Abstract
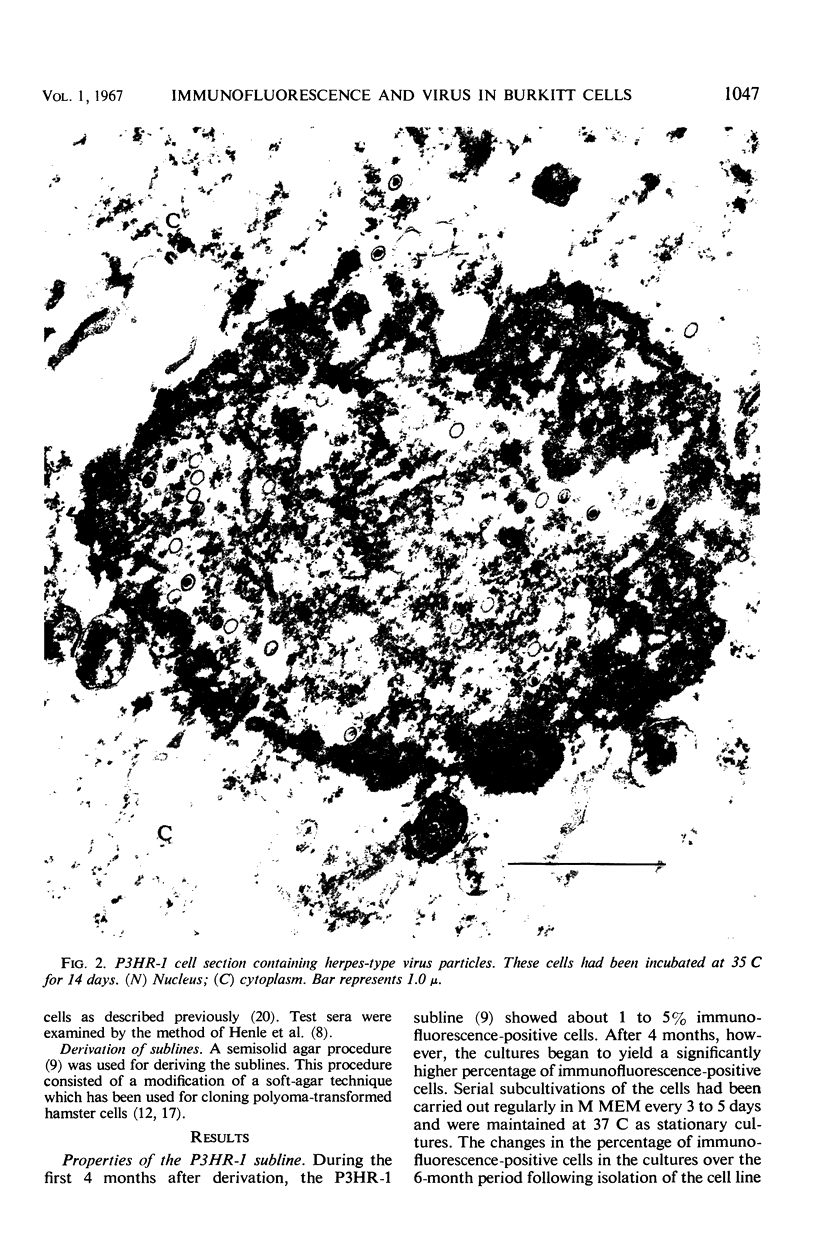
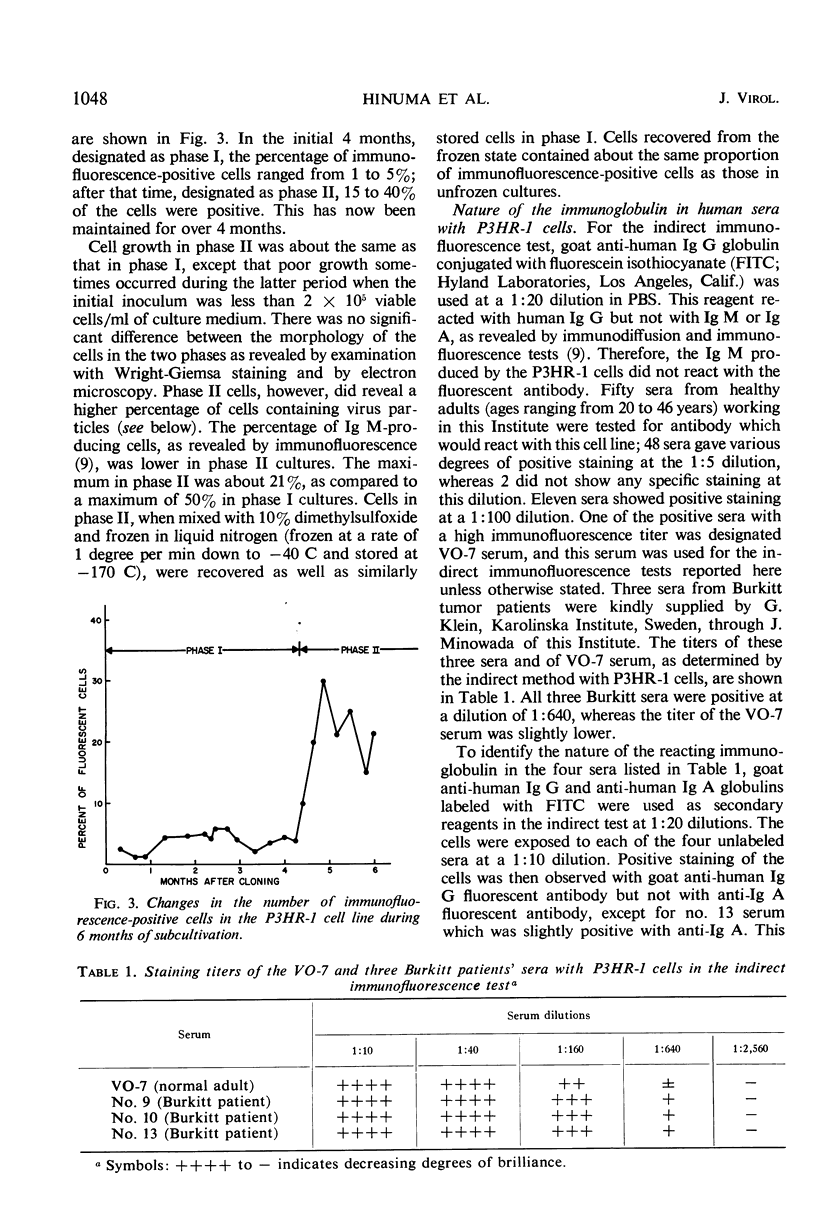
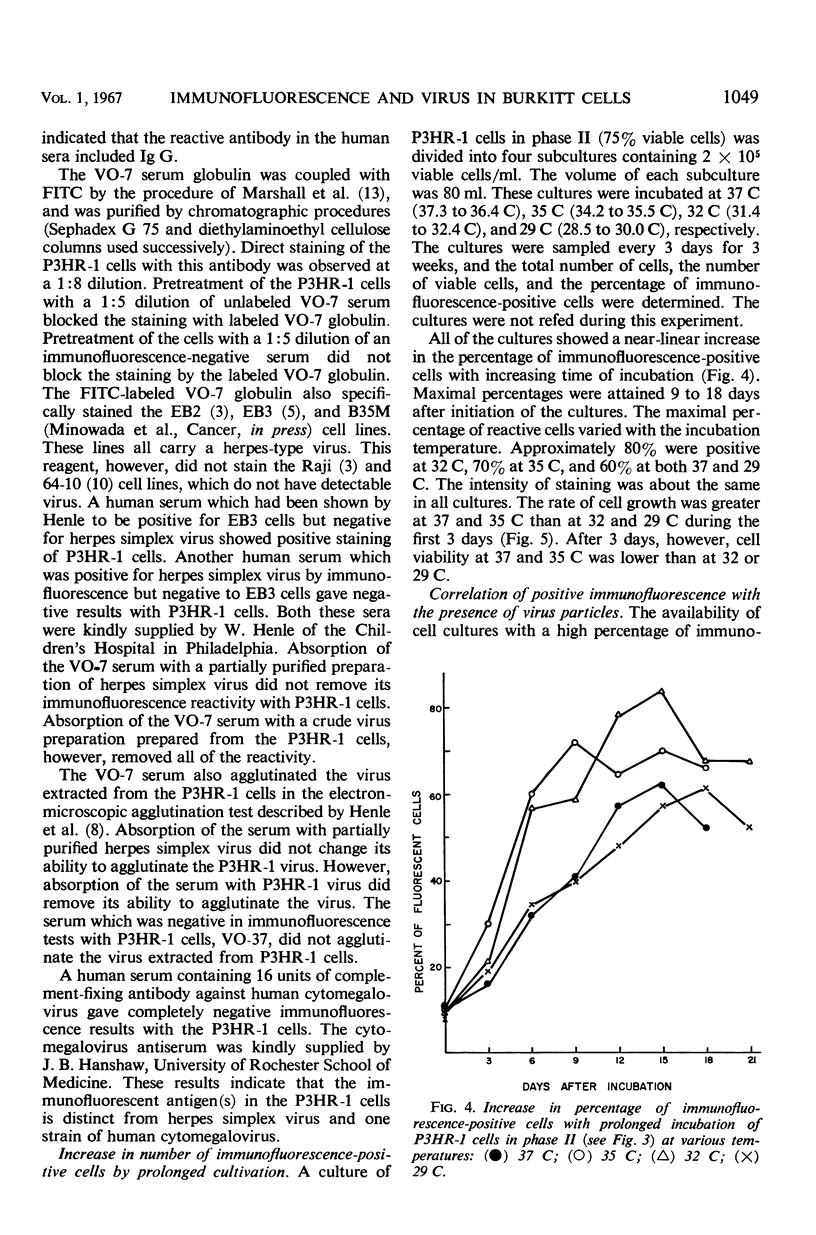
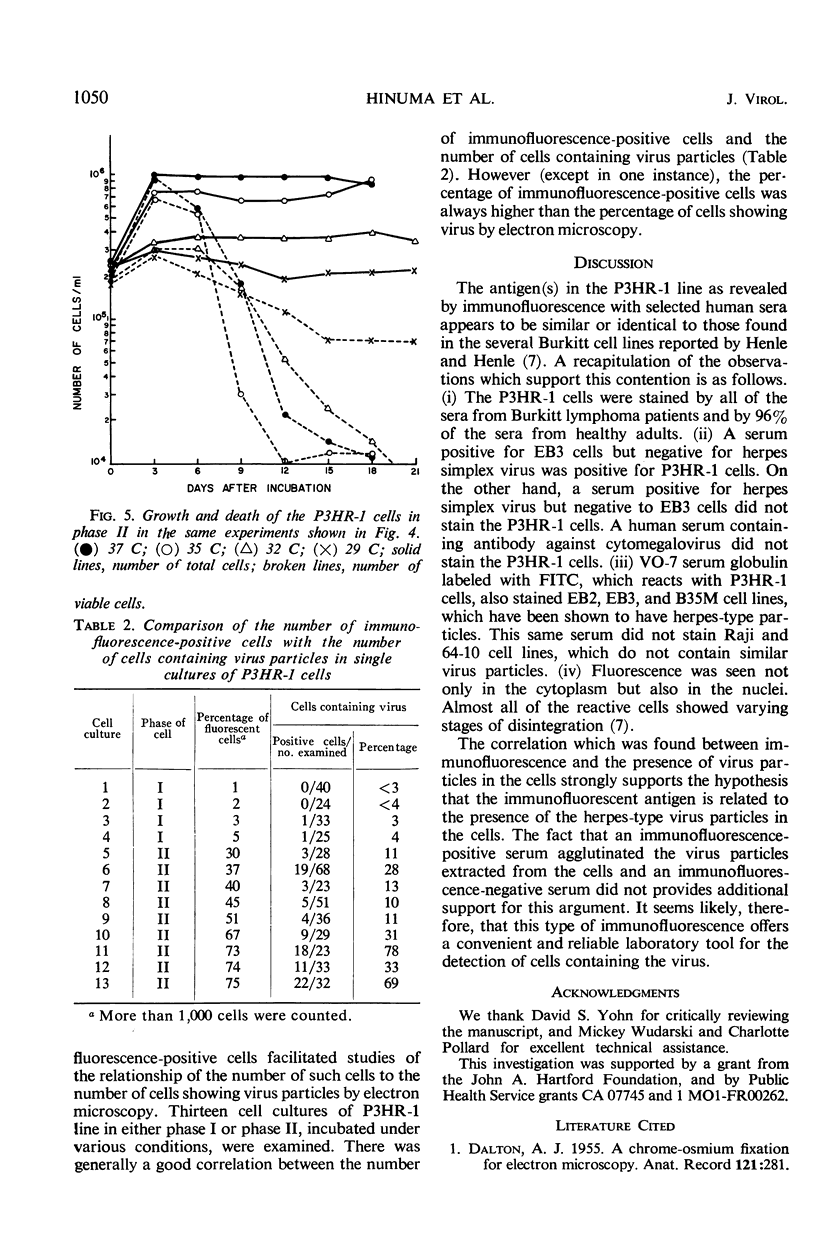
A subline of the P3 (Jijoye) Burkitt lymphoma cell line, designated P3HR-1, initially contained 1 to 5% of cells which were positive by indirect immunofluorescence with selected human sera. After 4 months of propagation, this cell line regularly showed 15 to 40% reactive cells. Antigen(s) in the cell line which was reactive by immunofluorescence was similar or identical to that found in several other Burkitt tumor cell lines in previous studies. When the cells were incubated at 35 or 32 C for 9 to 15 days without refeeding, more than 50% of the cells became immunofluorescence-positive. Thirteen different cultures of P3HR-1 cells, which contained up to 75% immunofluorescence-positive cells, were thin-sectioned and examined by electron microscopy. The percentage of cells containing herpes-type virus particles in the cultures varied from <3 to 78%. There was generally