Abstract
Background and Purpose
The hypothesis that endocannabinoids protect hearts against ventricular fibrillation (VF) induced by myocardial ischaemia and reperfusion was examined, and the concept that cannabinoids may represent a new class of anti-VF drug was tested.
Experimental Approach
In rat isolated hearts (Langendorff perfusion), VF evoked by reperfusion after 60 min regional ischaemia is known to be exacerbated by inhibitors of endogenous protectants such as nitric oxide. This preparation was used to assay the effects of cannabinoid agonists and antagonists, and the protocols were varied to examine mechanisms.
Key Results
Reperfusion-induced VF was not facilitated by relatively selective CB1 (1 μM AM251) or CB2 (1 μM AM630) antagonists. VF evoked during early (30 min) acute ischaemia was also unaffected. However, AM251 significantly increased the incidence of VF and the duration of VF episodes occurring during the later stage of acute ischaemia (30–60 min). AM630 had no such effects. In a separate study, cannabinoid perfusion (anandamide or 2-arachidonoylglycerol, both 0.01-1 μM) failed to reduce VF incidence concentration-dependently during 30 min ischaemia. In all these studies, changes in ancillary variables (QT, PR, heart rate) were unrelated to changes in VF.
Conclusions and Implications
Endocannabinoids are not endogenous anti-VF mediators during reperfusion, but may have a weak protective effect during the late stages of ischaemia, mediated via CB1 agonism. This does not suggest endocannabinoids are important endogenous protectants in these settings, or that CB1 (or CB2) receptors are useful novel targets for developing drugs for VF.
Keywords: acute myocardial infarction, 2-arachidonoylglycerol, anandamide, antiarrhythmic, arrhythmia, endocannabinoid, myocardial ischaemia, ventricular fibrillation, proarrhythmia, sudden cardiac death
Introduction
Sudden cardiac death (SCD) remains a challenging therapeutic target (Wannamethee et al., 1995; Zheng et al., 2001; Camm, 2012; John et al., 2012), with a preponderance of lethal ventricular fibrillation (VF) occurring unpredictably after the onset of ischaemia (Campbell, 1983; 1998). One possible approach to novel drug discovery is to exploit (by mimicry, facilitation or antagonism, as appropriate) the endogenous mediators that dampen or facilitate susceptibility to VF during ischaemia or reperfusion (Curtis et al., 1993; Parratt, 1993). Numerous studies have identified a range of possible candidate mediators that may facilitate VF (including thromboxane A2, 5-HT, histamine) and other endogenous substances that may dampen susceptibility such as adenosine and nitric oxide (NO) (Pabla et al., 1995; Curtis and Pabla, 1997; Clements-Jewery et al., 2009a), hereafter described as putative endogenous mediators and protectants, respectively. It has been a longstanding proposition that judicious inhibition of the actions of mediators, or mimicry or facilitation of the action of endogenous protectants may be exploited to form the basis of novel drug discovery for VF (Curtis et al., 1993; Parratt, 1993). However, this approach has yet to translate to novel therapy. There are numerous possible reasons for this including difficulties in achieving cardioselective- and disease-selective targeting by inhibitor or mimic drugs (Clements-Jewery et al., 2009a) and the possibility that there is a pathophysiological reserve for VF resistant to mediator/modulator-selective targeting (Yamada et al., 1990; Curtis, 1998). Additionally, it remains that there are putative endogenous mediator targets yet to be fully explored.
The present study focuses on cannabinoids (CBs). There is evidence that CBs may inhibit arrhythmias associated with reperfusion (Ugdyzhekova et al., 2001; Krylatov et al., 2002). Furthermore, a related substance, cannabidiol, reduces ventricular tachycardia (albeit its duration rather than its initiation/incidence) in rats in vivo (Walsh et al., 2010). Separately, there is some evidence that cannabinoids may ameliorate the pathophysiological progression of myocardial infarction (Underdown et al., 2005), increase resistance to arrhythmogenic effects of adrenaline (Ugdyzhekova et al., 2001) and ameliorate ischaemia-induced endothelial dysfunction (Bouchard et al., 2003). Furthermore, endogenous anandamide and 2-arachidonoylglycerol (2-AG) are not only present in rat hearts perfused in vitro, but levels are maintained during ischaemia and reperfusion (Wagner et al., 2006) which satisfies the most fundamental requirement for their having a possible role as mediators or modulators of cardiac dysfunction (Curtis et al., 1993).
In view of this suggestive, but limited evidence, we have examined the effects of CB1 and CB2 agonists and antagonists on VF evoked by regional ischaemia and by reperfusion in the isolated rat heart, a robust bioassay for such purposes (Curtis, 1998) in a comprehensive proof of concept study.
We selected three settings to examine. Early ischaemia (first 30 min of ischaemia) was chosen because numerous animal studies (data summarized in figures 4 of Curtis, 1998) and the best available clinical data (e.g. Campbell, 1983; 1998) have shown that this is the setting in which VF (and hence, in humans, SCD) is most prevalent after coronary obstruction, and this is the main reason why such a high proportion of SCD occurs as the first symptom of coronary artery disease (Huikuri et al., 2001). Later ischaemia (30–60 min) is associated with a much diminished susceptibility to VF and was included for separate subanalysis in the present study chiefly because novel effects specific to this interlude were observed. Reperfusion after sustained ischaemia (60 min) was included in the study in part because our previous work found that NO conferred endogenous protection in this scenario in rat and rabbit hearts (Curtis and Pabla, 1997). Separately, although spontaneous reperfusion-induced VF probably contributes little to the burden of SCD (see Curtis, 1998), and in animals reperfusion-induced VF is time-dependent and extremely rare when ischaemia is sustained beyond 60 min in control rat hearts (Ravingerova et al., 1995), there is an increasing trend for earlier elective reperfusion (which in humans means earlier than 3 h after symptom onset) in victims of coronary obstruction (Van de Werf, 2006). Thus VF induced by reperfusion after 60 min ischaemia (a target in human coronary artery disease) is worthy of study for its own sake.
