Abstract
Purpose.
To correlate changes between VEGF expression with systemic and retinal oxidative stress and inflammation in rodent models of obesity induced insulin resistance and diabetes.
Methods.
Retinal VEGF mRNA and protein levels were assessed by RT-PCR and VEGF ELISA, respectively. Urinary 8-hydroxydeoxyguanosine (8-OHdG), blood levels of C-reactive protein (CRP), malondialdehyde (MDA), and CD11b/c positive cell ratio were used as systemic inflammatory markers. Retinal expression of Nox2, Nox4, and p47phox mRNA levels were measured as oxidative stress markers. TNF-α, inter-cellular adhesion molecule-1 (ICAM-1), IL1β, and activation of nuclear factor κB (NF-κB) were used as retinal inflammatory markers.
Results.
Retinal VEGF mRNA and protein expression increased in Zucker diabetic fatty (ZDFfa/fa) rats and streptozotosin (STZ) induced diabetic Sprague-Dawley rats, after two months of disease, but not in Zucker fatty (ZF) rats. Systemic markers of oxidative stress and inflammation were elevated in insulin resistant and diabetic rats. Some oxidative stress and inflammatory markers (TNF-α, IL-6, ICAM-1, and IL1-β) were upregulated in the retina of ZDFfa/fa and STZ diabetic rats after 4 months of disease. In contrast, activation of NF-κB in the retina was observed in high fat fed nondiabetic and diabetic cis-NF-κBEGFP mice, ZF, ZDFfa/fa, and STZ-induced diabetic rats.
Conclusions.
Only persistent hyperglycemia and diabetes increased retinal VEGF expression. Some markers of inflammation and oxidative stress were elevated in the retina and systemic circulation of obese and insulin resistant rodents with and without diabetes. Induction of VEGF and its associated retinal pathologies by diabetes requires chronic hyperglycemia and factors in addition to inflammation and oxidative stress.
Only chronic diabetes induced late markers of inflammation and oxidative stress in the retina correlated with increased VEGF expression, but insulin resistance alone caused systemic and retinal inflammation and no increase in VEGF elevation.
Introduction
Diabetic retinopathy (DR) is observed in a majority of diabetic patients and is one of the most frequent causes of blindness in the United States.1,2 Chronic hyperglycemia is thought to be the primary cause of DR, as supported by the Diabetes Control and Complications Trial and the United Kingdom Prospective Diabetes Study.3,4 Sequential pathologic changes in DR include increased vascular permeability, pericyte apoptosis, acellular capillaries, and aberrant retinal new vessel growth, or neovascularization.5 VEGF is one of the most important endogenous angiogenic or permeability inducing polypeptides that respond to hypoxia under normal physiologic conditions.6,7 Elevated levels of VEGF in the vitreous have been shown to correlate with the development of diabetic macular edema and proliferative diabetic retinopathy (PDR).8,9 Further, treatments with VEGF inhibitors by intravitreous injections have been shown to protect visual acuity in diabetic macular edema and to regress neovascularization in PDR.10,11 These findings have established VEGF elevation in the ocular fluids and tissues as an important validated marker for severe DR.
Previous reports have indicated that the induction of oxidative stress and inflammation by abnormal metabolites induced by diabetes may play a significant role in increasing VEGF expression in the retina of diabetic patients or animals.12–14 The addition of reactive oxygen species (ROS) and inflammatory cytokines to vascular cells have also been shown to induce VEGF expression.15 However, it is also well established that obesity-induced insulin resistant states without hyperglycemia will increase oxidative stress and inflammation in many tissues and systemic circulation, yet vascular pathologies of DR are rarely observed in obesity/insulin resistant states without hyperglycemia.16,17 Thus, it is unclear whether elevation of ROS and inflammation are observed in the retina with obesity/insulin resistance and whether their elevation alone in the retina will increase VEGF expression without hyperglycemia.
In this study, we correlated the changes in systemic and retinal inflammation and oxidative stress with VEGF expression in the retina of various rodent models of diabetes and insulin resistance. The results suggested that inflammation or oxidation can occur early in both obesity and diabetes, but chronic exposure to hyperglycemia is essential for elevation of VEGF expression in the retina.
