Abstract
Background and Purpose
The role of inosine at the mammalian neuromuscular junction (NMJ) has not been clearly defined. Moreover, inosine was classically considered to be the inactive metabolite of adenosine. Hence, we investigated the effect of inosine on spontaneous and evoked ACh release, the mechanism underlying its modulatory action and the receptor type and signal transduction pathway involved.
Experimental Approach
End-plate potentials (EPPs) and miniature end-plate potentials (MEPPs) were recorded from the mouse phrenic-nerve diaphragm preparations using conventional intracellular electrophysiological techniques.
Key Results
Inosine (100 μM) reduced MEPP frequency and the amplitude and quantal content of EPPs; effects inhibited by the selective A3 receptor antagonist MRS-1191. Immunohistochemical assays confirmed the presence of A3 receptors at mammalian NMJ. The voltage-gated calcium channel (VGCC) blocker Cd2+, the removal of extracellular Ca2+ and the L-type and P/Q-type VGCC antagonists, nitrendipine and ω-agatoxin IVA, respectively, all prevented inosine-induced inhibition. In the absence of endogenous adenosine, inosine decreased the hypertonic response. The effects of inosine on ACh release were prevented by the Gi/o protein inhibitor N-ethylmaleimide, PKC antagonist chelerytrine and calmodulin antagonist W-7, but not by PKA antagonists, H-89 and KT-5720, or the inhibitor of CaMKII KN-62.
Conclusion and Implications
Our results suggest that, at motor nerve terminals, inosine induces presynaptic inhibition of spontaneous and evoked ACh release by activating A3 receptors through a mechanism that involves L-type and P/Q-type VGCCs and the secretory machinery downstream of calcium influx. A3 receptors appear to be coupled to Gi/o protein. PKC and calmodulin may be involved in these effects of inosine.
Keywords: inosine, A3 adenosine receptor, presynaptic inhibition, voltage-gated calcium channels, Ca2+- independent mechanism, PKC, calmodulin, mammalian neuromuscular junction
Introduction
At the neuromuscular junction (NMJ), it was demonstrated that adenine nucleotides as well as adenosine play an important role as modulators of transmitter release by activating different presynaptic ATP receptors and adenosine receptors respectively (reviewed by Burnstock, 2007).
It is known that ATP is co-released with acetylcholine (ACh) (Silinsky, 1975), and once in the synaptic cleft it is degraded to ADP, AMP and adenosine by a family of ecto-ATP/ADPases and ecto-5′-nucleotidases (revised by Robson et al., 2006). Furthermore, AMP can also be extracellularly deaminated into the metabolite IMP bypassing adenosine formation (Cunha and Sebastião, 1991; Magalhães-Cardoso et al., 2003). Both, adenosine and IMP are then metabolized to inosine; adenosine-deaminase is the enzyme that acts on the passage of adenosine to inosine (Barankiewicz and Cohen, 1985) and ecto-5′-nucleotidase is involved in the conversion of IMP to inosine (Magalhães-Cardoso et al., 2003). However, purines can also be released to the synaptic space from activated muscles fibres (Smith, 1991; Santos et al., 2003) and from perisynaptic Schwann cells (Liu et al., 2005).
Inosine has extensive effects on many cell types; it can stimulate degranulation of mast cells (Jin et al., 1997), reduce the production of pro-inflammatory cytokines TNF-α, IL-1, IL-12, and restrain the inflammatory response provoked by endotoxin (Haskó et al., 2000; Liaudet et al., 2002). It also inhibits the activation of human neutrophils (Marton et al., 2001) and reduces ischaemia-reperfusion injury in the rat heart transplantation model (Szabó et al., 2006). In the CNS, inosine was shown to enhance axon regeneration (Benowitz et al., 2002; Irwin et al., 2006; Zai et al., 2009) and to protect neurons and astrocytes against hypoxic injury (Litsky et al., 1999; Shen et al., 2005; Liu et al., 2006; Wu et al., 2008; Ma et al., 2011). At present, little is known about the role of inosine at the NMJ. Moreover, inosine was classically considered to be an inactive metabolite of adenosine since, in sartorius muscles of the frog, Ribeiro and Sebastião (1987) found that the nucleoside did not affect the amplitude of the end-plate potential (EPP). Hence, the aim of this study was (i) to investigate the effect of inosine upon spontaneous and evoked ACh release at mammalian NMJ; (ii) to determine the receptor through which inosine exerts its action; (iii) to elucidate the presynaptic mechanisms underlying the modulatory effect; (iv) to identify the signal transduction pathway involved in the inosine responses; and (v) to investigate the physiological relevance of these effects of inosine.
Methods
Preparations and solutions
Experiments were carried out on phrenic nerve-diaphragm preparations taken from 219 adult CF1 mice (30–40 g) of either sex. All animal procedures were performed under protocols approved by national guidelines, which are in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications no. 80-23) revised 1996. Mice were anaesthetized with sodium thiopental (50 mg·kg−1, i.p.) and left hemidiaphragms were excised and transferred to a 5 mL chamber superfused (3 mL·min−1) with Ringer Krebs solution (mM: NaCl 135, KCl 5, CaCl2 2, MgCl2 1, d-glucose 11, HEPES 5, pH 7.3–7.4, bubbled with O2). In some experiments, a saline solution containing 0 CaCl2, 2 mM MgCl2, and 1 mM EGTA (0Ca2+-EGTA) was employed in order to eliminate the inward Ca2+ gradient. When the KCl concentration of the Ringer Krebs solution was raised to 12–15 mM, an equal amount of NaCl was removed from the incubation medium to maintain the isotonicity. In experiments performed in 12 mM K+ – 0Ca2+-EGTA, 100 μM CdCl2 was added to prevent Ca2+ outflow from depolarized nerve terminals when the electrochemical Ca2+ gradient was reversed. Hyperosmotic media were freshly prepared by adding 100 mM sucrose to Krebs solutions and their osmolarity were checked with a Fiske osmometer before each experiment. When using nitrendipine, experiments were performed with extreme care to minimize exposure of drug solutions to light. All recordings were carried out at room temperature (22–23°C). All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010).
Electrophysiological recordings
Miniature end-plate potentials (MEPPs) and EPPs were recorded at the end-plate region from muscle fibres using borosilicate glass microelectrodes (WP Instruments, Sarasota, FL, USA) filled with 3 M KCl, with a resistance of 5–10 MΩ. Muscle fibres with a resting membrane potential less negative than –60 mV or MEPPs/EPPs with a rise time greater than 1 ms were rejected. For EPP recordings, the phrenic nerve was stimulated supramaximally at a frequency of 0.5 Hz (0.1 ms duration) using a suction electrode attached to a stimulus isolation unit (Grass SIU5, Grass Instruments, Quincy, MA, USA) and stimulator (Grass S48). Muscle twitches were prevented by a submaximal concentration (0.8–1.6 μM) of d-tubocurarine. MEPP/EPP amplitudes were normalized to a resting membrane potential of – 75 mV, using the formula Vc = [Vo × (–75)]/E, where Vc is the corrected MEPP/EPP amplitude, Vo is the observed MEPP/EPP amplitude, and E is the resting membrane potential. Quantal content of the EPP (m) was assessed using the failure method: m = ln(N/n0), where N is the total number of successive trials (100 at 0.5 Hz) and n0 is the number of trials in which the response fails (absence of EPP). In this case, twitches were prevented by increasing the concentration of magnesium (MgCl2 12–14 mM) in the bathing solution.
In the experiments where the hypertonic response was evaluated, 10 junctions were previously sampled in the isotonic solution and their values averaged. In each synapse, MEPP frequency was recorded for 100 s. Then, immediately after exposure to hyperosmotic solution, synapses were sampled repeatedly from the same small area of diaphragm over brief intervals for 30 min. An effort was made to keep the intervals between sampling as short as possible. In this case, MEPP frequency was recorded for 10 s in each synapse. Tetrodotoxin 10−6 M was added to hypertonic solutions to prevent the muscle from twitching violently.
All signals were amplified with Axoclamp 2B (Molecular Devices, Sunnyvale, CA, USA) and digitized with Digidata 1322 (Molecular Devices) and then analysed using pClamp 8.2 software (Molecular Devices).
Data analysis
In all cases, data are reported as mean ± SEM and n represents number of animals (only left hemidiaphragm was used from each mouse for a given experiment). Areas under the hypertonic curves were calculated using Prism (version 5.01). Statistical comparisons among three or more groups were performed using one-way anova followed by Tukey's or Dunnett's post-test. Two group comparisons were performed using Student's paired t-test. Differences were considered to be significant when P < 0.05.
Immunohistochemistry
Tissues
Diaphragm or gastrocnemius muscles were used. To obtain further insights into A3 receptor localization, in some experiments gastrocnemius muscles were denervated by cutting out a 0.3 cm portion of the right leg sciatic nerve. For this procedure, animals were anaesthetized with ketamine 45 mg·kg−1/xylazine 6 mg·kg−1 (injected i.p.) and, after the wound had been closed, the animals were allowed to recover for 7 days in an animal care facility under temperature- and light-controlled conditions (20–23°C, 12 h light/12 h dark cycle) with food and water provided ad libitum. Then, mice were anaesthetized with sodium thiopental (50 mg·kg−1) and the gastrocnemius muscles were removed. Contralateral leg muscles were used as controls.
All types of muscles were fixed for 3 min in 4% paraformaldehyde in phosphate buffer (PB, 0.1 M pH 7.4) at room temperature. Then, preparations were washed in PB for 1 min, permeabilized in 1% Triton X-100 for 5 min, washed again in PB for 1 min, and finally cryoprotected in 30% sucrose in PB for no longer than 72 h. Blocks of muscle were included in a sealed plastic tube with OCT Tissue-Tek (Sakura Finetek, Inc., Torrance, CA, USA) and then frozen in isopentane precooled in dry ice.
Frozen blocks of tissue were cut transversely (8–9 μm) with a cryostat microtome, and sections were thaw-mounted onto polylysine gelatin-coated slides, air dried for 15 min and stored at –20°C.