Figure 4.
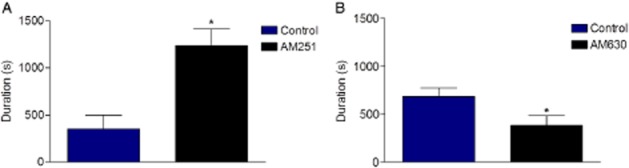
Duration of ischaemia-induced VF. Part A, AM251 versus control; part B, AM630 versus control. *P < 0.05 versus control.
It is important to examine VF mechanisms in more than one setting because even though acute ischaemia is likely to be the predominant cause of SCD, there is evidence that the underlying mechanisms of VF initiated during later ischaemia and during reperfusion differ (Curtis and Hearse, 1989a; Pabla et al., 1995; Clements-Jewery et al., 2005; Clements-Jewery et al., 2009b).
The data revealed a weak protection by endocannabinoid activation of CB1 receptors that does not support further work to develop cannabinoids as drugs for preventing SCD.
Methods
Rationale
To examine whether exogenously applied CB agonists possess antiarrhythmic activity, the region of myocardium subjected to ischaemia and/or reperfusion (conventionally known as the involved zone: IZ), was made deliberately large by the position on the coronary tree chosen for placement of the coronary ligature (Ridley et al., 1992; Curtis, 1998).
To examine whether endocannabinoids confer endogenous protection against VF, we tested whether CB antagonists could increase VF incidence from a low baseline susceptibility. This was achieved by reperfusion of the IZ after a sustained (60 min) episode of ischaemia, a scenario shown previously to be associated with a low incidence of VF (≍20% of hearts in a group), due in part to endogenous protection by NO (Pabla and Curtis, 1995).
Two antagonists were chosen for their relative selectivity for the CB1 versus CB2 receptor (Lan et al., 1999; Ross et al., 1999) defined in accordance with the nomenclature of the Guide to Receptors and Channels (Alexander et al., 2011). The rat heart was chosen because it expresses CB1 and CB2 receptors (Bouchard et al., 2003) and for its bioassay utility (Curtis, 1998). Coronary ligation in the rat heart produces uniform low flow regional ischaemia owing to a uniform low level of collateral anastomoses that renders regional flow in the rat IZ to below 5% of flow in the uninvolved zone in all hearts (Curtis and Hearse, 1989b). There is no scope for indirect protection against VF or myocardial infarction in the rat heart by coronary vasodilatation (Curtis, 1998) allowing the preparation to be used to evaluate direct (IZ flow-independent) drug actions, facilitating interpretation of data. Drug-induced bradycardia may delay ischaemia-induced injury and VF onset (Bernier et al., 1989) but if this occurs it may be controlled by atrial pacing (Tsuchihashi and Curtis, 1991) – found not to be needed in the present study.
The in vitro approach permits an uncluttered assessment of agonist and antagonist actions in the heart itself, free from the confounding influence of autonomic reflexes and other homeostatic processes, and the effects of metabolism on delivered drug concentrations (Ridley et al., 1992; Curtis, 1998). Promising results would justify use of more experimentally challenging and expensive in vivo approaches (Curtis, 1998).
General methods
All experiments were performed in accordance with the United Kingdom Home Office Guide on the Operation of the Animals (Scientific Procedures) Act 1986. The rat Langendorff left regional ischaemia procedure for induction of arrhythmias has been described in full (Curtis and Hearse, 1989a,b; Curtis, 1998). In accordance with the ARRIVE guidelines (Kilkenny et al., 2010) as interpreted for the British Journal of Pharmacology (McGrath et al., 2010), we report the following information concerning animal husbandry and anaesthesia. Male Wistar rats, 250–300 g (Bantin & Kingman, Hull, UK) were housed 5 to a cage and taken for experimentation a minimum of 3 days after delivery to the animal unit. No rats were left 1 to a cage at the end of any day. The RCI™ cages (NKP, Rochester, UK) were 1575 cm2 by 17 cm depth. Bedding was Select Aspen™ (Lillico, Betchworth, UK). A 12/12 h light/dark cycle was employed, with light change faded in and out over 1 h to simulate dawn and dusk. Full lights off occurred at 1800 h. Ambient temperature was regulated between 19 and 21°C. Ambient humidity was regulated between 45 and 65%. Animal feed was RMI™ (SDS, Witham, UK) and was provided ad libitum. The rats were anaesthetised with sodium pentobarbitone (80 mg·kg−1 i.p.) (Pentoject™, York, UK) and given heparin (1000 IU·kg−1 i.p.) (Sigma Aldrich, Poole, UK). This dose of pentobarbitone would be sufficient to arrest respiration and cause death, but once corneal reflexes were absent, indicative of a surgical level of general anaesthesia, animals were killed by cardiac excision and exsanguination. Coronary ligation was achieved using a traction-type ligature placed under the left main coronary artery (Curtis and Hearse, 1989a,b; Curtis, 1998). In all studies, an electrogram (ECG) was recorded from the middle of the IZ for determining rhythm, heart rate (HR) and ECG intervals (PR, QT). The QT interval was measured at 90% repolarization (Farkas and Curtis, 2003). Arrhythmias were defined according to the Lambeth Conventions (Curtis et al., 2013). Coronary flow (CF) was determined by timed collection of coronary effluent, and the IZ was quantified by an established dye exclusion technique (Curtis and Hearse, 1989a).
