Abstract
Background and Purpose
Because agonists at metabotropic glutamate receptors exert beneficial effects in schizophrenia, we have assessed the actions of Lu AF21934 and Lu AF32615, two chemically distinct, selective and brain-penetrant positive allosteric modulators (PAMs) of the mGlu4 receptor, in several tests reflecting positive, negative and cognitive symptoms of schizophrenia in rodents.
Experimental Approach
Hyperactivity induced by MK-801 or amphetamine and head twitches induced by 2,5-dimethoxy-4-iodoamphetamine (DOI) in mice were used as models for positive symptoms. Disruption of social interaction and spatial delayed alternation tests induced by MK-801 in rats were used as models for negative and cognitive symptoms of schizophrenia, respectively.
Key Results
Lu AF21934 (0.1–5 mg·kg−1) and Lu AF32615 (2–10 mg·kg−1) dose-dependently inhibited hyperactivity induced by MK-801 or amphetamine. They also antagonized head twitches and increased frequency of spontaneous excitatory postsynaptic currents (EPSCs) in brain slices, induced by DOI. In mice lacking the mGlu4 receptor (mGlu4−/−) mice, Lu AF21934 did not antagonize DOI-induced head twitches. MK-801-induced disruption in the social interaction test was decreased by Lu AF21934 at 0.5 mg·kg−1 and by Lu AF32615 at 10 mg·kg−1. In the delayed spatial alternation test, Lu AF21934 was active at 1 and 2 mg·kg−1, while Lu AF32615 was active at 10 mg·kg−1.
Conclusions and Implications
We propose that activation by PAMs of the mGlu4 receptor is a promising approach to the discovery of novel antipsychotic drugs.
Keywords: schizophrenia, Lu AF21934, Lu AF32615, mGlu receptors, positive allosteric modulation
Introduction
The glutamatergic theory of schizophrenia (Javitt, 1987) derives from the finding that NMDA receptor open channel blockers, such as phencyclidine (PCP), ketamine or dizocilpine (MK-801), potently induced a wide range of positive, negative and cognitive symptoms of psychosis in healthy volunteers. Therefore, pharmacological manipulation of this neurotransmitter may produce antipsychotic effects. The first clinical trials with agents acting on the glycine modulatory site on the NMDA receptor have been shown to improve cognition and decrease negative symptoms in schizophrenic subjects receiving standard antipsychotic therapy (Coyle and Tsai, 2004).
The identification of eight subtypes of metabotropic glutamate receptors (mGlu1–8) divided into three groups (Pin and Duvoisin, 1995), provided a wide range of options to modulate the activity of glutamatergic neurotransmission, constituting a potentially safer alternative to NMDA activators (receptor nomenclature follows Alexander et al., 2011). Support for the involvement of mGlu receptors in psychosis originated in the early clinical report of Patil et al. (2007) showing that treatment with LY2140023 (mGlu2/3 receptor agonist) was safe and well-tolerated, and effective in reducing both positive and negative symptoms compared to placebo. Unfortunately, follow-up clinical studies did not confirm the drug's efficacy (Kinon et al., 2011), and the trial was stopped recently (Lilly press release 08, 2012). However, despite this setback, ligands for the group II mGlu receptors provide a feasible new approach to antipsychotic therapy, with the particular feature of not targeting dopamine receptors (Weinberger, 2007). Indeed, the potential therapeutic efficacy in schizophrenia of ADX71149, a compound potentiating the mGlu2 receptor, through an allosteric mechanism (vide infra), is currently being investigated in the clinic (Addex Therapeutics website).
From a drug design perspective, the architecture of cell membrane mGlu receptors provides two distinct mechanisms to potentiate receptor function (Conn et al., 2009a). On the one hand, agonists acting at the orthosteric binding site compete with synaptic glutamate to effect receptor activation. In addition, in their transmembrane region, the mGlu receptors possess allosteric binding sites capable of interacting with lipophilic compounds. Following binding of ligands to the allosteric site, a change in receptor conformation is thought to lead to modification of receptor function (Sheffler et al., 2011). Positive allosteric modulators (PAMs) enhance the receptor functional response, whereas negative allosteric modulators (NAMs) reduce it. While both strategies (orthosteric agonists and PAMs) provide activation of mGlu receptors, the pharmacology elicited by these different types of ligands is not necessarily identical. Theoretically, PAMs produce an augmentation of endogenous glutamate-driven effects, whereas orthosteric agonists, in general, lead to receptor activation dependent on the individual pharmacokinetics and concentration at the site of action (Melancon et al., 2012).
Recent reports have demonstrated the preclinical antipsychotic-like activity of the non-selective orthosteric agonists of the group III mGlu receptors (mGlu4/6/7/8), ACPT-I (Figure 1, 4), and LSP1-2111 (Figure 1, 5) (Acher et al., 1997). Both compounds were shown to be active in animal models considered to be predictive of positive symptoms of schizophrenia (Pałucha-Poniewiera et al., 2008; Wierońska et al., 2012; 2013). However, these agonists lack subtype specificity, possibly activating more than one of the group III mGlu receptors, simultaneously. Therefore, in the present study, we evaluated the effects of two compounds selectively potentiating the mGlu4 receptor through allosteric mechanisms, Lu AF21934 (Figure 1, 1), (1S,2R)-N1-(3,4-dichlorophenyl)cyclohexane-1,2-dicarboxamide, Bennouar et al., 2013) and Lu AF32615 (Figure 1, 3), (East et al., 2010, Evotec/Boehringer Ingelheim). These compounds are structurally different, selective and known to cross the blood brain barrier. The enantiomer of Lu AF21934 (Lu AF21935, Figure 1, 2), inactive at the mGlu4 receptor, was also used as a control to support conclusions invoking mGlu4 receptor potentiation. We used a broad range of tests considered to be predictive of positive symptoms, such as MK-801- or amphetamine-induced hyperactivity, and head twitches induced by 2,5-dimethoxy-4-iodoamphetamine (DOI) (Geyer and Ellenbroek, 2003; Moghaddam and Jackson, 2003; Pałucha-Poniewiera et al., 2008). The social interaction test was used as a model of negative symptoms and the delayed alternation test was used as a model of cognitive disturbances. Electrophysiological studies were also conducted in brain slices from mice. Our results show that Lu AF21934 and Lu AF32615 induced antipsychotic-like effects in all of the tests used, suggesting their potential as an alternative to the presently used antipsychotic therapy.
Figure 1.
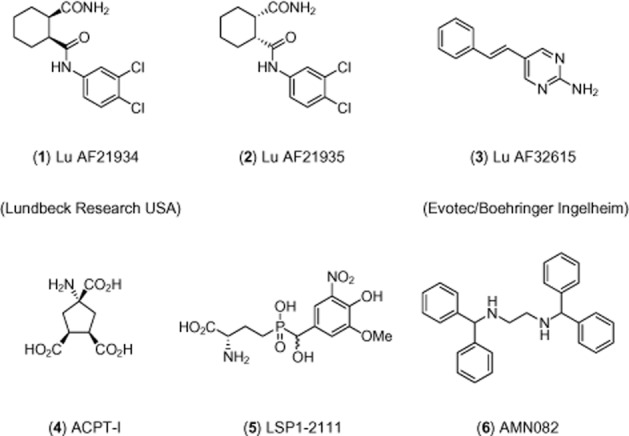
Chemical structures of mGlu4 receptor PAMs used in this work and relevant group III mGlu receptor ligands.
Methods
Animals and housing
All animal care and experimental procedures complied with the guidelines of the National Institutes of Health Animal Care and Use Committee and were approved by the Ethics Committee of the Institute of Pharmacology, Polish Academy of Sciences in Krakow and Lundbeck Research USA. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). A total of 968 animals (618 mice and 350 rats) were used in the experiments described here.
Male Albino Swiss (20–25 g) mice (Charles River Laboratory, Sulzfeld, Germany) were used to assess MK-801- and amphetamine-induced hyperlocomotion and DOI-induced head twitches. Mice lacking the mGlu4 receptor (mGlu4−/−) and wild type C57Bl/6J mice were used in DOI-induced head twitches. Heterozygous mGlu4−/+ mice were obtained from Abbot Laboratories (gift from Dr. Bespalov); mGlu4−/− and mGlu4+/+ mice were bred in our institute. The genotypes of newborn mice were analysed by PCR. The person responsible for breeding mGlu4−/− mice was M. Marciniak. Male Wistar rats weighing 250–300 g (Charles River Laboratory) were used in social interaction and spatial delayed alternation tests. All animals were kept under a 12:12 light–dark cycle at a room temperature of 19–21°C with free access to food and water. Each experimental group consisted of 8–10 animals per dose, and the animals were used only once in each test. All animals were experimentally naive prior to testing. The compounds were given in a volume of 10 mL·kg−1 (mice) or 1 mL·kg−1 (rats). All behavioural measurements were made by an observer unaware of the treatment.
Locomotor activity of habituated mice
The locomotor activity was recorded individually for each animal in OPTO-M3 locomotor activity cages (Columbus Instrument) linked online to a compatible PC. Each cage (13 cm × 23 cm × 15 cm) was surrounded with an array of photocell beams. Interruptions of these photobeams resulted in horizontal activity defined as ambulation scores. Mice were placed separately into activity cages for an acclimatization period of 30 min, then they were injected subcutaneously with Lu AF21934, Lu AF32615 or (2-hydropropyl)-β-cyclodextrin (HPBCD) and placed again into the same cages. After a further 60 min they were injected with saline (10 mL·kg−1). From this point on, the ambulation scores were measured for 80 min.
MK-801- or amphetamine-induced hyperactivity
The locomotor activity was recorded for each animal in locomotor activity cages (according to Rorick-Kehn et al., 2007a,b), with small modifications used in our previous studies (Pałucha-Poniewiera et al., 2008; Wierońska et al., 2012). The mice were placed individually into activity cages for an acclimatization period of 30 min; then they were injected s.c. with Lu AF21934, Lu AF32615 or HPBCD and placed again in the same cages. After 60 min all of the mice were injected i.p. with MK-801 at 0.3 mg·kg−1 or amphetamine at 3 mg·kg−1, and once again returned to the same cage. From then on, the ambulation scores were counted for 80 min. All of the groups were compared with the MK-801 or amphetamine control group. The experiment also included a control group treated with neither MK-801 nor amphetamine.
Head twitch test
The experiment was performed according to Pałucha-Poniewiera et al. (2008) and Wierońska et al. (2011; 2012; 2013). In order to habituate mice to the experimental environment, each animal was transferred to a 12 (diameter) × 20 cm (height) glass cage, lined with sawdust, 30 min before the treatment. The head twitches of the mice were induced by DOI (2.5 mg·kg−1, i.p). Immediately after the treatment, the number of head twitches was counted during a 20 min session. Lu AF21934 and Lu AF32615 were injected, 60 min before DOI; HPBCD was administered to the controls.
