Abstract
Anti-tumour therapies based on the use pro-apoptotic receptor agonists, including TNF-related apoptosis-inducing ligand (TRAIL) or monoclonal antibodies targeting TRAIL-R1 or TRAIL-R2, have been disappointing so far, despite clear evidence of clinical activity and lack of adverse events for the vast majority of these compounds, whether combined or not with conventional or targeted anti-cancer therapies. This brief review aims at discussing the possible reasons for the lack of apparent success of these therapeutic approaches and at providing hints in order to rationally design optimal protocols based on our current understanding of TRAIL signalling regulation or resistance for future clinical trials.
Linked Articles
This article is part of a themed section on Emerging Therapeutic Aspects in Oncology. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2013.169.issue-8
Keywords: TNF-receptor superfamily, TRAIL, Fas, therapy, apoptosis, tumour targeting, resistance
Introduction
Apoptosis is crucial for tissue homeostasis and normal development. Its dysregulation often occurs in cancers and has been implicated in many events relevant to the pathogenesis, progression or chemoresistance of tumours. Restoring sensitivity to and exploiting the induction of apoptosis as a mean to eradicate cancer cells has thus been considered attractive as a potential cure for patients for the last two decades (Kerr et al., 1994). The apoptotic machinery can be engaged by two main signalling pathways: the mitochondrial-dependent intrinsic pathway that is mostly engaged by conventional chemotherapeutic drugs (Fulda and Debatin, 2006), and the extrinsic pathway that can be triggered by ligands of the TNF or Toll superfamily, such as TNF-related apoptosis-inducing ligand (TRAIL), Fas, TNF-α or dsRNA (Ashkenazi and Dixit, 1998; Merino et al., 2007; Weber et al., 2010; Estornes et al., 2012).
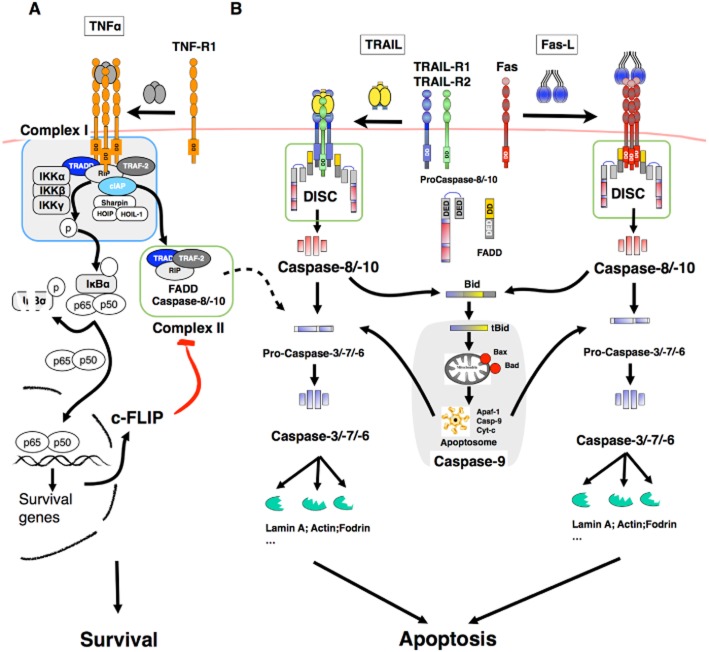
Unlike most chemotherapeutic drugs (Fridman and Lowe, 2003), ligands of the TNF family engage apoptosis in a p53-independent manner and have thus been considered as an alternative to conventional chemo- or radiotherapy owing to the fact that p53 mutations are often found in tumours, ranging from 10 to 80%, depending on the type (Nigro et al., 1989; Greenblatt et al., 1994). Binding of these ligands to their cognate receptors induces receptor aggregation and the formation of a macromolecular complex, coined DISC (death-inducing signalling complex), which, depending on the cellular context, may lead to apoptosis (Figure 1). The DISC forms due to homotypic interactions by means of the death domain (DD) and death effector domains of adaptor proteins such as FADD or TRADD and the initiator caspases, procaspase-8 and -10. DISC assembly ensures procaspase-8/10 oligomerization and activation, leading to subsequent cleavage and release of the active initiator caspase. While activation of caspase-8/10 within the DISC occurs at the plasma membrane upon Fas ligand and TRAIL stimulation, recruitment and activation of initiator caspases upon TNF-R1 engagement takes place in a cytosolic complex generated sequentially from TNF-R1 DISC (Figure 1), coined complex II, arising from the primary membrane-bound complex that contains TRADD, TRAF-2, RIP, cIAPs and IKKs but lacks FADD and caspase-8 (Micheau and Tschopp, 2003). Recently, it has been demonstrated that formation of complex II is negatively regulated through linear ubiquitination by LUBAC, a complex composed of HOIL-1, HOIP and sharpin, also recruited within complex I (Haas et al., 2009). Independently of whether initiator caspases are activated at the membrane or within the cytosolic compartment, their activation triggers a proteolytic cascade leading to apoptosis either directly or through an amplification loop involving mitochondria (Figure 1).
Figure 1.
Simplified schematic representation of Fas ligand, TNF-α and TRAIL-induced signalling: (A) Binding of TNF-α to TNF-RI induces the formation of a membrane bound-complex, composed of RIPK1, TRAF-2, TRADD, IAPs, LUBAC and IKKs that mainly triggers NF-κB activation and cell survival through the transcriptional regulation of the caspase-8 inhibitor c-FLIP. From complex I, a pro-apoptotic cytosolic complex (complex II) is generated, containing the initiator caspase-8 and the adaptor protein FADD. Complex II formation is partly regulated by the linear ubiquitin chain assembly complex, LUBAC, which contains HOIP, HOIL-1 and Sharpin and its pro-apoptotic function is inhibited by c-FLIP. Since the vast majority of cells are proficient for NF-kB activation, upon TNF-α stimulation, TNF-RI fails most of the time to trigger apoptosis. (B) TRAIL- and Fas ligand are potent apoptotic inducers. Binding of these ligands to their cognate pro-apoptotic receptors, TRAIL-R1 or TRAIL-R2 and Fas, respectively, trigger the formation of a membrane complex coined DISC, in which the adaptor protein FADD and the pro-caspase-8/-10 are recruited allowing strong caspase activation and apoptosis triggering. Apoptosis induced by these ligands is either induced through direct caspase-8-mediated caspase-3 activation or through an amplification loop involving the mitochondria and the cleavage of the BH3-only protein Bid by caspase-8.
TNF-α and Fas ligand
Clinical studies aiming at evaluating the anti-tumoral efficacy of TNF family members were initiated three decades ago with recombinant human TNF-α (for a recent review, see Roberts et al., 2011). Unfortunately, systemic TNF-α treatments induced severe adverse events (AE) including hepatotoxicity and hypotension. Clinical activity was rarely obtained, consistent with TNF-α's inability to trigger apoptosis or cell death in most tumour cells, unless the initial NF-kB pathway that is triggered by the membrane-bound complex fails to be activated (Figure 1 and Micheau et al., 2001). By contrast, TNF-α very efficiently triggers apoptosis of endothelial cells (Ruegg et al., 1998) and improves drug uptake by the tumour (van der Veen et al., 2000). These properties are now successfully used in clinical trials to treat limb-threatening soft tissue sarcomas (Deroose et al., 2012) or in-transit melanoma metastases (Rossi et al., 2010), in the setting of hyperthermic isolated limb perfusion with low dose of TNF and melphalan.
Like TNF-α, Fas ligand is highly cytotoxic towards primary hepatocytes and other non-transformed cells and has been shown to induce fulminant liver injuries in rodents (Ogasawara et al., 1993; Costelli et al., 2003). However, contrary to TNF-α, Fas ligand is a potent activator of apoptosis in tumour cells. Importantly, despite the fact that most ligands of the TNF family are naturally found as trimers, engagement of the apoptotic machinery by Fas can only be triggered by the use of hexameric ligands (Schneider et al., 1998) or by antibody-mediated trimeric ligand cross-linking (Berg et al., 2007). In line with these findings, a novel Fas ligand preparation coined APO010 has been generated (Holler et al., 2003) and is now being evaluated for its antitumoral properties (Figures 1 and 2A). This hexameric ligand, which has been obtained by fusing the collagen domain of adiponectin to Fas ligand extracellular domain (Holler et al., 2003), appears to be safe in vivo (Etter et al., 2007). APO010 was shown to be an effective anticancer agent in in vitro and in vivo preclinical studies (Verbrugge et al., 2009; 2010; Eisele et al., 2011). Locoregional administration of APO010 prolonged the survival of mice bearing peritoneal tumour xenografts (Etter et al., 2007). This preparation of Fas ligand is now being evaluated in a phase I dose-escalation study (NCT00437736), to determine its safety and tolerability after intravenous bolus injection in patients with solid tumours.
Figure 2.
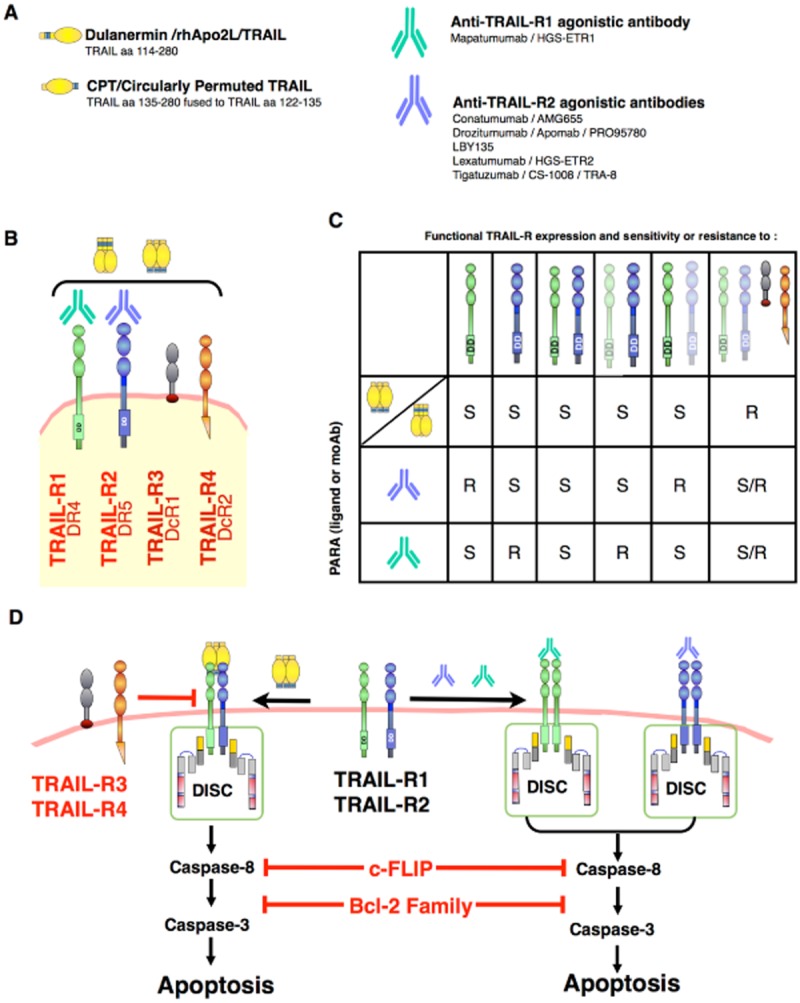
Fas ligand and TRAIL recombinant proteins or derivatives assessed in clinical trials: Schematic representation of (A) Fas ligand, TRAIL recombinant preparations and TRAIL agonistic monoclonal antibodies assessed in clinical trials and (B) the four main receptors to which TRAIL can bind, namely, TRAIL-R1, TRAIL-R2, TRAIL-R3 and TRAIL-R4. (C) Table representing potential pro-apoptotic capabilities of TRAIL preparations or monoclonal antibodies targeting TRAIL-R1 or TRAIL-R2 in tumour cells expressing variable amounts of TRAIL receptors. Receptors represented in light colour indicate poor engagement of the apoptotic machinery. S stands for potentially sensitive tumour cells; R, potentially resistant tumour cells; S/R, cells potentially sensitive or resistant to TRAIL-induced apoptosis depending on the selective or preferential engagement by TRAIL-R1 or TRAIL-R2. (D) Schematic representation of receptor complex formation and apoptosis induced by TRAIL recombinant preparations or monoclonal antibodies targeting TRAIL-R1 or TRAIL-R2. In all cases, overexpression of Bcl-2 family anti-apoptotic members or c-FLIP by tumour cells may impair TRAIL-induced cell death. TRAIL-R3 or TRAIL-R4 expression by tumour cells on the other hand can only impair apoptosis-induced by recombinant TRAIL preparations.
TRAIL and derivatives
TRAIL recombinant proteins
Within the TNF superfamily, TRAIL is the ligand that has as attracted the most interest in oncology owing to its ability to induce apoptosis in transformed cells but not in normal cells and to its lack of toxicity in animal models (Ashkenazi et al., 1999; Walczak et al., 1999; Nesterov et al., 2004; Drosopoulos et al., 2005; Nieminen et al., 2007). The first human recombinant TRAIL preparation, evaluated in clinical studies, was generated by Genentech (Figure 2A). This version of TRAIL, termed APO2L/TRAIL.0 and later on dulanermin, was obtained from bacteria as a non-tagged native recombinant protein encoding amino acid residues 114–281 of human TRAIL (Lawrence et al., 2001). Similar to TNF or Fas ligand, TRAIL is naturally found as a homotrimeric ligand (Hymowitz et al., 1999). However, TRAIL harbours a unique feature, a central zinc atom that binds to cysteine side chains from each trimer subunit and which displays important regulatory function. This coordinated zinc atom has been reported to play a crucial role not only for protein stability and solubility but also for TRAIL's biological activity (Bodmer et al., 2000; Hymowitz et al., 2000). Native TRAIL preparations were found superior to poly-histidine-tagged human TRAIL preparations in triggering cancer cell apoptosis, and importantly, contrary to his-tagged TRAIL, native TRAIL is poorly cytotoxic to hepatocytes (Jo et al., 2000). As reported from clinical studies (Herbst et al., 2006; 2010a; Ling et al., 2006), dulanermin can be safely administered intravenously in patients and is well tolerated up to 30 mg·kg−1 daily for 5 days every 3 weeks (Table 1). Common reported AE include fatigue, nausea, vomiting, fever, anaemia and constipation for 18 to 38% of the patients. Severe AE were reported in two patients. Most AE were related to disease progression or concomitant illness. Dulanermin's plasmatic concentrations were found to be compatible with most preclinical studies, ranging from 5 to 220 μg·mL−1 at 0.5 and 30 mg·kg−1 administration doses respectively. Pharmacokinetic parameters are not influenced by gender, race or enzymatic activities such as alkaline phosphatase or aspartate aminotransferase (Xin et al., 2008). The half-life of dulanermin, however, is relatively short and does not exceed 1 h, with no apparent accumulation in the serum (Herbst et al., 2010a). Despite clear evidence of biological activity as determined by the increase in serum caspase-3/7 activation levels in more than 50% of the patients treated with dulanermin (Pan et al., 2007; 2011), clinical antitumor activity was only evidenced in two patients with chondrosarcomas, who experienced partial response to dulanermin. These patients have been treated with dulanermin for 2 and more than 3 years, at the time of the submission of the manuscript (Herbst et al., 2010a). Because preclinical studies largely demonstrate that a chemotherapy regimen can overcome resistance to TRAIL-induced apoptosis (Gliniak and Le, 1999; Keane et al., 1999; Lacour et al., 2003; Singh et al., 2003; Ganten et al., 2004; Merino et al., 2007; Ashkenazi et al., 2008; Jacquemin et al., 2010; El Fajoui et al., 2011; Morizot et al., 2011; Jacquemin et al., 2012), with minimal toxicities (Evdokiou et al., 2002; Ravi et al., 2004; Ganten et al., 2006), several clinical studies have been designed to assess TRAIL anti-tumoral potential in combination with conventional or targeted anti-cancer therapies (Table 2). Dulanermin has been combined with rituximab in low-grade non-Hodgkin's lymphomas (Yee et al., 2007; Belada et al., 2010), to carboplatin, paclitaxel and bevacizumab in advanced tumours and first-line advanced stage III/IV non-small cell lung carcinomas (NSCLC; Blackhall et al., 2010; Soria et al., 2010; 2011), to FOLFOX and bevacizumab in untreated, advanced or recurrent metastatic colorectal cancers (Kozloff et al., 2012), to an anti-IGR1 in advanced refractory solid tumours (NCT00819169), to irinotecan and cetuximab or FOLFIRI in metastatic colorectal carcinomas (Yee et al., 2009), and to camptosar and erbitux or FOLFIRI plus or minus bevacizumab in previously untreated metastatic colorectal carcinomas (NCT00671372 and Kasubhai et al., 2012). Overall, with the exception of the anti-IGFR1, whose clinical trial was terminated for a reason that was not reported, combining dulanermin with these chemotherapeutic regimens appeared to be rather well tolerated by patients. Incidences of AE were relatively similar across the different treatments arms and most fatal AE were related to disease progression. However, combining dulanermin with these compounds did not improve objective response rates, neither overall patient survival. Reasons for this lack of efficacy are not clear for the moment, although some concerns may be pointed out regarding these combinations and the design of these studies which most of the time are poorly concordant with preclinical studies. One clinical study is still in progress to evaluate the efficacy of dulanermin combined with camptosar and erbitux or FOLFIRI in association or not with bevacizumab in previously untreated metastatic colorectal carcinomas (NCT00671372).
