Abstract
Background and Purpose
Excitatory amino acid transporters (EAATs) in the CNS contribute to the clearance of glutamate released during neurotransmission. The aim of this study was to explore the role of EAATs in the regulation of locus coeruleus (LC) neurons by glutamate.
Experimental Approach
We measured the effect of different EAAT subtype inhibitors/enhancers on glutamate- and KCl-induced activation of LC neurons in rat slices. EAAT2–3 expression in the LC was also characterized by immunohistochemistry.
Key Results
The EAAT2–5 inhibitor DL-threo-β-benzyloxaspartic acid (100 μM), but not the EAAT2, 4, 5 inhibitor L-trans-pyrrolidine-2,4-dicarboxylic acid (100 μM) or the EAAT2 inhibitor dihydrokainic acid (DHK; 100 μM), enhanced the glutamate- and KCl-induced activation of the firing rate of LC neurons. These effects were blocked by ionotropic, but not metabotrobic, glutamate receptor antagonists. DHK (100 μM) was the only EAAT inhibitor that increased the spontaneous firing rate of LC cells, an effect that was due to inhibition of EAAT2 and subsequent AMPA receptor activation. Chronic treatment with ceftriaxone (200 mg·kg−1 i.p., once daily, 7 days), an EAAT2 expression enhancer, increased the actions of glutamate and DHK, suggesting a functional impact of EAAT2 up-regulation on the glutamatergic system. Immuhistochemical data revealed the presence of EAAT2 and EAAT3 surrounding noradrenergic neurons and EAAT2 on glial cells in the LC.
Conclusions and Implications
These results remark the importance of EAAT2 and EAAT3 in the regulation of rat LC by glutamate. Neuronal EAAT3 would be responsible for terminating the action of synaptically released glutamate, whereas glial EAAT2 would regulate tonic glutamate concentrations in this nucleus.
Keywords: locus coeruleus, EAAT2, EAAT3, KCl, glutamate, firing
Introduction
Glutamate is the main excitatory neurotransmitter in the mammalian CNS. Excitatory amino acid transporters (EAATs) contribute to the clearance of glutamate released during neurotransmission so that extracellular glutamate concentrations can be maintained below excitotoxic levels. The specificity of excitatory synaptic signalling depends on the diffusion of glutamate away from active synapses and the robust uptake capacity of the transporters. Five EAAT subtypes have been cloned up to now: EAAT1 (SLC1A3), EAAT2 (SLC1A2), EAAT3 (SLC1A1), EAAT4 (SLC1A6) and EAAT5 (SLC1A7). In the rodent, EAAT1–4 are located in different brain areas whereas EAAT5 is restricted to the retina (Rothstein et al., 1994; Bunch et al., 2009). At the cellular level, EAAT1 and EAAT2 have been shown to be expressed primarily by astrocytes. EAAT3 is located in neurons throughout the brain and EAAT4 is almost exclusively expressed by cerebellar Purkinje cells (Fairman et al., 1995; Furuta et al., 1997). Recent interest on EAATs has emerged from the fact that regulation of extracellular glutamate levels by these transporters may affect some physiological functions (e.g. neuronal plasticity) and certain neuropsychiatric disorders including drug dependence (Sekiya et al., 2004; Fujio et al., 2005), epilepsy (Rakhade and Loeb, 2008) or Alzheimer's disease (Nieoullon et al., 2006).
The locus coeruleus (LC) is the primary noradrenergic nucleus in the CNS. It is involved in the control of different behavioural functions including attention, wake–sleep cycle or memory (Aston-Jones, 2005) and the mechanisms of various neuropsychiatric disorders (Berridge and Waterhouse, 2003). The main glutamatergic afferent to the LC arises from the lateral nucleus paragigantocellularis in the medulla (Ennis et al., 1992), which regulates the firing activity of these neurons. Different subunits of ionotropic glutamate receptors (iGluR) have been described in the LC (Sato et al., 1993; Wisden and Seeburg, 1993; Petralia et al., 1994), although glutamate seems to activate the LC majorly through AMPA/kainate iGluR (Cherubini et al., 1988; Ennis et al., 1992). Furthermore, a post-activation inhibition mediated by AMPA iGluR has been described in the LC (Zamalloa et al., 2009). LC neurons are known to fire slowly and regularly (tonic mode) when glutamatergic afferents to the LC are inactive, such as during vegetative behaviours. However, LC neurons are prone to discharge in bursts (phasic mode) when glutamatergic afferents are physiologically active, such as during vigilant and focused behaviours (Aston-Jones et al., 1986; Aston-Jones and Cohen, 2005). Hyperactivity of LC cells due to a glutamate up-regulation has been also linked to certain pathophysiological states, such as opiate withdrawal (Rasmussen et al., 1991; Van Bockstaele et al., 2001). Few data have been yet reported on the characterization of EAATs in the LC. Therefore, the aim of the present work was to characterize by single-unit extracellular techniques in vitro, the functional role of EAAT subtypes in the rat LC. We also studied, by immunohistochemical techniques, the expression of different EAAT subtypes in the LC.
Methods
Animals and treatments
A total of 108 adult male Sprague–Dawley rats weighing 200–300 g were housed under standard laboratory conditions (22°C, 12:12 h light/dark cycles) with free access to food and water. All experimental procedures reported in this manuscript were carried out in accordance with UK Animals (Scientific Procedures) Act, 1986, and associated guidelines (86/609/EEC) and approved by the Animal Care and Use Committee of the University of the Basque Country. All efforts were made to minimize the suffering of the animals and to reduce the number of animals used. Animals were obtained from the animal house of the University of the Basque Country (Leioa, Spain). To induce an increase in the expression of EAAT2, some rats were injected with ceftriaxone (200 mg·kg−1, i.p.) or its vehicle (saline) once daily for 7 days (Rothstein et al., 2005). All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010).
In vitro electrophysiology: brain slice preparation and extracellular recordings
Animals were anaesthetized with chloral hydrate (400 mg·kg−1 i.p.), and a block of tissue containing the brainstem was rapidly extracted. Coronal slices of 500–600 μm thickness containing the LC were cut using a vibratome. The tissue was allowed to recover from the slicing for 90 min in a modified Haas-type interface chamber continuously perfused with artificial CSF (aCSF) at 33°C, saturated with 95% O2/5% CO2 (final pH = 7.34), at a flow rate of 1.5 mL·min−1. The aCSF contained (in mM): NaCl 126, KCl 3, NaH2PO4 1.25, glucose 10, NaHCO3 25, CaCl2 2 and MgSO4 2. Single-unit extracellular recordings of LC cells were made as described (Mendiguren and Pineda, 2004). The recording electrode was an Omegadot glass micropipette (Sutter Instrument Co., Novato, CA, USA) pulled and filled with NaCl (0.05 M) (tip size of 2–5 μm, 3–5 MΩ). The electrode was placed in the LC, which was identified visually in the rostral pons as a dark oval area on the lateral borders of the central grey and the fourth ventricle, just anterior to the genu of the facial nerve. The extracellular signal from the electrode was passed through a high-input impedance amplifier and monitored on an oscilloscope and an audio unit. Individual neuronal spikes were isolated from the background noise with a window discriminator. The firing rate was analysed by means of a PC-based custom-made programme, which generated histogram bars representing the cumulative number of spikes in consecutive 10 s bins (HFCP®, Cibertec S.A., Madrid, Spain). Noradrenergic cells were identified by their spontaneous and regular discharge activity, the slow firing rate and the long-lasting, positive-negative waveform (Andrade and Aghajanian, 1984).
Pharmacological procedures
To characterize the functional role of EAATs in the total glutamate uptake, we tested the excitatory effect of glutamate (0.3 mM, 30 s). To explore the role of EAATs in the re-uptake of synaptically released glutamate, we measured the activation induced by the depolarizing agent KCl (30 mM, 30–60 s) in the presence of the GABAA receptor antagonist picrotoxin (100 μM) (Mendiguren and Pineda, 2007). In these assays, the aCSF contained a lower concentration of NaCl, which was equiosmotically substituted for KCl. As described, the duration of the perfusion was adjusted at the beginning of each experiment to obtain a reproducible effect of glutamate and KCl (Mendiguren and Pineda, 2007; Zamalloa et al., 2009). Finally, to study the importance of EAATs in the re-uptake of tonic or basal levels of glutamate, we monitored the spontaneous firing activity of LC cells. We performed some assays in the presence of 6-cyano-7-notroquinoxaline-2 (CNQX, a non-NMDA iGluR antagonist; 30 μM), D-(–)-2-amin-5-phosponopentanoic acid (D-AP5, an NMDA receptor antagonist; 100 μM), RS-methyl-4-carboxyphenylglycine [RS-MCPG, a non-selective metabotropic glutamate receptor (mGluR) antagonist; 0.5 mM] or 8-chloro-2-methyl-11H-imidazo[1,2-c][2,3]benzodiazepin-6-benzeneamine dihydrochloride (GYKI 52466, an AMPA receptor antagonist; 30 or 100 μM) to confirm the involvement of GluR in these effects (Zamalloa et al., 2009).