MK-801-induced deficits in social interaction test in rats
Social interaction tests were performed as described by Satow et al. (2009), using a circle made of wood, 90 cm in diameter divided into 10 × 10 cm squares by faint yellow lines. Each social interaction test between two rats was carried out during the light phase of the light/dark cycle. Rats were selected from separate housing cages to make a pair for the study. The body weights of the paired rats were matched, within 20 g. All rats were placed in an experimental room and the study was conducted 3.5 h after the s.c. injection of MK-801 (0.1 mg·kg−1). Sixty minutes before the test, Lu AF21934 or Lu AF32615 was given; HPBCD was given as a vehicle. The test box was wiped clean between each trial. Social interaction between two rats was determined as the total time spent participating in social behaviour such as sniffing, genital investigation, chasing and fighting each other. The total number of social episodes mentioned above was also measured. In addition, control experiments in animals not receiving MK-801 were also conducted, in order to establish if the drugs had any influence on social behaviour when given alone.
Spatial delayed alternation test in rats
The animals, deprived of water overnight, were trained and tested in four wooden T-mazes, which consisted of white and black end-arms (33 cm × 22 cm × 25 cm) and a grey starting arm (15 cm × 20 cm × 25 cm). The end-arms were equipped with a spout bottle located 9 cm above the floor and containing a 10% sucrose solution. The three arms were separated from each other by guillotine doors.
Adaptation
On the first 3 days, the animals were allowed freely to explore the whole T-maze for 10 min. On the next 2 days, they were confined to either of the two end-arms and allowed to drink the sucrose solution there for 10 min twice daily.
Training
On the next 2 weeks, the animals received once daily training sessions. Each session consisted of one forced trial (i.e. when one of the end-arm was closed) followed by 10 free choice trials. For each free choice trial, the animals were placed in the starting arm, the guillotine doors were raised, and when the rat entered one of the end-arm, the guillotine door was closed and the rat was allowed to drink the sucrose solution there for 5 s. Then the rat was gently returned to the starting arm, where it stayed for 10 s (delayed interval). After that time, the guillotine door was raised and the rat was allowed to enter the end-arms. If the end-arm chosen was opposite to that visited on the previous trial (a correct response), the sucrose solution was provided, and the drinking was allowed for 5 s. If the end-arm chosen was the same as on the previous trial an incorrect response was scored and the animal gently returned to the starting arm for 10 s (delayed interval). This training was continued until the animals reach performance criterion, which was defined as at least seven correct responses in 10 trials for two consecutive daily sessions.
Testing
The animals were injected with a drug and the above procedure was repeated; the rat was placed in the starting arm, the guillotine doors raised and the rat was allowed to enter the end-arms. If it chose the correct end-arm (i.e. opposite to that visited on the previous trial), the sucrose solution was provided, 5 s later the rat was returned to the starting arm for 10 s (delayed interval). If the end-arm selected was incorrect (i.e. the same as on the previous trial), and the rat was returned to the starting arm for 10 s. Such testing sessions were carried out once a week and were preceded by two daily training sessions.
Electrophysiological studies
Albino Swiss mice were decapitated, their frontal cortices were dissected and cut into slices (420 μm thick) in the frontal plane using a vibrating microtome. Slices were kept submerged in the artificial cerebrospinal fluid (ACSF) consisting of (in mM): 126 NaCl, 4 KCl, 2.5 CaCl2, 1.3 MgSO4, 1.25 KH2PO4, 26 NaHCO3 and 10 glucose, bubbled with 95% O2/5% CO2, pH = 7.4. A single slice was transferred to the recording chamber (volume 1 mL) and superfused with warmed (32°C) ACSF at 2 mL·min−1. Individual neurons were visualized using an upright microscope (Zeiss Axioskop 2FS) equipped with a long-range water immersion objective (40×) and an infrared camera. Recording micropipettes were pulled on a Flaming-Brown puller (P-87; Sutter Instruments, Novato, CA, USA) and had resistance 6–8 MΩ. Microelectrodes were filled with (in mM): 130 K-gluconate, 5 KCl, 0.3 CaCl2, 2 MgCl2, 1 EGTA, 10 HEPES, 5 Na2-ATP, 0.4 Na-GTP, osmolarity 290 mOsm, pH = 7.2. Whole-cell recordings were made from layer V pyramidal cells. After confirming the electrophysiological characteristics of the neurons in the current-clamp mode, cells were voltage-clamped at −76 mV and spontaneous EPSCs were recorded. Signals were acquired using the SEC 05 L amplifier (NPI, Germany) and digitized using Digidata 1322 interface (Molecular Devices, Sunnyvale, CA, USA). Drugs kept as concentrated stocks were diluted in ACSF just before the experiment and applied in the superfusate. After stable baseline recording for at least 15 min, DOI (10 μM) was applied for 10 min and spontaneous excitatory postsynaptic currents (EPSCs) were recorded (10 min). Next DOI was applied concurrently with Lu AF21934 or Lu AF32615 for 15 min and again spontaneous EPSCs were recorded. The measured parameter was the frequency of spontaneous EPSCs. The data were analysed off-line, using Mini Analysis program (Synaptosoft Inc. ver.6.0.3).
Data analysis
The data are presented as the means ± SEM. Statistical analysis of the data was performed using the Statistica 10 package (StatSoft Inc., OK, USA). One-way anova, followed by Dunnett's or Tukey's post hoc comparison test, was used in the analysis of the dose-dependent studies of Lu AF21934 and Lu AF32615. A P-value of <0.05 was considered as statistically significant.
Materials
The sources of the compounds used were as follows: Lu AF32615, Lu AF21934 (mGlu4 receptor PAMs) and Lu AF21935 (as mGlu4 receptor-inactive control) were provided by Lundbeck Research USA, and were characterized using H-1 and C-13 nuclear magnetic resonance spectroscopy and HPLC/mass-spectrometry methods. General in silico and in vitro properties of these compounds are shown in Table 1. The compounds were dosed using a suspension in 20% HPBCD and were injected s.c. 60 min before the tests. The administration schedules of Lu AF32615 and Lu AF21934 were planned based on brain, plasma and CSF concentration time course studies performed at Lundbeck Research USA (Bennouar et al., 2013), as well as according to the behavioural results we observed in our previous studies on anxiety (Sławińska et al., 2013) and in preliminary experiments. Brain, CSF and plasma exposures of Lu AF32615 were in agreement with literature reports (East et al., 2010). The psychostimulants, amphetamine (3 mg·kg−1), MK-801 (0.3 mg·kg−1, Sigma-Aldrich, St. Louis, MO, USA) or DOI (2.5 mg·kg−1) (4-iodo-2,5-dimethoxy-α-methylbenzene ethanamine hydrochloride) were dissolved in 0.9% NaCl, and the doses were selected on the basis of our previous work (Pałucha-Poniewiera et al., 2008; Wierońska et al., 2011; 2012) and that of the others (Geyer and Ellenbroek, 2003; Leite et al., 2008; Satow et al., 2009). Reference compounds clozapine (Tocris Biosciences, Bristol, UK) and risperidone (Tocris) were dispersed in 0.5% methylcellulose in 0.9% saline and haloperidol (5 mg·mL−1 ampoule; Warszawskie Zakłady Farmaceutyczne, Polfa) was diluted in 0.9% saline (which was used as the solvent in haloperidol ampoules). The reference compounds were injected i.p., 30 min before the test. All the solvents (including HPBCD) had no influence on the animals’ behaviour, when given to appropriate controls.
Table 1.
Characterization of Lu AF21934, Lu AF21935 and Lu AF32615
| Lu AF21934 (1) | Lu AF21935 (2) | Lu AF32615 (3) | ||
|---|---|---|---|---|
| Human mGlu4 receptor PAM EC50 | nM | 500 | >10 000 | 910 |
| %Modulation (EMAX) | % | 120 | 18 | 170 |
| Glutamate fold shift | – | 5 | – | 10 |
| Molecular weight | Amu | 315.2 | 315.2 | 197.2 |
| cLogP | – | 3.3 | 3.3 | 2.4 |
| LogD7.4 | – | 3.3 | 3.3 | n/a |
| tPSA | Å2 | 72.2 | 72.2 | 51.8 |
| Kinetic solubility pH 7.4 | μM | 120 | 120 | 170 |
| Optical rotation | [α]21D | +45c | −39c | Achiral |
| PAMPA PAPP | 10−6 cm·s−1 | 7 | 7 | 24 |
| MDCK PAPP | A→B; B→A, Ratio | 44; 30; 0.7 | n/a | 42; 28; 0.7 |
| rPPB | % | 95.6 | 87.6 | 88.3 |
| Rat brain unbound fraction UBBR | % | 3.0 | 1.9 | 1.9 |
| Rat CLint | mL·min−1 | 13 | 77 | 11 |
| Brain | ng·g−1 | 2422b | 740b | 822d |
| Plasma | ng·mL−1 | 2763b | 840b | 350d |
| Brain/Plasma ratio | – | 0.9b | 0.9ab | 2.3d |
| CSF | ng·mL−1 | 95b | 42b | n/a |
| mGlu receptor selectivity | FLIPR screens at 10 μM | mGlu6 receptor PAM; EC50 = 7 μM | No significant cross reactivity at 10 μM | Not active at mGlu1,2,3,5,7 in PAM, NAM and agonist mode at 10 μM |
| Broad cross reactivity panel | Binding inhibition | A2A antagonist Ki = 7 μM; 5-HT2B antagonist Ki = 2 μM | No significant cross reactivity at 10 μM | No significant cross reactivity at 10 μM |
a, Data for Lu AF21934 partly reported in Bennouar et al. (2013); b, Rat, SC dosing at 10 mg·kg−1, 1 h post-dose, formulated in 20% aqueous HPBCD; c, MeOH/CH2Cl2 (1:1); 2 mg·mL−1; d, PO, 10 mg·kg−1, 1 h post-dose, same formulation as b.
Results
Effect of Lu AF21934 and Lu AF32615 on locomotor activity in mice habituated to activity cages
In mice adapted to activity cages for 30 min, neither compound tested at the doses that were active in MK-801- or amphetamine-induced hyperactivities (Lu AF21934, 0.5–5 mg·kg−1; Lu AF32615, 2–10 mg·kg−1) had any significant effect on the locomotor activity measured over 80 min, 60 min after the s.c. injection of the compounds ( Figure 2A, B).
Figure 2.
