Abstract
Skin wound healing in mammals is a complex, multicellular process that depends on the precise supply of oxygen. Hypoxia-inducible factor (HIF) prolyl hydroxylase 2 (PHD2) serves as a crucial oxygen sensor and may therefore play an important role during reepithelialization. Hence, this study was aimed at understanding the role of PHD2 in cutaneous wound healing using different lines of conditionally deficient mice specifically lacking PHD2 in inflammatory, vascular, or epidermal cells. Interestingly, PHD2 deficiency only in keratinocytes and not in myeloid or endothelial cells was found to lead to faster wound closure, which involved enhanced migration of the hyperproliferating epithelium. We demonstrate that this effect relies on the unique expression of β3-integrin in the keratinocytes around the tip of the migrating tongue in an HIF1α-dependent manner. Furthermore, we show enhanced proliferation of these cells in the stratum basale, which is directly related to their attenuated transforming growth factor β signaling. Thus, loss of the central oxygen sensor PHD2 in keratinocytes stimulates wound closure by prompting skin epithelial cells to migrate and proliferate. Inhibition of PHD2 could therefore offer novel therapeutic opportunities for the local treatment of cutaneous wounds.
INTRODUCTION
Wound healing is a multicellular process comprising a series of events that involves inflammation, angiogenesis, and reepithelialization (1). Impaired or aberrant healing can eventually lead to major clinical problems. Understanding the precise molecular mechanisms of wound repair in vivo is therefore of utmost importance. Signaling networks controlling the healing process involve growth factors, proteinases, and adhesion molecules, including integrins, which are produced by different cell types. Integrins are cell surface receptors composed of noncovalently linked α and β subunits that mediate cell-matrix and cell-cell interactions and transduce signals that have an impact on various cell properties, including adhesion, migration, and invasion (2). α5β1-Integrin and several members of the αv-integrin family are thought to be involved in wound healing. Although β3-integrin was shown not to be expressed on keratinocytes (3, 4), αvβ5-, αvβ6-, and α5β1-integrins are upregulated in the epithelium during wound closure (5–7), whereas αvβ3-integrin is elevated in several other cell types, like macrophages, endothelial cells, and platelets (4, 8).
Apart from adhesion molecules, growth factors such as members of the transforming growth factor β (TGFβ) superfamily are critically involved in wound healing and repair. The three mammalian TGFβ isoforms (TGFβI to TGFβIII) are synthesized as latent precursors and mechanically or proteolytically activated (9), after which they exert their biological functions via binding to a heteromeric receptor complex, consisting of a type I and a type II receptor (10). In vitro, they have been shown to be mitogenic for fibroblasts, but they inhibit proliferation of most other cells, including keratinocytes (11). In line with this, it was shown that cutaneous wound healing is strongly accelerated in mice overexpressing a dominant negative TGFβII receptor (TGFβIIR) in the epidermis (12). In addition, loss of SMAD3, an intracellular effector protein of TGFβ signaling, shows accelerated reepithelialization in mice (13).
Importantly, a critical stimulus for wound healing is relative hypoxia (14). Although skin is naturally mildly hypoxic (15, 16), a skin wound becomes more hypoxic due to the vascular disruption and high oxygen consumption by the cells at the border of the wound (16, 17). The principal effector proteins during hypoxia are the hypoxia-inducible factors (HIFs), which are heterodimeric transcription factors consisting of an oxygen-sensitive α subunit and a constitutive β subunit. Of the most intensively studied HIFα genes, HIF1α has a ubiquitous pattern of expression in all tissues (18), whereas HIF2α expression is restricted to certain cell types (19, 20). Recently, using mice with a targeted HIF1α deficiency in keratinocytes, it was shown that HIF1α plays an important role in skin homeostasis during aging and that loss of HIF1α leads to a delay in wound healing (21). HIFα expression is tightly regulated by a family of enzymes known as the HIF prolyl hydroxylases (PHDs) (22). In the presence of oxygen, PHDs hydroxylate specific proline residues within the oxygen-sensitive α subunit of HIF, which is then tagged for proteasomal degradation (23). Under hypoxic conditions, HIFα is stabilized and activates transcription of its target genes in the nucleus. Three main isoforms of PHDs are currently known. Of these, PHD2 is the main enzyme controlling HIFα expression under normoxic or mild hypoxic conditions (24). It has been proposed that stabilization of HIF1α via the unspecific PHD inhibitor dimethyloxalylglycine (DMOG) improves healing of chronic wounds in diabetic mice (25). However, the detailed cellular and molecular mechanisms of these processes as well as the specific role of PHD2 in wound healing have not yet been elucidated.
Therefore, in order to investigate the involvement of PHD2 during tissue repair in different cellular compartments, we generated mice with conditional PHD2 deficiency in keratinocytes or myeloid or endothelial cells and subjected them to experimental skin wounds. In the current study, we demonstrate that deletion of PHD2 only in keratinocytes leads to a higher migratory rate, which is mediated by HIF1α-related induction of β3-integrin. Moreover, in wounds, these deficient keratinocytes exhibit enhanced proliferation in a TGFβ-dependent manner. Together, these findings describe a new function for PHD2 during wound healing and provide novel insights into the molecular mechanisms of tissue repair.
MATERIALS AND METHODS
Mice.
All mice were housed at the Experimental Centre at the University of Technology Dresden (Medical Faculty, University Hospital Carl-Gustav Carus) under specific-pathogen-free conditions. The PHD2f/f mouse strain was developed by our research group. HIF1αf/f mice were described earlier (26). All conditionally deficient mouse strains were backcrossed to C57BL/6 mice at least nine times and genotyped using genomic PCR. Mice used for wound healing experiments were born in a normal Mendelian distribution and exhibited a normal life span and no obvious defects. Mice double deficient for PHD2 and HIF1α in keratinocytes (CD68:cre-PHD2/HIF1αff/ff [conditional double knockout for PHD2 and HIF1α {cDKO1} mice]) started to die from week 5 after birth and as such were used only for the ex vivo experiments on primary keratinocytes (27). All animal experiments were in accordance with the facility guidelines on animal welfare and were approved by the Landesdirektion Dresden, Germany.
Skin wound healing.
Wound healing experiments were performed as previously described (28). Mice were anesthetized, dorsal hair was shaved, and the exposed skin was cleaned with 70% ethanol. Full-thickness excisional skin wounds were inflicted on either side of the dorsal middle line using a sterile 6-mm biopsy punch (Stiefel Laboratory, Bad Oldesloe, Germany). Four full-thickness dorsal wounds were made on each mouse, and healing was monitored by taking digital photographs at the indicated time points after removal of the remaining clot. Wound areas were defined and calculated using ImageJ software, version 1.44 (http://rsbweb.nih.gov/ij/). Wound closure was considered complete when the entire surface area was covered with tissue. For biochemical and histological analysis, animals were sacrificed by injection of an overdose of ketamine (Katanest; Pfizer, Berlin, Germany)-xylazine (Rompun Flakon; Bayer, Leverkusen, Germany). The wounds and the surrounding area were harvested and snap-frozen or embedded in a Tissue-Tec system (Sakura, Alphen aan den Rijn, Netherlands).
Cells.
Primary mouse keratinocytes and fibroblasts were isolated from newborn mice by incubating the skin with 250 mg/ml neutral protease (Dispase; Roche, Mannheim, Germany) overnight at 4°C. The epidermal layer was separated from the dermal layer and incubated in Accutase select enzyme (Sigma, St. Louis, MO) for 15 min at room temperature. The resulting single-cell suspension was cultured in CnT medium (Cell-n-Tech, Bern, Switzerland). glEND is an endothelial cell line known to constitutively express β3-integrin (A. Weidemann, J. Breyer, M. Rehm, K.-U. Eckardt, C. Daniel C, I. Cicha, K. Giehl, and M. Goppelt-Struebe, unpublished results). These cells were grown under standard cell culture conditions (Dulbecco modified Eagle medium, 10% bovine serum).
Ex vivo assays.
When the keratinocyte cultures were confluent, cells were removed from flasks via Accutase treatment and transferred to a 24-well plate (5 × 105 cells/well) supplied with a migration insert (Ibidi GmbH, Martinsried, Germany). After 12 h of cultivation, migration inserts were removed and migration of the cells was recorded by time-lapse video microscopy. For the αvβ3-integrin inhibition experiments, we added cyclo(Arg–Gly–Asp–d-Phe–Val) (RGDfV peptide; 0.01 ng/ml; Bachem, Bubendorf, Switzerland) at the time of keratinocyte seeding or just before the start of the migration experiment. Both conditions led to similar results, as described. For the TGFβ assay, keratinocytes were grown until they reached 70% confluence. Next, the medium was replaced with fresh CnT medium supplemented with TGFβ (11343161; 2 ng/ml; Immunotools) or vehicle. After 18 h of stimulation, cells were washed twice with phosphate-buffered saline (PBS) and collected, and RNA/proteins were isolated.
Time-lapse microscopy.
