Abstract
Impairment of the ubiquitin-proteasome system (UPS) has been implicated in the pathogenesis of human diseases, including neurodegenerative disorders. Thus, stimulating proteasome activity is a promising strategy to ameliorate these age-related diseases. Here we show that the protein kinase casein kinase 2 (CK2) regulates the transcriptional activity of Nrf1 to control the expression of the proteasome genes and thus the clearance of ubiquitinated proteins. We identify CK2 as an Nrf1-binding protein and find that the knockdown of CK2 enhances the Nrf1-dependent expression of the proteasome subunit genes. Real-time monitoring of proteasome activity reveals that CK2 knockdown alleviates the accumulation of ubiquitinated proteins upon proteasome inhibition. Furthermore, we identify Ser 497 of Nrf1 as the CK2 phosphorylation site and demonstrate that its alanine substitution (S497A) augments the transcriptional activity of Nrf1 and mitigates proteasome dysfunction and the formation of p62-positive juxtanuclear inclusion bodies upon proteasome inhibition. These results indicate that the CK2-mediated phosphorylation of Nrf1 suppresses the proteasome gene expression and activity and thus suggest that the CK2-Nrf1 axis is a potential therapeutic target for diseases associated with UPS impairment.
INTRODUCTION
Accumulation of misfolded and ubiquitinated proteins is a common pathological feature of various human diseases, such as amyotrophic lateral sclerosis (ALS), inclusion body myopathies, alcoholic and nonalcoholic steatohepatitis, and neurodegenerative disorders, including Alzheimer's, Parkinson's, and Huntington's disease (1–3). Multiple lines of evidence suggest that both the ubiquitin-proteasome system (UPS) and autophagy are responsible for the clearance of ubiquitinated proteins that would accumulate in these age-related diseases. It has been demonstrated that the 26S proteasome can degrade soluble ubiquitinated proteins but not the insoluble aggregates, which are targeted by the autophagy-lysosome pathway (4–7). Impairment of proteasome activity is known to cause proteins that are normally turned over by the UPS to aggregate and form inclusion bodies. Thus, it is expected that the upregulation of proteasome activity could prevent inclusion body formation and mitigate the progression of neurodegenerative and related diseases that are caused by the accumulation of abnormal proteins.
Nrf1 (nuclear factor E2-related factor 1 or Nfe2l1) is a member of the Cap‘n'Collar (CNC) family of basic leucine zipper (bZip) transcription factors, which also includes p45 NF-E2, Nrf2, and Nrf3 (8, 9). Nrf1 regulates its target gene expression through either the antioxidant response element (ARE) or the Maf recognition element (MARE) by heterodimerizing with small Maf proteins (8, 9). Several gene targeting studies have implicated Nrf1 in the regulation of cellular homeostasis in embryos, hepatocytes, and osteoclasts (10–14). Recent studies have revealed that Nrf1 also plays an essential role in maintaining neuronal cells and that the loss of Nrf1 induces neurodegeneration and abnormal accumulation of ubiquitinated protein aggregates in neurons (15, 16). The impairment of protein homeostasis that is induced by Nrf1 deficiency may be due to the decreased expression of proteasome subunits in these neurons (16). Indeed, Nrf1 controls the expression of proteasome subunit genes in mammalian cells under proteasome dysfunction (17, 18). Therefore, it is critically important to reveal the role of Nrf1 in the regulation of proteasome gene expression and to elucidate the molecular mechanisms underlying the regulation of Nrf1 activity.
In this study, we reveal that the vast majority of proteasome subunit genes and some proteasome-associated genes are under the transcriptional control of Nrf1. We identify the protein kinase casein kinase 2 (CK2) as an Nrf1-interacting protein and demonstrate that CK2 controls proteasome gene expression and activity by suppressing the transcriptional activity of Nrf1. A mutation of the CK2 phosphorylation site of Nrf1 enhances the proteasome activity and reduces the formation of juxtanuclear inclusion bodies. Thus, our work proposes that the CK2-Nrf1 axis could be a new regulatory target for the efficient clearance of ubiquitinated proteins.
MATERIALS AND METHODS
Antibodies.
The antibodies utilized in this study were normal rabbit IgG (Santa Cruz), anti-Flag (M2; Sigma), anti-α-tubulin (DM1A; Sigma), antihemagglutinin (anti-HA) (Y-11; Santa Cruz), anti-green fluorescent protein (anti-GFP) (B-2; Santa Cruz), anti-Nrf1 (H-285; Santa Cruz), anti-MafK (C-16; Santa Cruz), anti-CK2α (1AD9; Santa Cruz), anti-CK2α′ (ab10474; Abcam), anti-CK2β (6D5; Santa Cruz), anti-p62/SQSTM1 (PM045; MBL), antiubiquitin (P4D1; Santa Cruz), and anti-LC3 (PD014; MBL). The rabbit polyclonal antibodies directed against mouse Nrf1 that were used in chromatin immunoprecipitation (ChIP) experiments were raised by immunizing rabbits with a purified recombinant six-histidine (6×His)-tagged Nrf1 protein (residues 292 to 741) that was expressed in Escherichia coli. The resultant antibodies were subjected to affinity purification.
Plasmids and recombinant proteins.
The 3×Flag mouse Nrf1 expression plasmid was described previously (19). Human CK2α (hCK2α) and hCK2β cDNAs were subcloned into pcDNA3 (HA). The ubiquitin-fused luciferase reporter (Ub-FL) reporter plasmid was kindly provided by David Piwnica-Worms (20). The 3xPSMA4-ARE-Luc (Luc stands for luciferase) plasmid was kindly provided by Raymond J. Deshaies (17). The pRBGP2-Luciferase plasmid was described previously (21). The MafK expression plasmid was described previously (22). PCR-amplified Nrf1 fragments were subcloned into pET-15b. Recombinant 6× histidine-tagged Nrf1 fragments were expressed in E. coli and purified with nickel-nitrilotriacetic acid (Ni-NTA)−agarose (Qiagen). Recombinant CK2α was described previously (23).
Cell culture and transfection.
HeLa cells, COS7 cells, and MCF10A cells were cultured in Dulbecco's modified Eagle's medium (DMEM) (Wako) that was supplemented with 10% fetal calf serum (FCS) (Invitrogen), 4,500 mg/liter glucose, 40 μg/ml streptomycin, and 40 units/ml penicillin. Mouse embryonic fibroblasts (MEFs) were cultured in Iscove's modified Dulbecco's medium (IMDM) (Wako) that was supplemented with 10% FCS, 2 mM glutamine (Invitrogen), 40 μg/ml streptomycin, and 40 units/ml penicillin. The transfection of plasmid DNA and small interfering RNA (siRNA) was achieved using Lipofectamine Plus and Lipofectamine 2000 (Invitrogen), respectively.
siRNA knockdown experiment.
The cells were cultured for 24 h in medium without antibiotics. The cells were transfected twice with 40 nM siRNA (at 24 and 48 h after plating) using Lipofectamine 2000. The sequences of the siRNAs employed in the present study are listed in Table S3 in the supplemental material. Twenty-four hours after the last transfection, the cells were utilized for each experiment. For immunoblot analysis, the cells were lysed with an SDS sample buffer (50 mM Tris-HCl [pH 6.8], 10% glycerol, and 1% SDS), and the resultant whole-cell extracts were subjected to immunoblotting with the antibodies indicated in Fig. 1B, 2A and C, 5C, and 6C.
Fig 1.
Nrf1 regulates the expression of proteasome subunit genes that are induced by proteasome inhibition in HeLa cells. (A) siRNA-mediated knockdown of Nrf1. HeLa cells transfected with control (Ctrl) siRNA or Nrf1 siRNA were treated with DMSO or 1 μM MG132 for 16 h. mRNA expression levels of Nrf1 were determined by real-time quantitative PCR analysis. The values were normalized to 18S rRNA values and presented as the means plus standard deviations (SD) (error bars) (n = 3). (B) MG132 induces the accumulation of Nrf1 proteins. HeLa cell extracts were prepared at the indicated time points after 1 μM MG132 treatment and subjected to immunoblot analysis with anti-Nrf1 (αNrf1) (H-285) antibody. αα-tubulin, anti-α-tubulin antibody. (C) Nrf1-dependent induction of proteasome genes. The mRNA expression levels of the indicated genes were determined by real-time quantitative PCR. The expression level in the cells transfected with the control siRNA and treated with DMSO was set at 1. The values were normalized to 18S rRNA values and presented as the means plus SD (n = 3). (D) The heat map shows the mRNA expression levels of the indicated genes that correspond to the graphs in Fig. 1C and data not shown. The values were normalized to 18S rRNA values and presented as the means of at least three replicates. The N/C ratio is the ratio of the expression level in Nrf1 siRNA-treated cells to the expression level in control siRNA-treated cells with MG132 treatment. The color bar indicates the range of the expression ratios in log space. (E) Time course of expression of PSMC4, PSMA4, GSTA4, and NQO1 upon MG132 treatment. The values were normalized to 18S rRNA values and presented as the means ± SD (n = 5).