Methods
Animals and Induction of Diabetes
Nuclear factor κB (NF-κB)-dependent enhanced green fluorescent protein (GFP) transgenic mice (cis-NF-κBEGFP) were produced as described previously.18. Fourteen-week-old male Zucker diabetic fatty rats (ZDFfa/fa) were used as models of obesity associated with type 2 diabetes, and Zucker nondiabetic lean rats (ZDFfa/+) were used as their age-matched controls (Charles River Laboratories, Wilmington, MA). Six-month-old male Zucker fatty rats (ZF) and their age-matched lean controls (ZL) were used as models of insulin resistance (Charles River Laboratories). Diabetes was induced in C57/B6 mice and Sprague-Dawley (SD) rats by injection of streptozotosin (STZ; 55 mg/kg body weight), in 0.05 M/L citrate buffer (pH 4.5) or citrate buffer for controls, (Sigma-Aldrich, St. Louis, MO). Blood glucose levels were determined 2 days after the injections by glucose analyzer (Yellow Spring Instruments, Yellow Springs, OH). Glycemic levels of greater than 16.7 mM/L were defined as having diabetes. Obesity and insulin resistance in 8-week-old cis-NF-κBEGFP mice were induced by a high fat diet, 42% from fat, for an additional 8 weeks prior to sacrifice (Harlan Teklad, Indianapolis, IN). All procedures involving animals conformed to the Association for Research in Vision and Ophthalmology resolution on the use of animals in research and were approved by the Animal Use and Care Committee of Joslin Diabetes Center.
RT-PCR Analysis for Retinal RNA Expressions
Total RNA were isolated and purified from the retina by RNAeasy micro column with DNase treatment (Qiagen, Inc., Valencia, CA) and cDNA was synthesized using high capacity cDNA synthesis kit (Applied Biosystems, Foster City, CA) or SuperScript III reverse transcriptase (Invitrogen, Carlsbad, CA). The number of copies of transcript of VEGF (Forward: AGC CAG AAA ATC ACT GTG AGC C; Reverse: TTT AAC TCA AGC TGC CTC GCC; Probe: FAM-TGT TCC TGC AAA AAC ACA GAC TCG CGT TG-TAMRA), Nox2 (Forward: CAG TGC GTG TTG CTC AAC CAG AAT; Reverse: CAA TGG TGT GAA TGG CCG TGT GAA; Probe: FAM-TCG AAG ACA ACT GGA CAG GAA CCT TAC T-TAMRA), tumor necrosis factor-α (TNF-α) (Forward: TCT GTG CCT CAG CCT CTT CTC ATT; Reverse: AAC TGA TGA GAG GGA GCC CAT TTG; Probe: FAM-ATC GGT CCC AAC AAG GAG GAG AAG TT-TAMRA), ICAM-1 (Forward: ACA GCA GAC CAC TGT GCT TTG AGA; Reverse: ACT CGC TCT GGG AAC GAA TAC ACA; Probe: FAM-AGT CCT CGG CTT CTG CCA CCA TCA-TAMRA), and rat IL1β (Forward: TGCAGGCTTCGAGATGAAC; Reverse: GGGATTTTGTCGTTGCTTGTCR) in the retina were measured using Applied Biosystems (ABI) Taqman real-time PCR (Applied Biosystems). The p47phox (Forward: GTG AAG CCA TCG AGG TCA TTC; Reverse: CCC GCG GCT TCT AAT CTG T), Nox4 (Forward: GAA CCT CAA CTG CAG CCT GAT C; Reverse: CTT TTG TCC AAC AAT CTT CTT GTT CTC), IL-6 (Forward: CTT CAA TCC AGT TGC CTT CTT G; Reverse: AAT TAA GCC TCC GAC TTG TGA AG), gapdh (Forward: ATG TTC CAG TAT GAC TCC ACT CAC G; Reverse: GAA GAC ACC AGT AGA CTC CAC GAC A) mRNA expressions in the retina were evaluated by SYBR Green procedure (Applied Biosystems). Amplification and detection were performed using the ABI PRISM 7900 Sequence Detection System (Applied Biosystems).
