Abstract
The stomach is a target organ of the incretin hormone glucagon-like peptide-1 (GLP-1). However, the cellular expression and glandular distribution of its receptor (GLP-1R) in human gastric mucosa are not known. We determined the expression of GLP-1R in different regions of human stomach mucosa and its specific cellular association and distribution within gastric glands. Tissue samples from stomach body and antrum were obtained from 20 patients during routine esophagogastroduodenoscopy. mRNA encoding GLP-1R protein expression was evaluated by RT-PCR. Determination of cell types bearing GLP-1R, their localization, and their frequency in gastric glands in different gastric regions were estimated by immunohistochemical morphological analysis. Levels of GLP-1R mRNA were similar in body and antrum. GLP-1R immunoreactivity was found throughout the gastric mucosa in various types of glandular cells. The highest frequency of GLP-1R immunoreactive cells was found in the neck area of the principal glands in cells morphologically identified as parietal cells. GLP-1R immunostaining was also found on enteroendocrine-like cells in the pyloric glands. This study provides the first description of GLP-1R expression in human gastric glands and its specific cellular association. Our data suggest that GLP-1 may act directly on the gastric mucosa to modulate its complex functions.
Keywords: human gastric mucosa, gastric glands, GLP-1R immunoreactive cells
Introduction
The stomach is one of the major target organs of the gut hormone glucagon-like peptide-1 (GLP-1). Following food intake, this incretin hormone secreted by the small intestine endocrine cells regulates gastric motility and acid secretion (Näslund et al. 1999; Marathe et al. 2011). The activities of GLP-1 are mediated by a specific receptor, named GLP-1R. Most of the available data regarding expression of GLP-1R in the stomach are based on animal studies. GLP-1R was identified in the rat stomach by radioligand binding, RNase protection assay, RT-PCR, and in situ hybridization (Uttenthal and Blázquez 1990; Bullock and Habener 1996; Dunphy, Taylor, and Fuller 1998), and its sequences corresponded to the cloned islet GLP-1 receptor (Bullock and Habener 1996). Moreover, the receptor for GLP-1-(7-36) amide was detected in acid-secreting parietal cells in the rat stomach by northern blot, cross-linking, and radioligand binding assays (Schmidtler et al. 1994). Binding of GLP-1R to GLP-1 protein apparently mediated the direct effect of GLP-1 on rat parietal cell function, stimulating cAMP-dependent H+ production by these cells (Schmidtler et al. 1991). Very little data regarding GLP-1R expression in the human stomach are available. RNase protection assays demonstrated the presence of a pancreatic form of GLP-1R in tissue from the human stomach (Wei and Mojsov 1995, 1996), and GLP-1 binding sites have been identified on HGT-1 human gastric cancer cells (Hansen et al. 1988). These cells display the principal features of parietal cells (Carmosino et al. 2000). However, direct evidence demonstrating the presence of GLP-1R in human gastric mucosa is still lacking. In addition, the exact localization of GLP-1R within the gastric mucosa, its association with specific cell types of the gastric glands, and its distribution in the various gastric regions are not known. All these parameters may have a profound effect on gastric function in health and disease states. Therefore, we undertook to analyze the expression and distribution of GLP-1R in gastric mucosa of human patients. Apart from providing novel data, this analysis constitutes the first step before the role of this receptor in a disease state such as type 2 diabetes can be studied.
Materials & Methods
Patients
Twenty patients (50% females; mean age = 48.5 ± 16.94 years, range = 24–71 years), who were referred to the Institute of Gastroenterology at Assaf Harofeh Medical Center for routine esophagogastroduodenoscopy for nonspecific dyspeptic complaints or iron deficiency anemia between December 2008 and June 2010, were enrolled. All patients signed an informed consent and the study was approved by the local Helsinki committee (certificate no. 103/08*1, approved initiation date February 25, 2008). Four mucosal biopsies were taken from the body and two from the antrum regions of each patient and kept at −70C or immersed in formaldehyde for mRNA or immunohistochemical analysis, respectively.
Real-Time qPCR
Quantitative rather than qualitative PCR analysis was chosen in order to evaluate differences in GLP-1R mRNA expression in different stomach regions. Total RNA was extracted using the ZR RNA MicroPrep kit (Zymo Research; Irvine, CA) and cDNA was synthesized using the Verso cDNA synthesis kit (ABgene; Epsom, UK), both according to the manufacturer’s protocol. The primers used to amplify the GLP-1R gene were obtained from OriGene Technologies (Rockville, MD). The primers for GAPDH gene amplification were designed by Light Cycler Probe Design Software (Roche Applied Science; Mannheim, Germany) according to sequences found in the NCBI and Ensembl databases and synthesized by Integrated DNA Technologies (Coralville, IA). All primer sequences are listed in Table 1.
Table 1.
Primer Sequences for the Human GLP-1R and Housekeeping Gene.
| Gene Name | Forward Sequence | Reverse Sequence |
|---|---|---|
| Human GLP-1R | CTA CGC ACT CTC CTT CTC TGC T | CGG ACA ATG CTC GCA GGA TGA A |
| Human GAPDH | TCG GAG TCA ACG GAT TT | CCA CGA CGT ACT CAG C |
Abbreviation: GLP-1R, glucagon-like peptide-1 receptor.
Quantitative real-time PCR analysis was performed using a Rotor-Gene (Corbett Life Science; Sydney, Australia) thermocycler and SYBR FAST Universal Master Mix (Kapa Biosystems; Cape Town, South Africa). GLP-1R mRNA levels were normalized to the GAPDH mRNA level and expressed as relative units.