Fig 2.
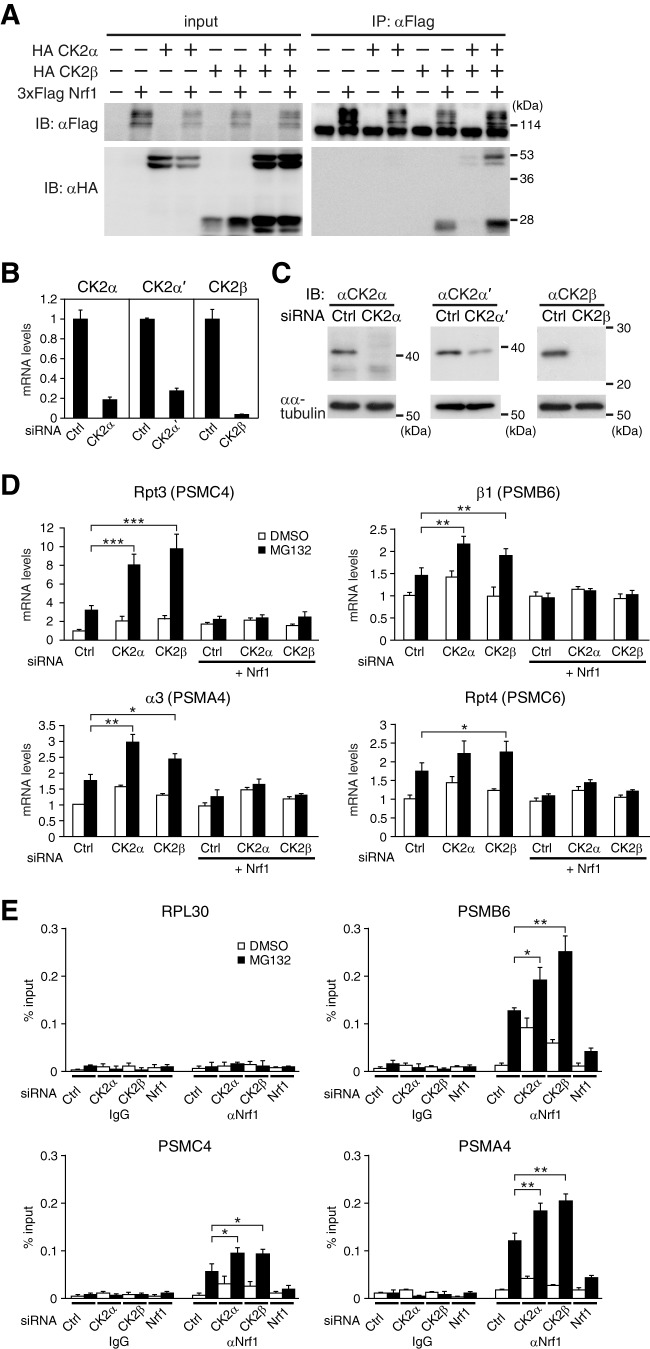
CK2 regulates the Nrf1-dependent expression of proteasome genes. (A) Physical interaction of Nrf1 with CK2. Whole-cell extracts of COS7 cells expressing 3×Flag-tagged Nrf1 (3×Flag Nrf1), HA-tagged CK2α (HA CK2α), and HA CK2β were subjected to immunoprecipitation (IP) with anti-Flag antibody (αFlag), followed by immunoblot (IB) analysis with the indicated antibodies. (B) siRNA-mediated knockdown of CK2 subunits. HeLa cells were transfected with the indicated siRNAs. The mRNA expression levels of the indicated genes were determined by real-time quantitative PCR. The values were normalized to 18S rRNA values and presented as the means plus SD (n = 3). (C) The siRNA-mediated knockdown of CK2α, CK2α′, and CK2β was determined by immunoblot analysis. (D) Knockdown of the CK2 subunits enhances the Nrf1-dependent induction of proteasome genes. HeLa cells transfected with control (Ctrl), both CK2α and α′ (CK2α), or CK2β siRNA were treated with Nrf1 siRNA (+ Nrf1) or left untreated and treated with DMSO or 1 μM MG132 for 16 h. The mRNA expression levels were determined by real-time quantitative PCR analysis. The values were normalized to 18S rRNA values and presented as the means plus SD (n = 3). Values that are significantly different are indicated by asterisks and bars as follows: ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001. (E) Knockdown of the CK2 subunits enhanced the recruitment of Nrf1 to the AREs of proteasome gene promoters. HeLa cells were transfected with the indicated siRNAs and treated with DMSO or 1 μM MG132 for 16 h. The cells were subjected to chromatin immunoprecipitation (ChIP) analysis using normal rabbit IgG (IgG) or anti-Nrf1 antibody (αNrf1). The recruitment of Nrf1 to the AREs of PSMB6, PSMC4, and PSMA4 was determined by real-time quantitative PCR. The promoter region of RPL30 served as a negative control. The values were presented as the means plus SD (n = 3). Values that are significantly different are indicated by asterisks and bars as follows: ∗, P < 0.05; ∗∗, P < 0.01.
Fig 5.
The Nrf1-S497A mutant has enhanced transcriptional activity and prevents the accumulation of ubiquitinated proteins. (A) The S497A mutant has enhanced transcriptional activity. COS7 cells transfected with either wild-type (WT) plasmid or S497A mutant plasmid in combination with a luciferase reporter containing three tandem copies of the AREs of PSMA4 or a pRBGP2 reporter. The levels of luciferase activity were normalized to the Renilla luciferase activity of an internal control pRL-TK and presented as the means plus SD (n = 3). Values that are significantly different (P < 0.05) are indicated by a bar and asterisk. (B) The S497A mutant upregulates expression of endogenous proteasome genes. HeLa cells were transfected with either wild-type (WT) or S497A mutant plasmid. The mRNA expression levels were determined by real-time quantitative PCR analysis. The values were normalized to 18S rRNA values and presented as the means plus SD (n = 5). Values that are significantly different (P < 0.05) are indicated by a bar and asterisk. Values that are not significantly different are indicated by a bar labeled n.s. (C) Protein stability is comparable in the wild type and the S497A mutant. COS7 cells transfected with the expression plasmid for the wild-type 3×Flag Nrf1 protein or the S497A mutant of 3×Flag Nrf1 in combination with the GFP expression plasmid were treated with 20 μg/ml cycloheximide (CHX). The cells were lysed at the indicated time points and subjected to immunoblot analysis with anti-Flag or anti-GFP antibodies. The data were normalized to cotransfected GFP values and are presented as the means ± standard errors (SE) (n = 3). (D and E) The S497A mutant has a greater ability to repress the accumulation of ubiquitinated proteins than wild-type Nrf1. (D) HeLa cells transfected with the Ub-FL reporter plasmid in combination with the indicated amount of wild-type or S497A mutant Nrf1 plasmid were treated with 10 nM epoxomicin and d-luciferin. Real-time monitoring of the reporter activity was performed using a photomultiplier, and representative data are shown. (E) HeLa cells transfected with the Ub-FL reporter plasmid and the control Renilla luciferase reporter in combination with the wild-type or S497A mutant plasmid were treated with DMSO or 10 nM epoxomicin with d-luciferin for 16 h. The cells were lysed and subjected to a luciferase assay. The values were normalized to the values for Renilla luciferase activity and presented as the means plus SD (n = 3). (F) CK2 suppresses the transcriptional activity of Nrf1-WT but not that of the Nrf1-S497A mutant. COS7 cells were transfected with the indicated plasmids in combination with the PSMA4-ARE reporter or a pRBGP2 reporter. The levels of luciferase activity were normalized to the values for Renilla luciferase activity of an internal control pRL-TK and presented as the means plus SD (n = 3). Statistical significance is indicated as follows: ∗, P < 0.05; n.s., not significant.
Fig 6.