To explore the involvement of EAAT subtypes on the glutamate and KCl-induced activation, we used the following EAAT inhibitors/enhancers: alphaxalone (EAAT3 enhancer; 10 μM), carbamazepine (EAAT3 enhancer; 10 μM), dihydrokainic acid (DHK, EAAT2 inhibitor; 10–100 μM), nicergoline (EAAT2 and EAAT3 enhancer; 10 μM), L-trans-pyrrolidine-2,4-dicarboxylic acid (t-PDC, EAAT2, 4, 5 inhibitor; 10–100 μM), riluzole (EAAT1-3 enhancer; 10 μM) and DL-threo-β-benzyloxaspartic acid (DL-TBOA, EAAT2-5 inhibitor; 10–100 μM). All drugs were perfused for at least 10 min before testing the effects of glutamate or KCl. Control assays were performed with equivalent volumes of the vehicles in which the drugs were dissolved.
Immunohistochemistry: surgery, EAAT immunolabelling and microscope studies
Animals were anaesthetized with an intramuscular injection of ketamine chlorhydrate (36 mg·kg−1) and xylazine (6.2 mg·kg−1, Rompun®, Bayer, Barcelona, Spain). Once analgesia was achieved, they were transcardially perfused with PBS 0.1 M, pH 7.4, followed by periodate-lysine-paraformaldehyde fixative solution (2% paraformaldehyde, 0.075 M lysine, 0.037 M sodium phosphate, 0.01 M periodate). Thereafter, the brain was removed, post-fixed during 4 h in the same fixative and then cryoprotected in 15% sucrose in PBS. From each brain block containing LC, coronal sections of 20 μm were made, and they were mounted on gelatin-coated slides. The sections were blocked for 1 h with 5% normal goat serum (NGS) in PBS followed by an overnight incubation at 4°C with primary antibodies rabbit anti-TH (1:500, AB152) or rabbit anti-glial fibrillary acidic protein (GFAP) (1:3000, Z0334), and guinea pig anti-EAAT2 (1:3000, AB1783) or mouse anti-EAAT3 (1 mg·mL−1, MAB1587) diluted in PBS containing 0.1% Triton X-100 and 2% NGS. Subsequently, the sections were washed in PBS and incubated with secondary antibody goat anti-rabbit Alexa488, goat anti-guinea pig Alexa594 or goat anti-mouse Alexa594, respectively, at a dilution of 1:200 in PBS. The sections were coverslipped with Vectastain mounting medium (Vector Laboratories, Burlingame, CA, USA) and were examined and photographed with a confocal laser microscope (SP2, Leica Microsystems, Buffalo Grove, IL, USA). The contrast and brightness of the figures were adjusted by using Adobe Photoshop CS3 Extended software (Adobe Systems Software, Dublin, Ireland).
Data analysis and statistics
The excitatory effect of glutamate or KCl was calculated by subtracting the average baseline firing rate (recorded during 2 min before drug application) from the peak firing rate value after drug perfusion. In each neuron, we calculated and then averaged the effects elicited by three consecutive applications of glutamate or KCl, before and after perfusion with the EAAT-targeted drugs. Analyses of the results were done by GraphPad Prism (version 5.0 for Windows, GraphPad Software, Inc., San Diego, CA, USA). Data are given as mean ± SEM. Statistical evaluation was carried out by a paired Student's t-test when the effects before and after drug application were compared within the same cell or by a two-sample Student's t-test when two independent groups were compared. The level of significance was set at P < 0.05.
Drugs and reagents
The following drugs were purchased from Tocris Bioscience (Bristol, UK): alphaxalone, D-AP5, 8-chloro-2-methyl-11H-imidazo[1,2-c][2,3]benzodiazepin-6-benzeneamine dihydrochloride (GYKI 52466), CNQX, DHK, RS-MCPG, nicergoline, DL-TBOA and t-PDC. The following drugs were purchased from Sigma-Aldrich Química S.A. (Madrid, Spain): AMPA, carbamazepine, chloral hydrate, L-glutamic acid, kainate, ketamine chlorhydrate, picrotoxin and riluzole. Ceftriaxone was obtained from Sala laboratories (Barcelona, Spain). NGS and Vectastain mounting medium were purchased from Vector Laboratories. Xylazine (Rompun) was obtained from Bayer. Primary rabbit anti-TH (AB152), guinea pig anti-EAAT2 (AB1783) and mouse anti-EAAT3 (MAB1587) were purchased from Millipore Iberica (Madrid, Spain) while anti-cow GFAP (Z0334) was purchased from DAKO (Glostrup, Denmark). Finally, secondary antibodies goat anti-rabbit Alexa488, goat anti-guinea pig Alexa594 and goat anti-mouse Alexa594 were obtained from Invitrogen (Barcelona, Spain). Picrotoxin, ceftriaxone and RS-MCPG were directly dissolved in aCSF and NaCl (0.9 %) respectively. Stock solutions were prepared in Milli-Q water, and then diluted in aCSF to their final concentration just before each application. Riluzole stock was prepared in dimethylsulfoxide (DMSO); the final concentration of DMSO in the perfusion fluid was <0.1 %.
Our drug/molecular target nomenclature conforms to the British Journal of Pharmacology's Guide to receptors and channels (Alexander et al., 2011).
Results
Effect of EAAT inhibitors on glutamate-induced activation of LC neurons
LC neurons discharged spontaneously at a regular and slow rate within the normal range of in vitro preparations (1.05 ± 0.05 Hz, n = 76). As expected, perfusion with glutamate (0.3 mM) strongly increased the firing rate of LC neurons (sevenfold increase, n = 25, P < 0.005); the effect was rapidly reversible and reproducible over time upon several applications of glutamate (Figure 1A). In order to evaluate the functional role of EAATs in the removal of glutamate in the LC, we studied the effect of the following EAAT inhibitors on glutamate-induced activation: DL-TBOA, t-PDC and DHK. Perfusion with the EAAT2–5 inhibitor DL-TBOA (10 and 100 μM) enhanced in a concentration-related manner (P < 0.005) the activation of LC neurons induced by glutamate (increase: 26 ± 10%, n = 4, P < 0.05 and 65 ± 7%, n = 4, P < 0.005, respectively) (Table 1; Figure 1A, C). The non-selective mGluR antagonist RS-MCPG (0.5 mM) failed to change the enhancing effect of DL-TBOA (100 μM) on glutamate-induced activation (increase: 49 ± 10%, n = 6, P < 0.005; non-significant vs. DL-TBOA effect without RS-MCPG) (Table 1). This indicates that mGluRs do not seem to be involved in the effect of EAAT inhibition, and only iGluRs would take part in this process. The EAAT2, 4, 5 inhibitor t-PDC (10–100 μM) enhanced by a lesser extent the glutamate-induced activation (increase: 11 ± 5%, n = 4, N.S. and 25 ± 5%, n = 5, P < 0.01, respectively) (Table 1; Figure 1C). Perfusion with the EAAT2 inhibitor DHK (10 and 100 μM) did not significantly change the glutamate-induced excitatory effect (n = 4 and n = 12, respectively) (Table 1; Figure 1C). These results support a role of specific EAATs in the clearance of high concentrations of glutamate in the LC.
Figure 1.
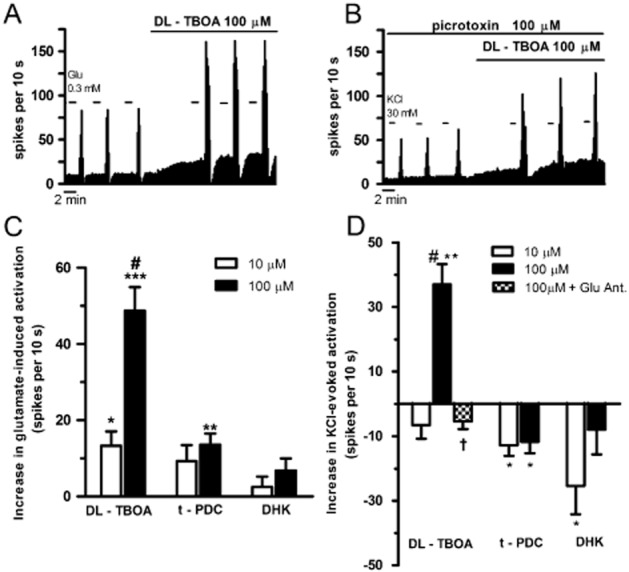
Effect of different EAAT inhibitors on glutamate-induced activation and KCl-evoked response on the firing rate of LC neurons. (A) Representative example of the firing rate recording of a neuron showing the enhancement of glutamate-induced activation (0.3 mM) by the EAAT2-5 inhibitor DL-TBOA (100 μM). (B) Representative example of the firing rate recording of a neuron showing the enhancement of KCl-evoked activation (30 mM) by DL-TBOA (100 μM) in the continuous presence of picrotoxin (100 μM). The vertical lines represent the integrated firing rates (spikes per 10 s). Drugs were bath applied at the concentration and for the time indicated by the horizontal bars. (C) Bar histograms representing the mean ± SEM of the increase in glutamate-induced activation by DL-TBOA (10 and 100 μM, n = 4 and n = 4, respectively), t-PDC (10 and 100 μM, n = 4 and n = 5, respectively) or DHK (10 and 100 μM, n = 4 and n = 12, respectively). *P < 0.05, **P < 0.01, ***P < 0.005 compared with glutamate-induced activation in the absence of EAAT inhibitors by a paired Student's t-test. #P < 0.005 compared with the enhancement of glutamate-induced activation produced by DL-TBOA (10 μM) by a two-sample Student's t-test. (D) Bar histograms showing the mean ± SEM of the increase in KCl-evoked activation of LC neurons (in the continuous presence of picrotoxin; 100 μM) after perfusion with DL-TBOA (10 and 100 μM, n = 3 and n = 5, respectively), DL-TBOA (100 μM) + D-AP5 (100 μM) + CNQX (30 μM) (n = 4), t-PDC (10 and 100 μM, n = 4 and n = 5, respectively) or DHK (10 and 100 μM, n = 5 and n = 4, respectively). *P < 0.05, **P < 0.005 compared with KCl-evoked activation before EAAT inhibitors by a paired Student's t-test. †P < 0.005 compared with KCl-evoked effect in the absence of iGluR antagonists D-AP5 (100 μM) and CNQX (30 μM) by a two-sample Student's t-test. #P < 0.005 compared with the enhancement of KCl-evoked activation produced by DL-TBOA (10 μM) (two-sample Student's t-test). Note that DL-TBOA increases in a concentration-dependent manner glutamate-induced and KCl-evoked activation of LC cells. The enhancement of KCl-evoked effect produced by DL-TBOA was blocked by iGluR antagonists.