Histological and Immunohistochemical Assays
Gastric mucosa biopsies were fixed in 10% neutral-buffered formalin and routinely processed. Paraffin wax-embedded sections were cut into 3- to 4-µm-thick serial sections. Hematoxylin and eosin (H&E) staining was performed for routine microscopic examination of each specimen. Primary monoclonal mouse anti-human whole GLP-1R antibodies (catalog no. MAb28141; R&D Systems, Minneapolis, MN) were used for immunohistochemical assays. This monoclonal antibody (mouse IgG2A) was produced from a hybridoma resulting from the fusion of a mouse myeloma with B cells obtained from a mouse immunized with NSO cells transfected with whole human GLP-1R (accession no. P43220). The IgG fraction of the tissue culture supernatant was purified by Protein G affinity chromotography. The specificity of this antibody was verified by its ability to stain GLP-1R-transfected CHO cells but not irrelevant transfectants, that is, positive and negative controls, respectively. Flow cytometry data demonstrate that this anti-GLP-1R antibody specifically recognizes GLP-1R in 96.7% of GLP-1R-transfected BAF/3 cells compared with isotype control antibodies (0.4%) and irrelevant BAF/3 CXCR6 transfectants (2.1%) (R&D System data—not shown). A concentration range of 3 to 8 μg/ml of this antibody, together with appropriate secondary reagents, was used for immunostaining of paraffin-embedded normal hippocampus human tissue sections (R&D Systems). Immunohistochemical staining of all mucosal specimens was performed using the Cell and Tissue Staining Mouse Kit HRP-DAB System (catalog no. CTS002; R&D Systems) based on the formation of avidin-biotin complex with primary IgG antibodies and recommended by R&D Systems for chromogenic detection of the GLP-1R labeling. Briefly, after deparaffinization and hydration of paraffin-embedded mucosal sections, the specimens were blocked with peroxidase blocking reagent (Part 865005) for 10 min, serum blocking reagent (Part 865004) for 15 min, avidin blocking reagent (Part 865009) for 15 min, and biotin blocking reagent (Part 865008) for 15 min. After washing, monoclonal primary mouse anti-human GLP-1R antibodies were diluted (3 μg/ml) in Dako antibody diluent (Dako; Carpinteria, CA), added to the specimens, and incubated overnight in a Petri dish wet chamber at 4C. Specimens were then incubated with secondary anti-mouse biotinylated antibodies (Part 865001) for 45 min at room temperature followed by incubation with high sensitivity streptavidin conjugates to horseradish peroxidase (HSS-HRP) (Part 865006) for 30 min. Visualization of enzymatic conversion of 3,3’-diaminobenzidine (DAB) chromogen substrate (Part 860001) into colored brown precipitate by HRP was performed under a microscope monitor. The stain development was stopped after 4 min by rinsing and washing. Mayers Haematoxylin (Pioneer Research Chemicals; Colchester, UK) was used for nuclear staining of the samples. After washing, dehydration and mounting procedures were performed. Sections immunostained without the primary GLP-1R antibodies were used as negative controls.
GLP-1R positive tissue controls were: normal stomach mucosa from a patient subjected to routine gastroscopy due to iron deficiency anemia with no clinical complaints and/or any macroscopic and microscopic gastric pathology and normal human pancreas (LEM Laboratory; Nes Ziona, Israel). GLP-1R negative tissue controls were: liver (ab4348; Abcam, Cambridge, UK), and adrenal gland (ab4282, Abcam) human specimens (Supplement 1, available at http://jhc.sagepub.com/supplemental).
Histological features of mucosal specimens were evaluated by microscopic observation of hematoxylin- and eosin-stained sections (Fig. 1A and 1F). The Sydney System method (Dixon et al. 1996) for standardized reporting of gastric biopsies was used. The following histological features, including the degree of active inflammation, chronic inflammation, atrophy, and intestinal and/or pyloric metaplasia and Helicobacter pylori density, were scored on a scale of 0 to 3 (0 = none, 1 = mild, 2 = moderate, and 3 = marked/severe). Semi-quantitative analysis of GLP-1R-immunostained cell intensity was carried out in high power fields of an Olympus BX51TF-5 microscope. Intensity of GLP-1R immunostaining was estimated by a progressive scale: 0 = negative staining (background level), 1 = mild, 2 = moderate, 3 = marked, and 4 = strong staining. In order to rule out the possible confounding effect of chronic gastritis (gastric inflammation, metaplasia, and atrophy) as well as the presence of H. pylori, we performed our analysis only in structurally intact areas of stomach mucosa devoid of these pathological features. Quantitative analysis was performed by counting the GLP-1R-immunoreactive and -non-immunoreactive glandular cells using Mac Biophotonic ImageJ (a Java program inspired by the National Institute of Mental Health, Bethesda, MD) in well-oriented, high-quality transversal cuts of principal glands (body) and pyloric glands (antrum). GLP-1R-immunoreactive cells in principal glands were counted separately in neck, mid-, and bottom gland regions. A minimum of 15 transversal cuts of principal glands (mean = 40 ± 17.8, range = 15–71) per patient and 10 cuts of pyloric glands (mean = 24 ± 11.5, range = 10–50) per patient was analyzed. Mean total glandular cell number counted per patient was 391 ± 143.8 (range = 177–633) and 328 ± 134.2 (range = 117–657) in body and antral mucosa, respectively. Results are expressed as a mean percentage of GLP-1R-immunoreactive cells among the total glandular cell number in each region of the stomach and in each glandular area. All histological specimens were examined by the same investigator in a blinded manner without reference to the clinical histories.
Figure 1.