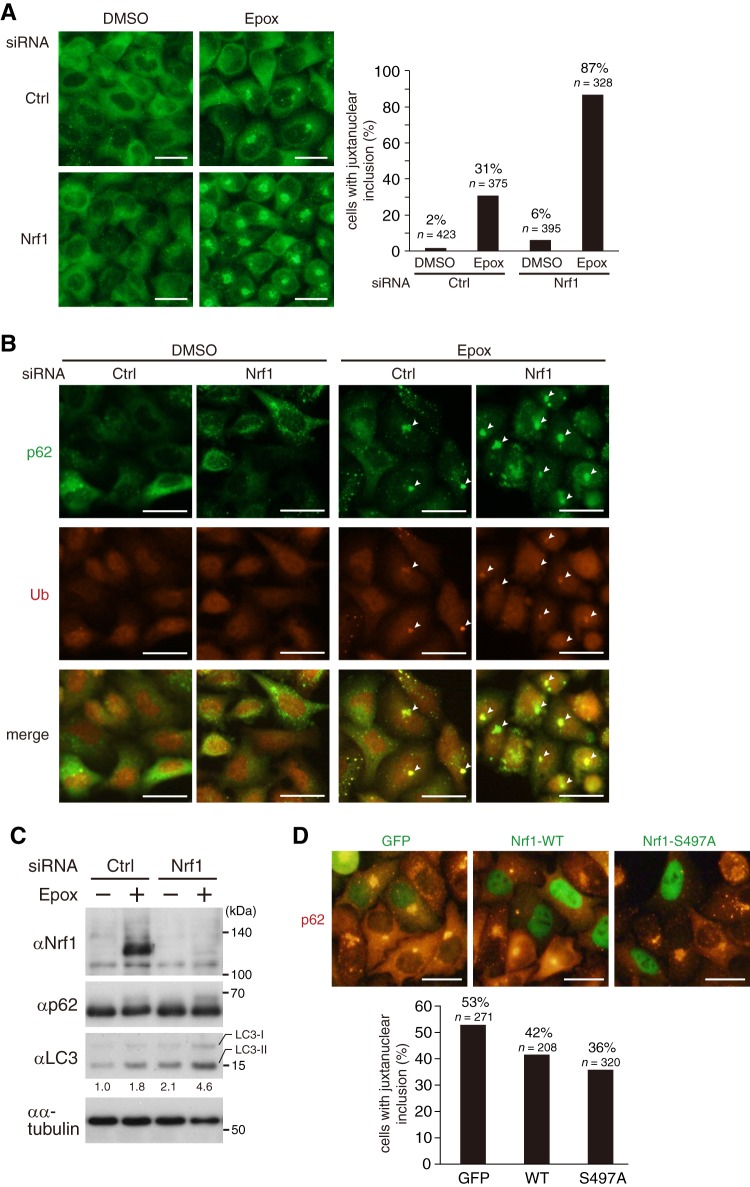
Nrf1 has the ability to ameliorate the formation of p62-positive inclusion bodies in HeLa cells. (A) Knockdown of Nrf1 causes enhanced formation of p62-positive inclusion bodies. HeLa cells transfected with control (Ctrl) or Nrf1 siRNA were treated with DMSO or 10 nM epoxomicin for 24 h. The cells were immunostained with anti-p62 antibody, and the percentage of cells containing juxtanuclear inclusion bodies were calculated. Bars, 50 μm. (B) p62-positive inclusion bodies formed by proteasome inhibition are ubiquitin positive. HeLa cells transfected with control (Ctrl) or Nrf1 siRNA were treated with DMSO or 10 nM epoxomicin for 24 h. The cells were immunostained with anti-p62 (green) and antiubiquitin (red) antibodies. Juxtanuclear inclusion bodies stained with both antibodies are indicated by small white arrowheads. Bars, 50 μm. (C) The cells treated as in panel A were lysed and subjected to immunoblot analysis with the indicated antibodies. The relative band intensities of LC3-II were quantified and normalized to α-tubulin values. (D) The S497A mutant has a greater ability to decrease the formation of p62-positive juxtanuclear inclusion bodies than wild-type Nrf1. HeLa cells transfected with the expression plasmid for GFP or wild-type or the S497A mutant of 3×Flag Nrf1 were treated with DMSO or 10 nM epoxomicin for 24 h. The cells were immunostained with anti-Flag and anti-p62 antibodies, and the percentage of cells containing juxtanuclear inclusion bodies out of total GFP- or 3×Flag Nrf1-expressing cells were calculated. Bars, 50 μm.
RNA extraction and real-time quantitative PCR.
Total RNA was extracted from cells with the RNeasy minikit (Qiagen) and subjected to cDNA synthesis with random hexamer primers and Moloney murine leukemia virus (M-MLV) reverse transcriptase (Invitrogen) according to the manufacturer's instructions. Real-time quantitative PCR was performed with FastStart Universal SYBR (Roche) and ABI Prism 7900 (Life Technologies). The PCR primers employed in the present study are listed in Table S4 in the supplemental material.
Immunoprecipitation and immunoblot analysis.
COS7 cells were treated with the proteasome inhibitor MG132 (Peptide Institute) at a concentration of 10 μM for 4 h and subjected to preparation as whole-cell extracts with lysis buffer (50 mM Tris-HCl [pH 8.0], 10% glycerol, 100 mM NaF, 50 mM NaCl, 2 mM EDTA, 2 mM sodium orthovanadate, 10 mM sodium pyrophosphate, 10 mM β-glycerophosphate, 0.1% NP-40, 1 mM phenylmethylsulfonyl fluoride (PMSF), and 1× protease inhibitor cocktail [Roche]). The whole-cell extracts were subjected to immunoprecipitation with anti-Flag M2 affinity gels (Sigma) at 4°C for 2 h. After the anti-Flag M2 affinity gels were washed with wash buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, and 0.1% NP-40) three times, the immunocomplexes were eluted by boiling in SDS sample buffer and subjected to immunoblot analysis using the antibodies indicated in the figures. The blots were treated with a horseradish peroxidase-conjugated secondary antibody (Invitrogen) and were developed with an enhanced chemiluminescence (ECL) kit (GE Healthcare).
Chromatin immunoprecipitation.
HeLa cells grown in a 100-mm dish were cross-linked in 1% formaldehyde for 10 min, followed by quenching with 1/10 volume of 1.25 M glycine solution and two washes with phosphate-buffered saline (PBS). The cells were lysed in cell lysis buffer (5 mM Tris-HCl [pH 8.0], 85 mM KCl, 0.5% NP-40, 1 mM PMSF, and 1× protease inhibitor cocktail). Nuclear extracts were prepared by treating the nuclear pellets with ChIP SDS lysis buffer (50 mM Tris-HCl [pH 8.0], 10 mM EDTA, 1% SDS, 1 mM PMSF, and 1× protease inhibitor cocktail), followed by sonication using a Bioruptor (Tosho Electric Co., Ltd.). Proteins were immunoprecipitated in ChIP dilution buffer (16.7 mM Tris-HCl [pH 8.0], 167 mM NaCl, 1.2 mM EDTA, 0.01% SDS, 1.1% Triton X-100, 1 mM PMSF, and 1× protease inhibitor cocktail) using the antibodies indicated in the figures and Dynabeads protein G (Invitrogen). The beads were washed with low-salt wash buffer (20 mM Tris-HCl [pH 8.0], 150 mM NaCl, 2 mM EDTA, 0.1% SDS, and 1% Triton X-100), high-salt wash buffer (20 mM Tris-HCl [pH 8.0], 500 mM NaCl, 2 mM EDTA, 0.1% SDS, and 1% Triton X-100), lithium wash buffer (10 mM Tris-HCl [pH 8.0], 250 mM LiCl, 1% deoxycholate, 1 mM EDTA, and 1% NP-40), and Tris-EDTA (TE) buffer. Cross-linking was reversed overnight at 65°C in ChIP elution buffer (1% SDS and 50 mM NaHCO3). ChIPed DNA was then treated with RNase A and proteinase K, purified with a QIAquick PCR purification kit (Qiagen), and analyzed by real-time quantitative PCR.
Bioluminescence recordings.
HeLa cells were transfected with a Ub-FL reporter in combination with the indicated siRNAs or 3× Flag Nrf1 (wild-type or S497A mutant) vectors using Lipofectamine 2000. Forty-eight hours after transfection, the cells were treated with 0.1 mM d-luciferin (Toyobo), and bioluminescence was measured and integrated for 1 min at 10-min intervals with a luminometer (AB-2550 Kronos Dio; Atto). Epoxomicin was added to the culture medium 1 to 2 h after the start of the measurement.
