link between inflammation and various chronic diseases, which include cancer, atherosclerosis, arthritis, diabetes, asthma, multiple sclerosis and Alzheimer's disease (Heidland et al., 2006). More than 200 different types of inflammatory disease have been described. When the name of a disease ends with ‘itis’, this means inflammation of the affected organ. Thus, arthritis is inflammation of the joints, whereas bronchitis, sinusitis, gastritis, oesophagitis, pancreatitis, meningitis, rhinitis and gingivitis are, respectively, inflammation of the bronchi, sinuses, stomach, oesophagus, pancreas, brain, nose and gums. Acute inflammation is thought to be therapeutic as it helps an organism to heal. Chronic inflammation, however, can lead to a disease; inflammation of the colon (colitis), for example, when persistant, for as long as 30 years, can finally lead to colon cancer.
During the past three decades, molecular mechanisms that lead to inflammation have been extensively examined. Various enzymes, cytokines, chemokines and polypeptide hormones have been identified, which can mediate inflammation. These include COX-2, 5-lipooxygenase (LOX), TNF-α, IL-1, IL-6, IL-8, Il-17, IL-21, IL-23 and monocyte chemotactic protein-1 (MCP-1). Among these, TNF-α is a major mediator of inflammation, which is the primary focus of this review.
Discovery of TNFs
TNF has at various times been called tumour necrosis serum, cachectin, lymphotoxin or monocyte cytotoxin based on work from our laboratory and others. It is now clear that TNF is a 25 kDa transmembrane protein (17 kDa when secreted) produced primarily by activated macrophages. The ability of tumours to undergo haemorrhagic necrosis after injection of endotoxin was first shown by Shear and Perrault (1944). O'Malley et al. (1962) reported that endotoxin injection into normal mice resulted in the appearance of tumour necrotizing activity in the circulating blood. This activity was renamed tumour necrosis factor by Carswell et al. (1975). The true chemical identity of TNF, however, was unclear until our group isolated two different molecules: one from macrophages, which we named TNF-α (Aggarwal et al., 1985b), and the other from lymphocytes, which we named TNF-β (Aggarwal et al., 1984). The current review primarily deals with TNF-α.
Because of the amino acid sequence homology between human TNF-α and endotoxin-induced murine cachectin, a protein linked to endotoxin-mediated cachexia and shock (Beutler et al., 1985), it became clear that TNF-α and cachectin were identical. Soon thereafter, numerous groups independently identified the same molecule by using a variety of approaches (Haranaka et al., 1984; Old, 1985; Wang et al., 1985; Fiers et al., 1986; Wallach, 1986). TNF-α is now known to bind to two different receptors, TNFRSF1A and TNFRSF1B, and to activate caspase-mediated apoptosis, NF-κB, activator protein-1 (AP-1), JNK, p38 MAPK and ERK signalling (Figure 1). Our group demonstrated that both TNF-α and TNF-β bind to identical receptors and with similar affinities (Aggarwal et al., 1985a). Although much is known about TNF-α, very little is understood about TNF-β (Aggarwal, 2003; Aggarwal et al., 2012). Both overlapping and non-overlapping activities of the two molecules have been reviewed (Stone-Wolff et al., 1984; Kuprash et al., 2002; Liepinsh et al., 2006).
Figure 1.
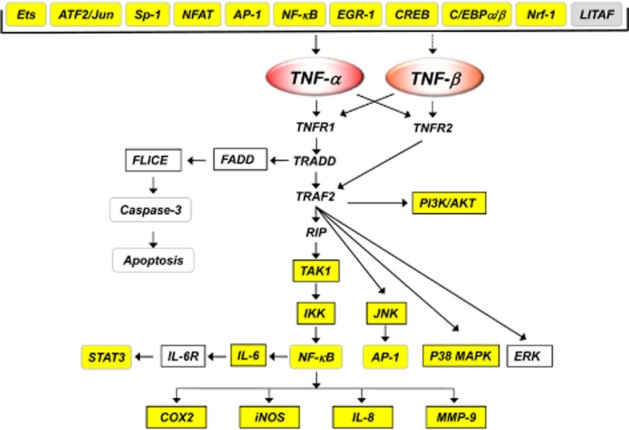
Regulation of the production and action of TNF by curcumin. TNFR1 and TNFR2 are TNF receptors TNFRSF1A and TNFRSF1B respectively. Targets highlighted as yellow are down modulated by curcumin.
Aside from originating in monocytes, it is now clear that TNF-α is also produced by a variety of other cell types including Kupffer cells in the liver, astrocytes in the brain, T-cells and beta cells in the immune system, and ovarian cells. In general, under appropriate conditions, most cell types have the potential to produce TNF-α.
TNF-α and inflammation
It is only within the past few decades that the mechanisms by which inflammation is mediated at the molecular level have become apparent. Although the role of macrophages in inflammation has been known for quite some time, the first indication of the pro-inflammatory activity of TNF emerged in 1985 when it was found to stimulate collagenase and PGE2 production by isolated human synovial cells and dermal fibroblasts (Dayer et al., 1985; Caput et al., 1986), thus suggesting that TNF may play a role in the tissue destruction and remodelling associated with inflammatory diseases.
TNF-associated diseases
TNF dysregulation has been linked to a wide variety of diseases including cancer, obesity, cardiovascular diseases, pulmonary diseases, metabolic diseases, neurological diseases, psychological diseases, skin diseases and autoimmune diseases (Aggarwal, 2003; Aggarwal et al., 2012). Thus, blockers of TNF have been approved for the treatment of various autoimmune disorders such as rheumatoid arthritis (RA), ankylosing spondylitis, Crohn's disease (CD), psoriasis, hidradenitis suppurativa and refractory asthma. The inhibition of TNF can be achieved with monoclonal antibodies such as infliximab (Remicade; Janssen Biotech Inc., Horsham, PA, USA), adalimumab (Humira; Abbott Laboratories, North Chicago, IL, USA), certolizumab pegol (Cimzia; UCB, Brussels, Belgium) and golimumab (Simponi; Janssen Biotech) or with a circulating receptor fusion protein such as etanercept (Enbrel; Amgen, Thousand Oaks, CA, USA). Their potential use in other pro-inflammatory diseases is currently being explored. Some of the important adverse effects most extensively associated with TNF blockers include lymphoma, infections, congestive heart failure, demyelinating disease, a lupus-like syndrome, induction of auto-antibodies, injection site reactions and systemic adverse effects (Scheinfeld, 2004).
Suppression of TNF-α production by curcumin in vitro
Numerous reports have suggested that the production of TNF from macrophages activated by various stimuli can be suppressed by curcumin (Table 1). Studies have supported findings that LPS is one of the major inducers of TNF-α in macrophages and monocytes and that curcumin can down-regulate the expression of TNF-α (Chan, 1995; Abe et al., 1999; Jang et al., 2001; Gao et al., 2004; Strasser et al., 2005; Woo et al., 2007; Liang et al., 2008; 2009; Cheung et al., 2009; Jain et al., 2009; Nishida et al., 2010; Zhao et al., 2010). Besides being expressed by myeloid cells, TNF is also expressed by microglial cells, adipocytes and other cell types. Curcumin, however, has been shown to down-regulate TNF expression (Jin et al., 2007; Lee et al., 2007; Zhang et al., 2008; 2010a). In addition to being induced by LPS, TNF is also upregulated by a variety of other stimuli including phorbol ester, palmitate and other inflammatory cytokines, and curcumin has been shown to block the expression of TNF induced by all of these stimuli (Abe et al., 1999; Lee et al., 2007; Jain et al., 2009; Wang et al., 2009).
Table 1.
Curcumin inhibits the production and action of TNF in vitro
| Production of TNF |
|---|
| • Inhibited LPS-induced TNF and IL-1 release from macrophages (Chan, 1995). |
| • Inhibited production of IL-8, MIP-1α, MCP-1, IL-1β and TNF-α by PMA- or LPS-stimulated human monocytes and alveolar macrophages (Abe et al., 1999). |
| • Inhibited LPS-induced TNF-α release from macrophages (Jang et al., 2001). |
| • Inhibited the expression/production of IL-12 and TNF-α by peritoneal macrophages (Gao et al., 2004). |
| • Decreased NF-κB activation and TNF-α secretion after LPS exposure in U-937 cells (Strasser et al., 2005). |
| • Inhibited the production of IL-1, IL-6 and TNF-α in LPS-stimulated BV2 microglia (Jin et al., 2007). |
| • Exhibited neuroprotective effects through suppression of NO, TNF-α, IL-1α and IL-6 from Abeta (25–35)/IFN-γ- and LPS-stimulated microglia cells (Lee et al., 2007). |
| • Inhibited inflammatory responses of adipose tissue in obesity by suppressing release of TNF-α, NO and MCP-1 from adipocytes (Woo et al., 2007). |
| • Inhibited NO and TNF-α production in rat primary microglia induced by LPS (Zhang et al., 2008). |
| • Inhibited LPS-induced TNF-α and IL-6 synthesis in macrophages (Liang et al., 2008). |
| • Down-regulated TNF, IL-1, NO and PGE2 in Raw 264.7 cells possibly through induction of phase II/antioxidant enzymes including HO-1 and NQO-1 (Cheung et al., 2009). |
| • Reversed palmitate-induced insulin resistance through suppression of NF-κB, TNF-α and IL-6 in adipocytes (Wang et al., 2009). |
| • Inhibited LPS-induced production of TNF-α, IL-1β, MCP-1, COX-2, iNOS and p65 NF-κB in the macrophages (Liang et al., 2009). |
| • Inhibited the high glucose-induced secretion of IL-6, IL-8, MCP-1 and TNF-α in U937 monocytes (Jain et al., 2009). |
| • Inhibited secretion of TNF-α and IL-6 in vitro (Tham et al., 2010). |
| • Inhibited the release of TNF-α and IL-6 in LPS-stimulated RAW 264.7 macrophages (Zhao et al., 2010). |
| • Inhibited IκB phosphorylation, NF-κB activation and TNF-α production induced by LPS in mouse macrophages (Nishida et al., 2010). |
| • Decreased LPS-induced TNF-α and IL-1β expression at both transcriptional and protein level in microglial cells (Zhang et al., 2010a). |
| Action of TNF-α |
|---|
| • Inhibited TNF-induced NF-κB activation in human myeloid cells (Singh and Aggarwal, 1995). |
| • Reduced TNF-induced endothelial tissue factor by inhibiting AP-1 and NF-κB in endothelial cells (Bierhaus et al., 1997). |
| • Blocked the activation of AP-1 and NF-κB induced by IL-1α and TNF-α in stromal cells (Xu et al., 1997). |
| • Inhibited TNF-α-induced expression of ICAM-1, VCAM-1 and E-selectin in HUVEC (Gupta and Ghosh, 1999; Kumar et al., 1998). |
| • Suppressed TNF-α-induced VEGF secretion in U937 and Raji cells. Reduced the expression of VEGF165 and VEGF121 mRNA induced by TNF-α (Chen et al., 2005). |
| • Inhibited TNF-mediated constitutive NF-κB activation linked to proliferation of mantle cell lymphoma cells (Shishodia et al., 2005). |
| • Blocked TNF-α-induced endothelial dysfunction in HUVEC (Nan et al., 2005). |
| • Down-regulated TNF-induced expression of cell proliferation and anti-apoptotic and metastatic gene products (Aggarwal et al., 2006). |
| • Inhibited TNF-α-stimulated Gb3 synthase (GalT6) mRNA expression in intestinal epithelial cells (Moon et al., 2006). |
| • Inhibited TNF-α-induced expression of IL-1β, IL-6, TNF-α and cyclin E, but not IL-8, in HaCaT cells (Cho et al., 2007). |
| • Inhibited TNF-α-induced NF-κB activation in MCF-7 cells by inhibiting the proteasomal activities (Yoon and Liu, 2007). |
| • Suppressed TNF-α-induced expression of ICAM-1 and VCAM-1, and secretion of IL-6, IL-8 and MCP-1 in HUVEC (Kim et al., 2007). |
| • Inhibited TNF-α-induced NF-κB activation in chronic myeloid leukaemia cells through modulation of redox status of the cells (Sandur et al., 2007). |
| • Down-regulated the expression of 29 out of 84 TNF-α-activated NF-κB-associated genes in leukaemia cells (Reuter et al., 2009). |
| • Inhibited TNF-induced NF-κB activation in leukaemia cells (Yadav et al., 2010). |
| • Inhibited TNF-α-induced cell migration, intracellular ROS generation, MMP-9 expression, MMP-9 activity and NF-κB in human aortic smooth muscle cells (Yu and Lin, 2010). |
| • Attenuated TNF-α-induced enhancement of TRPC1 expression, and COX-2-dependent PGE2 production in colonic myofibroblasts (Hai et al., 2011). |
| • Inhibited NF-κB-mediated inflammation in human tenocytes through suppression of the PI3K/Akt pathway (Buhrmann et al., 2011). |
AKT, AKT8 virus oncogene cellular homologue; AP-1, activator protein-1; HO-1, haeme oxygenase-1; ICAM-1, intercellular adhesion molecule-1; MCP-1, monocyte chemotactic protein-1; MIP-1α, macrophage inflammatory protein-1α; MMP-9, matrix metallopeptidase-9; NQO1, NADH quinone oxidoreductase 1; TRPC1, transient receptor potential channel 1; VCAM-1, vascular cell adhesion molecule-1.
How curcumin down-regulates TNF expression in different cell types and in response to a variety of stimuli has been examined extensively. TNF suppression primarily occurs at the transcriptional level. The factors known to be involved in TNF transcription include the transcription factor ETS (Kramer et al., 1995), activating transcription factor 2 (ATF2)/Jun (Leitman et al., 1991; Newell et al., 1994; Tsai et al., 1996a,b), Sp1 (Kramer et al., 1994), nuclear factor of activated T-cell transcription factor (NFAT) (McCaffrey et al., 1994; Tsai et al., 1996a,b), NF-κB (Udalova et al., 1998; Kuprash et al., 1999), early growth response protein-1 (Kramer et al., 1994), cAMP response element binding protein (CREB) (Geist et al., 1997), CCAAT/enhancer binding protein β (C/EBPβ) (Pope et al., 1994; Wedel et al., 1996; Zagariya et al., 1998), NF-E2-related factor 1 (Novotny et al., 1998; Prieschl et al., 1998) and LPS-induced TNF-α factor (LITAF) (Takashiba et al., 1995; Myokai et al., 1999) (Figure 1). Hence, different transcription factors appear to be involved in the stimulation of TNF expression by various stimuli and in different cell types. For instance, the transcription factor LITAF is involved in LPS-stimulated TNF expression. Whereas ATF2/Jun and NFATp are involved in TNF expression in activated beta and T-cells, C/EBPβ is involved in human monocytes. Several of these transcription factors have been shown to be modulated by curcumin.
Curcumin can mediate its effect on TNF expression by inhibiting p300/CREB-specific acetyl transferase, leading to repression of the acetylation of histone/non-histone proteins and histone acetyl transferase-dependent chromatin transcription (Balasubramanyam et al., 2004). It is also well known that curcumin can down-modulate the activation of NF-κB by a variety of agents (Singh and Aggarwal, 1995) and this down-regulation of NF-κB by curcumin plays a major role in suppressing the expression of TNF. In addition, in a number of studies it has been shown that methylation of a TNF promoter may affect the promoter's function (Kochanek et al., 1990; 1991; Muiznieks and Doerfler, 1994; Takei et al., 1996). Thus, curcumin could affect TNF expression by affecting the methylation of a TNF promoter (Reuter et al., 2011).
It is possible that the effects of curcumin on LPS-induced TNF production are mediated in part through its LPS signalling. Two of the toll-like receptors (TLRs), TLR2 and TLR4, mediate responsiveness to LPS. LPS-mediated TLR2 mRNA induction has been shown to be attenuated by pretreatment with curcumin (Matsuguchi et al., 2000). In addition, there is biochemical evidence indicating that curcumin can inhibit both ligand-induced and ligand-independent dimerization of TLR4 (Youn et al., 2006). The beneficial effect of curcumin is partly mediated by reducing the expression levels of TNF through inhibition of the expression of TLR2, TLR4 and TLR9 in mouse liver (Tu et al., 2012). Curcumin also binds with sub-micromolar affinity to the myeloid differentiation protein-2 (MD-2), which is the LPS-binding component of the endotoxin surface receptor complex MD-2/TLR4 (Gradisar et al., 2007). The binding site for curcumin overlaps with that of LPS; this results in the inhibition of MyD88-dependent and MyD88-independent signalling pathways of LPS signalling through TLR4, indicating that MD-2 is an important target of curcumin involved in its suppression of the innate immune response to bacterial infection.
Suppression of TNF-α-mediated signalling by curcumin in vitro
There are numerous reports suggesting that curcumin can not only block the production of TNF but also block the cell signalling mediated by TNF in a variety of cell types (Table 1). Our group was the first to show that curcumin can inhibit TNF-mediated NF-κB action in variety of cell types (Singh and Aggarwal, 1995). We also showed that TNF-mediated expression of various cell surface adhesion molecules in endothelial cells is down-regulated by curcumin (Kumar et al., 1998). Since then, a wide variety of cell signalling pathways activated by TNF have been shown to be down-regulated by curcumin; these include JNK, MAPK, PI3K/Akt.
In addition, curcumin has also been shown to modulate TNF-α function by directly binding to the ligand (Gupta et al., 2011). Wua et al. (2010) performed molecular docking studies with TNF-α and curcumin to predict and analyse the ability of curcumin to inhibit TNF-α by binding to it. The protein–ligand interactions were analysed by simulating the docking of the curcumin using Autodock 4.0. They identified three main binding regions for curcumin and found that curcumin is a potent inhibitor of TNF-α. They also observed that curcumin docked at the receptor-binding sites of TNF-α. Covalent π-π aromatic interactions or π–cation interactions were found between curcumin and TNF-α. The authors predicted that curcumin is a strong inhibitor of TNF-α because of the covalent bonds it forms with Cys129 in TNF-α. In contrast to its interaction with TNF-α, it is unclear whether curcumin can interact or affect the expression of TNF-β or lymphotoxin.