Urinary 8-OHdG, Plasma MDA, and Plasma CRP
Urinary 8-Hydroxydeoxyguanosine (8-OHdG) excretion was measured by new 8-OHdG Check ELISA Kit (Nikken Seil Co., Ltd., Shizuoka, Japan). Plasma malondialdehyde (MDA) and plasma C-reactive protein (CRP) concentrations were measured by Lipid Peroxidation Assay Kit (Oxi International, Inc., Foster City, CA) and by CRP, Rat Quantitative Kit (Helica, Fullerton, CA), respectively.
Monocyte Activation in Peripheral Blood
Peripheral blood was drawn from the abdominal large vein by heparinized syringe. Fresh drawn blood was treated by BD Pharm Lyse lysing solution (BD Biosciences, San Diego, CA) for 15 minutes to exclude red blood cells. Isolated leukocytes were used to analyze the monocyte activation by fluorescence-activated cell sorting (FACS) for the two color flow cytometry analysis, mouse PE-conjugated anti-rat CD11b/c monoclonal antibody, PE-conjugated mouse Immunoglobulin (IgG) 2a, κ isotype control (BD Biosciences) were used. Flow cytometry was performed on a BeckmanCoulter XL-MCL (BeckmanCoulter, Fullerton, CA). The percentage of population was calculated by the positive cell number in the total mononuclear cell number. Data were analyzed by Expo32 (BeckmanCoulter).
Immunoblot Analysis, VEGF ELISA, and NF-κB p65 DNA Binding Assay
Nuclear-specific proteins were isolated using the compartmental protein extraction kit (Millipore, Billerica, MA) according to the manufacturer's instructions. Samples were dissolved in 0.5% NP-40, used after optimization studies. Proteins were separated by SDS-PAGE. Blots were subsequently incubated with anti–NF-κB (p65) (Santa Cruz Biotechnologies, Santa Cruz, CA) and anti-PCNA (Cell Signaling, Beverly, MA). Labeled protein bands were identified with enhanced chemiluminescent system (Amersham Biosciences, Piscataway, NJ). VEGF protein was measured by VEGF ELISA kit (R&D Systems, Minneapolis, MN).
Retina was homogenized and extracted protein was used in VEGF ELISA assay. The data were normalized to protein level. NF-κB p65 activation (DNA binding activity) was assessed using NF-κB p65 Transcription Factor Kit as per manufacturer's instructions (Pierce Biotechnology, Rockford, IL).
Data Analysis
The data are expressed as mean ± SD. Comparison among more than two groups was performed by one-way ANOVA followed by the analysis with paired or unpaired t test to evaluate statistical significance between the two groups. All analyses were performed using StatView (SAS Institute, Cary, CA). Statistical significance was defined as P less than 0.05.
Results
Physiologic Characteristics of Experimental Groups
Body weights of ZF rats were greater than ZL rats by 2.1 ± 0.6-fold. Plasma insulin, cholesterol, free fatty acids, and triglyceride levels of ZF rats were elevated by 1.7 ± 0.2-fold, 5.3 ± 1.4-fold, 3.0 ± 0.7-fold, and 9.1 ± 3.8-fold, respectively, compared with ZL rats (P < 0.05, Table 1). In the ZDFfa/fa rats, a type 2 diabetic model, blood levels of glucose, cholesterol, and triglycerides were increased by 4.5 ± 1.5-fold, 1.8 ± 0.2-fold, and 7.2 ± 2.2-fold, respectively, compared with ZDFfa/+ nondiabetic rats (P < 0.05, Table 1). After induction of diabetes in Sprague-Dawley rats for 2 months, body weights of diabetic Sprague-Dawley rats were significantly less than the nondiabetic Sprague-Dawley rats by 20 ± 5% and insulin was decreased by 93 ± 10% in diabetic Sprague-Dawley rats compared with nondiabetic Sprague-Dawley rats (P < 0.05, Table 1). The final body weights of Sprague-Dawley diabetic rats were still greater than their initial weights indicating that the difference in body weights were not due to starvation. Blood glucose levels were significantly elevated in diabetic Sprague-Dawley rats at 559 ± 57 mg/dL and 473 ± 82 mg/dL at 2 and 4 months, respectively.
Table 1. .