Table 1.
Clinical trials of TRAIL and TRAIL derivatives
| Phase | Pts n (=) | Patients | Company/safety | Best response | Reference |
|---|---|---|---|---|---|
| rhTRAIL | |||||
| Dulanermin (rhApo2L) or AMG951 | Genentech/AMGEN | ||||
| Ia | 71 | Advanced or metastatic solid tumours | Safe and well tolerated up to 30 mg·kg−1 i.v. – half-life 1 h Peak plasmatic concentrations compatible with preclinical studies | SD (33) PR (2/5) chondrosarcoma | Herbst et al., 2010a Ling et al., 2006 Herbst et al., 2006 |
| Ia | 67 | Advanced solid tumours or NHL | Population pharmacokinetic: no influence of gender, race, albumin, alkaline phosphatase or aspartate aminotransferase | NR | Xin et al., 2008 |
| Ia | 71 | Advanced tumours | Serum caspase 3/7 and gDNA levels were observed in >50% of the pts treated with TRAIL | NA | Pan et al., 2011 Pan et al., 2007 |
| CPT (circularly permuted TRAIL) | Sunbio Biotech | ||||
| Ib | 27 | Relapsed/refractory multiple myeloma | Safe and well tolerated up to 15 mg·kg−1 i.v. – half-life 1 h – peak plasmatic concentrations compatible with preclinical studies | CR (1), PR (4) | Chen et al., 2012b |
| II | 27 | Severe AE in three pts – one with CPT-related liver injury | nCR (1), PR (8) ORR 33% | Chen et al., 2012c | |
| MoAb Anti-TRAIL-R1 | |||||
| Mapatumumab or TRM1 or HGS-ETR1 | GSK/HGS/Takeda | ||||
| Ia | 49 | Advanced solid tumours | Safe and well tolerated up to 20 mg·kg−1 i.v. – half-life 18–21 days Peak plasmatic concentrations compatible with preclinical studies | SD (19) | Tolcher et al., 2007 |
| Ia | 41 | Advanced solid tumours | SD (12) | Hotte et al., 2008 | |
| Ib/ II | 40 | Relapsed/refractory NHL | Three clinical responses out of 15 follicular lymphoma pts | CR (2) PR(1) SD (12) | NCT00094848 Younes et al., 2010 |
| II | 32 | Relapsed/refractory stage IIIb/IV or recurrent NSCLC | No AE, but no clinical activity demonstrated | SD (9) | NCT00092924 Greco et al., 2008 |
| II | 38 | Refractory colorectal cancer | SD (12) | Trarbach et al., 2010 | |
| MoAb Anti-TRAIL-R2 | |||||
| Conatumumab or AMG655 | AMGEN | ||||
| Ia | 37 | Advanced solid tumours | Safe and well tolerated up to 20 mg·kg−1 i.v. – half-life 13–19 days | evidence of activity PR (1-over 4 years) SD (14) | Herbst et al., 2010b LoRusso et al., 2007 |
| I | 18 | Advanced solid tumours | SD (9) | Doi et al., 2011 | |
| Drozitumab or apomab or PRO95780 | Genentech | ||||
| Ia | 50 | Advanced treatment or refractory solid tumours | Tolerated up to 20 mg·kg−1 i.v. but possible adverse hepatic events in four pts | SD (20) 23% tumour mass reduction in three pts | Camidge et al., 2010 |
| II | 128* | Untreated, advanced-stage NSCLC | Completed | NR | NCT00480831 |
| II | 90* | Advanced chondrosarcomas | Efficacy not evidenced for this population | Completed | NCT00543712 |
| LBY-135 | Novartis | ||||
| I | 32 | Advanced solid tumours | Safe and well tolerated up to 20 mg·kg−1 i.v. – half-life 10 days signs of clinical activity | Two pts decreased tumour markers (50 and 40%). | Sharma et al., 2008 |
| Lexatumumab or HGS-ETR2 | HGS/Kirin Brewery Co | ||||
| Ia | 37 | Advanced solid tumours | Safe and well tolerated up to 10 mg·kg−1 i.v. – half-life 2–18 days | SD (12) | Plummer et al., 2007 |
| Ia | 31 | Advanced solid tumours and lymphoma | Safe and well tolerated up to 10 mg·kg−1 i.v. | SD (10*) *1 mixed response | Wakelee et al., 2010 Patnaik et al., 2006 |
| I | 24 | Paediatric solid tumours | SD (5) – Clinical response observed two pts | NCT00428272 Merchant et al., 2012 | |
| Tigatuzumab or CS-1008 or TRA-8 | Daiichi Sankyo | ||||
| I | 17 | Relapsed/refractory solid tumours or lymphomas | Safe and well tolerated up to 8 mg·kg−1 i.v. – half-life 8–16 days | SD (7) | NCT00320827 Forero-Torres et al., 2010 |
Estimation/expected.
AE, adverse events; CR, complete response; NA, not applicable; nCR, near-complete response; NHL, non-Hodgkin lymphoma; NR, not reported; ORR, objective response rate; PR, partial response; pts, patients; SD, stable disease.
Table 2.
Clinical trials associating TRAIL and chemotherapy
| Combination | Phase | Pts n (=) | Tumour | Safety | Best response | Reference |
|---|---|---|---|---|---|---|
| Dulanermin or AMG951 | ||||||
| Rituximab | Ib | 7 | Low-grade NHL | Combination appears safe and shows evidence of activity | CR (2) PR (1) SD (1) | NCT00400764 Yee et al., 2007 Belada et al., 2010 |
| II R | 48 | Not better than rituximab alone | ||||
| Carboplatin, paclitaxel and bevacizumab | Ib | 24 | Advanced tumours | Dulanermin plus PCB was well tolerated with no occurrence of DLT | CR (1) PR (13) SD (9) | Soria et al., 2010 |
| Ib and II | 213 | Untreated advanced stage IIIb/IV NSCLC | Not better than PC or PCB PK appeared unaltered | NCT00508625 Blackhall et al., 2010 Soria et al., 2011 | ||
| FOLFOX bevacuzimab | Ib | 23 | Untreated, locally advanced, recurrent, or mCRC | No adverse interactions | PR (12 ± 3*) SD (7) | NCT00873756 Kozloff et al., 2012 |
| AMG479 (anti-IGFR1) | 1b/II | 89* | Advanced refractory solid tumours (NSCLC, CRC pancreatic ovarian and sarcomas) | NR | Terminated | NCT00819169 |
| Irinotecan and cetuximab or FOLFIRI | Ib | 30 | mCRC | Safe with irinotecan-regimen | NR | Yee et al., 2009 |
| Camptosar and Erbitux or FOLFIRI w/o bevacizumab | Ib | NR | Previously treated mCRC | NR | Ongoing, not recruiting | NCT00671372 |
| FOLFIRI bevacuzimab | Ib | 27 | Safe with FOLFIRI (± bevacuzimab) | PR (6) SD (17) | Kasubhai et al., 2012 | |
| CPT | ||||||
| Thalidomide | II | 43 | Relapsed/refractory multiple myeloma | Safe with thalidomide better than thalidomide alone | CR (2), nCR (3), PR (4) | Chen et al., 2012a |
Estimation/expected.
Camptosar, irinotecan HCL injection; CPT, circularly permuted TRAIL; CR, complete response; CRC, colorectal cancer; FOLFIRI, folinic acid, fluorouracil, irinotecan; FOLFOX, folinic acid, fluorouracil, oxaliplatin; NHL, non-Hodgkin lymphoma; NR, not reported; NSCLC, non-small cell lung carcinoma; PCB, paclitaxel, carboplatin, bevacizumab; PK, pharmacokinetics; PR, partial response; pts, patients; R, randomized; SD, stable disease.
More recently, another TRAIL preparation, consisting of the fusion of the human TRAIL amino acid residues 135–280 fused to residues 122–135 of (Figure 2A), also coined CPT or circularly permuted TRAIL, has entered clinical trials. Early preclinical studies evaluating CPT demonstrated its potential anti-tumoral properties alone and in association with chemotherapy in vitro and in vivo on several tumour types (Fang et al., 2005; Tang et al., 2005; 2008; Zhang et al., 2006; Wang et al., 2008). A phase Ib dose escalation study (Chen et al., 2012b) and a phase II multi-centre open-label single-arm study (Chen et al., 2012c) have been performed on relapsed or refractory multiple myeloma patients. CPT was either given intravenously for 5 consecutive days each 21 days for four cycles with increasing concentrations ranging from 5 to 15 mg·kg−1 per day or administered at 2.5 mg·kg−1 per day for 14 consecutive days of each 21-day cycle for two cycles. In the phase Ib study, CPT was found to be well tolerated up to 15 mg·kg−1 with no dose limiting toxicity and limited averse events, including fever, leucopenia, elevated aspartate amino transferase, fatigue and vomiting. Similar to dulanermin, CPT's half-life was estimated to 1 h. Of the 21 patients treated with CPT one achieved complete response and four underwent partial responses. In the phase II study, a similar overall response rate of 33% was found for the 27 patients enrolled, with one patient experiencing near-complete response and eight patients exhibiting partial responses. Of note, one patient experienced a severe AE, the occurrence of which was attributed to CPT-mediated liver injury. Interestingly, a third clinical study has been presented at the American Society of Haematology, associating CPT and thalidomide (Chen et al., 2012a). This multiple-centre, open-label, single-arm phase II study enrolled 43 second-line relapsed or refractory multiple myeloma patients resistant to thalidomide. Patients received thalidomide daily and CPT at 5, 8, or 10 mg·kg−1 per day during the first 5 days of a 21-day cycle, up to six cycles or until disease progression or intolerant advert events. Among the 41 patients that could be evaluated, two achieved complete response, three near-complete responses and four underwent partial responses. Of the three groups of patients, best responses were obtained at the highest CPT doses 10 mg·kg−1. No major AE was attributed to CPT. Overall, the authors of this study concluded the combination associating thalidomide and CPT was not only well tolerated, but displayed superior anti-tumoral properties and clinical activities as CPT alone.
Anti-TRAIL-R1 agonistic monocolonal antibody
In addition to TRAIL preparations, alternative therapeutic strategies based on agonistic monoclonal antibodies (MoAb), that specifically target TRAIL receptors TRAIL-R1 or TRAIL-R2 have been considered, to avoid TRAIL resistance induced by TRAIL binding to the two antagonistic receptors TRAIL-R3 and TRAIL-R4 (Marsters et al., 1997; Meng et al., 2000; Davidovich et al., 2004; Riccioni et al., 2005; Merino et al., 2006; Toscano et al., 2008). So far, only one anti-TRAIL-R1 agonistic MoAb has been evaluated in clinical studies. Mapatumumab, also coined TRM1 or HGS-ETR1 has been assessed in phase I and II clinical trials (Table 1), in advanced solid tumours (Tolcher et al., 2007), advanced hepatocellular carcinomas (Sun et al., 2011), refractory and relapsed non-Hodgkin's lymphomas (Younes et al., 2010), refractory colorectal carcinomas (Trarbach et al., 2010) and relapsed or recurrent stage III and IV NSCLC (Greco et al., 2008). From these studies, it was found that mapatumumab is safe and well tolerated up to 20 mg·kg−1 per day (Tolcher et al., 2007; Hotte et al., 2008; Trarbach et al., 2010; Younes et al., 2010). Mapatumumab-induced AE were mostly grade 1 and 2, including fatigue, hypotension and nausea. However, in a phase I study, two patients at the highest dose level experienced dose-limiting effects consisting of grade 3 transaminase and bilirubin elevations, probably associated with mapatumumab (Tolcher et al., 2007). Plasmatic concentrations were found to be largely compatible with preclinical studies ranging from 0.27 to 400 μg·mL−1 at the corresponding 0.01 to 20 mg·kg−1 doses. From these studies, however, mapatumumab only showed clinical activity in patients with refractory or relapsed non-Hodgkin's lymphomas and in particular in patients with follicular lymphomas. Out of 15 patients harbouring follicular lymphomas, two experienced a complete response and one a partial response (Younes et al., 2010). Clinical studies to evaluate the anti-tumoral efficacy of mapatumumab combined with chemotherapy have also been performed and some are still ongoing (Table 3). Of these, a phase I study associating mapatumumab with gemcitabine and cisplatin in patients with advanced solid tumours (Mom et al., 2009), reported that this anti-TRAIL-R1 MoAb could exhibit clinical activity. Likewise 26 out of 37 patients receiving mapatumumab experienced decreased tumour lesions, 12 patients achieved partial responses and 25 of them experienced stable disease. In another phase I study, the association of mapatumumab with paclitaxel and carboplatin (Leong et al., 2009) was also suggested to exhibit clinical activity with five patients achieving confirmed partial responses and 12 patients with stable disease. From these two studies, it was found that these combinations are rather safe and that the pharmacokinetic parameters of each of these compounds, alone, were apparently not affected by the combination (Chow et al., 2006). However, more recently, preliminary results of a phase II randomized study, performed on stage III and IV NSCLC patients receiving carboplatin and paclitaxel combined or not to mapatumumab as a first-line therapy, clearly suggest that mapatumumab did not improve the response rate nor the progression free survival of NSCLC patients treated with carboplatin and paclitaxel (Von Pawel et al., 2010). Moreover, a phase II randomized study in relapsed or refractory multiple myeloma demonstrated that mapatumumab combined with bortezomib was not better than bortezomib alone (Belch et al., 2010). Another platinum-based combination is currently recruiting patients in a phase Ib/II to assess the efficacy of first-line therapy associating mapatumumab, cisplatin and radiotherapy on cervical cancers (NCT01088347). In spite of these rather disappointing results, the use of TRAIL-R1 monoclonal antibodies may still be of interest for some tumour types, which respond to TRAIL-R1, such as lymphoid malignancies (Younes et al., 2010) or melanomas (Kurbanov et al., 2005).
Table 3.