Polyclonal antibodies and toxins
Specific primary antibodies for the A3 receptor were purchased from (Sigma Aldrich, St Louis, MO, USA). The polyclonal antibody was produced in rabbit using a synthetic peptide corresponding to the second extracellular loop of human A3 receptor as the immunogen. Double labelling was performed using goat anti-rabbit IgG coupled to Atto-488 (Sigma Aldrich) to identify the primary antibody and α-bungarotoxin coupled to tetramethylrhodamine (BgTx-R, Sigma Aldrich), to identify the postsynaptic ACh receptors. Antibody and toxin concentrations were as follows: anti-A3 receptor 1:200, secondary antibody 1:200 and BgTx-R 1:2000. Antibodies were diluted in 10 mM PBS containing 3% BSA, 0.1 M L-lysine and 0.075% Triton X-100, and the BgTx-R in 10 mM PBS.
Immunofluorescence
Tissue sections were processed simultaneously for double labelling by indirect immunofluorescence and direct staining with BgTx-R. All incubations were performed at room temperature, using 10 mM PBS pH 7.4 except where stated.
Sections were permeabilized with 0.1% Triton X-100 in PBS for 5 min, rinsed in PBS for 15 min and then incubated with the primary antibody at 4°C overnight (19–20 h).
After being successively washed with PBS for 30 min, with higher ionic concentration PBS (in mM 137 NaCl, 2.7 KCl, 4.3 Na2HPO4, 1.4 KH2PO4, pH 7.2) for 30 min and with PBS for 30 min, sections were incubated simultaneously with the secondary antibody and BgTx-R for 105 min, and washed in PBS for 40 min. Finally, the sections were mounted in 1:1 10 mM PBS:glycerol. The specificity of the A3 signal was further assessed by incubating the muscles in the absence of the primary antibody. No staining was observed in any control assays.
Microscopy and photography
Images were acquired with a Zeiss LSM 5 Pascal confocal microscope (Carl Zeiss, Oberkochen, Germany) equipped with an argon/HeNe-G laser, which allows simultaneous scanning and acquisition of the immunofluorescent sections (Plan-Apochromat 100x oil-immersion objective, numerical aperture 1.4). Assessment of co-localization of A3 receptor and ACh receptors immunoreactivity was performed using the Zeiss LSM Image Browser 4.2 software.
Chemicals
Chelerythrine, 8-cyclopentyl-1,3-dipropylxanthine (DPCPX), EGTA, inosine, (S)-5-isoquinolinesulfonic acid 4-[2-[(5-isoquinolinylsulfonyl) methylamino]-3-oxo-3-(4-phenyl-1-piperazinyl) propyl] phenyl ester 1-[N,O-bis(5-Isoquinolinesulfonyl)-N-methyl-L-tyrosyl]-4-phenylpiperazine (KN-62), (9S,10S,12R)-2,3,9,10,11,12-hexahydro-10-hydroxy-9-methyl-1-oxo-9,12-epoxy-1H-diindolo[1,2,3-fg:3′,2′,1′-kl]pyrrolo[3,4-i][1,6]benzodiazocine-10-carboxylic acid hexyl ester (KT-5720), α,β-methyleneadenosine 5′-diphosphate (αβ-MeADP), 3-ethyl-5 – benzyl – 2-methyl – 4 – phenylethynyl – 6 – phenyl-1, 4 – (±) – dihydropyridine – 3,5-, dicarboxylate (MRS-1191), N-ethylmaleimide (NEM), nitrendipine, 1-amino-4-[[4-[[4-chloro-6-[[3(or4)-sulfophenyl] amino]-1,3,5-triazin-2-yl]amino]-3-sulfophenyl] amino]-9,10-dihydro-9,10-dioxo-2-anthracenesulfonic acid (reactive blue-2), 8,8′-[carbonylbis [imino-3,1-phenylenecarbonylimino (4-methyl-3,1-phenylene) carbonylimino]]bis-1,3,5-naphthalenetrisulfonic acid hexasodium salt (suramin), and tetrodotoxin were purchased from Sigma-Aldrich Corp.; N-[2-[[3-(4-bromophenyl)-2-propenyl]amino]ethyl]-5-isoquinolinesulfonamide dihydrochloride (H-89), 1-[2-chloro – [[(3-iodophenyl) methyl] amino] – 9H – purin – 9 – yl]-1- deoxy-N-methyl – β- D -ribofuranuronamide (2-Cl-IB-MECA), 2-(2-furanyl)-7-(2-phenylethyl)-7H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin-5-amine (SCH-58261), and N-(6-aminohexil)-5-chloro-1-naphthalenesulfonamide hydrochloride (W-7) were obtained from Tocris Bioscience, Ellisville, MO, USA; and ω-agatoxin IVA (ω-Aga) and ω-conotoxin GVIA (ω-CgTx) were from Alomone Labs Ltd, Jerusalem, Israel. All other reagents were of the highest purity available. Aqueous dilutions of the stock solutions were made daily, and appropriate solvent controls were done.
The drug and molecular target nomenclature conform to BJP's Guide to Receptors and Channels (Alexander et al., 2011).
Results
Effect of inosine on spontaneous and evoked acetylcholine release
The results depicted in Figure 1A show that inosine induced a dose-related decrease in spontaneous ACh release (EC50 48.59 μM). The maximal decrease in MEPP frequency was obtained in the presence of 100 μM inosine (53.3 ± 2.0% of control values, P < 0.0001, n = 10, Figure 1B and C). These effects of inosine were completely reversible on washout with inosine-free medium, without any change in MEPP amplitude (control 0.94 ± 0.06 mV; after inosine 0.94 ± 0.04 mV, n = 6). When analysing the effect of inosine on evoked ACh release, we observed that the nucleoside decreased EPP amplitude to 64.4 ± 2.8% of control values (P < 0.0001, n = 7) and the EPP quantal content to 49.8 ± 9.0% of control values (P < 0.05, n = 4, Figure 1D, E and F). All these findings suggest a presynaptic action of the inosine.
Figure 1.
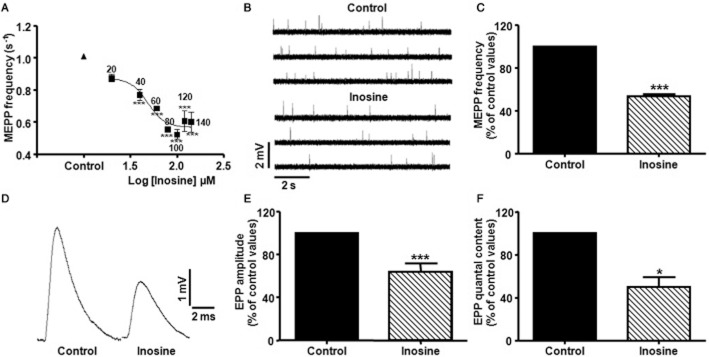
Inhibitory effect of inosine on spontaneous and evoked ACh release at the mouse NMJ. (A) Effect of inosine on MEPP frequency (s−1) as a function of its concentration. Each point represents mean ± SEM (n = 4), ***P < 0.001 versus control, anova followed by Dunnett's test; EC50: 58.59 μM. (B) Representative MEPPs recorded from diaphragm muscle fibres bathed with control solution (Vm:−74.9 mV), and with 100 μM inosine (Vm:−74.2 mV). Recordings were made from the same diaphragm preparation. (C) Summary bar graph showing the presynaptic inhibitory effect of 100 μM inosine on MEPP frequency (n = 10). Data (mean ± SEM) are expressed as percentage of control values. ***P < 0.0001, Student's paired t test. (D) Effect of 100 μM inosine on EPP amplitude at mammalian NMJ. Each representative tracing is the average of 30 EPPs at a stimulation frequency of 0.5 Hz recorded from diaphragm muscle fibres bathed with control solution (Vm:−72.1 mV), and with 100 μM inosine (Vm:−73.3 mV). Recordings were made from the same diaphragm preparation. (E,F) Summary bar graphs show the presynaptic inhibitory effect of 100 μM inosine on EPP amplitude (n = 7) and on EPP quantal content (n = 4), respectively. Data (mean ± SEM) are expressed as percentage of control values. ***P < 0.0001, *P < 0.05, Student's paired t test.
Inosine activates A3 adenosine receptors
Whereas the effects of adenosine are mediated by the combined action of the entire adenosine receptor family (A1, A2A, A2B, A3), no specific inosine receptor has been identified up to now. At the mammalian NMJ, presynaptic nerve terminals contain adenosine receptors as well as ATP receptors (revised by Burnstock, 2007). Thus, to investigate the receptor to which inosine binds to, we analysed its effect on spontaneous ACh release in the presence of antagonists of different purine receptors. We found that DPCPX (0.1 μM, selective A1 receptor antagonist), SCH-58261 (50 nM, selective A2A receptor antagonist), suramin (100 μM, a non-specific P2 receptor antagonist) and reactive blue-2 (5 μM, P2Y4,6,11,12,13 receptor antagonist) did not prevent the modulatory effect of inosine (Table 1). Conversely, pretreatment of the preparations with the specific A3 receptor antagonist MRS-1191 (5 μM, Jiang et al., 1996; Jacobson et al., 1997) prevented inosine-mediated presynaptic inhibition of MEPP frequency (MRS-1191 91.1 ± 3.6 (n = 4), MRS-1191 + inosine 95.5 ± 1.8, n = 4, Figure 2A and B). Moreover, the selective A3 adenosine receptor agonist 2-Cl-IB-MECA (200 nM) decreased spontaneous neurotransmitter secretion to 66.6 ± 0.9% of control values (P < 0.001, n = 3); and this effect was prevented by the A3 receptor antagonist (MRS-1191 97.7 ± 1.5%, MRS-1191 + 2-Cl-IB-MECA 102.3 ± 2.7%, n = 3).
Table 1.
Effect of A1, A2A and P2Y receptor antagonists on the inosine-mediated modulation of spontaneous ACh secretion
| Solution | MEPP frequency (% of control values) |
|---|---|
| DPCPX | 99.7 ± 3.8 (n = 4) |
| DPCPX + inosine | 57.8 ± 1.0 (n = 4)*** |
| SCH-58261 | 102.5 ± 1.6 (n = 4) |
| SCH-58261 + inosine | 65.8 ± 2.2 (n = 4)*** |
| Suramin | 99.6 ± 3.9 (n = 4) |
| Suramin + inosine | 61.7 ± 3.4 (n = 4)*** |
| Reactive blue-2 | 100.6 ± 3.3 (n = 4) |
| Reactive blue-2 + inosine | 65.0 ± 1.9 (n = 4)*** |
P < 0.001 versus control values and the antagonist without inosine. anova followed by Tukey's test.
Figure 2.