Table 1.
Glutamate-induced activation before and after administration of EAAT inhibitors/enhancers in LC neurons from rat brain slices
| Drug | Before drug (spikes per 10 s) | After drug (spikes per 10 s) | n |
|---|---|---|---|
| Inhibitor | |||
| DL-TBOA | |||
| 10 μM | 63.0 ± 12.2 | 76.3 ± 9.8* | 4 |
| 100 μM | 75.4 ± 7.1 | 124.0 ± 11.3*** | 4 |
| 100 μM + RS-MCPG (0.5 mM) | 85.6 ± 11.0 | 127.1 ± 16.5*** | 6 |
| t-PDC | |||
| 10 μM | 68.6 ± 19.4 | 77.8 ± 23.5 | 4 |
| 100 μM | 56.7 ± 10.6 | 70.3 ± 12.6** | 5 |
| DHK | |||
| 10 μM | 54.7 ± 4.9 | 57.2 ± 3.5 | 4 |
| 100 μM | 65.1 ± 7.5 | 71.9 ± 7.1 | 12 |
| Enhancer | |||
| Riluzole 10 μM | 80.8 ± 11.7 | 64.4 ± 8.2* | 4 |
| Alphaxalone 10 μM | 144.0 ± 14.7 | 147.3 ± 11.8 | 4 |
| Carbamacepine 10 μM | 102.4 ± 10.4 | 111.4 ± 17.3 | 3 |
| Nicergoline 10 μM | 87.1 ± 9.0 | 113.6 ± 23.5 | 2 |
Data represent the mean ± SEM of glutamate-induced activation before and after perfusion with several EAAT inhibitors or enhancers at different concentrations (10 and 100 μM).
P < 0.05,
P < 0.01,
P < 0.005 compared with glutamate-induced activation before EAAT inhibitor or EAAT enhancer perfusion by a paired Student's t-test.
Effect of EAAT inhibitors on KCl-evoked activation of LC neurons
To characterize the relevance of EAAT subtypes in removing the depolarization-released glutamate from the synaptic cleft, we studied the effect of DL-TBOA, t-PDC and DHK on KCl-evoked activation of LC cells in the presence of the GABAA receptor antagonist picrotoxin (100 μM) (Mendiguren and Pineda, 2007). KCl (30 mM) perfusion raised by about sixfold the firing rate of LC cells (n = 21, P < 0.005), an effect that was reversible and reproducible over three consecutive applications of KCl (Figure 1B). Perfusion with the highest concentration of the EAAT2–5 inhibitor DL-TBOA (100 μM), but not with the lower concentration (10 μM) (n = 3), enhanced the KCl-evoked activation (increase: 65 ± 14%, n = 5, P < 0.005) (Table 2; Figure 1B, D). The effect of DL-TBOA was likely due to glutamate and the subsequent activation of iGluR, because DL-TBOA (100 μM) failed to enhance KCl-evoked activation in the presence of the NMDA receptor and non-NMDA receptor antagonists D-AP5 (100 μM) and CNQX (30 μM) (KCl-evoked effect in the presence of iGluR antagonists, before DL-TBOA: 7.1 ± 2.0 Hz, and after DL-TBOA: 6.5 ± 2.2 Hz, n = 4, P = 0.11) (Figure 1D). Perfusion with t-PDC (10 and 100 μM) decreased the KCl-evoked activation, but this effect was small and unrelated to the concentration used (decrease: 23 ± 6%, n = 4, P < 0.05 and 24 ± 7%, n = 5, P < 0.05, respectively) (Table 2; Figure 1D). Likewise, a low concentration of the EAAT2 inhibitor DHK (10 μM), but not a high concentration (100 μM) (n = 4), decreased the KCl-evoked activation (decrease: 26 ± 7%, n = 5, P < 0.05) (Table 2; Figure 1D). These data suggest that specific EAATs may contribute to the clearance of synaptic concentrations of glutamate in the LC.
Table 2.
KCl-evoked activation before and after administration of EAAT inhibitors in LC neurons from rat brain slices
| Drug | Before drug (spikes per 10 s) | After drug (spikes per 10 s) | n |
|---|---|---|---|
| DL-TBOA | |||
| 10 μM | 78.9 ± 16.4 | 72.3 ± 16.8 | 3 |
| 100 μM | 62.3 ± 8.9 | 99.3 ± 7.4** | 5 |
| 100 μM + CNQX (30 μM) + | |||
| D-AP5 (100 μM) | 70.9 ± 20.4 | 65.5 ± 22.4† | 4 |
| t-PDC | |||
| 10 μM | 58.4 ± 4.7 | 45.6 ± 7.3* | 4 |
| 100 μM | 47.5 ± 4.8 | 35.8 ± 4.4* | 5 |
| DHK | |||
| 10 μM | 85.9 ± 15.0 | 60.5 ± 6.8* | 5 |
| 100 μM | 50.4 ± 5.8 | 42.4 ± 4.1 | 5 |
Data represent the mean ± SEM of KCl-evoked activation before and after perfusion with several EAAT inhibitors at different concentrations (10 and 100 μM).
P < 0.05, compared with KCl-induced activation before EAAT inhibitor perfusion by a paired Student's t-test.
P < 0.005, when analysed the difference values (i.e. activation data after – before the EAAT inhibitor) and compared these values with the corresponding group without the antagonist, by a two-sample Student's t-test.
Effect of EAAT inhibitors on the spontaneous firing rate of LC neurons
To study the contribution of EAATs in maintaining the tonic regulation of cell discharge by synaptic glutamate, we explored the effect of EAAT inhibitors on the spontaneous firing rate of LC neurons. DL-TBOA (100 μM) induced a 72% increase in the firing rate of LC cells (before DL-TBOA: 1.0 ± 0.1 Hz; after DL-TBOA: 1.6 ± 0.2 Hz, n = 4, P < 0.05), whereas a lower concentration (10 μM) failed to affect the firing activity (n = 4) (Figure 2C). The excitatory effect was not blocked by iGluR antagonists, so that DL-TBOA (100 μM) still increased the firing rate of LC cells in the presence of D-AP5 (100 μM) and CNQX (30 μM) (firing rate, before DL-TBOA: 1.4 ± 0.3 Hz; after DL-TBOA: 2.0 ± 0.4 Hz, n = 6, P < 0.05) (Figure 2C). Perfusion with t-PDC (10 or 100 μM) did not significantly change the firing rate of LC neurons (n = 5) (Figure 2C). However, the EAAT2 inhibitor DHK (100 μM) produced a strong increase in the firing rate of LC cells (before DHK: 1.1 ± 0.1 Hz; after DHK: 3.4 ± 0.6 Hz, n = 5, P < 0.05), which was completely blocked in the presence of D-AP5 (100 μM) and CNQX (30 μM) (n = 4, P < 0.05) (Figure 2A–C). These data suggest that DHK, but not DL-TBOA or t-PDC, increases the firing rate of LC neurons through a glutamate- and iGluR-dependent mechanism.
Figure 2.