Clinical trials associating mapatumumab and chemotherapy
| Combination | Phase | Pts n (=) | Tumour | Safety | Best response | Reference |
|---|---|---|---|---|---|---|
| Mapatumumab | ||||||
| Paclitaxel and carboplatin | I | 27 | Advanced solid tumours | Safe with paclitaxel and cisplatin up to 20 mg·kg−1 with no occurrence of DLT | PR (5) SD (12) | Leong et al., 2009 |
| Ib | 28 | Advanced solid tumours | PK profile of HGS-ETR1 not affected by paclitaxel and carboplatin | PR (6) SD (13) | Chow et al., 2006 | |
| Paclitaxel and carboplatin | II R | 111 | First-line advanced NSCLC | The results do not support further evaluation in combination with PC in pts with advanced NSCLC | Similar to PC alone | NCT00583830 Von Pawel et al., 2010 |
| Gemcitabine and cisplatin | Ib | 49 | Advanced solid tumours | Safe with gemcitabine and cisplatin at doses up to 30 mg·kg−1 | PR (12) SD (25) | Mom et al., 2009 |
| Cisplatin and radiotherapy | Ib/II | 42* | First-line advanced cervical cancer | Recruiting | NR | NCT01088347 |
| Sorafenib | Ib | 19 | Advanced hepatocellular carcinoma and chronic viral hepatitis | Safe with sorafenib at doses up to 30 mg·kg−1 | PR (2) SD (4) | NCT00712855 Sun et al., 2011 |
| II | 100* | First-line advanced hepatocellular carcinoma | Ongoing | NR | NCT01258608 | |
| Bortezomib | II R | 104 | Relapsed/refractory multiple myeloma | No adverse effects but no benefit | Similar to bortezomib alone | NCT00315757 Belch et al., 2010 |
Estimation/expected.
DLT, dose-limiting toxicity; NR, not reported; NSCLC, non-small cell lung carcinoma; PC, paclitaxel, carboplatin; PK, pharmacokinetics; PR, partial response; pts, patients; R, randomized; SD, stable disease.
Anti-TRAIL-R2 agonistic monocolonal antibodies
For an unknown reason, more agonistic monoclonal antibodies specifically targeting TRAIL-R2 have been generated than those for TRAIL-R1. So far, 5 anti-TRAIL-R2 MoAb have been assessed in clinical trials, namely conatumumab (or AMG655), drozitumumab (also coined Apomab or PRO95780), LBY135, lexatumumab (or HGS-TR2) and tigatuzumab (also called CS-1008 or TRA-8). Like TRAIL and mapatumumab, these agonistic anti-TRAIL-R2 antibodies have entered the clinical studies alone or in association with chemotherapeutic drugs. With the exception of drozitumumab that has been suspected to induce possible hepatic AEs in four patients out of 50, most TRAIL-R2 agonistic antibodies were found to be well tolerated by patients at doses ranging from 8 to 20 mg·kg−1 per day (Table 1). Their half-life was found to range from 8 to 19 days and clinical efficacies were reported for a large range of tumours.
Conatumumab
Conatumumab was suggested to exhibit anti-tumoral activity in advanced solid tumours (LoRusso et al., 2007; Herbst et al., 2010b; Doi et al., 2011). Combined studies report divergent results depending on the combination (Table 4). Some clinical activities of conatumumab were reported in association with ganitumab (an anti-IGFR1 monoclonal antibody) in advanced refractory solid tumours (Chawla et al., 2010), with FOLFIRI and ganitumab in second-line treatment KRAS mutant metastatic colorectal cancers (Cohn et al., 2012) and with gemcitabine associated or not with ganitumab in metastatic pancreatic cancers (Kindler et al., 2010). However, in unresectable soft tissue sarcomas, conatumumab was found to decrease the overall response rate of doxorubicin (Demetri et al., 2012). Four other clinical studies have been performed or are ongoing. Among these, it should be noted that one of these, evaluating conatumumab in association with bortezomib or vorinostat in relapsed or refractory lymphomas (NCT00791011), has been suspended and two others evaluating conatumumab in association with panitumumab (an anti-EGFR monoclonal antibody) in metastatic colorectal cancers NCT00630786), or paclitaxel and carboplatin in first-line advanced NSCLC (NCT00534027), have been completed, but the results have not been divulged. The last ongoing study is not recruiting patients and aims at evaluating conatumumab efficacy in association with FOLFOX6 and bevacizumab in first-line metastatic colorectal cancer patients (NCT00625651).
Table 4.
Clinical trials associating anti-TRAIL-R2 antibodies and chemotherapy
| Combination | Phase | Pts n (=) | Tumour | Safety | Best response | Reference |
|---|---|---|---|---|---|---|
| Conatumumab | ||||||
| Ganitumab (or AMG 479) | I | 9 | Advanced, refractory solid tumours | Safe with AMG 479 up to 15 mg·kg−1 | SD (3) | NCT00819169 Chawla et al., 2010 |
| Doxorubicin | II R | 128 | Unresectable soft tissue sarcomas | Safe with doxorubicin up to 15 mg·kg−1 Not better than doxorubicin alone | ORR decreased versus placebo 20 versus 24% | NCT00626704 Demetri et al., 2012 |
| mFOLFOX6 and Bevacizumab | Ib/2 | 202* | First-line mCRC | NR | Ongoing, not recruiting | NCT00625651 |
| FOLFIRI or ganitumab + FOLFIRI | II R | 155 | Second-line treatment mutant KRAS mCRC | CON+F tolerable in this population CON + F improved PFS | ORR was increased versus placebo 14 versus 2% | NCT00813605 Cohn et al., 2012 |
| Gemcitabine or gemcitabine + AMG 479 | II R | 125 | Metastatic pancreatic cancers | Safe with gemcitabine up to 10 mg·kg−1 | Some activity PR (1) SD (22) | NCT00630552 Kindler et al., 2010 |
| Carboplatin and paclitaxel | Ib/2 | 172* | First-line advanced NSCLC | NR | Completed | NCT00534027 |
| Panitumumab | Ib/2 | 53* | mCRC | NR | NR | NCT00630786 |
| Bortezomib or vorinostat | Ib | 62* | Relapsed or refractory lymphomas | NR | Suspended | NCT00791011 |
| Drozitumab | ||||||
| Rituximab | II | 40 | Relapsed NHL | Safe with rituximab up to 15 mg·kg−1. Not better than rituximab alone | CR (2) PR (18) | NCT00517049 Wittebol et al., 2010 |
| Carboplatin, paclitaxel ± bevacuzimab | II R | 124 | Previously untreated stage IIIb, IV NSCLC | The results do not support further evaluation with PC ± B | Similar to PC ± B alone | Karapetis et al., 2010 |
| Cetuximab and irinotecan or FOLFIRI ± bevacuzimab | Ib | 20 | First-line mCRC | Safe with irinotecan-regimens up to 15 mg·kg−1 | PR (3*) SD (13) | NCT00497497 Baron et al., 2011 |
| FOLFOX ± bevacuzimab | Ib | 9 | First-line mCRC | The results do not support further evaluation with FOLFOX ± B | PR (5*) SD (3) | NCT00851136 Rocha Lima et al., 2011; 2012 |
| LBY135 | ||||||
| Capecitabine | I | 24 | Advanced solid tumours | Tolerated with capecitabine at 20 mg·kg−1 but 2 grade 3 DLTs observed at 1 and 20 mg·kg−1 | Some activity PR (1) – two pts with decreased tumour vol. 60–73% | Sharma et al., 2008 |
| Lexatumumab | ||||||
| Gemcitabine, pemetrexed, doxorubicin or FOLFIRI | Ib | 41 | Advanced solid tumours | Tumour shrinkage observed, confirmed PRs in the FOLFIRI and doxorubicin arms | NR | Sikic et al., 2007 |
| IFNγ | I | 19 | Refractory paediatric solid tumours | NR | NCT01445093 | |
| Tigatuzumab | ||||||
| Carboplatin, paclitaxel | II R | 109* | Metastatic or unresectable NSCLC | NR | Completed | NCT00991796 |
| II | 24 | Locally advanced or metastatic ovarian cancer | NR | Completed | NCT00945191 | |
| Gemcitabine | II | 65* | Untreated and unresectable pancreatic cancer | NR | Completed | NCT00521404 |
| Irinotecan | II R | 8* | mCRC failed first-line treatment with oxaliplatin | NR | Completed | NCT00969033 |
| FOLFIRI | I | 20* | Patients who have failed other treatments | NR | Ongoing, not recruiting | NCT01124630 |
| Sorafenib | II R | 160* | Advanced liver cancer | NR | Ongoing, not recruiting | NCT01033240 |
| Abraxane (paclitaxel) | II R | 60* | Metastatic triple negative breast cancer | NR | Ongoing, not recruiting | NCT01307891 |
Estimation/expected.
CR, complete response; CRC, colorectal cancer; DLT, dose-limiting toxicity; FOLFIRI, folinic acid, fluorouracil, irinotecan; FOLFOX, folinic acid, fluorouracil, oxaliplatin; mCRC, metastatic colorectal carcinomas; NHL, non-Hodgkin lymphoma; NR, not reported; NSCLC, non-small cell lung carcinoma; ORR, objective response rate; PCB, paclitaxel, carboplatin, bevacizumab; PR, partial response; pts, patients; R, randomized; SD, stable disease.
Drozitumab
So far, no objective response has been attributed to the administration of drozitumab in patients with advanced solid tumours (Table 1). Only minor responses have been described in two patients with colorectal and granulosa cell ovarian cancers and one patient with a chondrosarcoma (Camidge et al., 2010). However, a terminated phase II study in patients with chondrosarcoma, suggests a lack of efficacy of drozitumab for this population (NCT00543712). A second phase II study in NSCLC patients has been terminated, but the results are still awaited (NCT00480831). With combined studies, results have also been disappointing. In association with rituximab in patients with relapsed non-Hodgkin's lymphoma (Wittebol et al., 2010) or with paclitaxel, carboplatin and bevacizumab in previously untreated stage IIIb and IV NSCLC patients (Karapetis et al., 2010), drozitumab failed to demonstrate clinical activity. In two other studies associating drozitumab with cetuximab and irinotecan or FOLFIRI and bevacizumab (Baron et al., 2011), or FOLFOX and bevacizumab in first-line metastatic colorectal cancer patients (Rocha Lima et al., 2011; 2012) some antitumor activity has been described (Table 4), yet drozitumab development appears to have been placed on hold, and currently, there are currently no additional clinical trials ongoing.
LBY135
LBY135 is another anti-TRAIL-R2 agonistic antibody that has been assessed in clinical trials (Tables 1 and 4). LBY135 alone in solid advanced tumours was shown to be safe and well tolerated up to 20 mg·kg−1 (Sharma et al., 2008). Some clinical activities were reported. One patient with sarcoma experienced a minor response and two patients with NSCLC or prostate cancer had a decrease in tumour markers of 50 and 40% respectively. Combined with capecitabin, a precursor of 5-FU, LBY135 induced two partial responses in ovarian and colorectal cancer patients and 60–73% tumour mass reduction in four patients with ovarian, colorectal and pancreatic cancers.
Lexatumumab
Despite the large number of preclinical studies demonstrating its anti-tumoral activity, lexatumumab has poorly been assessed in the clinic. Alone, lexatumumab was shown to be safe and well tolerated up to 10 mg·kg−1 per day and its half-life has been estimated to 2–18 days, depending on the study. In advanced solid tumours (Plummer et al., 2007), lymphomas (Wakelee et al., 2010) and paediatric solid tumours (Merchant et al., 2012), lexatumumab showed signs of clinical activity (Table 1). The most striking activity was achieved in a teenager with progressive, unresectable chest wall/lung osteosarcoma, who was treated for 2 years with lexatumumab and experienced resolution of symptoms, ossification of her lesion and loss of positron emission tomography activity. She remained symptom free with stable imaging for more than 1 year after cessation of therapy. Strikingly, combined studies associating lexatumumab and chemotherapy regimens have been scarce, compared to other anti-TRAIL-R2 agonistic monoclonal antibodies (Table 4). Evidence for clinical activity have only been reported in a study combining lexatumumab with gemcitabine, pemetrexed, doxorubicin or FOLFIRI in advanced solid tumours (Sikic et al., 2007). In this study, it was found that the combination induced some tumour shrinkage and partial responses in the FOLFIRI and doxorubicin arm. The second trial combining INFγ in refractory paediatric solid tumours (NCT01445093) has been completed, but so far, results have not been published.
Tigatuzumab
The only dose-escalating phase I reported for tigatuzumab enrolled 16 relapsed or refractory solid tumours or lymphoma patients (Forero-Torres et al., 2010). Tigatuzumab was well tolerated up to 8 mg·kg−1 per day with no dose limiting toxicity and a half-life estimated to 6–10 days. Tigatuzumab showed signs of clinical activity with seven patients experiencing stable disease, despite the fact that most of these patients have been heavily pretreated with chemotherapy regimens prior to enrolment. Remarkably, one patient entering the trial with progressive metastatic hepatocellular carcinoma, suffering from pain and having failed to respond to alkylating agents, topoisomerase and microtubule inhibitors, became pain free 6 weeks after the onset of the treatment and remained asymptomatic with stable disease for more than 26 months (Forero-Torres et al., 2010). Clinical studies combining tigatuzumab and chemotherapy regimens have been conducted but results have not yet been communicated (Table 4). Tigatuzumab has been assessed combined with paclitaxel and carboplatin in metastatic or unresectable NSCLC (NCT00991796), with gemcitabine in untreated and unresectable pancreatic cancers (NCT00945191) and with irinotecan in metastatic colorectal cancer patients who failed first-line treatments with oxaliplatin (NCT00521404). Three other studies are ongoing. A phase I study combining FOLFIRI and tigatuzumab in patients with metastatic colorectal cancer who have failed first-line treatments that were not based on irinotecan (NCT01124630) and two phase II randomized studies combining sorafenib in advanced liver cancer patients (NCT01033240) or paclitaxel in metastatic triple-negative breast cancers (NCT01307891). Results of these studies are eagerly awaited.
Study design and administration of TRAIL and derivatives: lessons from preclinical studies
With the exception of the newly designed recombinant TRAIL, CPT, clinical studies aiming at evaluating the efficacy of dulanermin or agonistic antibodies targeting TRAIL-R1 or TRAIL-R2, as single agents or combined with chemotherapy regimens, have been disappointing despite clear evidence of efficacy in a, so far, limited number of cases. Understanding how TRAIL signalling is regulated, in light of these results, appears more crucial than ever in order to design appropriate clinical trials aiming at evaluating the anti-tumoral properties of TRAIL preparations and TRAIL derivatives. The TRAIL system is probably the most complex system of the TNF superfamily owing to the ability of TRAIL to bind to five different receptors (Shirley et al., 2011). With the exception of osteoprotegerin, which displays low affinity to TRAIL, the four other receptors, namely, TRAIL-R1, TRAIL-R2, TRAIL-R3 and TRAIL-R4, bind TRAIL with high affinity. However, only TRAIL-R1 and TRAIL-R2 can engage apoptosis, owing to the presence within their intracellular domain of a particular motif, called the DD through which components of the DISC are assembled (Ashkenazi and Dixit, 1998).