Suppression of TNF by curcumin in vivo
The anti-inflammatory effect of curcumin was first demonstrated in acute and chronic models of inflammation in rats and mice (Srimal and Dhawan, 1973). The authors showed that curcumin (50–200 mg·kg−1) suppressed carrageenan-induced oedema in mice. Furthermore, curcumin was found to be as potent as phenylbutazone and exhibited minimal ulcerogenic activity. No mortality in mice was noted at doses as high as 2 g·kg−1 bodyweight (Srimal and Dhawan, 1973). In the same study, the authors showed that curcumin suppresses formaldehyde-induced arthritis in rats at a dose of 40 mg·kg−1 and inhibited granuloma formation at 80–160 mg·kg−1. However, the mechanism by which curcumin mediates these anti-inflammatory effects in animals was not revealed until several years later, when our group showed that curcumin can suppress TNF-induced NF-κB activation (Singh and Aggarwal, 1995) and other groups showed that curcumin blocked TNF production in cell culture (Chan, 1995) and the expression of pro-inflammatory genes (Jobin et al., 1999). Since then, numerous mechanisms by which curcumin can exhibit anti-inflammatory activity have been proposed (Figures 1 and 2).
Figure 2.
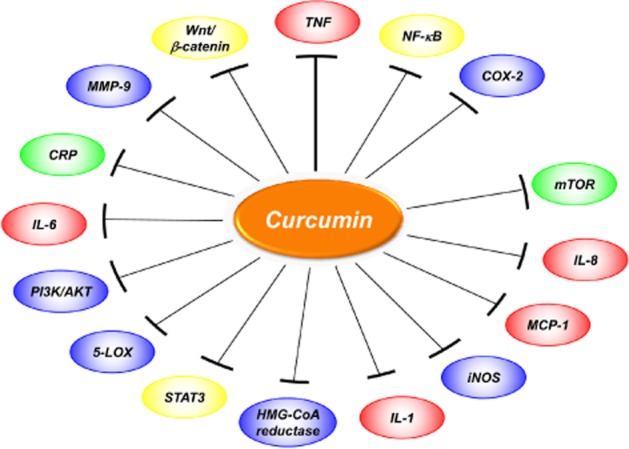
Inflammatory targets modulated by curcumin.
Numerous reports have been published suggesting that oral administration of curcumin down-regulates TNF-α expression both in the serum and in the tissue of animals (Nanji et al., 2003; Yao et al., 2004; Sharma et al., 2007a; Billerey-Larmonier et al., 2008; Larmonier et al., 2008; Ung et al., 2010; El-Moselhy et al., 2011; Gutierres et al., 2013) (Table 2). Attenuation of TNF-α levels by curcumin has been noted in mice (Leyon and Kuttan, 2003), rats (Siddiqui et al., 2006) and rabbits (Yao et al., 2004; Huang et al., 2008). A dose of curcumin of 50–500 mg·kg−1·day−1 was used for most of these studies. Endotoxin has been shown to induce septic shock in animals, in part, through the production of TNF, and this condition has been shown to be reversed by curcumin (Siddiqui et al., 2006; Chen et al., 2007; 2008; Huang et al., 2008; Nishida et al., 2010). Decreased TNF-α levels have also been noted in tumour-bearing animals treated with this polyphenol (Leyon and Kuttan, 2003). In addition to cancer, down-regulation of TNF-α by curcumin has been associated with protection from various pro-inflammatory diseases, including sub-chronic inflammation (Nandal et al., 2009; Nishida et al., 2010), cardiovascular diseases (Yao et al., 2004; 2005; Mito et al., 2011; Avci et al., 2012), diabetes (Jain et al., 2009; El-Azab et al., 2011; El-Moselhy et al., 2011), acute pancreatitis (Gulcubuk et al., 2006), enterocolitis (Jia et al., 2010), enteritis (Song et al., 2010), prostatitis (Zhang et al., 2010b), diabetic neuropathy (Sharma et al., 2007b), hepatic injury (Yun et al., 2010), Th1-type ileitis (Bereswill et al., 2010), hepatic fibrosis (Shu et al., 2007; Zeng et al., 2011), radiation-induced lung fibrosis (Lee et al., 2010), asthma (Ammar el et al., 2011), alcohol-induced liver disease (Nanji et al., 2003), non-alcoholic steatohepatitis (Ramirez-Tortosa et al., 2009), concanavalin A-induced liver injury (Tu et al., 2012), renal injury (Hashem et al., 2008; Pan et al., 2012), infection (Allam, 2009), fatigue (Gupta et al., 2009), bone turnover (Yang et al., 2011) and high-fat diet-induced hyperglycaemia (El-Moselhy et al., 2011). Curcumin has also been found to down-regulate NF-κB-regulated gene products such as inducible NOS (iNOS), IL-1, IL-6, IL-8, MCP-1, MMP-2 and MMP-9 in animals (Yao et al., 2004; Yeh et al., 2005; Jain et al., 2009). Curcumin also protects against the toxic effects of copper overload (Wan et al., 2007), dextran sulfate sodium (Arafa et al., 2009), p-dioxin (Ciftci et al., 2010) and aluminum (Sood et al., 2011) through down-modulation of TNF-α.
Table 2.
Curcumin inhibits TNF production in animals
| • Prevented alcohol-induced liver disease in rats by inhibiting the expression of NF-κB-dependent genes including TNF-α (Nanji et al., 2003). |
| • Reduced the serum level of TNF-α and NO in B16F-10 melanoma cells bearing C57BL/6 mice (Leyon and Kuttan, 2003). |
| • Suppressed the myocardial TNF-α and MMP-2 expression and improved left ventricular function in pressure overloaded rabbits (Yao et al., 2004). |
| • Decreased the elevations in plasma IL-8, IL-10 and TNF-α in rabbits after cardiopulmonary bypass and cardiac global ischaemia (Yeh et al., 2005). |
| • Significantly lowered the serum TNF-α and IL-6 levels in rat model of acute pancreatitis (Gulcubuk et al., 2006). |
| • Decreased the expression of TNF-α and reduced the mortality in rat model of sepsis (Siddiqui et al., 2006). |
| • Reduced the mortality rate of LPS-infused rats by decreasing the circulating TNF-α levels and the consumption of peripheral platelets and plasma fibrinogen (Chen et al., 2007). |
| • Significantly inhibited TNF-α and NO levels in rat model of diabetic neuropathy (Sharma et al., 2007b). |
| • Down-regulated the expressions of TNF-α and IL-8 in the copper-overloaded rats (Wan et al., 2007). |
| • Decreased the levels of NO, TGF-β1 and TNF-α in rat model of hepatic fibrosis (Shu et al., 2007). |
| • Inhibited expression of TNF-α and IL-1β stimulated by LPS in murine macrophages through inhibition of NF-κB pathway (Chen et al., 2008). |
| • Significantly reduced the LPS-induced overproduction of circulating TNF-α, IL-1β and IL-6, brain glutamate, PGE2, and hydroxyl radicals in rabbit (Huang et al., 2008). |
| • Significantly decreased TNF-α mRNA and caspase-8 that probably contributes to the protective role of the turmeric-based diet against renal injury in rat (Hashem et al., 2008). |
| • Reduced TNF-α levels in a rabbit model of non-alcoholic steatohepatitis (Ramirez-Tortosa et al., 2009). |
| • Decreased the levels of TNF-α in a rat model of subchronic inflammation (Nandal et al., 2009). |
| • Exhibited anti-fibrosis activity by decreasing the levels of TNF-α and TGF-β1 in serum and lung tissue of SiO2-induced fibrosis mice model (Jiang et al., 2009). |
| • Prevented the injurious effects of DSS and ameliorated release of TNF-α and NO in a rat model (Arafa et al., 2009). |
| • Decreased serum levels of IL-12 and TNF-α in mice infected with Schistosoma mansoni cercariae (Allam, 2009). |
| • Significantly attenuated oxidative stress and TNF-α levels in a mouse model of immunologically induced fatigue (Gupta et al., 2009). |
| • Significantly decreased the blood levels of IL-6, MCP-1, TNF-α, glucose, HbA1 and oxidative stress in streptozotocin-induced diabetic rat model (Jain et al., 2009). |
| • Decreased LPS-induced TNF-α production in lungs of mice. At 5% concentration, curcumin significantly improved survival of mice and decreased radiation-induced lung fibrosis (Lee et al., 2010). |
| • Exhibited protective effects against necrotizing enterocolitis in neonatal rats, possibly by inhibiting COX-2, reducing TNF-α and increasing IL-10 contents (Jia et al., 2010). |
| • Significantly decreased the levels of TNF-α and IL-8 in the serum and prostate tissues in a rat model of prostatitis (Zhang et al., 2010b). |
| • Significantly decreased the production of TNF-α in a mouse model of acute inflammation (Bansal and Chhibber, 2010). |
| • Protected mice from LPS/GalN-induced hepatic injury and inflammation by blocking TNF-α production (Yun et al., 2010). |
| • Increased IFN-γ, IL-12 and IL-13 levels, but decreased TNF-α level in rats intoxicated with 2,3,7,8-tetrachlorodibenzo-p-dioxin (Ciftci et al., 2010). |
| • Lowered the production of IL-23p19, IFN-γ, TNF-α, IL-6 and MCP-1 in a murine model of hyperacute Th1-type ileitis (Bereswill et al., 2010). |
| • Suppressed LPS stimulated TNF-α production in mice (Nishida et al., 2010). |
| • Reduced the aluminum-induced inflammatory response as indicated by down-regulation of NF-κB and TNF-α in glial cells (Sood et al., 2011). |
| • Improved the lipid metabolism and delayed the progression of hepatic fibrosis in rats with experimental steatohepatitis through suppression of TNF-α, NF-κB and HMG-CoA reductase (Zeng et al., 2011). |
| • Inhibited mRNA expression of TNF-α in a murine model of asthma (Ammar el et al., 2011). |
| • Suppressed inflammation by reducing levels of TNF-α, NF-κB and IL-6 in CCl4-treated rats (Bassiouny et al., 2011). |
| • Reduced cardiac inflammation through suppression of IL-1β, TNF-α, GATA-4 and NF-κB in a rat model of experimental autoimmune myocarditis (Mito et al., 2011). |
| • Suppressed serum levels of TNF-α and IL-1β in a streptozotocin-induced diabetic mouse model (El-Azab et al., 2011). |
| • Attenuated TNF-α levels and exhibited anti-hyperglycaemic effect and improved insulin sensitivity in high-fat diet-fed rats (El-Moselhy et al., 2011). |
| • Prevented deterioration of the bone structure and produced beneficial effects in bone turnover in transgenic mice possibly through modulation of TNF-α and IL-6 (Yang et al., 2011). |
| • Protected against ischaemia/reperfusion injury in rat skeletal muscle through inhibition of plasma TNF-α levels (Avci et al., 2012). |
| • Inhibited the high glucose-induced plasma TNF-α production and macrophage infiltration and prevented renal injury in diabetic rats (Pan et al., 2012). |
| • Attenuated concanavalin A-induced liver injury in mice by inhibition of TNF expression through TLR-2, TLR-4 and TLR-9 expression (Tu et al., 2012). |
CCl4, carbon tetrachloride; DSS, dextran sulfate sodium; GalN, d-galactosamine; HbA1, haemoglobin α1; HMG-CoA, 3-hydroxy-3-methylglutaryl-coenzyme A; MCP-1, monocyte chemotactic protein-1; SiO2, silicon dioxide.
A reduction in the production of iNOS mRNA was observed when BALB/c mouse peritoneal macrophages cultured ex vivo were treated with 1–20 μM curcumin (Chan et al., 1998); in vivo, two oral treatments of 0.5 mL of a 10 μM solution of curcumin (92 ng·g−1 bodyweight) reduced iNOS mRNA expression in the livers of LPS-injected mice by 50–70% (Chan et al., 1998). This suggests that curcumin is potent at nmol g−1 bodyweight. This efficacy was associated with two modifications of the schedule of dosing: firstly, an aqueous solution of curcumin was prepared by initially dissolving the compound in 0.5 N NaOH and then diluting it immediately with PBS; secondly, mice were fed curcumin at dusk after fasting. Inhibition of iNOS mRNA expression was not observed in the mice that were fed ad libitum, suggesting that food intake may interfere with the absorption of curcumin.
Oral bioavailability and safety of curcumin
Curcumin usually manifests its biological response when given orally to mice at about 50–500 mg·kg−1 bodyweight (Farombi and Ekor, 2006). These doses, however, are too low to detect significant levels of curcumin in the serum. The reason for this discrepancy is not clear; however, there are several possible explanations. Firstly, curcumin is known to bind to numerous proteins present in the serum including albumin (Gupta et al., 2011; Kim et al., 2012). Secondly, curcumin is rapidly transported across the cells and tissues (Anand et al., 2007). Thirdly, tetrahydrocurcumin, a metabolite of curcumin, was found to be more active than curcumin for treating chloroquine-induced hepatotoxicity in rats (Pari and Amali, 2005). Fourthly, in another study it was shown that when curcumin was dissolved in 0.1 N NaOH it manifested its effects in animals in the μg·kg−1 range (Chan et al., 1998).
In one recent study the potential of a novel solid lipid curcumin particle (SLCP) preparation to produce adverse effects in rats after acute and sub-chronic administration was investigated (Dadhaniya et al., 2011). The oral LD50 of the preparation in rats as well as in mice was found to be greater than 2000 mg·kg−1 bodyweight. In the sub-chronic toxicity study, 180, 360 and 720 mg·kg−1 bodyweight day−1 of SLCP preparation was administered via oral gavage to Wistar rats (10 per sex per group) for 90 days. Administration of the curcumin preparation did not result in any toxicologically significant treatment-related changes in clinical (including behavioural) observations, ophthalmic examinations, bodyweights, bodyweight gains, food consumption and organ weights. No adverse effects of the curcumin preparation were noted on the haematology, serum chemistry parameters and urinalysis. Terminal necropsy did not reveal any treatment-related gross or histopathology findings. On the basis of these study results, the no observed-adverse-effect level for this standardized novel curcumin preparation was determined as 720 mg·kg−1 bodyweight day−1.
In humans, as little as 150 mg of curcumin has been shown to be effective in reducing serum levels of pro-inflammatory cytokines (Usharani et al., 2008) (Table 3). The effect of curcumin administration, 500 mg of curcumin day−1 for 7 days, on serum levels of cholesterol and lipid peroxides was studied in 10 healthy human volunteers (Soni and Kuttan, 1992). A significant decrease in the level of serum lipid peroxides was noted, along with an increase in high-density lipoprotein cholesterol and a decrease in total serum cholesterol. In another study, curcumin was given orally at up to 8000 mg·day−1 to 25 patients (Cheng et al., 2001). The serum concentration of curcumin usually peaked at 1–2 h after oral intake of curcumin and gradually declined within 12 h. The average peak serum concentrations after oral intake of 4000, 6000 and 8000 mg of curcumin were 0.51 ± 0.11, 0.63 ± 0.06 and 1.77 ± 1.87 μM respectively. , However, urinary excretion of curcumin was undetectable.
Table 3.
Curcumin inhibits TNF production in humans
| • Improved endothelial function and reduced levels of malondialdehyde, IL-6, TNF-α and endothelin-1 in diabetic patients (Usharani et al., 2008). |
| • Had non-significant effects on the production of IL-8, IL-1β, TNF-α and COX-2 in gastric mucosa from Helicobacter pylori-infected gastritis patients (Koosirirat et al., 2010). |
| • Improved bodyweight, reduced serum TNF-α and induced p53 expression in patients with colorectal cancer (He et al., 2011). |
Vareed et al. (2008) examined the pharmacokinetics of a curcumin preparation in healthy human volunteers at 0.25–72 h after a single oral dose. Curcumin was administered at doses of 10 g (n = 6 subjects) and 12 g (n = 6 subjects). Using HPLC with a limit of detection of 50 ng·mL−1, only one subject had detectable free curcumin at any of the 14 time points assayed, but curcumin glucuronides and sulfates were detected in all subjects. Based on the pharmacokinetic model, the area under the curve for the 10 and 12 g doses was 35.33 ± 3.78 and 26.57 ± 2.97 μg·mL−1 × h, respectively, whereas Cmax was 2.30 ± 0.26 and 1.73 ± 0.19 μg·mL−1. The Tmax and t1/2 were estimated to be 3.29 ± 0.43 and 6.77 ± 0.83 h. The ratio of glucuronide to sulfate was 1.92:1. The curcumin conjugates were present as either glucuronide or sulfate, not as mixed conjugates. The group concluded that curcumin is absorbed after oral dosing in humans and can be detected as glucuronide and sulfate conjugates in plasma. Another study evaluated the efficacy of oral curcumin (4 g·day−1) in 26 patients with monoclonal gammopathy of undefined significance (Golombick et al., 2009). They found that oral curcumin was bioavailable as it decreased paraprotein load. Thus, all of these studies clearly demonstrate that although serum levels of curcumin administered orally are very low, it can still manifest its effect in vivo.
Suppression of TNF-α by curcumin in patients
At least two studies have suggested that orally administered curcumin can down-modulate the expression of TNF-α in patients (Usharani et al., 2008; He et al., 2011) (Table 3). In addition, several other pro-inflammatory biomarkers are decreased by curcumin in human subjects (Hanai and Sugimoto, 2009; Khajehdehi et al., 2011; Koosirirat et al., 2010). In most of these studies, 150–500 mg of curcumin was sufficient to manifest a response.