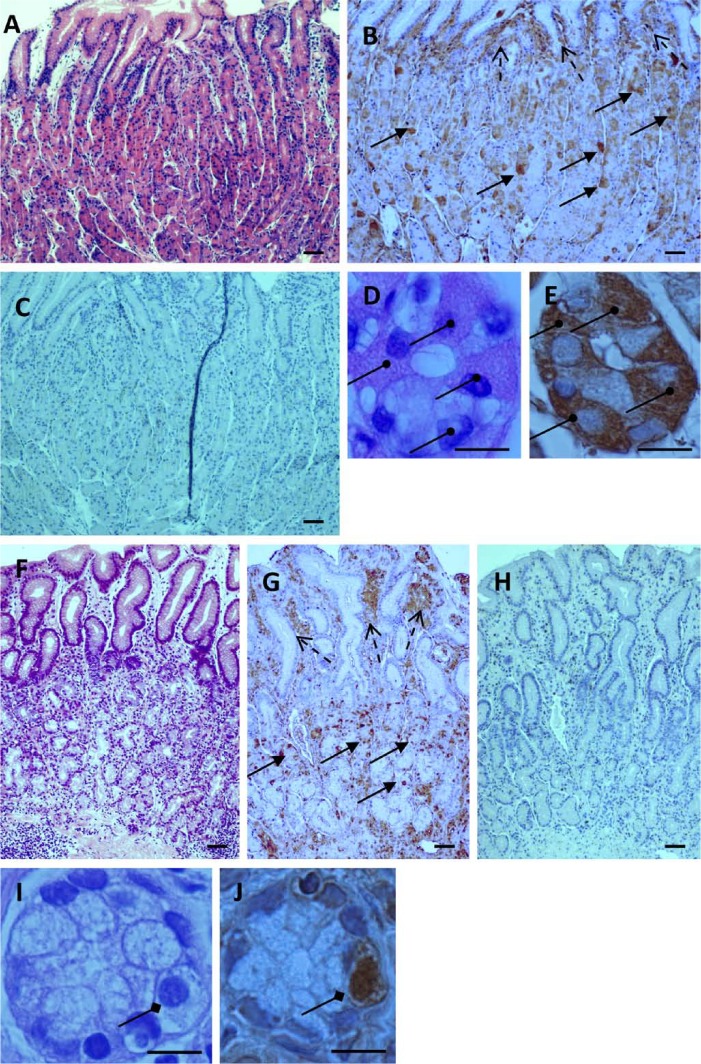
Glandular localization of glucagon-like peptide-1 receptor (GLP-1R)-immunoreactive cells in stomach mucosa. (A, F) Longitudinal gastric mucosa sections stained with hematoxylin and eosin (H&E) from body (A) and antrum (F) regions. (B, G) GLP-1R-immunostained sections of the same body (B) and antrum (G) regions. GLP-1R immunoreactive cells are visible in principal glands of body and pyloric glands of antrum (solid arrows) and in extra-glandular lamina propria (broken arrows). (C, H) Negative control immunostained sections of the same body (C) and antrum (H) regions, without primary antibody. (D, E, I, J) Transversal cuts of principal glands of body (D, E) and pyloric glands of antrum (I, J) (magnification × 1000). Parietal cells are clearly visible in H&E-stained (D) and GLP-1R immunostained (E) principal glands (round head arrows). Enteroendocrine-like cells are visible in H&E-stained (I) and GLP-1R-immunostained (J) pyloric gland sections (rhombus head arrows). Bars A–C, F–H = 100 µm; bars D, E, I, J = 10 µm.
Statistical Analysis
The data were analyzed by paired Student’s t-test. A p value ≤ 0.05 was considered significant.
Results
General Histomorphological Features of Gastric Mucosal Biopsies
Hematoxylin- and eosin-stained gastric mucosal biopsies revealed that 17 of 20 patients (85%) demonstrated histological features of chronic gastritis. Mild and moderate active chronic inflammation was found in 65% and 35% of patients, respectively. H. pylori infection was detected in 12 of 17 patients (71%), erosive areas of mucosa were found in 11 of 17 patients (65%), and areas of mucosa with ulcerations in 3 of 17 (18%). Mild atrophy was detected in the majority of the patients in the antrum and body at 75% and 70% of patients, respectively. A moderate atrophy was observed in the antrum and body of 6% and 12% of patients, respectively, and 18% of the patients had severe antral and corpus atrophy. Focal intestinal metaplasia in the antrum and body was found in 2 of 17 patients (12%). These data indicate that the majority of the study patients suffered from chronic gastritis most probably due to H. pylori infection.