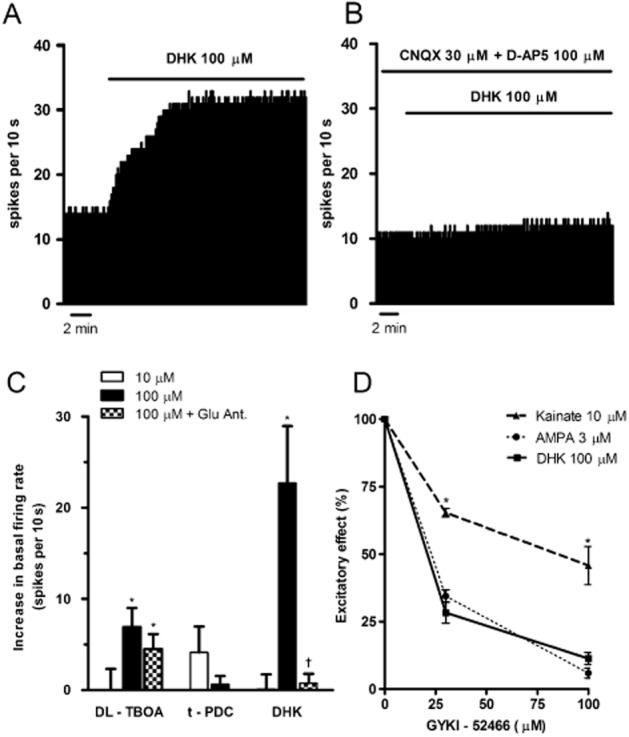
(A–B) Representative examples of the firing rate recording of a single LC neuron showing the excitatory effect of the EAAT2 inhibitor DHK (100 μM) on the basal firing rate (A) and the blockade of DHK (100 μM) effect in the presence of CNQX (30 μM) and D-AP5 (100 μM). (B) The vertical lines represent the integrated firing rates (spikes per 10 s). Drugs were bath applied at the concentration and for the time indicated by the horizontal bars. (C) Bar histograms show the increase in the basal firing rate after perfusion with DL-TBOA (10 μM, n = 4), DL-TBOA (100 μM, n = 4), DL-TBOA (100 μM) + D-AP5 (100 μM) + CNQX (30 μM, n = 6), t-PDC (10 μM, n = 5), t-PDC (100 μM, n = 5), DHK (10 μM, n = 4), DHK (100 μM, n = 5) or DHK (100 μM) + D-AP5 (100 μM) + CNQX (30 μM, n = 4). Each bar represents the mean ± SEM of n cells. *P < 0.05 compared with the basal firing rate in the absence of the inhibitors by a paired Student's t-test; †P < 0.05 compared with the effect produced by DHK (100 μM) in the absence of D-AP5 and CNQX by a two-sample Student's t-test. (D) Effect of the AMPA receptor antagonist GYKI 52466 (30 and 100 μM) on the excitatory effect of AMPA (3 μM, n = 5), kainate (10 μM, n = 5) and DHK (100 μM, n = 5) in the LC. Data points are the mean ± SEM of n cells at different concentrations of GYKI 52466. *P < 0.005 when compared with the excitatory effect of DHK (100 μM) by a two-sample Student's t-test. Note that DHK strongly increased the basal firing rate of LC cells, and that this effect was blocked by application of iGluR antagonists. The excitatory effect of DHK was also decreased in the presence of the AMPA antagonist GYKI 52466.
It has been shown that DHK interacts with kainate receptors at concentrations that inhibit the glutamate transporter (Bunch and Krogsgaard-Larsen, 2009). Therefore, in order to study the involvement of kainate receptors in DHK effect, we compared the activation induced by DHK (100 μM) with that of kainate (10 μM) and AMPA (3 μM) in the presence of the specific AMPA receptor antagonist GYKI 52466. Perfusion with GYKI 52466 (30 and 100 μM) failed to change the firing rate of LC cells, but it attenuated the excitatory effects of DHK (100 μM; reduction: 72%, n = 4, P < 0.01 and 89%, n = 4, P < 0.005, respectively), AMPA (3 μM; 66%, n = 4, P < 0.005 and 94%, n = 4, P < 0.01, respectively) and kainate (10 μM; 35%, n = 4, N.S. and 54%, n = 4, P < 0.05, respectively) (Figure 2D). Most importantly, GYKI 52466 was equally potent at antagonizing DHK and AMPA effects, but it was less potent at reducing the excitatory effect of kainate (P < 0.005 and P < 0.005 vs. DHK, respectively) (Figure 2D). This indicates that elevations in the firing rate produced by DHK (100 μM) may occur through AMPA receptors.
Effect of acute administration of EAAT enhancers on glutamate-induced activation of LC neurons
In order to further clarify the functional role of EAAT subtypes in the LC, we studied the effect of the following EAAT enhancers on glutamate-induced activation: alphaxalone (10 μM; for EAAT3), carbamazepine (10 μM; for EAAT3), nicergoline (10 μM; for EAAT2/EAAT3) and riluzole (10 μM; for EAAT1-3). As shown in Table 1, riluzole (10 μM) was the only drug able to modify the effect of glutamate on the LC (reduction: 20 ± 1%, n = 4 P < 0.05). Because riluzole may also inhibit postsynaptic currents mediated by AMPA receptors (Albo et al., 2004), we tested the excitatory effect of AMPA. Riluzole (10 μM) decreased by 18 ± 5% AMPA-induced activation (increase by AMPA, before riluzole: 11.5 ± 0.4 Hz; with riluzole: 9.5 ± 0.8 Hz; n = 4; P < 0.05), which suggests that riluzole effect on glutamate may be due to an antagonism of AMPA receptors rather than to regulation of glutamate uptake. Taken together, these data do not support the possibility of EAAT to be a functional target for enhancing glutamate uptake in the LC in vitro.
Effect of chronic treatment with the EAAT2 enhancer ceftriaxone on glutamate-induced activation of LC neurons
It has been demonstrated that chronic treatment with ceftriaxone increases EAAT2 transporter expression (Rothstein et al., 2005). To evaluate if a putative increase in the brain levels of EAAT2 may have consequences in the LC, we tested in vitro the effect of glutamate (0.3 and 1 mM) in animals that had been chronically treated with ceftriaxone (200 mg·kg−1 i.p., once daily, 7 days). The spontaneous firing rate of LC neurons in ceftriaxone-treated animals was not different from that in saline-treated animals (control: 0.8 ± 0.2 Hz, n = 7; and ceftriaxone: 1.0 ± 0.1 Hz, n = 7). However, the excitatory effect of glutamate (0.3 mM) in ceftriaxone-treated animals was a 54% higher than control (Figure 3A) (activation by glutamate, control: 7.7 ± 1.0 Hz, n = 7; and ceftriaxone group: 11.9 ± 1.6 Hz, n = 7; P < 0.05). When a higher concentration of glutamate (1 mM) was applied, no significant change in the effect was found in ceftriaxone-treated animals (Figure 3A). This indicates that chronic treatment with ceftriaxone supersensitizes glutamate responses, with an increase in its potency to activate LC cells without changing its maximal effect.
Figure 3.
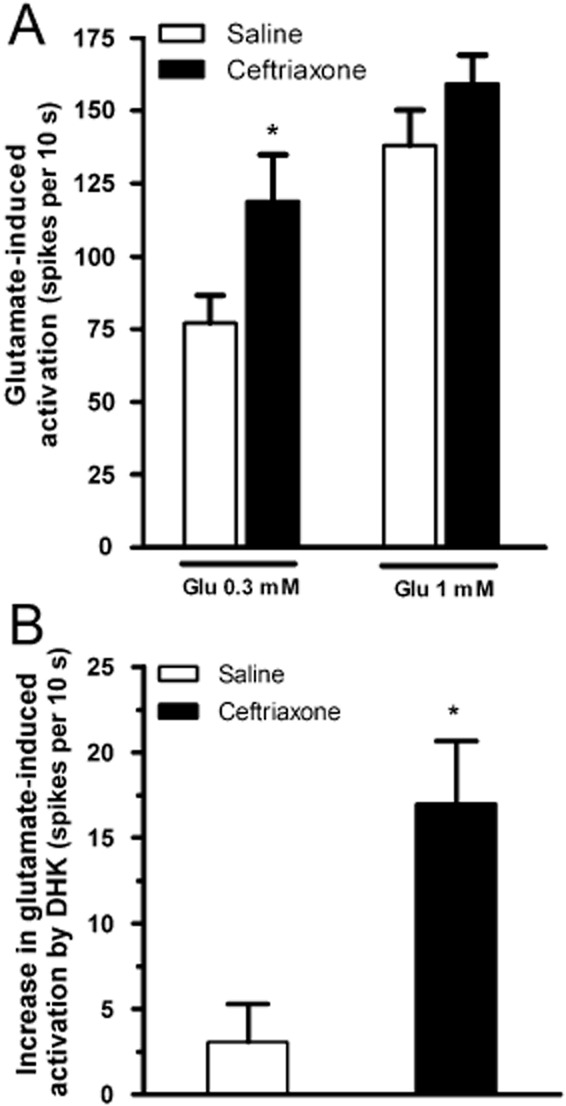
Effect of chronic treatment with ceftriaxone on glutamate-induced effect and on the increase of glutamate effect produced by DHK in LC neurons. (A) Bar histograms show the activation of LC cells induced by glutamate (0.3 and 1 mM) in saline (n = 7 and n = 4, respectively) and ceftriaxone group (n = 7 and n = 8, respectively). Each bar represents the mean ± SEM of n cells. *P < 0.05 compared with glutamate-induced activation in saline group by a two-sample Student's t-test. (B) Bar histograms show DHK-induced increase of glutamate effect (0.3 mM) in ceftriaxone group and in saline group. Each bar represents the mean ± SEM of n cells. *P < 0.05 compared with the increase of glutamate effect produced by DHK (100 μM) in saline group by a two-sample Student's t-test.