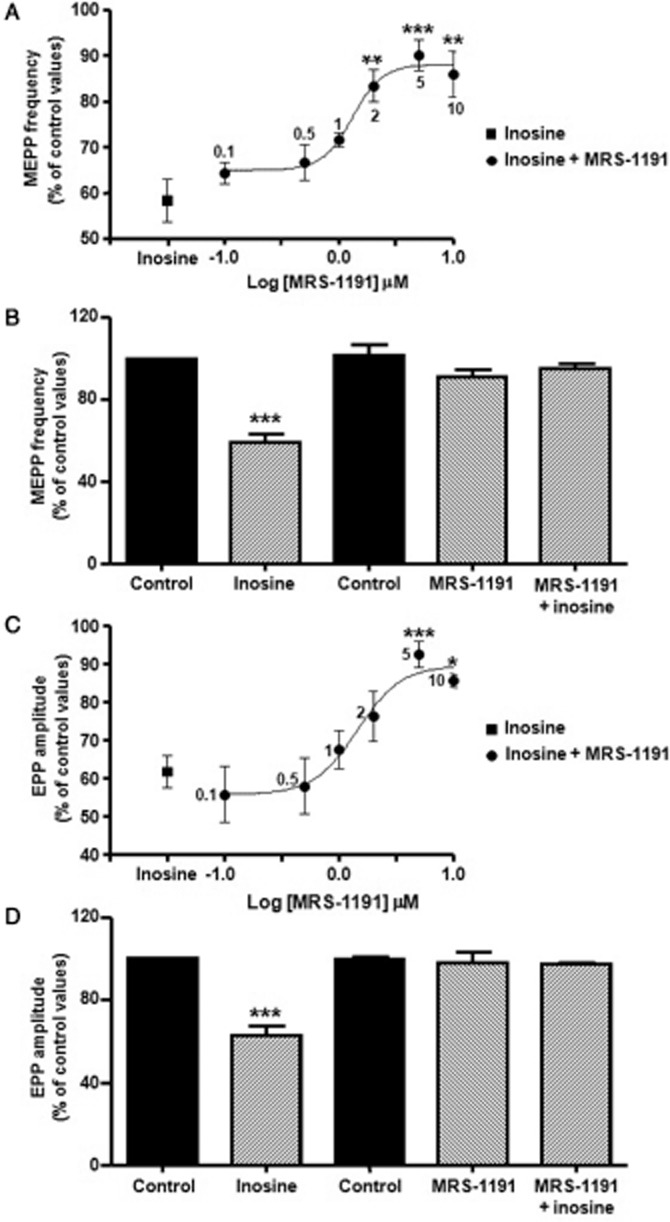
Inosine reduces ACh release when activating A3 adenosine receptors. (A) Effect of increasing concentration of MRS-1191 (selective antagonist of A3 receptors) regarding the effect of 100 μM inosine on MEPP frequency (expressed as % of control values). Each point represents mean ± SEM (n = 3), ***P < 0.001, **P < 0.01 versus inosine, anova followed by Dunnett's test, EC50: 1.31 μM. (B) MRS-1191 (5 μM) did not modify MEPP frequency, but prevented inosine effect on spontaneous secretion (n = 4). (C) Effect of increasing concentration of MRS-1191 on the effect of 100 μM inosine on EPP amplitude (expressed as % of control values). Each point represents mean ± SEM (n = 3), ***P < 0.001, *P < 0.05 versus inosine, anova followed by Dunnett's test, EC50: 1.45 μM. (D) MRS-1191 (5 μM) did not altered EPP amplitude, but suppressed the modulatory action of inosine on evoked ACh secretion (n = 4). In (B) and (D) data (mean ± SEM) are expressed as percentage of control values (black bar). ***P < 0.001, anova followed by Tukey's test.
As illustrated in Figure 2C and D, MRS-1191 also prevented the effect of inosine on EPP amplitude (MRS-1191 97.8 ± 5.3% of control values, MRS-1191 + inosine 97.3 ± 0.5%, n = 4), indicating that the modulatory action of inosine is obtained when presynaptic A3 receptors are activated.
To assess the specific distribution of A3 receptors at the NMJ, immunohistochemical studies were performed. Muscle cross-sections were dual-labelled with BgTX-R to identify postsynaptic ACh receptors at the motor end-plate region and, antibodies to A3 receptors followed by staining with goat anti-rabbit IgG conjugated with Atto-488 to visualize the location of A3 receptors. Figure 3 illustrates the co-staining of the diaphragm (A-C) and gastrocnemius (D-F) NMJs by BgTx-R and anti-A3 antibody. To show that anti-A3 antibodies bind to epitopes localized at the presynaptic membrane, immunostaining was performed in denervated gastrocnemius muscles (Figure 3G–I). In this case, BgTx-R labelled ACh receptors, whereas no labelling was observed with anti-A3 antibodies. The disappearance of A3 receptors in these sections is consistent with the degeneration of nerve terminals in response to denervation (Miledi and Slater, 1970). These results suggest that A3 receptors are present at the presynaptic membrane of motor nerve terminals.
Figure 3.
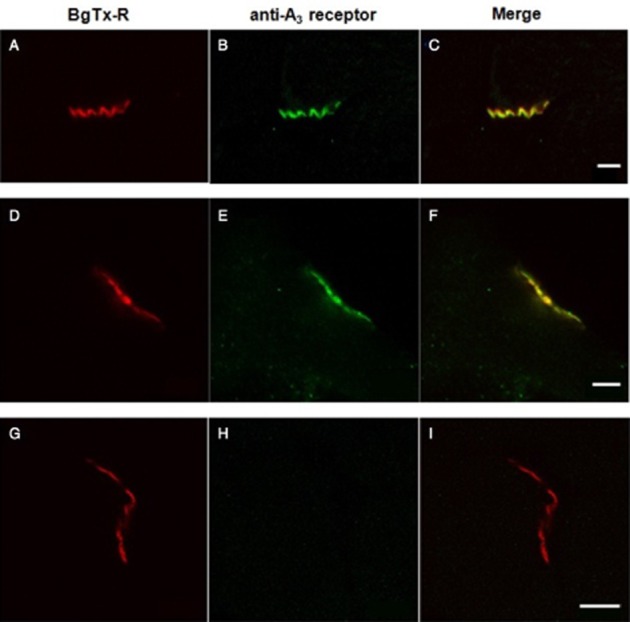
Distribution of A3 receptors at the mouse NMJ in transverse sections of diaphragm (A–C) and gastrocnemius (D–I) muscles. Sections were dual-labelled with BgTx-R (red) to identify ACh receptors at the NMJ (A,D,G) and with the specific A3 antibody, visualized with Atto-488 (green) conjugated secondary antibody (B,E,H). In innervated muscles (A–F), A3 receptors were localized at the NMJ (B,C,E,F), whereas in denervated muscles (G–I) no labelling was observed with the A3 antibody (H,I). Scale bar 5 μm.
Presynaptic mechanisms involved in inosine-mediated modulation of transmitter release
The next aim was to elucidate the mechanisms by which inosine decreases neurotransmitter release. One possibility was that activation of A3 receptors leads to a reduction in Ca2+ influx through the voltage-gated calcium channels (VGCCs) present at the presynaptic membrane of motor nerve terminals (P/Q-type, L-type and N-type VGCCs). Therefore, we first investigated the action of inosine on spontaneous ACh release in diaphragms previously incubated with the universal VGCC blocker Cd2+ (100 μM). As shown in Figure 4A, Cd2+ reduced MEPP frequency to 50.4 ± 2.6% of control values (P < 0.001, n = 4) and the addition of inosine to the bath solution did not induce any further reduction in MEPP frequency (57.4 ± 1.9% of control values). Since L-type and N-type VGCCs are involved in tonic secretion at the mammalian NMJ, (Losavio and Muchnik, 1997), we studied the effect of inosine in the presence of the specific channel blockers. The L-type VGCC blocker, nitrendipine (5 μM), reduced spontaneous secretion to 52.3 ± 3.3% of control values (P < 0.001, n = 4) and prevented inosine-induced presynaptic inhibition (53.8 ± 4.8% of control values). On reversing the order of administration, inosine decreased MEPP frequency to 52.1 ± 3.2% of control values (P < 0.001, n = 3), and the application of nitrendipine did not induce any additional effect (52.0 ± 4.8%, Figure 4B and C). In contrast, the specific N-type VGCC blocker ω-CgTx (5 μM) reduced MEPP frequency to 65.4 ± 3.0% of control values (P < 0.001, n = 4), but the addition of inosine to the solution induced a further decrease in spontaneous ACh release (42.9 ± 5.4% of control values, P < 0.001; ω-CgTx versus ω-CgTx + inosine, P < 0.01, Figure 4D).
Figure 4.