General Characteristics of the Rat Experimental Groups
|
|
ZL |
ZF |
ZDFfa/+ (Lean) |
ZDFfa/fa (Diabetic) |
Cont. (2M) |
STZ (2M) |
Cont. (4M) |
STZ (4M) |
| Number | 7 | 7 | 6 | 6 | 5 | 5 | 7 | 7 |
| Body weight, g | 400 ± 20 | 830 ± 130* | 385 ± 6 | 406 ± 5‡ | 283 ± 6 | 226 ± 13‖ | 475 ± 29 | 355 ± 20# |
| Blood glucose, g | 90 ± 10 | 99 ± 13 | 89 ± 11 | 402 ± 16§ | 151 ± 5 | 559 ± 57¶ | 128 ± 24 | 473 ± 82** |
| Insulin, ng/mL | 5.2 ± 2.6 | 36 ± 3† | 4.0 ± 0.9 | 2.4 ± 0.9 | 4.3 ± 1.6 | 0.3 ± 0.1¶ | ND | ND |
| FFA, mEq/L | 0.4 ± 0.2 | 1.3 ± 0.1† | ND | ND | ND | ND | ND | ND |
| T-Cho, mg/dL | 57 ± 7 | 474 ± 50† | 44 ± 14 | 75 ± 37‡ | 21 ± 12 | 38 ± 27 | ND | ND |
| TG, mg/dL | 131 ± 10 | 1198 ± 80† | 38 ± 2 | 275 ± 21§ | 0.9 ± 0.7 | 2.1 ± 2.3 | ND | ND |
Cont., nondiabetic Sprague-Dawley rats; ND, not done; FFA, free fatty acids; T-Cho, total cholesterol; TG, triglycerides. The data are expressed as the mean ± SD.
P < 0.05.
P < 0.001 versus ZL rats.
P < 0.05.
P < 0.001 versus ZDFfa/+ lean rats.
P < 0.05.
‡ P < 0.001 versus nondiabetic Sprague-Dawley rats (2M).
P < 0.05.
P < 0.001 versus nondiabetic Sprague-Dawley rats (4M).
VEGF Expression in the Retina
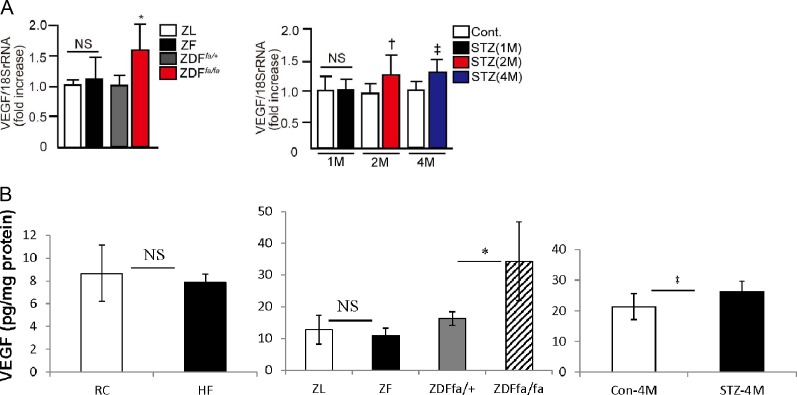
The expression of VEGF-β mRNA levels were elevated in the retina of diabetic ZDFfa/fa, which have had diabetes for 8 weeks compared with nondiabetic ZDFfa/− rats by 1.6 ± 0.2-fold (P < 0.05, Fig. 1). Retinal VEGF protein levels were 34.3 ± 12.4 pg/mg protein in diabetic ZDFfa/fa versus 16.2 ± 2.2 pg/mg in nondiabetic control ZDFfa/− rats (P < 0.05, Fig. 1B). In contrast, no differences in VEGF-β mRNA or protein expression were detected between ZL and ZF rats. In Sprague-Dawley rats, the induction of diabetes did not significantly increase VEGF-β mRNA expression until after 2 months of diabetes by 1.3 ± 0.3-fold (P < 0.05, Fig. 1), which persisted at 4 months of diabetes by 1.4 ± 0.2-fold (P < 0.05, Fig. 1). No significant increases in retinal VEGF-β mRNA expression were observed after 1 month of diabetes in Sprague-Dawley rats. Consistent with the mRNA levels, retinal VEGF protein significantly increased at 4 months (diabetic 26.2 ± 3.5 pg/mg versus nondiabetic rats 21.4 ± 4.2 pg/mg (P < 0.05, Fig. 1B).