Migrating keratinocytes were imaged with a Zeiss Axiovert 200 M inverted microscope (Carl Zeiss Vision, Jena, Germany) equipped with a motorized stage and incubator, humidifier, and carbon dioxide supply to maintain cell culture conditions (Visitron Systems). Bright-field images were acquired using a ×4 or ×20 objective and recorded with a Roper Scientific CoolSNAP ES charge-coupled-device camera using MetaMorph software. In each experiment, multiple fields were imaged in parallel at 15-min intervals for up to 32 h.
Western blotting.
Cell lysis was performed in PBS containing 0.02 M Tris, 0.125 M NaCl, 1% Triton, and protease inhibitor cocktail (Roche, Mannheim, Germany). Proteins were separated by electrophoresis using 10% or 4 to 12% gradient gels (Life Technologies GmbH, Darmstadt, Germany). The following primary antibodies were used: rabbit anti-PHD2 (Novus Biologicals/Acris Antibodies GmbH, Herford, Germany), rabbit anti-HIF1α (Cayman Chemicals or Transduction Laboratories, Ann Arbor, MI), rabbit anti-HIF2α (R&D, Wiesbaden-Nordenstadt, Germany), rabbit anti-β-tubulin (Thermo Scientific, Waltham, MA), and rabbit anti-β-actin (Sigma-Aldrich, St. Louis, MO). All secondary antibodies were from Santa Cruz (Santa Cruz, CA).
Immunofluorescence.
For frozen sections, samples were embedded in OCT Tissue-Tek specimen matrix compound, cut, and stored at −20°C. For immunofluorescence staining, sections were first fixed for 10 min in cold acetone and blocked with 5% goat serum in TNT buffer (20 mM Tris, pH 7.6, 0.9% NaCl, 0.05% Tween in PBS; Sigma-Aldrich, St. Louis, MO). Thereafter, sections were incubated with the following primary antibodies either at 37°C for 1 h or at 4°C overnight: rat anti-PECAM, rat anti-CD11b (BD Pharmingen, Heidelberg, Germany), rabbit anti-keratin 6 (anti-K6; Covance, Munster Germany), rabbit anti-NG2 (Millipore, Schwalbach, Germany), rat anti-Ki67 (Dako, Hamburg, Germany), pSMAD2 (Millipore, Schwalbach, Germany), β3-integrin (Abcam, Cambridge, United Kingdom), and rabbit anti-HIF1α (Cayman Chemicals or Transduction Laboratories, Ann Arbor, MI). Secondary antibodies labeled with Alexa Fluor 488 or Alexa Fluor 594 (Molecular Probes/Life Technologies GmbH, Darmstadt, Germany) for 30 min at room temperature were subsequently used for visualization. Cell nuclei were stained with DAPI (4′,6-diamidino-2-phenylindole). Slides were mounted with fluorescent mounting medium (Dako, Hamburg, Germany). The numbers of CD11b-, K6-, and Ki67-positive (Ki67+) cells in 3 directly neighboring fields were counted. Microvessel density was determined by counting the cells in the PECAM-positive endothelial lining within the wound granulation tissue.
Microscopy.
Fluorescent images were acquired using an Axioplan-2 imaging microscope and plan Apochromat lenses (Carl Zeiss Mikroscopy, Jena, Germany). The cameras and acquisition software were from Axiocam MRc5. Confocal images were acquired using a Zeiss LSM 700 confocal system (Carl Zeiss Mikroscopy, Jena, Germany). Images were taken as either 1.2-μm (×40) or 0.9-μm (×63) single optical sections. Images taken as tile scans were stitched together using ZEN software (Carl Zeiss Mikroscopy, Jena, Germany). Image processing and analysis were done using ImageJ software and the ImageJ distribution package Fiji (http://pacific.mpi-cbg.de/wiki/index.php/Fiji). Z stacks of images were projected into one plane (maximum-intensity projection).
Expression analysis.
RNA was isolated from wounds or sorted cells using an RNeasy minikit (Qiagen, Hilden, Germany), TRIzol (Life Technologies GmbH, Darmstadt, Germany), or a universal RNA purification kit (Roboklon, Berlin, Germany). cDNA was synthesized using random primers (Roche, Mannheim, Germany) and SuperScript II reverse transcriptase (Invitrogen//Life Technologies GmbH, Darmstadt, Germany). Expression levels were determined by performing quantitative real-time PCR using Maxima SYBR green quantitative PCR (qPCR) master mix (Fermentas, St. Leon-Rot, Germany) on an iCycler iQ apparatus (Bio-Rad, Munich, Germany). Expression levels were normalized with the ΔΔCT (where CT is threshold cycle) method using the genes for MDM2 binding protein (mTBP) and elongation factor 2 (EF-2) as household reference genes. The sequences of the primers are available from the corresponding author.
Statistics.
Data and graphs represent means ± standard errors of the means (SEMs) of representative experiments. Statistical significance was calculated as two tailed (unless explicitly stated otherwise) by Student's t test (Prism software, v5.04; GraphPad Software, La Jolla, CA), with a P value of <0.05 considered statistically significant.
RESULTS
Characterization of three different conditionally PHD2-deficient mice.
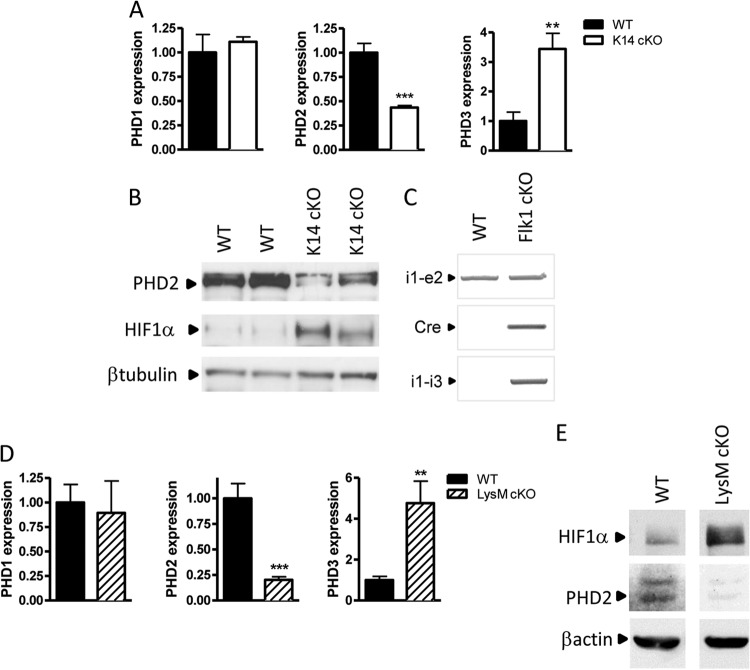
The direct impact of HIF prolyl hydroxylase 2 (PHD2) or HIFα stabilization on the individual phases of tissue repair is unexplored. As cutaneous wound healing is attributed to several cell types, we generated three strains with conditional knockout (cKO) of PHD2 in keratinocytes (K14:cre-PHD2f/f [K14 cKO cells]) (29), myeloid cells (LysM:cre-PHD2f/f [LysM cKO cells]) (30), and endothelial cells (Flk1:cre-PHD2f/f [Flk1 cKO cells]) (31) using a PHD2f/f mouse line developed by our research group (32). K14 cKO keratinocytes isolated from 1- to 2-day-old pups showed a significant reduction in the PHD2 transcript and a compensatory upregulation of PHD3 (Fig. 1A). Additionally, we found a clear loss of the PHD2 protein in keratinocyte cell lysates and the consequent stabilization of HIF1α even under normoxic conditions, which indicates that PHD3 induction is not sufficient to entirely reduce HIF1α levels (Fig. 1B). These mice were born in a normal Mendelian ratio, displayed normal erythrocyte counts (hematocrits) and hemoglobin values, and showed no abnormalities or differences in the vessel density in the skin, which is in clear contrast to the findings for the K14:cre-VHLf/f mice (33). Furthermore, keratinocyte differentiation markers (keratins 5 and 6 and loricrin) were detected in the epidermis of all of the mice (data not shown). In Flk1 cKO mice, we showed a high genomic PHD2 recombination efficiency in the aorta (Fig. 1C). Thioglycolate-elicited neutrophils from LysM cKO mice displayed a high knockout efficiency for PHD2 mRNA and a compensatory induction of PHD3 expression and HIF1α stabilization (Fig. 1D and E).
Fig 1.