The first requirement to assess TRAIL-based anti-tumoral efficacy would certainly deserve, whenever possible, evaluation of TRAIL receptor expression levels in tumours biopsies. The lack of expression of these receptors for a given patient would obviously lead to resistance. While this might appear trivial, this is not an easy goal to achieve. Likewise, most immunohistochemical studies performed so far to evaluate TRAIL receptor expression in primary tumour tissues are poorly informative due to a lack of specific evaluation of membrane-bound expression levels of these receptors. Nonetheless, heterogeneous expression levels of TRAIL receptors have been found in a large variety of tumours (Strater et al., 2002; Min et al., 2004; McCarthy et al., 2005; van Geelen et al., 2006; Kuijlen et al., 2006; Cooper et al., 2008; Granci et al., 2008; Ganten et al., 2009; Leithner et al., 2009; Macher-Goeppinger et al., 2009; Pordzik et al., 2011). However, their expression level is probably overestimated by the fact that most of these studies revealed mainly cytoplasmic expression. In line with this concern, it has been demonstrated that membrane expression levels of TRAIL-R1 and TRAIL-R2 in acute myeloid leukaemia did not exceed 50 and 12.5% respectively (Riccioni et al., 2005). In hepatocellular carcinomas (HCC), a particularly well-designed and informative study demonstrates that TRAIL-R1 and TRAIL-R2 expression were found restricted to the membrane in 30 and 15% of HCC primary tumours, respectively, but an additional fraction of these tumours, 36 and 3% expressed TRAIL-R1 and TRAIL-R2, respectively, both at the membrane and in the cytosol, (Kriegl et al., 2010). As compared to surrounding non-tumorous cells, overall expression levels decreased in HCC with nearly 3% of the tumours lacking TRAIL-R1 and 46% lacking TRAIL-R2, and almost 30% of them expressing TRAIL-R1 and TRAIL-R2 only in the cytoplasm. From this study, it would be anticipated that almost 33 to 82% of these primary tumours would have lost or exhibit non-functional TRAIL-R1 and TRAIL-R2. Furthermore, it is becoming clear that selective engagement of either TRAIL-R1 or TRAIL-R2 can occur in a variety of tumours. Likewise, it has been demonstrated that chronic lymphocytic leukaemia and mantle cell lymphoma (MacFarlane et al., 2005a,b; Natoni et al., 2007) or pancreatic carcinomas (Lemke et al., 2010; Stadel et al., 2010) signal to apoptosis almost exclusively through TRAIL-R1, regardless of TRAIL-R2 membrane expression levels, whereas apoptosis in human glioma (Nagane et al., 2010) or p53 wt myeloma cells (Surget et al., 2012) mainly involves TRAIL-R2. Thus, since we are still unable to predict which TRAIL receptors are functional for a given tumour, these findings are likely to provide a satisfactory answer to the limited efficacy of some of the pro-apoptotic receptor agonist (PARAs) used alone in clinical trial, and in particular to the poor efficacy of mapatumumab. Moreover, the efficacy of dulanermin or CPT may specifically be compromised in tumours cells expressing TRAIL-R3 or TRAIL-R4 (Figure 2). We and others have shown that these antagonistic receptors negatively regulate apoptosis induced by recombinant TRAIL preparations (Meng et al., 2000; Bouralexis et al., 2003; Merino et al., 2006; Sanlioglu et al., 2007; Jacquemin et al., 2012) while sparing that induced by selective anti-TRAIL-R1 or -TRAIL-R2 agonistic antibodies or peptidomimetics targeting TRAIL-R2 (Pavet et al., 2010). However, like TRAIL-R1 and TRAIL-R2, immunohistochemical studies describing high TRAIL-R3 and TRAIL-R4 expression levels in primary tumours mostly document cytoplasm expression but rarely membrane expression levels. Yet, the study performed by Riccioni et al., assessing TRAIL receptor expression levels at the membrane in acute myeloid leukaemia demonstrates that TRAIL-R3 and TRAIL-R4 are highly expressed in more than 60% of cases (Riccioni et al., 2005). Whether these receptors are also expressed at the cell surface in other types of tumours certainly remains to be determined more carefully since the latter, although not exclusively, are likely to compromise anti-tumoral therapies based on the use of recombinant TRAIL preparations.
In addition to their spontaneous expression by primary tumours, expression of these antagonistic receptors at the membrane is likely to be induced by some chemotherapy regimens, prior enrolment or during combined studies. We have recently demonstrated that whilst oxaliplatin is able to synergize with TRAIL in p53-deficient or mutated colorectal tumour cells (El Fajoui et al., 2011), this compound triggers a p53-dependent up-regulation of TRAIL-R3 that impairs the benefit of the combination in wild type p53 colorectal tumour cells, without affecting expression levels of the other receptors (Toscano et al., 2008). The reasons for the selective up-regulation of TRAIL-R3 by oxaliplatin in these cells remains unclear since it has been demonstrated that the promoters of all four TRAIL receptors contain functional p53 sites Wu et al., 1997; Takimoto and El-Deiry, 2000; Ruiz de Almodovar et al., 2002; Liu et al., 2004; Liu et al., 2005). Nonetheless, enforced p53 activation using adenoviruses induces TRAIL-R4 expression and cross-resistance to chemotherapy-induced cell death (Meng et al., 2000; Liu et al., 2005). Likewise, ectopic expression of TRAIL-R4 in some tumour cells impairs apoptosis induced by chemotherapy (Lalaoui et al., 2011). In agreement with these studies, it has been found that inactivation of TRAIL-R2 or mouse TRAIL-R expression conferred resistance to chemotherapy (Wang and El-Deiry, 2004) and radiotherapy (Finnberg et al., 2005). It therefore cannot be excluded that some chemotherapy regimens may lead to TRAIL resistance or that selection of resistant tumours induced by TRAIL treatments may give rise to cross-resistance to chemotherapy through progressive acquisition of antagonistic receptor expression (Bouralexis et al., 2003) or loss of agonistic TRAIL receptors (Wang and El-Deiry, 2004; Finnberg et al., 2005).
TRAIL pro-apoptotic signalling pathway is regulated by three main checkpoints that can cooperate to inhibit full execution of the machinery (Figure 2D). The first checkpoint, at the membrane, is highly specific and tightly controlled by receptor expression levels, whether by loss of TRAIL-R1 or TRAIL-R2 expression, overexpression of the antagonistic receptors, TRAIL-R3 or TRAIL-R4 or by post-translational modifications through O-glycosylation. The second, less specific but nonetheless potent, checkpoint at the DISC level is mostly under the control of c-FLIP, the endogenous inhibitor of the initiator caspases-8 and -10, and is also involved in regulating apoptosis-induced by the other receptors of the TNF superfamily and TLR3. Last but not least, the third checkpoint, which is also involved in chemotherapy-induced cell death, occurs at the mitochondrial level.
As discussed above, one possible explanation for the lack of efficacy of these PARAs could reside in the poor expression level of TRAIL-R1 or TRAIL-R2 in some tumours (Ganten et al., 2009; Duiker et al., 2010; Kriegl et al., 2010), and/or result from selective engagement of either TRAIL-R1 or TRAIL-R2 (MacFarlane et al., 2005a,b; Nagane et al., 2010; Stadel et al., 2010; Surget et al., 2012) or regulation of the antagonistic receptors TRAIL-R3 or TRAIL-R4 and/or downstream inhibitors of TRAIL signalling by chemotherapeutic drugs (Bouralexis et al., 2003; Davidovich et al., 2004; Liu et al., 2005; Nguyen et al., 2006; Toscano et al., 2008; Lalaoui et al., 2011; Morizot et al., 2011). TRAIL receptor expression in patients having received first-line chemotherapy based on DNA-damaging drugs or in protocols associating TRAIL and DNA-damage compounds should thus be assessed carefully to evaluate the efficacy of these combinations in clinical studies.
Alternatively, lack of efficacy of some of these PARAs may be explained by their poor efficiency in triggering apoptosis. It has been demonstrated for example that some humanized anti-TRAIL receptor antibodies require cross-linking to achieve optimal activity in in vitro studies, and that most of them display lower pro-apoptotic activity as compared to recombinant TRAIL preparations (Chuntharapai et al., 2001; Jin et al., 2008; Yada et al., 2008; Dobson et al., 2009; Zinonos et al., 2009; Kang et al., 2011). In line with this hypothesis is the demonstration that the newly formulated TRAIL preparation CPT, which displays stronger pro-apoptotic activity than dulanermin (Fang et al., 2005), appeared to be more efficient than dulanermin or monoclonal antibodies targeting TRAIL-R1 or TRAIL-R2 in early clinical trials, either alone or combined with chemotherapy. However, so far, CPT has only been assessed in relapsed or refractory multiple myeloma in association or not with thalidomide, and additional clinical trials may be necessary to fully demonstrate that CPT preparations are superior to dulanermin or anti-TRAIL receptor antibodies. Nonetheless, these results are encouraging and suggest that it may be possible to engineer TRAIL derivatives that could display higher anti-tumoral properties and that may be of interest for oncologists (van der Sloot et al., 2006; Reis et al., 2009; Szegezdi et al., 2012). In addition, although it had been demonstrated that engagement of apoptosis and DISC formation by dulanermin requires TRAIL receptor O-glycosylation, and that these post-translational modifications have no impact on TRAIL binding to TRAIL-R2 (Wagner et al., 2007), it remains unclear whether these modifications are required for monoclonal antibodies targeting TRAIL receptors to engage apoptosis and whether they might compromise binding of some of these antibodies to their target. Less specifically, poor clinical activities may also be explained by high expression levels of c-FLIP or anti-apoptotic proteins of the bcl-2 family, both of which are found to be highly expressed in a large number of tumours (Garcia et al., 2002; Tolcher, 2005; Bagnoli et al., 2009; Du et al., 2009; Duiker et al., 2010; McLornan et al., 2013).
Notwithstanding the molecular mechanisms that are required to efficiently engage TRAIL-induced cell death machinery in primary tumour cells, inappropriate administration schemes most likely provide the best explanation for the poor efficacy of PARAs combined with chemotherapy regimens in clinical studies. It has been found in the past that chemotherapy and TRAIL, used simultaneously, can afford restoration of apoptosis in a large number of TRAIL- or chemoresistant tumour cells in vitro and in vivo (Gliniak and Le, 1999; Keane et al., 1999; Walczak et al., 2000; Cuello et al., 2001; Lacour et al., 2003; Ohtsuka et al., 2003; Jin et al., 2004; Shankar and Srivastava, 2004; Fiveash et al., 2008), but these synergies were shown to critically rely on mitochondrial activation (von Haefen et al., 2004; Nguyen et al., 2006). Unfortunately, a large proportion of tumours, and not only haematological malignancies, spontaneously overexpress Bcl-2 anti-apoptotic family members or, to a lesser extent, display loss-of-function mutations of Bax (Meijerink et al., 1998; Garcia et al., 2002; Pepper et al., 2008; Likui et al., 2009) and in vitro studies demonstrate that simultaneous treatments are unable to overcome TRAIL resistance induced by a deficiency of Bax or the overexpression of Bcl-2 (Fulda et al., 2002; LeBlanc et al., 2002; von Haefen et al., 2004). Because recombinant TRAIL or moAb targeting TRAIL-R1 or TRAIL-R2 have been administered simultaneously starting from day 1 of each cycle with the chemotherapeutic compounds of interest, in most if not all clinical studies, the lack of efficacy of these combinations may be attributed to their inability to overcome the mitochondrial block (Ganten et al., 2004; von Haefen et al., 2004; Ndozangue-Touriguine et al., 2008; El Fajoui et al., 2011; Morizot et al., 2011; Jacquemin et al., 2012).
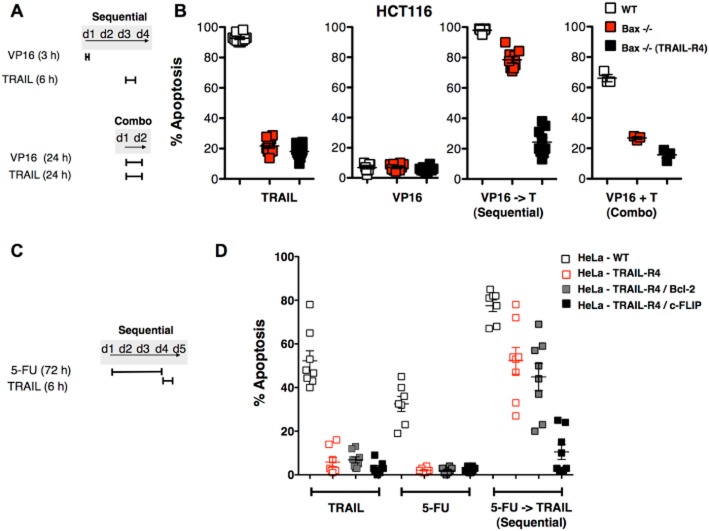
However, some chemotherapeutic drugs applied sequentially are able to overcome resistance induced by one or even two TRAIL signalling checkpoints, including those acting at the mitochondrial level (Singh et al., 2003; Galligan et al., 2005; Shankar et al., 2005; Ivanov et al., 2007; Morizot et al., 2011). As illustrated in Figure 3, while simultaneous treatment with TRAIL and etoposide (VP16) fails to cooperate to induce apoptosis in the colon cancer cell line HCT116, deficient for Bax (Bax-/-), sequential administration of TRAIL and VP16 overcomes Bax deficiency (Figure 3, adapted from Morizot et al., 2011). Yet, when Bax deficiency is associated with the ectopic expression of TRAIL-R4, this combination fails to restore apoptosis induced by TRAIL (Figure 3). However, when other chemotherapeutic regimens, such as the metabolic inhibitor 5-FU, are used sequentially, they can afford TRAIL-induced cell death restoration in Bax-deficient HCT116 cells expressing TRAIL-R4 ectopically (Figure 3), owing to 5-FU's ability to inhibit c-FLIP expression (Galligan et al., 2005; Morizot et al., 2011). Similarly, in the cervical adenocarcinoma cell line HeLa, sequential treatment with 5-FU and TRAIL can overcome resistance induced by ectopic expression of TRAIL-R4 alone or TRAIL-R4 and Bcl-2, but fails to restore sensitivity to TRAIL-induced cell death when TRAIL-R4 is expressed together with the caspase-8 inhibitor c-FLIP (Figure 3).
Figure 3.
Differential TRAIL-induced apoptosis following combined versus sequential chemotherapy. (A) Schematic representation of the treatment protocols used panel B. (B) TRAIL-induced apoptosis in HCT116 WT cells (empty squares), HCT116 Bax deficient (Bax-/-) (grey squares) or HCT16 Bax deficient expressing ectopically TRAIL-R4 [Bax-/-(TRAIL-R4)] cells (black squares), stimulated either sequentially with etoposide (VP16) or simultaneously (combo). For sequential treatments, cells were first incubated for 3 h in the presence of 10 μM VP16, washed, allowed to recover at 37°C for 45 h and then stimulated with 500 ng·mL−1 TRAIL for 6 h. Alternatively, cells were stimulated simultaneously with TRAIL and VP16 (combo), or with single agents for 24 or 48 h respectively. Apoptosis was measured by Hoechst staining. (C) Schematic representation of the treatment protocols used panel D. (D) Apoptosis induced by TRAIL, 5-FU or sequential treatments associating 5-FU and TRAIL in HeLa WT cells (empty squares) or HeLa cells expressing TRAIL-R4 ectopically (empty red squares), TRAIL-R4 and Bcl-2 (grey squares) or TRAIL-R4 and c-FLIP (black squares). HeLa cells were stimulated or not for 72 h with 1 μM 5-FU, then treated or not with 500 ng·mL−1 TRAIL for 6 h and apoptosis was monitored by Hoechst staining. Modified from Morizot et al. (2011).