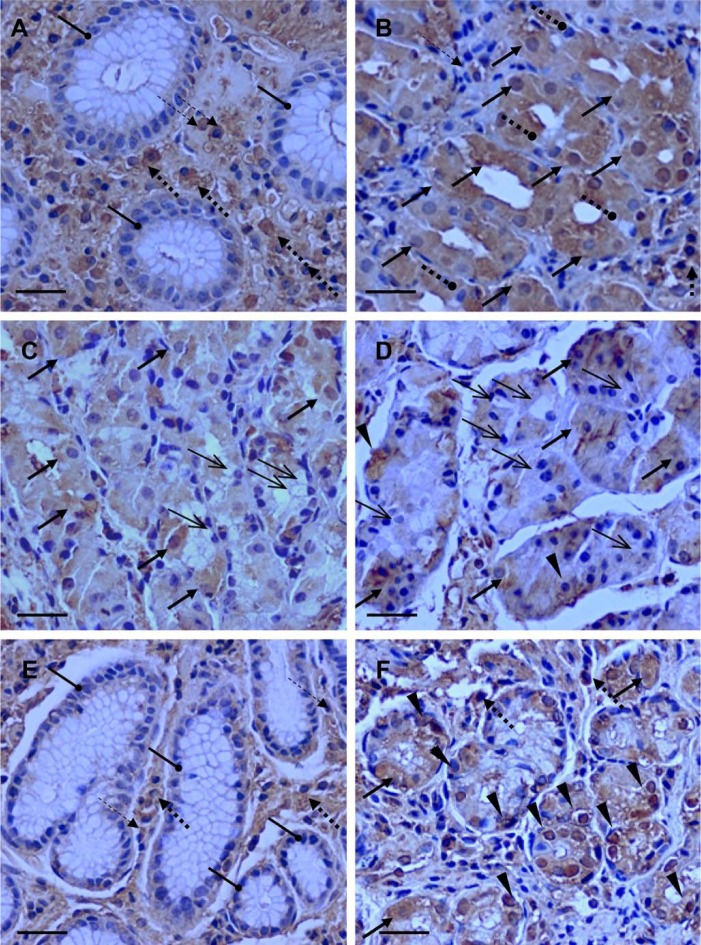
GLP-1R is expressed in glandular and extra-glandular cells of the gastric mucosa. Analysis of longitudinal histological sections demonstrated that GLP-1R-bearing cells are detected in glandular and extra-glandular areas of the stomach body and antrum (Figs. 1B and 1G). Positive GLP-1R immunostaining was observed in several cellular compartments including the cell membrane, cytoplasm, and nucleus. Transversal sections showed no expression of GLP-1R by mucus-producing cells, which formed the superficial layer of the mucosa (Goldstein, Brothers, and Davis 1969), in both the stomach body and antrum (Figs. 2A and 2E). The GLP-1R-immunoreactive cells were detectable under the superficial layer in all areas of principal glands located in the stomach body (Figs. 2B, 2C, and 2D) and in antrum pyloric glands (Fig. 2F). GLP-1R immunoreactivity was not restricted to cells within the gastric glands but was also detectable in various extra-glandular cells such as lymphocytes and plasma cells, but not in polymorphonuclear leukocytes within the loose connective tissue lamina propria (Figs. 2A, 2B, 2E, and 2F). Thus, GLP-1R is expressed on different glandular and extra-glandular cell types of human gastric mucosa.
Figure 2.
Distribution of glucagon-like peptide-1 receptor (GLP-1R)-immunoreactive cells in gastric mucosa. Transversal GLP-1R-immunostained mucosal sections of the body (A, B, C, D) and antrum (E, F) of the stomach. (A, E) Mucus-secreting cells in superficial layer of mucosa (roundhead arrows) in the stomach body (A) and antrum (E) demonstrate no GLP-1R immunoreactivity. Extra-glandular lymphocytes (thin broken arrows) and plasma cells (thick broken arrows) in the lamina propria demonstrate GLP-1R immunoreactivity (A, E, F). Most GLP-1R-immunoreactive glandular parietal cells (thick solid arrows) are detectable in the neck area of principal glands (stomach body) where these acid-secreting cells are predominant. (B) Mucus-secreting neck cells (roundhead broken arrows), which are localized in this area, do not demonstrate GLP-1R immunoreactivity. A lesser frequency of GLP-1R-immunoreactive cells (thick solid arrows) is detectable in the mid (C) and bottom (D) areas. Chief cells (thin solid arrows) located in the mid and bottom areas of the principal glands do not demonstrate GLP-1R immunoreactivity (C, D). GLP-1R-immunoreactive, predominantly enteroendocrine-like cells (arrow heads) and few parietal cells (thick solid arrows) and extra-glandular plasma cells (thick broken arrows) are detectable in pyloric glands of antrum (F). Bars = 20 µm.
Quantitative Analysis of GLP-1R Expression in Different Gastric Mucosa Regions
Two different methodologies were used to determine the expression and distribution of GLP-1R in the gastric mucosa, namely, mRNA levels and immunohistochemical analysis. As shown in Fig. 3A, GLP-1R mRNA was detected in the body and antrum of all patients. Mean levels of GLP-1R mRNA were comparable in the body and antrum regions of the stomach. To further estimate the presence and distribution of this receptor in the gastric mucosa, we employed a semi-quantitative as well as a quantitative immunohistochemical morphological analysis. As shown in Fig. 3B, the intensity of GLP-1R staining in glandular cells was slightly reduced in the antrum region as compared to the stomach body. However, this difference was not statistically significant. Quantitative analysis shown in Fig. 3C demonstrates that the mean frequency of GLP-1R-positive cells among all glandular cells was similar in both principal (stomach body) and pyloric (antrum) glands. These results demonstrate that GLP-1R is equally expressed in the body and antral regions of gastric mucosa.
Figure 3.