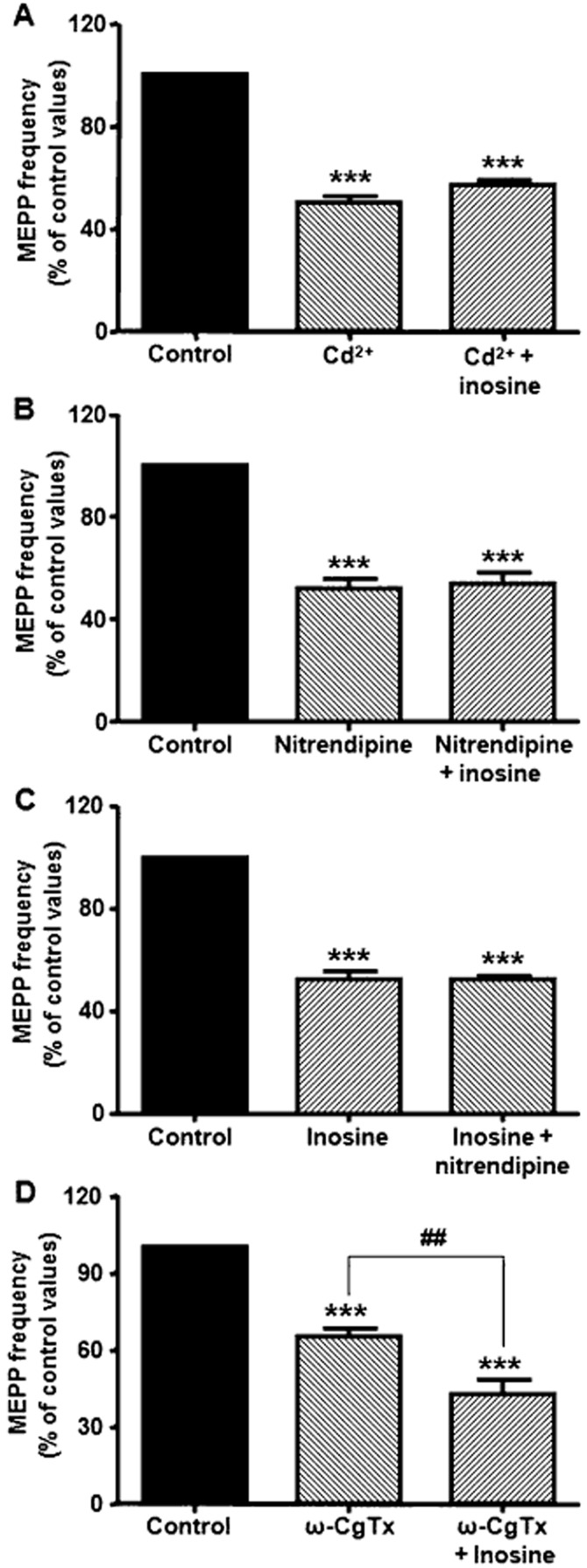
Effect of 100 μM inosine on VGCCs associated with spontaneous ACh secretion. (A) The universal VGCC blocker Cd2+ (100 μM, n = 4) diminished MEPP frequency and prevented the effect of inosine. (B) The L-type VGCC blocker nitrendipine (5 μM, n = 4) decreased spontaneous secretion and prevented the effect of inosine. (C) Nitrendipine had no effect when preparations were pre-incubated with inosine (n = 3). (D) The N-type VGCC blocker ω-CgTx (5 μM, n = 4) reduced MEPP frequency and did not prevent the modulatory effect of inosine on L-type VGCCs. Data (mean ± SEM) are expressed as percentage of control values. ***P < 0.001, ##P < 0.01, anova followed by Tukey's test.
The evoked release of ACh from mature mammalian motor nerve relies on Ca2+entry through P/Q-type VGCCs (Protti and Uchitel, 1993). Since treatment of preparations with Cd2+ or the P/Q-type VGCC blocker would suppress responses induced by electrical stimulation (EPPs), we analysed the effects of inosine in preparations exposed to high K+ concentrations, a situation in which the increase in MEPP frequency also depends on Ca2+ influx via P/Q-type VGCCs (Protti and Uchitel, 1993; Losavio and Muchnik, 1997). When preparations were exposed to 15 mM K+, MEPP frequency increased to 780.8 ± 31.1% of control values (P < 0.001, n = 5). Interestingly, the addition of inosine to preparations in 15 mM K+ did not provoke a significant modulation of asynchronic ACh secretion (701.2 ± 36.1% of control values, Figure 5A). To evaluate the possibility that this result is due to the extracellular accumulation of endogenous adenosine in the synaptic space occupying A3 receptors, we studied the action of inosine at high K+ in the presence of 100 μM αβ-MeADP (inhibitor of ecto-5′-nucleotidase, the enzyme that acts at the final step in the conversion from ATP to adenosine). In these conditions, inosine did decrease ACh secretion (15 mM K+ + αβ-MeADP 888.5 ± 62.3% of control values, n = 4; 15 mM K+ + αβ-MeADP + inosine 623.1 ± 42.3%, P < 0.05, Figure 5B). Similarly, the inhibition of A3 receptors with the antagonist MRS-1191 (5 μM) in 15 mM K+ provoked an increase in asynchronous ACh release (15 mM K+ 830.4 ± 55.5% of control values, 15 mM K+ + MRS-1191 1067.0 ± 78.0%, n = 4, P < 0.05, Figure 5C), suggesting that, at high K+, endogenous nucleosides modulate neurotransmitter secretion by activating A3 receptors. Next, we decided to investigate the action of inosine in preparations incubated with 12 mM K+, a concentration at which the modulatory effect of inosine could be observed directly (12 mM K+ 432.1 ± 27.3% of control values, n = 4; 12 mM K+ + inosine 279.8 ± 36.7%, P < 0.01, Figure 6A). The non-selective VGCC blocker Cd2+ decreased K+-evoked neurotransmitter secretion and prevented this presynaptic action induced by inosine (12 mM K+ 391.6 ± 53.1% of control values, n = 3; 12 mM K+ + Cd2+ 106.2 ± 13.2%, P < 0.001 vs. 12 mM K+; 12 mM K+ + Cd2+ + inosine 113.8 ± 12.0%), as shown in Figure 6. The same behaviour was observed when extracellular Ca2+ was eliminated from the bathing solutions (0Ca2+-EGTA-Cd2+): 12 mM K+ + 0Ca2+-EGTA-Cd2+ 43.1 ± 3.3% of control values, n = 3; 12 mM K+ + 0Ca2+-EGTA-Cd2+ + inosine 42.7 ± 3.8% of control values (Figure 6C) or when preparations were pre-incubated with 100 nM ω-Aga, a specific P/Q-type VGCC blocker (12 mM K+ 395.5 ± 13.5% of control values, n = 4; 12 mM K+ + ω-Aga 110.5 ± 9.5%, P < 0.001 vs. 12 mM K+; 12 mM K+ + ω-Aga + inosine 108.1 ± 1.7%, Figure 6D).
Figure 5.
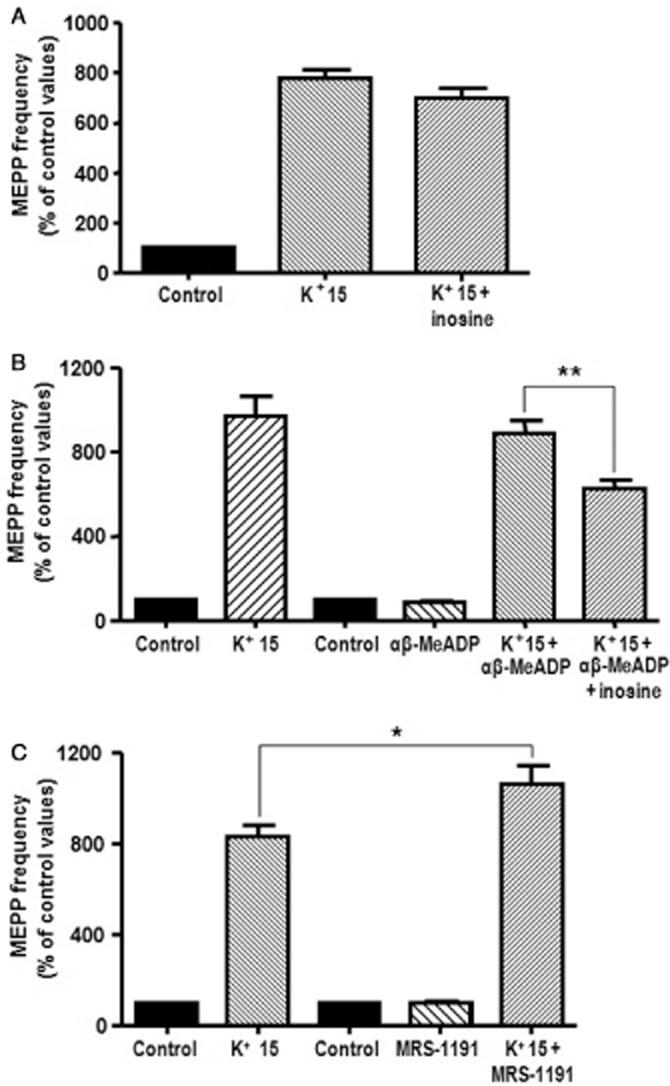
Activation of A3 receptors by endogenous adenosine prevented the effect of inosine in 15 mM K+. (A) Inosine (100 μM) failed to reduce asynchronous ACh release induced by 15 mM K+ (n = 5). (B) The inhibition of the production of adenosine by 100 μM αβ-MeADP (inhibitor of ecto-5′-nucleotidase, n = 4) allowed the effect of 100 μM inosine on 15 mM K+-evocked ACh release. (C) Blockade of A3 receptors with the selective antagonist MRS-1191 (5 μM) prevented the inhibitory action of endogenous nucleosides and further increased MEPP frequency in 15 mM K+ (n = 4). Data (mean ± SEM) are expressed as percentage of control values. **P < 0.01, *P < 0.05, anova followed by Tukey's test.
Figure 6.
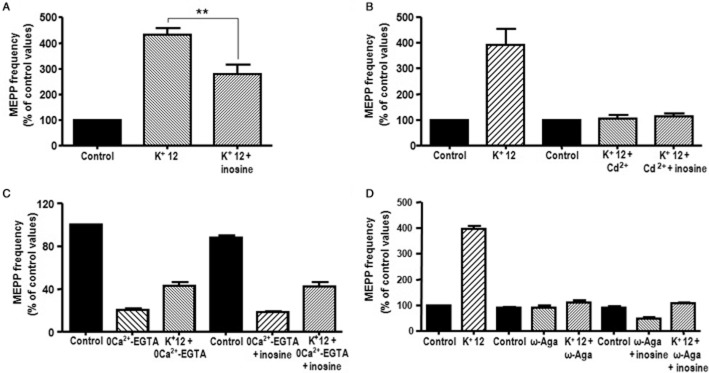
Inosine-mediated modulation of asynchronous ACh secretion is associated with Ca2+ influx through P/Q-type VGCC. (A) Inosine (100 μM) reduced asynchronous ACh release induced by 12 mM K+ (n = 4). (B,C,D) The modulatory effect of inosine on 12 mM K+-evoked ACh release was not observed when preparations were pre-incubated with the universal VGCC blocker Cd2+ (100 μM, n = 3), 0 Ca2+-EGTA (n = 3) or the specific P/Q-type VGCC blocker ω-Aga (100 nM, n = 4), respectively. In (D) it is interesting to note that 100 μM inosine decreased spontaneous ACh secretion when MEPP frequency was assessed in the presence of ω-Aga and control K+ (5 mM), a situation not dependent on Ca2+ influx through P/Q-type VGCCs (n = 4, P < 0.01). Data (mean ± SEM) are expressed as percentage of control values. **P < 0.01, anova followed by Tukey's test.
Taken together, these results suggested that the activation of A3 receptors leads to a modulation of the L-type and P/Q-type VGCCs associated with the spontaneous and evoked release of ACh respectively.