It is unclear why sequential treatments are superior to combined treatments and why some chemotherapeutic drugs are able to bypass two checkpoints while others only manage to circumvent one at a time. Nonetheless, similar concepts have recently been documented for targeted therapies combined with DNA-damaging agents. It has been demonstrated for example that time-staggered EGFR inhibition, but not simultaneous coadministration, sensitized triple-negative breast cancer cells to genotoxic drugs (Lee et al., 2012). As far as TRAIL is concerned, we and others have demonstrated that sequential treatments with some therapeutic agents induce an increase in DISC formation and caspase-8 activation at the membrane (Lacour et al., 2003; Ganten et al., 2004; Morizot et al., 2011), while others, including polyphenol derivatives or oxaliplatin, act mainly at the mitochondrial level (El Fajoui et al., 2011; Jacquemin et al., 2012). Some compounds, including the metabolic inhibitor 5-FU are able to enhance DISC formation and inhibit c-FLIP at the same time (Morizot et al., 2011). As a matter of fact, inhibition of c-FLIP expression by 5-FU requires much more time than TRAIL to induce DISC formation and caspase activation, which takes place within minutes. Thus, simultaneous stimulations are unlikely to provide enough time to inhibit c-FLIP expression or to allow molecular events leading to enhanced TRAIL DISC formation. This increase in DISC formation and in caspase-8 activation by some chemotherapeutic compounds is essential to bypass the mitochondrial block (Ndozangue-Touriguine et al., 2008; Morizot et al., 2011). Alternatively, many chemotherapeutic compounds have been described to enhance TRAIL-induced cell death mediated by up-regulating TRAIL-R1 or TRAIL-R2 (Baritaki et al., 2007; Liu et al., 2007; Son et al., 2007; David et al., 2008; Kwon et al., 2008; Hori et al., 2010; Zhu et al., 2010; Ding et al., 2011).
The hope that TRAIL or its derivatives could be used as single agents to treat patients suffering from cancer is clearly over, but there is still a possibility that TRAIL-based antitumoral therapies, in association with targeted or conventional chemotherapy, will be of interest in oncology. Understanding more precisely the molecular mechanisms underlying TRAIL signalling checkpoint regulation by conventional chemotherapy or targeted anti-cancer compounds, is required more than ever before designing novel clinical trials aiming at evaluating the efficacy of these PARAs in the clinic. Whatever the molecular mechanism required, future clinical studies, besides monitoring TRAIL receptor, c-FLIP and Bcl-2 family members expression levels, in order to select patients that may mostly benefit from these combinations, should also consider administrating TRAIL sequentially after chemotherapy, to overcome resistance induced at the mitochondrial level. Sequential therapies should prove valuable, provided that the combination acts cooperatively and is not deleterious for TRAIL signalling.
Acknowledgments
OM laboratory is supported by grants of the Conseil Regional de Bourgogne, Cancéropôle Grand-Est, ANR (Agence Nationale de la Recherche, ANR-07-PCV-0031 and SphingoDR). F. D. was supported by ANR (SphingoDR). We are indebted to Nicolas Isambert for critical reading of the manuscript.
Glossary
- DISC
death-inducing signalling complex
- PARAs
pro-apoptotic receptor agonists
- TRAIL
TNF-related apoptosis-inducing ligand
Conflict of interest
None.
References
- Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999;104:155–162. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashkenazi A, Holland P, Eckhardt SG. Ligand-based targeting of apoptosis in cancer: the potential of recombinant human apoptosis ligand 2/tumor necrosis factor-related apoptosis-inducing ligand (rhApo2L/TRAIL) J Clin Oncol. 2008;26:3621–3630. doi: 10.1200/JCO.2007.15.7198. [DOI] [PubMed] [Google Scholar]
- Bagnoli M, Ambrogi F, Pilotti S, Alberti P, Ditto A, Barbareschi M, et al. c-FLIPL expression defines two ovarian cancer patient subsets and is a prognostic factor of adverse outcome. Endocr Relat Cancer. 2009;16:443–453. doi: 10.1677/ERC-08-0218. [DOI] [PubMed] [Google Scholar]
- Baritaki S, Huerta-Yepez S, Sakai T, Spandidos DA, Bonavida B. Chemotherapeutic drugs sensitize cancer cells to TRAIL-mediated apoptosis: up-regulation of DR5 and inhibition of Yin Yang 1. Mol Cancer Ther. 2007;6:1387–1399. doi: 10.1158/1535-7163.MCT-06-0521. [DOI] [PubMed] [Google Scholar]
- Baron AD, O'Bryant C, Choi Y, Royer-Joo S, Portera CC. Phase Ib study of drozitumab combined with cetuximab (CET) plus irinotecan (IRI) or with FOLFIRI with or without bevacizumab (BV) in previously treated patients (pts) with metastatic colorectal cancer (mCRC) J Clin Oncol. 2011;29(15s):abstr 3581. [Google Scholar]
- Belada D, Mayer J, Czuczman MS, Flinn IW, Durbin-Johnson B, Bray GL. Phase II study of dulanermin plus rituximab in patients with relapsed follicular non-Hodgkin's lymphoma (NHL) J Clin Oncol. 2010;28(15s):abstr 8104. [Google Scholar]
- Belch A, Sharma A, Spencer A, Tarantolo S, Bahlis N, Doval D, et al. Results of an international, randomized phase II clinical trial of bortezomib ± Mapatumumab (TRAIL-R1 agonist monoclonal antibody) for the treatment of relapsed/refractory multiple myeloma. Haematologica. 2010;96(3s):abstr 1006. [Google Scholar]
- Berg D, Lehne M, Muller N, Siegmund D, Munkel S, Sebald W, et al. Enforced covalent trimerization increases the activity of the TNF ligand family members TRAIL and CD95L. Cell Death Differ. 2007;14:2021–2034. doi: 10.1038/sj.cdd.4402213. [DOI] [PubMed] [Google Scholar]
- Blackhall FH, Márk Z, Zatloukal P, Szima B, Albert I, Juhász E, et al. A randomized phase II study of paclitaxel (P) and carboplatin (C) ± bevacizumab (B) ± dulanermin (D) in non-small cell lung cancer (NSCLC) J Clin Oncol. 2010;28(15s):abstr 7534. doi: 10.1200/JCO.2011.37.2623. [DOI] [PubMed] [Google Scholar]
- Bodmer JL, Meier P, Tschopp J, Schneider P. Cysteine 230 is essential for the structure and activity of the cytotoxic ligand TRAIL. J Biol Chem. 2000;275:20632–20637. doi: 10.1074/jbc.M909721199. [DOI] [PubMed] [Google Scholar]
- Bouralexis S, Findlay DM, Atkins GJ, Labrinidis A, Hay S, Evdokiou A. Progressive resistance of BTK-143 osteosarcoma cells to Apo2L/TRAIL-induced apoptosis is mediated by acquisition of DcR2/TRAIL-R4 expression: resensitisation with chemotherapy. Br J Cancer. 2003;89:206–214. doi: 10.1038/sj.bjc.6601021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camidge DR, Herbst RS, Gordon MS, Eckhardt SG, Kurzrock R, Durbin B, et al. A phase I safety and pharmacokinetic study of the death receptor 5 agonistic antibody PRO95780 in patients with advanced malignancies. Clin Cancer Res. 2010;16:1256–1263. doi: 10.1158/1078-0432.CCR-09-1267. [DOI] [PubMed] [Google Scholar]
- Chawla SP, Tabernero J, Kindler HL, Chiorean EG, LoRusso PM, Hsu M, et al. Phase I evaluation of the safety of conatumumab (AMG 655) in combination with AMG 479 in patients (pts) with advanced, refractory solid tumors. J Clin Oncol. 2010;28(15s):abstr 3102. [Google Scholar]
- Chen W, Hou J, Zhao Y, Qiu L, Ke X, Wang Z, et al. 2012a. Circularly permuted TRAIL (CPT) combined with thalidomide for the treatment of relapsed or refractory multiple myeloma: an open-label, multicenter phase II clinical trial. 54th ASH annual meeting abstr 2958.
- Chen W, Qiu L, Hou J, Zhang X, Ke X, Wang Z, et al. 2012b. Phase Ib study of recombinant circularly permuted TRAIL (CPT) in relapsed or refractory multiple myeloma patients. 54th ASH annual meeting abstr 1857.
- Chen W, Qiu L, Hou J, Zhao Y, Pan L, Yang S, et al. 2012c. Recombinant circularly permuted TRAIL (CPT) for the treatment of relapsed or refractory multiple myeloma: an open-label, multicenter phase II clinical trial. 54th ASH annual meeting abstr 78.
- Chow LQ, Eckhardt G, Gustafson DL, O'Bryant C, Hariharan S, Diab S, et al. HGS-ETR1, an antibody targeting TRAIL-R1, in combination with paclitaxel and carboplatin in patients with advanced solid malignancies: results of a phase 1 and PK study. J Clin Oncol. 2006;24(18s):abstr 2515. [Google Scholar]
- Chuntharapai A, Dodge K, Grimmer K, Schroeder K, Marsters SA, Koeppen H, et al. Isotype-dependent inhibition of tumor growth in vivo by monoclonal antibodies to death receptor 4. J Immunol. 2001;166:4891–4898. doi: 10.4049/jimmunol.166.8.4891. [DOI] [PubMed] [Google Scholar]
- Cohn AL, Tabernero J, Maurel J, Nowara E, Dubey S, Baker N, et al. Conatumumab (CON) plus FOLFIRI (F) or ganitumab (GAN) plus F for second-line treatment of mutant (MT) KRAS metastatic colorectal cancer (mCRC) J Clin Oncol. 2012;30(4s):abstr 534. [Google Scholar]
- Cooper WA, Kohonen-Corish MR, Zhuang L, McCaughan B, Kennedy C, Screaton G, et al. Role and prognostic significance of tumor necrosis factor-related apoptosis-inducing ligand death receptor DR5 in nonsmall-cell lung cancer and precursor lesions. Cancer. 2008;113:135–142. doi: 10.1002/cncr.23528. [DOI] [PubMed] [Google Scholar]
- Costelli P, Aoki P, Zingaro B, Carbo N, Reffo P, Lopez-Soriano FJ, et al. Mice lacking TNFalpha receptors 1 and 2 are resistant to death and fulminant liver injury induced by agonistic anti-Fas antibody. Cell Death Differ. 2003;10:997–1004. doi: 10.1038/sj.cdd.4401281. [DOI] [PubMed] [Google Scholar]
- Cuello M, Ettenberg SA, Nau MM, Lipkowitz S. Synergistic induction of apoptosis by the combination of trail and chemotherapy in chemoresistant ovarian cancer cells. Gynecol Oncol. 2001;81:380–390. doi: 10.1006/gyno.2001.6194. [DOI] [PubMed] [Google Scholar]
- David E, Sinha R, Chen J, Sun SY, Kaufman JL, Lonial S. Perifosine synergistically enhances TRAIL-induced myeloma cell apoptosis via up-regulation of death receptors. Clin Cancer Res. 2008;14:5090–5098. doi: 10.1158/1078-0432.CCR-08-0016. [DOI] [PubMed] [Google Scholar]
- Davidovich IA, Levenson AS, Levenson Chernokhvostov VV. Overexpression of DcR1 and survivin in genetically modified cells with pleiotropic drug resistance. Cancer Lett. 2004;211:189–197. doi: 10.1016/j.canlet.2004.03.026. [DOI] [PubMed] [Google Scholar]
- Demetri GD, Le Cesne A, Chawla SP, Brodowicz T, Maki RG, Bach BA, et al. First-line treatment of metastatic or locally advanced unresectable soft tissue sarcomas with conatumumab in combination with doxorubicin or doxorubicin alone: a phase I/II open-label and double-blind study. Eur J Cancer. 2012;48:547–563. doi: 10.1016/j.ejca.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Deroose JP, van Geel AN, Burger JW, Eggermont AM, Verhoef C. Isolated limb perfusion with TNF-alpha and melphalan for distal parts of the limb in soft tissue sarcoma patients. J Surg Oncol. 2012;105:563–569. doi: 10.1002/jso.22121. [DOI] [PubMed] [Google Scholar]
- Ding L, Yuan C, Wei F, Wang G, Zhang J, Bellail AC, et al. Cisplatin restores TRAIL apoptotic pathway in glioblastoma-derived stem cells through up-regulation of DR5 and down-regulation of c-FLIP. Cancer Invest. 2011;29:511–520. doi: 10.3109/07357907.2011.605412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson CL, Main S, Newton P, Chodorge M, Cadwallader K, Humphreys R, et al. Human monomeric antibody fragments to TRAIL-R1 and TRAIL-R2 that display potent in vitro agonism. mAbs. 2009;1:552–562. doi: 10.4161/mabs.1.6.10057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi T, Murakami H, Ohtsu A, Fuse N, Yoshino T, Yamamoto N, et al. Phase 1 study of conatumumab, a pro-apoptotic death receptor 5 agonist antibody, in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol. 2011;68:733–741. doi: 10.1007/s00280-010-1544-1. [DOI] [PubMed] [Google Scholar]
- Drosopoulos KG, Roberts ML, Cermak L, Sasazuki T, Shirasawa S, Andera L, et al. Transformation by oncogenic RAS sensitizes human colon cells to TRAIL-induced apoptosis by up-regulating death receptor 4 and death receptor 5 through a MEK-dependent pathway. J Biol Chem. 2005;280:22856–22867. doi: 10.1074/jbc.M412483200. [DOI] [PubMed] [Google Scholar]
- Du X, Bao G, He X, Zhao H, Yu F, Qiao Q, et al. Expression and biological significance of c-FLIP in human hepatocellular carcinomas. J Exp Clin Cancer Res. 2009;28:24. doi: 10.1186/1756-9966-28-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duiker EW, van der Zee AG, de Graeff P, Boersma-van Ek W, Hollema H, de Bock GH, et al. The extrinsic apoptosis pathway and its prognostic impact in ovarian cancer. Gynecol Oncol. 2010;116:549–555. doi: 10.1016/j.ygyno.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Eisele G, Roth P, Hasenbach K, Aulwurm S, Wolpert F, Tabatabai G, et al. APO010, a synthetic hexameric CD95 ligand, induces human glioma cell death in vitro and in vivo. Neuro-oncol. 2011;13:155–164. doi: 10.1093/neuonc/noq176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Fajoui Z, Toscano F, Jacquemin G, Abello J, Scoazec JY, Micheau O, et al. Oxaliplatin sensitizes human colon cancer cells to TRAIL through JNK-dependent phosphorylation of Bcl-xL. Gastroenterology. 2011;141:663–673. doi: 10.1053/j.gastro.2011.04.055. [DOI] [PubMed] [Google Scholar]
- Estornes Y, Toscano F, Virard F, Jacquemin G, Pierrot A, Vanbervliet B, et al. dsRNA induces apoptosis through an atypical death complex associating TLR3 to caspase-8. Cell Death Differ. 2012;19:1482–1494. doi: 10.1038/cdd.2012.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etter AL, Bassi I, Germain S, Delaloye JF, Tschopp J, Sordat B, et al. The combination of chemotherapy and intraperitoneal MegaFas Ligand improves treatment of ovarian carcinoma. Gynecol Oncol. 2007;107:14–21. doi: 10.1016/j.ygyno.2007.05.030. [DOI] [PubMed] [Google Scholar]