Drug delivery
Because the study used lipophylic drugs the following drug delivery procedure for constant pressure perfusion was used. High concentrations of drug were stably dissolved in solvent, then delivered at a low flow rate (through the shortest feasible length of tubing) to enter and mix close to the heart in the main stream of gravity-fed perfusate. The agonist solutions were made up on the day of use for each individual heart from frozen stock solutions and added to a 50 mL syringe (BD Plastipak, Oxford, UK) in a volume calculated as 150% the approximate volume needed for each heart perfused. A 25 G blunted needle (BD Microlance, Oxford, UK) was attached to the syringe and securely connected to a short length of small diameter fine polyethelene tubing. The syringe was then placed in the infusion pump (KD Scientific, Holliston, MA, USA) and the fine tubing was fed into a side arm of the main aortic cannula. The tubing tip was located distal to the ports feeding into the terminal section of the aortic cannula to ensure adequate mixing of drug/vehicle solution in the main perfusion solution by the flow's turbulence. The drug/vehicle solution was prepared at 20 times the desired final concentration and infused at 1/20th of the rate of total CF, with the rate adjusted at 5 min intervals throughout the experiment, to sustain delivery of the desired concentration. The ability of the drug delivery system to achieve its objective (steady delivery of drug at its desired concentration) was validated with a drug with a known effect on CF, verapamil (Sigma Aldrich, Poole, UK), which at a nominal 600 nM increased CF (Figure 1) to the level expected for precisely 600 nM verapamil when delivered dissolved in the main perfusion reservoir in the rat perfused heart (Farkas et al., 1999).
Figure 1.
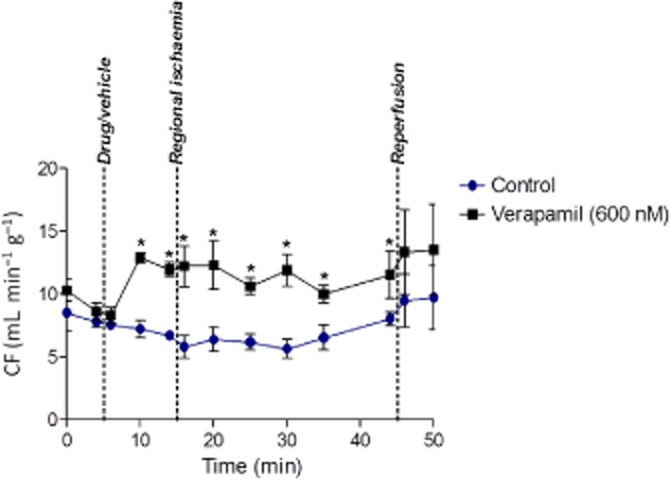
Validation of the infusion pump delivery method using verapamil. Verapamil increased coronary flow (CF) significantly within the 5 min of the start of infusion and this was maintained all the while the drug was delivered. Values are expressed as flow per gram perfused tissue. Data are mean ± SEM. n = 3. *P < 0.05 versus control.
Protocols
In all studies hearts were initially perfused with Krebs solution (modified to contain 1.4 mM Ca2+ and 3 mM K+, at 37°C and pH 7.4) to obtain baseline readings prior to blinded and randomized switch to test solution (drug or vehicle). Once solution was switched the test solution was delivered thereafter and, 10 min after solution switch, the coronary ligature was tightened to induce regional ischaemia lasting for 30 or 60 min.
In the first part of the study, hearts were subjected to 60 min ischaemia in order to examine possible facilitation by CB antagonists of VF when baseline susceptibility is ordinarily low in controls in this preparation (i) during reperfusion (Pabla and Curtis, 1995) and (ii) during the latter stages of ischaemia, that is, >30 min after coronary ligation (Clements-Jewery et al., 2006).
In view of the outcome of the first part of the study (see results), a separate experiment was undertaken in which hearts were subjected to 30 min of ischaemia to study early ischaemia-induced arrhythmias, also known as ‘phase 1’ arrhythmias (Curtis, 1998; Clements-Jewery et al., 2005) and their possible suppression by CB agonists.
Exclusions
Bradycardia delays the onset of VF, and hence reduces the incidence of VF during the early stages of acute ischaemia (Bernier et al., 1989). For this reason if HR was less than 250 beats per min 5 min prior to switching to the drug/vehicle solution the heart was excluded. A high CF is indicative of a torn aorta. A low baseline CF is indicative of heart damage sustained in the excision procedure, especially accidental incursion of an air bubble, trapped in the coronary tree. A torn aorta renders CF values meaningless. A low CF may lead to global low-flow ischaemia. Therefore, hearts with a CF of more than 23 mL·min−1·g−1 or less than 7.5 mL·min−1·g−1 in the 5 min period prior to switching to test solution were excluded as in previous studies (Clements-Jewery et al., 2006).
Drugs
To distinguish between CB1- and CB2-mediated endogenous protection, we chose AM251, an antagonist that is 300-fold selective for CB1 versus CB2 receptors, and AM630 which is 160-fold selective for CB2 versus CB1 receptors (Lan et al., 1999; Ross et al., 1999). The choice of drug concentration was based on published studies in which actions in the cardiovascular system, in particular antagonism of effects of CB agonists, were obtained with 1 μM AM251 and 1 μM AM630 (Ford et al., 2002; Currie et al., 2008). AM251 and AM630 were dissolved in 100% DMSO to make up 10 mM stock solutions. The final concentrations of drugs and vehicle delivered to hearts were 1 μM AM251 and 1 μM AM630 in 0.01% DMSO in Krebs solution. The vehicle, 0.01% DMSO, was used in control ‘test’ solution. DMSO vehicle has been used previously without evidence of effects on arrhythmias or other variables (Rees and Curtis, 1995). For studies with anandamide and 2-AG, the drugs were dissolved in their standard vehicle, Tocrisolve™, and each of the various final drug concentrations were delivered in 0.035% Tocrisolve in Krebs solution. As shown later, the incidence of VF during ischaemia in Tocrisolve controls was close to 100% indicating that this vehicle, like DMSO, does not affect arrhythmia susceptibility. Drugs and Tocrisolve were bought from Tocris Bioscience (Bristol, UK). In agonist/antagonist combination studies, vehicle was 0.01% DMSO plus 0.035% Tocrisolve in Krebs solution.