Luciferase reporter assay.
Cells expressing the reporters indicated in the legends for Fig. 3A and B, 5A and D to F, and 6 were lysed, and the luciferase activities were measured with the PicaGene luciferase assay system (Toyo Ink) and a Berthold Lumat LB9507 luminometer.
Fig 3.
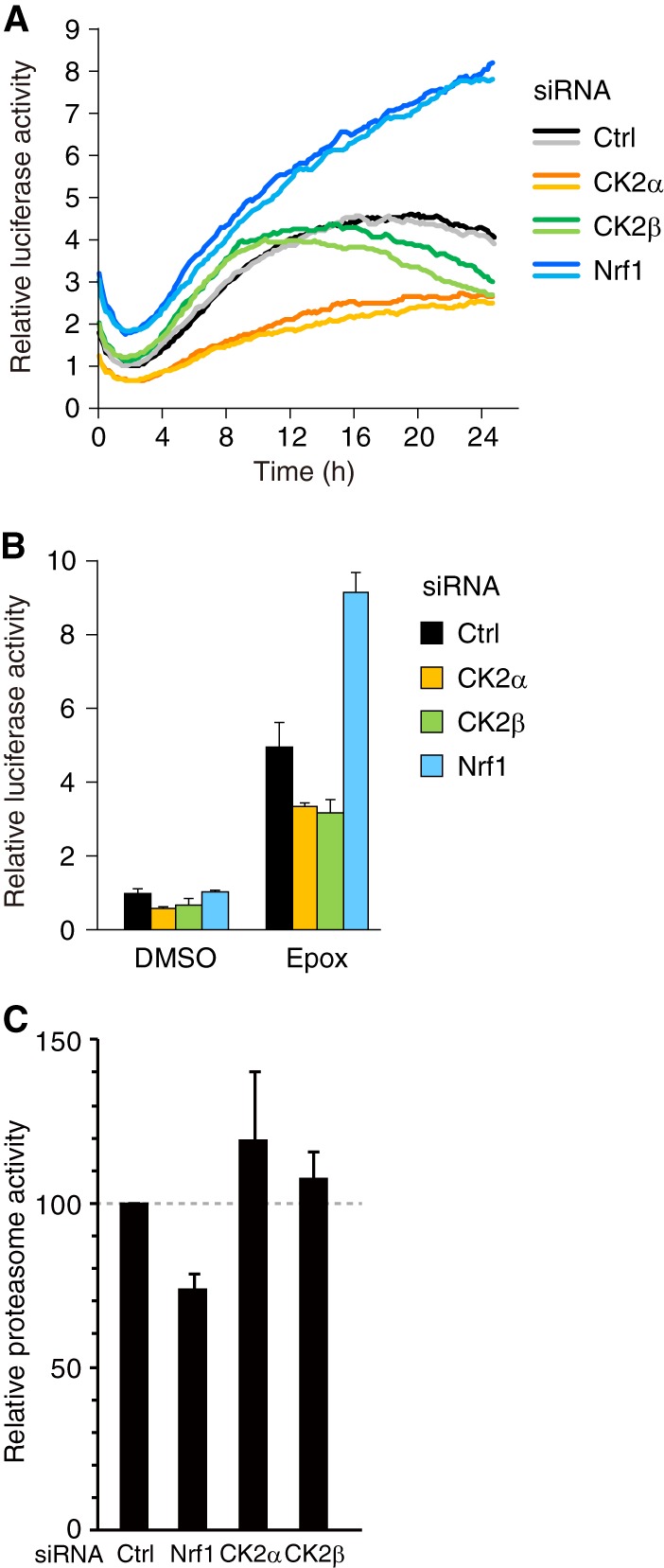
Knockdown of the CK2 subunits or Nrf1 affects degradation of ubiquitinated proteins. (A) HeLa cells transfected with the Ub-FL reporter plasmid and the indicated siRNAs were treated with 0.1 mM d-luciferin. Real-time monitoring of the reporter activity was performed using a photomultiplier. Epoxomicin was added to the culture medium 1 to 2 h after the start of measurement. The darker and lighter lines indicate duplicate traces of two independent samples. Representative data are shown. (B) HeLa cells transfected with the Ub-FL reporter plasmid, the control Renilla luciferase reporter, and the indicated siRNAs were treated with DMSO or 10 nM epoxomicin (Epox) with d-luciferin for 16 h. The cells were lysed and subjected to a luciferase assay. The values were normalized to Renilla luciferase activity values and presented as the means plus SD (n = 3). (C) HeLa cells transfected with control (Ctrl), Nrf1, CK2α, or CK2β siRNA were treated with 10 nM epoxomicin for 24 h. The proteasome activity was determined by using a luminogenic substrate Suc-LLVY-aminoluciferin for the chymotrypsin-like activity. The values were shown as the percent changes of proteasome activity over the control siRNA-treated cells (means plus SD, n = 9).
Measurement of proteasome activity.
HeLa cells transfected with the siRNAs indicated in the figures were treated with 10 nM epoxomicin for 24 h. The proteasome activity was determined by measuring chymotrypsin activity with Proteasome-Glo chymotrypsin-like cell-based assay (Promega) according to the manufacturer's instructions.
In vitro kinase assay.
Purified Nrf1 fragments were incubated with or without 100 ng of recombinant CK2α in kinase reaction buffer (50 mM Tris-HCl [pH 7.5], 200 mM NaCl, 10 mM MgCl2, 15 mM β-glycerophosphate, 2 mM EGTA, 1 mM dithiothreitol [DTT], and 50 μM ATP) supplemented with 0.1 MBq of [γ-32P]ATP for 15 min at 37°C. The reaction was stopped by the addition of SDS sample buffer. After resolution by SDS-PAGE, substrate phosphorylation was detected with a bioimaging analyzer (BAS-2500; Fujifilm).
Cycloheximide chase experiment.
COS7 cells that were transfected with the plasmids indicated in the figures were treated with 20 μg/ml of cycloheximide, and the whole-cell extracts were prepared at the time points indicated in the figures and subjected to immunoblot analysis with the antibodies indicated in the figures.
Immunocytochemical staining.
The cells were fixed with 4% formaldehyde for 10 min, washed twice with PBS, and permeabilized with 0.5% Triton X-100 in PBS for 5 min. The cells were washed twice with PBS and treated with the antibodies indicated in the figures for 1 h at room temperature. After the cells were washed three times with PBS, they were incubated with Alexa Fluor 488- or Alexa Fluor 546-conjugated secondary antibodies (Invitrogen) for 30 min at room temperature. The nuclei were stained with 4′,6′-diamidino-2-phenylindole (DAPI). After the cells were washed three times with PBS, they were sealed with a drop of fluorescence mounting medium (Dako). Fluorescent images were captured with an Olympus LX71 fluorescence microscope.
RESULTS
Nrf1 regulates the expression of almost all proteasome subunits and several proteasome-related genes.
We first evaluated the importance of Nrf1 in the transcriptional induction of proteasome subunits and proteasome-related genes upon inhibition of the proteasome in HeLa cells. To this end, HeLa cells were treated with the proteasome inhibitor MG132 for 16 h in the presence or absence of siRNA that targets Nrf1. The Nrf1 mRNA expression level was significantly repressed by the Nrf1-specific siRNA (Fig. 1A). MG132 stabilized the Nrf1 protein as previously reported (17–19), and the siRNA-mediated knockdown of Nrf1 efficiently suppressed the accumulation of Nrf1 protein (Fig. 1B). Upon inhibition of the proteasome, Nrf1 is reported to stimulate the expression of a large set of proteasome subunit genes and the proteasome maturation factor POMP (18). Thus, we first examined the mRNA expression profiles of all the proteasome subunits and well-characterized proteasome-related genes. We found that most of the proteasome subunit genes were upregulated by MG132 in an Nrf1-dependent manner (Fig. 1C and D) and that the expression of almost all the base and lid subunit genes was significantly induced by proteasome inhibition in HeLa cells. In addition, among the many proteasome-related genes, the proteasome activator PA200, the proteasome-associated deubiquitinating enzyme Usp14, and POMP were markedly upregulated by MG132 in an Nrf1-dependent manner (Fig. 1C and D). These results reemphasize the importance of Nrf1 in the strong coordination of proteasome biogenesis. A time profile of proteasome subunit gene expression was correlated with that of Nrf1 accumulation in cells that were treated with MG132 (Fig. 1B and E).