- Evdokiou A, Bouralexis S, Atkins GJ, Chai F, Hay S, Clayer M, et al. Chemotherapeutic agents sensitize osteogenic sarcoma cells, but not normal human bone cells, to Apo2L/TRAIL-induced apoptosis. Int J Cancer. 2002;99:491–504. doi: 10.1002/ijc.10376. [DOI] [PubMed] [Google Scholar]
- Fang F, Wang AP, Yang SF. Antitumor activity of a novel recombinant mutant human tumor necrosis factor-related apoptosis-inducing ligand. Acta Pharmacol Sin. 2005;26:1373–1381. doi: 10.1111/j.1745-7254.2005.00206.x. [DOI] [PubMed] [Google Scholar]
- Finnberg N, Gruber JJ, Fei P, Rudolph D, Bric A, Kim SH, et al. DR5 knockout mice are compromised in radiation-induced apoptosis. Mol Cell Biol. 2005;25:2000–2013. doi: 10.1128/MCB.25.5.2000-2013.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiveash JB, Gillespie GY, Oliver PG, Zhou T, Belenky ML, Buchsbaum DJ. Enhancement of glioma radiotherapy and chemotherapy response with targeted antibody therapy against death receptor 5. Int J Radiat Oncol Biol Phys. 2008;71:507–516. doi: 10.1016/j.ijrobp.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forero-Torres A, Shah J, Wood T, Posey J, Carlisle R, Copigneaux C, et al. Phase I trial of weekly tigatuzumab, an agonistic humanized monoclonal antibody targeting death receptor 5 (DR5) Cancer Biother Radiopharm. 2010;25:13–19. doi: 10.1089/cbr.2009.0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridman JS, Lowe SW. Control of apoptosis by p53. Oncogene. 2003;22:9030–9040. doi: 10.1038/sj.onc.1207116. [DOI] [PubMed] [Google Scholar]
- Fulda S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006;25:4798–4811. doi: 10.1038/sj.onc.1209608. [DOI] [PubMed] [Google Scholar]
- Fulda S, Meyer E, Debatin KM. Inhibition of TRAIL-induced apoptosis by Bcl-2 overexpression. Oncogene. 2002;21:2283–2294. doi: 10.1038/sj.onc.1205258. [DOI] [PubMed] [Google Scholar]
- Galligan L, Longley DB, McEwan M, Wilson TR, McLaughlin K, Johnston PG. Chemotherapy and TRAIL-mediated colon cancer cell death: the roles of p53, TRAIL receptors, and c-FLIP. Mol Cancer Ther. 2005;4:2026–2036. doi: 10.1158/1535-7163.MCT-05-0262. [DOI] [PubMed] [Google Scholar]
- Ganten TM, Haas TL, Sykora J, Stahl H, Sprick MR, Fas SC, et al. Enhanced caspase-8 recruitment to and activation at the DISC is critical for sensitisation of human hepatocellular carcinoma cells to TRAIL-induced apoptosis by chemotherapeutic drugs. Cell Death Differ. 2004;11(Suppl. 1):S86–S96. doi: 10.1038/sj.cdd.4401437. [DOI] [PubMed] [Google Scholar]
- Ganten TM, Koschny R, Sykora J, Schulze-Bergkamen H, Buchler P, Haas TL, et al. Preclinical differentiation between apparently safe and potentially hepatotoxic applications of TRAIL either alone or in combination with chemotherapeutic drugs. Clin Cancer Res. 2006;12:2640–2646. doi: 10.1158/1078-0432.CCR-05-2635. [DOI] [PubMed] [Google Scholar]
- Ganten TM, Sykora J, Koschny R, Batke E, Aulmann S, Mansmann U, et al. Prognostic significance of tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) receptor expression in patients with breast cancer. J Mol Med. 2009;87:995–1007. doi: 10.1007/s00109-009-0510-z. [DOI] [PubMed] [Google Scholar]
- Garcia EJ, Lawson D, Cotsonis G, Cohen C. Hepatocellular carcinoma and markers of apoptosis (bcl-2, bax, bcl-x): prognostic significance. Appl Immunohistochem Mol Morphol. 2002;10:210–217. doi: 10.1097/00129039-200209000-00004. [DOI] [PubMed] [Google Scholar]
- van Geelen CM, Westra JL, de Vries EG, Boersma-van Ek W, Zwart N, Hollema H, et al. Prognostic significance of tumor necrosis factor-related apoptosis-inducing ligand and its receptors in adjuvantly treated stage III colon cancer patients. J Clin Oncol. 2006;24:4998–5004. doi: 10.1200/JCO.2006.06.8809. [DOI] [PubMed] [Google Scholar]
- Gliniak B, Le T. Tumor necrosis factor-related apoptosis-inducing ligand's antitumor activity in vivo is enhanced by the chemotherapeutic agent CPT-11. Cancer Res. 1999;59:6153–6158. [PubMed] [Google Scholar]
- Granci V, Bibeau F, Kramar A, Boissiere-Michot F, Thezenas S, Thirion A, et al. Prognostic significance of TRAIL-R1 and TRAIL-R3 expression in metastatic colorectal carcinomas. Eur J Cancer. 2008;44:2312–2318. doi: 10.1016/j.ejca.2008.06.042. [DOI] [PubMed] [Google Scholar]
- Greco FA, Bonomi P, Crawford J, Kelly K, Oh Y, Halpern W, et al. Phase 2 study of mapatumumab, a fully human agonistic monoclonal antibody which targets and activates the TRAIL receptor-1, in patients with advanced non-small cell lung cancer. Lung Cancer. 2008;61:82–90. doi: 10.1016/j.lungcan.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Greenblatt MS, Bennett WP, Hollstein M, Harris CC. Mutations in the p53 tumor suppressor gene: clues to cancer etiology and molecular pathogenesis. Cancer Res. 1994;54:4855–4878. [PubMed] [Google Scholar]
- Haas TL, Emmerich CH, Gerlach B, Schmukle AC, Cordier SM, Rieser E, et al. Recruitment of the linear ubiquitin chain assembly complex stabilizes the TNF-R1 signaling complex and is required for TNF-mediated gene induction. Mol Cell. 2009;36:831–844. doi: 10.1016/j.molcel.2009.10.013. [DOI] [PubMed] [Google Scholar]
- von Haefen C, Gillissen B, Hemmati PG, Wendt J, Guner D, Mrozek A, et al. Multidomain Bcl-2 homolog Bax but not Bak mediates synergistic induction of apoptosis by TRAIL and 5-FU through the mitochondrial apoptosis pathway. Oncogene. 2004;23:8320–8332. doi: 10.1038/sj.onc.1207971. [DOI] [PubMed] [Google Scholar]
- Herbst RS, Mendolson DS, Ebbinghaus S, Gordon MS, O'Dwyer M, Lieberman G, et al. A phase I safety and pharmacokinetic (PK) study of recombinant Apo2L/TRAIL, an apoptosis-inducing protein in patients with advanced cancer. J Clin Oncol. 2006;24(18S):abstr 3013. [Google Scholar]
- Herbst RS, Eckhardt SG, Kurzrock R, Ebbinghaus S, O'Dwyer PJ, Gordon MS, et al. Phase I dose-escalation study of recombinant human Apo2L/TRAIL, a dual proapoptotic receptor agonist, in patients with advanced cancer. J Clin Oncol. 2010a;28:2839–2846. doi: 10.1200/JCO.2009.25.1991. [DOI] [PubMed] [Google Scholar]
- Herbst RS, Kurzrock R, Hong DS, Valdivieso M, Hsu CP, Goyal L, et al. A first-in-human study of conatumumab in adult patients with advanced solid tumors. Clin Cancer Res. 2010b;16:5883–5891. doi: 10.1158/1078-0432.CCR-10-0631. [DOI] [PubMed] [Google Scholar]
- Holler N, Tardivel A, Kovacsovics-Bankowski M, Hertig S, Gaide O, Martinon F, et al. Two adjacent trimeric Fas ligands are required for Fas signaling and formation of a death-inducing signaling complex. Mol Cell Biol. 2003;23:1428–1440. doi: 10.1128/MCB.23.4.1428-1440.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori T, Kondo T, Kanamori M, Tabuchi Y, Ogawa R, Zhao QL, et al. Nutlin-3 enhances tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis through up-regulation of death receptor 5 (DR5) in human sarcoma HOS cells and human colon cancer HCT116 cells. Cancer Lett. 2010;287:98–108. doi: 10.1016/j.canlet.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Hotte SJ, Hirte HW, Chen EX, Siu LL, Le LH, Corey A, et al. A phase 1 study of mapatumumab (fully human monoclonal antibody to TRAIL-R1) in patients with advanced solid malignancies. Clin Cancer Res. 2008;14:3450–3455. doi: 10.1158/1078-0432.CCR-07-1416. [DOI] [PubMed] [Google Scholar]
- Hymowitz SG, Christinger HW, Fuh G, Ultsch M, O'Connell M, Kelley RF, et al. Triggering cell death: the crystal structure of Apo2L/TRAIL in a complex with death receptor 5. Mol Cell. 1999;4:563–571. doi: 10.1016/s1097-2765(00)80207-5. [DOI] [PubMed] [Google Scholar]
- Hymowitz SG, O'Connell MP, Ultsch MH, Hurst A, Totpal K, Ashkenazi A, et al. A unique zinc-binding site revealed by a high-resolution X-ray structure of homotrimeric Apo2L/TRAIL. Biochemistry. 2000;39:633–640. doi: 10.1021/bi992242l. [DOI] [PubMed] [Google Scholar]
- Ivanov VN, Zhou H, Hei TK. Sequential treatment by ionizing radiation and sodium arsenite dramatically accelerates TRAIL-mediated apoptosis of human melanoma cells. Cancer Res. 2007;67:5397–5407. doi: 10.1158/0008-5472.CAN-07-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemin G, Shirley S, Micheau O. Combining naturally occurring polyphenols with TNF-related apoptosis-inducing ligand: a promising approach to kill resistant cancer cells. Cell Mol Life Sci. 2010;67:3115–3130. doi: 10.1007/s00018-010-0407-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemin G, Granci V, Gallouet AS, Lalaoui N, Morle A, Iessi E, et al. Quercetin-mediated Mcl-1 and survivin downregulation restores TRAIL-induced apoptosis in non-Hodgkin's lymphoma B cells. Haematologica. 2012;97:38–46. doi: 10.3324/haematol.2011.046466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Yang R, Fong S, Totpal K, Lawrence D, Zheng Z, et al. Apo2 ligand/tumor necrosis factor-related apoptosis-inducing ligand cooperates with chemotherapy to inhibit orthotopic lung tumor growth and improve survival. Cancer Res. 2004;64:4900–4905. doi: 10.1158/0008-5472.CAN-04-0408. [DOI] [PubMed] [Google Scholar]
- Jin H, Yang R, Ross J, Fong S, Carano R, Totpal K, et al. Cooperation of the agonistic DR5 antibody apomab with chemotherapy to inhibit orthotopic lung tumor growth and improve survival. Clin Cancer Res. 2008;14:7733–7740. doi: 10.1158/1078-0432.CCR-08-0670. [DOI] [PubMed] [Google Scholar]
- Jo M, Kim TH, Seol DW, Esplen JE, Dorko K, Billiar TR, et al. Apoptosis induced in normal human hepatocytes by tumor necrosis factor-related apoptosis-inducing ligand. Nat Med. 2000;6:564–567. doi: 10.1038/75045. [DOI] [PubMed] [Google Scholar]
- Kang Z, Chen JJ, Yu Y, Li B, Sun SY, Zhang B, et al. Drozitumab, a human antibody to death receptor 5, has potent antitumor activity against rhabdomyosarcoma with the expression of caspase-8 predictive of response. Clin Cancer Res. 2011;17:3181–3192. doi: 10.1158/1078-0432.CCR-10-2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karapetis CS, Clingan PR, Leighl NB, Durbin-Johnson B, O'Neill V, Spigel DR. Phase II study of PRO95780 plus paclitaxel, carboplatin, and bevacizumab (PCB) in non-small cell lung cancer (NSCLC) J Clin Oncol. 2010;28(15s):abstr 7535. [Google Scholar]
- Kasubhai SM, Bendell JC, Kozloff M, Kapp AM, Ashkenazi A, Royer-Joo S, et al. Phase Ib study of dulanermin combined with FOLFIRI (with or without bevacizumab [BV]) in previously treated patients (Pts) with metastatic colorectal cancer (mCRC) J Clin Oncol. 2012;30(15s) abstr 3543. [Google Scholar]
- Keane MM, Ettenberg SA, Nau MM, Russell EK, Lipkowitz S. Chemotherapy augments TRAIL-induced apoptosis in breast cell lines. Cancer Res. 1999;59:734–741. [PubMed] [Google Scholar]
- Kerr JF, Winterford CM, Harmon BV. Apoptosis. Its significance in cancer and cancer therapy. Cancer. 1994;73:2013–2026. doi: 10.1002/1097-0142(19940415)73:8<2013::aid-cncr2820730802>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Kindler HL, Richards DA, Stephenson J, Garbo LE, Rocha Lima CS, Safran H, et al. A placebo-controlled, randomized phase II study of conatumumab (C) or AMG 479 (A) or placebo (P) plus gemcitabine (G) in patients (pts) with metastatic pancreatic cancer (mPC) J Clin Oncol. 2010;28(15s):abstr 4035. doi: 10.1093/annonc/mds142. [DOI] [PubMed] [Google Scholar]
- Kozloff M, Messersmith WA, Kapp AV, Ashkenazi A, Royer-Joo S, Portera CC, et al. Phase Ib study of dulanermin combined with first-line FOLFOX plus bevacizumab (BV) in patients (Pts) with metastatic colorectal cancer (mCRC) J Clin Oncol. 2012;30(15s):abstr 3552. [Google Scholar]
- Kriegl L, Jung A, Engel J, Jackstadt R, Gerbes AL, Gallmeier E, et al. Expression, cellular distribution, and prognostic relevance of TRAIL receptors in hepatocellular carcinoma. Clin Cancer Res. 2010;16:5529–5538. doi: 10.1158/1078-0432.CCR-09-3403. [DOI] [PubMed] [Google Scholar]
- Kuijlen JM, Mooij JJ, Platteel I, Hoving EW, van der Graaf WT, Span MM, et al. TRAIL-receptor expression is an independent prognostic factor for survival in patients with a primary glioblastoma multiforme. J Neurooncol. 2006;78:161–171. doi: 10.1007/s11060-005-9081-1. [DOI] [PubMed] [Google Scholar]
- Kurbanov BM, Geilen CC, Fecker LF, Orfanos CE, Eberle J. Efficient TRAIL-R1/DR4-mediated apoptosis in melanoma cells by tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) J Invest Dermatol. 2005;125:1010–1019. doi: 10.1111/j.0022-202X.2005.23900.x. [DOI] [PubMed] [Google Scholar]
- Kwon D, Choi K, Choi C, Benveniste EN. Hydrogen peroxide enhances TRAIL-induced cell death through up-regulation of DR5 in human astrocytic cells. Biochem Biophys Res Commun. 2008;372:870–874. doi: 10.1016/j.bbrc.2008.05.148. [DOI] [PubMed] [Google Scholar]
- Lacour S, Micheau O, Hammann A, Drouineaud V, Tschopp J, Solary E, et al. Chemotherapy enhances TNF-related apoptosis-inducing Ligand DISC assembly in HT29 human colon cancer cells. Oncogene. 2003;22:1807–1816. doi: 10.1038/sj.onc.1206127. [DOI] [PubMed] [Google Scholar]
- Lalaoui N, Morle A, Merino D, Jacquemin G, Iessi E, Morizot A, et al. TRAIL-R4 promotes tumor growth and resistance to apoptosis in cervical carcinoma HeLa cells through AKT. PLoS ONE. 2011;6:e19679. doi: 10.1371/journal.pone.0019679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence D, Shahrokh Z, Marsters S, Achilles K, Shih D, Mounho B, et al. Differential hepatocyte toxicity of recombinant Apo2L/TRAIL versions. Nat Med. 2001;7:383–385. doi: 10.1038/86397. [DOI] [PubMed] [Google Scholar]