Group sizes and statistics
In order to achieve statistical power when examining arrhythmia facilitation from a low control baseline, we used a minimum of n = 20 hearts per group as in previous studies (Pabla and Curtis, 1995). Some inequalities arose owing to the need to replace hearts that had developed sustained ischaemia-induced VF that persisted at the moment of reperfusion. Replaced hearts were incorporated into the data set for ischaemia-induced VF, resulting in larger group sizes for that part of the study. In order to avoid needless animal sacrifices, studies were unblinded, analysed, and assessed after 12 hearts per group was achieved. The study was stopped if there was a clear-cut outcome. If a weak drug effect or trend was observed, to avoid type 1 error, we added hearts in a blinded and randomized fashion (to obtain final group sizes of n = 20 hearts and greater statistical power). On this basis, studies with 2-AG were terminated after initial unblinding whereas studies conducted with anandamide were continued to n = 20 hearts per group. Variation in group sizes resulted from these design considerations. Gaussian distributed variables were subjected to unpaired t tests (for comparing two groups) or anova followed by Dunnett's post hoc test (for comparing three or more groups) where appropriate (Rees and Curtis, 1995). Where a Gaussian distribution could not be assumed (e.g. duration of VF episodes) the Mann–Whitney test was used. Values are expressed as mean ± SEM. Non-parametric analyses of binomially distributed variables such as VF incidence was achieved with chi2. A P value of <0.05 was considered significant. GraphPad Prism 5 or Mainland's contingency tables (Mainland et al., 1965) were used for all statistical assessments.
Results
Actions of CB antagonists during 60 min ischaemia and during reperfusion
In the first part of the study, total incidence of VF during 60 min ischaemia was predictably high in controls and this was not affected by either drug, with values among groups ranging from 16/25 to 22/26 (P = NS). When the 60 min period of ischaemia was subdivided into sequential 30 min time blocks, AM251 had no effect on VF incidence during the first 30 min of ischaemia but was found to significantly increase the incidence of VF during the later stages of ischaemia (30–60 min) from 5/28 to 17/29 (P < 0.05). The lack of effect during the first 30 min indicates this was an increase in late susceptibility rather than a timeshift in early susceptibility. In contrast, AM630 had no such effect (9/24 vs. 8/23 in controls in the 30–60 min period). Accompanying haemodynamic and ECG changes, assessed to give clues to mechanisms, included a marked increase in CF with AM251 that was not replicated by AM630 (Figure 2). However, this effect was transient and no longer present during the later 30–60 min period when facilitation of ischaemia-induced VF occurred.
Figure 2.
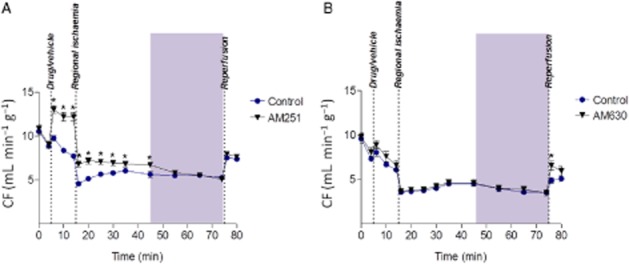
Coronary flow (CF) before and during 60 min regional ischaemia. Part A AM251 versus control, part B AM630 versus control. *P < 0.05 versus control.
AM251 increased QT interval, and although this effect was small, it was significant and restricted to the relevant 30–60 min period (Figure 3A) during which ischaemia-induced VF incidence was increased. However, AM630 had a similar weak effect on QT during the same time interval (Figure 3B) despite its lack of effect on VF incidence.
Figure 3.
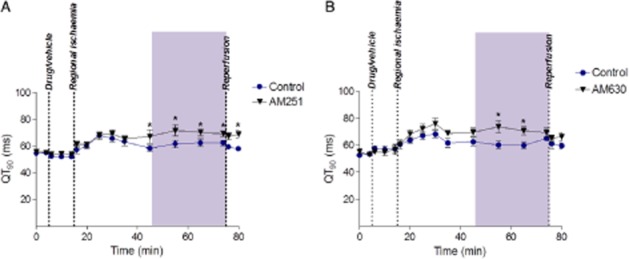
QT interval before and during 60 min ischaemia. Part A, AM251 versus control; part B, AM630 versus control. *P < 0.05 versus control.
Typically, drugs that prolong QT without affecting VF incidence can nevertheless make VF transient (i.e. self terminate after short runs) in the rat isolated heart (Tsuchihashi and Curtis, 1991). Although AM630 was found to reduce the average duration of episodes of ischaemia-induced VF (P < 0.05) as predicted by its QT effect, AM251 actually made individual VF episodes be sustained for longer periods (P < 0.05) (Figure 4) despite having similar effects on QT to AM630 (Figure 3). Neither drug affected HR, and PR interval changes were insubstantial (data not shown).
The other objective of this first part of the study was to examine possible facilitation of VF in hearts reperfused after sustained (60 min) ischaemia. There was no facilitation; neither AM 251 (2/20 hearts in VF vs. 1/20 controls), nor AM 630 (4/20 vs. 4/20) increased VF incidence during reperfusion (all P = NS).
Actions of exogenously administered endocannabinioids
The first part of the study suggests that endogenous CB1 agonism may exert an endogenous protective effect during the 30–60 min period of ischaemia. This raises the possibility that exogenously applied CB1 agonists may (more usefully) protect against the more severe earlier (phase 1) VF that occurs during the first 30 min of ischaemia. VF incidence during 30 min ischaemia was 11/12, 11/12, 11/12 and 10/12 in the control, 0.01, 0.1 and 1 μM 2-AG groups respectively (all P > 0.05). 2-AG had no significant effects on any measured variable (i.e. HR, CF, PR or QT interval; data not shown). However, anandamide had a trend towards a significant effect on ischaemia-induced VF incidence at 0.1 μM so the n number was increased in each group to 20 in a randomized and blinded manner in order to ensure avoidance of a type 1 error. A concentration-independent and weak inhibition of VF was found to reach statistical significance, with incidences of 18/20, 18/20, 11/20* and 14/20 in the control, 0.01, 0.1 and 1 μM anandamide groups respectively (*P < 0.05 for the 0.1 μM group only).