To directly explore whether a putative elevation of EAAT2 by chronic ceftriaxone may change the clearance of glutamate through this transporter, we tested the effect of the EAAT2 inhibitor DHK (100 μM) on the firing activity and glutamate-induced (0.3 and 1 mM) activation in animals chronically treated with ceftriaxone. As expected (mentioned earlier), in the saline-treated group, perfusion with DHK (100 μM) significantly increased the spontaneous firing rate of LC cells (increase: 87 ± 17%, n = 7, P < 0.001) but failed to change glutamate-induced (0.3 mM) activation (n = 7) (Figure 3B). In the ceftriaxone group, DHK (100 μM) also increased the firing rate of LC neurons (increase: 81 ± 11%, n = 7, P < 0.001), and the extent of this activation was not different from that in saline group. However, unlike the control, DHK (100 μM) significantly enhanced glutamate-induced (0.3 mM) activation in ceftriaxone group (glutamate effect, before DHK: 11.9 ± 1.6 Hz; after DHK: 13.6 ± 1.6 Hz; n = 7, P < 0.05) (Figure 3B). Finally, the effects induced by a higher concentration of glutamate (1 mM) were not modified in either control (n = 4) or ceftriaxone (n = 8) groups. These data suggest that chronic ceftriaxone does not alter the effect of DHK on the firing activity but it potentiates DHK effect on glutamate-induced activation, probably due to an enhanced contribution of EAAT2 to remove high concentrations of glutamate from the synaptic cleft.
Immunohistochemistry of EAAT2 and EAAT3
Double immunofluorescence was performed within the LC in order to study the expression of EAAT2 and EAAT3 in TH-immunopositive noradrenergic neurons or in GFAP-immunoreactive astroglial cells. Positive controls were first carried out to confirm staining in the rat striatum for anti-EAAT2 and the rat cerebral cortex for anti-EAAT3. Negative controls in which primary antibody was omitted resulted in lack of staining. As shown in a low magnification image (Figure 4), immunofluorescence for EAAT3 and TH shaped the LC near the lateral wall of the fourth ventricle.
Figure 4.
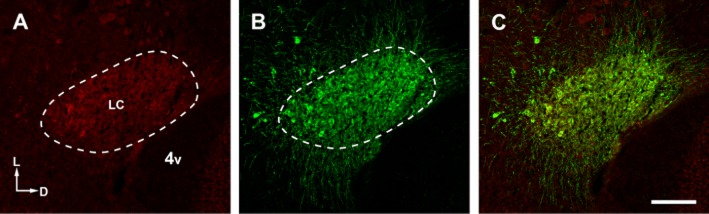
Immunofluorescence labelling of EAAT3 and TH in the rat's left LC. Low magnification photomicrographs show that both EAAT3 (A) and TH (B) labelling (merged in C) are present in the LC near the lateral wall of the fourth ventricle. The areas were taken at a middle level of the rostrocaudal axes of LC (bregma −9.8 mm to −10.04 mm) and at a distance of 125–150 μm from the lateral wall of the fourth ventricle, approximately in the central area of LC. LC, locus coeruleus; 4v, fourth ventricle; L, lateral; D, dorsal. Scale bar: (A,B,C) 200 μm.
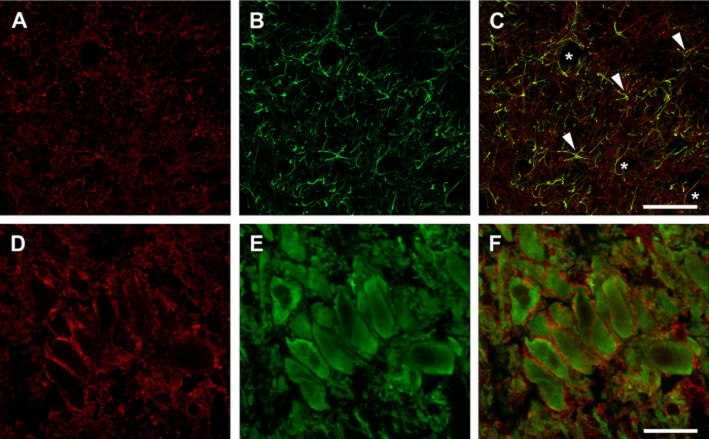
Characterization of EAAT2 immunoreactivity by a higher magnification of the LC revealed many labelled puncta and short fragments of immunopositive cellular processes homogeneously distributed in the whole extension of the nucleus (Figure 5A, D). High-resolution confocal analysis about the coexpression of GFAP and EAAT2 in the same cells corroborated that these EAAT2-positive structures corresponded to astroglial processes because practically all labelled astroglial cells were also immunopositive for EAAT2 (Figure 5B, C). These results indicate the intrinsic origin of expression of EAAT2 transporter in astrocytes located within the LC. On the other hand, noradrenergic neurons were strongly TH immunoreactive, in such a way that the intense labelling refilled neuronal somata and the most proximal portions of the main dendritic trunks (Figure 5E). When double immunofluorescence experiments were carried out to analyse the possible expression of EAAT2 by LC neurons, EAAT2-labelled puncta were found surrounding the TH-immunopositive noradrenergic neuronal somata (Figure 5F). These EAAT2-labelled puncta were not immunoreactive neither for TH nor for GFAP. This distribution suggests that positive puncta could belong to presynaptic boutons localized in glutamatergic afferents to the noradrenergic cells.
Figure 5.
Fluorescence immunolabelling of EAAT2, GFAP and TH in the LC. A–C and D–F are views of consecutive coronal sections immunolabelled against EAAT2 and GFAP or EAAT2 and TH, respectively. (A–C) EAAT2-immunopositive puncta are in red (A), while astrocytic processes immunopositives for GFAP appear marked in green (B). Double immunolabelling (C) shows that the overwhelming majority of GFAP-immunopositive cells are also EAAT2 immunoreactive (arrowheads) because astroglial cells, some of them surrounding capillaries (asterisk), appear marked in yellow. (D–F) Double immunofluorescence against EAAT2 (single labelling showed in D) and TH (single labelling showed in E) shows no colocalization of this glutamate transporter with TH, where EAAT2-immunopositive puncta appear outlining noradrenergic cells somata (F). Scale bar: (A,B,C) 50 μm; (D,E,F) 25 μm.
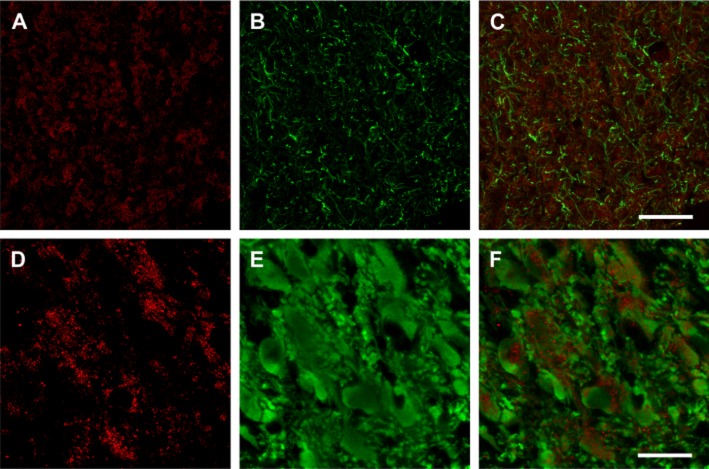
EAAT3 immunohistochemistry showed a pattern of immunolabelling similar to that found for the EAAT2. Thus, a high density of positive puncta appeared distributed in the whole extension of LC (Figure 6A, D), although their size was smaller than that found for EAAT2 boutons. However, in contrast to EAAT2 double immunofluorescence experiments, there was no colocalization of EAAT3 and GFAP expression, because astrocytes were not immunoreactive for this glutamate transporter (Figure 6B, C). When the colocalization of EAAT3 and TH expression was evaluated in noradrenergic cells, we found that EAAT3-positive puncta also formed baskets surrounding these neurons (Figure 6E, F) as it was described for the EAAT2 labelling. These EAAT3 labelled puncta were not immunoreactive either for TH or for GFAP. These results suggest an extrinsic origin for the glutamate transporter EAAT3 in the LC.
Figure 6.
Fluorescence immunolabelling of EAAT3, GFAP and TH in the LC. A–C and D–F are views of consecutive coronal sections immunolabelled against EAAT3 and GFAP, and EAAT3 and TH, respectively. (A–C) EAAT3-immunolabelled puncta are labelled in red (A), and astrocytic processes immunopositives for GFAP are labelled in green (B). Double immunolabelling (C) shows no colocalization of EAAT3 with astrocytic marker GFAP. (D–F) Confocal analysis of parallel sections immunolabelled for EAAT3 (D), TH (E) and EAAT3 + TH (F) shows that noradrenergic cells do not express the EAAT3 glutamate transporter, but EAAT3-immunopositive puncta appear surrounding their somata (F). Scale bar: (A,B,C) 50 μm; (D,E,F) 25 μm.
Discussion and conclusions
The present work was undertaken to characterize the functional role of EAATs in the regulation of glutamate actions in the LC. Our results reveal that the EAAT2–5 inhibitor DL-TBOA enhances both glutamate-induced and KCl-evoked effects on LC cells, but it fails to affect directly the spontaneous firing activity of these neurons. On the contrary, the EAAT2 inhibitor DHK increased the firing activity of LC cells without changing the excitatory effects of glutamate or KCl. Chronic treatment with the EAAT2 enhancer ceftriaxone potentiated the glutamate-induced activation of LC cells and unmasked an enhancing effect of DHK on glutamate-induced activation. Finally, our study shows the presence of EAAT2 and EAAT3 immunolabelling around TH-positive noradrenergic somata and EAAT2 immunolabelling on GFAP-positive astroglial cells.