- LeBlanc H, Lawrence D, Varfolomeev E, Totpal K, Morlan J, Schow P, et al. Tumor-cell resistance to death receptor-induced apoptosis through mutational inactivation of the proapoptotic Bcl-2 homolog Bax. Nat Med. 2002;8:274–281. doi: 10.1038/nm0302-274. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Ye AS, Gardino AK, Heijink AM, Sorger PK, MacBeath G, et al. Sequential application of anticancer drugs enhances cell death by rewiring apoptotic signaling networks. Cell. 2012;149:780–794. doi: 10.1016/j.cell.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leithner K, Stacher E, Wurm R, Ploner F, Quehenberger F, Wohlkoenig C, et al. Nuclear and cytoplasmic death receptor 5 as prognostic factors in patients with non-small cell lung cancer treated with chemotherapy. Lung Cancer. 2009;65:98–104. doi: 10.1016/j.lungcan.2008.10.015. [DOI] [PubMed] [Google Scholar]
- Lemke J, Noack A, Adam D, Tchikov V, Bertsch U, Roder C, et al. TRAIL signaling is mediated by DR4 in pancreatic tumor cells despite the expression of functional DR5. J Mol Med. 2010;88:729–740. doi: 10.1007/s00109-010-0619-0. [DOI] [PubMed] [Google Scholar]
- Leong S, Cohen RB, Gustafson DL, Langer CJ, Camidge DR, Padavic K, et al. Mapatumumab, an antibody targeting TRAIL-R1, in combination with paclitaxel and carboplatin in patients with advanced solid malignancies: results of a phase I and pharmacokinetic study. J Clin Oncol. 2009;27:4413–4421. doi: 10.1200/JCO.2008.21.7422. [DOI] [PubMed] [Google Scholar]
- Likui W, Qun L, Wanqing Z, Haifeng S, Fangqiu L, Xiaojun L. Prognostic role of myeloid cell leukemia-1 protein (Mcl-1) expression in human gastric cancer. J Surg Oncol. 2009;100:396–400. doi: 10.1002/jso.21344. [DOI] [PubMed] [Google Scholar]
- Ling J, Herbst RS, Mendelson DS, Eckhardt SG, O'Dwyer P, Ebbinghaus S, et al. Apo2L/TRAIL pharmacokinetics in a phase 1a trial in advanced cancer and lymphoma. J Clin Oncol. 2006;24(18s):abstr 3047. [Google Scholar]
- Liu X, Yue P, Khuri FR, Sun SY. p53 upregulates death receptor 4 expression through an intronic p53 binding site. Cancer Res. 2004;64:5078–5083. doi: 10.1158/0008-5472.CAN-04-1195. [DOI] [PubMed] [Google Scholar]
- Liu X, Yue P, Khuri FR, Sun SY. Decoy receptor 2 (DcR2) is a p53 target gene and regulates chemosensitivity. Cancer Res. 2005;65:9169–9175. doi: 10.1158/0008-5472.CAN-05-0939. [DOI] [PubMed] [Google Scholar]
- Liu X, Yue P, Chen S, Hu L, Lonial S, Khuri FR, et al. The proteasome inhibitor PS-341 (bortezomib) up-regulates DR5 expression leading to induction of apoptosis and enhancement of TRAIL-induced apoptosis despite up-regulation of c-FLIP and survivin expression in human NSCLC cells. Cancer Res. 2007;67:4981–4988. doi: 10.1158/0008-5472.CAN-06-4274. [DOI] [PubMed] [Google Scholar]
- LoRusso PM, Hong DS, Heath E, Kurzrock R, Wang D, Hsu M, et al. First-in-human study of AMG 655, a pro-apoptotic TRAIL receptor-2 agonist, in adult patients with advanced solid tumors. J Clin Oncol. 2007;25(18s):abstr 3534. [Google Scholar]
- McCarthy MM, Sznol M, DiVito KA, Camp RL, Rimm DL, Kluger HM. Evaluating the expression and prognostic value of TRAIL-R1 and TRAIL-R2 in breast cancer. Clin Cancer Res. 2005;11:5188–5194. doi: 10.1158/1078-0432.CCR-05-0158. [DOI] [PubMed] [Google Scholar]
- MacFarlane M, Inoue S, Kohlhaas SL, Majid A, Harper N, Kennedy DB, et al. Chronic lymphocytic leukemic cells exhibit apoptotic signaling via TRAIL-R1. Cell Death Differ. 2005a;12:773–782. doi: 10.1038/sj.cdd.4401649. [DOI] [PubMed] [Google Scholar]
- MacFarlane M, Kohlhaas SL, Sutcliffe MJ, Dyer MJ, Cohen GM. TRAIL receptor-selective mutants signal to apoptosis via TRAIL-R1 in primary lymphoid malignancies. Cancer Res. 2005b;65:11265–11270. doi: 10.1158/0008-5472.CAN-05-2801. [DOI] [PubMed] [Google Scholar]
- Macher-Goeppinger S, Aulmann S, Tagscherer KE, Wagener N, Haferkamp A, Penzel R, et al. Prognostic value of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and TRAIL receptors in renal cell cancer. Clin Cancer Res. 2009;15:650–659. doi: 10.1158/1078-0432.CCR-08-0284. [DOI] [PubMed] [Google Scholar]
- McLornan D, Hay J, McLaughlin K, Holohan C, Burnett AK, Hills RK, et al. Prognostic and therapeutic relevance of c-FLIP in acute myeloid leukaemia. Br J Haematol. 2013;160:188–198. doi: 10.1111/bjh.12108. [DOI] [PubMed] [Google Scholar]
- Marsters SA, Sheridan JP, Pitti RM, Huang A, Skubatch M, Baldwin D, et al. A novel receptor for Apo2L/TRAIL contains a truncated death domain. Curr Biol. 1997;7:1003–1006. doi: 10.1016/s0960-9822(06)00422-2. [DOI] [PubMed] [Google Scholar]
- Meijerink JP, Mensink EJ, Wang K, Sedlak TW, Sloetjes AW, de Witte T, et al. Hematopoietic malignancies demonstrate loss-of-function mutations of BAX. Blood. 1998;91:2991–2997. [PubMed] [Google Scholar]
- Meng RD, McDonald ER, 3rd, Sheikh MS, Fornace AJ, Jr, El-Deiry WS. The TRAIL decoy receptor TRUNDD (DcR2, TRAIL-R4) is induced by adenovirus-p53 overexpression and can delay TRAIL-, p53-, and KILLER/DR5-dependent colon cancer apoptosis. Mol Ther. 2000;1:130–144. doi: 10.1006/mthe.2000.0025. [DOI] [PubMed] [Google Scholar]
- Merchant MS, Geller JI, Baird K, Chou AJ, Galli S, Charles A, et al. Phase I trial and pharmacokinetic study of lexatumumab in pediatric patients with solid tumors. J Clin Oncol. 2012;30:4141–4147. doi: 10.1200/JCO.2012.44.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino D, Lalaoui N, Morizot A, Schneider P, Solary E, Micheau O. Differential inhibition of TRAIL-mediated DR5-DISC formation by decoy receptors 1 and 2. Mol Cell Biol. 2006;26:7046–7055. doi: 10.1128/MCB.00520-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino D, Lalaoui N, Morizot A, Solary E, Micheau O. TRAIL in cancer therapy: present and future challenges. Expert Opin Ther Targets. 2007;11:1299–1314. doi: 10.1517/14728222.11.10.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- Micheau O, Lens S, Gaide O, Alevizopoulos K, Tschopp J. NF-kappaB signals induce the expression of c-FLIP. Mol Cell Biol. 2001;21:5299–5305. doi: 10.1128/MCB.21.16.5299-5305.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min YJ, Lee JH, Choi SJ, Chi HS, Lee JS, Kim WK, et al. Prognostic significance of Fas (CD95) and TRAIL receptors (DR4/DR5) expression in acute myelogenous leukemia. Leuk Res. 2004;28:359–365. doi: 10.1016/j.leukres.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Mom CH, Verweij J, Oldenhuis CN, Gietema JA, Fox NL, Miceli R, et al. Mapatumumab, a fully human agonistic monoclonal antibody that targets TRAIL-R1, in combination with gemcitabine and cisplatin: a phase I study. Clin Cancer Res. 2009;15:5584–5590. doi: 10.1158/1078-0432.CCR-09-0996. [DOI] [PubMed] [Google Scholar]
- Morizot A, Merino D, Lalaoui N, Jacquemin G, Granci V, Iessi E, et al. Chemotherapy overcomes TRAIL-R4-mediated TRAIL resistance at the DISC level. Cell Death Differ. 2011;18:700–711. doi: 10.1038/cdd.2010.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagane M, Shimizu S, Mori E, Kataoka S, Shiokawa Y. Predominant antitumor effects by fully human anti-TRAIL-receptor 2 (DR5) monoclonal antibodies in human glioma cells in vitro and in vivo. Neuro-oncol. 2010;12:687–700. doi: 10.1093/neuonc/nop069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natoni A, MacFarlane M, Inoue S, Walewska R, Majid A, Knee D, et al. TRAIL signals to apoptosis in chronic lymphocytic leukaemia cells primarily through TRAIL-R1 whereas cross-linked agonistic TRAIL-R2 antibodies facilitate signalling via TRAIL-R2. Br J Haematol. 2007;139:568–577. doi: 10.1111/j.1365-2141.2007.06852.x. [DOI] [PubMed] [Google Scholar]
- Ndozangue-Touriguine O, Sebbagh M, Merino D, Micheau O, Bertoglio J, Breard J. A mitochondrial block and expression of XIAP lead to resistance to TRAIL-induced apoptosis during progression to metastasis of a colon carcinoma. Oncogene. 2008;27:6012–6022. doi: 10.1038/onc.2008.197. [DOI] [PubMed] [Google Scholar]
- Nesterov A, Nikrad M, Johnson T, Kraft AS. Oncogenic Ras sensitizes normal human cells to tumor necrosis factor-alpha-related apoptosis-inducing ligand-induced apoptosis. Cancer Res. 2004;64:3922–3927. doi: 10.1158/0008-5472.CAN-03-2219. [DOI] [PubMed] [Google Scholar]
- Nguyen DM, Yeow WS, Ziauddin MF, Baras A, Tsai W, Reddy RM, et al. The essential role of the mitochondria-dependent death-signaling cascade in chemotherapy-induced potentiation of Apo2L/TRAIL cytotoxicity in cultured thoracic cancer cells: amplified caspase 8 is indispensable for combination-mediated massive cell death. Cancer J. 2006;12:257–273. doi: 10.1097/00130404-200607000-00004. [DOI] [PubMed] [Google Scholar]
- Nieminen AI, Partanen JI, Hau A, Klefstrom J. c-Myc primed mitochondria determine cellular sensitivity to TRAIL-induced apoptosis. EMBO J. 2007;26:1055–1067. doi: 10.1038/sj.emboj.7601551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigro JM, Baker SJ, Preisinger AC, Jessup JM, Hostetter R, Cleary K, et al. Mutations in the p53 gene occur in diverse human tumour types. Nature. 1989;342:705–708. doi: 10.1038/342705a0. [DOI] [PubMed] [Google Scholar]
- Ogasawara J, Watanabe-Fukunaga R, Adachi M, Matsuzawa A, Kasugai T, Kitamura Y, et al. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364:806–809. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- Ohtsuka T, Buchsbaum D, Oliver P, Makhija S, Kimberly R, Zhou T. Synergistic induction of tumor cell apoptosis by death receptor antibody and chemotherapy agent through JNK/p38 and mitochondrial death pathway. Oncogene. 2003;22:2034–2044. doi: 10.1038/sj.onc.1206290. [DOI] [PubMed] [Google Scholar]
- Pan Y, Xu R, Peach M, Huang C, Branstetter D, Durbin B, et al. Application of pharmacodynamic assays in a phase Ia trial of Apo2L/TRAIL in patients with advanced tumors. J Clin Oncol. 2007;25(18s):abstr 3535. [Google Scholar]
- Pan Y, Xu R, Peach M, Huang CP, Branstetter D, Novotny W, et al. Evaluation of pharmacodynamic biomarkers in a Phase 1a trial of dulanermin (rhApo2L/TRAIL) in patients with advanced tumours. Br J Cancer. 2011;105:1830–1838. doi: 10.1038/bjc.2011.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patnaik A, Wakelee HA, Mita M, Fitzgerald A, Hill M, Fox NL, et al. HGS-ETR2 – a fully human monoclonal antibody to TRAIL-R2: Results of a phase I trial in patients with advanced solid tumors. J Clin Oncol. 2006;24(18s) abstr 3012. [Google Scholar]
- Pavet V, Beyrath J, Pardin C, Morizot A, Lechner MC, Briand JP, et al. Multivalent DR5 peptides activate the TRAIL death pathway and exert tumoricidal activity. Cancer Res. 2010;70:1101–1110. doi: 10.1158/0008-5472.CAN-09-2889. [DOI] [PubMed] [Google Scholar]
- Pepper C, Lin TT, Pratt G, Hewamana S, Brennan P, Hiller L, et al. Mcl-1 expression has in vitro and in vivo significance in chronic lymphocytic leukemia and is associated with other poor prognostic markers. Blood. 2008;112:3807–3817. doi: 10.1182/blood-2008-05-157131. [DOI] [PubMed] [Google Scholar]
- Plummer R, Attard G, Pacey S, Li L, Razak A, Perrett R, et al. Phase 1 and pharmacokinetic study of lexatumumab in patients with advanced cancers. Clin Cancer Res. 2007;13:6187–6194. doi: 10.1158/1078-0432.CCR-07-0950. [DOI] [PubMed] [Google Scholar]
- Pordzik S, Petrovici K, Schmid C, Kroell T, Schweiger C, Kohne CH, et al. Expression and prognostic value of FAS receptor/FAS ligand and TrailR1/TrailR2 in acute myeloid leukemia. Hematology. 2011;16:341–350. doi: 10.1179/102453311X13127324303353. [DOI] [PubMed] [Google Scholar]
- Ravi R, Jain AJ, Schulick RD, Pham V, Prouser TS, Allen H, et al. Elimination of hepatic metastases of colon cancer cells via p53-independent cross-talk between irinotecan and Apo2 ligand/TRAIL. Cancer Res. 2004;64:9105–9114. doi: 10.1158/0008-5472.CAN-04-2488. [DOI] [PubMed] [Google Scholar]
- Reis CR, van der Sloot AM, Szegezdi E, Natoni A, Tur V, Cool RH, et al. Enhancement of antitumor properties of rhTRAIL by affinity increase toward its death receptors. Biochemistry. 2009;48:2180–2191. doi: 10.1021/bi801927x. [DOI] [PubMed] [Google Scholar]
- Riccioni R, Pasquini L, Mariani G, Saulle E, Rossini A, Diverio D, et al. TRAIL decoy receptors mediate resistance of acute myeloid leukemia cells to TRAIL. Haematologica. 2005;90:612–624. [PubMed] [Google Scholar]
- Roberts NJ, Zhou S, Diaz LA, Jr, Holdhoff M. Systemic use of tumor necrosis factor alpha as an anticancer agent. Oncotarget. 2011;2:739–751. doi: 10.18632/oncotarget.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha Lima CM, Bayraktar S, Flores AM, MacIntyre J, Montero A, Baranda JC, et al. Phase Ib study of drozitumab combined with first-line mFOLFOX6 plus bevacizumab in patients with metastatic colorectal cancer. Cancer Invest. 2012;30:727–731. doi: 10.3109/07357907.2012.732163. [DOI] [PubMed] [Google Scholar]
- Rocha Lima CS, Baranda JC, Wallmark J, Choi Y, Royer-Joo S, Portera CC. Phase Ib study of drozitumab combined with first-line FOLFOX plus bevacizumab (BV) in patients (pts) with metastatic colorectal cancer (mCRC) J Clin Oncol. 2011;29(4S):abstr 546. [Google Scholar]
- Rossi CR, Pasquali S, Mocellin S, Vecchiato A, Campana LG, Pilati P, et al. Long-term results of melphalan-based isolated limb perfusion with or without low-dose TNF for in-transit melanoma metastases. Ann Surg Oncol. 2010;17:3000–3007. doi: 10.1245/s10434-010-1104-2. [DOI] [PubMed] [Google Scholar]