The study was therefore extended with additional groups of hearts to explore which receptor mediated the protection afforded by anandamide. The ‘effective’ anadamide concentration was combined in concurrent co-perfusion with 1 μM AM251 or AM630, or both drugs. However, disappointingly the protective effect of anandamide was not reproducible (Figure 5), suggesting that the initial observation was a chance and false finding (type II error). AM251 combined with anandamide, or combined with anandamide plus AM630 increased CF before the onset of ischaemia, as also seen in the first study (data not shown). Although anandamide alone had no effect on QT, the QT prolongation seen previously with AM251 and AM630 did not occur in anandamide co-perfused hearts (data not shown). There were no effects of any drug combination on HR (data not shown).
Figure 5.
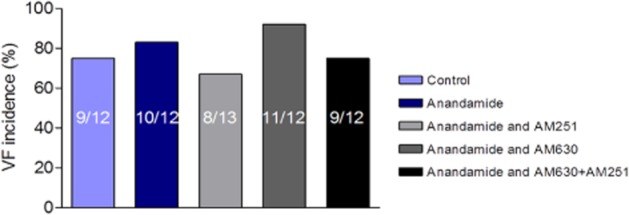
Effect of 0.1 μM anandamide on incidence of phase 1 ischaemia-induced VF in the absence and presence of co-perfusion with 1 μM AM251, 1 μM AM630 or the combination.
Discussion
Bioassay of endogenous cardioprotection against VF
The model and approaches used in the present study were chosen for their suitability for proof-of-concept studies on endogenous modulation of ischaemia- and reperfusion-induced VF (Tsuchihashi and Curtis, 1991; Rees and Curtis, 1995; Curtis, 1998). A protocol with a known low susceptibility to VF (reperfusion after 60 min ischaemia) was selected first to test whether CB antagonists would unmask VF. This approach has been used previously to show that NO confers endogenous protection against VF (Pabla and Curtis, 1995). The precise concentrations of endocannabinoids to test was uncertain due in part to the uncertainty concerning the concentrations present in ischaemic hearts. Measured concentrations appear to vary during the course of ischaemia, and therefore cannot be precisely replicated (Wagner et al., 2006). In view of this, we used a concentration-response design with endocannabinoids as a conservative approach.
Key findings
The study failed to identify VF facilitation during reperfusion, but identified a weak facilitation of VF susceptibility during the latter 30 min of a 60 min episode of regional ischaemia by the CB1 antagonist, AM251. This suggests that endocannabinoids may exert a weak protective influence during this time via CB1 agonism. In separate follow-up studies, perfusion with anandamide (but not 2-AG) revealed a protective effect against earlier (phase 1) ischaemia-induced VF, but the effect was weak, not concentration-dependent, and not reproducible in a subsequent study to probe the receptor mechanisms. Thus, any benefit that cannabinoids may have against ischaemia-induced VF appears to be very weak, and no benefit against reperfusion-induced VF was found.
Mechanisms
Given the limited extent of benefit observed, the mechanisms responsible deserve only limited consideration. Cannabinoids have a variety of pharmacological effects at the level of cells and isolated tissues, but whether these translate to the intact heart is unclear. Some of these actions will affect arrhythmias if sufficient in magnitude. For example, Li et al. (2009) showed that CB1 antagonism blocks L-channel activating effects of anandamide. Blockade of cardiac L channels increases CF, widens PR interval and inhibits ischaemia-induced arrhythmias in the rat isolated heart through parallel independent L channel mediated effects (Farkas et al., 1999). Therefore if the CB1 antagonist AM251 has substantial effects on an endocannabinoid-mediated L channel activating effect in isolated rat hearts, AM251 should increase CF, widen PR interval and suppress arrhythmias, whereas anandamide should have the opposite effects. Clearly, this was not the case in the present study, meaning the weak effects observed are not likely to be mediated via modulation of L channels. The transient increase in CF with AM251 in the present study is not novel. It has been published that AM251 can apparently dilate coronary arteries in rat isolated hearts. Ford et al. (2002) showed that coronary perfusion pressure was unaffected by anandamide, but when AM251 was added perfusion pressure fell from 65 ± 9 to 54 ± 4 mmHg. This was not reported as significant or remarked upon but it is in the same ballpark as the effects we saw, and likely represents an unknown off target action.
Drugs that block certain K+ currents (Ito, IKATP, IK1) prolong QT in the rat heart (Tsuchihashi and Curtis, 1991; Rees and Curtis, 1995; 1996). AM251 and AM630 both increased QT interval in the present study during the later period of ischaemia (30–60 min). Evidently, the relevance of this to the facilitation of ischaemia-induced VF by AM251 is likely to be nil, in part because AM630 had no effect on VF incidence, and in part because the effect was small compared with that seen previously with K+ current-blocking drugs that affect VF in this model (Rees and Curtis, 1996). Drugs that prolong QT interval by blocking Ito or IKATP make VF transient (non-sustained) in the rat heart. AM251 and AM630 had opposite effects on the ability of VF to sustain after it had begun despite both drugs prolonging QT.
Quite separately, it is known that the agonists, anandamide and 2-AG, block Ito in nM range via CB1 and CB2 receptor-independent effects (Barana et al., 2009). Such actions, if present in the present study, were insubstantial since anandamide and 2-AG did not cause QT prolongation, in contrast with known effects of K+ current-blocking drugs in this model (Rees and Curtis, 1996).
Finally, Ito blockade can slow HR (Tsuchihashi and Curtis, 1991). Bradycardia can delay the time-dependent diminution in susceptibility to VF occurring during the later stage of acute ischaemia (30–60 min) (Bernier et al., 1989). However, neither AM251 nor AM 630 affected HR.