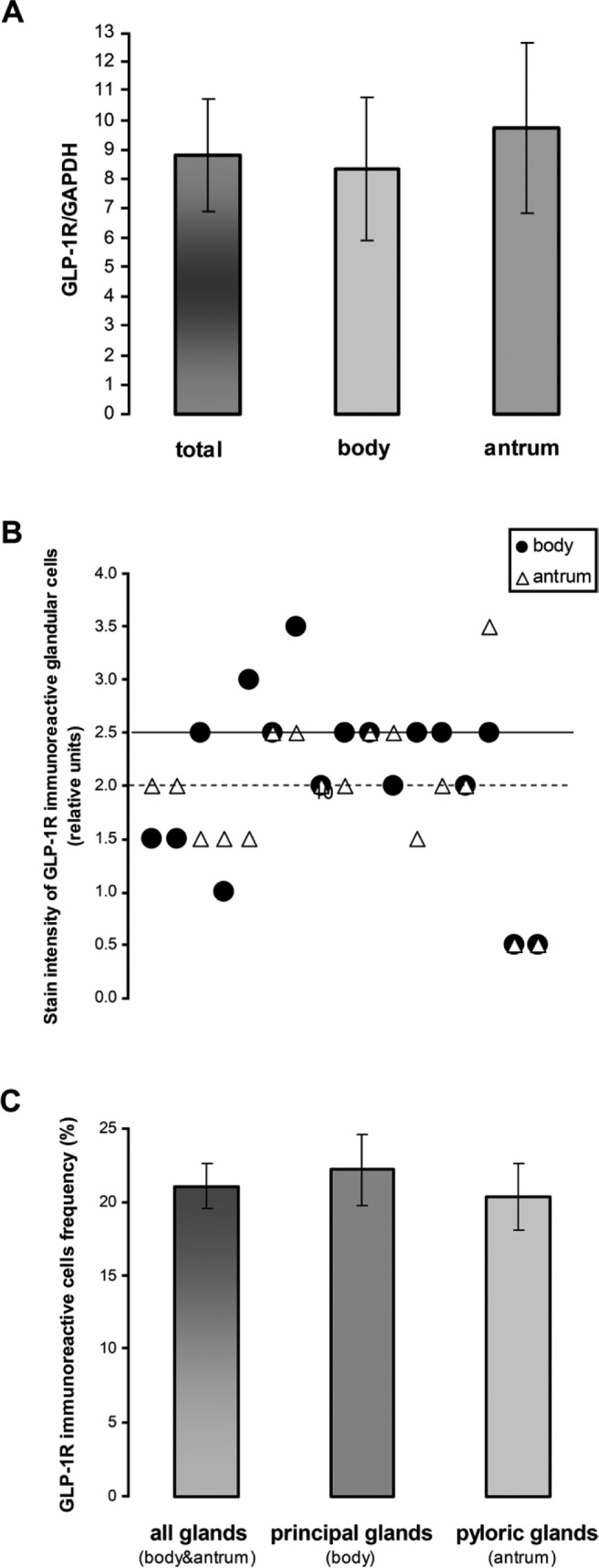
Expression of glucagon-like peptide-1 receptor (GLP-1R) in the body and antrum of the human stomach. (A) Levels of mRNA encoding GLP-1R protein expression in mucosal biopsies from the body and antrum of the stomach were evaluated by RT-PCR. The ratio between GLP-1R and housekeeping gene (GAPDH) was calculated and expressed as the mean ± standard error (SE). (B) Staining intensity of GLP-1R-immunoreactive cells in principal glands of the stomach body and in the antrum pyloric glands was evaluated by a semi-quantitative method. Each symbol represents an individual patient. Median levels of body (solid line) and antrum (dashed line) are shown. (C) Frequency of GLP-1R-immunostained cells in the principal (body) and pyloric (antrum) glands. Frequency was calculated as percentage of the total number of glandular cells and expressed as the mean ± standard error (SE).
Distribution and Frequency of GLP-1R Immunoreactive Cells in Different Areas of the Principal and Pyloric Glands
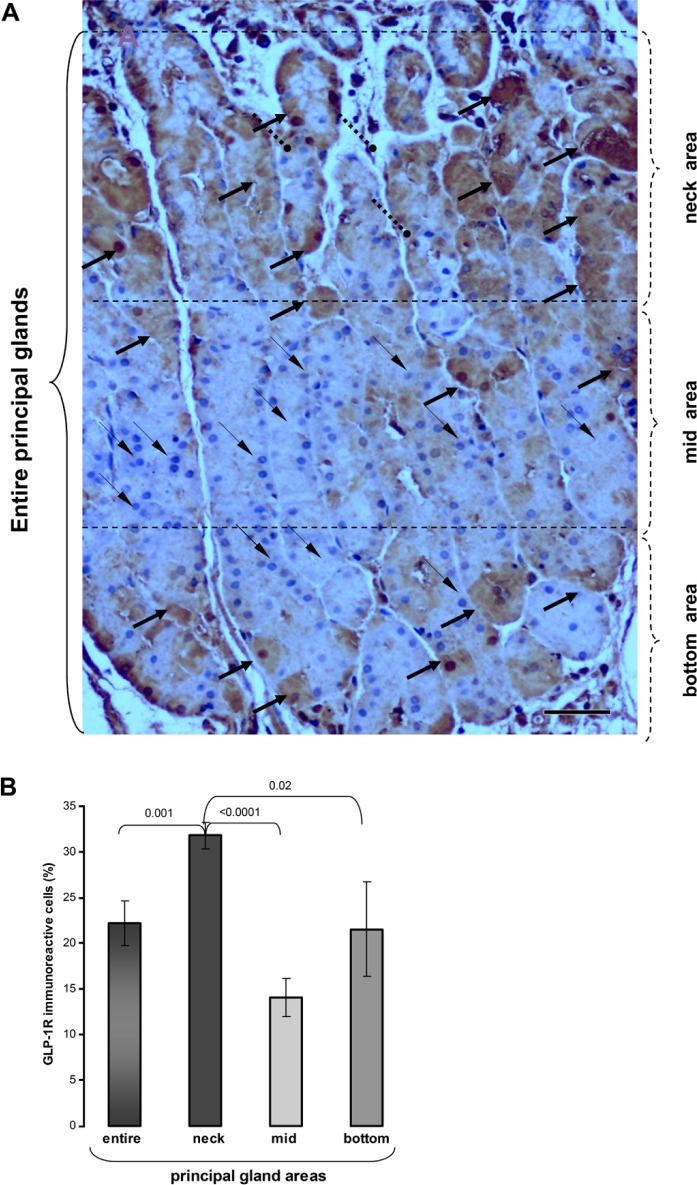
To shed light on the role of GLP-1R in human gastric mucosa, we determined its relative frequency within the complex structure of the principal gastric glands. These glands are located at the stomach body and commonly subdivided into three morphologically different areas: neck, middle, and bottom areas. Each area contains predominantly different cells: acid-secreting parietal cells are predominant in the neck area whereas the pepsinogen-secreting chief cells prevail in the middle area. The bottom area contains a variety of different cells including enteroendocrine cells. We determined, therefore, whether GLP-1R is expressed in accordance with these functionally different glandular areas. As shown in Fig. 4A, the majority of GLP-1R-immunostained cells are located at the neck region of the gastric principal glands. The frequency of GLP-1R-bearing cells was 2-fold higher in the neck as compared with that in the area (p<0.0001) and also significantly higher as compared to the bottom area (p<0.02) (Fig. 4B). These neck GLP-1R-bearing cells are morphologically identified as parietal cells (Figs. 1D and 1E). In contrast, mucus-producing cells, which are found in the neck area, did not express GLP-1R (Figs. 2B and 4A). Pepsinogen-secreting chief cells located in the mid area and bottom of the principal glands did not express GLP-1R (Figs. 2C, 2D, and 4A). It is interesting that a variety of cells, most probably enteroendocrine cells according to their morphology, were also positively stained with anti-GLP-1R antibodies in the bottom area of the principal glands (Fig. 2D). GLP-1R was also identified in two different cell types of the pyloric glands: large parietal cells and glandular cells of smaller size, most likely enteroendocrine cells according to their morphology (Figs. 1I, 1J, and 2F). The overall frequency of GLP-1R positively stained cells in pyloric glands was significantly lower as compared with the neck area of principal glands (p<0.02) (data not shown). Thus, the neck area of principal glands in the stomach body containing the acid-secreting parietal cells displays the highest cellular GLP-1R immunoreactivity.