It has been reported that dysfunction of the proteasome leads to the induction of molecular chaperones and several autophagy-related genes, including Hsp70, p62/SQSTM1, and Bag3 (24–26). These genes contribute to protein quality control and the activation of selective autophagy, another degradation pathway for ubiquitinated proteins (27, 28). We examined whether Nrf1 is involved in the expression of autophagy-related genes that are induced by proteasome inhibition. Treatment of the cells with MG132 significantly induced these genes, and knockdown of Nrf1 had little impact on this induction (Fig. 1D and data not shown). We also examined the expression of other candidate genes for Nrf1 targets, GSTA4 and NQO1, which are well-known ARE-regulated genes. As a result, expression of GSTA4 but not NQO1 was induced by MG132 in an Nrf1-dependent manner (Fig. 1E). These results indicate that, upon proteasome inhibition, Nrf1 specifically upregulates proteasome-related genes along with a subset of antioxidant response genes. As expression of several proteasome genes was also induced in an Nrf1-dependent manner in another human cell line, MCF10A, it is likely that Nrf1-dependent induction of proteasome genes is a general mechanism utilized by various types of cells, although there is some variation among cell lines in response to proteasome inhibition (data not shown).
Identification of CK2 as a suppressor of Nrf1-mediated transcription.
It is conceivable that regulating the transcriptional activity of Nrf1 is of critical importance in controlling cellular proteasome activity. To understand regulatory mechanisms of Nrf1 activity, we conducted a mass spectrometric analysis and identified a wide variety of proteins as Nrf1-binding proteins (see Table S1 in the supplemental material) (19). We focused on the protein kinase CK2 because protein kinases are often the critical regulators for diverse transcription factors. CK2 was the only protein kinase identified in our analysis, and both the catalytic α subunit and the regulatory β subunit of CK2 were identified (see Tables S1 and S2 in the supplemental material). CK2 is known to form a heterotetrameric complex composed of two α (and/or α′) subunits and two β subunits (29). To further investigate the interaction of Nrf1 with the CK2 holoenzyme, we performed coimmunoprecipitation assays with COS7 cells. The results clearly demonstrate that CK2β was coimmunoprecipitated with Nrf1 (Fig. 2A). Of note, CK2α was coimmunoprecipitated with Nrf1 only in the presence of CK2β (Fig. 2A). Thus, Nrf1 may bind to the CK2 holoenzyme through its regulatory β subunit. To examine whether CK2 regulates the transcriptional activity of Nrf1, we assessed the effect of an siRNA-mediated knockdown of the CK2 subunits on the Nrf1-dependent expression of the proteasome subunit genes. siRNAs for CK2α, CK2α′, a paralogous isoform of CK2α, and CK2β efficiently downregulated the expression of their target mRNAs (Fig. 2B). Efficient knockdown of CK2α, CK2α′, and CK2β was also confirmed by Western blotting (Fig. 2C). The siRNA-mediated knockdown of both CK2α and CK2α′ enhanced the MG132-induced expression of proteasome subunit genes such as PSMC4, PSMB6, PSMA4, and PSMC6 (Fig. 2D). Knockdown of CK2β had similar effects on the expression of these genes (Fig. 2D). These enhancements were not observed when Nrf1 was downregulated simultaneously by siRNA (Fig. 2D). These results indicate that CK2 suppresses the Nrf1-dependent expression of proteasome subunit genes that are induced by proteasome inhibition. To further evaluate whether Nrf1 mediates the effect of CK2 knockdown on the transcription of proteasome genes, we performed chromatin immunoprecipitation (ChIP) assays using an anti-Nrf1 antibody. We examined the recruitment of Nrf1 to the AREs, which are located in the proximal promoter of PSMC4 and PSMB6 and in the first intron of PSMA4 (16, 17). siRNA-mediated knockdown of CK2α and CK2β significantly augmented the recruitment of Nrf1 to the AREs, both with and without MG132 treatment (Fig. 2E; see Discussion). The 5′-upstream region of RPL30 served as a negative control of Nrf1 binding. As siRNA-mediated knockdown of Nrf1 markedly decreased the amount of precipitated ARE regions of the proteasome subunit promoters (Fig. 2E), the observed ChIP signals should reflect Nrf1-ARE binding. These data collectively indicate that CK2 suppresses the transcriptional activity of Nrf1 by regulating the recruitment of Nrf1 to its target AREs.
The Nrf1-mediated transcriptional regulation of the proteasome subunit genes should control the total cellular proteasome activity. To investigate the effect of a CK2 knockdown on the detailed time profile of proteasome activity under conditions of proteasome inhibition, we performed real-time monitoring of proteasome activity in living cells. We utilized a ubiquitin-fused luciferase reporter (Ub-FL) as the indicator of endogenous proteasome activity (20). In this system, high reporter activity corresponds to low proteasome activity. The addition of the proteasome inhibitor epoxomicin resulted in a gradual increase of the reporter activity, which was greatly enhanced by siRNA-mediated knockdown of Nrf1 (Fig. 3A). In contrast, the knockdown of either CK2α or CK2β suppressed the epoxomicin-induced stabilization of the reporter protein (Fig. 3A). Similar results were obtained by measuring the properly normalized reporter activity in cell lysates that were prepared 16 h after the addition of epoxomicin (Fig. 3B). We also measured endogenous proteasome activity by using a luminogenic substrate Suc-LLVY-aminoluciferin for the chymotrypsin-like activity. The result demonstrates that Nrf1 knockdown cells show lower proteasome activity than control cells 24 h after epoxomicin treatment (Fig. 3C). On the other hand, CK2α or CK2β knockdown cells show slightly higher proteasome activity than control cells, although the difference in proteasome activity is moderate compared with the results in Fig. 3A and B. These results suggest that CK2-mediated suppression of Nrf1 activity leads to the downregulation of proteasome activity in cells.
CK2 phosphorylates Nrf1 at a specific serine residue.
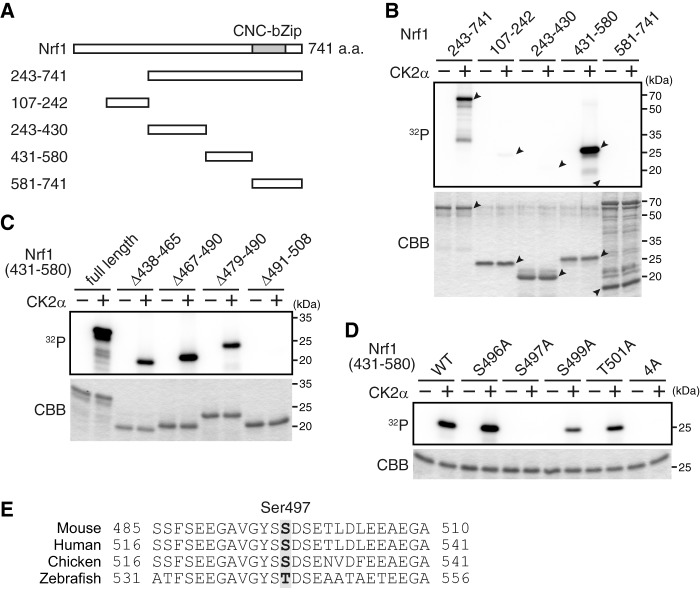
Next, we focused on the underlying mechanisms of the CK2 regulation of Nrf1. We assumed that CK2 directly phosphorylates Nrf1 and regulates its transcriptional activity. To test this hypothesis, we performed an in vitro kinase assay using recombinant CK2α and a series of Nrf1 fragments (Fig. 4A). Among the constructed fragments, Nrf1 (residues 431 to 580) was specifically phosphorylated by CK2α (Fig. 4B). Additional experiments narrowed the phosphorylation site to a small region (residues 491 to 508) of Nrf1 (Fig. 4C). We introduced an alanine substitution at each of the four candidate phosphoacceptor sites (Ser 496, Ser 497, Ser 499, and Thr 501). Of the constructed mutants, only the Ser 497 to Ala (S497A) mutant was not phosphorylated by CK2α in vitro (Fig. 4D). Thus, we concluded that Ser 497 of Nrf1 is the primary target for phosphorylation by CK2α in vitro (Fig. 4E).
Fig 4.