a good correlation between the number of immunofluorescent cells and the number of cells containing virus particles. The number of virus particles per cell section ranged from 1 to more than 100. These results strongly support the hypothesis that the immunofluorescent antigen is related to the presence of the herpes-type virus particle in the cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- EPSTEIN M. A., ACHONG B. G., BARR Y. M. VIRUS PARTICLES IN CULTURED LYMPHOBLASTS FROM BURKITT'S LYMPHOMA. Lancet. 1964 Mar 28;1(7335):702–703. doi: 10.1016/s0140-6736(64)91524-7. [DOI] [PubMed] [Google Scholar]
- EPSTEIN M. A., BARR Y. M., ACHONG B. G. A SECOND VIRUS-CARRYING TISSUE CULTURE STRAIN (EB2) OF LYMPHOBLASTS FROM BURKITT'S LYMPHOMA. Pathol Biol. 1964 Dec;12:1233–1234. [PubMed] [Google Scholar]
- EPSTEIN M. A., HENLE G., ACHONG B. G., BARR Y. M. MORPHOLOGICAL AND BIOLOGICAL STUDIES ON A VIRUS IN CULTURED LYMPHOBLASTS FROM BURKITT'S LYMPHOMA. J Exp Med. 1965 May 1;121:761–770. doi: 10.1084/jem.121.