Figure 4.
Distribution of glucagon-like peptide-1 receptor (GLP-1R)-immunostained cells within the principal glands of the stomach body. (A) GLP-1R-immunostained section of human stomach body mucosa. The highest number of GLP-1R-immunostained cells, which are morphologically compatible with parietal cells, are located in neck area of the principal glands (thick solid arrows). Mucus-secreting cells (roundhead broken arrows) do not demonstrate GLP-1R-immunostained. A lesser number of GLP-1R-immunostained cells is seen in mid and bottom areas. Chief cells located in these areas do not demonstrate GLP-1R immunoreactivity (thin solid arrows). (B) The frequency of GLP-1R immunoreactive cells in neck, mid, and bottom areas of principal glands was calculated as a percentage of the total number of glandular cells and presented as mean ± standard error (SE). Bars = 50 µm.
Discussion
This study provides for the first time direct evidence for the presence of GLP-1R in gastric glands of human stomach mucosa. Furthermore, it provides new data regarding the distribution of this major incretin receptor within the gastric glands, its localization throughout the stomach regions, and its association with different cell types of the human stomach. Our data demonstrate that GLP-1R was equally expressed in the body and antrum regions of the human stomach as evidenced by both mRNA and immunohistochemical analysis. However, quantitative analysis of GLP-1R cellular expression revealed significant differences in its expression within the gastric glands and the cellular compartments. It is noteworthy that positive immunostaining for GLP-1R was observed in several cellular compartments including the cell membrane, cytoplasm, and nucleus. This is not surprising as similar findings were previously reported for a variety of hormonal receptors, such as steroid and thyroid receptors, depending on the type of hormone, cell type, and receptor internalization with the hormone (Jenster, Trapman, and Brinkmann 1993; Razandi et al. 1999; Kawata 2001). Moreover, it has been demonstrated in resting human embryonic kidney 293 and insulinoma MIN6 cells that a fully functional green fluorescent protein-tagged GLP-1R was localized both at the cell membrane and in highly mobile intracellular compartments (Syme, Zhang, and Bisello 2006). It is of interest that signaling at the cell nucleus has been previously reported for several G-protein-coupled receptors implicated in genomic regulation but not GLP-1R (Gobeil et al. 2006). Whether GLP-1R has also an independent nuclear role remains to be determined. The highest frequency of GLP-1R-immunostained cells was found at the neck area of the principal glands of the stomach body. The GLP-1R-positive staining appears in cells that clearly exhibit morphological features of parietal cells, which are thought to be the predominant cell type in this glandular area (Samuelson 2006). Several reports support a role for the incretin hormones in the regulation of acid secretion in animals and humans. Schmidtler et al. (1991) demonstrated that GLP-1 constitutes a potent cAMP-dependent stimulus of rat parietal cell H+ production. Numerous functional studies in human patients have shown that GLP-1 has an inhibitory effect on acid secretion (Schjoldager et al. 1989; O’Halloran et al. 1990; Wettergren et al. 1993, 1997). This effect is dependent at least in part on intact vagal stimulation (Wettergren et al. 1997). However, the direct mechanism(s) mediating this inhibition have not been elucidated. Thus, the finding of GLP-1R on parietal cells in our patients constitutes direct evidence of the involvement of this receptor in the regulation of acid secretion in the human stomach. Three receptors are commonly thought to directly regulate acid secretion in the human stomach, namely, the H2 histamine receptor, the M3 muscarinic receptor, and the CCKB gastrin receptor. Taken together with the above-mentioned functional studies, our data indicate that GLP-1R should also be included with these groups of receptors that regulate acid secretion in humans. Our findings also suggest that GLP-1R is involved in the direct regulation of acid secretion in the human stomach. It is of interest that GLP-1 can induce heteromerization of its own receptor and gastric inhibitory polypeptide (GIP) receptors to promote a biological action (Schelshorn et al. 2012). It is conceivable, therefore, that GLP-1R can act in concert with other established receptors to modulate the complex process of acid secretion. Further studies are necessary to determine its relative role and integration with other established receptors. Expression of GLP-1R was not restricted to parietal glandular cells but was also detected in a variety of other intra- and extra-glandular cells such as non-parietal enteroendocrine-like cells, predominantly in the antrum, and immune cells in the loose connective tissue such as lymphocytes in the lamina propria. These findings are compatible with other reports demonstrating the expression of this receptor in immunocompetent cells (Hadjiyanni