Overall, this means that off-target K+ channel blocking effects have no likely relevance to modulation of VF in the present study.
The most likely explanation for the observations is that the QT prolongation evoked by the two drugs was endocannabinoid receptor-independent and too insubstantial to reduce VF incidence, whereas in the increase in VF incidence and duration caused by AM251 was the consequence of inhibition of endogenous antiarrhythmic effects of endocannabinoids and unrelated to the weak effects on QT interval. The mechanism by which endocannabinoids inhibit VF cannot be ascertained from the present study, albeit modulation of repolarization (and hence K+ currents) can be ruled out. Likewise, there was no evidence of L type calcium channel or sodium channel mediated effects (no bradycardia or PR interval alteration). It is debatable whether the mechanism responsible for apparently subtle CB1 receptor-mediated effects on electrophysiology and the modest resultant alteration of late ischaemia-induced VF incidence and duration warrant further investigation.
Limitations
Cannabinoid agonists were studied primarily to examine their properties as putative cardiac protectants. Antagonist drugs were used as tools in the present study because the necessary conditional cardiac specific knockouts required to study mechanisms are not readily available in rats. This pharmacological approach is potentially limited by the specificity and selectivity of the chosen tools. For example, it is possible that we failed to detect a real endocannabinoid mediated contribution to the low susceptibility to reperfusion-induced VF when hearts were reperfused after 60 min ischaemia, because the antagonists we used failed to block the relevant receptors. The feeble antiarrhythmic effects of anandamide and 2-AG during ischaemia would suggest this is unlikely, but endocannabinoid-mediated protection during reperfusion operating via non-CB1 or CB2 receptors cannot be discounted. The complex and contradictory readout from the present study may result from limitations in drug selectivity, although the drugs selected for use have been selected by many groups for equivalent purposes in other settings (Lan et al., 1999; Ross et al., 1999; Pertwee, 2006) and represent the most cost and time efficient entry level approach to the type of question we sought to address. To provide greater and more reliable clarity would require use of suitable transgenic rats, and since these are not readily available this would necessitate considerable work. Mice are available (Ravinet Trillou et al., 2004; Buckley, 2008) but the mouse regional ischaemia model is not well-established as a method for reliable generation of ischaemia-induced VF (Stables and Curtis, 2009).
Conclusion
A possible endogenous cardioprotective effect of endocannabinoids was unmasked by the CB1 receptor antagonist, AM251, in the isolated rat heart. This satisfies one of the criteria set out by Parratt (1993) for a substance acting as an endogenous modulator of ischaemia-induced VF (drugs that block the receptors activated by the putative mediator must increase the susceptibility to VF). However, the effects were weak and restricted to the interlude (30–60 min) after the main period of susceptibility to VF (‘phase 1’, the first 30 min of ischaemia). Phase 1 VF was unaffected by CB1 and CB2 agonists and antagonists. If drugs have no effect on phase 1 VF they have little likely potential value for suppression of SCD. We therefore conclude that development of CB1 or CB2 agonists as antiarrhythmics does not appear to be justified. This knowledge is useful since it allows the community to focus elsewhere for leads to generate new therapies for SCD.
Acknowledgments
The experiments were funded by a generous grant from the Burton Fund.
Glossary
- 2-AG
2-arachidonoylglycerol
- CF
coronary flow
- HR
heart rate
- IZ
involved zone
- SCD
sudden cardiac death
- VF
ventricular fibrillation
Conflict of interest
None.
References
- Alexander S, Mathie A, Peters J. Guide to receptors and channels (GRAC), 5th edition. Br J Pharmacol. 2011;164:1–324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barana A, Amorós I, Caballero R, Gómez R, Osuna L, Lillo MP, et al. Endocannabinoids and cannabinoid analogues block cardiac hKv1.5 channels in a cannabinoid receptor-independent manner. Cardiovasc Res. 2009;85:56–67. doi: 10.1093/cvr/cvp284. [DOI] [PubMed] [Google Scholar]
- Bernier M, Curtis MJ, Hearse DJ. Ischemia-induced and reperfusion-induced arrhythmias: importance of heart rate. Am J Physiol. 1989;256:H21–H31. doi: 10.1152/ajpheart.1989.256.1.H21. [DOI] [PubMed] [Google Scholar]
- Bouchard JF, Lépicier P, Lamontagne D. Contribution of endocannabinoids in the endothelial protection afforded by ischemic preconditioning in the isolated rat heart. Life Sci. 2003;72:1859–1870. doi: 10.1016/s0024-3205(02)02474-8. [DOI] [PubMed] [Google Scholar]
- Buckley N. The peripheral cannabinoid receptor knockout mice: an update. Br J Pharmacol. 2008;153:309–318. doi: 10.1038/sj.bjp.0707527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camm AJ. Cardiac arrhythmias – trials and tribulations. Lancet. 2012;380:1448–1451. doi: 10.1016/S0140-6736(12)61773-5. [DOI] [PubMed] [Google Scholar]
- Campbell RWF. Treatment and prophylaxis of ventricular arrhythmias in acute myocardial infarction. Am J Cardiol. 1983;52:55C–59C. doi: 10.1016/0002-9149(83)90633-1. [DOI] [PubMed] [Google Scholar]