In order to investigate whether inosine-mediated inhibition is also associated with the modulation of a step in the secretory machinery down-stream of Ca2+ influx, we studied the effect of inosine on hypertonicity-induced enhancement of MEPP frequency, a situation that has been shown to be independent of Ca2+ (Furshpan, 1956; Hubbard et al., 1968; Rosenmund and Stevens, 1996; Losavio and Muchnik, 1997; Kashani et al., 2001). As shown in Figure 7A, B and C, when hypertonic solution was applied to diaphragm muscles, MEPP frequency increased from a value of 0.90 ± 0.05 s−1 in isotonic condition to a peak of 8.75 ± 0.62 s−1 (n = 4), and declined gradually during the continuous application of the hypertonic solution. The area under the curve was 116.9 ± 3.8 (n = 4). After washout with isotonic solution MEPP frequency returned to control values. The addition of inosine to the preparations reduced MEPP frequency to 0.55 ± 0.04 s−1 (62.1 ± 2.7% of the control responses, p < 0.001) in isotonic solution, but did not affect the hypertonic response, since MEPP frequency at the peak of the response was 8.13 ± 0.75 s−1 (93.8 ± 6.7% of the control responses) and the area under the curve was 109.0 ± 6.4 (93.7 ± 6.8% of the control responses). To rule out the possibility that endogenous adenosine, generated during the hypertonic response, may be occupying A3 receptors and preventing the binding of inosine, we assessed the effect of inosine in the presence of 100 μM αβ-MeADP. In this case, inosine was able to decrease the hypertonic response (peak of the response: αβ-MeADP 98.5 ± 10.7% of control response; αβ-MeADP + inosine 67.4 ± 8.9%, P < 0.05; area under the curve: αβ-MeADP 97.0 ± 10.4%; αβ-MeADP + inosine 64.3 ± 3.1%, P < 0.01, n = 7, Figure 7D, E, and F). This finding suggests that activation of A3 receptors also modulates the transmitter-releasing machinery in a Ca2+ independent manner.
Figure 7.
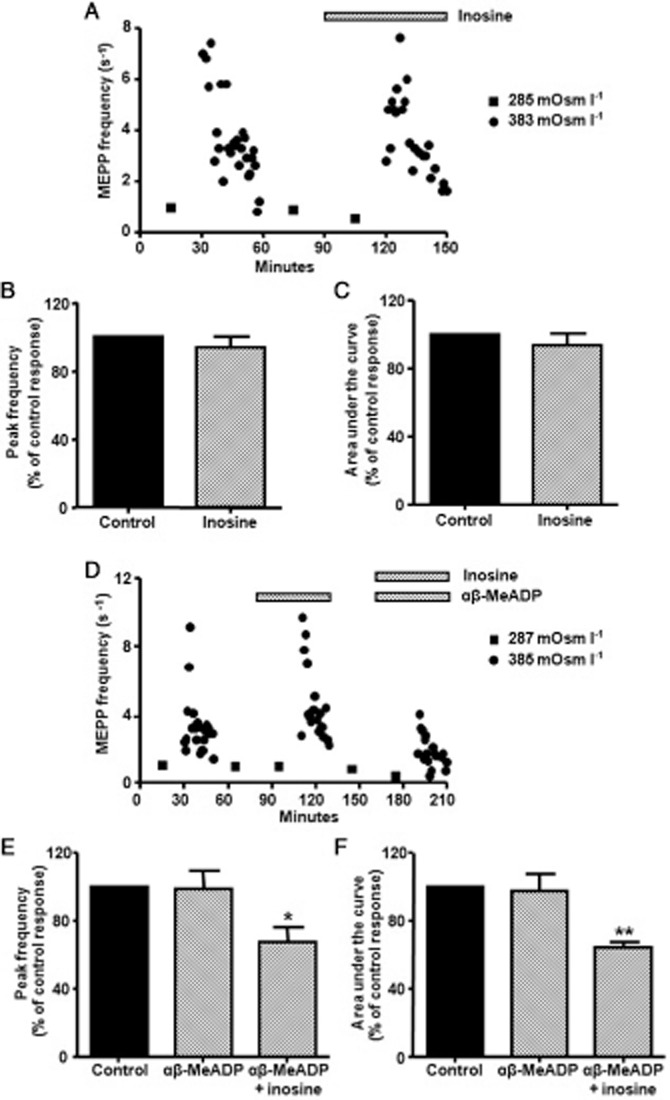
Effect of 100 μM inosine on the hypertonic response. (A) Effect of inosine on ACh release when a diaphragm muscle was exposed to isotonic and hypertonic conditions. (B,C) Summary bar graphs show the lack of a modulatory effect of inosine on the peak frequency and area under the curve of the hypertonic response (n = 4). (D) Effect of inosine on ACh release when a diaphragm muscle was exposed to isotonic and hypertonic conditions in the presence of 100 μM αβ-MeADP. (E,F) Summary bar graphs showing the modulatory effect of inosine on the peak frequency and area under the curve of the hypertonic response (n = 7) when the production of adenosine was inhibited by αβ-MeADP. In (A) and (D), square symbols indicate mean values from 10 synapses obtained after exposing the preparations to isotonic condition and circles represent the time course of hypertonic response (each point represents averaged value of MEPP frequency recorded from a single synapse). In (B), (C), (E), and (F), data (mean ± SEM) are expressed as percentage of control values. **P < 0.01, *P < 0.05, anova followed by Tukey's test.
Intracellular pathways associated with the activation of A3 adenosine receptors
It is known that A3 receptors couple predominantly to G proteins of the Gi/o family, leading to inhibition of adenylyl cyclase. Furthermore, activation of A3 receptors can also stimulate PLC activity via Gq proteins (Zhou et al., 1992; Ramkumar et al., 1993; Abbracchio et al., 1995; Palmer et al., 1995). To determine whether A3 receptors at the NMJ are coupled to Gi/o proteins, we investigated the effect of inosine in preparations pre-incubated with NEM, a sulphydryl-alkylating reagent that interferes with Gi/o protein-mediated second messenger pathways (Hoshino et al., 1990; Shapiro et al., 1994). We found that 10 μM NEM prevented the effect of inosine on MEPP frequency (NEM 125.7 ± 13.9% of control values, NEM + inosine 123.8 ± 6.6%, n = 5) suggesting that A3 receptors are linked to Gi/o.
In an attempt to elucidate the transduction mechanisms associated with A3 receptor activation, we investigated the action of inosine in the presence of inhibitors of several pathways (Table 2). The specific PKA inhibitors, H-89 (1 μM) and KT-5720 (500 nM), did not modify spontaneous ACh release or alter (mimick or block) the effect of inosine on MEPP frequency, indicating that inosine-mediated modulation was not associated with an effect on the cAMP cascade. The concentration of H-89 used in these experiments was shown to inhibit PKA in our system (De Lorenzo et al., 2004; Veggetti et al., 2008). When analysing the possible participation of PKC in the intracellular pathway activated by inosine, we observed that chelerythrine (5 μM), a specific inhibitor of PKC, completely prevented the inhibitory effect of inosine on spontaneous ACh secretion. Similar results were obtained when the sequence of application of drugs was reversed. These data suggest that PKC is involved in the presynaptic inhibition induced by inosine.
Table 2.
Effect of inhibitors of second-messenger pathways on the inosine-mediated effect
| Solution values | MEPP frequency (% of control values) | EPP amplitude (% of control) |
|---|---|---|
| H-89 | 101.8 ± 5.0 (n = 4) | 92.9 ± 0.9 (n = 4) |
| H-89 + inosine | 67.9 ± 5.0 (n = 4)*** | 63.4 ± 2.4 (n = 4)*** |
| KT-5720 | 98.4 ± 2.4 (n = 3) | |
| KT-5720 + inosine | 66.4 ± 4.8 (n = 3)*** | |
| Chelerythrine | 102.7 ± 9.3 (n = 4) | 102.9 ± 9.8 (n = 4) |
| Chelerythrine + inosine | 105.9 ± 10.7 (n = 4) | 109.3 ± 9.2 (n = 4) |
| Inosine | 65.5 ± 5.7 (n = 3)*** | |
| Inosine + chelerythrine | 93.9 ± 1.5 (n = 3) | |
| W-7 | 97.8 ± 1.7 (n = 4) | 107.4 ± 9.1 (n = 4) |
| W-7 + inosine | 97.6 ± 7.5 (n = 4) | 101.4 ± 8.9 (n = 4) |
| KN-62 | 104.4 ± 7.9 (n = 4) | 104.3 ± 7.5 (n = 6) |
| KN-62 + inosine | 68.0 ± 4.9 (n = 4)** | 67.9 ± 8.1 (n = 6)** |
P < 0.001 and
P < 0.01 versus control values and the inhibitor without inosine.
anova followed by Tukey's test.
In our previous research, we showed that activation of A1 receptors and P2Y receptors decreases ACh release by a Ca2+-calmodulin-dependent mechanism, since this presynaptic inhibitory effect was prevented by the calmodulin antagonist W-7 (De Lorenzo et al., 2004; 2006; Veggetti et al., 2008). Hence, we examined the possibility that the above mechanism might be involved in the effect of inosine and found that 50 μM W-7 prevented the effects of inosine. However, pretreatment of the preparations with the specific inhibitor of calcium/calmodulin-dependent protein kinase II (CAMKII) KN-62 (10 μM) did not affect inosine's action, indicating that it is independent of the phosphorylation induced by the CAMKII.
Consistent with the above results, the effect of inosine on EPP amplitude was also blocked by 5 μM chelerythrine and 50 μM W-7, but not by 1 μM H-89 and 10 μM KN-62, suggesting that the intracellular pathways related to PKC and calmodulin are also involved with this effect of inosine (Table 2).
Discussion and conclusions
In this study, we have demonstrated that, contrary to what was classically believed, inosine is able to modulate ACh release at the mouse NMJ. We found that 100 μM inosine depressed MEPP frequency without affecting MEPP amplitude and reduced EPP amplitude as well as its quantum content by activating A3 receptors. This result differs from that observed by Ribeiro and Sebastião (1987) at the frog sartorius NMJ, where they showed that 100 μM inosine had virtually no effect on EPP amplitude. These discrepancies might reflect different sensitivities to inosine itself or a different density or distribution of A3 receptors at the presynaptic membrane in these species.