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein M. A., Achong B. G., Barr Y. M., Zajac B., Henle G., Henle W. Morphological and virological investigations on cultured Burkitt tumor lymphoblasts (strain Raji). J Natl Cancer Inst. 1966 Oct;37(4):547–559. [PubMed] [Google Scholar]
- Epstein M. A., Barr Y. M., Achong B. G. Studies with Burkitt's lymphoma. Wistar Inst Symp Monogr. 1965 Sep;4:69–82. [PubMed] [Google Scholar]
- Henle G., Henle W. Immunofluorescence in cells derived from Burkitt's lymphoma. J Bacteriol. 1966 Mar;91(3):1248–1256. doi: 10.1128/jb.91.3.1248-1256.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle W., Hummeler K., Henle G. Antibody coating and agglutination of virus particles separated from the EB3 line of Burkitt lymphoma cells. J Bacteriol. 1966 Jul;92(1):269–271. doi: 10.1128/jb.92.1.269-271.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinuma Y., Grace J. T., Jr Cloning of immunoglobulin-producing human leukemic and lymphoma cells in long-term cultures. Proc Soc Exp Biol Med. 1967 Jan;124(1):107–111. doi: 10.3181/00379727-124-31677. [DOI] [PubMed] [Google Scholar]
- Levy J. A., Henle G. Indirect immunofluorescence tests with sera from African children and cultured Burkitt lymphoma cells. J Bacteriol. 1966 Jul;92(1):275–276. doi: 10.1128/jb.92.1.275-276.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACPHERSON I., MONTAGNIER L. AGAR SUSPENSION CULTURE FOR THE SELECTIVE ASSAY OF CELLS TRANSFORMED BY POLYOMA VIRUS. Virology. 1964 Jun;23:291–294. doi: 10.1016/0042-6822(64)90301-0. [DOI] [PubMed] [Google Scholar]
- MARSHALL J. D., EVELAND W. C., SMITH C. W. Superiority of fluorescein isothiocyanate (Riggs) for fluorescent-antibody technic with a modification of its application. Proc Soc Exp Biol Med. 1958 Aug-Sep;98(4):898–900. doi: 10.3181/00379727-98-24222. [DOI] [PubMed] [Google Scholar]
- MOLLENHAUER H. H. PLASTIC EMBEDDING MIXTURES FOR USE IN ELECTRON MICROSCOPY. Stain Technol. 1964 Mar;39:111–114. [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabson A. S., O'Conor G. T., Baron S., Whang J. J., Legallais F. Y. Morphologic, cytogenetic and virologic studies in vitro of a malignant lymphoma from an African child. Int J Cancer. 