The interest in curcumin research in human participants has increased markedly over the years (Table 4). To date, over 60 clinical trials have evaluated the safety and efficacy of this polyphenol in humans, whereas another 35 clinical trials are further evaluating its efficacy. Curcumin was found to be effective in TNF-associated human diseases such as cancer, cardiovascular diseases, metabolic diseases, neurological diseases, skin diseases, RA, CD and psoriasis. However, whether curcumin exerts its effects through modulation of TNF in these patients is, at present, unclear. Given the fact that most of the currently available TNF blockers produce adverse effects in patients and are very expensive, this orally bioavailable polyphenol represents an important therapeutic for TNF-associated diseases.
Table 4.
Chronological listing of curcumin studies in human participants
| Year | Disease | Reference |
|---|---|---|
| 1937 | Cholecystitis | Oppenheimer, 1937 |
| 1972 | Diabetes | Srinivasan, 1972 |
| 1980 | Rheumatoid arthritis | Deodhar et al., 1980 |
| 1986 | Post-operative inflammation | Satoskar et al., 1986 |
| 1987 | Cancer lesions | Kuttan et al., 1987 |
| 1992 | Lung cancer | Polasa et al., 1992 |
| 1992 | Atherosclerosis | Soni and Kuttan, 1992 |
| 1993 | Gastric ulcer | Kositchaiwat et al., 1993 |
| 1996 | Acquired immunodeficiency syndrome | James, 1996 |
| 1997 | Cancer lesions | Hastak et al., 1997 |
| 1999 | Biliary dyskinesia | Niederau and Gopfert, 1999 |
| Gallbladder contraction | Rasyid and Lelo, 1999 | |
| Chronic anterior uveitis | Lal et al., 1999 | |
| 2000 | Idiopathic orbital inflammatory pseudotumour | Lal et al., 2000 |
| Psoriasis | Heng et al., 2000 | |
| 2001 | Cancer lesions | Cheng et al., 2001 |
| Colorectal cancer | Sharma et al., 2001 | |
| Peptic ulcer | Prucksunand et al., 2001 | |
| 2004 | Colorectal cancer | Sharma et al., 2004 |
| Irritable bowel syndrome | Bundy et al., 2004 | |
| 2005 | Colorectal cancer | Garcea et al., 2005 |
| Pancreatic cancer | Durgaprasad et al., 2005 | |
| Crohn's disease | Holt et al., 2005 | |
| Ulcerative proctitis | Holt et al., 2005 | |
| Alzheimer's disease | Ringman et al., 2005 | |
| Renal transplantation | Shoskes et al., 2005 | |
| 2006 | Colorectal cancer | Cruz-Correa et al., 2006 |
| Ulcerative colitis | Hanai et al., 2006 | |
| 2007 | Cancer lesions | Chainani-Wu et al., 2007 |
| Multiple myeloma | Vadhan-Raj et al., 2007 | |
| Helicobacter pylori infection | Di Mario et al., 2007 | |
| 2008 | Pancreatic cancer | Dhillon et al., 2008 |
| Psoriasis | Kurd et al., 2008 | |
| Alzheimer disease | Baum et al., 2008 | |
| Acute coronary syndrome | Alwi et al., 2008 | |
| Diabetes | Usharani et al., 2008 | |
| Hepatoprotection | Adhvaryu et al., 2008 | |
| 2009 | Multiple myeloma | Golombick et al., 2009 |
| Irritable bowel syndrome | Shimouchi et al., 2009 | |
| Dejerine–Sottas disease | Burns et al., 2009 | |
| Recurrent respiratory tract infections | Zuccotti et al., 2009 | |
| Chronic bacterial prostatitis | Cai et al., 2009 | |
| 2010 | Cancer lesions | Rai et al., 2010 |
| Pancreatic cancer | Epelbaum et al., 2010 | |
| Breast cancer | Bayet-Robert et al., 2010 | |
| Prostate cancer | Ide et al., 2010 | |
| Inflammatory bowel disease | Epstein et al., 2010 | |
| Recurrent anterior uveitis | Allegri et al., 2010 | |
| Helicobacter pylori infection | Koosirirat et al., 2010 | |
| Osteoarthritis | Belcaro et al., 2010a; Belcaro et al., 2010b | |
| Diabetes | Wickenberg et al., 2010 | |
| Vitiligo | Asawanonda and Klahan, 2010 | |
| β-Thalassaemia | Kalpravidh et al., 2010 | |
| Chronic arsenic exposure | Biswas et al., 2010 | |
| Osteosarcoma | Gota et al., 2010 | |
| 2011 | Colorectal cancer | Carroll et al., 2011; He et al., 2011 |
| Pancreatic cancer | Kanai et al., 2011 | |
| Head and neck cancer | Kim et al., 2011 | |
| Ulcerative colitis | Lahiff and Moss, 2011 | |
| Diabetic nephropathy | Khajehdehi et al., 2011 | |
| Diabetic microangiopathy | Appendino et al., 2011 | |
| Alcohol intoxication | Sasaki et al., 2011 | |
| 2012 | Rheumatoid arthritis | Chandran and Goel, 2012 |
| Diabetes | Chuengsamarn et al., 2012 | |
| Lupus nephritis | Khajehdehi et al., 2012 |
In addition to its efficacy in TNF-associated human diseases, curcumin has been found to be effective in a number of other human diseases. Readers interested in such studies should refer to one of the recent reviews published from this laboratory (Gupta et al., 2013).
Role of curcumin in TNF-related diseases
Rheumatoid arthritis
Numerous reports have suggested that TNF plays a major role in RA. Thus, TNF blockers have been found to be beneficial for patients with RA. Therefore, curcumin has been tested as a treatment for RA. One of the earliest indications of its potential efficacy was obtained in 1973, when curcumin was found to suppress formaldehyde-induced arthritis in rats at a dose of 40 mg·kg−1 and inhibit granuloma formation at 80–160 mg·kg−1 (Srimal and Dhawan, 1973). Later, Joe et al. (1997) showed that curcumin can lower the elevated serum acidic glycoprotein levels present in adjuvant-induced arthritis. Also, oral administration of curcumin has been shown to prevent streptococcal cell wall–induced arthritis in mice (Funk et al., 2006) and to suppress MMP-1 and MMP-3 production and attenuate the inflammatory response in a collagen-induced arthritis model in mice (Moon et al., 2010). In addition, curcumin has been found to down-regulate the expression of TNF-α and IL-1β in ankle joints and decrease NF-κB activity, PGE2 production, COX-2 expression and MMP secretion in synoviocytes. Furthermore, curcumin has been shown to have a synergistic effect with methotrexate in decreasing adjuvant-induced arthritis in mice and in minimizing liver damage (Banji et al., 2011).
Other in vitro findings indicate that the protective effects of curcumin against RA are mediated through inhibition of neutrophil activation, suppression of synoviocyte proliferation and inhibition of angiogenesis as suggested by curcumin's ability to inhibit collagenase and stromelysin in chondrocytes (Jackson et al., 2006). Further, the suppression of NF-κB by curcumin has been found to be associated with its inhibition of the expression of COX-2, NO, PGE2, IL-1β, IL-6, IL-8, MMP-3 and MMP-9 in human chondrocytes (Shakibaei et al., 2007; Mathy-Hartert et al., 2009). Curcumin has also been found to suppress IL-8 expression in human synovial fibroblasts (Tong et al., 2008).
One of the earliest studies demonstrating that curcumin has anti-rheumatic activity in humans appeared almost three decades ago (Deodhar et al., 1980). In a more recent, the efficacy of a proprietary complex of curcumin with soy phosphatidylcholine (Meriva®; Throne Research Inc., Dover, ID, USA) was investigated in 50 patients with osteoarthritis (OA) at a dosage of 200 mg of curcumin day−1 (Belcaro et al., 2010b). OA symptoms were evaluated by the use of Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scores. After 3 months of treatment with this complex, the global WOMAC score was found to be decreased by 58% (P < 0.05), walking distance in the treadmill test was prolonged from 76 to 332 m (P < 0.05) and C-reactive protein levels decreased from 168 ± 18 to 11.3 ± 4.1 mg·L−1 in the subpopulation with high C-reactive protein levels. In comparison, the control group experienced only a modest improvement in these parameters. These results show that curcumin is clinically effective in the management and treatment of OA. In another study, the same investigator examined the efficacy and safety of Meriva in 100 patients with OA after long-term administration (8 months) (Belcaro et al., 2010a). The clinical end points were WOMAC score, Karnofsky performance scale index score and treadmill walking performance, and were complemented by the evaluation of a series of inflammatory markers including IL-1β, IL-6, sCD40L, soluble vascular cell adhesion molecule-1 and erythrocyte sedimentation rate. Significant improvements in both the clinical and the biochemical end points were observed for the Meriva group compared with the control group.
In another randomized pilot study, the efficacy of curcumin alone and in combination with diclofenac sodium was assessed in patients with active RA (Chandran and Goel, 2012). Forty-five patients diagnosed as having RA were randomized into three groups: patients receiving curcumin alone (500 mg), those receiving diclofenac sodium alone (50 mg) and those receiving combinations of curcumin and diclofenac sodium. The primary end points were reduction in Disease Activity Score (DAS) 28. The secondary end points included American College of Rheumatology (ACR) criteria for reduction in tenderness and swelling of joint scores. Patients in the curcumin group showed the highest percentage of improvement in overall DAS and ACR scores, which were significantly better than those of patients in the diclofenac sodium group. To our knowledge, this is the first evidence showing the potential of curcumin as a therapeutic for patients with active RA.
Inflammatory bowel disease
Inflammatory bowel disease (IBD) consists of two separate diseases, CD and ulcerative colitis (UC); both characterized by chronic recurrent ulceration of the bowel (Kozuch and Hanauer, 2008). It is likely that the pathogenesis of these diseases involves genetic, environmental and immunological factors (Hanauer, 1996). The expression of several cytokines, including TNF-α, IL-1β, IL-6, IL-8 and chemokines, all regulated by NF-κB, is increased in IBD (Ferretti et al., 1994; Jijon et al., 2000; Yamamoto et al., 2000; Jobin, 2008). All of these critical proteins are up-regulated by NF-κB and suppression of NF-κB by anti-sense can attenuate experimental colitis in mice (Neurath et al., 1996). Among the various gut immune factors, TNF-α is a major pro-inflammatory cytokine in IBD (Brown and Mayer, 2007; Louis, 2001). Mucosal levels of TNF-α are elevated in patients with IBD (Murch et al., 1991; Braegger et al., 1992), and its inhibition (Papadakis and Targan, 2000) or neutralization can improve both UC (Jarnerot, 1989) and CD (Ardizzone and Bianchi Porro, 2005). Conventional therapies for UC include sulfasalazine, 5-aminosalicylic acid, salazosulfapyridine, azathioprine, mercaptopurines, cyclosporine, corticosteroids and TNF blockers (Kozuch and Hanauer, 2008; Ng and Kamm, 2009). All of these treatments have significant toxic side effects and are partly or completely ineffective in a significant number of patients.
Now there are numerous lines of evidence to suggest that curcumin has enormous potential against both CD and UC (Table 5). Firstly, epidemiological studies indicate that turmeric (which contains 2–8% curcuminoids) may contribute to the lower incidence of cancer, especially large-bowel cancers in Indians (Mohandas and Desai, 1999; Sinha et al., 2003). Secondly, curcumin, when administered orally in the diet, prevented trinitrobenzene sulfonic acid (TNBS)- or dinitrobenzene sulfonic acid (DNBS)-induced colitis in mice (Sugimoto et al., 2002; Salh et al., 2003; Venkataranganna et al., 2007; Ung et al., 2010). Thirdly, curcumin improves both the wasting and histopathological signs of colonic inflammation. Fourthly, curcumin inhibits CD4+ T-cell infiltration and NF-κB activation in colonic mucosa. Fifthly, curcumin manifests its effects against colitis by suppressing the expression of inflammatory cytokines such as TNF-α (Camacho-Barquero et al., 2007; Mouzaoui et al., 2012), IFN-γ (Ung et al., 2010), IL-17 (Ung et al., 2010) and enzymes, such as p38 MAPK, iNOS, COX-2, myeloperoxidase and MMP-9, in the colonic mucosa (Camacho-Barquero et al., 2007). Sixthly, curcumin has a therapeutic effect on DNBS-induced colitis in mice induced by its agonistic action on the vanilloid receptor TRPV1 (Martelli et al., 2007). Seventhly, curcumin suppresses colonic inflammation induced by deletion of the mdr1 gene in mice (Nones et al., 2009). The effects of curcumin in TNBS-induced colitis in mice were found to be strain-dependent: BALB/c mice were protected, whereas SJL/J mice were not protected (Billerey-Larmonier et al., 2008). The effect of curcumin against colitis was also limited in Th1-driven colitis in IL-10-deficient mice (Larmonier et al., 2008; Ung et al., 2010). Curcumin failed to inhibit NF-κB in these mice, but when combined with IL-10, curcumin inhibited NF-κB quite effectively. Eighthly, curcumin decreases TNF-α-induced oxidative stress and colitis in mice (Mouzaoui et al., 2012). Ninthly, curcumin combined with resveratrol and simvastatin decreases acute small intestinal inflammation in mice by down-regulating the Th1-type immune response (Bereswill et al., 2010). Tenthly, curcumin suppresses TNBS-induced colonic inflammation in mice by down-regulation of NF-κB, TLR4 and MyD88 (Lubbad et al., 2009a). Eleventhly, curcumin has been shown to inhibit colitis by inducing the production of tolerogenic dendritic cells that promote differentiation of T-cells into Treg, which include CD4+CD25+Foxp3+Treg and IL-10-producing Tr1 cells, and by producing TGF-β (Cong et al., 2009). Twelfthly, curcumin can reverse TNF-α-mediated reduction in Phex protein in mice, which is responsible for the inhibition of osteoblast mineralization linked to the abnormal bone metabolism associated with IBD (Uno et al., 2006). For this, the authors examined calvaria of 6- to 7-week-old mice given TNBS with or without neutralizing TNF-α antibody, dietary curcumin or systemically with recombinant TNF-α. They found that compared to control animals, Phex mRNA expression decreased by 40–50% in both TNBS colitis and TNF-α-injected mice. Dietary curcumin and TNF-α antibody counteracted these detrimental effects of TNBS on Phex gene expression.
Table 5.
Effect of curcumin on models of inflammatory bowel disease
| • Prevented TNBS-induced colitis in mice; inhibited CD4+ T-cell infiltration and NF-κB activation, and expression of TNF-α, IFN-γ, IL-6 and IL-12 in colonic mucosa (Sugimoto et al., 2002). |
| • Inhibited DNB-induced colitis in mice, prevented tissue damage, reduced MPO and IL-1β expression and inhibited NF-κB activation in the mucosal tissue (Salh et al., 2003). |
| • Inhibited mucosal injury in TNBS-induced colitis in mice, reduced NO and ROS levels, inhibited neutrophil infiltration and inactivated NF-κB in colonic mucosa (Ukil et al., 2003). |
| • Inhibited TNBS-induced colitis in rats, inhibited IL-1 expression, increased IL-10 expression in colonic mucosa and decreased NF-κB activation (Jian et al., 2004). |
| • Attenuated TNBS-induced chronic colitis through inhibition of MPO and COX-2 in rats and improved survival (Jiang et al., 2006). |
| • Prevented TNBS-induced chronic colitis, decreased Th1 (IL-12, IFN-γ/TNF-α, IL-1) and increased Th2 (IL-4 and IL-10) cytokines in colon mucosa; and increased IL-4 and IFN-γ in splenocytes and circulation (Zhang et al., 2008). |
| • Reversed TNF-α-mediated reduction in Phex protein responsible for the inhibition of osteoblast mineralization linked abnormal bone metabolism in IBD (Uno et al., 2006). |
| • Prevented the development of DSS-induced experimental colitis in BALB/c mice through inhibition of MPO and NF-κB (Deguchi et al., 2007). |
| • Protected against DNCB-induced colitis through down-regulation of MPO, ALP, LPO, NF-κB and iNOS (Venkataranganna et al., 2007). |
| • Prevented DNBS-induced colitis in mice by interaction with vanilloid receptor TRPV1 (Martelli et al., 2007). |
| • Attenuated the TNBS-induced colitis in rats through inhibition of MPO, TNF-α, COX-2, iNOS, p38 MAPK in colonic mucosa (Camacho-Barquero et al., 2007). |
| • Attenuated the TNBS-induced colitis in rats through inhibition of down-regulation of hepatic CYP3A2 (Masubuchi et al., 2008). |
| • Inhibited the TNBS-induced colitis and splenocyte proliferation in BALB/c mice but not in NKT-deficient SJL/J mice (Billerey-Larmonier et al., 2008). |
| • Exhibited protective effect on Th1-driven colitis in IL-10 deficient mice, with no effect on NF-κB (Larmonier et al., 2008). |
| • Attenuated the TNBS-induced colitis in rats through reversal of carbachol-induced contraction of the colon and modulating NF-κB activation (Lubbad et al., 2009b). |
| • Attenuated the TNBS-induced colitis in rats through suppression of expression in TLR-4, MyD88 and NF-κB proteins in inflamed tissue (Lubbad et al., 2009a). |
| • Prevented the DSS-induced colitis in mice through suppression of serum TNF-α levels, NO and colonic MPO expression (Arafa et al., 2009). |
| • Protected from IBD in mdr1a-KO mice through inhibition of TNF-α, IFN-γ, chemokine, p38, TLR2, CD14 and up-regulation of xenobiotic metabolism (Nones et al., 2009). |
| • Protected from IBD by inducing the tolerogenic dendritic cell that promotes differentiation of intestine-protective regulatory T-cells in vivo (Cong et al., 2009). |
| • Inhibited pro-inflammatory cytokine release in the IL-10-deficient mouse model of IBD (Ung et al., 2010). |
| • Ameliorated small intestinal inflammation by down-regulating Th1 cell-associated cytokines (IFN-γ, TNF-α, IL-6, MCP-1) (Bereswill et al., 2010). |
| • Protected intestinal mucosal barrier function of rat enteritis via activation of MKP-1 and attenuation of p38 and NF-κB activation (Song et al., 2010). |
| • Inhibited DSS-induced colitis in mice via inhibition of beta catenin translocation, and down-regulation of TNF-α and IFN-γ levels (Villegas et al., 2011). |
| • Attenuated TNF-α-induced oxidative stress, acute colitis and hepatotoxicity in mice (Mouzaoui et al., 2012). |
| • Suppressed p38, reduced IL-1β and MMP-3, and enhanced IL-10 in mucosa of children and adults with IBD (Epstein et al., 2010). |
ALP, alkaline phosphatase; DNBS, dinitrobenzene sulfonic acid; DNCB, 2,4-dinitrochlorobenzene; DSS, dextran sulfate sodium; IBD, inflammatory bowel disease; LPO, lipid peroxidation; MCP-1, monocyte chemotactic protein-1; MKP-1, mitogen-activated protein kinase phosphatase-1; MPO, myeloperoxidase; MyD88, myeloid differentiation primary response gene 88; NKT, natural killer T-cells; Phex, phosphate regulating gene with homologies to endopeptidases on the X chromosome; Th, T helper cell type; TLR, toll-like receptor; TNBS, trinitrobenzene sulfonic acid.