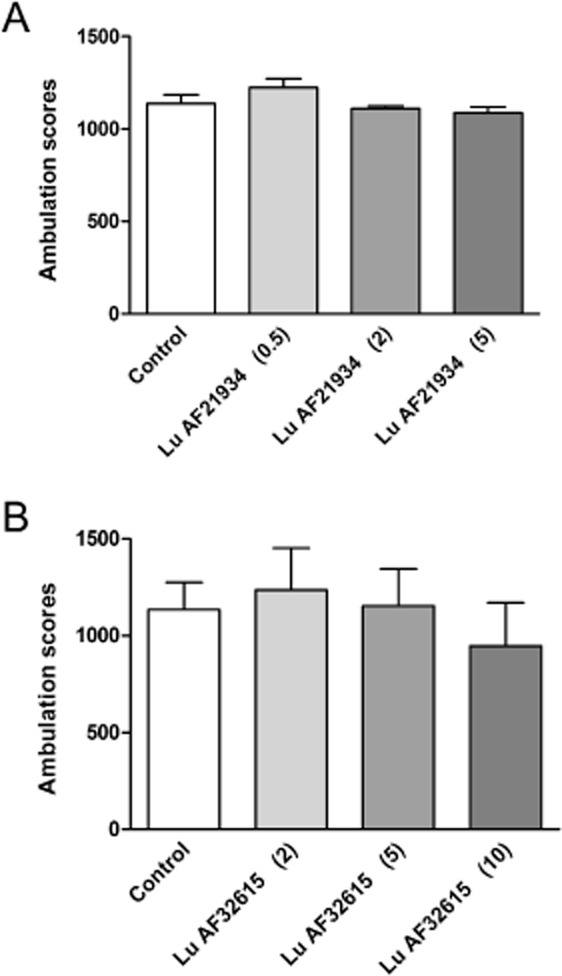
Effect of Lu AF21934 (A) and Lu AF32615 (B) on the locomotor activity of mice habituated to activity cages. The compounds were given s.c 60 min before the test. Locomotor activity was measured during 80 min time session. Data expressed as mean ± SEM were evaluated by one-way anova. Values in parentheses represent the doses of the compounds in mg·kg−1.
The effect of MK-801 on locomotor activity in mice
MK-801 (0.3 mg·kg−1) increased [F(1,35) = 33.98, P < 0.0001] the ambulation scores within 80 min of the experimental session and this increase was reversed by the reference compounds, haloperidol (0.25 mg·kg−1; P < 0.001) or clozapine (10 mg·kg−1; P < 0.001) (Figure 3A). Lu AF21934 in the dose range of 0.5-2 mg·kg−1 reduced the MK-801-induced effect [F(5,42) = 6.458; P < 0.0002] and lower doses (0.1 mg·kg−1) or higher doses (5 and 10 mg·kg−1) were not effective (Figure 3B). Lu AF32615 reversed MK-801-induced locomotor activity [F(3.28) = 5.149, P = 0.005] only at the highest dose (10 mg·kg−1; Figure 3C). The inactive enantiomer Lu AF21935 (0.1, 0.5 or 2 mg·kg−1) did not influence the MK-801 induced effect, at any dose. (Figure 4).
Figure 3.
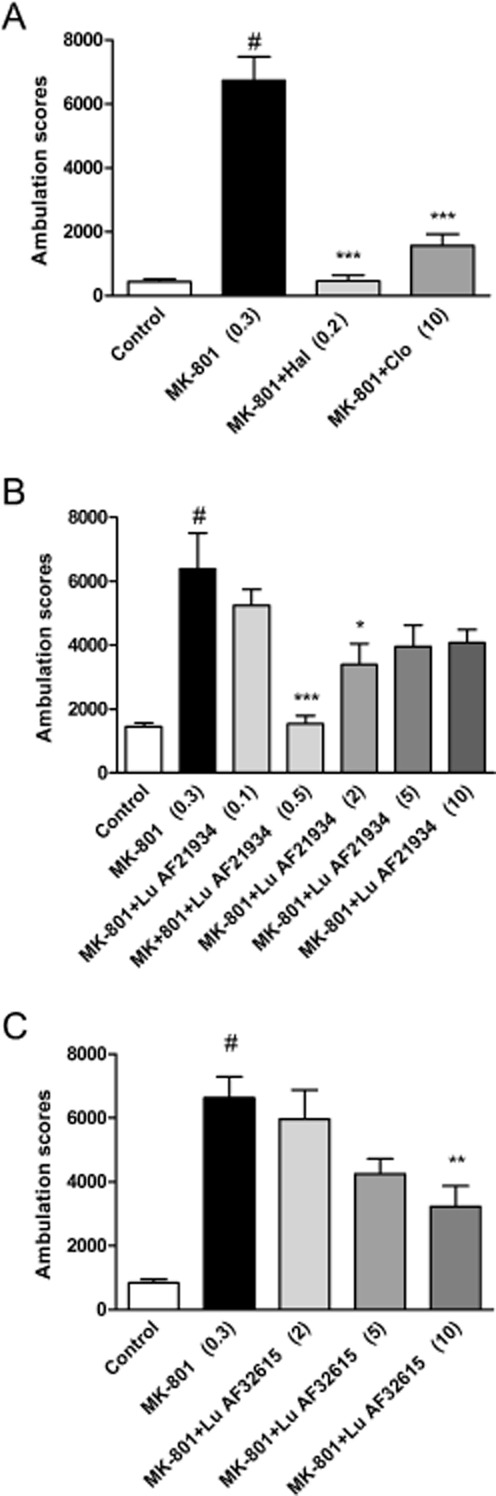
Effect of reference compounds haloperidol and clozapine (A), Lu AF21934 (B) and Lu AF32615 (C) on MK-801–induced hyperactivity in Albino Swiss mice. The reference compounds were given 30 min before the test. The test compounds were given 60 min before MK-801 administration. Locomotor activity was monitored over an 80 min session immediately following an injection of psychostimulant agent. Values in parentheses represent the doses of the compounds in mg·kg−1. The data are presented as mean ± SEM #P < 0.001 versus control group and ***P < 0.001, **P < 0.01 and *P < 0.05 versus MK-801-treated group; one-way anova and Dunnett's test.
Figure 4.
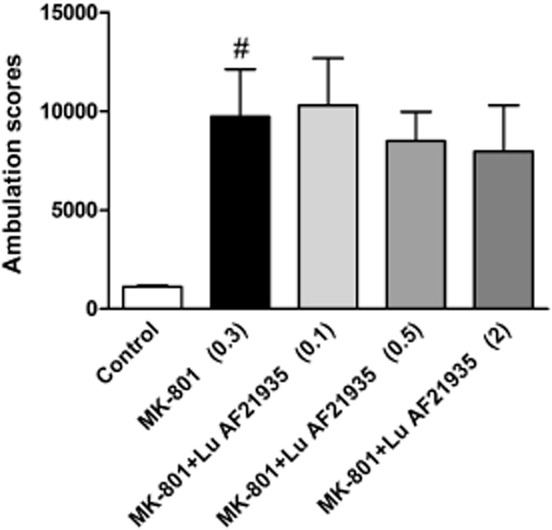
Effect of Lu AF21935, the enantiomer of Lu AF21934 devoid of measurable in vitro mGlu4 receptor PAM activity, on MK-801–induced hyperactivity in Albino Swiss mice. The compound was given 60 min before MK-801 administration. Locomotor activity was monitored over an 80 min session immediately following an injection of MK-801. Values in parentheses represent the doses of the compounds in mg·kg−1. The data are presented as mean ± SEM #P < 0.001 versus control group; one-way anova.
Amphetamine-induced hyperactivity in mice
In the vehicle treated group, amphetamine (3 mg·kg−1) produced a robust increase in the ambulation scores (Figure 5A, B, C). This effect of amphetamine was abolished by haloperidol (0.25 mg·kg−1) and clozapine (10 mg·kg−1), used as reference compounds [F(1.35) = 20.1, P < 0.001]. Both PAMs of the mGlu4 receptor decreased the amphetamine-induced effect. Lu AF21934 was effective at 0.1, 0.5 and 2 mg·kg−1 [F(5.,54) = 8.275, P < 0.0001], but not at 5 and 10 mg·kg−1. Lu AF32615 was effective only at 10 mg·kg−1 [F(3.28) = 5.922, P < 0.003].
Figure 5.
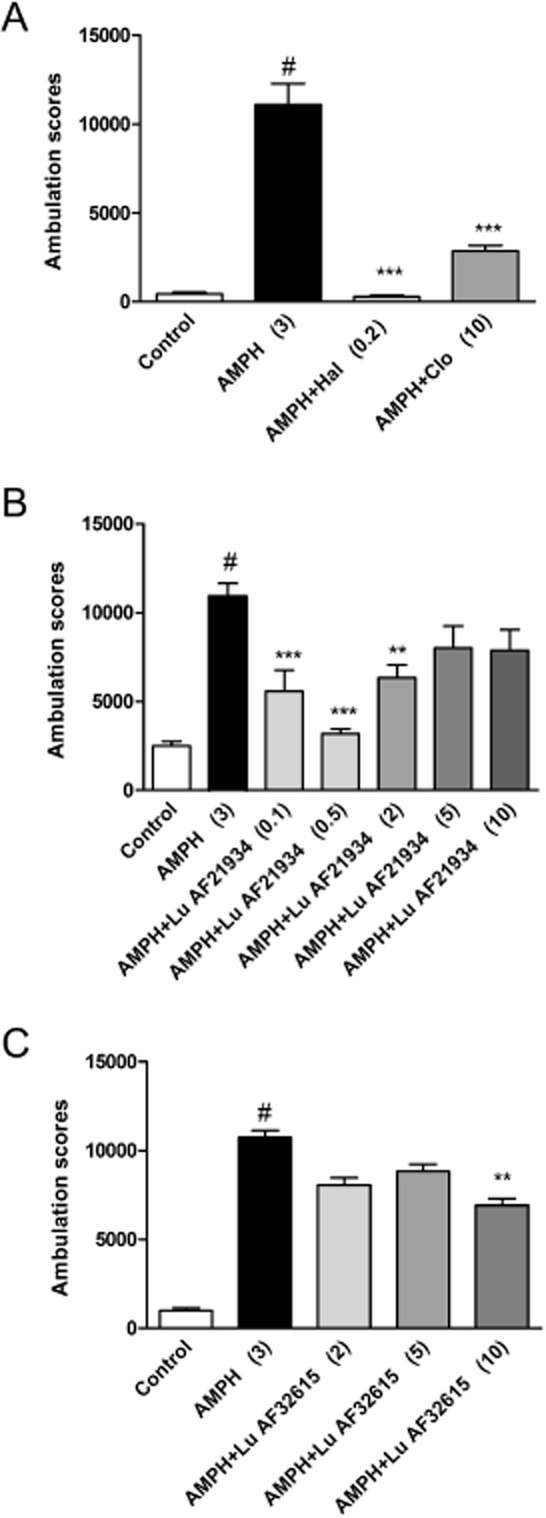
Effect of reference compounds haloperidol and clozapine (A), Lu AF21934 (B) and Lu AF32615 (C) on amphetamine–induced hyperactivity in Albino Swiss mice. The compounds were given 30 or 60 min before amphetamine administration. Locomotor activity was monitored over an 80 min session immediately following an injection of amphetamine. Values in parentheses represent the doses of the compounds in mg·kg−1.The data are presented as mean ± SEM. #P < 0.001 versus control group and ***P < 0.001, **P < 0.01 versus amphetamine-treated group; one-way anova and Dunnett's test.