CK2 phosphorylates Nrf1 at Ser 497. (A) Schematic structures of the Nrf1 fragments. a.a., amino acids. (B) CK2 phosphorylates residues 431 to 580 of Nrf1. The purified Nrf1 fragments were incubated in the presence of [γ-32P]ATP with (+) or without (−) recombinant CK2α. The autoradiograph was analyzed with a phosphorimager. The indicated input proteins were analyzed by Coomassie brilliant blue (CBB) staining. The positions of input proteins are indicated by black arrowheads. (C) CK2 phosphorylates residues 491 to 501 of Nrf1. The Nrf1 fragments (residues 431 to 580) with the indicated internal deletions were subjected to an in vitro phosphorylation assay. (D) CK2 phosphorylates Ser 497 of Nrf1. The Nrf1 fragments (residues 431 to 580) with the indicated point mutations were subjected to an in vitro phosphorylation assay. WT, wild type; 4A, all four of the candidate serine/threonine residues were replaced by alanine. (E) Conservation of Nrf1 sequences among the species around the CK2-mediated phosphorylation site.
The CK2 phosphorylation site mutant enhances the transcriptional activity of Nrf1.
To investigate the effect of the CK2-mediated phosphorylation of Nrf1, we compared the transcriptional activity of the S497A mutant with wild-type Nrf1 (Nrf1-WT). We used a luciferase reporter that was driven by three tandem copies of the ARE from the PSMA4 promoter (17). Forced expression of Nrf1 increased the reporter activity in a dose-dependent manner (Fig. 5A). Notably, the S497A mutant exhibited enhanced transcriptional activity compared to wild-type Nrf1 (Fig. 5A). Similar results were obtained with the reporter assay using pRBGP2 luciferase reporter that was driven by three tandem copies of the MARE (Fig. 5A) (21). Furthermore, forced expression of the Nrf1-S497A mutant increased expression of endogenous proteasome genes such as PSMC4 and PSMA4 much more than Nrf1-WT, although expression of PSMB6 did not increase significantly (Fig. 5B). As the steady-state level and the degradation rates of these proteins were comparable (Fig. 5C), the enhanced activity of the S497A mutant is not merely due to the elevated expression and/or stabilization of the Nrf1 proteins. In addition, there was no difference in the subcellular localization and physical interaction with MafK, a heterodimerization partner of Nrf1, between wild-type Nrf1 and the S497A mutant (data not shown). We next examined whether the enhanced transcriptional activity of the S497A mutant could affect endogenous proteasome activity. The Ub-FL reporter plasmid with either wild-type Nrf1 or the S497A mutant expression plasmid was transfected into HeLa cells, and the reporter activity was monitored in cells treated with epoxomicin. The forced expression of wild-type Nrf1 prevented the increase in reporter activity that was induced by epoxomicin in a dose-dependent manner (Fig. 5D). This result suggests that the upregulation of the proteasome activity by the Nrf1-mediated induction of proteasome expression confers tolerance for proteasome inhibition on cells. Importantly, compared to wild-type Nrf1, the S497A mutant had a greater ability to prevent the increase in reporter activity (Fig. 5D). Similar results were obtained by measuring the properly normalized reporter activity in cell lysates prepared 16 h after the addition of epoxomicin (Fig. 5E). These results clearly demonstrate that the CK2 phosphorylation site mutant of Nrf1 has a greater ability to upregulate proteasome expression and activity than wild-type Nrf1. To evaluate the importance of phosphorylation at Ser 497 in the regulation by CK2, we examined whether CK2 affects the transcriptional activity of Nrf1-WT and Nrf1-S497A. The results demonstrate that CK2 suppresses the PSMA4 reporter activity induced by Nrf1-WT but not by the Nrf1-S497A mutant (Fig. 5F, left graph). Similar results were obtained using the pRBGP2 reporter (Fig. 5F, right graph). These results collectively suggest that CK2 suppresses the transcriptional activity of Nrf1 via phosphorylation of Nrf1 at Ser 497.
The CK2 phosphorylation site mutant of Nrf1 suppresses the formation of p62-positive inclusion bodies.
Proteasome dysfunction is associated with the formation of juxtanuclear inclusion bodies such as aggresomes (1). These inclusion bodies contain p62 and function to sequester unfolded and misfolded proteins that could not be degraded by the proteasome (30). The phosphorylation state of Ser 497 of Nrf1 may affect the efficiency of the formation of such inclusion bodies. To assess this possibility, we examined the effect of forced expression of the S497A mutant of Nrf1 on the formation of p62-positive inclusion bodies. Treatment of cells with epoxomicin led to the formation of p62-positive juxtanuclear inclusion bodies in HeLa cells (Fig. 6A). Similar results were obtained when MG132 was used instead of epoxomicin (data not shown). Strikingly, the siRNA-mediated knockdown of Nrf1 markedly enhanced the inclusion body formation (Fig. 6A). This result indicates that Nrf1 plays an important role in preventing the formation of juxtanuclear inclusion bodies. These p62-positive inclusion bodies were also ubiquitin positive, indicating the accumulation of ubiquitinated proteins (Fig. 6B). Knockdown of Nrf1 did not affect the total amount of p62 but caused an increase in the autophagic marker LC3-II (Fig. 6C). This finding suggests that the downregulation of Nrf1 leads to the accumulation of autophagosomes that are involved in an alternative degradation pathway for misfolded proteins. We tested the effect of Nrf1 overexpression on inclusion body formation. Forced expression of wild-type Nrf1 decreased the efficiency of formation of the p62-positive juxtanuclear inclusion bodies that were induced by epoxomicin (Fig. 6D). Importantly, forced expression of the S497A mutant reduced inclusion body formation more effectively than that of wild-type Nrf1 (Fig. 6D). These results suggest that the phosphorylation state of Ser 497 affects the formation efficiency of the p62-positive juxtanuclear inclusion bodies that are induced by proteasome inhibition.
DISCUSSION
Significance of CK2 as a regulator of Nrf1 transcriptional activity.
In this study, we demonstrate that CK2 interacts with and phosphorylates Nrf1 and suppresses its transcriptional activity, thereby regulating the expression of proteasome subunits. Nrf1 is localized to the endoplasmic reticulum (ER) membrane and is constitutively degraded by the proteasome under normal conditions (18, 19). Inhibition or dysfunction of the proteasome may induce the stabilization and nuclear translocation of Nrf1 proteins. Our results have implicated CK2 in the regulatory mechanisms of Nrf1 activity. Given that knockdown of the CK2 subunits facilitates the recruitment of Nrf1 to the AREs of their promoters and increases the expression of Nrf1 target genes even without proteasome inhibition (Fig. 2D and E), CK2 may suppress the transcriptional activity of Nrf1 and thus prevent the unnecessary expression of Nrf1 target genes under physiological conditions. As we have no data indicating that phosphorylation of Ser 497 is altered in response to proteasome inhibition, it remains to be elucidated whether CK2-mediated phosphorylation of Nrf1 is regulated during Nrf1 activation. Our preliminary data demonstrate that the expression of CK2α is reduced upon inhibition of the proteasome (data not shown). Although the mechanism underlying the decrease in CK2α expression is unclear, it is possible that Nrf1 phosphorylation is decreased upon proteasome inhibition, resulting in efficient transcriptional activation of stabilized Nrf1 proteins.
Nrf1 exists as multiple isoforms, including TCF11 (transcription factor 11), a longer isoform found in humans (31). The regulatory mechanism for TCF11 might differ from that of Nrf1, as TCF11 has the nuclear export signal that is not present in Nrf1. In this study, we used the expression plasmid for mouse Nrf1 but not the expression plasmid for TCF11. Thus, it remains to be elucidated in future studies whether human TCF11 can be regulated by CK2 and whether there is any difference in CK2-mediated regulation between mouse Nrf1 and human TCF11. In addition to a known proteasome-mediated regulation of Nrf1 activity, we propose that CK2-mediated phosphorylation of Nrf1 acts as another layer of Nrf1 regulation to fine-tune its transcriptional activity.
Effect of CK2-mediated direct phosphorylation of Nrf1.
The phosphorylation of Nrf1 Ser 497 is likely to enhance its transcriptional activity but not affect its stability, subcellular localization, or ability to bind MafK proteins. Our data indicate that Ser 497 has an important role in CK2-dependent suppression of Nrf1 transcriptional activity. Given that CK2 knockdown enhances the recruitment of Nrf1 to its target AREs, it is likely that CK2-mediated phosphorylation of Ser 497 controls the recruitment of Nrf1 to the AREs of the target promoters. However, it remains to be investigated how phosphorylation of Ser 497 suppresses Nrf1 recruitment. As Ser 497 resides in the Neh6-like domain and are located next to the CNC-bZip domain, phosphorylation may induce a conformational change of the Nrf1 protein, compromising its binding to the target DNA.