There are no receptors known to be specific for inosine. Although the nucleoside usually binds to A3 adenosine receptors to promote its actions (Jin et al., 1997; Tilley et al., 2000; Gomez and Sitkovsky, 2003), it has been shown to bind to other adenosine receptors (Gomez and Sitkovsky, 2003; Nascimento et al., 2010) and even to produce its effects by a GPCR independent of adenosine receptors (Idzko et al., 2004). Our results suggest that inosine binds to A3 receptors since MRS-1191 was the only purine receptor antagonist that prevented the inosine-mediated presynaptic inhibition of spontaneous and evoked ACh secretion. The presence of A3 receptors at motor nerve terminals was proposed by Ribeiro and Sebastião (1986), but this is the first time that the existence of these receptors has been demonstrated in pharmacological and immunohistochemical studies.
At the mammalian NMJ, activation of A1 receptors reduces action potential-evoked ACh secretion by a mechanism that decreases P/Q-type Ca2+ currents (Hamilton and Smith, 1991; Silinsky, 2004) and spontaneous secretion by affecting the nitrendipine-sensitive component of MEPP frequency (De Lorenzo et al., 2004). Furthermore, a Ca2+-independent step in the cascade of the exocytotic process also appears to be involved in this response (Silinsky, 2005; Veggetti et al., 2008). The present results provide evidence that activation of A3 receptors interfere with calcium-dependent mechanisms, since incubation of the preparations with the universal VGCC blocker Cd2+ or removal of extracellular Ca2+ (0Ca2+-EGTA), abolished the effect of inosine. We found that inosine reduced spontaneous ACh release by modulating L-type VGCCs, without affecting N-type VGCCs since nitrendipine prevented inosine effect, whereas in the presence of ω-CgTx, inosine induced a further reduction in MEPP frequency. Likewise, the specific antagonist of P/Q-type VGCCs, ω-Aga, abolished the modulatory action of inosine on 12 mM K+-induced ACh release. Hence, similar to A1 receptors, it is likely that activation of A3 receptors at the mouse NMJ, induces presynaptic inhibition of spontaneous and evoked neurotransmitter secretion by reducing Ca2+ influx through L-type and P/Q-type VGCC respectively.
However, it is also possible that inosine directly modulates a step in the secretory machinery downstream of Ca2+ entry by an effect independent of that on VGCCs. Transmitter release can be induced by raising the tonicity of the superfusing solution, a condition known to be independent of [Ca2+]o but this mechanism appears to share the major elements of the basic Ca2+-triggered vesicle fusion (Dreyer et al., 1987; Gansel et al., 1987; Aravamudan et al., 1999). Our results suggest that activation of A3 receptors reduces ACh secretion by acting on a step downstream of Ca2+ entry, since inosine decreases the enhancement of neurotransmitter release induced by hypertonicity (peak and area under the curve of the hypertonic response), although this effect was only observed when the conversion from AMP to adenosine was inhibited by αβ-MeADP (see below for discussion). We have previously demonstrated that, at mammalian NMJ, hypertonic responses are not affected by the specific VGCC blockers nifedipine, ω-CgTx or ω-Aga (Losavio and Muchnik, 1997). Therefore, the decrease in the hypertonic response induced by inosine is not the result of a lower availability of intracellular Ca2+ provoked by the action of the nucleoside on VGCCs. As presynaptic VGCCs are intimately coupled to key elements of the synaptic vesicle docking and fusion processes (Khanna et al., 2007), it is possible that the action of an A3 agonist on strategic components of the secretory apparatus could decrease the activation of the VGCCs. In this regard, Silinsky (2005) showed in the mouse, that cleavage of the presynaptic membrane SNARE syntaxin with botulinum toxin type C decreased the inhibitory effect of adenosine on calcium currents. Further experiments are needed to clarify whether the action of inosine on presynaptic VGCCs is associated with an effect on the secretory machinery downstream of Ca2+ influx or whether they are individual targets.
A3 receptors couple primarily to proteins of the Gαi class and to a lesser extent to Gαq/11, although additional intracellular pathways have recently been shown to be involved in receptor signalling (revised by Gessi et al., 2008). Our data showed that, at the mouse NMJ, A3 receptors are coupled to Gi/o protein, since incubation with NEM prevented the effect of inosine. Although the downstream mechanism of A3 receptors is commonly based on inhibition of adenylyl cyclase resulting in a reduction in intracellular cAMP levels-transduction pathway (Zhou et al., 1992), our results indicate that this is not the primary transduction pathway by which stimulation of A3 receptors produced its physiological effects, since the specific inhibitors of PKA, H-89 or KT-5720, neither mimicked nor occluded the effect of inosine. When evaluating the involvement of PKC, we found that the PKC inhibitor chelerythrine prevented the response to inosine. It has been suggested that βγ subunits, released by Gi-o proteins, stimulate the PLC-diacylglycerol-PKC pathway (Dickenson and Hill, 1998; Selbie and Hill, 1998). Hence, in our experiments activation of PKC by inosine could phosphorylate presynaptic VGCCs leading to a decrease in Ca2+ influx, as found in cerebellar granule cells (Perroy et al., 2000) and in cardiac myocytes (Zhang et al., 1997; McHugh et al., 2000). Alternatively, PKC might phosphorylate some of the proteins involved in the exocytotic process. In particular, phosphorylation of SNAP-25 and Munc-18 by PKC has been demonstrated to reduce the affinity of these proteins with syntaxin (Fujita et al., 1996; Shimazaki et al., 1996). PKC-induced phosphorylation of SNAP-25 at Ser187 which modulates calcium dynamics by inhibiting VGCCs (Pozzi et al., 2008).
Our results also suggest that calmodulin is involved in the inosine-induced presynaptic inhibition, since its antagonist W-7 prevented this effect of inosine. Application of the CaMKII inhibitor KN62 did not modify the effect of inosine, demonstrating that CaMKII was not involved in this effect of calmodulin. It is known that calmodulin associates with presynaptic VGCCs including P/Q-type and L-type VGCCs (Lee et al., 1999; Dick et al., 2008). Moreover, Ivanina et al. (2000) found that at basal cellular levels of Ca2+, G protein βγ subunits have an inhibitory effect on L-type VGCC dependent on calmodulin. Furthermore, calmodulin can interact with proteins associated with exocytosis, for example, the GTP-bound form of Rab3 has to interact with Ca2+-calmodulin in order to inhibit secretion (Coppola et al., 1999).
Another interesting finding in our study was that inosine failed to exert any modulatory effect in preparations exposed to 15 mM K+ or on hypertonic responses (See Figures 5A and 7A–C). This lack of effect may be due to the extracellular accumulation of endogenous adenosine at the synaptic cleft, generated as result of the increased ACh secretion induced by a high K+ concentration or hypertonicity. Indeed, we found that inhibiting the production of adenosine by addition of αβ-MeADP, allowed the activation of A3 receptors by inosine and its modulatory effects. On the other hand, since inosine and adenosine access the intracellular space through the same equilibrative nucleoside transporters (Pastor-Anglada et al., 2001), it is possible that the addition of exogenous inosine might impair adenosine uptake into the cells via the equilibrative transporters increasing adenosine concentration in the synaptic cleft. Alternatively, adenosine may also be released as such from stimulated motor nerve terminals, skeletal muscle fibres and perisynaptic Schwann cell (Smith, 1991; Santos et al., 2003). In all cases, adenosine could occupy the presynaptic A3 receptors preventing the effect of inosine. In previous studies, we demonstrated that endogenous adenosine is able to activate A1 receptors and to modulate neurotransmitter secretion when muscles are exposed to high K+ concentration (15 and 20 mM) or to hypertonicity (De Lorenzo et al., 2004; Veggetti et al., 2008). In the present study, tonic activation of A3 receptors by endogenously generated adenosine was revealed when, under depolarizing conditions, the blockade of A3 receptors by the selective antagonist MRS-1191 induced a further increase in ACh secretion, endorsing the above hypothesis.
Of the four adenosine receptors subtypes identified, A1 and A2A receptors are activated by submicromolar concentrations of adenosine (Zhou et al., 1992), whereas A2B and A3 receptors are only activated by micromolar concentrations of this nucleoside (Olah and Stiles,1995). Inosine has been found to activate rat and guinea pig A3 receptors with Ki values in the range of 15–25 μM (Jin et al., 1997), but this nucleoside accumulates to even higher levels than adenosine in ischaemic tissues (Roth et al., 1997; Linden, 2001; Kékesi et al., 2002; Shen et al., 2005; Takahashi et al., 2010). Hence, even though A3 receptors may not have a very high affinity for inosine, interstitial concentrations of the nucleoside may be high enough to activate these receptors in ischaemic conditions. This might be the case at the NMJ. Neurotransmitter release is affected by a deficit of oxygen (Hirsch and Gibson, 1984) and its failure produces muscular weakness during ischaemia (Eccles et al., 1966). So, an increase in the concentration of inosine in the synaptic cleft, coming from the deamination of adenosine, or from intracellular sources through equilibrative transporters, might provide a modulatory effect on ischaemic tissues, as occurs in the CNS.
In conclusion, at mammalian NMJ, inosine induces presynaptic inhibition of spontaneous and evoked ACh release by activating A3 receptors, through a mechanism that involves L-type and P/Q-type VGCCs, and a Ca2+-independent step in the cascade of the exocytotic process. We found that A3 receptors are coupled to Gi/o protein and that PKC and calmodulin might be involved in the action of this nucleoside. Further experiments are needed to provide information about the relative contribution of inosine to the modulatory role of purines at the NMJ, especially during hypoxia.
Acknowledgments
The authors thank Mrs. María Fernanda Rodriguez for technical assistance. This research was supported by grants from CONICET (PIP 112200901003595919 to A L).
Glossary
- αβ-MeADP
α,β-methyleneadenosine 5′-diphosphate
- ω-CgTx
ω-conotoxin GVIA
- ACh
acetylcholine
- BgTx-R
α-bungarotoxin coupled to tetramethylrhodamine
- CAMKII
calcium/calmodulin-dependent protein kinase II
- DPCPX
8-cyclopentyl-1,3-dipropylxanthine
- NEM
N-ethylmaleimide, NMJ, neuromuscular junction
- VGCC
voltage-gated calcium channel
- ω-Aga
ω-agatoxin IVA
Conflict of interest
The authors declare no conflict of interest.