To characterize the role of EAATs in the LC, we used the EAAT inhibitors DL-TBOA, t-PDC and DHK in view of their affinities for EAAT subtypes. DL-TBOA has a higher affinity for EAAT2, 3, 4 and 5 than for EAAT1 (Shimamoto et al., 2000; Shigeri et al., 2001; Jensen and Brauner-Osborne, 2004) and it has been used at similar or identical concentrations to block EAATs in slice preparations (Sharifullina and Nistri, 2006; Thomson et al., 2006). DL-TBOA does not have any significant effect on iGluR or mGluR and does not induce non-specific currents (Shimamoto et al., 1998). t-PDC is selective for EAAT2, 4 and 5 (Bridges and Esslinger, 2005) and DHK is a potent and selective inhibitor of EAAT2 (Jensen and Brauner-Osborne, 2004; Bunch et al., 2009). We designed three approaches to study EAATs in the LC. First, we tested the response of LC cells to exogenous application of glutamate, which would reveal the importance of EAAT subtypes in removing high concentrations of glutamate. Second, we studied the KCl-evoked activation of LC cells (in the presence of a GABAA receptor antagonist), which would characterize EAAT subtypes responsible for uptaking synaptically released glutamate. We have previously demonstrated that after blocking GABAA receptors, more than 50% of LC cell activation evoked by KCl is caused by released glutamate (Mendiguren and Pineda, 2007). Third, we explored the spontaneous firing rate of LC cells, which would identify the EAAT subtype maintaining the steady-state levels of glutamate.
In the present study, we found that DL-TBOA, but not same concentrations of t-PDC or DHK, enhanced the effect of exogenously applied glutamate. This effect was concentration dependent. As mentioned earlier, DL-TBOA is the only drug used herein that blocks the neuronal glutamate transporter EAAT3 (Jensen and Brauner-Osborne, 2004). Therefore, the EAAT3 would be the main re-uptake system for removing high concentrations of glutamate after it is synaptically released in the LC. The specific involvement of EAAT3 in the removal of neuronally released glutamate agrees with the fact that EAAT2s are mostly expressed in glial cells and that the EAAT4 and 5 are restricted to the cerebellum and the retina respectively (Fairman et al., 1995; Bunch et al., 2009). Moreover, DL-TBOA was the only EAAT inhibitor that enhanced the activation of LC cells by KCl, and this effect was blocked by a combination of iGluR antagonists, which shows the specificity of the effect. Therefore, the glutamate-mediated effect of KCl on the LC may be regulated by EAAT3 but not EAAT2, 4, 5 transporters. This confirms the role of EAAT3 in removing synaptic glutamate and, hence, in the prevention of glutamate accumulation that would activate postsynaptic iGluR in the LC. Other authors have suggested that postsynaptic responses via iGluRs are not shaped by EAATs because neuronal EAATs are positioned to regulate the amount of glutamate that escapes the synapse rather than being poised to influence the synaptic glutamate concentrations (Amara and Fontana, 2002). This proposal is based on studies measuring EPSCs and EPSPs evoked by focal or monosynaptic stimuli of afferent fibres in which iGluRs within the cleft may be saturated by the high glutamate concentration reached after the release. In the present work, we used external perfusions applied to extracellular recording techniques, which measure the global postsynaptic GluR activation throughout the whole somatodendritic area of the neuron. Under these conditions, we may not be able to isolate the time-course of synaptic from extrasynaptic GluR activation. On the other hand, the concentration of glutamate reached at the active sites after glutamate or KCl perfusions was not high enough to fully saturate the GluR and, thereby, an enhancement of firing activation is observed after EAAT inhibition. This increase could be the result of a higher synaptic and extrasynaptic glutamate concentration or a delayed washout of the same glutamate concentration from the active sites after EAAT inhibitor administration.
To confirm the involvement of iGluR rather than mGluR in the effect of EAAT inhibition, we performed some assays with the non-selective mGluR antagonist RS-MCPG, which has been previously used in the LC (Dubé and Marshall, 1997; Zamalloa et al., 2009). In the presence of RS-MCPG, the enhancing effect of DL-TBOA on glutamate effect was not altered, suggesting that only iGluRs may contribute to this process. This is in contrast with the ability of RS-MCPG to block the effect of EAAT inhibition on glutamatergic synaptic currents in cultured hippocampal neurons, which are mediated by presynaptic mGluR located extrasynaptically (Maki et al., 1994). However, our data are in agreement with a previous study by Dubé and Marshall (1997) showing that group I, II and III mGluR agonists do not modify glutamate responses in the LC.
In our study, administration of the EAAT2 inhibitor DHK increased the spontaneous firing activity of LC cells, effect that was blocked by iGluR antagonists. DL-TBOA also increased the firing rate of LC neurons, but this effect was not blocked by iGluR antagonists. Although DHK has activity at kainate receptors (Bunch and Krogsgaard-Larsen, 2009), the excitatory effect of DHK was prevented by the AMPA receptor antagonist GYKI 52466 at concentrations that blocked AMPA but not kainate effects. This finding indicates that DHK may activate LC cells by glutamate-induced activation of AMPA receptors, probably due to an accumulation of synaptic glutamate as the consequence of EAAT2 inhibition, rather than as a result of a direct kainate receptor activation.
It has been proposed by extracellular recordings that tonic glutamate activity in the LC in vitro is very low and, hence, iGluR antagonists fail to modify the cell firing rate (Olpe et al., 1989; Singewald and Philippu, 1998; see Results). This has been explained by the fact that long glutamate inputs to the LC are removed when coronal slices are prepared (Alreja and Aghajanian, 1995) and only impulse-independent nerve leaking and cellular metabolism are sources of glutamate. In contrast, there seems to be intrinsic glutamate release in vivo, which is responsible for certain degree of LC cell activation during tonic and phasic periods (Torrecilla et al., 2007; also see Introduction). Therefore, our results with DHK on the firing rate support a role for the glial transporter system, specifically the EAAT2, in maintaining a tonic low activity of synaptic glutamate.
In immunohistochemical assays, we found expression of EAAT2-positive fragments colocalized with GFAP staining in the LC. However, colocalization of EAAT3-positive puncta and GFAP immunostaining was not detected in the LC. This suggests the presence of EAAT2 but not EAAT3 on glial cells of the LC, in consonance with other authors that reported the expression of mRNA for EAAT2 in astrocytes in the LC (Berger and Hediger, 1998). EAAT2 is distributed in astrocytes throughout the whole brain and spinal cord (Rothstein et al., 1994; Danbolt, 2001; Bridges and Esslinger, 2005). On the other hand, we observed the presence of EAAT2 and EAAT3 labelled puncta surrounding TH-immunopositive cells in the LC. This finding indicates that both glutamate transporters, EAAT2 and EAAT3, may be located on nerve terminals apposing noradrenergic neurons in the LC. EAAT3 is the most widely distributed neuronal glutamate transporter in the CNS. It has been reported to be present in different parts of the neuron including the nerve terminal, where it plays a functional role in removing glutamate from the synaptic cleft (He et al., 2000; Nieoullon et al., 2006). Therefore, both morphological and functional results (presented earlier) support the importance of the neuronal transporter EAAT3 for terminating glutamate action in the LC when it is synaptically released. The EAAT3 would prevent accumulation of high or sustained concentrations of glutamate and damage of neurons against oxidative stress (Nieoullon et al., 2006), contributing to the normal activity of glutamate receptors in the LC. As discussed earlier, the glial transporter EAAT2 would have a greater importance when low glutamate concentrations are present in the synaptic cleft; it would be responsible for the re-uptake of basal glutamate and maintaining the tonic low activity of synaptic glutamate.
Functional and molecular studies in vitro and in vivo have shown that administration of the β-lactamic antibiotic ceftriaxone enhances the transcription of glial EAAT2, increases the glutamate re-uptake by glial membranes and reduces the extracellular concentration of glutamate in the brain (Rothstein et al., 2005; Lee et al., 2008; Verma et al., 2010; Rasmussen et al., 2011). In the present study, chronic treatment with ceftriaxone enhanced the excitatory effect of glutamate in the LC, which suggests the induction of iGluR sensitization. This may be due to the up-regulation of EAAT2 transporters by ceftriaxone and the consequent increase in glutamate uptake and reduction in synaptic glutamate. Similar receptor supersensitivity has been reported with other receptors in the CNS after reduction of the endogenous neurotransmission (e.g. see Ugedo et al., 1993). In ceftriaxone-treated animals, as opposed to control, DHK was able to enhance the glutamate-induced activation of LC cells, whereas the direct effect of DHK on the firing rate was unchanged. One explanation for these results is that EAAT2 does not contribute to the main re-uptake of high concentrations of glutamate under control situation, but it becomes involved in glutamate removal after up-regulation of the transporter. Therefore, EAAT2 would be more efficacious in removing synaptic glutamate in certain pathophysiological or pharmacological conditions when glial glutamate transporters are up-regulated.