Ser 497 of mouse Nrf1 is conserved in mouse Nrf2 (Ser 365), but it is not known whether CK2 can phosphorylate Nrf2 at Ser 365. It has been reported that Nrf2 is phosphorylated by CK2 and that this phosphorylation regulates the transcriptional activity of Nrf2 (32, 33). The effect of the CK2-mediated phosphorylation on Nrf2 activity is controversial, but several reports have shown that CK2 inhibition results in Nrf2 inactivation (32, 34, 35). The CK2-mediated phosphorylation of Nrf2 facilitates its nuclear translocation and the upregulation of Nrf2 target gene expression. Thus, it seems likely that the role of CK2-dependent regulation in Nrf2 may be different from that in Nrf1.
The CK2-Nrf1 axis as a new therapeutic target for diseases associated with proteasome dysfunction.
The regulation of proteasome activity is an established strategy for cancer treatment, and it is expected to be a promising approach to ameliorate some age-related disorders, such as neurodegenerative diseases. Supporting this notion, a previous study has demonstrated that a small-molecule inhibitor of USP14, a proteasome-associated deubiquitinating enzyme, increases the proteasome activity and enhances the degradation of several neurodegenerative disease-associated proteins such as tau and TDP-43 (36). Thus, the upregulation of proteasome activity should alleviate the accumulation of aggregate-prone proteins. In addition, it has been reported that overexpression of a proteasome subunit gene increases proteasome activity, decreases the accumulation of ubiquitinated proteins, and ameliorates the response to oxidative stress (37–39). Therefore, the fact that Nrf1 regulates the expression of most of the proteasome subunit genes may provide a new approach, transcriptional upregulation of the proteasome, to treat human diseases associated with the accumulation of abnormal proteins. Our findings that Nrf1-S497A exerts a more significant effect on the inhibition of p62-positive inclusion body formation than wild-type Nrf1 does strongly suggest that the CK2-mediated phosphorylation of Nrf1 is one of the potential targets for treating proteinopathies such as neurodegenerative diseases.
CK2 has an array of substrate proteins and functions in diverse cellular processes, including cell growth and proliferation (40, 41). Recent studies have also implicated CK2 in neuronal functions and the progression of neurodegenerative diseases (42, 43). It has been reported that CK2 phosphorylates several neurodegenerative disease-related proteins, such as α-synuclein, synphilin-1, and apolipoprotein E, to enhance aggregate formation (44–46). Thus, the regulation of CK2 activity may represent a possible target for therapeutic intervention. Indeed, CK2 inhibition has been shown to exert a protective effect on neurons (44, 47, 48). In contrast, a recent report has shown that CK2 phosphorylates p62 and stimulates the clearance of protein aggregates via the autophagy-lysosome pathway (49). This finding raises the possibility that CK2 inhibition or knockdown results in the impairment of aggregate clearance. Therefore, there seem to be pros and cons to modulating CK2 activity with regard to ameliorating proteinopathies. Nevertheless, our data strongly suggest that the CK2-mediated regulation of Nrf1 can be a novel target for the treatment of diseases associated with proteasome dysfunction.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to David Piwnica-Worms and Raymond J. Deshaies for the Ub-FL plasmid and the 3xPSMA4-ARE-Luc plasmid, respectively. We also thank Noriko Noguchi and Akiko Matsumoto for research support.
This work was supported in part by grants-in-aid (A.K. and Y.T.) and the Strategic Research Foundation at Private Universities (2012 to 2016) (A.K.) from the Ministry of Education, Sports, Science and Technology, the Mochida Memorial Foundation (A.K.), the Naito Foundation (A.K.), the Suzuken Memorial Foundation (A.K.), the Takeda Science Foundation (A.K.), the Uehara Memorial Foundation (A.K.), Astellas Foundation for Research on Metabolic Disorders (A.K.), and the Inamori Foundation (Y.T.).
Footnotes
Published ahead of print 1 July 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/MCB.01271-12.
REFERENCES
- 1.Kopito RR. 2000. Aggresomes, inclusion bodies and protein aggregation. Trends Cell Biol. 10:524–530 [DOI] [PubMed] [Google Scholar]
- 2.Ross CA, Poirier MA. 2004. Protein aggregation and neurodegenerative disease. Nat. Med. 10:S10–S17 [DOI] [PubMed] [Google Scholar]
- 3.Strnad P, Zatloukal K, Stumptner C, Kulaksiz H, Denk H. 2008. Mallory-Denk-bodies: lessons from keratin-containing hepatic inclusion bodies. Biochim. Biophys. Acta 1782:764–774 [DOI] [PubMed] [Google Scholar]
- 4.Glickman MH, Ciechanover A. 2002. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol. Rev. 82:373–428 [DOI] [PubMed] [Google Scholar]
- 5.Levine B, Kroemer G. 2008. Autophagy in the pathogenesis of disease. Cell 132:27–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. 2008. Autophagy fights disease through cellular self-digestion. Nature 451:1069–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubinsztein DC. 2006. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature 443:780–786 [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi M, Yamamoto M. 2006. Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv. Enzyme Regul. 46:113–140 [DOI] [PubMed] [Google Scholar]
- 9.Sykiotis GP, Bohmann D. 2010. Stress-activated cap‘n'collar transcription factors in aging and human disease. Sci. Signal. 3:re3. 10.1126/scisignal.3112re3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan JY, Kwong M, Lu R, Chang J, Wang B, Yen TS, Kan YW. 1998. Targeted disruption of the ubiquitous CNC-bZIP transcription factor, Nrf-1, results in anemia and embryonic lethality in mice. EMBO J. 17:1779–1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim J, Xing W, Wergedal J, Chan JY, Mohan S. 2010. Targeted disruption of nuclear factor erythroid-derived 2-like 1 in osteoblasts reduces bone size and bone formation in mice. Physiol. Genomics 40:100–110 [DOI] [PubMed] [Google Scholar]
- 12.Ohtsuji M, Katsuoka F, Kobayashi A, Aburatani H, Hayes JD, Yamamoto M. 2008. Nrf1 and Nrf2 play distinct roles in activation of antioxidant response element-dependent genes. J. Biol. Chem. 283:33554–33562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xing W, Singgih A, Kapoor A, Alarcon CM, Baylink DJ, Mohan S. 2007. Nuclear factor-E2-related factor-1 mediates ascorbic acid induction of osterix expression via interaction with antioxidant-responsive element in bone cells. J. Biol. Chem. 282:22052–22061 [DOI] [PubMed] [Google Scholar]
- 14.Xu Z, Chen L, Leung L, Yen TS, Lee C, Chan JY. 2005. Liver-specific inactivation of the Nrf1 gene in adult mouse leads to nonalcoholic steatohepatitis and hepatic neoplasia. Proc. Natl. Acad. Sci. U. S. A. 102:4120–4125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobayashi A, Tsukide T, Miyasaka T, Morita T, Mizoroki T, Saito Y, Ihara Y, Takashima A, Noguchi N, Fukamizu A, Hirotsu Y, Ohtsuji M, Katsuoka F, Yamamoto M. 2011. Central nervous system-specific deletion of transcription factor Nrf1 causes progressive motor neuronal dysfunction. Genes Cells 16:692–703 [DOI] [PubMed] [Google Scholar]
- 16.Lee CS, Lee C, Hu T, Nguyen JM, Zhang J, Martin MV, Vawter MP, Huang EJ, Chan JY. 2011. Loss of nuclear factor E2-related factor 1 in the brain leads to dysregulation of proteasome gene expression and neurodegeneration. Proc. Natl. Acad. Sci. U. S. A. 108:8408–8413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radhakrishnan SK, Lee CS, Young P, Beskow A, Chan JY, Deshaies RJ. 2010. Transcription factor Nrf1 mediates the proteasome recovery pathway after proteasome inhibition in mammalian cells. Mol. Cell 38:17–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steffen J, Seeger M, Koch A, Kruger E. 2010. Proteasomal degradation is transcriptionally controlled by TCF11 via an ERAD-dependent feedback loop. Mol. Cell 40:147–158 [DOI] [PubMed] [Google Scholar]