- Campbell RWF. Ventricular fibrillation: facts, fiction, and the future. In: Hearse DJ, Manning A, Janse M, editors. Life-Threatening Arrhythmias during Ischemia and Infarction. New York: Raven Press; 1998. pp. 1–9. [Google Scholar]
- Clements-Jewery H, Hearse DJ, Curtis MJ. Phase 2 ventricular arrhythmias in acute myocardial infarction: a neglected target for therapeutic antiarrhythmic drug development and for safety pharmacology evaluation. Br J Pharmacol. 2005;145:551–564. doi: 10.1038/sj.bjp.0706231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements-Jewery H, Kanaganayagam GS, Kabra R, Curtis MJ. Actions of flecainide on susceptibility to phase-2 ventricular arrhythmias during infarct evolution in rat isolated perfused hearts. Br J Pharmacol. 2006;147:468–475. doi: 10.1038/sj.bjp.0706633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements-Jewery H, Andrag E, Curtis MJ. Druggable targets for sudden cardiac death prevention: lessons from the past and strategies for the future. Curr Opin Pharmacol. 2009a;9:146–153. doi: 10.1016/j.coph.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Clements-Jewery H, Andrag E, Hearse DJ, Curtis MJ. Complex adrenergic and inflammatory mechanisms contribute to phase 2 ventricular arrhythmias in anaesthetized rats. Br J Pharmacol. 2009b;156:444–453. doi: 10.1111/j.1476-5381.2008.00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie S, Rainbow RD, Ewart M-A, Kitson S, Pliego EH, Kane KA, et al. IP3R-mediated Ca2+ release is modulated by anandamide in isolated cardiac nuclei. J Mol Cell Cardiol. 2008;45:804–811. doi: 10.1016/j.yjmcc.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Curtis MJ. Characterisation, utilisation and clinical relevance of isolated perfused heart models of ischaemia-induced ventricular fibrillation. Cardiovasc Res. 1998;39:194–215. doi: 10.1016/s0008-6363(98)00083-2. [DOI] [PubMed] [Google Scholar]
- Curtis MJ, Hearse DJ. Ischaemia-induced and reperfusion-induced arrhythmias differ in their sensitivity to potassium: implications for mechanisms of initiation and maintenance of ventricular fibrillation. J Mol Cell Cardiol. 1989a;21:21–40. doi: 10.1016/0022-2828(89)91490-9. [DOI] [PubMed] [Google Scholar]
- Curtis MJ, Hearse DJ. Reperfusion-induced arrhythmias are critically dependent upon occluded zone size: relevance to the mechanism of arrhythmogenesis. J Mol Cell Cardiol. 1989b;21:625–637. doi: 10.1016/0022-2828(89)90828-6. [DOI] [PubMed] [Google Scholar]
- Curtis MJ, Pabla R. Nitric oxide supplementation or synthesis block- which is the better approach to treatment of heart disease? Trends Pharmacol Sci. 1997;18:239–244. doi: 10.1016/s0165-6147(97)01080-8. [DOI] [PubMed] [Google Scholar]
- Curtis MJ, Pugsley MK, Walker MJ. Endogenous chemical mediators of ventricular arrhythmias in ischaemic heart disease. Cardiovasc Res. 1993;27:703–719. doi: 10.1093/cvr/27.5.703. [DOI] [PubMed] [Google Scholar]
- Curtis MJ, Hancox JC, Farkas A, Wainwright CL, Stables CL, Saint DA, et al. The Lambeth Conventions (II): guidelines for the study of animal and human ventricular and supraventricular arrhythmias. Pharmacol Ther. 2013 doi: 10.1016/j.pharmthera.2013.04.008. DOI: 10.1016/j.pharmthera.2013.04.008. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Farkas A, Curtis MJ. Does QT widening in the Langendorff-perfused rat heart represent the effect of repolarization delay or conduction slowing? J Cardiovasc Pharmacol. 2003;42:612–621. doi: 10.1097/00005344-200311000-00006. [DOI] [PubMed] [Google Scholar]
- Farkas A, Qureshi A, Curtis MJ. Inadequate ischaemia-selectivity limits the antiarrhythmic efficacy of mibefradil during regional ischaemia and reperfusion in the rat isolated perfused heart. Br J Pharmacol. 1999;128:41–50. doi: 10.1038/sj.bjp.0702778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford WR, Honan SA, White R, Hiley CR. Evidence of a novel site mediating anandamide-induced negative inotropic and coronary vasodilatator responses in rat isolated hearts. Br J Pharmacol. 2002;135:1191–1198. doi: 10.1038/sj.bjp.0704565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. N Engl J Med. 2001;345:1473–1482. doi: 10.1056/NEJMra000650. [DOI] [PubMed] [Google Scholar]
- John R, Tedrow U, Koplan B, Albert C, Epstein L, Sweeney M, et al. Ventricular arrhythmias and sudden cardiac death. Lancet. 2012;380:1520–1529. doi: 10.1016/S0140-6736(12)61413-5. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8:e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krylatov AV, Uzhachenko RV, Maslov LN, Bernatskaya NA, Makriyannis A, Mechoulam R, et al. Endogenous cannabinoids improve myocardial resistance to arrhythmogenic effects of coronary occlusion and reperfusion: a possible mechanism. Bull Exp Biol Med. 2002;133:122–124. doi: 10.1023/a:1015574100494. [DOI] [PubMed] [Google Scholar]
- Lan R, Liu Q, Fan P, Lin S, Fernando SR, McCallion D, et al. Structure-activity relationships of pyrazole derivatives as cannabinoid receptor antagonists. J Med Chem. 1999;42:769–776. doi: 10.1021/jm980363y. [DOI] [PubMed] [Google Scholar]