Thus, the above findings in animals clearly indicate that orally administered curcumin has the potential to protect from the development of IBD. Additional evidence suggests its potential in humans. Firstly, as little as 150 mg of curcumin twice daily can suppress the levels of expression of inflammatory cytokines TNF-α and IL-6 in serum (Usharani et al., 2008; He et al., 2011). Secondly, a dose of 2 g·day−1 of curcumin can suppress NF-κB activation in human peripheral blood mononuclear cells (Vadhan-Raj et al., 2007). Thirdly, curcumin was found to suppress p38 MAPK, reduce IL-1β and MMP-3, and enhance IL-10 in mucosa of children and adults with IBD (Epstein et al., 2010). Fourthly, more than 65 different trials have been conducted with orally administered curcumin in humans. Fifthly, promising results were obtained from a small open-label study examining the use of curcumin to treat IBD (Holt et al., 2005). A pure curcumin preparation was administered to five patients with ulcerative proctitis and to five patients with CD. All patients with proctitis improved, and four had their concomitant medications reduced; four of the five CD patients had lowered CDAI scores and sedimentation rates. Sixthly, in a randomized multicentre double-blind, placebo-controlled trial, curcumin was examined as a maintenance therapy for UC and found to produce favourable effects (Hanai et al., 2006). Of 89 patients with UC, 45 received 1 g of curcumin after breakfast and 1 g after their evening meal, plus sulfasalazine and mesalamine for 6 months. Of the 43 patients who received curcumin, 2 (4.65%) experienced relapse during the 6 months of therapy, whereas 8 (20.51%) of the 39 patients in the placebo group experienced relapse. Recurrence rates in the curcumin-treated and placebo groups were significantly different. Furthermore, curcumin use resulted in an improvement in both the clinical activity index and the endoscopic index and suppressed UC-associated morbidity. Thus, curcumin appears to be a promising and safe medication for maintaining remission in patients with quiescent UC. These two small studies have shown promising results for IBD. Seventhly, orally administered curcumin was found to have a therapeutic effect against colorectal cancer (He et al., 2011). In an open-label study in which 126 patients were treated with 360 mg, p.o., curcumin three times a day, curcumin's effects were noted within 10–30 days.
Psoriasis
Like IBD and RA, psoriasis is a common chronic inflammatory disease of the skin and joints that affects about 2% of the general population is another indication for which TNF blockers have been approved. Depending on the stage of disease, current treatment options include UVB, UVA plus psoralen, methotrexate, acitretin, cyclosporine, infliximab, etanercept, adalimumab, efalizumab and alefacept. Whereas infliximab, etanercept and adalimumab are specific TNF blockers, all of the others are immunosuppressive agents that could increase the risk of infections and malignancies, especially with long-term use (Greaves and Weinstein, 1995; Kurd et al., 2007). Psoriasis is a chronic disease, which requires long-term treatment and 51% of patients with psoriasis use complementary and alternative therapies (Fleischer et al., 1996). Thus, safe, affordable and effective agents are needed to treat this condition.
There are several reasons to believe that curcumin may have potential for treating psoriasis. Firstly, on irradiation with visible light, curcumin has been proven to be phototoxic for Salmonella typhimurium and Escherichia coli, even at very low concentrations (Tonnesen et al., 1987). This observed phototoxicity makes curcumin a potential photosensitizing drug, which could be used in phototherapy of psoriasis. Secondly, when curcumin was tested as an anti-psoriatic drug in the modified mouse tail test, an animal model of psoriasis, it exhibited some activity (Bosman, 1994). Thirdly, curcumin has been shown to inhibit the proliferation of human keratinocytes through suppression of pro-inflammatory pathways (Pol et al., 2003; Cho et al., 2007). Curcumin inhibited the expression of TNF-α-induced IL-1β, IL-6, TNF-α, cyclin E, MAPKs (JNK, p38 MAPK and ERK) and NF-κB in HaCaT cells. Because curcumin can reverse the anti-apoptotic function of TNF-α in skin cells, it may have potential for the treatment of psoriasis (Sun et al., 2012). Fourthly, as TNF blockers have been successfully used to treat psoriasis and since curcumin can block both the production and the action of TNF, curcumin may have potential as a treatment of psoriasis. Fifthly, our laboratory has shown that curcumin is a potent inhibitor of phosphorylase kinase (PhK) activity (Reddy and Lokesh, 1996), the elevation in which has been correlated with psoriatic activity (Heng et al., 2000).
Heng et al. (2000) investigated whether the anti-psoriatic activity of curcumin in patients is due to suppression of PhK activity. In this study, PhK activity was assayed in four groups of 10 subjects each: (i) active untreated psoriasis; (ii) resolving psoriasis treated by calcipotriol, a vitamin D3 analogue and indirect inhibitor of PhK; (iii) curcumin treatment (1% in gel); and (iv) 10 normal non-psoriatic subjects. PhK activity, from highest to lowest, was as follows: the active untreated psoriasis group, the calcipotriol-treated group, the curcumin-treated group and the non-psoriatic subjects. The decrease in PhK activity in the curcumin-treated and calcipotriol-treated psoriasis groups was associated with a decrease in the expression of the keratinocyte transferrin receptor, a reduced severity of parakeratosis and a reduction in the density of epidermal CD8+ T-cells. The authors of this study concluded that drug-induced suppression of PhK activity is associated with resolution of psoriatic activity and that the anti-psoriatic activity of curcumin may be achieved through its modulation of PhK.
The safety and efficacy of oral curcumin in patients with moderate to severe psoriasis has been investigated in a prospective phase II, open-label, Simon's two-stage clinical trial (Kurd et al., 2008). Twelve patients with chronic plaque psoriasis were enrolled in this study and were given a 4.5 g curcumin capsule per day for 12 weeks; this was followed by a 4 week observation period. Curcumin was well tolerated and all participants completed the study. However, the response rate was low and possibly caused by a placebo effect or the natural history of psoriasis. Nevertheless, two patients who responded to the treatment showed 83–88% improvement at 12 weeks of treatment. There were no study-related adverse events that necessitated participant withdrawal. Small sample size and the lack of a control (placebo) group were the limitations of the study.
Refractory asthma
Patients' asthma is considered refractory when they experience persistent symptoms, frequent asthma attacks and/or low lung function despite taking asthma medications. Some patients with refractory asthma have to take oral steroids such as prednisone to manage their symptoms. TNF-α has been shown to have a pathobiological role in asthma, mainly in severe refractory asthma and in chronic obstructive pulmonary disease (COPD) (Matera et al., 2010). Thus, TNF-α inhibitors (infliximab, golimumab and etanercept) are now regarded as potential new medications in asthma and COPD management.
Numerous rodent studies suggest that curcumin may also have potential for treatment of asthma. Firstly, curcumin (at 20 mg·kg−1 bodyweight) was reported to attenuate allergen-induced airway hyper-responsiveness in sensitized guinea pigs (Ram et al., 2003). When administered to mice, it was found to prevent ovalbumin-induced airway inflammation by regulating NO (Moon et al., 2008) and, in a more recent study, to diminish the development of allergic airway inflammation and hyper-responsiveness, possibly through inhibition of NF-κB activation in asthmatic lung tissue (Oh et al., 2011). For these studies, BALB/c mice were sensitized to ovalbumin, allowing analysis of the effects of curcumin administration (200 mg·kg−1 bodyweight per day, i.p.) on airway hyper-responsiveness, inflammatory cell number and IgE levels in bronchoalveolar lavage fluid. Ammar el et al. (2011) also examined the anti-inflammatory activity of curcumin in a murine model of asthma and showed it down-modulated the serum levels of IgE, iNOS, transforming growth factor β1 and mRNA expression of TNF-α. Secondly, curcumin has been shown to have therapeutic potential for controlling allergic responses. Animals exposed to latex showed enhanced serum IgE; latex-specific IgG1, IL-4, IL-5 and IL-13; eosinophils; and inflammation in the lungs (Kurup et al., 2007). Intragastric treatment of latex-sensitized mice with curcumin demonstrated a diminished Th2 response with a concurrent reduction in lung inflammation. Eosinophilia in the curcumin-treated mice was markedly reduced, as was the expression of the co-stimulatory molecules (CD80, CD86 and OX40L) on antigen-presenting cells, and expression of MMP-9, ornithine amino transferase and thymic stromal lymphopoietin genes was also attenuated. Thirdly, curcumin was found to reverse corticosteroid resistance in monocytes exposed to oxidants by maintaining histone deacetylase-2 activity (Meja et al., 2008). Although no clinical data are available yet, all of these pre-clinical studies suggest that curcumin has potential as a therapeutic for asthma.
Conclusions
Overall, all these studies suggest that curcumin can suppress pro-inflammatory pathways linked with most chronic diseases. It can block both the production and the action of TNF. Curcumin also binds to TNF directly. Evidence for curcumin as a TNF blocker has been obtained in both in vitro and in vivo studies. However, only a few studies have demonstrated that curcumin is effective at inhibiting TNF production in humans. Unlike most other TNF blockers, curcumin can be given orally. In addition, it is quite safe and affordable. However, more studies are needed in humans to prove that curcumin has the ability to be an effective treatment of various pro-inflammatory conditions.
Acknowledgments
We thank Tamara Locke and MD Anderson's Department of Scientific Publications for carefully editing the manuscript and providing valuable comments. Dr Aggarwal is the Ransom Horne, Jr., Professor of Cancer Research.
Glossary
- ACR
American College of Rheumatology
- AP-1
activator protein-1
- ATF2
activating transcription factor 2
- C/EBP
CCAAT/enhancer binding protein
- CD
Crohn's disease
- COPD
chronic obstructive pulmonary disease
- CREB
cAMP response element binding protein
- DAS
Disease Activity Score
- DNBS
dinitrobenzene sulfonic acid
- IBD
inflammatory bowel disease
- LITAF
LPS-induced TNF-α factor
- MCP-1
monocyte chemotactic protein-1
- MD-2
myeloid differentiation protein-2
- NFAT
nuclear factor of activated T-cell transcription factor
- OA
osteoarthritis
- PhK
phosphorylase kinase
- RA
rheumatoid arthritis
- SLCP
solid lipid curcumin particle
- TLRs
toll-like receptors
- TNBS
trinitrobenzene sulfonic acid
- UC
ulcerative colitis
Conflict of interest
The authors declare no conflicts of interest.
References
- Abe Y, Hashimoto S, Horie T. Curcumin inhibition of inflammatory cytokine production by human peripheral blood monocytes and alveolar macrophages. Pharmacol Res. 1999;39:41–47. doi: 10.1006/phrs.1998.0404. [DOI] [PubMed] [Google Scholar]
- Adhvaryu MR, Reddy N, Vakharia BC. Prevention of hepatotoxicity due to anti tuberculosis treatment: a novel integrative approach. World J Gastroenterol. 2008;14:4753–4762. doi: 10.3748/wjg.14.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- Aggarwal BB, Moffat B, Harkins RN. Human lymphotoxin. Production by a lymphoblastoid cell line, purification, and initial characterization. J Biol Chem. 1984;259:686–691. [PubMed] [Google Scholar]
- Aggarwal BB, Eessalu TE, Hass PE. Characterization of receptors for human tumour necrosis factor and their regulation by gamma-interferon. Nature. 1985a;318:665–667. doi: 10.1038/318665a0. [DOI] [PubMed] [Google Scholar]
- Aggarwal BB, Kohr WJ, Hass PE, Moffat B, Spencer SA, Henzel WJ, et al. Human tumor necrosis factor. Production, purification, and characterization. J Biol Chem. 1985b;260:2345–2354. [PubMed] [Google Scholar]
- Aggarwal BB, Gupta SC, Kim JH. Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey. Blood. 2012;119:651–665. doi: 10.1182/blood-2011-04-325225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal S, Ichikawa H, Takada Y, Sandur SK, Shishodia S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates expression of cell proliferation and antiapoptotic and metastatic gene products through suppression of IkappaBalpha kinase and Akt activation. Mol Pharmacol. 2006;69:195–206. doi: 10.1124/mol.105.017400. [DOI] [PubMed] [Google Scholar]
- Allam G. Immunomodulatory effects of curcumin treatment on murine schistosomiasis mansoni. Immunobiology. 2009;214:712–727. doi: 10.1016/j.imbio.2008.11.017. [DOI] [PubMed] [Google Scholar]
- Allegri P, Mastromarino A, Neri P. Management of chronic anterior uveitis relapses: efficacy of oral phospholipidic curcumin treatment. Long-term follow-up. Clin Ophthalmol. 2010;4:1201–1206. doi: 10.2147/OPTH.S13271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alwi I, Santoso T, Suyono S, Sutrisna B, Suyatna FD, Kresno SB, et al. The effect of curcumin on lipid level in patients with acute coronary syndrome. Acta Med Indones. 2008;40:201–210. [PubMed] [Google Scholar]
- Ammar el SM, Gameil NM, Shawky NM, Nader MA. Comparative evaluation of anti-inflammatory properties of thymoquinone and curcumin using an asthmatic murine model. Int Immunopharmacol. 2011;11:2232–2236. doi: 10.1016/j.intimp.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- Appendino G, Belcaro G, Cornelli U, Luzzi R, Togni S, Dugall M, et al. Potential role of curcumin phytosome (Meriva) in controlling the evolution of diabetic microangiopathy. A pilot study. Panminerva Med. 2011;53:43–49. [PubMed] [Google Scholar]
- Arafa HM, Hemeida RA, El-Bahrawy AI, Hamada FM. Prophylactic role of curcumin in dextran sulfate sodium (DSS)-induced ulcerative colitis murine model. Food Chem Toxicol. 2009;47:1311–1317. doi: 10.1016/j.fct.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Ardizzone S, Bianchi Porro G. Biologic therapy for inflammatory bowel disease. Drugs. 2005;65:2253–2286. doi: 10.2165/00003495-200565160-00002. [DOI] [PubMed] [Google Scholar]
- Asawanonda P, Klahan SO. Tetrahydrocurcuminoid cream plus targeted narrowband UVB phototherapy for vitiligo: a preliminary randomized controlled study. Photomed Laser Surg. 2010;28:679–684. doi: 10.1089/pho.2009.2637. [DOI] [PubMed] [Google Scholar]
- Avci G, Kadioglu H, Sehirli AO, Bozkurt S, Guclu O, Arslan E, et al. Curcumin protects against ischemia/reperfusion injury in rat skeletal muscle. J Surg Res. 2012;172:e39–e46. doi: 10.1016/j.jss.2011.08.021. [DOI] [PubMed] [Google Scholar]
- Balasubramanyam K, Varier RA, Altaf M, Swaminathan V, Siddappa NB, Ranga U, et al. Curcumin, a novel p300/CREB-binding protein-specific inhibitor of acetyltransferase, represses the acetylation of histone/nonhistone proteins and histone acetyltransferase-dependent chromatin transcription. J Biol Chem. 2004;279:51163–51171. doi: 10.1074/jbc.M409024200. [DOI] [PubMed] [Google Scholar]