Generation of three different mouse strains conditionally deficient in PHD2. (A) Keratinocytes from newborn K14:cre-PHD2f/f (K14 cKO) mice and their WT littermates were isolated, expanded, and tested for PHD1, -2, and -3. Apart from a significant reduction of PHD2, we found also a 3-fold induction of PHD3 (n = 4). (B) In addition, we detected a clear reduction of PHD2 at the protein level and a consequent HIF1α stabilization. (C) PCR of genomic DNA isolated from the aorta of Flk1:cre-PHD2f/f (Flk1 cKO) mice and their WT (PHD2f/f) littermates. In PHD2f/f mice, two loxP sites encompass exons 2 and 3. A positive PCR band with primers in intron 1 and exon 2 (i1-e2) represents the situation in which no recombination has taken place (WT situation). A positive PCR band obtained with primers in intron 1 and intron 3 (i1-i3) represents the situation after recombination. (D) qPCR of mRNA from thioglycolate-elicited polymorphonuclear leukocytes from LysM:cre-PHD2f/f (LysM cKO) mice and their WT littermates showed downregulation of PHD2 similar to that seen for K14 cKO mice (n = 4). (E) Bone marrow-derived macrophages of WT and LysM cKO mice were cultivated for 24 h under conditions of normoxia (21% O2) or hypoxia (1% O2), and cell lysates were immunoblotted for HIF1α and PHD2 using β-actin as a loading control. Results are given as means ± SEMs. **, P < 0.01; ***, P < 0.001.
Loss of PHD2 in keratinocytes accelerates wound healing.
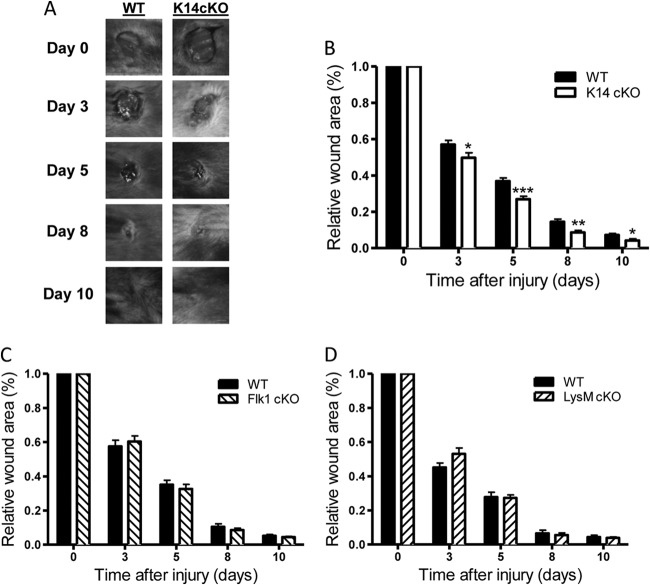
To analyze the kinetics of skin wound healing in these three conditionally PHD2-deficient mice, we induced 6-mm wounds in male mice from all three conditionally deficient strains and the respective wild-type (WT) littermates. All wounds from the conditionally deficient mice as well as the WT mice typically closed by day 11 or 12. Notably, we found that wound closure in K14 cKO mice was significantly faster than that in their WT littermates (Fig. 2A and B). This acceleration was observed from day 3 onwards and was sustained throughout the study. The differences in relative wound areas between K14 cKO and WT mice were −13% at day 3, −28% at day 5, −38% at day 8, and −40% at day 10. Interestingly, Flk1 cKO and LysM cKO mice showed no such continuous differences during the entire healing process compared to their WT controls (Fig. 2C and D). These results strongly suggest that PHD2 in keratinocytes is a negative regulator of skin wound healing.
Fig 2.
Loss of PHD2 in keratinocytes results in accelerated wound healing of the skin. (A) Representative images of cutaneous wounds from WT and K14 cKO mice at different time points throughout the healing process. (B to D) Relative wound area of WT and cKO mice measured immediately after wounding (day 0) and on days 3, 5, 8, and 10 for K14 cKO (B), Flk1 cKO (C), and LysM cKO (D) mice. The wound area is expressed as the percentage of wound closure for each genotype. The graphs represent data from at least two independent experiments (n = 12 to 32). Results are given as means ± SEMs. ***, P < 0.001; **, P < 0.01; *, P < 0.05.
Enhanced wound healing in cKO mice is associated with extended reepithelialization.
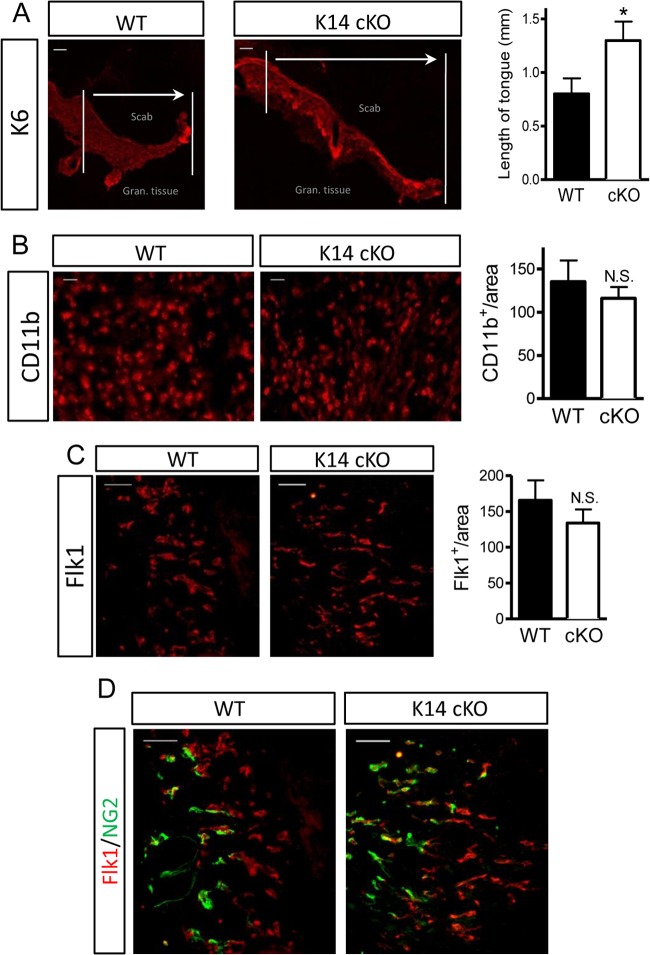
Although a significant difference in healing was observed as early as day 3, we concentrated our analysis on samples taken 5 days after initiation of the skin wound, as we observed the largest difference in wound size at this time point. To assess the progress of reepithelialization microscopically, we measured the length of the hyperproliferating keratinocyte tongue (K6 positive [K6+]). K14 cKO mice showed a significantly longer tongue than their WT littermates, which confirmed our macroscopic observations (Fig. 3A).
Fig 3.
Accelerated wound reepithelialization in cKO PHD2 mice. (A) Representative images of wound sections showing hyperproliferating keratinocytes (anti-keratin 6 [K6]) forming the tongue underneath the scab and above the granulation tissue (wound area). Vertical lines highlight the margins of the wound; arrows indicate the direction of keratinocyte migration and the length of the tongue. The hyperproliferative keratinocyte tongue is >60% longer in K14 cKO mouse wounds than WT control mouse wounds at day 5 after wounding (n = 7). (B) CD11b staining for myeloid cells in the granulation tissue shows no significant difference between the two genotypes (n = 8). (C) Granulation tissue stained for vascular endothelial growth factor receptor 2 (Flk1) shows actively proliferating endothelial cells, but the difference between the genotypes was not significant (n = 6). (D) Maturation/normalization of the new vessels (Flk1+ [red]) was ongoing in both genotypes, and costaining for pericytes (NG2+ [green]) revealed no difference at this stage. Data are given as means ± SEMs. *, P < 0.05 by Student's t test; N.S., not significant by Student's t test. Bars, 100 μm (A) and 50 μm (B to D).
To determine whether the loss of PHD2 in keratinocytes could also influence other phases of healing, we stained the wound sections for myeloid cells (CD11b) and angiogenic vessels (Flk1-positive [Flk1+] cells) in the wound bed but detected no significant differences in the total amount of cells per unit area (Fig. 3B and C). Maturation/normalization of these new vessels was ongoing, but costaining with pericytes (NG2-positive [NG2+] cells) revealed no difference at this stage (Fig. 3D). Staining for myofibroblasts (alpha smooth muscle actin) at this early point was negative for both genotypes (data not shown). These observations suggest that keratinocyte-specific PHD2 deficiency results in a cell-intrinsic effect during wound healing and does not alter other cellular compartments.
Loss of PHD2 enhances keratinocyte migration in an HIF1α-dependent manner.