et al. 2010; Marx et al. 2010; Drucker and Rosen 2011; Hogan et al. 2011) and could be linked with chronic inflammation, which was present in a considerable number of our patients. The non-parietal glandular cells expressing GLP-1R, which were mostly found in the antrum, are morphologically similar to gastric enteroendocrine cells. However, in the absence of double immunostaining analysis, the exact nature of these unidentified glandular cells remains elusive. It is interesting that GLP-1R-dependent stimulation of rabbit fundic D-cells increased somatostatin release from these enteroendocrine cells in a dose-dependent manner in vitro (Beales and Calam 1996, 2003). The authors suggest that the inhibitory effect of GLP-1 on gastric acid secretion is mediated in part via the paracrine release of somatostatin by D-cells rather than by directly inhibiting the acid-secreted parietal cells. It is also known that not only D-cells but also G- and ECL-enteroendocrine cells are located in conjunction with acid-secreting parietal cells and control acid secretion (Joseph et al. 2003). These data together with our current results suggest that enteroendocrine cells may also express the GLP-1R and take part in the multifunctional actions of GLP-1 in the stomach mucosa. The exact types of glandular enteroendocrine cells expressing the GLP-1R and their specific roles in the regulation of the GLP-1 inhibiting effect of postprandial gastric acid secretion in the human stomach remain to be elucidated. It should also be noted that most of our patients were not entirely healthy as indicated by their clinical symptoms and the presence of histological features of chronic inflammation. In addition, the limited number of the examined patients and the nonspecific nature of their clinical symptoms did not allow determining a correlation between the expression of glandular GLP-1R and clinical or histological characteristics.
It should also be pointed out that all reported animal models used for the study of GLP-1R in gastric mucosa were conducted in parietal cells of rat stomach mucosa specimens (Bullock and Habener 1996) and in isolated rat parietal cells (Schmidtler et al. 1994). These tissues were devoid of inflammation or H. pylori infection. Thus, the effect of chronic inflammation and/or H. pylori infection could not be evaluated. Therefore, the extrapolation of our findings to normal human physiology and health should be made with caution.
In conclusion, our novel pilot findings of GLP-1R expression in human gastric mucosa suggest that GLP-1 may act directly on the gastric mucosa to modulate its complex functions. They also provide the necessary data that can be used for future comparative studies in various other diseases affecting gastric function such as diabetes mellitus.
Supplementary Material
Acknowledgments
This study was supported in part by a 2-year project grant from the Assisted Studies Program of Merck. The opinions expressed in this article are those of the authors and do not necessarily represent those of Merck.
Footnotes
Supplementary material for this article is available on the Journal of Histochemistry & Cytochemistry Web site at http://jhc.sagepub.com/supplemental.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Merck research grant for investigator initiative research.
References
- Beales ILP, Calam J. 1996. Truncated glucagon-like peptide 1 and oxyntomodulin stimulate somatostatin release from rabbit fundic D-cells in primary culture. Exp Physiol. 81:1039–1041 [DOI] [PubMed] [Google Scholar]
- Beales ILP, Calam J. 2003. Regulation of amylin release from cultured rabbit gastric fundic mucosal cells. BMC Physiol. 3:13–2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock BP, Habener JF. 1996. Tissue distribution of messenger ribonucleic acid encoding the rat glucagon-like peptide 1 receptor. Endocrinology. 137:2968–2978 [DOI] [PubMed] [Google Scholar]
- Carmosino M, Procino G, Casavola V, Svelto M, Valenti G. 2000. The cultured human gastric cells HGT-1 express the principal transporters involved in acid secretion. Pflugers Arch. 440:871–880 [DOI] [PubMed] [Google Scholar]
- Dixon MF, Genta RM, Yardley JH, Correa P. 1996. Classification and grading of gastritis: the updated Sydney System. Am J Surg Pathol. 20:1161–1181 [DOI] [PubMed] [Google Scholar]
- Drucker DJ, Rosen CF. 2011. Glucagon-like peptide-1(GLP-1) receptor agonists, obesity and psoriasis: diabetes meets dermatology. Diabetologia. 54:2741–2744 [DOI] [PubMed] [Google Scholar]
- Dunphy JL, Taylor RG, Fuller PJ. 1998. Tissue distribution of rat glucagon receptor and GLP-1 receptor gene expression. Mol and Cell Endocrinol. 141:179–186 [DOI] [PubMed] [Google Scholar]
- Gobeil F, Fortier A, Zhu T, Bossolasco M, Leduc M, Grandbois M, Heveker N, Bkaily G, Chemtob S, Barbaz D. 2006. G-protein-coupled receptor signaling at the cell nucleus: an emerging paradigm. Can J Physiol Pharmacol. 84:287–297 [DOI] [PubMed] [Google Scholar]
- Goldstein AMB, Brothers MR, Davis EA. 1969. The architecture of the superficial layer of the gastric mucosa. J Anat. 104:539–551 [PMC free article] [PubMed] [Google Scholar]