- Banji D, Pinnapureddy J, Banji OJ, Saidulu A, Hayath MS. Synergistic activity of curcumin with methotrexate in ameliorating Freund's Complete Adjuvant induced arthritis with reduced hepatotoxicity in experimental animals. Eur J Pharmacol. 2011;668:293–298. doi: 10.1016/j.ejphar.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Bansal S, Chhibber S. Curcumin alone and in combination with augmentin protects against pulmonary inflammation and acute lung injury generated during Klebsiella pneumoniae B5055-induced lung infection in BALB/c mice. J Med Microbiol. 2010;59:429–437. doi: 10.1099/jmm.0.016873-0. [DOI] [PubMed] [Google Scholar]
- Bassiouny AR, Zaky A, Kandeel KM. Alteration of AP-endonuclease1 expression in curcumin-treated fibrotic rats. Ann Hepatol. 2011;10:516–530. [PubMed] [Google Scholar]
- Baum L, Lam CW, Cheung SK, Kwok T, Lui V, Tsoh J, et al. Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer disease. J Clin Psychopharmacol. 2008;28:110–113. doi: 10.1097/jcp.0b013e318160862c. [DOI] [PubMed] [Google Scholar]
- Bayet-Robert M, Kwiatkowski F, Leheurteur M, Gachon F, Planchat E, Abrial C, et al. Phase I dose escalation trial of docetaxel plus curcumin in patients with advanced and metastatic breast cancer. Cancer Biol Ther. 2010;9:8–14. doi: 10.4161/cbt.9.1.10392. [DOI] [PubMed] [Google Scholar]
- Belcaro G, Cesarone MR, Dugall M, Pellegrini L, Ledda A, Grossi MG, et al. Efficacy and safety of Meriva(R), a curcumin-phosphatidylcholine complex, during extended administration in osteoarthritis patients. Altern Med Rev. 2010a;15:337–344. [PubMed] [Google Scholar]
- Belcaro G, Cesarone MR, Dugall M, Pellegrini L, Ledda A, Grossi MG, et al. Product-evaluation registry of Meriva®, a curcumin-phosphatidylcholine complex, for the complementary management of osteoarthritis. Panminerva Med. 2010b;52:55–62. [PubMed] [Google Scholar]
- Bereswill S, Munoz M, Fischer A, Plickert R, Haag LM, Otto B, et al. Anti-inflammatory effects of resveratrol, curcumin and simvastatin in acute small intestinal inflammation. PLoS ONE. 2010;5:e15099. doi: 10.1371/journal.pone.0015099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B, Greenwald D, Hulmes JD, Chang M, Pan YC, Mathison J, et al. Identity of tumour necrosis factor and the macrophage-secreted factor cachectin. Nature. 1985;316:552–554. doi: 10.1038/316552a0. [DOI] [PubMed] [Google Scholar]
- Bierhaus A, Zhang Y, Quehenberger P, Luther T, Haase M, Muller M, et al. The dietary pigment curcumin reduces endothelial tissue factor gene expression by inhibiting binding of AP-1 to the DNA and activation of NF-kappa B. Thromb Haemost. 1997;77:772–782. [PubMed] [Google Scholar]
- Billerey-Larmonier C, Uno JK, Larmonier N, Midura AJ, Timmermann B, Ghishan FK, et al. Protective effects of dietary curcumin in mouse model of chemically induced colitis are strain dependent. Inflamm Bowel Dis. 2008;14:780–793. doi: 10.1002/ibd.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas J, Sinha D, Mukherjee S, Roy S, Siddiqi M, Roy M. Curcumin protects DNA damage in a chronically arsenic-exposed population of West Bengal. Hum Exp Toxicol. 2010;29:513–524. doi: 10.1177/0960327109359020. [DOI] [PubMed] [Google Scholar]
- Bosman B. Testing of lipoxygenase inhibitors, cyclooxygenase inhibitors, drugs with immunomodulating properties and some reference antipsoriatic drugs in the modified mouse tail test, an animal model of psoriasis. Skin Pharmacol. 1994;7:324–334. doi: 10.1159/000211314. [DOI] [PubMed] [Google Scholar]
- Braegger CP, Nicholls S, Murch SH, Stephens S, MacDonald TT. Tumour necrosis factor alpha in stool as a marker of intestinal inflammation. Lancet. 1992;339:89–91. doi: 10.1016/0140-6736(92)90999-j. [DOI] [PubMed] [Google Scholar]
- Brown SJ, Mayer L. The immune response in inflammatory bowel disease. Am J Gastroenterol. 2007;102:2058–2069. doi: 10.1111/j.1572-0241.2007.01343.x. [DOI] [PubMed] [Google Scholar]
- Buhrmann C, Mobasheri A, Busch F, Aldinger C, Stahlmann R, Montaseri A, et al. Curcumin modulates nuclear factor kappaB (NF-kappaB)-mediated inflammation in human tenocytes in vitro: role of the phosphatidylinositol 3-kinase/Akt pathway. J Biol Chem. 2011;286:28556–28566. doi: 10.1074/jbc.M111.256180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundy R, Walker AF, Middleton RW, Booth J. Turmeric extract may improve irritable bowel syndrome symptomology in otherwise healthy adults: a pilot study. J Altern Complement Med. 2004;10:1015–1018. doi: 10.1089/acm.2004.10.1015. [DOI] [PubMed] [Google Scholar]
- Burns J, Joseph PD, Rose KJ, Ryan MM, Ouvrier RA. Effect of oral curcumin on Dejerine–Sottas disease. Pediatr Neurol. 2009;41:305–308. doi: 10.1016/j.pediatrneurol.2009.04.030. [DOI] [PubMed] [Google Scholar]
- Cai T, Mazzoli S, Bechi A, Addonisio P, Mondaini N, Pagliai RC, et al. Serenoa repens associated with Urtica dioica (ProstaMEV) and curcumin and quercitin (FlogMEV) extracts are able to improve the efficacy of prulifloxacin in bacterial prostatitis patients: results from a prospective randomised study. Int J Antimicrob Agents. 2009;33:549–553. doi: 10.1016/j.ijantimicag.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Camacho-Barquero L, Villegas I, Sanchez-Calvo JM, Talero E, Sanchez-Fidalgo S, Motilva V, et al. Curcumin, a Curcuma longa constituent, acts on MAPK p38 pathway modulating COX-2 and iNOS expression in chronic experimental colitis. Int Immunopharmacol. 2007;7:333–342. doi: 10.1016/j.intimp.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Caput D, Beutler B, Hartog K, Thayer R, Brown-Shimer S, Cerami A. Identification of a common nucleotide sequence in the 3′-untranslated region of mRNA molecules specifying inflammatory mediators. Proc Natl Acad Sci U S A. 1986;83:1670–1674. doi: 10.1073/pnas.83.6.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll RE, Benya RV, Turgeon DK, Vareed S, Neuman M, Rodriguez L, et al. Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prev Res (Phila) 2011;4:354–364. doi: 10.1158/1940-6207.CAPR-10-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975;72:3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chainani-Wu N, Silverman S, Jr, Reingold A, Bostrom A, Mc Culloch C, Lozada-Nur F, et al. A randomized, placebo-controlled, double-blind clinical trial of curcuminoids in oral lichen planus. Phytomedicine. 2007;14:437–446. doi: 10.1016/j.phymed.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Chan MM. Inhibition of tumor necrosis factor by curcumin, a phytochemical. Biochem Pharmacol. 1995;49:1551–1556. doi: 10.1016/0006-2952(95)00171-u. [DOI] [PubMed] [Google Scholar]
- Chan MM, Huang HI, Fenton MR, Fong D. In vivo inhibition of nitric oxide synthase gene expression by curcumin, a cancer preventive natural product with anti-inflammatory properties. Biochem Pharmacol. 1998;55:1955–1962. doi: 10.1016/s0006-2952(98)00114-2. [DOI] [PubMed] [Google Scholar]
- Chandran B, Goel A. A randomized, pilot study to assess the efficacy and safety of curcumin in patients with active rheumatoid arthritis. Phytother Res. 2012;26:1719–1725. doi: 10.1002/ptr.4639. [DOI] [PubMed] [Google Scholar]
- Chen D, Nie M, Fan MW, Bian Z. Anti-inflammatory activity of curcumin in macrophages stimulated by lipopolysaccharides from Porphyromonas gingivalis. Pharmacology. 2008;82:264–269. doi: 10.1159/000161127. [DOI] [PubMed] [Google Scholar]
- Chen HW, Kuo HT, Chai CY, Ou JL, Yang RC. Pretreatment of curcumin attenuates coagulopathy and renal injury in LPS-induced endotoxemia. J Endotoxin Res. 2007;13:15–23. doi: 10.1177/0968051907078605. [DOI] [PubMed] [Google Scholar]
- Chen WH, Chen Y, Cui GH. Effects of TNF-alpha and curcumin on the expression of VEGF in Raji and U937 cells and on angiogenesis in ECV304 cells. Chin Med J (Engl) 2005;118:2052–2057. [PubMed] [Google Scholar]
- Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–2900. [PubMed] [Google Scholar]
- Cheung KL, Khor TO, Kong AN. Synergistic effect of combination of phenethyl isothiocyanate and sulforaphane or curcumin and sulforaphane in the inhibition of inflammation. Pharm Res. 2009;26:224–231. doi: 10.1007/s11095-008-9734-9. [DOI] [PubMed] [Google Scholar]
- Cho JW, Lee KS, Kim CW. Curcumin attenuates the expression of IL-1beta, IL-6, and TNF-alpha as well as cyclin E in TNF-alpha-treated HaCaT cells; NF-kappaB and MAPKs as potential upstream targets. Int J Mol Med. 2007;19:469–474. [PubMed] [Google Scholar]
- Chuengsamarn S, Rattanamongkolgul S, Luechapudiporn R, Phisalaphong C, Jirawatnotai S. Curcumin extract for prevention of type 2 diabetes. Diabetes Care. 2012;35:2121–2127. doi: 10.2337/dc12-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciftci O, Tanyildizi S, Godekmerdan A. Protective effect of curcumin on immune system and body weight gain on rats intoxicated with 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) Immunopharmacol Immunotoxicol. 2010;32:99–104. doi: 10.3109/08923970903164318. [DOI] [PubMed] [Google Scholar]
- Cong Y, Wang L, Konrad A, Schoeb T, Elson CO. Curcumin induces the tolerogenic dendritic cell that promotes differentiation of intestine-protective regulatory T cells. Eur J Immunol. 2009;39:3134–3146. doi: 10.1002/eji.200939052. [DOI] [PubMed] [Google Scholar]
- Cruz-Correa M, Shoskes DA, Sanchez P, Zhao R, Hylind LM, Wexner SD, et al. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin Gastroenterol Hepatol. 2006;4:1035–1038. doi: 10.1016/j.cgh.2006.03.020. [DOI] [PubMed] [Google Scholar]
- Dadhaniya P, Patel C, Muchhara J, Bhadja N, Mathuria N, Vachhani K, et al. Safety assessment of a solid lipid curcumin particle preparation: acute and subchronic toxicity studies. Food Chem Toxicol. 2011;49:1834–1842. doi: 10.1016/j.fct.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Dayer JM, Beutler B, Cerami A. Cachectin/tumor necrosis factor stimulates collagenase and prostaglandin E2 production by human synovial cells and dermal fibroblasts. J Exp Med. 1985;162:2163–2168. doi: 10.1084/jem.162.6.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguchi Y, Andoh A, Inatomi O, Yagi Y, Bamba S, Araki Y, et al. Curcumin prevents the development of dextran sulfate sodium (DSS)-induced experimental colitis. Dig Dis Sci. 2007;52:2993–2998. doi: 10.1007/s10620-006-9138-9. [DOI] [PubMed] [Google Scholar]
- Deodhar SD, Sethi R, Srimal RC. Preliminary study on antirheumatic activity of curcumin (diferuloyl methane) Indian J Med Res. 1980;71:632–634. [PubMed] [Google Scholar]
- Dhillon N, Aggarwal BB, Newman RA, Wolff RA, Kunnumakkara AB, Abbruzzese JL, et al. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res. 2008;14:4491–4499. doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- Di Mario F, Cavallaro LG, Nouvenne A, Stefani N, Cavestro GM, Iori V, et al. A curcumin-based 1-week triple therapy for eradication of Helicobacter pylori infection: something to learn from failure? Helicobacter. 2007;12:238–243. doi: 10.1111/j.1523-5378.2007.00497.x. [DOI] [PubMed] [Google Scholar]
- Durgaprasad S, Pai CG, Vasanthkumar AJF, Namitha S. A pilot study of the antioxidant effect of curcumin in tropical pancreatitis. Indian J Med Res. 2005;122:315–318. [PubMed] [Google Scholar]
- El-Azab MF, Attia FM, El-Mowafy AM. Novel role of curcumin combined with bone marrow transplantation in reversing experimental diabetes: effects on pancreatic islet regeneration, oxidative stress, and inflammatory cytokines. Eur J Pharmacol. 2011;658:41–48. doi: 10.1016/j.ejphar.2011.02.010. [DOI] [PubMed] [Google Scholar]
- El-Moselhy MA, Taye A, Sharkawi SS, El-Sisi SF, Ahmed AF. The antihyperglycemic effect of curcumin in high fat diet fed rats. Role of TNF-alpha and free fatty acids. Food Chem Toxicol. 2011;49:1129–1140. doi: 10.1016/j.fct.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Epelbaum R, Schaffer M, Vizel B, Badmaev V, Bar-Sela G. Curcumin and gemcitabine in patients with advanced pancreatic cancer. Nutr Cancer. 2010;62:1137–1141. doi: 10.1080/01635581.2010.513802. [DOI] [PubMed] [Google Scholar]
- Epstein J, Docena G, MacDonald TT, Sanderson IR. Curcumin suppresses p38 mitogen-activated protein kinase activation, reduces IL-1beta and matrix metalloproteinase-3 and enhances IL-10 in the mucosa of children and adults with inflammatory bowel disease. Br J Nutr. 2010;103:824–832. doi: 10.1017/S0007114509992510. [DOI] [PubMed] [Google Scholar]
- Farombi EO, Ekor M. Curcumin attenuates gentamicin-induced renal oxidative damage in rats. Food Chem Toxicol. 2006;44:1443–1448. doi: 10.1016/j.fct.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Ferretti M, Casini-Raggi V, Pizarro TT, Eisenberg SP, Nast CC, Cominelli F. Neutralization of endogenous IL-1 receptor antagonist exacerbates and prolongs inflammation in rabbit immune colitis. J Clin Invest. 1994;94:449–453. doi: 10.1172/JCI117345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiers W, Brouckaert P, Devos R, Fransen L, Leroux-Roels G, Remaut E, et al. Lymphokines and monokines in anti-cancer therapy. Cold Spring Harb Symp Quant Biol. 1986;51((Pt 1)):587–595. doi: 10.1101/sqb.1986.051.01.071. [DOI] [PubMed] [Google Scholar]
- Fleischer AB, Jr, Feldman SR, Rapp SR, Reboussin DM, Exum ML, Clark AR. Alternative therapies commonly used within a population of patients with psoriasis. Cutis. 1996;58:216–220. [PubMed] [Google Scholar]
- Funk JL, Oyarzo JN, Frye JB, Chen G, Lantz RC, Jolad SD, et al. Turmeric extracts containing curcuminoids prevent experimental rheumatoid arthritis. J Nat Prod. 2006;69:351–355. doi: 10.1021/np050327j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Kuo J, Jiang H, Deeb D, Liu Y, Divine G, et al. Immunomodulatory activity of curcumin: suppression of lymphocyte proliferation, development of cell-mediated cytotoxicity, and cytokine production in vitro. Biochem Pharmacol. 2004;68:51–61. doi: 10.1016/j.bcp.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Garcea G, Berry DP, Jones DJ, Singh R, Dennison AR, Farmer PB, et al. Consumption of the putative chemopreventive agent curcumin by cancer patients: assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidemiol Biomarkers Prev. 2005;14:120–125. [PubMed] [Google Scholar]
- Geist LJ, Hopkins HA, Dai LY, He B, Monick MM, Hunninghake GW. Cytomegalovirus modulates transcription factors necessary for the activation of the tumor necrosis factor-alpha promoter. Am J Respir Cell Mol Biol. 1997;16:31–37. doi: 10.1165/ajrcmb.16.1.8998076. [DOI] [PubMed] [Google Scholar]
- Golombick T, Diamond TH, Badmaev V, Manoharan A, Ramakrishna R. The potential role of curcumin in patients with monoclonal gammopathy of undefined significance – its effect on paraproteinemia and the urinary N-telopeptide of type I collagen bone turnover marker. Clin Cancer Res. 2009;15:5917–5922. doi: 10.1158/1078-0432.CCR-08-2217. [DOI] [PubMed] [Google Scholar]
- Gota VS, Maru GB, Soni TG, Gandhi TR, Kochar N, Agarwal MG. Safety and pharmacokinetics of a solid lipid curcumin particle formulation in osteosarcoma patients and healthy volunteers. J Agric Food Chem. 2010;58:2095–2099. doi: 10.1021/jf9024807. [DOI] [PubMed] [Google Scholar]
- Gradisar H, Keber MM, Pristovsek P, Jerala R. MD-2 as the target of curcumin in the inhibition of response to LPS. J Leukoc Biol. 2007;82:968–974. doi: 10.1189/jlb.1206727. [DOI] [PubMed] [Google Scholar]
- Greaves MW, Weinstein GD. Treatment of psoriasis. N Engl J Med. 1995;332:581–588. doi: 10.1056/NEJM199503023320907. [DOI] [PubMed] [Google Scholar]
- Gulcubuk A, Altunatmaz K, Sonmez K, Haktanir-Yatkin D, Uzun H, Gurel A, et al. Effects of curcumin on tumour necrosis factor-alpha and interleukin-6 in the late phase of experimental acute pancreatitis. J Vet Med A Physiol Pathol Clin Med. 2006;53:49–54. doi: 10.1111/j.1439-0442.2006.00786.x. [DOI] [PubMed] [Google Scholar]