DOI-induced head twitches in mice
Both clozapine and haloperidol diminished the number of head twitches induced by DOI [F(2,21) = 9.63, P < 0.01 and F(2,21) = 14.33, P < 0.01, respectively]. Lu AF21934 administered s.c. at 2, 5, 10 but not 0.5 mg·kg−1, significantly decreased the number of DOI-induced head twitches in mice (45, 38.5 and 35%, respectively) [F(4.38) = 6.632, P = 0.0004]. Lu AF32615 also reduced number of head twitches at 5 and 10 mg·kg−1 (49,5% and 68%) [F(3.36) = 13.99, P < 0.0001], but the lower dose of this compound (2 mg·kg−1) was not effective (Figure 6A, B, C).
Figure 6.
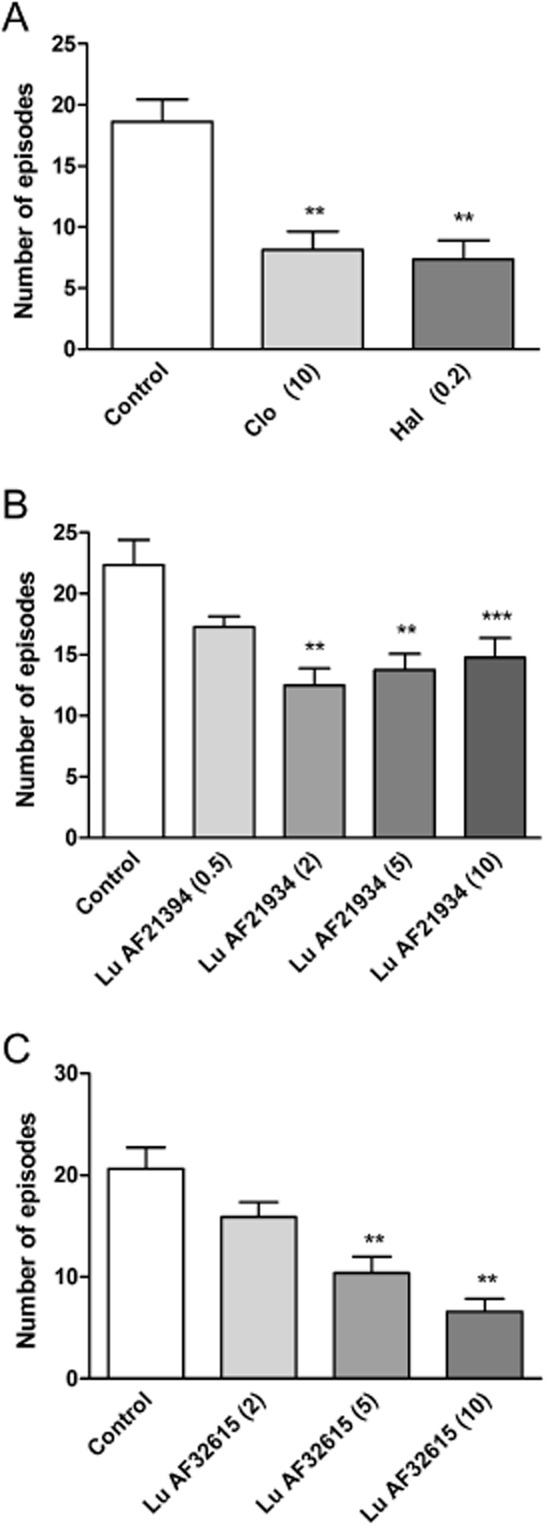
The suppression of DOI-induced head twitches by reference compounds haloperidol and clozapine (A), Lu AF21934 (B) and Lu AF32615 (C). The compounds were given 30 (A) or 60 min before DOI (2.5 mg·kg−1) administration, and immediately after DOI the test begun. The values represent the number of head twitches (mean ± SEM) during a 20-min session. Values in parentheses represent the doses of the compounds in mg·kg−1.The data are presented as means ± SEM. ***P < 0.001 and **P < 0.01 versus DOI-treated group; one-way anova and Dunnett's test.
Lu AF21934 was not active when tested in mGlu4−/− animals (Figure 7A). These mice showed a decrease in number of head twitches observed [F(1.20) = 12; P < 0.01], relative to the wild type mice (Figure 7A, B). However, clozapine (5 mg·kg−1) used as the reference compound was active in both wild type and mGlu4-/- animals [F(1.20) = 12.8; P < 0.01] (Figure 7B).
Figure 7.
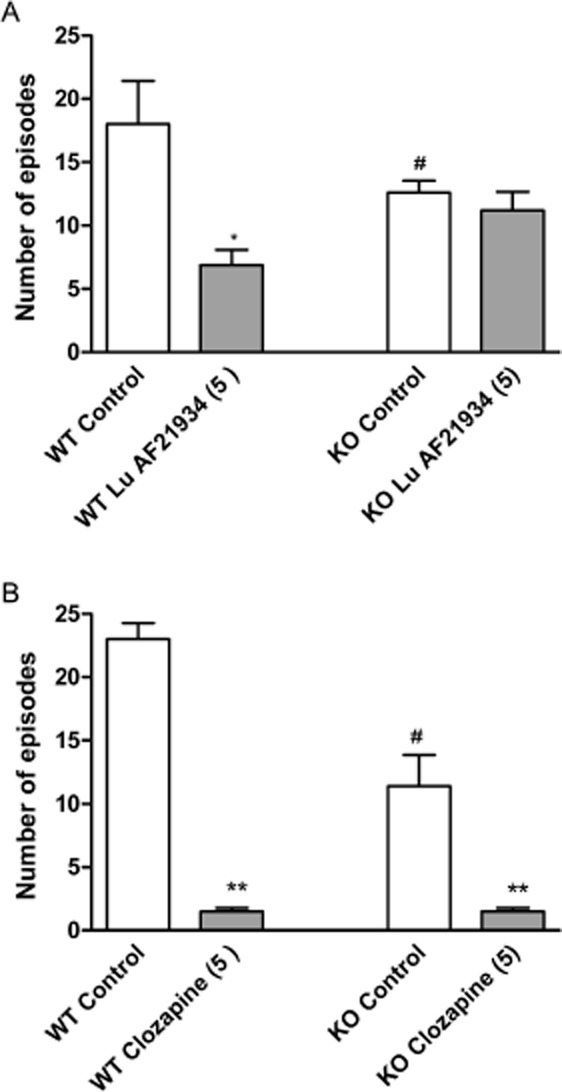
The suppression of DOI-induced head twitches by Lu AF21934 (A) and reference compound clozapine (B) in wild type (WT) and mGlu4−/− (KO) mice. The compounds were given 60 min before DOI (2.5 mg·kg−1) administration, and immediately after DOI the test begun. The values represent the number of head twitches (mean ± SEM) during a 20-min session. Values in parentheses represent the doses of the compounds in mg·kg−1. The data is presented as means ± SEM. #P < 0.05 versus DOI-treated WT mice, *P < 0.05 versus DOI-treated WT mice and **P < 0.01 versus DOI-treated WT group; one-way anova and Dunnett's test.
DOI-induced spontaneous EPSCs
To investigate the effects of DOI on spontaneous excitatory postsynaptic currents (sEPSCs), voltage-clamp recordings were made from layer V cortical cells in the presence of picrotoxin (30 μM) to block GABAA receptor-mediated currents. All recorded cells (n = 69) had electrophysiological characteristics of regular spiking pyramidal neurons (tested in current clamp; McCormick et al., 1985). Their mean resting membrane potential (RMP) was −74 ± 5 mV (±SEM) and the mean input resistance (Rin) was 252 ± 27 MΩ (±SEM). The mean basal frequency of spontaneous synaptic activity ranged between 2.9 and 7.5 Hz (±SEM; 4.9 ± 0.3 Hz) and its mean amplitude was 9.77 ± 0.3 pA (±SEM). Spontaneous postsynaptic currents were blocked by the non-NMDA glutamatergic receptor antagonist CNQX (5 μM; n = 5, data not shown), indicating that they represented excitatory currents. The application of DOI (10 μM) increased the mean sEPSCs frequency to: 147 ± 5% (± SEM) of baseline. The measurements performed on a separate group of five neurons demonstrated that the effect of DOI on sEPSCs did not desensitize over a 40 min continuous application of DOI (Figure 8).
Figure 8.
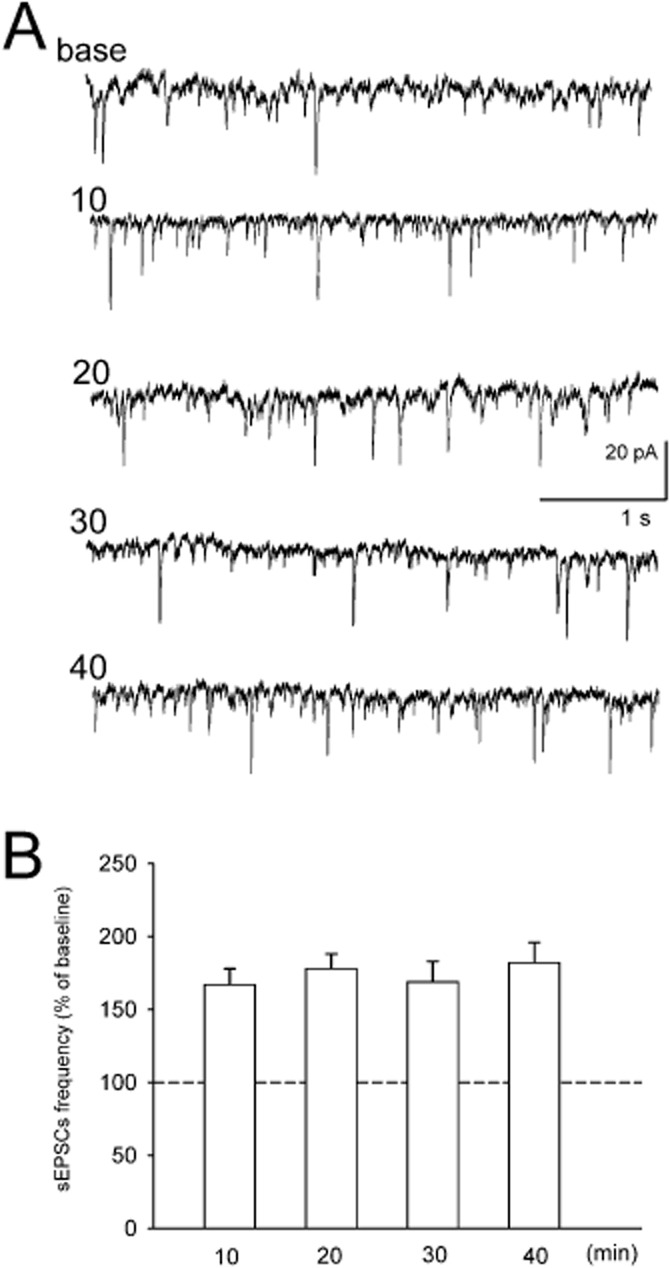
(A). Examples of sEPSCs recordings from brain slices. Base – recording before DOI application. Numbers denote time after the beginning of DOI application (minutes). (B) Mean (± SEM) frequency of sEPSCs over 5 min periods beginning at indicated time points. Data are normalized to baseline recording obtained before 10 uM DOI application (n = 4).