- Ruegg C, Yilmaz A, Bieler G, Bamat J, Chaubert P, Lejeune FJ. Evidence for the involvement of endothelial cell integrin alphaVbeta3 in the disruption of the tumor vasculature induced by TNF and IFN-gamma. Nat Med. 1998;4:408–414. doi: 10.1038/nm0498-408. [DOI] [PubMed] [Google Scholar]
- Ruiz de Almodovar C, Lopez-Rivas A, Redondo JM, Rodriguez A. Transcription initiation sites and promoter structure of the human TRAIL-R3 gene. FEBS Lett. 2002;531:304–308. doi: 10.1016/s0014-5793(02)03544-5. [DOI] [PubMed] [Google Scholar]
- Sanlioglu AD, Karacay B, Koksal IT, Griffith TS, Sanlioglu S. DcR2 (TRAIL-R4) siRNA and adenovirus delivery of TRAIL (Ad5hTRAIL) break down in vitro tumorigenic potential of prostate carcinoma cells. Cancer Gene Ther. 2007;14:976–984. doi: 10.1038/sj.cgt.7701087. [DOI] [PubMed] [Google Scholar]
- Schneider P, Holler N, Bodmer JL, Hahne M, Frei K, Fontana A, et al. Conversion of membrane-bound Fas(CD95) ligand to its soluble form is associated with downregulation of its proapoptotic activity and loss of liver toxicity. J Exp Med. 1998;187:1205–1213. doi: 10.1084/jem.187.8.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar S, Srivastava RK. Enhancement of therapeutic potential of TRAIL by cancer chemotherapy and irradiation: mechanisms and clinical implications. Drug Resist Updat. 2004;7:139–156. doi: 10.1016/j.drup.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Shankar S, Chen X, Srivastava RK. Effects of sequential treatments with chemotherapeutic drugs followed by TRAIL on prostate cancer in vitro and in vivo. Prostate. 2005;62:165–186. doi: 10.1002/pros.20126. [DOI] [PubMed] [Google Scholar]
- Sharma S, de Vries E, Infante JR, Oldenhuis CN, Chiang L, Bilic S, et al. Phase I trial of LBY135, a monoclonal antibody agonist to DR5, alone and in combination with capecitabine in advanced solid tumors. J Clin Oncol. 2008;26(20s):abstr 3538. [Google Scholar]
- Shirley S, Morizot A, Micheau O. Regulating TRAIL receptor-induced cell death at the membrane: a deadly discussion. Recent Pat Anticancer Drug Discov. 2011;6:311–323. doi: 10.2174/157489211796957757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikic BI, Wakelee HA, von Mehren M, Lewis D, Calvert H, Plummer R, et al. A phase Ib study to assess the safety of lexatumumab, a human monoclonal antibody that activates TRAIL-R2, in combination with gemcitabine, pemetrexed, doxorubicin or FOLFIRI. J Clin Oncol. 2007;25(18s):abstr 14006. [Google Scholar]
- Singh TR, Shankar S, Chen X, Asim M, Srivastava RK. Synergistic interactions of chemotherapeutic drugs and tumor necrosis factor-related apoptosis-inducing ligand/Apo-2 ligand on apoptosis and on regression of breast carcinoma in vivo. Cancer Res. 2003;63:5390–5400. [PubMed] [Google Scholar]
- van der Sloot AM, Tur V, Szegezdi E, Mullally MM, Cool RH, Samali A, et al. Designed tumor necrosis factor-related apoptosis-inducing ligand variants initiating apoptosis exclusively via the DR5 receptor. Proc Natl Acad Sci U S A. 2006;103:8634–8639. doi: 10.1073/pnas.0510187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son YG, Kim EH, Kim JY, Kim SU, Kwon TK, Yoon AR, et al. Silibinin sensitizes human glioma cells to TRAIL-mediated apoptosis via DR5 up-regulation and down-regulation of c-FLIP and survivin. Cancer Res. 2007;67:8274–8284. doi: 10.1158/0008-5472.CAN-07-0407. [DOI] [PubMed] [Google Scholar]
- Soria JC, Smit E, Khayat D, Besse B, Yang X, Hsu CP, et al. Phase 1b study of dulanermin (recombinant human Apo2L/TRAIL) in combination with paclitaxel, carboplatin, and bevacizumab in patients with advanced non-squamous non-small-cell lung cancer. J Clin Oncol. 2010;28:1527–1533. doi: 10.1200/JCO.2009.25.4847. [DOI] [PubMed] [Google Scholar]
- Soria JC, Mark Z, Zatloukal P, Szima B, Albert I, Juhasz E, et al. Randomized phase II study of dulanermin in combination with paclitaxel, carboplatin, and bevacizumab in advanced non-small-cell lung cancer. J Clin Oncol. 2011;29:4442–4451. doi: 10.1200/JCO.2011.37.2623. [DOI] [PubMed] [Google Scholar]
- Stadel D, Mohr A, Ref C, MacFarlane M, Zhou S, Humphreys R, et al. TRAIL-induced apoptosis is preferentially mediated via TRAIL receptor 1 in pancreatic carcinoma cells and profoundly enhanced by XIAP inhibitors. Clin Cancer Res. 2010;16:5734–5749. doi: 10.1158/1078-0432.CCR-10-0985. [DOI] [PubMed] [Google Scholar]
- Strater J, Hinz U, Walczak H, Mechtersheimer G, Koretz K, Herfarth C, et al. Expression of TRAIL and TRAIL receptors in colon carcinoma: TRAIL-R1 is an independent prognostic parameter. Clin Cancer Res. 2002;8:3734–3740. [PubMed] [Google Scholar]
- Sun W, Nelson D, Alberts SR, Poordad F, Leong S, Teitelbaum UR, et al. Phase Ib study of mapatumumab in combination with sorafenib in patients with advanced hepatocellular carcinoma (HCC) and chronic viral hepatitis. J Clin Oncol. 2011;29(4s):abstr 261. [Google Scholar]
- Surget S, Chiron D, Gomez-Bougie P, Descamps G, Menoret E, Bataille R, et al. Cell death via DR5, but not DR4, is regulated by p53 in myeloma cells. Cancer Res. 2012;72:4562–4573. doi: 10.1158/0008-5472.CAN-12-0487. [DOI] [PubMed] [Google Scholar]
- Szegezdi E, van der Sloot AM, Mahalingam D, O'Leary L, Cool RH, Munoz IG, et al. Kinetics in signal transduction pathways involving promiscuous oligomerizing receptors can be determined by receptor specificity: apoptosis induction by TRAIL. Mol Cell Proteomics. 2012;11:M111.013730. doi: 10.1074/mcp.M111.013730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takimoto R, El-Deiry WS. Wild-type p53 transactivates the KILLER/DR5 gene through an intronic sequence-specific DNA-binding site. Oncogene. 2000;19:1735–1743. doi: 10.1038/sj.onc.1203489. [DOI] [PubMed] [Google Scholar]
- Tang CH, Liu XQ, Yang SF, Zhu B, Gao HJ. Inhibitory effects of a combination of rmhTRAIL and gemcitabine on human non-small cell lung cancer cell line NCI-H460 cells in vitro and in vivo. Zhonghua Zhong Liu Za Zhi. 2008;30:808–812. [PubMed] [Google Scholar]
- Tang YM, Yang SF, Zhu B, Cui JS. Therapeutic effects of recombinant mutant human tumor necrosis factor-related apoptosis-inducing ligand on non-small lung cell cancer: an experimental with rats. Zhonghua Yi Xue Za Zhi. 2005;85:2021–2025. [PubMed] [Google Scholar]
- Tolcher AW. Targeting Bcl-2 protein expression in solid tumors and hematologic malignancies with antisense oligonucleotides. Clin Adv Hematol Oncol. 2005;3:635–642, 662. [PubMed] [Google Scholar]
- Tolcher AW, Mita M, Meropol NJ, von Mehren M, Patnaik A, Padavic K, et al. Phase I pharmacokinetic and biologic correlative study of mapatumumab, a fully human monoclonal antibody with agonist activity to tumor necrosis factor-related apoptosis-inducing ligand receptor-1. J Clin Oncol. 2007;25:1390–1395. doi: 10.1200/JCO.2006.08.8898. [DOI] [PubMed] [Google Scholar]
- Toscano F, Fajoui ZE, Gay F, Lalaoui N, Parmentier B, Chayvialle JA, et al. P53-mediated upregulation of DcR1 impairs oxaliplatin/TRAIL-induced synergistic anti-tumour potential in colon cancer cells. Oncogene. 2008;27:4161–4171. doi: 10.1038/onc.2008.52. [DOI] [PubMed] [Google Scholar]
- Trarbach T, Moehler M, Heinemann V, Kohne CH, Przyborek M, Schulz C, et al. Phase II trial of mapatumumab, a fully human agonistic monoclonal antibody that targets and activates the tumour necrosis factor apoptosis-inducing ligand receptor-1 (TRAIL-R1), in patients with refractory colorectal cancer. Br J Cancer. 2010;102:506–512. doi: 10.1038/sj.bjc.6605507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Veen AH, de Wilt JH, Eggermont AM, van Tiel ST, Seynhaeve AL, Ten Hagen TL. TNF-alpha augments intratumoural concentrations of doxorubicin in TNF-alpha-based isolated limb perfusion in rat sarcoma models and enhances anti-tumour effects. Br J Cancer. 2000;82:973–980. doi: 10.1054/bjoc.1999.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbrugge I, Wissink EH, Rooswinkel RW, Jongsma J, Beltraminelli N, Dupuis M, et al. Combining radiotherapy with APO010 in cancer treatment. Clin Cancer Res. 2009;15:2031–2038. doi: 10.1158/1078-0432.CCR-08-2125. [DOI] [PubMed] [Google Scholar]
- Verbrugge I, Maas C, Heijkoop M, Verheij M, Borst J. Radiation and anticancer drugs can facilitate mitochondrial bypass by CD95/Fas via c-FLIP downregulation. Cell Death Differ. 2010;17:551–561. doi: 10.1038/cdd.2009.141. [DOI] [PubMed] [Google Scholar]
- Von Pawel J, Harvey JH, Spigel DR, Dediu M, Reck M, Cebotaru CL, et al. A randomized phase II trial of mapatumumab, a TRAIL-R1 agonist monoclonal antibody, in combination with carboplatin and paclitaxel in patients with advanced NSCLC. J Clin Oncol. 2010;28(18s):abstr LBA7501. doi: 10.1016/j.cllc.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Wagner KW, Punnoose EA, Januario T, Lawrence DA, Pitti RM, Lancaster K, et al. Death-receptor O-glycosylation controls tumor-cell sensitivity to the proapoptotic ligand Apo2L/TRAIL. Nat Med. 2007;13:1070–1077. doi: 10.1038/nm1627. [DOI] [PubMed] [Google Scholar]
- Wakelee HA, Patnaik A, Sikic BI, Mita M, Fox NL, Miceli R, et al. Phase I and pharmacokinetic study of lexatumumab (HGS-ETR2) given every 2 weeks in patients with advanced solid tumors. Ann Oncol. 2010;21:376–381. doi: 10.1093/annonc/mdp292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M, et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999;5:157–163. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- Walczak H, Bouchon A, Stahl H, Krammer PH. Tumor necrosis factor-related apoptosis-inducing ligand retains its apoptosis-inducing capacity on Bcl-2- or Bcl-xL-overexpressing chemotherapy-resistant tumor cells. Cancer Res. 2000;60:3051–3057. [PubMed] [Google Scholar]
- Wang S, El-Deiry WS. Inducible silencing of KILLER/DR5 in vivo promotes bioluminescent colon tumor xenograft growth and confers resistance to chemotherapeutic agent 5-fluorouracil. Cancer Res. 2004;64:6666–6672. doi: 10.1158/0008-5472.CAN-04-1734. [DOI] [PubMed] [Google Scholar]
- Wang YR, Wen SP, Wang FX, Wen L, Yang BY, Yang JC, et al. Apoptosis of the adriamycin-resistant leukemia cell line induced by the recombinant mutant human TNF-related apoptosis-inducing ligand combined with arsenic trioxide. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2008;16:1055–1059. [PubMed] [Google Scholar]
- Weber A, Kirejczyk Z, Besch R, Potthoff S, Leverkus M, Hacker G. Proapoptotic signalling through Toll-like receptor-3 involves TRIF-dependent activation of caspase-8 and is under the control of inhibitor of apoptosis proteins in melanoma cells. Cell Death Differ. 2010;17:942–951. doi: 10.1038/cdd.2009.190. [DOI] [PubMed] [Google Scholar]
- Wittebol S, Ferrant A, Wickham NW, Fehrenbacher L, Durbin-Johnson B, Bray G. Phase II study of PRO95780 plus rituximab in patients with relapsed follicular non-Hodgkin's lymphoma (NHL) J Clin Oncol. 2010;28(15s):abstr e18511. [Google Scholar]
- Wu GS, Burns TF, McDonald ER, 3rd, Jiang W, Meng R, Krantz ID, et al. KILLER/DR5 is a DNA damage-inducible p53-regulated death receptor gene. Nat Genet. 1997;17:141–143. doi: 10.1038/ng1097-141. [DOI] [PubMed] [Google Scholar]
- Xin Y, Tohnya TM, Herbst RS, Mendelson DS, Eckhardt SG, O'Dwyer PJ, et al. Population pharmacokinetic (PPK) analysis of recombinant human Apo2L/TRAIL (rhApo2L/TRAIL) in a Phase 1a Study in advanced cancer and lymphoma. J Clin Oncol. 2008;26(20S):abstr 2525. [Google Scholar]
- Yada A, Yazawa M, Ishida S, Yoshida H, Ichikawa K, Kurakata S, et al. A novel humanized anti-human death receptor 5 antibody CS-1008 induces apoptosis in tumor cells without toxicity in hepatocytes. Ann Oncol. 2008;19:1060–1067. doi: 10.1093/annonc/mdn015. [DOI] [PubMed] [Google Scholar]
- Yee L, Fanale M, Dimick K, Calvert S, Robins C, Ing J, et al. A phase IB safety and pharmacokinetic (PK) study of recombinant human Apo2L/TRAIL in combination with rituximab in patients with low-grade non-Hodgkin lymphoma. J Clin Oncol. 2007;25(18S):abstr 8078. [Google Scholar]
- Yee L, Burris HA, Kozloff M, Wainberg Z, Pao M, Skettino S, et al. Phase Ib study of recombinant human Apo2L/TRAIL plus irinotecan and cetuximab or FOLFIRI in metastatic colorectal cancer (mCRC) patients (pts): preliminary results. J Clin Oncol. 2009;27(15s):abstr 4129. [Google Scholar]
- Younes A, Vose JM, Zelenetz AD, Smith MR, Burris HA, Ansell SM, et al. A Phase 1b/2 trial of mapatumumab in patients with relapsed/refractory non-Hodgkin's lymphoma. Br J Cancer. 2010;103:1783–1787. doi: 10.1038/sj.bjc.6605987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XJ, Wen L, Wang FX, Luo JM, Pan L, Liu XJ, et al. Combined effect of recombinant mutant human TRAIL and daunorubicin in inducing apoptosis of leukemia cell and its mechanism. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2006;14:1123–1128. [PubMed] [Google Scholar]
- Zhu H, Liu XW, Ding WJ, Xu DQ, Zhao YC, Lu W, et al. Up-regulation of death receptor 4 and 5 by celastrol enhances the anti-cancer activity of TRAIL/Apo-2L. Cancer Lett. 2010;297:155–164. doi: 10.1016/j.canlet.2010.04.030. [DOI] [PubMed] [Google Scholar]
- Zinonos I, Labrinidis A, Lee M, Liapis V, Hay S, Ponomarev V, et al. Apomab, a fully human agonistic antibody to DR5, exhibits potent antitumor activity against primary and metastatic breast cancer. Mol Cancer Ther. 2009;8:2969–2980. doi: 10.1158/1535-7163.MCT-09-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]