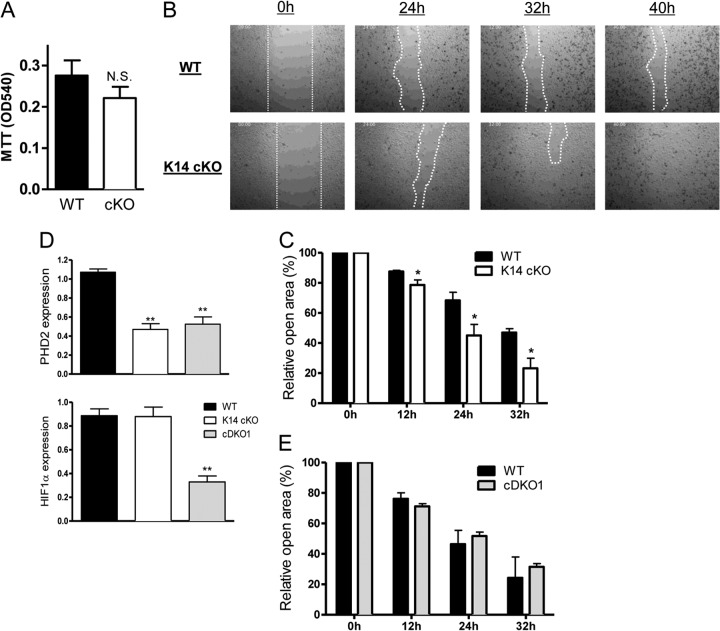
Given the longer epithelial tongue in wounds in K14 cKO mice, we analyzed the migration efficiency of primary keratinocytes isolated from the skin of newborn K14 cKO mice and their WT littermates. First, we defined the proliferation rate of these cells but could not find any obvious difference between the genotypes in this ex vivo setting (Fig. 4A). Next, we performed a modified ex vivo migration assay as described in Materials and Methods and found that K14 cKO keratinocytes displayed significantly faster closure of the wound, suggesting that enhanced healing of K14 cKO mouse wounds is at least in part dependent on faster migration (Fig. 4B and C).
Fig 4.
Loss of PHD2 induces migration of primary keratinocytes ex vivo. (A) Primary keratinocytes from newborn mice were isolated, and the growth rate was defined via the 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay. No significant difference was found between WT and K14 cKO cells (n = 3). OD540, optical density at 540 nm. (B) Representative pictures of primary keratinocytes isolated from K14 cKO and WT mice during ex vivo wound healing assays (modified scratch assay). (C) The remaining open area (in percent) at different time points after wound induction, as indicated in panel B. WT cells needed significantly more time to close. Although fluctuations in the final closing time between three independent experiments were apparent, the difference between the genotypes was always significant throughout the individual experiments (n = 5 to 6). (D) Keratinocytes from newborn K14:cre-PHD2f/f (K14 cKO) mice, CD68:cre-PHD2/HIF1αff/ff (cDKO1) mice, and their WT littermates were isolated, expanded, and tested for PHD2 and HIF1α expression using qPCR (n = 4). (E) cDKO1 cells, deficient for PHD2 and HIF1α, lost the ability to migrate faster than those of their WT littermates (n = 5). Results are given as means ± SEMs. *, P < 0.05; **P < 0.01; N.S., not significant.
HIF1α is the most important target of PHD2; in addition, it was recently suggested that knockdown of HIF1α in human keratinocytes inhibits their migration (34). In order to define the involvement of HIF1α in this phenotype, we used our recently described mice double deficient for PHD2 and HIF1α (conditional double knockout for PHD2 and HIF1α [cDKO1]) (27), isolated primary keratinocytes from newborns (Fig. 4D), and examined the extent of ex vivo wound closure in these cells. Interestingly, we found that the enhanced migration seen in PHD2-deficient (K14 cKO) cells was completely abolished in the double-deficient cells (cDKO1), suggesting that the migration effect is entirely HIF1α dependent (Fig. 4E).
β3-Integrin is expressed in PHD2-deficient keratinocytes.
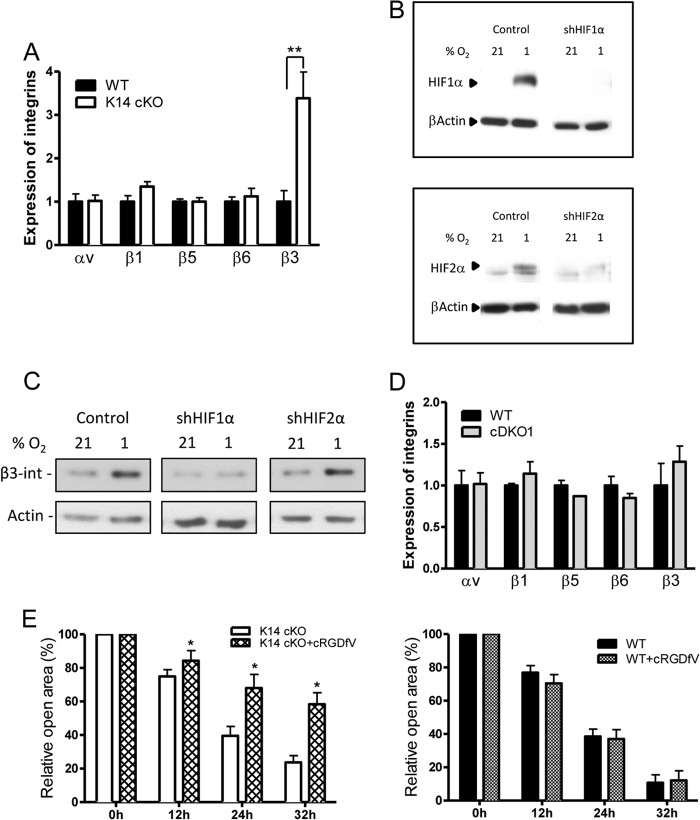
Several members of the αv-integrin family are upregulated in the epidermis and are known to be involved in wound healing (e.g., αvβ5-integrin and αvβ6-integrin) (6, 7). However, β3-integrin, although very important during this process, has been shown not to be expressed on wild-type keratinocytes (3, 4). However, we demonstrate that primary WT keratinocytes cultivated ex vivo can contain low levels of β3-integrin mRNA, whereas the expression in cells lacking PHD2 is significantly induced. Other integrins show no significant induction compared to WT cells, although β1-integrin has been shown before to be induced by HIF in fibroblasts (35) (Fig. 5A). β3-Integrin has been described before to be a protein which is induced by hypoxia on tumor cells (36). To assess whether β3-integrin induction is dependent on HIF1α or HIF2α, we used an endothelial cell line (glEND) constitutively expressing β3-integrin under normoxic and hypoxic conditions and transfected it with short-hairpin HIF1α (shHIF1α) or shHIF2α (Fig. 5B). Interestingly, the induction of β3-integrin after hypoxia was abolished only in the presence of shHIF1α and not in the presence of shHIF2α, implicating HIF1α as the central mediator (Fig. 5C). Moreover, in primary cDKO1 keratinocytes double deficient for PHD2 and HIF1α, β3-integrin expression was similar to that in control cells, emphasizing the direct role of HIF1α in the regulation of this particular integrin (Fig. 5D).
Fig 5.
β3-Integrin is induced by HIF1α. (A) qPCR experiments on primary keratinocytes of K14 cKO mice demonstrate that only β3-integrin is significantly induced compared to the proteins induced in WT cells (n = 5). (B) glEND cells were transfected with either shScrambled (control), shHIF1α (up), or shHIF2α (down) and grown under normoxic or hypoxic conditions for 24 h. Western blotting confirmed the downregulation of HIF1α or HIF2α expression. (C) A Western blot for β3-integrin clearly shows an HIF1α-dependent induction of the protein after hypoxic growth. (D) PHD2/HIF1α double-deficient keratinocytes showing no β3-integrin induction compared to that for their WT littermates (n = 5). (E) The influence of β3-integrin on the closure of the ex vivo wound was investigated using a specific RGD peptide that blocks αvβ3-integrin. Compared to K14 cKO keratinocytes, the closure of RGD-treated K14 cKO mouse wounds was significantly retarded (n = 5 to 6). On the other hand, WT cells treated with or without the inhibitor showed no significant difference (n = 4). Results are given as means ± SEMs. *, P < 0.05; **, P < 0.01.
Next, we wanted to verify if the HIF1α-driven migration of the keratinocytes ex vivo, which we observed before (Fig. 4B to E), is indeed β3-integrin dependent. Therefore, in an ex vivo wound healing experiment, we selectively inhibited αvβ3-integrin with an RGD peptide, cyclo(Arg–Gly–Asp–d-Phe–Val) (37), which resulted in significantly slower closure of wounds in K14 cKO mice than in mice with mock-treated K14 cKO keratinocytes (Fig. 5E). In addition, the wounds of WT mice treated with the same inhibitor closed as fast as those of their controls (Fig. 5E), further underscoring the finding that β3-integrin is responsible for the enhanced migration of K14 cKO keratinocytes.