We have tested the effect of the EAAT3 enhancers alphaxalone and carbamazepine and the EAAT2/EAAT3 enhancer, nicergoline, on the excitation of LC cells by glutamate, but they failed to modify it. The EAAT1–3 enhancer, riluzole, decreased glutamate-induced excitation. However, this effect could be due to an antagonism of postsynaptic AMPA receptors rather than an action on EAAT transporters, because riluzole reduced the excitation induced directly by AMPA in the LC (see Results) and in other CNS areas (Albo et al., 2004). We can speculate that the lack of effect of EAAT enhancers on the glutamate-induced activation may be explained by the fact that these drugs act through indirect mechanisms (Lee et al., 2005; Ryu et al., 2009). Alternatively, when extracellular glutamate reaches high concentrations, as expected in our system, the efficiency of glutamate uptake by EAAT is near maximal and cannot be acutely enhanced.
EAAT2 and EAAT3 have been postulated to be potential therapeutic targets in glutamate related pathologies (see Introduction). In the present study, it is concluded that at least two different subtypes of glutamate transporters are present in the rat LC: EAAT2 and EAAT3. We speculate that neuronal EAAT3 is located at nerve terminals apposing noradrenergic neurons in the LC, and would be responsible for terminating glutamate action when it is synaptically released at high concentrations. The glial transporter EAAT2 would play a prominent role in the regulation of tonic, low concentrations of glutamate, although it would also regulate synaptic glutamate concentrations after pharmacological manipulations such as ceftriaxone treatment. This could explain why ceftriaxone administration decreases morphine dependence (Nakagawa et al., 2001; Rawls et al., 2010), because ceftriaxone induces overexpression of EAAT2 (presented earlier; Rothstein et al., 2005), which would counteract the enhanced glutamate release in the LC during opiate withdrawal (Koob et al., 1992; Aghajanian et al., 1994). Similar results have been reported by gene transfer of the GLT-1 (i.e. EAAT2) into the LC with recombinant adenoviruses (Ozawa et al., 2004). On the other hand, raising the glutamate levels by EAAT3 inhibition increases morphine withdrawal and morphine-induced conditioned place preference (Sekiya et al., 2004). Therefore, our results highlight the interest of glutamate modulation to prevent states that result from an excessive glutamatergic activation such as morphine dependence.
Acknowledgments
This work was supported by Ministerio de Ciencia e Innovación (Grant SAF2008-03612), Fondo de Investigación Sanitaria (FIS, PS09/00476) and the University of the Basque Country (UPV/EHU) (Grant GIU11/27). Pineda's research group takes part in a network unit supported by the University of the Basque Country (UFI 11/35). M. C. M. was supported by a predoctoral fellowship from the Basque Government.
Glossary
- aCSF
artificial CSF
- CNQX
6-cyano-7-notroquinoxaline-2, 3-dione
- D-AP5
D-(–)-2-amin-5-phosponopentanoic acid
- DHK
dihydrokainic acid
- DL-TBOA
DL-threo-β-benzyloxaspartic acid
- DMSO
dimethylsulfoxide
- EAAT
excitatory amino acid transporter
- GFAP
glial fibrillary acidic protein
- GYKI 52466
8-chloro-2-methyl-11H-imidazo[1,2-c][2,3]benzodiazepin-6-benzeneamine dihydrochloride
- iGluR
ionotropic glutamate receptor
- LC
locus coeruleus
- mGluR
metabotropic glutamate receptor
- NGS
normal goat serum
- RS-MCPG
RS-methyl-4-carboxyphenylglycine
- t-PDC
L-trans-pyrrolidine-2,4-dicarboxylic acid
Conflict of interest
None of the authors have any conflicts of interest to disclose relating to this work.
References
- Aghajanian GK, Kogan JH, Moghaddam B. Opiate withdrawal increases glutamate and aspartate efflux in the locus coeruleus: an in vivo microdialysis study. Brain Res. 1994;636:126–130. doi: 10.1016/0006-8993(94)90186-4. [DOI] [PubMed] [Google Scholar]
- Albo F, Pieri M, Zona C. Modulation of AMPA receptors in spinal motor neurons by the neuroprotective agent riluzole. J Neurosci Res. 2004;78:200–207. doi: 10.1002/jnr.20244. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 5th edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alreja M, Aghajanian GK. Intracellular application of macromolecules through patch pipettes in brain slices. In: Schurr A, Rigor BM, editors. Brain Slices in Basic and Clinical Research. London: CRC Press; 1995. pp. 117–130. [Google Scholar]
- Amara SG, Fontana ACK. Excitatory amino acid transporters: keeping up with glutamate. Neurochem Intern. 2002;41:313–318. doi: 10.1016/s0197-0186(02)00018-9. [DOI] [PubMed] [Google Scholar]
- Andrade R, Aghajanian GK. Locus coeruleus activity in vitro: intrinsic regulation by a calcium- dependent potassium conductance but not alpha 2-adrenoceptors. J Neurosci. 1984;4:161–170. doi: 10.1523/JNEUROSCI.04-01-00161.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G. Brain structures and receptors involved in alertness. Sleep Med. 2005;6(Suppl. 1):S3–S7. doi: 10.1016/s1389-9457(05)80002-4. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. Adaptive gain and the role of the locus coeruleus-norepinephrine system in optimal performance. J Comp Neurol. 2005;493:99–110. doi: 10.1002/cne.20723. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Ennis M, Pieribone VA, Nickell WT, Shipley MT. The brain nucleus locus coeruleus: restricted afferent control of a broad efferent network. Science. 1986;234:734–737. doi: 10.1126/science.3775363. [DOI] [PubMed] [Google Scholar]
- Berger UV, Hediger MA. Comparative analysis of glutamate transporter expression in rat brain using differential double in situ hybridization. Anat Embryol (Berl) 1998;198:13–30. doi: 10.1007/s004290050161. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Bridges RJ, Esslinger CS. The excitatory amino acid transporters: pharmacological insights on substrate and inhibitor specificity of the EAAT subtypes. Pharmacol Ther. 2005;107:271–285. doi: 10.1016/j.pharmthera.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Bunch L, Krogsgaard-Larsen P. Subtype selective kainic acid receptor agonists: discovery and approaches to rational design. Med Res Rev. 2009;29:3–28. doi: 10.1002/med.20133. [DOI] [PubMed] [Google Scholar]
- Bunch L, Erichsen MN, Jensen AA. Excitatory amino acid transporters as potential drug targets. Expert Opin Ther Targets. 2009;13:719–731. doi: 10.1517/14728220902926127. [DOI] [PubMed] [Google Scholar]
- Cherubini E, North RA, Williams JT. Synaptic potentials in rat locus coeruleus neurones. J Physiol. 1988;406:431–442. doi: 10.1113/jphysiol.1988.sp017389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Dubé GR, Marshall KC. Modulation of excitatory synaptic transmission in locus coeruleus by multiple presynaptic metabotropic glutamate receptors. Neuroscience. 1997;80:511–521. doi: 10.1016/s0306-4522(97)00004-3. [DOI] [PubMed] [Google Scholar]
- Ennis M, Aston-Jones G, Shiekhattar R. Activation of locus coeruleus neurons by nucleus paragigantocellularis or noxious sensory stimulation is mediated by intracoerulear excitatory amino acid neurotransmission. Brain Res. 1992;598:185–195. doi: 10.1016/0006-8993(92)90182-9. [DOI] [PubMed] [Google Scholar]
- Fairman WA, Vandenberg RJ, Arriza JL, Kavanaugh MP, Amara SG. An excitatory amino-acid transporter with properties of a ligand-gated chloride channel. Nature. 1995;375:599–603. doi: 10.1038/375599a0. [DOI] [PubMed] [Google Scholar]
- Fujio M, Nakagawa T, Sekiya Y. Gene transfer of GLT-1, a glutamate transporter, into the nucleus accumbens shell attenuates methamphetamine- and morphine-induced conditioned place preference in rats. Eur J Neurosci. 2005;22:2744–2754. doi: 10.1111/j.1460-9568.2005.04467.x. [DOI] [PubMed] [Google Scholar]
- Furuta A, Martin LJ, Lin CL, Dykes-Hoberg M, Rothstein JD. Cellular and synaptic localization of the neuronal glutamate transporters excitatory amino acid transporter 3 and 4. Neuroscience. 1997;81:1031–1042. doi: 10.1016/s0306-4522(97)00252-2. [DOI] [PubMed] [Google Scholar]
- He Y, Janssen WG, Rothstein JD, Morrison JH. Differential synaptic localization of the glutamate transporter EAAC1 and glutamate receptor subunit GluR2 in the rat hippocampus. J Comp Neurol. 2000;418:255–269. [PubMed] [Google Scholar]