1966 Jan;1(1):89–106. doi: 10.1002/ijc.2910010112. [DOI] [PubMed] [Google Scholar]
- SANDERS F. K., BURFORD B. O. ASCITES TUMOURS FROM BHK.21 CELLS TRANSFORMED IN VITRO BY POLYOMA VIRUS. Nature. 1964 Feb 22;201:786–789. doi: 10.1038/201786a0. [DOI] [PubMed] [Google Scholar]
- STEWART S. E., LOVELACE E., WHANG J. J., NGU V. A. BURKITT TUMOR: TISSUE CULTURE, CYTOGENETIC AND VIRUS STUDIES. J Natl Cancer Inst. 1965 Feb;34:319–327. doi: 10.1093/jnci/34.2.319. [DOI] [PubMed] [Google Scholar]
- Toplin I., Schidlovsky G. Partial purification and electron microscopy of virus in the EB-3 cell line derived from a Burkitt lymphoma. Science. 1966 May 20;152(3725):1084–1085. doi: 10.1126/science.152.3725.1084. [DOI] [PubMed] [Google Scholar]
- Yamaguchi J., Hinuma Y., Grace J. T., Jr Structure of virus particles extracted from a Burkitt lymphoma cell line. J Virol. 1967 Jun;1(3):640–642. doi: 10.1128/jvi.1.3.640-642.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]