References
- Abbracchio MP, Brambilla R, Ceruti S, Kim HO, von Lubitz DK, Jacobson KA, et al. G protein-dependent activation of phospholipase C by adenosine A3 receptors in rat brain. Mol Pharmacol. 1995;48:1038–1045. [PubMed] [Google Scholar]
- Alexander S, Harmar A, McGrath I. New updated GRAC Fifth Edition with searchable online version Launch of new portal Guide to Pharmacology in association with NC-IUPHAR Transporter-Themed Issue. Br J Pharmacol. 2011;164:1749–1750. doi: 10.1111/j.1476-5381.2011.01751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravamudan B, Fergestad T, Davis WS, Rodesch CK, Broadie K. Drosophila Unc-13 is essential for synaptic transmission. Nat Neurosci. 1999;2:965–971. doi: 10.1038/14764. [DOI] [PubMed] [Google Scholar]
- Barankiewicz J, Cohen A. Purine nucleotide metabolism in resident and activated rat macrophages in vitro. Eur J Immunol. 1985;15:627–631. doi: 10.1002/eji.1830150618. [DOI] [PubMed] [Google Scholar]
- Benowitz LI, Goldberg DE, Irwin N. Inosine stimulates axon growth in vitro and in the adult CNS. Prog Brain Res. 2002;137:389–399. doi: 10.1016/s0079-6123(02)37030-4. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- Coppola T, Perret-Menoud V, Lüthi S, Farnsworth CC, John A, Glomset JA, et al. Disruption of Rab3–calmodulin interaction, but not other effector interactions, prevents Rab3 inhibition of exocytosis. EMBO J. 1999;18:5885–5891. doi: 10.1093/emboj/18.21.5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha RA, Sebastião AM. Extracellular metabolism of adenine nucleotides and adenosine in the innervated skeletal muscle of the frog. Eur J Pharmacol. 1991;197:83–92. doi: 10.1016/0014-2999(91)90368-z. [DOI] [PubMed] [Google Scholar]
- De Lorenzo S, Veggeti M, Muchnik S, Losavio A. Presynaptic inhibition of spontaneous acetylcholine release induced by adenosine at the mouse neuromuscular junction. Br J Pharmacol. 2004;142:113–124. doi: 10.1038/sj.bjp.0705656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lorenzo S, Veggetti M, Muchnik S, Losavio A. Presynaptic inhibition of spontaneous acetylcholine release mediated by P2Y receptors at the mouse neuromuscular junction. Neuroscience. 2006;142:71–85. doi: 10.1016/j.neuroscience.2006.05.062. [DOI] [PubMed] [Google Scholar]
- Dick IE, Tadross MR, Liang H, Tay LH, Yang W, Yue DT. A modular switch for spatial Ca2+ selectivity in the calmodulin regulation of CaV channels. Nature. 2008;451:830–834. doi: 10.1038/nature06529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickenson JM, Hill SJ. Involvement of G-protein βγ subunits in coupling the adenosine A1 receptor to phospholipase C in transfected CHO cells. Eur J Pharmacol. 1998;355:85–93. doi: 10.1016/s0014-2999(98)00468-3. [DOI] [PubMed] [Google Scholar]
- Dreyer F, Rosenberg F, Becker C, Bigalke H, Penner R. Differential effects of various secretagogues on quantal transmitter release from mouse motor nerve terminals treated with botulinum A and tetanus toxin. Naunyn Schmiedebergs Arch Pharmacol. 1987;335:1–7. doi: 10.1007/BF00165027. [DOI] [PubMed] [Google Scholar]
- Eccles RM, Løyning Y, Oshima T. Effects of hypoxia on the monosynaptic reflex pathway in the cat spinal cord. J Neurophysiol. 1966;29:315–332. doi: 10.1152/jn.1966.29.2.315. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Sasaki T, Fukui K, Kotani H, Kimura T, Hata Y, et al. Phosphorylation of Munc-18/n- Sec1/rbSec1 by protein kinase C. Its implication in regulating the interaction of Munc-18/n-Sec1/rbSec1 with syntaxin. J Biol Chem. 1996;271:7265–7268. doi: 10.1074/jbc.271.13.7265. [DOI] [PubMed] [Google Scholar]
- Furshpan EJ. The effects of osmotic pressure changes on the spontaneous activity at motor nerve endings. J Physiol. 1956;134:689–697. doi: 10.1113/jphysiol.1956.sp005675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gansel M, Penner R, Dreyer F. Distinct sites of action of clostridial neurotoxins revealed by double-poisoning of mouse motor nerve terminals. Pflügers Arch. 1987;409:533–539. doi: 10.1007/BF00583812. [DOI] [PubMed] [Google Scholar]
- Gessi S, Merighi S, Varani K, Leung E, Mac Lennan S, Borea PA. The A3 adenosine receptor: an enigmatic player in cell biology. Pharmacol Ther. 2008;117:123–140. doi: 10.1016/j.pharmthera.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Gomez G, Sitkovsky MV. Differential requirement for A2a and A3 adenosine receptors for the protective effect of inosine in vivo. Blood. 2003;102:4472–4478. doi: 10.1182/blood-2002-11-3624. [DOI] [PubMed] [Google Scholar]
- Hamilton BR, Smith DO. Autoreceptor-mediated purinergic and cholinergic inhibition of motor nerve terminal calcium currents in the rat. J Physiol. 1991;432:327–341. doi: 10.1113/jphysiol.1991.sp018387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskó G, Kuhel DG, Nemeth ZH, Mabley JG, Stachlewitz RF, Virag L, et al. Inosine inhibits inflammatory cytokine production by a posttranscriptional mechanism and protects against endotoxin-induced shock. J Immunol. 2000;164:1013–1019. doi: 10.4049/jimmunol.164.2.1013. [DOI] [PubMed] [Google Scholar]
- Hirsch JA, Gibson GE. Selective alteration of neurotransmitter release by low oxygen in vitro. Neurochem Res. 1984;9:1039–1049. doi: 10.1007/BF00964800. [DOI] [PubMed] [Google Scholar]
- Hoshino S-I, Kikkawa S, Takahashi K, Itoh H, Kaziro Y, Kawasaki H, et al. Identification of sites for alkylation by N-ethylmaleimide and pertussis toxin-catalyzed ADP ribosylation on GTP-binding proteins. FEBS Lett. 1990;276:227–231. doi: 10.1016/0014-5793(90)80548-w. [DOI] [PubMed] [Google Scholar]
- Hubbard JI, Jones SF, Landau EM. An examination of the effects of osmotic pressure changes upon transmitter release from mammalian motor nerve terminals. J Physiol. 1968;197:639–657. doi: 10.1113/jphysiol.1968.sp008579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idzko M, Panther E, Bremer HC, Windisch W, Sorichter S, Herouy Y, et al. Inosine stimulates chemotaxis, Ca2+-transients and actin polymerization in immature human dendritic cells via a pertussis toxin-sensitive mechanism independent of adenosine receptors. J Cell Physiol. 2004;199:149–156. doi: 10.1002/jcp.10431. [DOI] [PubMed] [Google Scholar]
- Irwin N, Li YM, O'Toole JE, Benowitz LI. Mst3b, a purine sensitive Ste20-like protein kinase, regulates axon outgrowth. Proc Natl Acad Sci USA. 2006;103:18320–18325. doi: 10.1073/pnas.0605135103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanina T, Blumenstein Y, Shistik E, Barzilai R, Dascal N. Modulation of L-type Ca2+ channels by Gβγ and calmodulin via interactions whit N and C termini of α1c. J Biol Chem. 2000;275:39846–39854. doi: 10.1074/jbc.M005881200. [DOI] [PubMed] [Google Scholar]
- Jacobson KA, Park K-S, Jiang J-L, Kim Y-C, Olah ME, Stiles GL, et al. Pharmacological characterization of novel A3 adenosine receptor-selective antagonists. Neuropharmacology. 1997;36:1157–1165. doi: 10.1016/s0028-3908(97)00104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J-L, van Rhee AM, Melman N, Ji XD, Jacobson KA. 6-Phenyl-1,4-dihydropyridine derivatives as potent and selective A3 adenosine receptor antagonists. J Med Chem. 1996;39:4667–4675. doi: 10.1021/jm960457c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Shepherd RK, Duling BR, Linden J. Inosine binds to A3 adenosine receptors and stimulates mast cell degranulation. J Clin Invest. 1997;100:2849–2857. doi: 10.1172/JCI119833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashani AH, Chen B-M, Grinnell AD. Hypertonic enhancement of transmitter release from frog motor nerve terminals: Ca2+ independence and role of integrins. J Physiol. 2001;530:243–252. doi: 10.1111/j.1469-7793.2001.0243l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kékesi V, Zima E, Barat E, Huszar E, Nagy A, Losonczi L, et al. Pericardial concentrations of adenosine, inosine and hypoxanthine in an experimental canine model of spastic ischaemia. Clin Sci. 2002;48:198S–201S. doi: 10.1042/CS103S198S. [DOI] [PubMed] [Google Scholar]
- Khanna R, Li Q, Bewersdorf J, Stanley EF. The presynaptic CaV2.2 channel–transmitter release site core complex. Eur J Neurosci. 2007;26:547–559. doi: 10.1111/j.1460-9568.2007.05680.x. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. NC3Rs Reporting Guidelines Working Group. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Wong ST, Gallagher D, Li B, Storm DR, Scheuer T, et al. Ca2+/calmodulin binds to and modulates P/Q-type calcium channels. Nature. 1999;99:155–159. doi: 10.1038/20194. [DOI] [PubMed] [Google Scholar]
- Liaudet L, Mabley JG, Pacher P, Virag L, Soriano FG, Marton A, et al. Inosine exerts a broad range of anti-inflammatory effects in a murine model of acute lung injury. Ann Surg. 2002;235:568–578. doi: 10.1097/00000658-200204000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden J. Molecular approach to adenosine receptors: receptor-mediated mechanisms of tissue protection. Annu Rev Pharmacol Toxicol. 2001;41:775–787. doi: 10.1146/annurev.pharmtox.41.1.775. [DOI] [PubMed] [Google Scholar]
- Litsky ML, Hohl CM, Lucas JH, Jurkowitz MS. Inosine and guanosine preserve neuronal and glial cell viability in mouse spinal cord cultures during chemical hypoxia. Brain Res. 1999;821:426–432. doi: 10.1016/s0006-8993(99)01086-0. [DOI] [PubMed] [Google Scholar]