- Hadjiyanni I, Siminovitch KA, Danska JS, Drucker DJ. 2010. Glucagon-like peptide-1 receptor signaling selectively regulates murine lymphocyte proliferation and maintenance of peripheral regulatory T cells. Diabetologia. 53:730–740 [DOI] [PubMed] [Google Scholar]
- Hansen AB, Gespach CP, Rosselin GE, Holst JJ. 1988. Effect of truncated glucagon-like peptide 1 on cAMP in rat gastric glands and HGT-1 human gastric cancer cells. FEBS Letters. 236:119–122 [DOI] [PubMed] [Google Scholar]
- Hogan AE, Tobin AM, Ahern T, Corrigan MA, Gaoatswe G, Jackson R, O’Reilly V, Lynch L, Doherty DG, Moynagh PN, et al. 2011. Glucagon-like peptide-1 (GLP-1) and the regulation of human invariant natural killer T cells: lessons from obesity, diabetes and psoriasis. Diabetologia. 54:2745–2754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenster G, Trapman J, Brinkmann AO. 1993. Nuclear import of the human androgen receptor. Biochem J. 293:761–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph IMP, Zavros Y, Merchant JL, Kirschner D. 2003. A model for integrative study of human gastric acid secretion. J Appl Physiol. 94:1602–1618 [DOI] [PubMed] [Google Scholar]
- Kawata M. 2001. Subcellular steroid/nuclear receptor dynamics. Arch Histol Cytol. 64(4):353–368 [DOI] [PubMed] [Google Scholar]
- Marathe SC, Rayner CK, Jones KL, Horowitz M. 2011. Effects of GLP-1 and incretin-based therapies on gastrointestinal motor function. Experimental Diabetes Research. 10.1155/2011/279530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx N, Burgmaier M, Heinz P, Ostertag M, Hausauer A, Bach H, Durst R, Hombach V, Walcher D. 2010. Glucagon-like peptide-1(1-37) inhibits chemokine-induced migration of human CD-4 positive lymphocytes. Cell Mol Life Sci. 67:3549–3555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näslund E, Bogefors J, Skogar S, Grybäck P, Jacobsson H, Holst JJ, Hellström PM. 1999. GLP-1 slows solid gastric emptying and inhibits insulin, glucagon, and PYY release in humans. Am J Physiol. 277:R910–R916 [DOI] [PubMed] [Google Scholar]
- O’Halloran DJ, Nikou GC, Kreymann B, Ghatei MA, Bloom SR. 1990. Glucagon-like peptide-1 (7-36)-NH2: a physiological inhibitor of gastric acid secretion in man. J Endocrinol. 126:169–173 [DOI] [PubMed] [Google Scholar]
- Razandi M, Pedram A, Greene GL, Levin ER. 1999. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ER alpha and ER beta expressed in Chinese hamster ovary cells. Mol Endocrinol. 13(2):307–319 [DOI] [PubMed] [Google Scholar]
- Samuelson LC. 2006. Genetically engineered mouse models of gastric physiology. In: Johnson LR, editor. Physiology of the gastrointestinal tract, 4th ed., Volume 2 Waltham, MA: Academic Press; p. 1293–1312 [Google Scholar]
- Schelshorn DW, Joly F, Mutel S, Hample C, Breton B, Mutel V, Lutjens R. 2012. Lateral allosterism in the glucagon receptor family: GLP-1 induces GPCR heterodimer formation. Mol Pharmacol. 81:309–331 [DOI] [PubMed] [Google Scholar]
- Schjoldager BGT, Mortensen PE, Christiansen J, Orskov C, Holst JJ. 1989. GLP-1 (glucagon-like peptide 1) and truncated GLP-1, fragments of human proglucagon, inhibit gastric acid secretion in humans. Dig Dis Sci. 34:703–708 [DOI] [PubMed] [Google Scholar]
- Schmidtler J, Dehne K, Allescher HD, Schusdziarra V, Classen M, Holst JJ, Polack A, Schepp W. 1994. Rat parietal cell receptors for GLP-1-(7-36) amide: Northern blot, cross-linking, and radioligand binding. Am J Physiol. 267:G423–G432 [DOI] [PubMed] [Google Scholar]
- Schmidtler J, Schepp W, Janczewska I, Weigert N, Furlinger C, Schusdziarra V, Classen M. 1991. Glucagon-like peptide-1 (GLP-1): potent cAMP-dependent stimulus of rat parietal cell H+ production. Am J Physiol. 260:G940–G950 [DOI] [PubMed] [Google Scholar]
- Syme A, Zhang L, Bisello A. 2006. Caveolin-1 regulates cellular trafficking and function of the glucagon-like peptide 1 receptor. Mol Endocrinol. 20(12):3400–3411 [DOI] [PubMed] [Google Scholar]
- Uttenthal LO, Blázquez E. 1990. Characterization of high-affinity receptors for truncated glucagon-like peptide-1 in rat gastric glands. FEBS Letters. 262:139–141 [DOI] [PubMed] [Google Scholar]
- Wei Y, Mojsov S. 1995. Tissue-specific expression of the human receptor for glucagon-like peptide-1: brain, heart and pancreatic forms have the same deduced amino acid sequences. FEBS Letters. 358:219–224 [DOI] [PubMed] [Google Scholar]
- Wei Y, Mojsov S. 1996. Distribution of GLP-1 and PACAP receptors in human tissues. Acta Physiol Scand. 157:355–357 [DOI] [PubMed] [Google Scholar]
- Wettergren A, Schjoldager B, Mortensen PE, Myhre J, Christiansen J, Holst JJ. 1993. Truncated GLP-1 (proglucagon 78-107-amide) inhibits gastric and pancreatic functions in man. Dig Dis Sci. 38:665–673 [DOI] [PubMed] [Google Scholar]
- Wettergren A, Wojdemann M, Meisner S, Stadil F, Holst JJ. 1997. The inhibitory effect of glucagon-like peptide-1 (GLP-1) 7-36 amide on gastric acid secretion in humans depends on intact vagal innervation of the stomach. Gut. 40:597–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.