- Gupta A, Vij G, Sharma S, Tirkey N, Rishi P, Chopra K. Curcumin, a polyphenolic antioxidant, attenuates chronic fatigue syndrome in murine water immersion stress model. Immunobiology. 2009;214:33–39. doi: 10.1016/j.imbio.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Gupta B, Ghosh B. Curcuma longa inhibits TNF-alpha induced expression of adhesion molecules on human umbilical vein endothelial cells. Int J Immunopharmacol. 1999;21:745–757. doi: 10.1016/s0192-0561(99)00050-8. [DOI] [PubMed] [Google Scholar]
- Gupta SC, Prasad S, Kim JH, Patchva S, Webb LJ, Priyadarsini IK, et al. Multitargeting by curcumin as revealed by molecular interaction studies. Nat Prod Rep. 2011;28:1937–1955. doi: 10.1039/c1np00051a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J. 2013;15:195–218. doi: 10.1208/s12248-012-9432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierres VO, Pinheiro CM, Assis RP, Vendramini RC, Pepato MT, Brunetti IL. Curcumin-supplemented yoghurt improves physiological and biochemical markers of experimental diabetes. Br J Nutr. 2012;108:440–448. doi: 10.1017/S0007114511005769. [DOI] [PubMed] [Google Scholar]
- Hai L, Kawarabayashi Y, Imai Y, Honda A, Inoue R. Counteracting effect of TRPC1-associated Ca2+ influx on TNF-alpha-induced COX-2-dependent prostaglandin E2 production in human colonic myofibroblasts. Am J Physiol Gastrointest Liver Physiol. 2011;301:G356–G367. doi: 10.1152/ajpgi.00354.2010. [DOI] [PubMed] [Google Scholar]
- Hanai H, Sugimoto K. Curcumin has bright prospects for the treatment of inflammatory bowel disease. Curr Pharm Des. 2009;15:2087–2094. doi: 10.2174/138161209788489177. [DOI] [PubMed] [Google Scholar]
- Hanai H, Iida T, Takeuchi K, Watanabe F, Maruyama Y, Andoh A, et al. Curcumin maintenance therapy for ulcerative colitis: randomized, multicenter, double-blind, placebo-controlled trial. Clin Gastroenterol Hepatol. 2006;4:1502–1506. doi: 10.1016/j.cgh.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Hanauer SB. Inflammatory bowel disease. N Engl J Med. 1996;334:841–848. doi: 10.1056/NEJM199603283341307. [DOI] [PubMed] [Google Scholar]
- Haranaka K, Satomi N, Sakurai A. Antitumor activity of murine tumor necrosis factor (TNF) against transplanted murine tumors and heterotransplanted human tumors in nude mice. Int J Cancer. 1984;34:263–267. doi: 10.1002/ijc.2910340219. [DOI] [PubMed] [Google Scholar]
- Hashem RM, Soliman HM, Shaapan SF. Turmeric-based diet can delay apoptosis without modulating NF-kappaB in unilateral ureteral obstruction in rats. J Pharm Pharmacol. 2008;60:83–89. doi: 10.1211/jpp.60.1.0011. [DOI] [PubMed] [Google Scholar]
- Hastak K, Lubri N, Jakhi SD, More C, John A, Ghaisas SD, et al. Effect of turmeric oil and turmeric oleoresin on cytogenetic damage in patients suffering from oral submucous fibrosis. Cancer Lett. 1997;116:265–269. doi: 10.1016/s0304-3835(97)00205-x. [DOI] [PubMed] [Google Scholar]
- He ZY, Shi CB, Wen H, Li FL, Wang BL, Wang J. Upregulation of p53 expression in patients with colorectal cancer by administration of curcumin. Cancer Invest. 2011;29:208–213. doi: 10.3109/07357907.2010.550592. [DOI] [PubMed] [Google Scholar]
- Heidland A, Klassen A, Rutkowski P, Bahner U. The contribution of Rudolf Virchow to the concept of inflammation: what is still of importance? J Nephrol. 2006;19(Suppl. 10):S102–S109. [PubMed] [Google Scholar]
- Heng MC, Song MK, Harker J, Heng MK. Drug-induced suppression of phosphorylase kinase activity correlates with resolution of psoriasis as assessed by clinical, histological and immunohistochemical parameters. Br J Dermatol. 2000;143:937–949. doi: 10.1046/j.1365-2133.2000.03767.x. [DOI] [PubMed] [Google Scholar]
- Holt PR, Katz S, Kirshoff R. Curcumin therapy in inflammatory bowel disease: a pilot study. Dig Dis Sci. 2005;50:2191–2193. doi: 10.1007/s10620-005-3032-8. [DOI] [PubMed] [Google Scholar]
- Huang WT, Niu KC, Chang CK, Lin MT, Chang CP. Curcumin inhibits the increase of glutamate, hydroxyl radicals and PGE2 in the hypothalamus and reduces fever during LPS-induced systemic inflammation in rabbits. Eur J Pharmacol. 2008;593:105–111. doi: 10.1016/j.ejphar.2008.07.017. [DOI] [PubMed] [Google Scholar]
- Ide H, Tokiwa S, Sakamaki K, Nishio K, Isotani S, Muto S, et al. Combined inhibitory effects of soy isoflavones and curcumin on the production of prostate-specific antigen. Prostate. 2010;70:1127–1133. doi: 10.1002/pros.21147. [DOI] [PubMed] [Google Scholar]
- Jackson JK, Higo T, Hunter WL, Burt HM. The antioxidants curcumin and quercetin inhibit inflammatory processes associated with arthritis. Inflamm Res. 2006;55:168–175. doi: 10.1007/s00011-006-0067-z. [DOI] [PubMed] [Google Scholar]
- Jain SK, Rains J, Croad J, Larson B, Jones K. Curcumin supplementation lowers TNF-alpha, IL-6, IL-8, and MCP-1 secretion in high glucose-treated cultured monocytes and blood levels of TNF-alpha, IL-6, MCP-1, glucose, and glycosylated hemoglobin in diabetic rats. Antioxid Redox Signal. 2009;11:241–249. doi: 10.1089/ars.2008.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James JS. Curcumin: clinical trial finds no antiviral effect. AIDS Treat News. 1996:1–2. [PubMed] [Google Scholar]
- Jang MK, Sohn DH, Ryu JH. A curcuminoid and sesquiterpenes as inhibitors of macrophage TNF-alpha release from Curcuma zedoaria. Planta Med. 2001;67:550–552. doi: 10.1055/s-2001-16482. [DOI] [PubMed] [Google Scholar]
- Jarnerot G. Newer 5-aminosalicylic acid based drugs in chronic inflammatory bowel disease. Drugs. 1989;37:73–86. doi: 10.2165/00003495-198937010-00005. [DOI] [PubMed] [Google Scholar]
- Jia SH, Wei H, Yu JL, Wei XD, Zhang XP, Li JC. [Protective effects of curcumin on neonatal rats with necrotizing enterocolitis] Zhongguo Dang Dai Er Ke Za Zhi. 2010;12:132–136. [PubMed] [Google Scholar]
- Jian YT, Wang JD, Mai GF, Zhang YL, Lai ZS. [Modulation of intestinal mucosal inflammatory factors by curcumin in rats with colitis] Di Yi Jun Yi Da Xue Xue Bao. 2004;24:1353–1358. [PubMed] [Google Scholar]
- Jiang H, Deng CS, Zhang M, Xia J. Curcumin-attenuated trinitrobenzene sulphonic acid induces chronic colitis by inhibiting expression of cyclooxygenase-2. World J Gastroenterol. 2006;12:3848–3853. doi: 10.3748/wjg.v12.i24.3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang ZY, Zou L, Shi SS, Lu YR, Dong J, Yang CH, et al. [Effects of curcumin on TNF-alpha and TGF-beta1 in serum and lung tissue of SiO2-induced fibrosis in mice] Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2009;25:399–401. [PubMed] [Google Scholar]
- Jijon HB, Churchill T, Malfair D, Wessler A, Jewell LD, Parsons HG, et al. Inhibition of poly(ADP-ribose) polymerase attenuates inflammation in a model of chronic colitis. Am J Physiol Gastrointest Liver Physiol. 2000;279:G641–G651. doi: 10.1152/ajpgi.2000.279.3.G641. [DOI] [PubMed] [Google Scholar]
- Jin CY, Lee JD, Park C, Choi YH, Kim GY. Curcumin attenuates the release of pro-inflammatory cytokines in lipopolysaccharide-stimulated BV2 microglia. Acta Pharmacol Sin. 2007;28:1645–1651. doi: 10.1111/j.1745-7254.2007.00651.x. [DOI] [PubMed] [Google Scholar]
- Jobin C. Nf-kappa B signaling cascade and IBD: turn it down? Inflamm Bowel Dis. 2008;14(Suppl. 2):S108–S109. doi: 10.1002/ibd.20717. [DOI] [PubMed] [Google Scholar]
- Jobin C, Bradham CA, Russo MP, Juma B, Narula AS, Brenner DA, et al. Curcumin blocks cytokine-mediated NF-kappa B activation and proinflammatory gene expression by inhibiting inhibitory factor I-kappa B kinase activity. J Immunol. 1999;163:3474–3483. [PubMed] [Google Scholar]
- Joe B, Rao UJ, Lokesh BR. Presence of an acidic glycoprotein in the serum of arthritic rats: modulation by capsaicin and curcumin. Mol Cell Biochem. 1997;169:125–134. doi: 10.1023/a:1006877928703. [DOI] [PubMed] [Google Scholar]
- Kalpravidh RW, Siritanaratkul N, Insain P, Charoensakdi R, Panichkul N, Hatairaktham S, et al. Improvement in oxidative stress and antioxidant parameters in beta-thalassemia/Hb E patients treated with curcuminoids. Clin Biochem. 2010;43:424–429. doi: 10.1016/j.clinbiochem.2009.10.057. [DOI] [PubMed] [Google Scholar]
- Kanai M, Yoshimura K, Asada M, Imaizumi A, Suzuki C, Matsumoto S, et al. A phase I/II study of gemcitabine-based chemotherapy plus curcumin for patients with gemcitabine-resistant pancreatic cancer. Cancer Chemother Pharmacol. 2011;68:157–164. doi: 10.1007/s00280-010-1470-2. [DOI] [PubMed] [Google Scholar]
- Khajehdehi P, Pakfetrat M, Javidnia K, Azad F, Malekmakan L, Nasab MH, et al. Oral supplementation of turmeric attenuates proteinuria, transforming growth factor-beta and interleukin-8 levels in patients with overt type 2 diabetic nephropathy: a randomized, double-blind and placebo-controlled study. Scand J Urol Nephrol. 2011;45:365–370. doi: 10.3109/00365599.2011.585622. [DOI] [PubMed] [Google Scholar]
- Khajehdehi P, Zanjaninejad B, Aflaki E, Nazarinia M, Azad F, Malekmakan L, et al. Oral supplementation of turmeric decreases proteinuria, hematuria, and systolic blood pressure in patients suffering from relapsing or refractory lupus nephritis: a randomized and placebo-controlled study. J Ren Nutr. 2012;22:50–57. doi: 10.1053/j.jrn.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Kim HG, Lee JH, Lee SJ, Oh JH, Shin E, Jang YP, et al. The increased cellular uptake and biliary excretion of curcumin by quercetin: a possible role of albumin binding interaction. Drug Metab Dispos. 2012;40:1452–1455. doi: 10.1124/dmd.111.044123. [DOI] [PubMed] [Google Scholar]
- Kim SG, Veena MS, Basak SK, Han E, Tajima T, Gjertson DW, et al. Curcumin treatment suppresses IKKbeta kinase activity of salivary cells of patients with head and neck cancer: a pilot study. Clin Cancer Res. 2011;17:5953–5961. doi: 10.1158/1078-0432.CCR-11-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Ahn Y, Hong MH, Joo SY, Kim KH, Sohn IS, et al. Curcumin attenuates inflammatory responses of TNF-alpha-stimulated human endothelial cells. J Cardiovasc Pharmacol. 2007;50:41–49. doi: 10.1097/FJC.0b013e31805559b9. [DOI] [PubMed] [Google Scholar]
- Kochanek S, Toth M, Dehmel A, Renz D, Doerfler W. Interindividual concordance of methylation profiles in human genes for tumor necrosis factors alpha and beta. Proc Natl Acad Sci U S A. 1990;87:8830–8834. doi: 10.1073/pnas.87.22.8830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanek S, Radbruch A, Tesch H, Renz D, Doerfler W. DNA methylation profiles in the human genes for tumor necrosis factors alpha and beta in subpopulations of leukocytes and in leukemias. Proc Natl Acad Sci U S A. 1991;88:5759–5763. doi: 10.1073/pnas.88.13.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koosirirat C, Linpisarn S, Changsom D, Chawansuntati K, Wipasa J. Investigation of the anti-inflammatory effect of Curcuma longa in Helicobacter pylori-infected patients. Int Immunopharmacol. 2010;10:815–818. doi: 10.1016/j.intimp.2010.04.021. [DOI] [PubMed] [Google Scholar]
- Kositchaiwat C, Kositchaiwat S, Havanondha J. Curcuma longa Linn. in the treatment of gastric ulcer comparison to liquid antacid: a controlled clinical trial. J Med Assoc Thai. 1993;76:601–605. [PubMed] [Google Scholar]
- Kozuch PL, Hanauer SB. Treatment of inflammatory bowel disease: a review of medical therapy. World J Gastroenterol. 2008;14:354–377. doi: 10.3748/wjg.14.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer B, Meichle A, Hensel G, Charnay P, Kronke M. Characterization of an Krox-24/Egr-1-responsive element in the human tumor necrosis factor promoter. Biochim Biophys Acta. 1994;1219:413–421. doi: 10.1016/0167-4781(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Kramer B, Wiegmann K, Kronke M. Regulation of the human TNF promoter by the transcription factor Ets. J Biol Chem. 1995;270:6577–6583. doi: 10.1074/jbc.270.12.6577. [DOI] [PubMed] [Google Scholar]
- Kumar A, Dhawan S, Hardegen NJ, Aggarwal BB. Curcumin (Diferuloylmethane) inhibition of tumor necrosis factor (TNF)-mediated adhesion of monocytes to endothelial cells by suppression of cell surface expression of adhesion molecules and of nuclear factor-kappaB activation. Biochem Pharmacol. 1998;55:775–783. doi: 10.1016/s0006-2952(97)00557-1. [DOI] [PubMed] [Google Scholar]
- Kuprash DV, Udalova IA, Turetskaya RL, Kwiatkowski D, Rice NR, Nedospasov SA. Similarities and differences between human and murine TNF promoters in their response to lipopolysaccharide. J Immunol. 1999;162:4045–4052. [PubMed] [Google Scholar]
- Kuprash DV, Alimzhanov MB, Tumanov AV, Grivennikov SI, Shakhov AN, Drutskaya LN, et al. Redundancy in tumor necrosis factor (TNF) and lymphotoxin (LT) signaling in vivo: mice with inactivation of the entire TNF/LT locus versus single-knockout mice. Mol Cell Biol. 2002;22:8626–8634. doi: 10.1128/MCB.22.24.8626-8634.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurd SK, Richardson SK, Gelfand JM. Update on the epidemiology and systemic treatment of psoriasis. Expert Rev Clin Immunol. 2007;3:171–185. doi: 10.1586/1744666X.3.2.171. [DOI] [PubMed] [Google Scholar]
- Kurd SK, Smith N, VanVoorhees A, Troxel AB, Badmaev V, Seykora JT, et al. Oral curcumin in the treatment of moderate to severe psoriasis vulgaris: a prospective clinical trial. J Am Acad Dermatol. 2008;58:625–631. doi: 10.1016/j.jaad.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurup VP, Barrios CS, Raju R, Johnson BD, Levy MB, Fink JN. Immune response modulation by curcumin in a latex allergy model. Clin Mol Allergy. 2007;5:1. doi: 10.1186/1476-7961-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuttan R, Sudheeran PC, Josph CD. Turmeric and curcumin as topical agents in cancer therapy. Tumori. 1987;73:29–31. doi: 10.1177/030089168707300105. [DOI] [PubMed] [Google Scholar]
- Lahiff C, Moss AC. Curcumin for clinical and endoscopic remission in ulcerative colitis. Inflamm Bowel Dis. 2011;17:E66. doi: 10.1002/ibd.21710. [DOI] [PubMed] [Google Scholar]
- Lal B, Kapoor AK, Asthana OP, Agrawal PK, Prasad R, Kumar P, et al. Efficacy of curcumin in the management of chronic anterior uveitis. Phytother Res. 1999;13:318–322. doi: 10.1002/(SICI)1099-1573(199906)13:4<318::AID-PTR445>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Lal B, Kapoor AK, Agrawal PK, Asthana OP, Srimal RC. Role of curcumin in idiopathic inflammatory orbital pseudotumours. Phytother Res. 2000;14:443–447. doi: 10.1002/1099-1573(200009)14:6<443::aid-ptr619>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Larmonier CB, Uno JK, Lee KM, Karrasch T, Laubitz D, Thurston R, et al. Limited effects of dietary curcumin on Th-1 driven colitis in IL-10 deficient mice suggest an IL-10-dependent mechanism of protection. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1079–G1091. doi: 10.1152/ajpgi.90365.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Jung KK, Cho JY, Rhee MH, Hong S, Kwon M, et al. Neuroprotective effect of curcumin is mainly mediated by blockade of microglial cell activation. Pharmazie. 2007;62:937–942. [PubMed] [Google Scholar]
- Lee JC, Kinniry PA, Arguiri E, Serota M, Kanterakis S, Chatterjee S, et al. Dietary curcumin increases antioxidant defenses in lung, ameliorates radiation-induced pulmonary fibrosis, and improves survival in mice. Radiat Res. 2010;173:590–601. doi: 10.1667/RR1522.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitman DC, Ribeiro RC, Mackow ER, Baxter JD, West BL. Identification of a tumor necrosis factor-responsive element in the tumor necrosis factor alpha gene. J Biol Chem. 1991;266:9343–9346. [PubMed] [Google Scholar]
- Leyon PV, Kuttan G. Studies on the role of some synthetic curcuminoid derivatives in the inhibition of tumour specific angiogenesis. J Exp Clin Cancer Res. 2003;22:77–83. [PubMed] [Google Scholar]