Lu AF21934, when applied concurrently with DOI, reversibly suppressed DOI-induced increase in the frequency, but did not affect the mean amplitude of sEPSCs. The dose dependency of Lu AF21934 effect was U shaped with the highest decrease of sEPSCs frequency observed at 1 μM (P < 0.05) (Figure 9A). The effects of the second compound, Lu AF32615, was concentration-dependent from 2.5 to 10 μM (Figure 9B).
Figure 9.
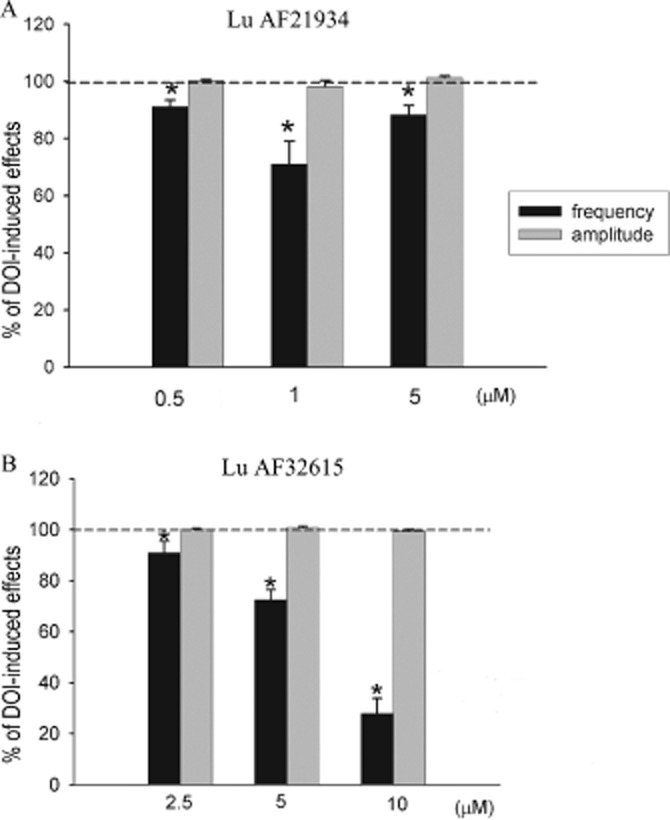
Suppression by Lu AF21934 (A) or Lu AF32615 (B) of the increase in sEPSCs activity induced by DOI recording after 10 min incubation with DOI and after 10 min incubation with Lu AF21934 or Lu AF32615 in the continuous presence of DOI. *P < 0.05 versus DOI effect; paired t-test.
Social interaction test
MK-801 (0.1 mg·kg−1) disrupted both the number of social episodes between rats and the total time of interaction, decreasing these behaviours to 25.8% and to 22.4% of control level, respectively. The reference compound, clozapine (7.5 mg·kg−1, i.p), increased the number of social episodes [F(2.24) = 22.97; P < 0.05] and duration of contact [F(2.24) = 27.23; P < 0.01], impaired by MK-801 (Figure 10), having no effect by itself (data not shown). Similar attenuation of the number of episodes and time of interactions were observed after Lu AF21934 administration at 0.5 mg·kg−1 [F(4.40) = 5.44; P < 0.05 and F(4.40) = 5.66; P < 0.01, respectively] but not at the two other doses (0.2 and 1 mg·kg−1) (Figure 11A, B). Control experiments revealed that the PAM did not change the behaviour of rats when administered alone (Figure 11C, D).
Figure 10.
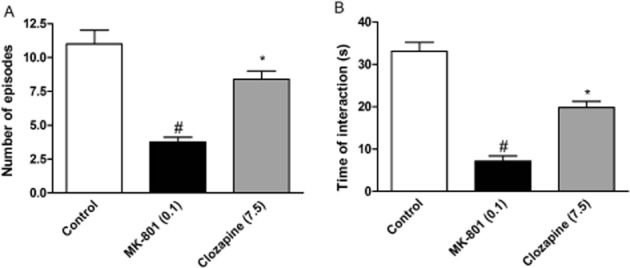
Effects of reference compound, clozapine, on the MK-801-induced deficits in social interaction test. The graphs represent the disruption in the number of social episodes (A) and in the total duration of social episodes (B). Clozapine was given 60 min before the test, and MK-801 was administered 3.5 h before the test. Values in parentheses represent the doses of the compounds in mg·kg−1. Data are presented as means ± SEM. #P < 0.01 versus control group *P < 0.05, and **P < 0.01 versus MK-801-treated group; one-way anova and Tukey's post hoc test.
Figure 11.
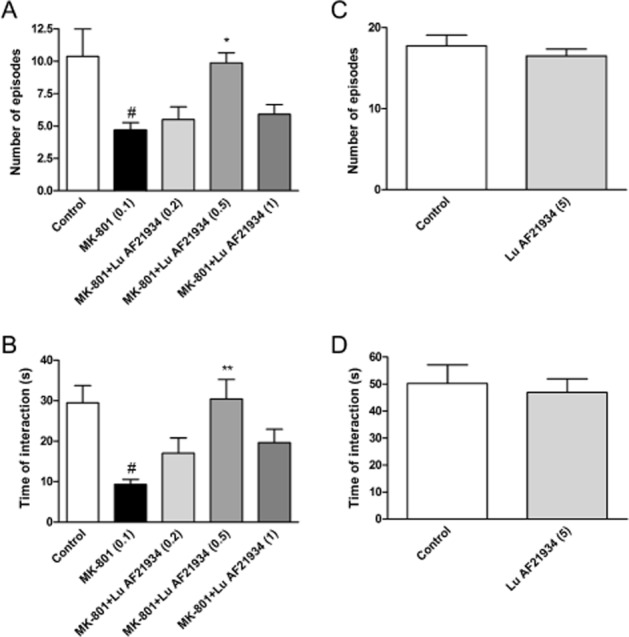
Effects of Lu AF21934 on the MK-801-induced deficits in social interaction test. The graphs represent the disruption in the number of social episodes (A) and in the total duration of social episodes (B). Lu AF21934 was given 60 min before the test, and MK-801 was administered 3.5 h before the test. Values in parentheses represent the doses of the compounds in mg·kg−1. Data are presented as mean ± SEM. #P < 0.01 versus control group, *P < 0.05 and **P < 0.01 versus MK-801-treated group; one-way anova analysis followed by Tukey's post hoc tests. The control experiments without MK-801 administration are represented by the panels C and D. The control experiments without MK-801 administration are represented by the panels C and D.
The second compound, Lu AF32615, was effective at 10 mg·kg−1, but not at 2 and 5 mg·kg−1, increasing the time of interaction [F(4.43) = 6.55; P < 0.01], and the number of episodes [F(4.43) = 7.19; P < 0.01] (Figure 12A, B). Control experiments revealed that the drug had no influence on the social behaviour of animals when given alone (Figure 12C, D).
Figure 12.
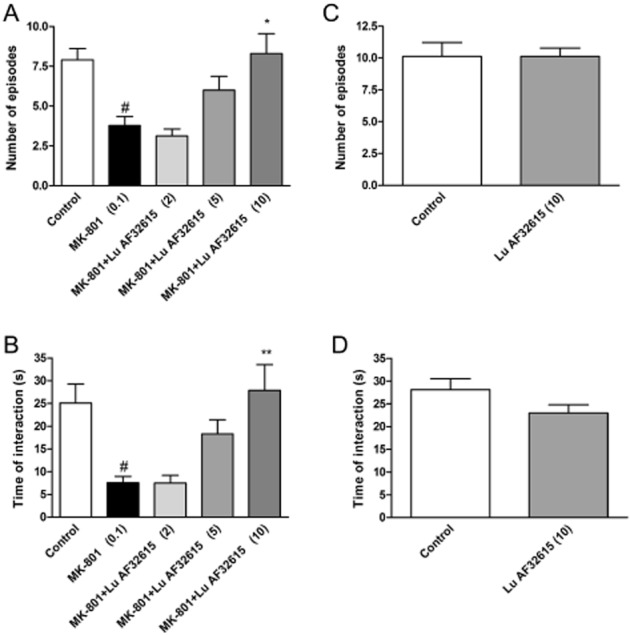
Effects of Lu AF32615 on MK-801-induced deficits in social interaction test. The graphs represent the disruption in the number of social episodes (A) and in the total duration of social episodes (B). Lu AF32615 was given 60 min before the test, and MK-801 was given 3.5 h before the test. Values in parentheses represent the doses of the compounds in mg·kg−1. Data are presented as mean ± SEM. #P < 0.01 versus control group and *P < 0.05 and **P < 0.01 versus MK-801-treated group; one-way anova analysis followed by Tukey's post hoc test. The control experiments without MK-801 administration are represented by the panels C and D.
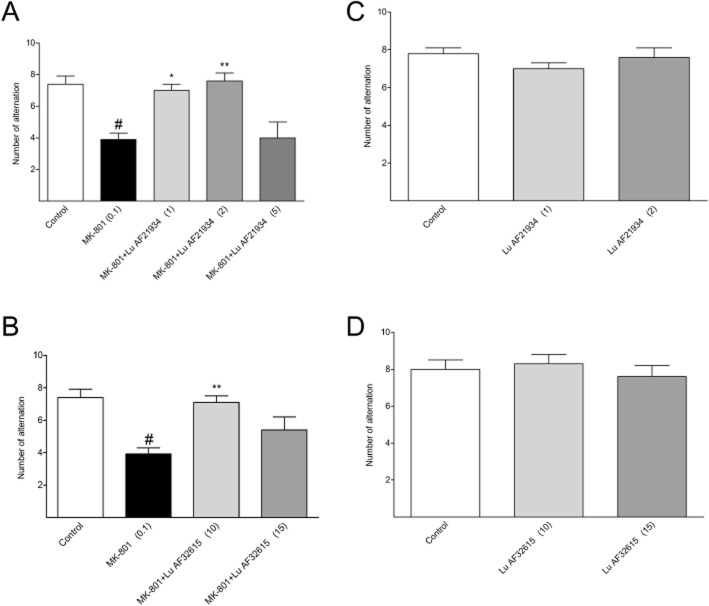
Delayed spatial alternation task
MK-801 (0.1 mg·kg−1) impaired this behaviour, that is, it decreased the number of alternations, an effect reversed by the reference compound, risperidone [F(2.24) = 20.17; P < 0.01] (Figure 13). Lu AF21934 was effective only at 1 or 2 mg·kg−1 [F(4.42) = 6.7; P < 0.01] (Figure 14A). The second compound, Lu AF32615 was tested over a higher dose range (5–15 mg·kg−1) and was effective only at 10 mg·kg−1 [F(3.35) = 6.17; P < 0.05] (Figure 14B). Control experiments revealed that neither risperidone (not shown) nor the PAMs had any effect on the behaviour of animals when given alone (Figure 14C, D).