- Liu F, You SW, Yao LP, Liu HL, Jiao XY, Shi M, et al. Secondary degeneration reduced by inosine after spinal cord injury in rats. Spinal Cord. 2006;44:421–426. doi: 10.1038/sj.sc.3101878. [DOI] [PubMed] [Google Scholar]
- Liu GJ, Werry EL, Bennett MR. Secretion of ATP from Schwann cells in response to uridine triphosphate. Eur J Neurosci. 2005;21:151–160. doi: 10.1111/j.1460-9568.2004.03831.x. [DOI] [PubMed] [Google Scholar]
- Losavio A, Muchnik S. Spontaneous acetylcholine release in mammalian neuromuscular junction. Am J Physiol. 1997;273:C1835–C1841. doi: 10.1152/ajpcell.1997.273.6.C1835. [DOI] [PubMed] [Google Scholar]
- Ma QR, Yang H, Zhao XH, Zhang YK, Yao AH, Cheng P, et al. The protective effects of inosine against chemical hypoxia on cultured rat oligodendrocytes. Cell Mol Neurobiol. 2011;31:1171–1186. doi: 10.1007/s10571-011-9719-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh D, Sharp EM, Scheuer T, Catterall WA. Inhibition of cardiac L-type calcium channels by protein kinase C phosphorylation of two sites in the N-terminal domain. Proc Natl Acad Sci USA. 2000;97:12334–12338. doi: 10.1073/pnas.210384297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhães-Cardoso MT, Pereira MF, Oliveira L, Ribeiro JA, Cunha RA, Correia-de-Sá P. Ecto-AMP deaminase blunts the ATP-derived adenosine A2A receptor facilitation of acetylcholine release at rat motor nerve endings. J Physiol. 2003;549:399–408. doi: 10.1113/jphysiol.2003.040410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marton A, Pacher P, Murthy KG, Nemeth ZH, Hasko G, Szabo C. Anti-inflammatory effects of inosine in human monocytes, neutrophils and epithelial cells in vitro. Int J Mol Med. 2001;8:617–621. [PubMed] [Google Scholar]
- Miledi R, Slater CR. On the degeneration of rat neuromuscular junctions after nerve section. J Physiol. 1970;207:507–528. doi: 10.1113/jphysiol.1970.sp009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento FP, Figueredo SM, Marcon R, Martins DF, Macedo SJ, Jr, Lima DA, et al. Inosine reduces pain-related behavior in mice: involvement of adenosine A1 and A2A receptor subtypes and protein kinase C pathways. J Pharmacol Exp Ther. 2010;334:590–598. doi: 10.1124/jpet.110.166058. [DOI] [PubMed] [Google Scholar]
- Olah ME, Stiles GL. Adenosine receptor subtypes: characterization and therapeutic regulation. Annu Rev Pharmacol Toxicol. 1995;35:581–606. doi: 10.1146/annurev.pa.35.040195.003053. [DOI] [PubMed] [Google Scholar]
- Palmer TM, Gettys TW, Stiles GL. Differential interaction with and regulation of multiple G-proteins by the rat A3 adenosine receptor. J Biol Chem. 1995;270:16895–16902. doi: 10.1074/jbc.270.28.16895. [DOI] [PubMed] [Google Scholar]
- Pastor-Anglada M, Casado FJ, Valdes R, Mata J, Garcia-Manteiga J, Molina M. Complex regulation of nucleoside transporter expression in epithelial and immune system cells. Mol Membr Biol. 2001;18:81–85. doi: 10.1080/096876800110033783. [DOI] [PubMed] [Google Scholar]
- Perroy J, Prezeau L, De Waard M, Shigemoto R, Bockaert J, Fagni L. Selective blockade of P/Q-type calcium channels by the metabotropic glutamate receptor type 7 involves a phospholipase C pathway in neurons. J Neurosci. 2000;20:7896–7904. doi: 10.1523/JNEUROSCI.20-21-07896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzi D, Condliffe S, Bozzi Y, Chikhladze M, Grumelli C, Proux-Gillardeaux V, et al. Activity-dependent phosphorylation of Ser187 is required for SNAP-25-negative modulation of neuronal voltage-gated calcium channels. Proc Natl Acad Sci USA. 2008;105:323–328. doi: 10.1073/pnas.0706211105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protti DA, Uchitel OD. Transmitter release and presynaptic Ca2+ currents blocked by the spider toxin omega-Aga-IVA. Neuroreport. 1993;5:333–336. doi: 10.1097/00001756-199312000-00039. [DOI] [PubMed] [Google Scholar]
- Ramkumar V, Stiles GL, Beaven MA, Ali H. The A3 adenosine receptor is the unique adenosine receptor which facilitates release of allergic mediators in mast cells. J Biol Chem. 1993;268:16887–16890. [PubMed] [Google Scholar]
- Ribeiro JA, Sebastião AM. Adenosine receptors and calcium: basis for proposing a third (A3) adenosine receptor. Prog Neurobiol. 1986;26:179–209. doi: 10.1016/0301-0082(86)90015-8. [DOI] [PubMed] [Google Scholar]
- Ribeiro JA, Sebastião AM. On the role, inactivation, and origin of endogenous adenosine at the frog neuromuscular junction. J Physiol. 1987;384:571–585. doi: 10.1113/jphysiol.1987.sp016470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson SC, Sévigny J, Zimmermann H. The E-NTPDase family of ectonucleotidases: structure function relationships and pathophysiological significance. Purinergic Signal. 2006;2:409–430. doi: 10.1007/s11302-006-9003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmund C, Stevens CF. Definition of the readily releasable pool of vesicles at hippocampal synapses. Neuron. 1996;16:1197–1207. doi: 10.1016/s0896-6273(00)80146-4. [DOI] [PubMed] [Google Scholar]
- Roth S, Rosenbaum PS, Osinski J, Park SS, Toledano AY, Li B, et al. Ischemia induces significant changes in purine nucleoside concentration in the retina-choroid in rats. Exp Eye Res. 1997;65:771–779. doi: 10.1006/exer.1997.0391. [DOI] [PubMed] [Google Scholar]
- Santos DA, Salgado AI, Cunha RA. ATP is released from nerve terminals and from activated muscle fibres on stimulation of the rat phrenic nerve. Neurosci Lett. 2003;338:225–228. doi: 10.1016/s0304-3940(02)01419-2. [DOI] [PubMed] [Google Scholar]
- Selbie LA, Hill SJ. G protein-coupled-receptor cross-talk: the fine-tuning of multiple receptor-signalling pathways. Trends Pharmacol Sci. 1998;19:87–93. doi: 10.1016/s0165-6147(97)01166-8. [DOI] [PubMed] [Google Scholar]
- Shapiro MS, Wollmuth LP, Hille B. Modulation of Ca2+channels by PTX-Sensitive G-Proteins is blocked by N-ethylmaleimide in rat sympathetic neurons. J Neurosci. 1994;14:7109–7116. doi: 10.1523/JNEUROSCI.14-11-07109.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Chen GJ, Harvey BK, Bickford PC, Wang Y. Inosine reduces ischemic brain injury in rats. Stroke. 2005;36:654–659. doi: 10.1161/01.STR.0000155747.15679.04. [DOI] [PubMed] [Google Scholar]
- Shimazaki Y, Nishiki T, Omori A, Sekiguchi M, Kamata Y, Kozaki S, et al. Phosphorylation of 25-kDa synaptosome-associated protein. Possible involvement in protein kinase C-mediated regulation of neurotransmitter release. J Biol Chem. 1996;271:14548–14553. doi: 10.1074/jbc.271.24.14548. [DOI] [PubMed] [Google Scholar]
- Silinsky EM. On the association between transmitter secretion and the release of adenine nucleotides from mammalian motor nerve terminals. J Physiol. 1975;247:145–162. doi: 10.1113/jphysiol.1975.sp010925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky EM. Adenosine decreases both presynaptic calcium currents and neurotransmitter release at the mouse neuromuscular junction. J Physiol. 2004;558:389–401. doi: 10.1113/jphysiol.2004.061457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky EM. Modulation of calcium currents is eliminated after cleavage of a strategic component of the mammalian secretory apparatus. J Physiol. 2005;566:681–688. doi: 10.1113/jphysiol.2005.090647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DO. Sources of adenosine released during neuromuscular transmission in the rat. J Physiol. 1991;432:343–354. doi: 10.1113/jphysiol.1991.sp018388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó G, Stumpf N, Radovits T, Sonnenberg K, Gero D, Hagl S, et al. Effects of inosine on reperfusion injury after heart transplantation. Eur J Cardiothorac Surg. 2006;30:96–102. doi: 10.1016/j.ejcts.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Otsuguro K, Ohta T, Ito S. Adenosine and inosine release during hypoxia in the isolated spinal cord of neonatal rats. Br J Pharmacol. 2010;161:1806–1816. doi: 10.1111/j.1476-5381.2010.01002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilley SL, Wagoner VA, Salvatore CA, Jacobson MA, Koller BH. Adenosine and inosine increase cutaneous vasopermeability by activating A(3) receptors on mast cells. J Clin Invest. 2000;105:361–367. doi: 10.1172/JCI8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veggetti M, Muchnik S, Losavio A. Effect of purines on calcium-independent acetylcholine release at the mouse neuromuscular junction. Neuroscience. 2008;154:1324–1336. doi: 10.1016/j.neuroscience.2008.04.056. [DOI] [PubMed] [Google Scholar]
- Wu ZL, Liu F, Wang YZ, Jiao XY, Shi M, Zhao QB, et al. Reduced cell death by inosine pretreatment after photochemically induced cerebral ischemia in adult rats. Prog Nat Sci. 2008;18:1513–1518. [Google Scholar]
- Zai L, Ferrari C, Subbaiah S, Havton LA, Coppola G, Strittmatter S, et al. Inosine alters gene expression and axonal projections in neurons contralateral to a cortical infarct and improves skilled use of the impaired limb. J Neurosci. 2009;29:8187–8197. doi: 10.1523/JNEUROSCI.0414-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z-H, Johnson JA, Chen L, El-Sherif N, Mochly-Rosen D, Boutjdir M. C2 region-derived peptides of beta-protein kinase C regulate cardiac Ca2+ channels. Circ Res. 1997;80:720–729. doi: 10.1161/01.res.80.5.720. [DOI] [PubMed] [Google Scholar]
- Zhou QY, Li C, Olah ME, Johnson RA, Stiles GL, Civelli O. Molecular cloning and characterization of an adenosine receptor: the A3 adenosine receptor. Proc Natl Acad Sci USA. 1992;89:7432–7436. doi: 10.1073/pnas.89.16.7432. [DOI] [PMC free article] [PubMed] [Google Scholar]