- Liang G, Li X, Chen L, Yang S, Wu X, Studer E, et al. Synthesis and anti-inflammatory activities of mono-carbonyl analogues of curcumin. Bioorg Med Chem Lett. 2008;18:1525–1529. doi: 10.1016/j.bmcl.2007.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang G, Zhou H, Wang Y, Gurley EC, Feng B, Chen L, et al. Inhibition of LPS-induced production of inflammatory factors in the macrophages by mono-carbonyl analogues of curcumin. J Cell Mol Med. 2009;13:3370–3379. doi: 10.1111/j.1582-4934.2009.00711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepinsh DJ, Grivennikov SI, Klarmann KD, Lagarkova MA, Drutskaya MS, Lockett SJ, et al. Novel lymphotoxin alpha (LTalpha) knockout mice with unperturbed tumor necrosis factor expression: reassessing LTalpha biological functions. Mol Cell Biol. 2006;26:4214–4225. doi: 10.1128/MCB.01751-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis E. The immuno-inflammatory reaction in Crohn's disease and ulcerative colitis: characterisation, genetics and clinical application. Focus on TNF alpha. Acta Gastroenterol Belg. 2001;64:1–5. [PubMed] [Google Scholar]
- Lubbad A, Oriowo MA, Khan I. Curcumin attenuates inflammation through inhibition of TLR-4 receptor in experimental colitis. Mol Cell Biochem. 2009a;322:127–135. doi: 10.1007/s11010-008-9949-4. [DOI] [PubMed] [Google Scholar]
- Lubbad AS, Oriowo MA, Khan I. Curcumin reverses attenuated carbachol-induced contraction of the colon in a rat model of colitis. Scand J Gastroenterol. 2009b;44:187–194. doi: 10.1080/00365520802449302. [DOI] [PubMed] [Google Scholar]
- McCaffrey PG, Goldfeld AE, Rao A. The role of NFATp in cyclosporin A-sensitive tumor necrosis factor-alpha gene transcription. J Biol Chem. 1994;269:30445–30450. [PubMed] [Google Scholar]
- Martelli L, Ragazzi E, di Mario F, Martelli M, Castagliuolo I, Dal Maschio M, et al. A potential role for the vanilloid receptor TRPV1 in the therapeutic effect of curcumin in dinitrobenzene sulphonic acid-induced colitis in mice. Neurogastroenterol Motil. 2007;19:668–674. doi: 10.1111/j.1365-2982.2007.00928.x. [DOI] [PubMed] [Google Scholar]
- Masubuchi Y, Enoki K, Horie T. Down-regulation of hepatic cytochrome P450 enzymes in rats with trinitrobenzene sulfonic acid-induced colitis. Drug Metab Dispos. 2008;36:597–603. doi: 10.1124/dmd.107.018754. [DOI] [PubMed] [Google Scholar]
- Matera MG, Calzetta L, Cazzola M. TNF-alpha inhibitors in asthma and COPD: we must not throw the baby out with the bath water. Pulm Pharmacol Ther. 2010;23:121–128. doi: 10.1016/j.pupt.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Mathy-Hartert M, Jacquemond-Collet I, Priem F, Sanchez C, Lambert C, Henrotin Y. Curcumin inhibits pro-inflammatory mediators and metalloproteinase-3 production by chondrocytes. Inflamm Res. 2009;58:899–908. doi: 10.1007/s00011-009-0063-1. [DOI] [PubMed] [Google Scholar]
- Matsuguchi T, Musikacharoen T, Ogawa T, Yoshikai Y. Gene expressions of Toll-like receptor 2, but not Toll-like receptor 4, is induced by LPS and inflammatory cytokines in mouse macrophages. J Immunol. 2000;165:5767–5772. doi: 10.4049/jimmunol.165.10.5767. [DOI] [PubMed] [Google Scholar]
- Meja KK, Rajendrasozhan S, Adenuga D, Biswas SK, Sundar IK, Spooner G, et al. Curcumin restores corticosteroid function in monocytes exposed to oxidants by maintaining HDAC2. Am J Respir Cell Mol Biol. 2008;39:312–323. doi: 10.1165/rcmb.2008-0012OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mito S, Watanabe K, Harima M, Thandavarayan RA, Veeraveedu PT, Sukumaran V, et al. Curcumin ameliorates cardiac inflammation in rats with autoimmune myocarditis. Biol Pharm Bull. 2011;34:974–979. doi: 10.1248/bpb.34.974. [DOI] [PubMed] [Google Scholar]
- Mohandas KM, Desai DC. Epidemiology of digestive tract cancers in India. V. Large and small bowel. Indian J Gastroenterol. 1999;18:118–121. [PubMed] [Google Scholar]
- Moon DO, Jin CY, Lee JD, Choi YH, Ahn SC, Lee CM, et al. Curcumin decreases binding of Shiga-like toxin-1B on human intestinal epithelial cell line HT29 stimulated with TNF-alpha and IL-1beta: suppression of p38, JNK and NF-kappaB p65 as potential targets. Biol Pharm Bull. 2006;29:1470–1475. doi: 10.1248/bpb.29.1470. [DOI] [PubMed] [Google Scholar]
- Moon DO, Kim MO, Lee HJ, Choi YH, Park YM, Heo MS, et al. Curcumin attenuates ovalbumin-induced airway inflammation by regulating nitric oxide. Biochem Biophys Res Commun. 2008;375:275–279. doi: 10.1016/j.bbrc.2008.08.025. [DOI] [PubMed] [Google Scholar]
- Moon DO, Kim MO, Choi YH, Park YM, Kim GY. Curcumin attenuates inflammatory response in IL-1beta-induced human synovial fibroblasts and collagen-induced arthritis in mouse model. Int Immunopharmacol. 2010;10:605–610. doi: 10.1016/j.intimp.2010.02.011. [DOI] [PubMed] [Google Scholar]
- Mouzaoui S, Rahim I, Djerdjouri B. Aminoguanidine and curcumin attenuated tumor necrosis factor (TNF)-alpha-induced oxidative stress, colitis and hepatotoxicity in mice. Int Immunopharmacol. 2012;12:302–311. doi: 10.1016/j.intimp.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Muiznieks I, Doerfler W. The impact of 5′-CG-3′ methylation on the activity of different eukaryotic promoters: a comparative study. FEBS Lett. 1994;344:251–254. doi: 10.1016/0014-5793(94)00394-7. [DOI] [PubMed] [Google Scholar]
- Murch SH, Lamkin VA, Savage MO, Walker-Smith JA, MacDonald TT. Serum concentrations of tumour necrosis factor alpha in childhood chronic inflammatory bowel disease. Gut. 1991;32:913–917. doi: 10.1136/gut.32.8.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myokai F, Takashiba S, Lebo R, Amar S. A novel lipopolysaccharide-induced transcription factor regulating tumor necrosis factor alpha gene expression: molecular cloning, sequencing, characterization, and chromosomal assignment. Proc Natl Acad Sci U S A. 1999;96:4518–4523. doi: 10.1073/pnas.96.8.4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nan B, Lin P, Lumsden AB, Yao Q, Chen C. Effects of TNF-alpha and curcumin on the expression of thrombomodulin and endothelial protein C receptor in human endothelial cells. Thromb Res. 2005;115:417–426. doi: 10.1016/j.thromres.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Nandal S, Dhir A, Kuhad A, Sharma S, Chopra K. Curcumin potentiates the anti-inflammatory activity of cyclooxygenase inhibitors in the cotton pellet granuloma pouch model. Methods Find Exp Clin Pharmacol. 2009;31:89–93. doi: 10.1358/mf.2009.31.2.1357705. [DOI] [PubMed] [Google Scholar]
- Nanji AA, Jokelainen K, Tipoe GL, Rahemtulla A, Thomas P, Dannenberg AJ. Curcumin prevents alcohol-induced liver disease in rats by inhibiting the expression of NF-kappa B-dependent genes. Am J Physiol Gastrointest Liver Physiol. 2003;284:G321–G327. doi: 10.1152/ajpgi.00230.2002. [DOI] [PubMed] [Google Scholar]
- Neurath MF, Pettersson S, Meyer zum Buschenfelde KH, Strober W. Local administration of antisense phosphorothioate oligonucleotides to the p65 subunit of NF-kappa B abrogates established experimental colitis in mice. Nat Med. 1996;2:998–1004. doi: 10.1038/nm0996-998. [DOI] [PubMed] [Google Scholar]
- Newell CL, Deisseroth AB, Lopez-Berestein G. Interaction of nuclear proteins with an AP-1/CRE-like promoter sequence in the human TNF-alpha gene. J Leukoc Biol. 1994;56:27–35. doi: 10.1002/jlb.56.1.27. [DOI] [PubMed] [Google Scholar]
- Ng SC, Kamm MA. Therapeutic strategies for the management of ulcerative colitis. Inflamm Bowel Dis. 2009;15:935–950. doi: 10.1002/ibd.20797. [DOI] [PubMed] [Google Scholar]
- Niederau C, Gopfert E. [The effect of chelidonium- and turmeric root extract on upper abdominal pain due to functional disorders of the biliary system. Results from a placebo-controlled double-blind study] Med Klin (Munich) 1999;94:425–430. doi: 10.1007/BF03044726. [DOI] [PubMed] [Google Scholar]
- Nishida M, Nishiumi S, Mizushina Y, Fujishima Y, Yamamoto K, Masuda A, et al. Monoacetylcurcumin strongly regulates inflammatory responses through inhibition of NF-kappaB activation. Int J Mol Med. 2010;25:761–767. doi: 10.3892/ijmm_00000402. [DOI] [PubMed] [Google Scholar]
- Nones K, Dommels YE, Martell S, Butts C, McNabb WC, Park ZA, et al. The effects of dietary curcumin and rutin on colonic inflammation and gene expression in multidrug resistance gene-deficient (mdr1a-/-) mice, a model of inflammatory bowel diseases. Br J Nutr. 2009;101:169–181. doi: 10.1017/S0007114508009847. [DOI] [PubMed] [Google Scholar]
- Novotny V, Prieschl EE, Csonga R, Fabjani G, Baumruker T. Nrf1 in a complex with fosB, c-jun, junD and ATF2 forms the AP1 component at the TNF alpha promoter in stimulated mast cells. Nucleic Acids Res. 1998;26:5480–5485. doi: 10.1093/nar/26.23.5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley WE, Achinstein B, Shear MJ. Action of bacterial polysaccharide on tumors. II. Damage of sarcoma 37 by serum of mice treated with serratia marcescens polysaccharide, and induced tolerance. J Natl Cancer Inst. 1962;29:1169–1175. [Google Scholar]
- Oh SW, Cha JY, Jung JE, Chang BC, Kwon HJ, Lee BR, et al. Curcumin attenuates allergic airway inflammation and hyper-responsiveness in mice through NF-kappaB inhibition. J Ethnopharmacol. 2011;136:414–421. doi: 10.1016/j.jep.2010.07.026. [DOI] [PubMed] [Google Scholar]
- Old LJ. Tumor necrosis factor (TNF) Science. 1985;230:630–632. doi: 10.1126/science.2413547. [DOI] [PubMed] [Google Scholar]
- Oppenheimer A. Turmeric (curcumin) in biliary diseases. Lancet. 1937;229:619–621. [Google Scholar]
- Pan Y, Wang Y, Cai L, Cai Y, Hu J, Yu C, et al. Inhibition of high glucose-induced inflammatory response and macrophage infiltration by a novel curcumin derivative prevents renal injury in diabetic rats. Br J Pharmacol. 2012;166:1169–1182. doi: 10.1111/j.1476-5381.2012.01854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadakis KA, Targan SR. Role of cytokines in the pathogenesis of inflammatory bowel disease. Annu Rev Med. 2000;51:289–298. doi: 10.1146/annurev.med.51.1.289. [DOI] [PubMed] [Google Scholar]
- Pari L, Amali DR. Protective role of tetrahydrocurcumin (THC) an active principle of turmeric on chloroquine induced hepatotoxicity in rats. J Pharm Pharm Sci. 2005;8:115–123. [PubMed] [Google Scholar]
- Pol A, Bergers M, Schalkwijk J. Comparison of antiproliferative effects of experimental and established antipsoriatic drugs on human keratinocytes, using a simple 96-well-plate assay. In Vitro Cell Dev Biol Anim. 2003;39:36–42. doi: 10.1290/1543-706x(2003)039<0036:coaeoe>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Polasa K, Raghuram TC, Krishna TP, Krishnaswamy K. Effect of turmeric on urinary mutagens in smokers. Mutagenesis. 1992;7:107–109. doi: 10.1093/mutage/7.2.107. [DOI] [PubMed] [Google Scholar]
- Pope RM, Leutz A, Ness SA. C/EBP beta regulation of the tumor necrosis factor alpha gene. J Clin Invest. 1994;94:1449–1455. doi: 10.1172/JCI117482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieschl EE, Novotny V, Csonga R, Jaksche D, Elbe-Burger A, Thumb W, et al. A novel splice variant of the transcription factor Nrf1 interacts with the TNFalpha promoter and stimulates transcription. Nucleic Acids Res. 1998;26:2291–2297. doi: 10.1093/nar/26.10.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prucksunand C, Indrasukhsri B, Leethochawalit M, Hungspreugs K. Phase II clinical trial on effect of the long turmeric (Curcuma longa Linn) on healing of peptic ulcer. Southeast Asian J Trop Med Public Health. 2001;32:208–215. [PubMed] [Google Scholar]
- Rai B, Kaur J, Jacobs R, Singh J. Possible action mechanism for curcumin in pre-cancerous lesions based on serum and salivary markers of oxidative stress. J Oral Sci. 2010;52:251–256. doi: 10.2334/josnusd.52.251. [DOI] [PubMed] [Google Scholar]
- Ram A, Das M, Ghosh B. Curcumin attenuates allergen-induced airway hyperresponsiveness in sensitized guinea pigs. Biol Pharm Bull. 2003;26:1021–1024. doi: 10.1248/bpb.26.1021. [DOI] [PubMed] [Google Scholar]
- Ramirez-Tortosa MC, Ramirez-Tortosa CL, Mesa MD, Granados S, Gil A, Quiles JL. Curcumin ameliorates rabbits's steatohepatitis via respiratory chain, oxidative stress, and TNF-alpha. Free Radic Biol Med. 2009;47:924–931. doi: 10.1016/j.freeradbiomed.2009.06.015. [DOI] [PubMed] [Google Scholar]
- Rasyid A, Lelo A. The effect of curcumin and placebo on human gall-bladder function: an ultrasound study. Aliment Pharmacol Ther. 1999;13:245–249. doi: 10.1046/j.1365-2036.1999.00464.x. [DOI] [PubMed] [Google Scholar]
- Reddy AC, Lokesh BR. Effect of curcumin and eugenol on iron-induced hepatic toxicity in rats. Toxicology. 1996;107:39–45. doi: 10.1016/0300-483x(95)03199-p. [DOI] [PubMed] [Google Scholar]
- Reuter S, Charlet J, Juncker T, Teiten MH, Dicato M, Diederich M. Effect of curcumin on nuclear factor kappaB signaling pathways in human chronic myelogenous K562 leukemia cells. Ann N Y Acad Sci. 2009;1171:436–447. doi: 10.1111/j.1749-6632.2009.04731.x. [DOI] [PubMed] [Google Scholar]
- Reuter S, Gupta SC, Park B, Goel A, Aggarwal BB. Epigenetic changes induced by curcumin and other natural compounds. Genes Nutr. 2011;6:93–108. doi: 10.1007/s12263-011-0222-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringman JM, Frautschy SA, Cole GM, Masterman DL, Cummings JL. A potential role of the curry spice curcumin in Alzheimer's disease. Curr Alzheimer Res. 2005;2:131–136. doi: 10.2174/1567205053585882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salh B, Assi K, Templeman V, Parhar K, Owen D, Gomez-Munoz A, et al. Curcumin attenuates DNB-induced murine colitis. Am J Physiol Gastrointest Liver Physiol. 2003;285:G235–G243. doi: 10.1152/ajpgi.00449.2002. [DOI] [PubMed] [Google Scholar]
- Sandur SK, Ichikawa H, Pandey MK, Kunnumakkara AB, Sung B, Sethi G, et al. Role of pro-oxidants and antioxidants in the anti-inflammatory and apoptotic effects of curcumin (diferuloylmethane) Free Radic Biol Med. 2007;43:568–580. doi: 10.1016/j.freeradbiomed.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki H, Sunagawa Y, Takahashi K, Imaizumi A, Fukuda H, Hashimoto T, et al. Innovative preparation of curcumin for improved oral bioavailability. Biol Pharm Bull. 2011;34:660–665. doi: 10.1248/bpb.34.660. [DOI] [PubMed] [Google Scholar]
- Satoskar RR, Shah SJ, Shenoy SG. Evaluation of anti-inflammatory property of curcumin (diferuloyl methane) in patients with postoperative inflammation. Int J Clin Pharmacol Ther Toxicol. 1986;24:651–654. [PubMed] [Google Scholar]
- Scheinfeld N. Adalimumab (Humira): a brief review for dermatologists. J Dermatolog Treat. 2004;15:348–352. doi: 10.1080/09546630410017284. [DOI] [PubMed] [Google Scholar]
- Shakibaei M, John T, Schulze-Tanzil G, Lehmann I, Mobasheri A. Suppression of NF-kappaB activation by curcumin leads to inhibition of expression of cyclo-oxygenase-2 and matrix metalloproteinase-9 in human articular chondrocytes: implications for the treatment of osteoarthritis. Biochem Pharmacol. 2007;73:1434–1445. doi: 10.1016/j.bcp.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Sharma RA, McLelland HR, Hill KA, Ireson CR, Euden SA, Manson MM, et al. Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin Cancer Res. 2001;7:1894–1900. [PubMed] [Google Scholar]
- Sharma RA, Euden SA, Platton SL, Cooke DN, Shafayat A, Hewitt HR, et al. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res. 2004;10:6847–6854. doi: 10.1158/1078-0432.CCR-04-0744. [DOI] [PubMed] [Google Scholar]
- Sharma RA, Steward WP, Gescher AJ. Pharmacokinetics and pharmacodynamics of curcumin. Adv Exp Med Biol. 2007a;595:453–470. doi: 10.1007/978-0-387-46401-5_20. [DOI] [PubMed] [Google Scholar]
- Sharma S, Chopra K, Kulkarni SK. Effect of insulin and its combination with resveratrol or curcumin in attenuation of diabetic neuropathic pain: participation of nitric oxide and TNF-alpha. Phytother Res. 2007b;21:278–283. doi: 10.1002/ptr.2070. [DOI] [PubMed] [Google Scholar]