Figure 13.
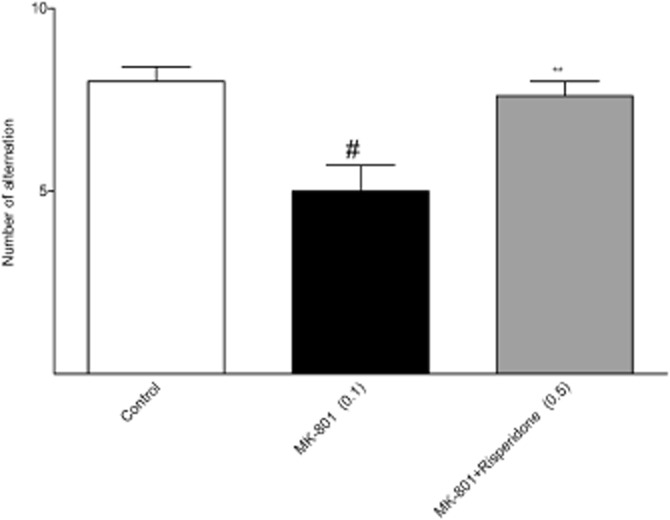
Effects of reference compound risperidone on MK-801-induced deficits in delayed spatial alternation task. Risperidone was given 30 min before the test. Values in parentheses represent the doses of the compounds in mg·kg−1. Data are presented as mean ± SEM. #P < 0.01 versus control group and *P < 0.05 and **P < 0.01 versus MK-801-treated group; one-way anova analysis followed by Tukey's post hoc test.
Figure 14.
Effects of Lu AF21934 (A) and Lu AF32615 (B) on MK-801-induced deficits in delayed spatial alternation task. Compounds were given 60 min before the test. Values in parentheses represent the doses of the compounds in mg·kg−1. Data are presented as mean ± SEM. #P < 0.01 versus control group and *P < 0.05 and **P < 0.01 versus MK-801-treated group; one-way anova analysis followed by Tukey's post hoc test. The control experiments without MK-801 administration are represented by the panels C and D.
Discussion
In the present work, we explored the antipsychotic-like activity of Lu AF21934 and Lu AF32615, two selective, structurally distinct PAMs of the mGlu4 receptor, using in vivo behavioural and ex vivo electrophysiological assessments. These tool compounds have been thoroughly characterized in terms of physicochemical and in vitro pharmacological properties, as well as brain penetration in rat (East et al., 2010; Bennouar et al., 2013). Their properties are presented in Table 1. While Lu AF21934 shows very weak activity at the mGlu6 receptor (PAM EC50 = 7 μM, 14-fold selectivity vs. mGlu4 receptors), the narrow and extra-CNS localization of this receptor suggests that this cross-reactivity will not interfere with effects of action at sites within the CNS (Nakanishi et al., 1998). The antipsychotic-like activity of these compounds was evaluated with respect to the positive, negative and cognitive symptoms of schizophrenia using well-characterized preclinical tests. The experimental schedules used in the present study were adapted from previous reports from our group (Pałucha-Poniewiera et al., 2008; Wierońska et al., 2012; 2013).
The reversal of MK-801- or amphetamine-induced hyperlocomotion are widely used animal models for detecting the effectiveness of drugs in reversing positive symptoms of schizophrenia (Geyer and Ellenbroek, 2003; Jones et al., 2011a,b). Lu AF21934 reversed MK-801- and amphetamine-induced hyperlocomotions with a U-shaped dose-responsive effect, while the action of Lu AF32615 was monotonic, within the restricted dose range studied (up to 10 mg·kg−1). Moreover, the actions of Lu AF21934 were consistently seen at ca. 10-fold lower doses than for Lu AF32615, which is in agreement with its improved in vitro potency (twofold), brain free fraction (1.6-fold) and absolute brain concentrations (threefold, see Table 1) (Hammarlund-Udenaes, 2010). In addition, its enantiomer lacking mGlu4 receptor PAM activity, Lu AF21935, was inactive in MK-801-induced hyperlocomotion when tested at comparable doses. Lu AF21934 was effective at doses similar to that described earlier when its anxiolytic-like properties were demonstrated (Sławińska et al., 2013).
The biphasic dose-behavioural response relationship observed here for Lu AF21934 was demonstrated earlier for other mGlu4 receptor agonists in a variety of experimental settings. Thus, a number of group III mGlu receptor agonists, such as APCPr, ACPT-I, LSP1-2111, exhibited biphasic dose-response relationships when tested in vivo in the reversal of haloperidol-induced catalepsy test (Sibille et al., 2007; Beurrier et al., 2009; Lopez et al., 2012). The group III-selective agonist L-AP4 also generated biphasic concentration-response curves during whole cell patch-clamp recordings from neurons in the globus pallidus (Valenti et al., 2003). Thus, our observations are consistent with previous observations on the pharmacology of mGlu4 receptor activation. Two hypotheses have been put forward to explain these biphasic responses: opposing pharmacology caused by the activation of mGlu7 or mGlu8 receptors at high agonist concentrations or agonist-driven mGlu4 receptor desensitization. The latter is the prevailing explanation at this time (Duty, 2010).
To further investigate the hypothesis of antipsychotic activity of mGlu4 receptor PAMs in models predictive of positive symptoms of schizophrenia, we used the 5-HT receptor agonist, DOI. In humans, activation of the 5-HT2A receptors (with LSD for example) induces hallucinogenic effects (Jacobs and Trulson, 1979). In rodents, an injection of DOI (a 5-HT2A receptor agonist) induces so-called head twitches (Vickers et al., 2001), that are reversed by compounds with both typical and atypical neuroleptic efficacy in the clinic (see Results). We found that both Lu AF21934 and Lu AF32615 attenuated this DOI-induced effect, as did agonists of group III metabotropic receptors (Pałucha-Poniewiera et al., 2008; Wierońska et al., 2012), and again the action of Lu AF21934 was more evident at low doses. The compound was also not active in mGlu4−/− animals, although the reference compound clozapine showed strong significant activity, confirming the specificity of Lu AF21934 for the mGlu4 receptor. However, due to limited number of available mGlu4−/− mice, Lu AF32615 was not investigated. Moreover, DOI effects appeared to be reduced in mGlu4-/- animals, and it does not align well with the effects of mGlu4 receptor activators in this model, and may be a result of compensatory mechanisms. Analogous effects were observed previously for mGlu7 knockout mice, which showed antidepressant-like phenotype, and similar antidepressant-like efficacy was observed for a mGlu7 PAM, AMN082 (see Pałucha-Poniewiera et al., 2008). There are other studies showing that knockout animals may behave differently from WT animals treated with antagonists. For example, mice that lack the 5-HT transporter exhibit increased anxio-depressive-like behaviour, although blockade of that transporter is currently the most common way to treat depression (Holmes et al., 2003; Lira et al., 2003).
A prominent negative feature of schizophrenia is social withdrawal, manifested as reduced social behaviour that often precedes the onset of the first psychotic episode and is strongly associated with poor psychosocial function (Cramer et al., 1992; Mueser and McGurk, 2004). Generally, in rodents social withdrawal can be obtained by the administration of NMDA antagonists, but not through activation of the dopaminergic system (Sams-Dodd, 1997). This effect can be reversed after administration of atypical, but not typical neuroleptics (Corbett et al., 1993; 1995); therefore clozapine was selected as a reference compound. The social withdrawal in the social interaction paradigm was induced by MK-801 and the number of social episodes and time of interaction between two rats was investigated in a novel environment, such as open field apparatus (Sams-Dodd, 1995; 1996). We adapted the modification of the method from the work of Satow et al., 2009, in which the positive effect of CFMTI, an mGlu1 receptor antagonist, was established. Both compounds investigated in this report reversed MK-801-induced social withdrawal and, as observed in earlier experiments, the action of Lu AF21934 was U-shaped and the activity of Lu AF32615 was evident in a linear manner within the dose range tested. Control experiments revealed that the drugs did not influence the social behaviour of rats when given alone.
In the last part of our investigations, we used the spatial delayed alternation task (DAT) as a model predictive of cognitive disturbances. Similarly to earlier described negative symptoms, efficacy of orthosteric agonists of the group III mGlu receptors towards cognitive disturbances had not been investigated previously. The DAT is based on the tendency of rodents to choose alternative maze arms or locations when they are re-exposed to an apparatus (Dudchenko, 2004) and is considered as working memory task because the animals must remember their initial response in order to select an alternative response. In our experimental schedule, we used 10% sucrose solution which helped the rats to make the resolution. A number of earlier studies established that NMDA receptor antagonists (PCP, MK-801, ketamine) resulted in chance-level performance in the DAT (Verma and Moghaddam, 1996; Wedzony et al., 2000; Aultman and Moghaddam, 2001). In our studies, we used risperidone as a reference compound, as haloperidol is ineffective in this paradigm and the effectiveness of clozapine is not evident. Both mGlu4 receptor PAMs reversed the MK-801 induced deficit in the dose ranges similar to those effective in previous behavioural models. The results again confirm that the mGlu4 receptor activation may constitute a promising alternative to currently used antipsychotic therapy. This aspect of our work seems to be of particular interest. The efficacy of the compounds towards not only positive, but also negative and cognitive symptoms of schizophrenia may constitute an advantage of the activators of the mGlu4 receptors over presently used antipsychotic therapy.
Considering the mechanisms of the behavioural effects described here, we would cite the hypothesis of Conn et al. (2009b). The psychotic symptoms evoked by MK-801 antagonists are thought to result from an over-activation of thalamocortical glutamatergic neurons that provide excitatory input to pyramidal neurons in the prefrontal cortex, leading to an enhanced glutamate release and the excessive activation of postsynaptic structures in the prefrontal cortex. The mGlu4 receptors, localized on glutamatergic terminals in the prefrontal and pyriform cortex (Benítez et al., 2000; Wierońska et al., 2007), as well as in thalamocortical circuitry (Wang et al., 2005) regulate glutamate release. The stimulation of those presynaptic mGlu4 receptors may counteract the MK-801-induced dysfunctions, by inhibiting the abnormal glutamate efflux ( Figure 15). Mechanistic support for this line of thinking was further gained through electrophysiological experiments performed in this studies, in which exposure to DOI increased the frequency and the amplitude of spontaneous EPSCs in layer V pyramidal neurons in the mice cortical slices, confirming earlier data (Kłodzinska et al., 2002; Pałucha-Poniewiera et al., 2008; Wierońska et al., 2012). Lu AF21934 and Lu AF32615 suppressed only the frequency, and not the amplitude of the spontaneous EPSCs, suggesting that their actions could be attributed to a presynaptic, rather than a postsynaptic, site (van der Kloot, 1991). The modulation by mGlu4 receptors of synaptic currents induced by 5-HT2A receptor activation in the prefrontal cortex/neocortex mimics the effects of the selective mGlu2/3 receptor agonists LY354740 and LY379268 (Marek et al., 2000; Schoepp and Marek, 2002; Wischhof et al., 2013). Therefore, this may constitute a common neuronal mechanism of antipsychotic effects of those compounds, which is also evident for atypical neuroleptics, such as clozapine (Schoepp and Marek, 2002).