- 19.Tsuchiya Y, Morita T, Kim M, Iemura S, Natsume T, Yamamoto M, Kobayashi A. 2011. Dual regulation of the transcriptional activity of Nrf1 by β-TrCP- and Hrd1-dependent degradation mechanisms. Mol. Cell. Biol. 31:4500–4512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luker GD, Pica CM, Song J, Luker KE, Piwnica-Worms D. 2003. Imaging 26S proteasome activity and inhibition in living mice. Nat. Med. 9:969–973 [DOI] [PubMed] [Google Scholar]
- 21.Igarashi K, Itoh K, Motohashi H, Hayashi N, Matuzaki Y, Nakauchi H, Nishizawa M, Yamamoto M. 1995. Activity and expression of murine small Maf family protein MafK. J. Biol. Chem. 270:7615–7624 [DOI] [PubMed] [Google Scholar]
- 22.Igarashi K, Kataoka K, Itoh K, Hayashi N, Nishizawa M, Yamamoto M. 1994. Regulation of transcription by dimerization of erythroid factor NF-E2 p45 with small Maf proteins. Nature 367:568–572 [DOI] [PubMed] [Google Scholar]
- 23.Miyata Y, Nishida E. 2005. CK2 binds, phosphorylates, and regulates its pivotal substrate Cdc37, an Hsp90-cochaperone. Mol. Cell. Biochem. 274:171–179 [DOI] [PubMed] [Google Scholar]
- 24.Kuusisto E, Suuronen T, Salminen A. 2001. Ubiquitin-binding protein p62 expression is induced during apoptosis and proteasomal inhibition in neuronal cells. Biochem. Biophys. Res. Commun. 280:223–228 [DOI] [PubMed] [Google Scholar]
- 25.Wang HQ, Liu HM, Zhang HY, Guan Y, Du ZX. 2008. Transcriptional upregulation of BAG3 upon proteasome inhibition. Biochem. Biophys. Res. Commun. 365:381–385 [DOI] [PubMed] [Google Scholar]
- 26.Zhou M, Wu X, Ginsberg HN. 1996. Evidence that a rapidly turning over protein, normally degraded by proteasomes, regulates hsp72 gene transcription in HepG2 cells. J. Biol. Chem. 271:24769–24775 [DOI] [PubMed] [Google Scholar]
- 27.Gamerdinger M, Hajieva P, Kaya AM, Wolfrum U, Hartl FU, Behl C. 2009. Protein quality control during aging involves recruitment of the macroautophagy pathway by BAG3. EMBO J. 28:889–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johansen T, Lamark T. 2011. Selective autophagy mediated by autophagic adapter proteins. Autophagy 7:279–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niefind K, Guerra B, Ermakowa I, Issinger OG. 2001. Crystal structure of human protein kinase CK2: insights into basic properties of the CK2 holoenzyme. EMBO J. 20:5320–5331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Komatsu M, Waguri S, Koike M, Sou YS, Ueno T, Hara T, Mizushima N, Iwata J, Ezaki J, Murata S, Hamazaki J, Nishito Y, Iemura S, Natsume T, Yanagawa T, Uwayama J, Warabi E, Yoshida H, Ishii T, Kobayashi A, Yamamoto M, Yue Z, Uchiyama Y, Kominami E, Tanaka K. 2007. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell 131:1149–1163 [DOI] [PubMed] [Google Scholar]
- 31.Husberg C, Murphy P, Bjørgo E, Kalland KH, Kolstø AB. 2003. Cellular localisation and nuclear export of the human bZIP transcription factor TCF11. Biochim. Biophys. Acta 1640:143–151 [DOI] [PubMed] [Google Scholar]
- 32.Apopa PL, He X, Ma Q. 2008. Phosphorylation of Nrf2 in the transcription activation domain by casein kinase 2 (CK2) is critical for the nuclear translocation and transcription activation function of Nrf2 in IMR-32 neuroblastoma cells. J. Biochem. Mol. Toxicol. 22:63–76 [DOI] [PubMed] [Google Scholar]
- 33.Pi J, Bai Y, Reece JM, Williams J, Liu D, Freeman ML, Fahl WE, Shugar D, Liu J, Qu W, Collins S, Waalkes MP. 2007. Molecular mechanism of human Nrf2 activation and degradation: role of sequential phosphorylation by protein kinase CK2. Free Radic. Biol. Med. 42:1797–1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Afonyushkin T, Oskolkova OV, Binder BR, Bochkov VN. 2011. Involvement of CK2 in activation of electrophilic genes in endothelial cells by oxidized phospholipids. J. Lipid Res. 52:98–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ivanov AV, Smirnova OA, Ivanova ON, Masalova OV, Kochetkov SN, Isaguliants MG. 2011. Hepatitis C virus proteins activate NRF2/ARE pathway by distinct ROS-dependent and independent mechanisms in HUH7 cells. PLoS One 6:e24957. 10.1371/journal.pone.0024957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee BH, Lee MJ, Park S, Oh DC, Elsasser S, Chen PC, Gartner C, Dimova N, Hanna J, Gygi SP, Wilson SM, King RW, Finley D. 2010. Enhancement of proteasome activity by a small-molecule inhibitor of USP14. Nature 467:179–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chondrogianni N, Gonos ES. 2007. Overexpression of hUMP1/POMP proteasome accessory protein enhances proteasome-mediated antioxidant defence. Exp. Gerontol. 42:899–903 [DOI] [PubMed] [Google Scholar]
- 38.Chondrogianni N, Tzavelas C, Pemberton AJ, Nezis IP, Rivett AJ, Gonos ES. 2005. Overexpression of proteasome beta5 assembled subunit increases the amount of proteasome and confers ameliorated response to oxidative stress and higher survival rates. J. Biol. Chem. 280:11840–11850 [DOI] [PubMed] [Google Scholar]
- 39.Tonoki A, Kuranaga E, Tomioka T, Hamazaki J, Murata S, Tanaka K, Miura M. 2009. Genetic evidence linking age-dependent attenuation of the 26S proteasome with the aging process. Mol. Cell. Biol. 29:1095–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allende JE, Allende CC. 1995. Protein kinases. 4. Protein kinase CK2: an enzyme with multiple substrates and a puzzling regulation. FASEB J. 9:313–323 [DOI] [PubMed] [Google Scholar]
- 41.Meggio F, Pinna LA. 2003. One-thousand-and-one substrates of protein kinase CK2? FASEB J. 17:349–368 [DOI] [PubMed] [Google Scholar]
- 42.Blanquet PR. 2000. Casein kinase 2 as a potentially important enzyme in the nervous system. Prog. Neurobiol. 60:211–246 [DOI] [PubMed] [Google Scholar]
- 43.Perez DI, Gil C, Martinez A. 2011. Protein kinases CK1 and CK2 as new targets for neurodegenerative diseases. Med. Res. Rev. 31:924–954 [DOI] [PubMed] [Google Scholar]
- 44.Lee G, Tanaka M, Park K, Lee SS, Kim YM, Junn E, Lee SH, Mouradian MM. 2004. Casein kinase II-mediated phosphorylation regulates α-synuclein/synphilin-1 interaction and inclusion body formation. J. Biol. Chem. 279:6834–6839 [DOI] [PubMed] [Google Scholar]
- 45.Okochi M, Walter J, Koyama A, Nakajo S, Baba M, Iwatsubo T, Meijer L, Kahle PJ, Haass C. 2000. Constitutive phosphorylation of the Parkinson's disease associated α-synuclein. J. Biol. Chem. 275:390–397 [DOI] [PubMed] [Google Scholar]
- 46.Raftery M, Campbell R, Glaros EN, Rye KA, Halliday GM, Jessup W, Garner B. 2005. Phosphorylation of apolipoprotein-E at an atypical protein kinase CK2 PSD/E site in vitro. Biochemistry 44:7346–7353 [DOI] [PubMed] [Google Scholar]
- 47.Chen-Roetling J, Li Z, Regan RF. 2008. Hemoglobin neurotoxicity is attenuated by inhibitors of the protein kinase CK2 independent of heme oxygenase activity. Curr. Neurovasc. Res. 5:193–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moreno H, Yu E, Pigino G, Hernandez AI, Kim N, Moreira JE, Sugimori M, Llinás RR. 2009. Synaptic transmission block by presynaptic injection of oligomeric amyloid beta. Proc. Natl. Acad. Sci. U. S. A. 106:5901–5906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matsumoto G, Wada K, Okuno M, Kurosawa M, Nukina N. 2011. Serine 403 phosphorylation of p62/SQSTM1 regulates selective autophagic clearance of ubiquitinated proteins. Mol. Cell 44:279–289 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.