- Shear MJ, Perrault A. Chemical treatment of tumors. IX. Reactions of mice with primary subcutaneous tumors to injection of a hemorrhage-producing bacterial polysaccharide. J Natl Cancer Inst. 1944;4:461–476. [Google Scholar]
- Shimouchi A, Nose K, Takaoka M, Hayashi H, Kondo T. Effect of dietary turmeric on breath hydrogen. Dig Dis Sci. 2009;54:1725–1729. doi: 10.1007/s10620-008-0550-1. [DOI] [PubMed] [Google Scholar]
- Shishodia S, Amin HM, Lai R, Aggarwal BB. Curcumin (diferuloylmethane) inhibits constitutive NF-kappaB activation, induces G1/S arrest, suppresses proliferation, and induces apoptosis in mantle cell lymphoma. Biochem Pharmacol. 2005;70:700–713. doi: 10.1016/j.bcp.2005.04.043. [DOI] [PubMed] [Google Scholar]
- Shoskes D, Lapierre C, Cruz-Correa M, Muruve N, Rosario R, Fromkin B, et al. Beneficial effects of the bioflavonoids curcumin and quercetin on early function in cadaveric renal transplantation: a randomized placebo controlled trial. Transplantation. 2005;80:1556–1559. doi: 10.1097/01.tp.0000183290.64309.21. [DOI] [PubMed] [Google Scholar]
- Shu JC, Wang HL, Ye GR, He YJ, Lu X, Fang L. [The study of therapeutic effects of curcumin on hepatic fibrosis and variation of correlated cytokine] Zhong Yao Cai. 2007;30:1421–1425. [PubMed] [Google Scholar]
- Siddiqui AM, Cui X, Wu R, Dong W, Zhou M, Hu M, et al. The anti-inflammatory effect of curcumin in an experimental model of sepsis is mediated by up-regulation of peroxisome proliferator-activated receptor-gamma. Crit Care Med. 2006;34:1874–1882. doi: 10.1097/01.CCM.0000221921.71300.BF. [DOI] [PubMed] [Google Scholar]
- Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) [corrected] J Biol Chem. 1995;270:24995–25000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- Sinha R, Anderson DE, McDonald SS, Greenwald P. Cancer risk and diet in India. J Postgrad Med. 2003;49:222–228. [PubMed] [Google Scholar]
- Song WB, Wang YY, Meng FS, Zhang QH, Zeng JY, Xiao LP, et al. Curcumin protects intestinal mucosal barrier function of rat enteritis via activation of MKP-1 and attenuation of p38 and NF-kappaB activation. PLoS ONE. 2010;5:e12969. doi: 10.1371/journal.pone.0012969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soni KB, Kuttan R. Effect of oral curcumin administration on serum peroxides and cholesterol levels in human volunteers. Indian J Physiol Pharmacol. 1992;36:273–275. [PubMed] [Google Scholar]
- Sood PK, Nahar U, Nehru B. Curcumin attenuates aluminum-induced oxidative stress and mitochondrial dysfunction in rat brain. Neurotox Res. 2011;20:351–361. doi: 10.1007/s12640-011-9249-8. [DOI] [PubMed] [Google Scholar]
- Srimal RC, Dhawan BN. Pharmacology of diferuloyl methane (curcumin), a non-steroidal anti-inflammatory agent. J Pharm Pharmacol. 1973;25:447–452. doi: 10.1111/j.2042-7158.1973.tb09131.x. [DOI] [PubMed] [Google Scholar]
- Srinivasan M. Effect of curcumin on blood sugar as seen in a diabetic subject. Indian J Med Sci. 1972;26:269–270. [PubMed] [Google Scholar]
- Stone-Wolff DS, Yip YK, Kelker HC, Le J, Henriksen-Destefano D, Rubin BY, et al. Interrelationships of human interferon-gamma with lymphotoxin and monocyte cytotoxin. J Exp Med. 1984;159:828–843. doi: 10.1084/jem.159.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser EM, Wessner B, Manhart N, Roth E. The relationship between the anti-inflammatory effects of curcumin and cellular glutathione content in myelomonocytic cells. Biochem Pharmacol. 2005;70:552–559. doi: 10.1016/j.bcp.2005.05.030. [DOI] [PubMed] [Google Scholar]
- Sugimoto K, Hanai H, Tozawa K, Aoshi T, Uchijima M, Nagata T, et al. Curcumin prevents and ameliorates trinitrobenzene sulfonic acid-induced colitis in mice. Gastroenterology. 2002;123:1912–1922. doi: 10.1053/gast.2002.37050. [DOI] [PubMed] [Google Scholar]
- Sun J, Han J, Zhao Y, Zhu Q, Hu J. Curcumin induces apoptosis in tumor necrosis factor-alpha-treated HaCaT cells. Int Immunopharmacol. 2012;13:170–174. doi: 10.1016/j.intimp.2012.03.025. [DOI] [PubMed] [Google Scholar]
- Takashiba S, Van Dyke TE, Shapira L, Amar S. Lipopolysaccharide-inducible and salicylate-sensitive nuclear factor(s) on human tumor necrosis factor alpha promoter. Infect Immun. 1995;63:1529–1534. doi: 10.1128/iai.63.4.1529-1534.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei S, Fernandez D, Redford A, Toyoda H. Methylation status of 5′-regulatory region of tumor necrosis factor alpha gene correlates with differentiation stages of monocytes. Biochem Biophys Res Commun. 1996;220:606–612. doi: 10.1006/bbrc.1996.0450. [DOI] [PubMed] [Google Scholar]
- Tham CL, Liew CY, Lam KW, Mohamad AS, Kim MK, Cheah YK, et al. A synthetic curcuminoid derivative inhibits nitric oxide and proinflammatory cytokine synthesis. Eur J Pharmacol. 2010;628:247–254. doi: 10.1016/j.ejphar.2009.11.053. [DOI] [PubMed] [Google Scholar]
- Tong KM, Shieh DC, Chen CP, Tzeng CY, Wang SP, Huang KC, et al. Leptin induces IL-8 expression via leptin receptor, IRS-1, PI3K, Akt cascade and promotion of NF-kappaB/p300 binding in human synovial fibroblasts. Cell Signal. 2008;20:1478–1488. doi: 10.1016/j.cellsig.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Tonnesen HH, de Vries H, Karlsen J, Beijersbergen van Henegouwen G. Studies on curcumin and curcuminoids. IX: investigation of the photobiological activity of curcumin using bacterial indicator systems. J Pharm Sci. 1987;76:371–373. doi: 10.1002/jps.2600760506. [DOI] [PubMed] [Google Scholar]
- Tsai EY, Jain J, Pesavento PA, Rao A, Goldfeld AE. Tumor necrosis factor alpha gene regulation in activated T cells involves ATF-2/Jun and NFATp. Mol Cell Biol. 1996a;16:459–467. doi: 10.1128/mcb.16.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai EY, Yie J, Thanos D, Goldfeld AE. Cell-type-specific regulation of the human tumor necrosis factor alpha gene in B cells and T cells by NFATp and ATF-2/JUN. Mol Cell Biol. 1996b;16:5232–5244. doi: 10.1128/mcb.16.10.5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu CT, Han B, Yao QY, Zhang YA, Liu HC, Zhang SC. Curcumin attenuates Concanavalin A-induced liver injury in mice by inhibition of Toll-like receptor (TLR) 2, TLR4 and TLR9 expression. Int Immunopharmacol. 2012;12:151–157. doi: 10.1016/j.intimp.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Udalova IA, Knight JC, Vidal V, Nedospasov SA, Kwiatkowski D. Complex NF-kappaB interactions at the distal tumor necrosis factor promoter region in human monocytes. J Biol Chem. 1998;273:21178–21186. doi: 10.1074/jbc.273.33.21178. [DOI] [PubMed] [Google Scholar]
- Ukil A, Maity S, Karmakar S, Datta N, Vedasiromoni JR, Das PK. Curcumin, the major component of food flavour turmeric, reduces mucosal injury in trinitrobenzene sulphonic acid-induced colitis. Br J Pharmacol. 2003;139:209–218. doi: 10.1038/sj.bjp.0705241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ung VY, Foshaug RR, MacFarlane SM, Churchill TA, Doyle JS, Sydora BC, et al. Oral administration of curcumin emulsified in carboxymethyl cellulose has a potent anti-inflammatory effect in the IL-10 gene-deficient mouse model of IBD. Dig Dis Sci. 2010;55:1272–1277. doi: 10.1007/s10620-009-0843-z. [DOI] [PubMed] [Google Scholar]
- Uno JK, Kolek OI, Hines ER, Xu H, Timmermann BN, Kiela PR, et al. The role of tumor necrosis factor alpha in down-regulation of osteoblast Phex gene expression in experimental murine colitis. Gastroenterology. 2006;131:497–509. doi: 10.1053/j.gastro.2006.05.020. [DOI] [PubMed] [Google Scholar]
- Usharani P, Mateen AA, Naidu MU, Raju YS, Chandra N. Effect of NCB-02, atorvastatin and placebo on endothelial function, oxidative stress and inflammatory markers in patients with type 2 diabetes mellitus: a randomized, parallel-group, placebo-controlled, 8-week study. Drugs R D. 2008;9:243–250. doi: 10.2165/00126839-200809040-00004. [DOI] [PubMed] [Google Scholar]
- Vadhan-Raj S, Weber D, Wang M, Giralt S, Alexanian R, Thomas S, et al. Curcumin downregulates NF-κB and related genes in patients with multiple myeloma: results of a phase 1/2 study. Blood. 2007;110:357a. (Abstract 1177) [Google Scholar]
- Vareed SK, Kakarala M, Ruffin MT, Crowell JA, Normolle DP, Djuric Z, et al. Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol Biomarkers Prev. 2008;17:1411–1417. doi: 10.1158/1055-9965.EPI-07-2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkataranganna MV, Rafiq M, Gopumadhavan S, Peer G, Babu UV, Mitra SK. NCB-02 (standardized Curcumin preparation) protects dinitrochlorobenzene-induced colitis through down-regulation of NFkappa-B and iNOS. World J Gastroenterol. 2007;13:1103–1107. doi: 10.3748/wjg.v13.i7.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villegas I, Sanchez-Fidalgo S, de la Lastra CA. Chemopreventive effect of dietary curcumin on inflammation-induced colorectal carcinogenesis in mice. Mol Nutr Food Res. 2011;55:259–267. doi: 10.1002/mnfr.201000225. [DOI] [PubMed] [Google Scholar]
- Wallach D. Cytotoxins (tumour necrosis factor, lymphotoxin and others): molecular and functional characteristics and interactions with interferons. Interferon. 1986;7:89–124. [PubMed] [Google Scholar]
- Wan XH, Li YW, Luo XP. [Curcumin attenuated the lipid peroxidation and apoptotic liver injury in copper-overloaded rats] Zhonghua Er Ke Za Zhi. 2007;45:604–608. [PubMed] [Google Scholar]
- Wang AM, Creasey AA, Ladner MB, Lin LS, Strickler J, Van Arsdell JN, et al. Molecular cloning of the complementary DNA for human tumor necrosis factor. Science. 1985;228:149–154. doi: 10.1126/science.3856324. [DOI] [PubMed] [Google Scholar]
- Wang SL, Li Y, Wen Y, Chen YF, Na LX, Li ST, et al. Curcumin, a potential inhibitor of up-regulation of TNF-alpha and IL-6 induced by palmitate in 3T3-L1 adipocytes through NF-kappaB and JNK pathway. Biomed Environ Sci. 2009;22:32–39. doi: 10.1016/S0895-3988(09)60019-2. [DOI] [PubMed] [Google Scholar]
- Wedel A, Sulski G, Ziegler-Heitbrock HW. CCAAT/enhancer binding protein is involved in the expression of the tumour necrosis factor gene in human monocytes. Cytokine. 1996;8:335–341. doi: 10.1006/cyto.1996.0046. [DOI] [PubMed] [Google Scholar]
- Wickenberg J, Ingemansson SL, Hlebowicz J. Effects of Curcuma longa (turmeric) on postprandial plasma glucose and insulin in healthy subjects. Nutr J. 2010;9:43. doi: 10.1186/1475-2891-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo HM, Kang JH, Kawada T, Yoo H, Sung MK, Yu R. Active spice-derived components can inhibit inflammatory responses of adipose tissue in obesity by suppressing inflammatory actions of macrophages and release of monocyte chemoattractant protein-1 from adipocytes. Life Sci. 2007;80:926–931. doi: 10.1016/j.lfs.2006.11.030. [DOI] [PubMed] [Google Scholar]
- Wua ST, Sun J, Lee K, Sun Y. Docking prediction for tumor necrosis factor-α and five herbal inhibitors. Int J Eng Sci Technol. 2010;2:4263–4277. [Google Scholar]
- Xu YX, Pindolia KR, Janakiraman N, Chapman RA, Gautam SC. Curcumin inhibits IL1 alpha and TNF-alpha induction of AP-1 and NF-kB DNA-binding activity in bone marrow stromal cells. Hematopathol Mol Hematol. 1997;11:49–62. [PubMed] [Google Scholar]
- Yadav VR, Prasad S, Kannappan R, Ravindran J, Chaturvedi MM, Vaahtera L, et al. Cyclodextrin-complexed curcumin exhibits anti-inflammatory and antiproliferative activities superior to those of curcumin through higher cellular uptake. Biochem Pharmacol. 2010;80:1021–1032. doi: 10.1016/j.bcp.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yamamoto M, Yoshizaki K, Kishimoto T, Ito H. IL-6 is required for the development of Th1 cell-mediated murine colitis. J Immunol. 2000;164:4878–4882. doi: 10.4049/jimmunol.164.9.4878. [DOI] [PubMed] [Google Scholar]
- Yang MW, Wang TH, Yan PP, Chu LW, Yu J, Gao ZD, et al. Curcumin improves bone microarchitecture and enhances mineral density in APP/PS1 transgenic mice. Phytomedicine. 2011;18:205–213. doi: 10.1016/j.phymed.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Yao QH, Wang DQ, Cui CC, Yuan ZY, Chen SB, Yao XW, et al. Curcumin ameliorates left ventricular function in rabbits with pressure overload: inhibition of the remodeling of the left ventricular collagen network associated with suppression of myocardial tumor necrosis factor-alpha and matrix metalloproteinase-2 expression. Biol Pharm Bull. 2004;27:198–202. doi: 10.1248/bpb.27.198. [DOI] [PubMed] [Google Scholar]
- Yeh CH, Chen TP, Wu YC, Lin YM, Jing Lin P. Inhibition of NFkappaB activation with curcumin attenuates plasma inflammatory cytokines surge and cardiomyocytic apoptosis following cardiac ischemia/reperfusion. J Surg Res. 2005;125:109–116. doi: 10.1016/j.jss.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Yoon H, Liu RH. Effect of selected phytochemicals and apple extracts on NF-kappaB activation in human breast cancer MCF-7 cells. J Agric Food Chem. 2007;55:3167–3173. doi: 10.1021/jf0632379. [DOI] [PubMed] [Google Scholar]
- Youn HS, Saitoh SI, Miyake K, Hwang DH. Inhibition of homodimerization of Toll-like receptor 4 by curcumin. Biochem Pharmacol. 2006;72:62–69. doi: 10.1016/j.bcp.2006.03.022. [DOI] [PubMed] [Google Scholar]
- Yu YM, Lin HC. Curcumin prevents human aortic smooth muscle cells migration by inhibiting of MMP-9 expression. Nutr Metab Cardiovasc Dis. 2010;20:125–132. doi: 10.1016/j.numecd.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Yun SS, Kim SP, Kang MY, Nam SH. Inhibitory effect of curcumin on liver injury in a murine model of endotoxemic shock. Biotechnol Lett. 2010;32:209–214. doi: 10.1007/s10529-009-0153-8. [DOI] [PubMed] [Google Scholar]
- Zagariya A, Mungre S, Lovis R, Birrer M, Ness S, Thimmapaya B, et al. Tumor necrosis factor alpha gene regulation: enhancement of C/EBPbeta-induced activation by c-Jun. Mol Cell Biol. 1998;18:2815–2824. doi: 10.1128/mcb.18.5.2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng CH, Zeng P, Deng YH, Shen N, Peng ML, Liu Q, et al. [The effects of curcumin derivative on experimental steatohepatitis] Zhonghua Gan Zang Bing Za Zhi. 2011;19:454–459. [PubMed] [Google Scholar]
- Zhang L, Wu C, Zhao S, Yuan D, Lian G, Wang X, et al. Demethoxycurcumin, a natural derivative of curcumin attenuates LPS-induced pro-inflammatory responses through down-regulation of intracellular ROS-related MAPK/NF-kappaB signaling pathways in N9 microglia induced by lipopolysaccharide. Int Immunopharmacol. 2010a;10:331–338. doi: 10.1016/j.intimp.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Zhang LJ, Wu CF, Meng XL, Yuan D, Cai XD, Wang QL, et al. Comparison of inhibitory potency of three different curcuminoid pigments on nitric oxide and tumor necrosis factor production of rat primary microglia induced by lipopolysaccharide. Neurosci Lett. 2008;447:48–53. doi: 10.1016/j.neulet.2008.09.067. [DOI] [PubMed] [Google Scholar]
- Zhang M, Deng CS, Zheng JJ, Xia J. Curcumin regulated shift from Th1 to Th2 in trinitrobenzene sulphonic acid-induced chronic colitis. Acta Pharmacol Sin. 2006;27:1071–1077. doi: 10.1111/j.1745-7254.2006.00322.x. [DOI] [PubMed] [Google Scholar]
- Zhang QY, Mo ZN, Liu XD. [Reducing effect of curcumin on expressions of TNF-alpha, IL-6 and IL-8 in rats with chronic nonbacterial prostatitis] Zhonghua Nan Ke Xue. 2010b;16:84–88. [PubMed] [Google Scholar]
- Zhao C, Yang J, Wang Y, Liang D, Yang X, Li X, et al. Synthesis of mono-carbonyl analogues of curcumin and their effects on inhibition of cytokine release in LPS-stimulated RAW 264.7 macrophages. Bioorg Med Chem. 2010;18:2388–2393. doi: 10.1016/j.bmc.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Zuccotti GV, Trabattoni D, Morelli M, Borgonovo S, Schneider L, Clerici M. Immune modulation by lactoferrin and curcumin in children with recurrent respiratory infections. J Biol Regul Homeost Agents. 2009;23:119–123. [PubMed] [Google Scholar]