Figure 15.
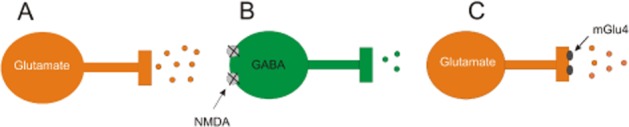
Schematic representation of the possible mechanisms by which mGlu4 receptor PAMs exert their antipsychotic action. Blockade of the NMDA receptors on midbrain inhibitory GABAergic neurons, which are normally under excitatory control from glutamatergic afferents (A), results in decreased excitation of GABAergic inhibitory neurons (B) and the loss of inhibitory control on excitatory glutamatergic thalamocortical neurons (C) that project to pyramidal neurons in the prefrontal cortex. This decreased activity of GABAergic inhibitory neurons leads to overactivation of thalamocortical glutamatergic neurons that normally provide excitatory input to pyramidal neurons in the prefrontal cortex.. This results in an enhanced release of glutamate and the excessive activation in postsynaptic structures in the prefrontal cortex, including pyramidal neurons. The mGlu4 receptors are expressed presynaptically at the thalamocortical pyramidal neurons and their activation may inhibit this abnormal glutamate release (see Conn et al., 2009b).
The simultaneous anti-Parkinsonian effectiveness of mGlu4 receptor PAMs may be puzzling, as both of these compounds have shown dose-dependent efficacy reversing haloperidol-induced catalepsy (East et al., 2010; Bennouar et al., 2013). The efficacy of Lu AF21934 potentiating the positive motor effects of L-DOPA in the 6-OHDA rat model has also been documented (Bennouar et al., 2013). The symptomatic effects in Parkinson's disease are derived from a re-balancing of the direct and indirect striatopallidal pathways (Lindsley and Hopkins, 2012). The key GABAergic nucleus in the basal ganglia is the globus pallidus, which receives an inhibitory striatal projection (see Valenti et al., 2003). Modulation of this striatopallidal synapse by mGlu4 receptors expressed on GABAergic terminals (Corti et al., 2002) could decrease the excessive inhibition within the globus pallidus that has been proposed in Parkinson's disease (Valenti et al., 2003; Marino et al., 2006). Therefore, the anti-Parkinsonian, as distinct from the antipsychotic, activity of mGlu4 receptor PAMs may be related to the different mechanisms and aetiologies of these diseases and the receptor localization in different brain regions. The efficacy of mGlu4 receptor PAMs in schizophrenia is thought to derive from a reduction of the excessive glutamatergic tone within thalamocortical circuits that characterizes this disease, while the anti-Parkinsonian effects would be a result of decreasing abnormal GABA efflux in the globus pallidus (GP).
In summary, the results of the present study constitute another step in the progress of ligands for metabotropic glutamate receptors from drug design to clinical efficacy. These initial results show that the effectiveness of mGlu4 receptor PAMs was evident in a broad spectrum of animal models of schizophrenia, thought to be predictive of positive, negative and cognitive disturbances. The current working hypothesis is based on the inhibition of glutamate release due to stimulation of presynaptic receptors localized on glutamatergic axons. The exact mechanism of action of these ligands is still under investigation.
Acknowledgments
This study is supported by project National Science Centre, 2012/06/A/NZ7/00014 given to A.P.
Glossary
- DAT
delayed alternation task
- DOI
2,5-dimethoxy-4-iodoamphetamine
- EPSCs
excitatory postsynaptic currents
- HPBCD
(2-hydropropyl)-β-cyclodextrin
- NAM
negative allosteric modulator
- PAM
positive allosteric modulator
Conflict of interest
DD is an employee of Lundbeck.
References
- Acher FC, Tellier FJ, Azerad R, Brabet IN, Fagni L, Pin JP. Synthesis and pharmacological characterization of aminocyclopentanetricarboxylic acids: new tools to discriminate between metabotropic glutamate receptor subtypes. J Med Chem. 1997;40:3119–3129. doi: 10.1021/jm970207b. [DOI] [PubMed] [Google Scholar]
- Addex Therapeutics website. ADX 71149. Available at: http://www.addextherapeutics.com/rd/pipeline/adx71149-for-schizophrenia (accessed September 7, 2012)
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th edn. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aultman JM, Moghaddam B. Distinct contributions of glutamate and dopamine receptors to temporal aspects of rodent working memory using a clinically relevant task. Psychopharmacology (Berl) 2001;153:353–364. doi: 10.1007/s002130000590. [DOI] [PubMed] [Google Scholar]
- Benítez R, Fernández-Capetillo O, Lázaro E, Mateos JM, Osorio A, Elezgarai I, et al. Immunocytochemical localization of the metabotropic glutamate receptor mGluR4a in the piriform cortex of the rat. J Comp Neurol. 2000;417:263–274. [PubMed] [Google Scholar]
- Bennouar KE, Uberti MA, Melon C, Bacolod MD, Jimenez HN, Cajina M, et al. Synergy between l-DOPA and a novel positive allosteric modulator of metabotropic glutamate receptor 4: implications for Parkinson's disease treatment and dyskinesia. Neuropharmacology. 2013;66:158–169. doi: 10.1016/j.neuropharm.2012.03.022. [DOI] [PubMed] [Google Scholar]
- Beurrier C, Lopez S, Révy D, Selvam C, Goudet C, Lhérondel M, et al. Electrophysiological and behavioral evidence that modulation of metabotropic glutamate receptor 4 with a new agonist reverses experimental parkinsonism. FASEB J. 2009;23:3619–3628. doi: 10.1096/fj.09-131789. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Christopoulos A, Lindsley CW. Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat Rev Drug Discov. 2009a;8:41–54. doi: 10.1038/nrd2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Lindsley CW, Jones CK. Activation of metabotropic glutamate receptors as a novel approach for the treatment of schizophrenia. Trends Pharmacol Sci. 2009b;30:25–31. doi: 10.1016/j.tips.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett R, Hartman H, Kerman LL, Woods AT, Strupczewski JT, Helsley GC, et al. Effects of atypical antipsychotic agents on social behavior in rodents. Pharmacol Biochem Behav. 1993;45:9–17. doi: 10.1016/0091-3057(93)90079-9. [DOI] [PubMed] [Google Scholar]
- Corbett R, Camacho F, Woods AT, Kerman LL, Fishkin RJ, Brooks K, et al. Antipsychotic agents antagonize non-competitive N-methyl-D-aspartate antagonist-induced behaviors. Psychopharmacology (Berl) 1995;120:67–74. doi: 10.1007/BF02246146. [DOI] [PubMed] [Google Scholar]
- Corti C, Aldegheri L, Somogyi P, Ferraguti F. Distribution and synaptic localisation of the metabotropic glutamate receptor 4 (mGluR4) in the rodent CNS. Neuroscience. 2002;110:403–420. doi: 10.1016/s0306-4522(01)00591-7. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Tsai G. The NMDA receptor glycine modulatory site: a therapeutic target for improving cognition and reducing negative symptoms in schizophrenia. Psychopharmacology (Berl) 2004;174:32–38. doi: 10.1007/s00213-003-1709-2. [DOI] [PubMed] [Google Scholar]
- Cramer P, Bowen J, O'Neill M. Schizophrenics and social judgement. Why do schizophrenics get it wrong? Br J Psychiatry. 1992;160:481–487. doi: 10.1192/bjp.160.4.481. [DOI] [PubMed] [Google Scholar]
- Dudchenko PA. An overview of the tasks used to test working memory in rodents. Neurosci Biobehav Rev. 2004;28:699–709. doi: 10.1016/j.neubiorev.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Duty S. Therapeutic potential of targeting group III metabotropic glutamate receptors in the treatment of Parkinson's disease. Br J Pharmacol. 2010;161:271–287. doi: 10.1111/j.1476-5381.2010.00882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- East SP, Bamford S, Dietz MG, Eickmeier C, Flegg A, Ferger B, et al. An orally bioavailable positive allosteric modulator of the mGlu4 receptor with efficacy in an animal model of motor dysfunction. Bioorg Med Chem Lett. 2010;20:4901–4905. doi: 10.1016/j.bmcl.2010.06.078. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Ellenbroek B. Animal behavior models of the mechanisms underlying antipsychotic atypicality. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1071–1079. doi: 10.1016/j.pnpbp.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Hammarlund-Udenaes M. Active-site concentrations of chemicals – are they a better predictor of effect than plasma/organ/tissue concentrations? Basic Clin Pharmacol Toxicol. 2010;106:215–220. doi: 10.1111/j.1742-7843.2009.00517.x. [DOI] [PubMed] [Google Scholar]
- Holmes A, Lit Q, Murphy DL, Gold E, Crawley JN. Abnormal anxiety-related behavior in serotonin transporter null mutant mice: the influence of genetic background. Genes Brain Behav. 2003;2:365–380. doi: 10.1046/j.1601-1848.2003.00050.x. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Trulson ME. Mechanisms of action of LSD. Am Sci. 1979;67:396–404. [PubMed] [Google Scholar]
- Javitt DC. Negative schizophrenic symptomatology and the PCP (phencyclidine) model of schizophrenia. Hillside J Clin Psychiatry. 1987;9:12–35. [PubMed] [Google Scholar]
- Jones CA, Brown AM, Auer DP, Fone KC. The mGluR2/3 agonist LY379268 reverses post-weaning social isolation-induced recognition memory deficits in the rat. Psychopharmacology (Berl) 2011a;214:269–283. doi: 10.1007/s00213-010-1931-7. [DOI] [PubMed] [Google Scholar]
- Jones CA, Watson DJG, Fone KCF. Animal models of schizophrenia. Br J Pharmacol. 2011b;164:1162–1194. doi: 10.1111/j.1476-5381.2011.01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. NC3Rs Reporting Guidelines Working Group. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinon BJ, Zhang L, Millen BA, Osuntokun OO, Williams JE, Kollack-Walker S, et al. A multicenter, inpatient, phase 2, double-blind, placebo-controlled dose-ranging study of LY2140023 monohydrate in patients with DSM-IV schizophrenia. J Clin Psychopharmacol. 2011;31:349–355. doi: 10.1097/JCP.0b013e318218dcd5. [DOI] [PubMed] [Google Scholar]