- Jensen AA, Brauner-Osborne H. Pharmacological characterization of human excitatory amino acid transporters EAAT1, EAAT2 and EAAT3 in a fluorescence-based membrane potential assay. Biochem Pharmacol. 2004;67:2115–2127. doi: 10.1016/j.bcp.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. NC3Rs Reporting Guidelines Working Group. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Maldonado R, Stinus L. Neural substrates of opiate withdrawal. Trends Neurosci. 1992;15:186–191. doi: 10.1016/0166-2236(92)90171-4. [DOI] [PubMed] [Google Scholar]
- Lee G, Huang Y, Washington JM, Briggs NW, Zuo Z. Carbamazepine enhances the activity of glutamate transporter type 3 via phosphatidylinositol 3-kinase. Epilepsy Res. 2005;66:145–153. doi: 10.1016/j.eplepsyres.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Lee SG, Su ZZ, Emdad L, Gupta P, Sarkar D, Borjabad A, et al. Mechanism of ceftriaxone induction of excitatory amino acid transporter-2 expression and glutamate uptake in primary human astrocytes. J Biol Chem. 2008;283:13116–13123. doi: 10.1074/jbc.M707697200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki R, Robinson MB, Dichter MA. The Glutamate Uptake inhibitor L- Trans-pyrrolidine-2,4-dicarboxylate depresses excitatory synaptic transmission via a presynaptic mechanism in cultured hippocampal neurons. J Neurosci. 1994;14:6754–6762. doi: 10.1523/JNEUROSCI.14-11-06754.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendiguren A, Pineda J. Cannabinoids enhance N-methyl-D-aspartate-induced excitation of locus coeruleus neurons by CB1 receptors in rat brain slices. Neurosci Lett. 2004;363:1–5. doi: 10.1016/j.neulet.2004.02.073. [DOI] [PubMed] [Google Scholar]
- Mendiguren A, Pineda J. CB (1) cannabinoid receptors inhibit the glutamatergic component of KCl-evoked excitation of locus coeruleus neurons in rat brain slices. Neuropharmacology. 2007;52:617–625. doi: 10.1016/j.neuropharm.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Ozawa T, Shige K, Yamamoto R, Minami M, Satoh M, et al. Inhibition of morphine tolerance and dependence by MS-153, a glutamate transporter activator. Eur J Pharmacol. 2001;419:39–45. doi: 10.1016/s0014-2999(01)00965-7. [DOI] [PubMed] [Google Scholar]
- Nieoullon A, Canolle B, Masmejean F, Guillet B, Pisano P, Lortet S. The neuronal excitatory amino acid transporter EAAC1/EAAT3: does it represent a major actor at the brain excitatory synapse? J Neurochem. 2006;98:1007–1018. doi: 10.1111/j.1471-4159.2006.03978.x. [DOI] [PubMed] [Google Scholar]
- Olpe HR, Steinmann MW, Brugger F, Pozza MF. Excitatory amino acid receptors in rat locus coeruleus. An extracellular in vitro study. Naunyn Schmiedeberg's Arch Pharmacol. 1989;339:312–315. doi: 10.1007/BF00173584. [DOI] [PubMed] [Google Scholar]
- Ozawa T, Nakagawa T, Sekiya Y, Minami M, Satoh M. Effect of gene transfer of GLT-1, a glutamate transporter, into the locus coeruleus by recombinant adenoviruses on morphine physical dependence in rats. Eur J Neurosci. 2004;19:221–226. doi: 10.1111/j.1460-9568.2004.03101.x. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Yokotani N, Wenthold RJ. Light and electron microscope distribution of the NMDA receptor subunit NMDAR1 in the rat nervous system using a selective anti-peptide antibody. J Neurosci. 1994;14:667–696. doi: 10.1523/JNEUROSCI.14-02-00667.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakhade SN, Loeb JA. Focal reduction of neuronal glutamate transporters in human neocortical epilepsy. Epilepsia. 2008;49:226–236. doi: 10.1111/j.1528-1167.2007.01310.x. [DOI] [PubMed] [Google Scholar]
- Rasmussen BA, Baron DA, Kim JK, Unterwald EM, Rawls SM. Beta-lactam antibiotic produces a sustained reduction in extracellular glutamate in the nucleus accumbens of rats. Amino Acids. 2011;40:761–764. doi: 10.1007/s00726-010-0589-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen K, Krystal JH, Aghajanian GK. Excitatory amino acids and morphine withdrawal: differential effects of central and peripheral kynurenic acid administration. Psychopharmacology (Berl) 1991;105:508–512. doi: 10.1007/BF02244371. [DOI] [PubMed] [Google Scholar]
- Rawls SM, Baron DA, Kim J. Beta-Lactam antibiotic inhibits development of morphine physical dependence in rats. Behav Pharmacol. 2010;21:161–164. doi: 10.1097/FBP.0b013e328337be10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein JD, Martin L, Levey AI, Bristol LA, Jin L, Kuncl RW, et al. Localization of neuronal and glial glutamate transporters. Neuron. 1994;13:713–725. doi: 10.1016/0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, et al. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- Ryu J, Cheong IY, Do SH, Zuo Z. Alphaxalone, a neurosteroid anaesthetic, increases the activity of the glutamate transporter type 3 expressed in Xenopus oocytes. Eur J Pharmacol. 2009;602:23–27. doi: 10.1016/j.ejphar.2008.10.063. [DOI] [PubMed] [Google Scholar]
- Sato K, Kiyama H, Tohyama M. The differential expression patterns of messenger RNAs encoding non-N-methyl-D-aspartate glutamate receptor subunits (GluR1-4) in the rat brain. Neuroscience. 1993;52:515–539. doi: 10.1016/0306-4522(93)90403-3. [DOI] [PubMed] [Google Scholar]
- Sekiya Y, Nakagawa T, Ozawa T, Minami M, Satoh M. Facilitation of morphine withdrawal symptoms and morphine-induced conditioned place preference by a glutamate transporter inhibitor DL-threo-beta-benzyloxyaspartate in rats. Eur J Pharmacol. 2004;485:201–210. doi: 10.1016/j.ejphar.2003.11.062. [DOI] [PubMed] [Google Scholar]
- Sharifullina E, Nistri A. Glutamate uptake block triggers deadly rhythmic bursting of neonatal rat hypoglossal motoneurons. J Physiol. 2006;572:407–423. doi: 10.1113/jphysiol.2005.100412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeri Y, Shimamoto K, Yasuda-Kamatani Y, Seal RP, Yumoto N, Nakajima T, et al. Effects of threo-β-hydroxyaspartate derivatives on excitatory amino acid transporters (EAAT4 and EAAT5) J Neurochem. 2001;79:297–302. doi: 10.1046/j.1471-4159.2001.00588.x. [DOI] [PubMed] [Google Scholar]
- Shimamoto K, Lebrun B, Yasuda-Kamatani Y, Sakaitani M, Shigeri Y, Yumoto N, et al. DL-threo-beta-benzyloxyaspartate, a potent blocker of excitatory amino acid transporters. Mol Pharmacol. 1998;53:195–201. doi: 10.1124/mol.53.2.195. [DOI] [PubMed] [Google Scholar]
- Shimamoto K, Shigeri Y, Yasuda-Kamatani Y, Lebrun B, Yumoto N, Nakajima T. Syntheses of optically pure beta-hydroxyaspartate derivatives as glutamate transporter blockers. Bioorg Med Chem Lett. 2000;10:2407–2410. doi: 10.1016/s0960-894x(00)00487-x. [DOI] [PubMed] [Google Scholar]
- Singewald N, Philippu A. Release of neurotransmitters in the locus coeruleus. Prog Neurobiol. 1998;56:237–267. doi: 10.1016/s0301-0082(98)00039-2. [DOI] [PubMed] [Google Scholar]
- Thomson LM, Zeng J, Terman GW. Differential effect of glutamate transporter inhibition on EPSCs in the morphine näıve and morphine tolerant neonatal spinal cord slice. Neurosci Lett. 2006;407:64–69. doi: 10.1016/j.neulet.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Torrecilla M, Ruiz-Ortega JA, Ugedo L, Pineda J. Excitatory regulation of noradrenergic neurons by L-arginine/nitric oxide pathway in the rat locus coeruleus in vivo. Naunyn Schmiedebergs Arch Pharmacol. 2007;375:337–347. doi: 10.1007/s00210-007-0163-9. [DOI] [PubMed] [Google Scholar]
- Ugedo L, Garro MA, Pineda J, Giralt MT, Miralles A, Olmos G, et al. Acute and chronic effects of reserpine on biochemical and functional parameters of central and peripheral alpha 2-adrenoceptors. Eur J Pharmacol. 1993;239:149–157. doi: 10.1016/0014-2999(93)90988-t. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Menko AS, Drolet G. Neuroadaptive responses in brainstem noradrenergic nuclei following chronic morphine exposure. Mol Neurobiol. 2001;23:155–171. doi: 10.1385/mn:23:2-3:155. [DOI] [PubMed] [Google Scholar]
- Verma R, Mishra V, Sasmal D, Raghubir R. Pharmacological evaluation of glutamate transporter 1 (GLT-1) mediated neuroprotection following cerebral ischemia/reperfusion injury. Eur J Pharmacol. 2010;638:65–71. doi: 10.1016/j.ejphar.2010.04.021. [DOI] [PubMed] [Google Scholar]
- Wisden W, Seeburg PH. A complex mosaic of high-affinity kainate receptors in rat brain. J Neurosci. 1993;13:38–44. doi: 10.1523/JNEUROSCI.13-08-03582.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamalloa T, Bailey CP, Pineda J. Glutamate-induced post-activation inhibition of locus coeruleus neurons is mediated by AMPA/kainate receptors and sodium-dependent potassium currents. Br J Pharmacol. 2009;156:649–661. doi: 10.1111/j.1476-5381.2008.00004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]