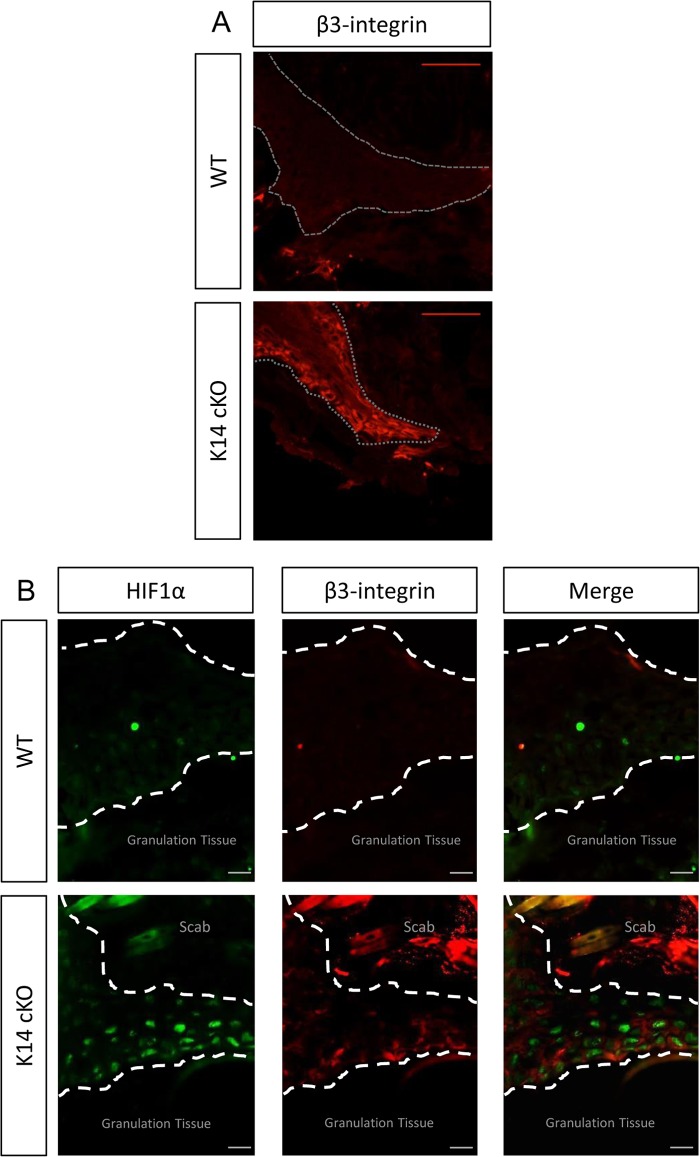
Finally, we performed immunohistochemistry (IHC) for β3-integrin on 5-day-old wounds and detected profound staining in the K14 cKO mouse wounds, especially on the keratinocytes forming the tip of the tongue (Fig. 6A). In addition and in clear contrast to the results for the WT littermates, PHD2-deficient epithelial cells located in the tip also showed strong nuclear HIF1α staining, which colocalized with β3-integrin staining (Fig. 6B).
Fig 6.
β3-Integrin expressed in the PHD2-deficient keratinocyte tongue. IHC of 5-day-old wounds showed enhanced β3-integrin expression in the tip of the tongue of K14 cKO mouse wounds (red) but not in WT mouse wounds (A) and colocalization of β3-integrin staining with high HIF1α nuclear staining (green) (B). Dotted line, keratinocyte tongue. Bars, 50 μm.
Taken together, loss of PHD2 in keratinocytes induces HIF1α stabilization and subsequent induction of β3-integrin in vivo and ex vivo, thus triggering the keratinocytes to migrate faster and invade the wounded tissue.
Induced keratinocyte proliferation in vivo is accompanied by reduced TGFβ activity.
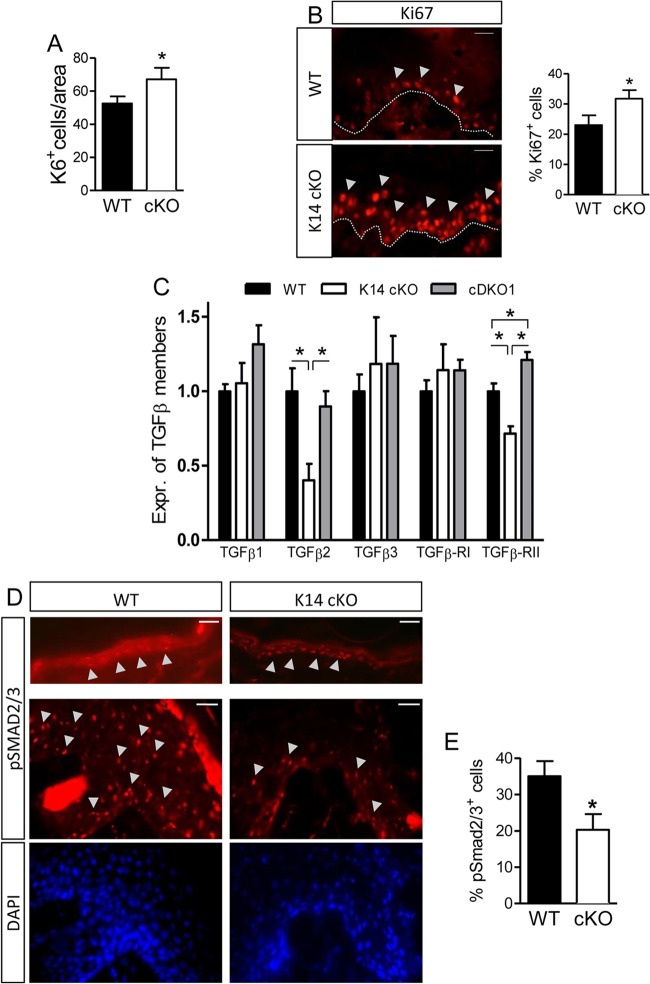
Given that the keratinocyte tongue is longer in the K14 cKO cells, we also checked for enhanced proliferation of these cells and indeed could demonstrate that there was a slight but significant increase in their absolute numbers (Fig. 7A). Furthermore, we stained wound sections for Ki67 and confirmed the induced proliferation (Fig. 7B), which is in contrast to the data obtained in the ex vivo keratinocyte cultures (Fig. 4A). Interestingly, Ki67+ cells in the K14 cKO mouse wounds were not only restricted to the single layer of the stratum basale, as in WT mice, but were also present in the next layer(s) of cells (Fig. 7B).
Fig 7.
Enhanced proliferation of keratinocytes in K14 cKO mouse wounds related to the TGFβ pathway. (A) The number of newly formed keratinocytes 5 days after wounding was assessed by quantification of K6+ cells (n = 5 to 8). (B) The proliferation marker Ki67 was stained and found to be expressed in the basal layer (stratum basale) of the keratinocyte tongue of both genotypes. In K14 cKO mice, Ki67+ cells extensively exceeded this layer, suggesting more proliferating cells (n = 7). (C) Isolated keratinocytes were tested for the levels of expression (Expr.) of several TGFβ family members via qPCR (n = 3 to 5). (D) IHC for pSMAD2/3 was performed on unwounded skin sections (top) and 5-day-old wounds (middle). (E) Quantification of pSMAD2/3-positive cells in wounds of both genotypes (n = 5 to 6). Results are given as means ± SEMs. *, P < 0.05. Bars, 25 μm (D, top) and 50 μm (D, middle and bottom).
To elucidate the molecular mechanism of the increased proliferation in vivo, we examined the role of the TGFβ family members, as they are known to inhibit proliferation of most cells in the skin, including keratinocytes (13, 38, 39). For this purpose, we first performed qPCR analysis on keratinocytes isolated from newborn mice and found a significant reduction in TGFβII and TGFβIIR mRNA in K14 cKO versus WT cells, which was abolished in cDKO1 cells (Fig. 7C).
These results therefore urged us to investigate the activation of the canonical TGFβ pathway in the wound sections by means of IHC for phosphorylated SMAD2/3 (pSMAD2/3) (40, 41). Although there was sustained pSMAD2/3 staining in the keratinocytes of unwounded skin of both genotypes, we observed that the epidermis of the wounds of K14 cKO mice contained significantly less pSMAD2/3-positive (pSMAD2/3+) keratinocytes than the epidermis of the wounds of their WT littermates (Fig. 7D and E). This suggests that TGFβ-mediated signaling is indeed reduced in epithelial cells deficient for PHD2 in these cells.
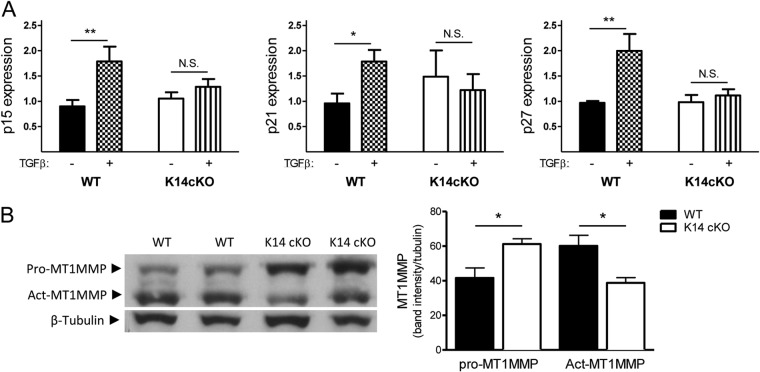
To confirm this, we studied the expression of the downstream cell cycle inhibitors p15INK4B, p21CIP1/WAF1, and p27KIP1 in keratinocytes. Interestingly, although we found no difference in the expression of these regulators in untreated cells from mice of both genotypes, TGFβ treatment significantly induced the transcription of all three inhibitors in WT cells but not in PHD2-deficient keratinocytes (Fig. 8A), suggesting that this effect might be regulated at least in part by the reduced TGFβIIR expression.