- Kłodzinska A, Bijak M, Tokarski K, Pilc A. Group II mGlu receptor agonists inhibit behavioural and electrophysiological effects of DOI in mice. Pharmacol Biochem Behav. 2002;73:327–332. doi: 10.1016/s0091-3057(02)00845-6. [DOI] [PubMed] [Google Scholar]
- Leite JV, Guimarães FS, Moreira FA. Aripiprazole, an atypical antipsychotic, prevents the motor hyperactivity induced by psychotomimetics and psychostimulants in mice. Eur J Pharmacol. 2008;578:222–227. doi: 10.1016/j.ejphar.2007.09.016. [DOI] [PubMed] [Google Scholar]
- Lindsley CW, Hopkins CR. Metabotropic glutamate receptor 4 (mGlu4)-positive allosteric modulators for the treatment of Parkinson's disease: historical perspective and review of the patent literature. Expert Opin Ther Pat. 2012;22:461–481. doi: 10.1517/13543776.2012.679437. [DOI] [PubMed] [Google Scholar]
- Lira A, Zhou M, Castanon N, Ansorge MS, Gordon JA, Francis JH, et al. Altered depression-related behaviors and functional changes in the dorsal raphe nucleus of serotonin transporter-deficient mice. Biol Psychiatry. 2003;54:960–971. doi: 10.1016/s0006-3223(03)00696-6. [DOI] [PubMed] [Google Scholar]
- Lopez S, Jouve L, Turle-Lorenzo N, Kerkerian-Legoff L, Salin P, Amalric M. Antiparkinsonian action of a selective group III mGlu receptor agonist is associated with reversal of subthalamonigral overactivity. Neurobiol Dis. 2012;46:69–77. doi: 10.1016/j.nbd.2011.12.045. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Connors BW, Lighthall JW, Prince DA. Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J Neurophysiol. 1985;54:782–806. doi: 10.1152/jn.1985.54.4.782. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek GJ, Wright RA, Schoepp DD, Monn JA, Aghajanian GK. Physiological antagonism between 5-hydroxytryptamine(2A) and group II metabotropic glutamate receptors in prefrontal cortex. J Pharmacol Exp Ther. 2000;292:76–87. [PubMed] [Google Scholar]
- Marino MJ, Williams DL, Jr, O'Brien JA, Valenti O, McDonald TP, Clements MK, et al. Allosteric modulation of group III metabotropic glutamate receptor 4: a potential approach to Parkinson's disease treatment. Proc Natl Acad Sci U S A. 2006;100:13668–13673. doi: 10.1073/pnas.1835724100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melancon BJ, Hopkins CR, Wood MR, Emmitte KA, Niswender CM, Christopoulos A, et al. Allosteric modulation of seven transmembrane spanning receptors: theory, practice, and opportunities for central nervous system drug discovery. J Med Chem. 2012;55:1445–1464. doi: 10.1021/jm201139r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Jackson ME. Glutamatergic animal models of schizophrenia. Ann N Y Acad Sci. 2003;1003:131–137. doi: 10.1196/annals.1300.065. [DOI] [PubMed] [Google Scholar]
- Mueser KT, McGurk SR. Schizophrenia. Lancet. 2004;363:2063–2072. doi: 10.1016/S0140-6736(04)16458-1. [DOI] [PubMed] [Google Scholar]
- Nakanishi S, Nakajima Y, Masu M, Ueda Y, Nakahara K, Watanabe D, et al. Glutamate receptors: brain function and signal transduction. Brain Res Brain Res Rev. 1998;26:230–235. doi: 10.1016/s0165-0173(97)00033-7. [DOI] [PubMed] [Google Scholar]
- Pałucha-Poniewiera A, Kłodzińska A, Stachowicz K, Tokarski K, Hess G, Schann S, et al. Peripheral administration of group III mGlu receptor agonist ACPT-I exerts potential antipsychotic effects in rodents. Neuropharmacology. 2008;55:517–524. doi: 10.1016/j.neuropharm.2008.06.033. [DOI] [PubMed] [Google Scholar]
- Patil ST, Zhang L, Martenyi F, Lowe SL, Jackson KA, Andreev BV, et al. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized phase 2 clinical trial. Nat Med. 2007;13:1102–1107. doi: 10.1038/nm1632. [DOI] [PubMed] [Google Scholar]
- Pin JP, Duvoisin R. The metabotropic glutamate receptors: structure and functions. Neuropharmacology. 1995;34:1–26. doi: 10.1016/0028-3908(94)00129-g. [DOI] [PubMed] [Google Scholar]
- Rorick-Kehn LM, Johnson BG, Knitowski KM, Salhoff CR, Witkin JM, Perry KW, et al. In vivo pharmacological characterization of the structurally novel, potent, selective mGlu2/3 receptor agonist LY404039 in animal models of psychiatric disorders. Psychopharmacology (Berl) 2007a;193:121–136. doi: 10.1007/s00213-007-0758-3. [DOI] [PubMed] [Google Scholar]
- Rorick-Kehn LM, Johnson BG, Burkey JL, Wright RA, Calligaro DO, Marek GJ, et al. Pharmacological and pharmacokinetic properties of a structurally novel, potent, and selective metabotropic glutamate 2/3 receptor agonist: in vitro characterization of agonist (-)-(1R,4S,5S,6S)-4-amino-2-sulfonylbicyclo[3.1.0]-hexane-4,6-dicarboxylic acid (LY404039) J Pharmacol Exp Ther. 2007b;321:308–317. doi: 10.1124/jpet.106.110809. [DOI] [PubMed] [Google Scholar]
- Sams-Dodd F. Distinct effects of d-amphetamine and phencyclidine on the social behaviour of rats. Behav Pharmacol. 1995;6:55–65. [PubMed] [Google Scholar]
- Sams-Dodd F. Phencyclidine-induced stereotyped behaviour and social isolation in rats: a possible animal model of schizophrenia. Behav Pharmacol. 1996;7:3–23. [PubMed] [Google Scholar]
- Sams-Dodd F. Effect of novel antipsychotic drugs on phencyclidine-induced stereotyped behaviour and social isolation in the rat social interaction test. Behav Pharmacol. 1997;8:196–215. [PubMed] [Google Scholar]
- Satow A, Suzuki G, Maehara S, Hikichi H, Murai T, Murai T, et al. Unique antipsychotic activities of the selective metabotropic glutamate receptor 1 allosteric antagonist 2-cyclopropyl-5-[1-(2-fluoro-3-pyridinyl)-5-methyl-1H-1,2,3-triazol-4-yl]-2,3-dihydro-1H-isoindol-1-one. J Pharmacol Exp Ther. 2009;330:179–190. doi: 10.1124/jpet.109.151118. [DOI] [PubMed] [Google Scholar]
- Schoepp DD, Marek GJ. Preclinical pharmacology of mGlu2/3 receptor agonists: novel agents for schizophrenia? Curr Drug Targets CNS Neurol Disord. 2002;1:215–225. doi: 10.2174/1568007024606177. [DOI] [PubMed] [Google Scholar]
- Sheffler DJ, Gregory KJ, Rook JM, Conn PJ. Allosteric modulation of metabotropic glutamate receptors. Adv Pharmacol. 2011;62:37–77. doi: 10.1016/B978-0-12-385952-5.00010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibille P, Lopez S, Brabet I, Valenti O, Oueslati N, Gaven F, et al. Synthesis and biological evaluation of 1-amino-2-phosphonomethylcyclopropanecarboxylic acids, new group III metabotropic glutamate receptor agonists. J Med Chem. 2007;50:3585–3595. doi: 10.1021/jm070262c. [DOI] [PubMed] [Google Scholar]
- Sławińska A, Wierońska JM, Stachowicz K, Pałucha-Poniewiera A, Uberti MA, Bacolod MA, et al. Anxiolytic- but not antidepressant-like activity of Lu AF21934, a novel, selective positive allosteric modulator of the mGlu(4) receptor. Neuropharmacology. 2013;66:225–235. doi: 10.1016/j.neuropharm.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Valenti O, Marino MJ, Wittmann M, Lis E, DiLella AG, Kinney GG, et al. Group III metabotropic glutamate receptor-mediated modulation of the striatopallidal synapse. J Neurosci. 2003;23:7218–7226. doi: 10.1523/JNEUROSCI.23-18-07218.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Kloot W. The regulation of quantal size. Prog Neurobiol. 1991;36:93–130. doi: 10.1016/0301-0082(91)90019-w. [DOI] [PubMed] [Google Scholar]
- Verma A, Moghaddam B. NMDA receptor antagonists impair prefrontal cortex function as assessed via spatial delayed alternation performance in rats: modulation by dopamine. J Neurosci. 1996;16:373–379. doi: 10.1523/JNEUROSCI.16-01-00373.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers SP, Easton N, Malcolm CS, Allen NH, Porter RH, Bickerdike MJ, et al. Modulation of 5-HT(2A) receptor-mediated head-twitch behaviour in the rat by 5-HT(2C) receptor agonists. Pharmacol Biochem Behav. 2001;69:643–652. doi: 10.1016/s0091-3057(01)00552-4. [DOI] [PubMed] [Google Scholar]
- Wang X, Ai J, Hampson DR, Snead OC., 3rd Altered glutamate and GABA release within thalamocortical circuitry in metabotropic glutamate receptor 4 knockout mice. Neuroscience. 2005;134:1195–1203. doi: 10.1016/j.neuroscience.2005.05.033. [DOI] [PubMed] [Google Scholar]
- Wedzony K, Maćkowiak M, Zajaczkowski W, Fijał K, Chocyk A, Czyrak A. WAY 100135, an antagonist of 5-HT1A serotonin receptors, attenuates psychotomimetic effects of MK-801. Neuropsychopharmacology. 2000;23:547–559. doi: 10.1016/S0893-133X(00)00150-0. [DOI] [PubMed] [Google Scholar]
- Weinberger DR. Schizophrenia drug says goodbye to dopamine. Nat Med. 2007;13:1018–1019. doi: 10.1038/nm0907-1018. [DOI] [PubMed] [Google Scholar]
- Wierońska JM, Kłak K, Pałucha A, Brański P, Pilc A. Citalopram influences mGlu7, but not mGlu4 receptors’ expression in the rat brain hippocampus and cortex. Brain Res. 2007;1184:88–95. doi: 10.1016/j.brainres.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Wierońska JM, Kusek M, Tokarski K, Wabno J, Froestl W, Pilc A. The GABA B receptor agonist CGP44532 and the positive modulator GS39783 reverse some behavioural changes related to positive syndromes of psychosis in mice. Br J Pharmacol. 2011;163:1034–1047. doi: 10.1111/j.1476-5381.2011.01301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierońska JM, Stachowicz K, Acher F, Lech T, Pilc A. Opposing efficacy of group III mGlu receptor activators, LSP1-2111 and AMN082, in animal models of positive symptoms of schizophrenia. Psychopharmacology (Berl) 2012;220:481–494. doi: 10.1007/s00213-011-2502-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierońska JM, Acher FC, Sławińska A, Gruca P, Łasoń-Tyburkiewicz M, Papp M, et al. The antipsychotic-like effects of the mGlu group III orthosteric agonist, LSP1-2111, involves 5-HT1A signaling. Psychopharmacology (Berl) 2013;227:711–725. doi: 10.1007/s00213-013-3005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wischhof L, Aho HE, Koch M. DOI-induced deficits in prepulse inhibition in Wistar rats are reversed by mGlu2/3 receptor stimulation. Pharmacol Biochem Behav. 2012;102:6–12. doi: 10.1016/j.pbb.2012.03.011. [DOI] [PubMed] [Google Scholar]