- Li Q, Ma HJ, Zhang H, Qi Z, Guan Y, Zhang Y. Electrophysiological effects of anandamide on rat myocardium. Br J Pharmacol. 2009;158:2022–2029. doi: 10.1111/j.1476-5381.2009.00518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath JC, Drummond GB, McLachlan EM, Kilkenny C, Wainwright CL. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainland D, Herrera L, Sutcliffe M, editors. Statistical Tables for Use with Binomial Samples– Contingency Tests, Confidence Limits and Sample Size Estimates. New York: New York Medical College of Medicine Publications; 1965. [Google Scholar]
- Pabla R, Curtis MJ. Effects of NO modulation on cardiac arrhythmias in the rat isolated heart. Circ Res. 1995;77:984–992. doi: 10.1161/01.res.77.5.984. [DOI] [PubMed] [Google Scholar]
- Pabla R, Bland-Ward P, Moore PK, Curtis MJ. An endogenous protectant effect of cardiac cGMP against reperfusion-induced ventricular fibrillation in the rat heart. Br J Pharmacol. 1995;116:2923–2930. doi: 10.1111/j.1476-5381.1995.tb15946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parratt J. Endogenous myocardial protective (antiarrhythmic) substances. Cardiovasc Res. 1993;27:693–702. doi: 10.1093/cvr/27.5.693. [DOI] [PubMed] [Google Scholar]
- Pertwee R. The pharmacology of cannabinoid receptors and their ligands: an overview. Int J Obes. 2006;30:S13–S18. doi: 10.1038/sj.ijo.0803272. [DOI] [PubMed] [Google Scholar]
- Ravinet Trillou C, Delgorge C, Menet C, Arnone M, Soubrie P. CB1 cannabinoid receptor knockout in mice leads to leanness, resistance to diet-induced obesity and enhanced leptin sensitivity. Int J Obes Relat Metab Disord. 2004;28:640–648. doi: 10.1038/sj.ijo.0802583. [DOI] [PubMed] [Google Scholar]
- Ravingerova T, Tribulova N, Slezak J, Curtis MJ. Brief, intermediate and prolonged ischemia in the isolated crystalloid perfused rat heart: relationship between susceptibility to arrhythmias and degree of ultrastructural injury. J Mol Cell Cardiol. 1995;27:1937–1951. doi: 10.1016/0022-2828(95)90016-0. [DOI] [PubMed] [Google Scholar]
- Rees SA, Curtis MJ. Pharmacological analysis in rat of the role of the ATP-sensitive potassium channel as a potential target for antifibrillatory intervention in acute myocardial ischaemia. J Cardiovasc Pharmacol. 1995;26:280–288. doi: 10.1097/00005344-199508000-00014. [DOI] [PubMed] [Google Scholar]
- Rees SA, Curtis MJ. Which cardiac potassium channel subtype is the preferable target for suppression of ventricular arrhythmias? Pharmacol Ther. 1996;69:199–217. doi: 10.1016/0163-7258(95)02063-2. [DOI] [PubMed] [Google Scholar]
- Ridley PD, Yacoub MH, Curtis MJ. A modified model of global ischaemia: application to the study of syncytial mechanisms of arrhythmogenesis. Cardiovasc Res. 1992;26:309–315. doi: 10.1093/cvr/26.4.309. [DOI] [PubMed] [Google Scholar]
- Ross RA, Brockie HC, Stevenson LA, Murphy VL, Templeton F, Makriyannis A, et al. Agonist-inverse agonist characterization at CB1 and CB2 cannabinoid receptors of L759633, L759656, and AM630. Br J Pharmacol. 1999;126:665–672. doi: 10.1038/sj.bjp.0702351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stables CL, Curtis MJ. Development and characterization of a mouse in vitro model of ischaemia-induced ventricular fibrillation. Cardiovasc Res. 2009;83:397–404. doi: 10.1093/cvr/cvp068. [DOI] [PubMed] [Google Scholar]
- Tsuchihashi K, Curtis MJ. Influence of tedisamil on the initiation and maintenance of ventricular fibrillation: chemical defibrillation by Ito blockade? J Cardiovasc Pharmacol. 1991;18:445–456. doi: 10.1097/00005344-199109000-00018. [DOI] [PubMed] [Google Scholar]
- Ugdyzhekova DS, Bernatskaya NA, Stefano JB, Graier VF, Tam SW, Mechoulam R. Endogenous cannabinoid anandamide increases heart resistance to arrhythmogenic effects of epinephrine: role of CB 1 and CB 2 receptors. Bull Exp Biol Med. 2001;131:251–253. doi: 10.1023/a:1017651432193. [DOI] [PubMed] [Google Scholar]
- Underdown NJ, Hiley CR, Ford WR. Anandamide reduces infarct size in rat isolated hearts subjected to ischaemia–reperfusion by a novel cannabinoid mechanism 1. Br J Pharmacol. 2005;146:809–816. doi: 10.1038/sj.bjp.0706391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Werf FJ. Fine-tuning the selection of a reperfusion strategy. Circulation. 2006;114:2002–2003. doi: 10.1161/CIRCULATIONAHA.106.658252. [DOI] [PubMed] [Google Scholar]
- Wagner JA, Abesser M, Harvey-white J, Ertl G. 2-Arachidonylglycerol acting on CB1 cannabinoid receptors mediates delayed cardioprotection induced by nitric oxide in rat isolated hearts. J Cardiovasc Pharmacol. 2006;47:650–655. doi: 10.1097/01.fjc.0000211752.08949.eb. [DOI] [PubMed] [Google Scholar]
- Walsh SK, Hepburn CY, Kane KA, Wainwright CL. Acute administration of cannabidiol in vivo suppresses ischaemia-induced cardiac arrhythmias and reduces infarct size when given at reperfusion. Br J Pharmacol. 2010;160:1234–1242. doi: 10.1111/j.1476-5381.2010.00755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wannamethee G, Shaper AG, Macfarlane PW, Walker M. Risk factors for Sudden Cardiac Death in middle-aged British men. Circulation. 1995;91:1749–1756. doi: 10.1161/01.cir.91.6.1749. [DOI] [PubMed] [Google Scholar]
- Yamada M, Hearse DJ, Curtis MJ. Reperfusion and readmission of oxygen: pathophysiological relevance to arrhythmogenesis. Circ Res. 1990;67:1211–1223. doi: 10.1161/01.res.67.5.1211. [DOI] [PubMed] [Google Scholar]
- Zheng Z-J, Croft JB, Giles WH, Mensah GA. Sudden Cardiac Death in the United States, 1989 to 1998. Circulation. 2001;104:2158–2163. doi: 10.1161/hc4301.098254. [DOI] [PubMed] [Google Scholar]