Fig 8.
The TGFβ pathway is less active in K14 cKO keratinocytes. (A) Isolated keratinocytes were treated with or without TGFβ for 18 h and subsequently tested via qPCR for the cell cycle inhibitors p15, p21, and p27. Only WT cells showed significant induction of the inhibitors after TGFβ stimulation (n = 4 to 9). (B) Western blotting of wound lysates from WT and cKO mice 5 days after wounding for inactive MT1MMP (pro-MT1MMP) and activated MT1MMP (Act-MT1MMP). cKO mouse wounds contained more inactive MT1MMP but significantly less of the active form than WT mouse wounds (n = 4). Results are given as means ± SEMs *, P < 0.05; **, P < 0.01; N.S., not significant.
In addition, latent TGFβ must be released extracellularly by proteases like the matrix metalloproteinases (MMPs) before it can exert its function (42, 43). Although we found no difference in the amounts of active gelatinases (MMP2 and MMP9) in wound lysates (data not shown), we observed that the 5-day-old wounds of K14 cKO mice contained significantly less active membrane type 1 metalloprotease (MT1MMP), which could be a direct link to reduced TGFβ signaling, in addition to diminished expression of TGF family members (Fig. 8B).
DISCUSSION
In the current study, we used a genetic approach to investigate in detail the biological role of PHD2 in keratinocytes and endothelial and myeloid cells during skin wound healing. We demonstrate that wounds in mice deficient for PHD2 only in the epidermis are able to heal faster due to enhanced migration and proliferation of the keratinocytes. In contrast, PHD2 knockout in myeloid or endothelial cells does not affect wound healing in our model. Our results strongly suggest that the enhanced migration phenotype in keratinocytes directly relates to the, until now, unknown induction of HIF1α-dependent β3-integrin, whereas the higher proliferation rate is directly related to the reduced activity of the canonical TGFβ pathway.
Following cutaneous injury, a well-orchestrated cascade of events is activated to restore tissue structure and homeostasis. Infiltration of inflammatory cells, reepithelialization, and vascular remodeling are the keystones of this process (44). In addition to being the main transcription factor involved in oxygen sensing, HIF1α has been observed in healing wounds and has been found to participate in tissue repair (21, 45, 46). Moreover, Rezvani and colleagues recently demonstrated that the loss of HIF1α in keratinocytes resulted in a substantial delay in wound healing in aged mice (34). However, the role of PHD2, the main HIF1α regulator, in skin repair has not been clearly elucidated.
We compared multiple parameters of wound healing in wild-type mice and mice lacking PHD2 in the endothelium, myeloid cells, or keratinocytes. Interestingly, only the genetic deletion of PHD2 in the keratinocytes resulted in a dramatic increase in reepithelialization without altering the inflammatory components in granulation tissue, neoangiogenesis, or collagen deposition in these wounds. On the other hand, in a study in which HIF1α was overexpressed in keratinocytes, the dermal vasculature was expanded, suggesting control of blood vessel growth by epidermal cells (47). The dissimilarity in phenotypes observed in our mice could come from the difference in the levels of HIF1α, since mice overexpressing HIF1α already display an increase in the number of dermal blood vessels during development.
During the reepithelialization phase of wound healing, keratinocytes undergo a dramatic phenotypic conversion to become migratory, hyperproliferative, and invasive. In order to understand the migration properties of the PHD2-deficient keratinocytes, we conducted ex vivo experiments with primary mouse cells isolated from the skin of these mice. Our results clearly demonstrate that loss of PHD2 leads to HIF1α-dependent accelerated closure of the induced wounds independently of proliferation. Although previous studies have shown that HIF1α can modulate the expression of β-integrin subunits (35, 48), we found no evidence of induction of the expression of the common keratinocyte-related integrins (αv-, β1-, β5-, or β6-integrin) in K14 cKO mouse wounds. However, in contrast to its expression in WT cells, β3-integrin was highly expressed in these cKO keratinocytes, and we show that β3-integrin is the direct mediator of enhanced migration of these epidermal cells. In line with this, we also detected a profound induction of β3-integrin expression on the migratory keratinocyte membrane, which was very obvious around the tip of the tongue of K14 cKO mouse wounds and colocalized with nuclear HIF1α staining. These findings are novel since it was shown before that the β3-integrin subunit is not expressed on keratinocytes in vitro or during wound healing in WT mice (3, 4). Moreover, our data strongly suggest that the induction of β3-integrin is HIF1α and not HIF2α driven, a finding that we confirmed using PHD2/HIF1α double-deficient keratinocytes and stable cell lines deficient for HIF1α or HIF2α. This result is in agreement with previously published data showing HIF-induced β3-integrin expression on trophoblast stem cells and melanoma cells (36). Moreover, we also found a potential hypoxia-responsive element (HRE) 15.1 kb upstream of the start codon of the mouse β3-integrin gene, consisting of an HIFα binding site (HBS) and an HIFα ancillary sequence (HAS) (Fig. 9A). The fact that β3-integrin in keratinocytes enhances migration during wound healing is, as such, not surprising, as wound healing actually shares many common pathways with tumor development (49, 50). Indeed, in cutaneous squamous cell carcinomas (51) or metastatic melanoma cells (52), the overexpression of αvβ3-integrin has been shown to support adhesion and invasive migration of tumor cells. Recently, β3-integrin has also been shown to be expressed on primary mammary epithelial cells, where they are similarly involved in cell migration but not proliferation (53), further substantiating our observations.
Fig 9.
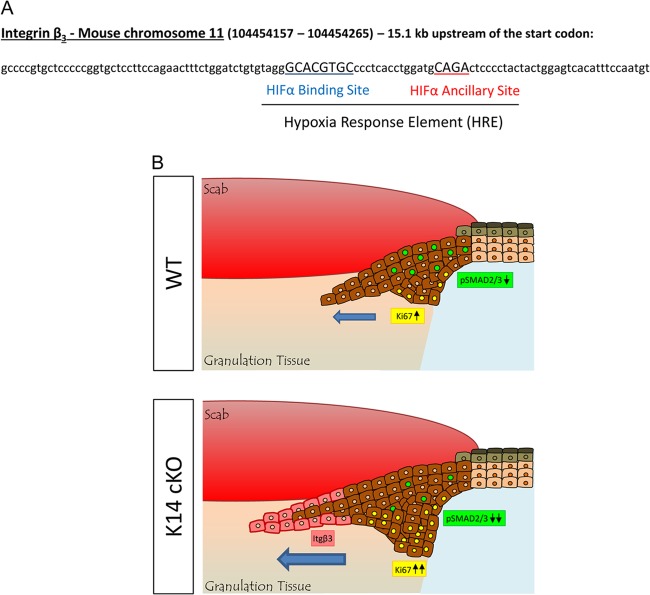
β3-Integrin expression in keratinocytes is HIF1α regulated and enhances wound healing. (A) Potential HRE for β3-integrin. Mouse β3-integrin is located on chromosome 11, which contains a potential HRE 15.1 kb upstream of the start codon, consisting of an HIFα binding site (HBS) and an HIFα ancillary sequence (HAS) in its promoter. (B) Model of enhanced reepithelialization of skin wounds in K14 cKO mice compared to that in their WT littermates, representing the β3-integrin (Itgβ3)-induced migration in the tip of the keratinocyte tongue (blue arrow, direction of migration) and reduced TGFβ activity, leading to more proliferation.
In the phase of reepithelialization, epidermal cells also start to proliferate behind the actively migrating cells (54). Indeed, our results also suggest augmented proliferation of the epidermis in K14 cKO mice due to the slightly higher number of activated K6+ keratinocytes. Moreover, we detected an increased amount of Ki67+ keratinocytes located behind the migrating tongue. These cells extended beyond the single layer of the stratum basale in the K14 cKO mouse epidermis. One of the major modulators of cell proliferation during skin tissue repair is the TGFβ family. Although its activity has been shown to induce proliferation of fibroblasts, contrarily, it is known to inhibit proliferation of keratinocytes (11). In line with this, we found that PHD2-deficient keratinocytes contain less TGFβII and TGFβIIR than WT cells. Furthermore, we detected significantly fewer pSMAD2/3+ cells in the keratinocyte tongue of 5-day-old wounds of K14 cKO mice, suggesting diminished TGFβ activity and induced proliferation. Our data are also in accordance with previous data from transgenic mice overexpressing a dominant negative TGFβIIR, which led to the loss of TGFβ signaling in keratinocytes and accelerated reepithelialization (12). Furthermore, since these differences were abolished or even reverted in cDKO1 cells, an HIF1α effect on the proliferation capacity of K14 cKO keratinocytes is strongly suggested. Our findings were also underscored by an ex vivo experiment where we stimulated keratinocytes with exogenous TGFβ. Interestingly, we found that only WT cells were able to induce the expression of three cell cycle inhibitors, suggesting that the cellular pathway is silenced and, therefore, proliferation is slower. One hypothesis is that HIF1α-related β3-integrin induction can act as an inhibitor on these particular TGFβ family members and influence downstream signaling. In that respect, the enhanced reepithelialization observed in β3-integrin-null mice has been shown to be associated with elevated TGFβIR and TGFβIIR expression in dermal fibroblasts, indicating that αvβ3-integrin can indeed suppress TGFβ-mediated signaling during wound healing (4). Moreover, trans-dominant inhibition of β3-integrin has also been described for other integrins (55) or growth factor receptors, such as Flk-1 (56).
The reason why the proliferation phenotype was seen only in the wounds and not in the ex vivo migration assay might be directly related to the fact that latent TGFβ must be mechanically or proteolytically released by active proteases like the matrix metalloproteinases (MMPs) (42, 43). In vivo, we observed that wounds of K14 cKO mice indeed contained significantly less active MT1MMP, which would add to the reduced TGFβ signaling and enhanced proliferation of keratinocytes.
In summary, we show that PHD2 in epidermal cells plays a central role in their reepithelialization during skin wound healing in mice. We further elucidate that loss of this oxygen sensor leads to enhanced keratinocyte migration, which is directly triggered by the induction of β3-integrin in an HIF1α-dependent manner. Furthermore, our data suggest that enhanced proliferation of K14 cKO keratinocytes is associated with reduced TGFβ activity (Fig. 9B). Thus, our findings shed new light on the molecular mechanisms during skin wound healing and offer new therapeutic opportunities for the use of specific PHD inhibitors in the treatment of cutaneous wounds.
ACKNOWLEDGMENTS
J.K., K.F., S.M., R.P.S., and A.M. are supported by the Emmy Noether program (the Deutsche Forschungsgemeinschaft [DFG], Germany). B.W. is an Emmy Noether fellow. This work was supported by grants (to B.W.) from the MeDDrive-Programm (TU Dresden, Germany) and DFG, Germany (WI 3291/1-1 and SPP 1190, The Tumor-Vessel Interface). A.W. is supported by a grant from the DFG (WE4275/3-1).
We thank the team of Roland Jung for excellent technical support, Vineeth Surendranath for HRE mapping, and Johannes Schödel and Vasuprada Iyengar for helpful discussions.
The work was performed as a collaborative project within the COST Action TD0901 HypoxiaNet.
We declare that we have no conflicts of interest.
Footnotes
Published ahead of print 24 June 2013
REFERENCES
- 1.Shaw TJ, Martin P. 2009. Wound repair at a glance. J. Cell Sci. 122:3209–3213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lowell CA, Mayadas TN. 2012. Overview: studying integrins in vivo. Methods Mol. Biol. 757:369–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gailit J, Welch MP, Clark RA. 1994. TGF-beta 1 stimulates expression of keratinocyte integrins during re-epithelialization of cutaneous wounds. J. Invest. Dermatol. 103:221–227 [DOI] [PubMed] [Google Scholar]
- 4.Reynolds LE, Conti FJ, Lucas M, Grose R, Robinson S, Stone M, Saunders G, Dickson C, Hynes RO, Lacy-Hulbert A, Hodivala-Dilke K. 2005. Accelerated re-epithelialization in beta3-integrin-deficient-mice is associated with enhanced TGF-beta1 signaling. Nat. Med. 11:167–174 [DOI] [PubMed] [Google Scholar]
- 5.Grose R, Hutter C, Bloch W, Thorey I, Watt FM, Fassler R, Brakebusch C, Werner S. 2002. A crucial role of beta 1 integrins for keratinocyte migration in vitro and during cutaneous wound repair. Development 129:2303–2315 [DOI] [PubMed] [Google Scholar]
- 6.Larjava H, Salo T, Haapasalmi K, Kramer RH, Heino J. 1993. Expression of integrins and basement membrane components by wound keratinocytes. J. Clin. Invest. 92:1425–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zambruno G, Marchisio PC, Marconi A, Vaschieri C, Melchiori A, Giannetti A, De Luca M. 1995. Transforming growth factor-beta 1 modulates beta 1 and beta 5 integrin receptors and induces the de novo expression of the alpha v beta 6 heterodimer in normal human keratinocytes: implications for wound healing. J. Cell Biol. 129:853–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shattil SJ. 1995. Function and regulation of the beta 3 integrins in hemostasis and vascular biology. Thromb. Haemost. 74:149–155 [PubMed] [Google Scholar]
- 9.Hynes RO. 2009. The extracellular matrix: not just pretty fibrils. Science 326:1216–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moustakas A, Heldin CH. 2009. The regulation of TGFbeta signal transduction. Development 136:3699–3714 [DOI] [PubMed] [Google Scholar]
- 11.Werner S, Grose R. 2003. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 83:835–870 [DOI] [PubMed] [Google Scholar]
- 12.Amendt C, Mann A, Schirmacher P, Blessing M. 2002. Resistance of keratinocytes to TGFbeta-mediated growth restriction and apoptosis induction accelerates re-epithelialization in skin wounds. J. Cell Sci. 115:2189–2198 [DOI] [PubMed] [Google Scholar]
- 13.Ashcroft GS, Yang X, Glick AB, Weinstein M, Letterio JL, Mizel DE, Anzano M, Greenwell-Wild T, Wahl SM, Deng C, Roberts AB. 1999. Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat. Cell Biol. 1:260–266 [DOI] [PubMed] [Google Scholar]
- 14.Lokmic Z, Musyoka J, Hewitson TD, Darby IA. 2012. Hypoxia and hypoxia signaling in tissue repair and fibrosis. Int. Rev. Cell Mol. Biol. 296:139–185 [DOI] [PubMed] [Google Scholar]
- 15.Bedogni B, Welford SM, Cassarino DS, Nickoloff BJ, Giaccia AJ, Powell MB. 2005. The hypoxic microenvironment of the skin contributes to Akt-mediated melanocyte transformation. Cancer Cell 8:443–454 [DOI] [PubMed] [Google Scholar]
- 16.Varghese MC, Balin AK, Carter DM, Caldwell D. 1986. Local environment of chronic wounds under synthetic dressings. Arch. Dermatol. 122:52–57 [PubMed] [Google Scholar]
- 17.Ninikoski J, Heughan C, Hunt TK. 1972. Oxygen tensions in human wounds. J. Surg. Res. 12:77–82 [DOI] [PubMed] [Google Scholar]
- 18.Stroka DM, Burkhardt T, Desbaillets I, Wenger RH, Neil DA, Bauer C, Gassmann M, Candinas D. 2001. HIF-1 is expressed in normoxic tissue and displays an organ-specific regulation under systemic hypoxia. FASEB J. 15:2445–2453 [DOI] [PubMed] [Google Scholar]
- 19.Rankin EB, Biju MP, Liu Q, Unger TL, Rha J, Johnson RS, Simon MC, Keith B, Haase VH. 2007. Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. J. Clin. Invest. 117:1068–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiesener MS, Jurgensen JS, Rosenberger C, Scholze CK, Horstrup JH, Warnecke C, Mandriota S, Bechmann I, Frei UA, Pugh CW, Ratcliffe PJ, Bachmann S, Maxwell PH, Eckardt KU. 2003. Widespread hypoxia-inducible expression of HIF-2alpha in distinct cell populations of different organs. FASEB J. 17:271–273 [DOI] [PubMed] [Google Scholar]
- 21.Rezvani HR, Ali N, Nissen LJ, Harfouche G, de Verneuil H, Taieb A, Mazurier F. 2011. HIF-1alpha in epidermis: oxygen sensing, cutaneous angiogenesis, cancer, and non-cancer disorders. J. Invest. Dermatol. 131:1793–1805 [DOI] [PubMed] [Google Scholar]
- 22.Kaelin WG, Jr, Ratcliffe PJ. 2008. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol. Cell 30:393–402 [DOI] [PubMed] [Google Scholar]
- 23.Maxwell PH, Ratcliffe PJ. 2002. Oxygen sensors and angiogenesis. Semin. Cell Dev. Biol. 13:29–37 [DOI] [PubMed] [Google Scholar]
- 24.Takeda K, Ho VC, Takeda H, Duan LJ, Nagy A, Fong GH. 2006. Placental but not heart defects are associated with elevated hypoxia-inducible factor alpha levels in mice lacking prolyl hydroxylase domain protein 2. Mol. Cell. Biol. 26:8336–8346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Botusan IR, Sunkari VG, Savu O, Catrina AI, Grunler J, Lindberg S, Pereira T, Yla-Herttuala S, Poellinger L, Brismar K, Catrina SB. 2008. Stabilization of HIF-1alpha is critical to improve wound healing in diabetic mice. Proc. Natl. Acad. Sci. U. S. A. 105:19426–19431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryan HE, Poloni M, McNulty W, Elson D, Gassmann M, Arbeit JM, Johnson RS. 2000. Hypoxia-inducible factor-1alpha is a positive factor in solid tumor growth. Cancer Res. 60:4010–4015 [PubMed] [Google Scholar]
- 27.Franke K, Kalucka J, Mamlouk S, Singh RP, Muschter A, Weidemann A, Iyengar V, Jahn S, Wieczorek K, Geiger K, Muders M, Sykes AM, Poitz DM, Ripich T, Otto T, Bergmann S, Breier G, Baretton G, Fong GH, Greaves DR, Bornstein S, Chavakis T, Fandrey J, Gassmann M, Wielockx B. 2013. HIF-1alpha is a protective factor in conditional PHD2-deficient mice suffering from severe HIF-2alpha-induced excessive erythropoiesis. Blood 121:1436–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hattori N, Mochizuki S, Kishi K, Nakajima T, Takaishi H, D'Armiento J, Okada Y. 2009. MMP-13 plays a role in keratinocyte migration, angiogenesis, and contraction in mouse skin wound healing. Am. J. Pathol. 175:533–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hafner M, Wenk J, Nenci A, Pasparakis M, Scharffetter-Kochanek K, Smyth N, Peters T, Kess D, Holtkotter O, Shephard P, Kudlow JE, Smola H, Haase I, Schippers A, Krieg T, Muller W. 2004. Keratin 14 Cre transgenic mice authenticate keratin 14 as an oocyte-expressed protein. Genesis 38:176–181 [DOI] [PubMed] [Google Scholar]
- 30.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. 1999. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 8:265–277 [DOI] [PubMed] [Google Scholar]
- 31.Licht AH, Raab S, Hofmann U, Breier G. 2004. Endothelium-specific Cre recombinase activity in flk-1-Cre transgenic mice. Dev. Dyn. 229:312–318 [DOI] [PubMed] [Google Scholar]
- 32.Singh RP, Franke K, Kalucka J, Mamlouk S, Muschter A, Gembarska A, Grinenko T, Willam C, Naumann R, Anastassiadis K, Stewart AF, Bornstein S, Chavakis T, Breier G, Waskow C, Wielockx B. 10 May 2013. HIF-prolyl hydroxylase 2 (PHD2) is a critical regulator of hematopoietic stem cell maintenance during steady-state and stress. Blood [Epub ahead of print.] 10.1182/blood-2012-12-471185 [DOI] [PubMed] [Google Scholar]
- 33.Boutin AT, Weidemann A, Fu Z, Mesropian L, Gradin K, Jamora C, Wiesener M, Eckardt KU, Koch CJ, Ellies LG, Haddad G, Haase VH, Simon MC, Poellinger L, Powell FL, Johnson RS. 2008. Epidermal sensing of oxygen is essential for systemic hypoxic response. Cell 133:223–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rezvani HR, Ali N, Serrano-Sanchez M, Dubus P, Varon C, Ged C, Pain C, Cario-Andre M, Seneschal J, Taieb A, de Verneuil H, Mazurier F. 2011. Loss of epidermal hypoxia-inducible factor-1alpha accelerates epidermal aging and affects re-epithelialization in human and mouse. J. Cell Sci. 124:4172–4183 [DOI] [PubMed] [Google Scholar]
- 35.Keely S, Glover LE, MacManus CF, Campbell EL, Scully MM, Furuta GT, Colgan SP. 2009. Selective induction of integrin beta1 by hypoxia-inducible factor: implications for wound healing. FASEB J. 23:1338–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cowden Dahl KD, Robertson SE, Weaver VM, Simon MC. 2005. Hypoxia-inducible factor regulates alphavbeta3 integrin cell surface expression. Mol. Biol. Cell 16:1901–1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brooks PC, Montgomery AM, Rosenfeld M, Reisfeld RA, Hu T, Klier G, Cheresh DA. 1994. Integrin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell 79:1157–1164 [DOI] [PubMed] [Google Scholar]
- 38.Amendt C, Schirmacher P, Weber H, Blessing M. 1998. Expression of a dominant negative type II TGF-beta receptor in mouse skin results in an increase in carcinoma incidence and an acceleration of carcinoma development. Oncogene 17:25–34 [DOI] [PubMed] [Google Scholar]
- 39.Frank S, Madlener M, Werner S. 1996. Transforming growth factors beta1, beta2, and beta3 and their receptors are differentially regulated during normal and impaired wound healing. J. Biol. Chem. 271:10188–10193 [DOI] [PubMed] [Google Scholar]
- 40.Faler BJ, Macsata RA, Plummer D, Mishra L, Sidawy AN. 2006. Transforming growth factor-beta and wound healing. Perspect. Vasc. Surg. Endovasc. Ther. 18:55–62 [DOI] [PubMed] [Google Scholar]
- 41.Rahimi RA, Leof EB. 2007. TGF-beta signaling: a tale of two responses. J. Cell. Biochem. 102:593–608 [DOI] [PubMed] [Google Scholar]
- 42.Tatti O, Vehvilainen P, Lehti K, Keski-Oja J. 2008. MT1-MMP releases latent TGF-beta1 from endothelial cell extracellular matrix via proteolytic processing of LTBP-1. Exp. Cell Res. 314:2501–2514 [DOI] [PubMed] [Google Scholar]
- 43.Wang M, Zhao D, Spinetti G, Zhang J, Jiang LQ, Pintus G, Monticone R, Lakatta EG. 2006. Matrix metalloproteinase 2 activation of transforming growth factor-beta1 (TGF-beta1) and TGF-beta1-type II receptor signaling within the aged arterial wall. Arterioscler. Thromb. Vasc. Biol. 26:1503–1509 [DOI] [PubMed] [Google Scholar]
- 44.Singer AJ, Clark RA. 1999. Cutaneous wound healing. N. Engl. J. Med. 341:738–746 [DOI] [PubMed] [Google Scholar]
- 45.Biswas S, Roy S, Banerjee J, Hussain SR, Khanna S, Meenakshisundaram G, Kuppusamy P, Friedman A, Sen CK. 2010. Hypoxia inducible microRNA 210 attenuates keratinocyte proliferation and impairs closure in a murine model of ischemic wounds. Proc. Natl. Acad. Sci. U. S. A. 107:6976–6981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elson DA, Ryan HE, Snow JW, Johnson R, Arbeit JM. 2000. Coordinate up-regulation of hypoxia inducible factor (HIF)-1alpha and HIF-1 target genes during multi-stage epidermal carcinogenesis and wound healing. Cancer Res. 60:6189–6195 [PubMed] [Google Scholar]
- 47.Elson DA, Thurston G, Huang LE, Ginzinger DG, McDonald DM, Johnson RS, Arbeit JM. 2001. Induction of hypervascularity without leakage or inflammation in transgenic mice overexpressing hypoxia-inducible factor-1alpha. Genes Dev. 15:2520–2532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kong T, Eltzschig HK, Karhausen J, Colgan SP, Shelley CS. 2004. Leukocyte adhesion during hypoxia is mediated by HIF-1-dependent induction of beta2 integrin gene expression. Proc. Natl. Acad. Sci. U. S. A. 101:10440–10445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dvorak HF. 1986. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N. Engl. J. Med. 315:1650–1659 [DOI] [PubMed] [Google Scholar]
- 50.Schäfer M, Werner S. 2008. Cancer as an overhealing wound: an old hypothesis revisited. Nat. Rev. Mol. Cell Biol. 9:628–638 [DOI] [PubMed] [Google Scholar]
- 51.Kremser ME, Przybylo M, Hoja-Lukowicz D, Pochec E, Amoresano A, Carpentieri A, Bubka M, Litynska A. 2008. Characterisation of alpha3beta1 and alpha (v) beta3 integrin N-oligosaccharides in metastatic melanoma WM9 and WM239 cell lines. Biochim. Biophys. Acta 1780:1421–1431 [DOI] [PubMed] [Google Scholar]
- 52.Hudson LG, Gale JM, Padilla RS, Pickett G, Alexander BE, Wang J, Kusewitt DF. 2010. Microarray analysis of cutaneous squamous cell carcinomas reveals enhanced expression of epidermal differentiation complex genes. Mol. Carcinog. 49:619–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jeanes AI, Wang P, Moreno-Layseca P, Paul N, Cheung J, Tsang R, Akhtar N, Foster FF, Brennan K, Streuli CH. 2012. Specific beta-containing integrins exert differential control on proliferation and 2D collective cell migration in mammary epithelial cells. J. Biol. Chem. 287:24103–24112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arwert EN, Hoste E, Watt FM. 2012. Epithelial stem cells, wound healing and cancer. Nat. Rev. Cancer 12:170–180 [DOI] [PubMed] [Google Scholar]
- 55.Diaz-Gonzalez F, Forsyth J, Steiner B, Ginsberg MH. 1996. Trans-dominant inhibition of integrin function. Mol. Biol. Cell 7:1939–1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reynolds LE, Wyder L, Lively JC, Taverna D, Robinson SD, Huang X, Sheppard D, Hynes RO, Hodivala-Dilke KM. 2002. Enhanced pathological angiogenesis in mice lacking beta3 integrin or beta3 and beta5 integrins. Nat. Med. 8:27–34 [DOI] [PubMed] [Google Scholar]