Figure 1. .
Expression of VEGF mRNA and protein in the retina. VEGF mRNA (A) and protein expression (B) in the retina of ZL, ZF, ZDFfa/+ lean, ZDFfa/fa, nondiabetic Sprague-Dawley and STZ-induced diabetic Sprague-Dawley rats after several months of disease duration. *P < 0.05 versus ZDFfa/+ lean rats. †P < 0.05 versus nondiabetic Sprague-Dawley rats (2M). ‡P < 0.05 versus nondiabetic Sprague-Dawley rats (4M). One of three independent experiments is shown. These data are expressed as mean ± SD. NS, not significant; STZ, STZ-induced diabetic Sprague-Dawley rats; M, months.
Systemic Oxidative Stress and Inflammation in the Experimental Groups
Systemic markers of oxidative stress and inflammation were assessed in fatty and diabetic rats and compared with their respective controls. Oxidative stress markers as measured by plasma MDA levels were significantly increased in ZF, ZDFfa/fa, and diabetic Sprague-Dawley rats with 2 months of diabetes by 1.6 ± 0.3-fold, 2.1 ± 0.8-fold, and 1.7 ± 0.4-fold, respectively, when compared with ZL, ZDFfa/+ lean and nondiabetic Sprague-Dawley rat controls (P < 0.05, Table 1). Similar to MDA, urinary oxidative stress markers 8-OHdG excretion was also increased in ZF, ZDFfa/fa, and diabetic Sprague-Dawley rats with 2 months of diabetes by 2.6 ± 1.2-fold, 8.0 ± 2.1-fold, and 4.4 ± 1.3-fold, respectively, when compared with ZL, ZDFfa/+ lean and nondiabetic Sprague-Dawley rats (P < 0.05, Table 2).
Table 2. .
Systemic Oxidative Stress and Inflammatory Markers
|
|
ZL |
ZF |
ZDFfa/+ (lean) |
ZDFfa/fa (diabetic) |
Cont. (2M) |
STZ (2M) |
| Number | 7 | 7 | 6 | 6 | 5 | 5 |
| MDA, μM | 0.7 ± 0.1 | 1.1 ± 0.3* | 1.2 ± 0.5 | 2.5 ± 0.6† | 0.9 ± 0.2 | 1.6 ± 0.5‡ |
| CRP, mg/mL | 303 ± 45 | 385 ± 83* | 334 ± 55 | 392 ± 55† | 252 ± 57 | 290 ± 70 |
| CD11b/c positive population, % | 15.3 ± 1.5 | 39.7 ± 8.6* | 15.6 ± 1.7 | 29.5 ± 3.8† | 13.1 ± 3.5 | 23.8 ± 6.6‡ |
| Urinary 8-OHdG, ng/d | 144 ± 64 | 381 ± 107* | 325 ± 113 | 2603 ± 1130† | 267 ± 119 | 1188 ± 334‡ |
The data are expressed as the mean ± SD.
P < 0.05, versus ZL rats.
P < 0.05, versus ZDFfa/+ lean rats.
P < 0.05 versus nondiabetic Sprague-Dawley rats (2M).
Several systemic inflammatory markers were assessed including circulating monocyte activation using CD11b/c labeling and plasma CRP levels. Circulating monocytes positive for CD11b/c, as measured by FACS, were increased in ZF, ZDFfa/fa, and diabetic Sprague-Dawley rats by 1.9 ± 0.4-fold, 2.6 ± 0.2-fold, and 1.8 ± 0.5-fold, respectively, when compared with ZL, ZDFfa/+ lean and nondiabetic Sprague-Dawley rats with 2 months of diabetes (P < 0.05, Table 1). Similarly, plasma levels of CRP were also increased in ZF and ZDFfa/fa rats by 1.3 ± 0.5-fold and 1.2 ± 0.1-fold, respectively, when compared with ZL and ZDFfa/+ lean rats. In contrast, plasma CRP levels were not significantly increased in Sprague-Dawley rats with 2 months of diabetes compared with Sprague-Dawley nondiabetic rats (P < 0.05, Table 1).
Characterization of Inflammatory Markers in the Retina
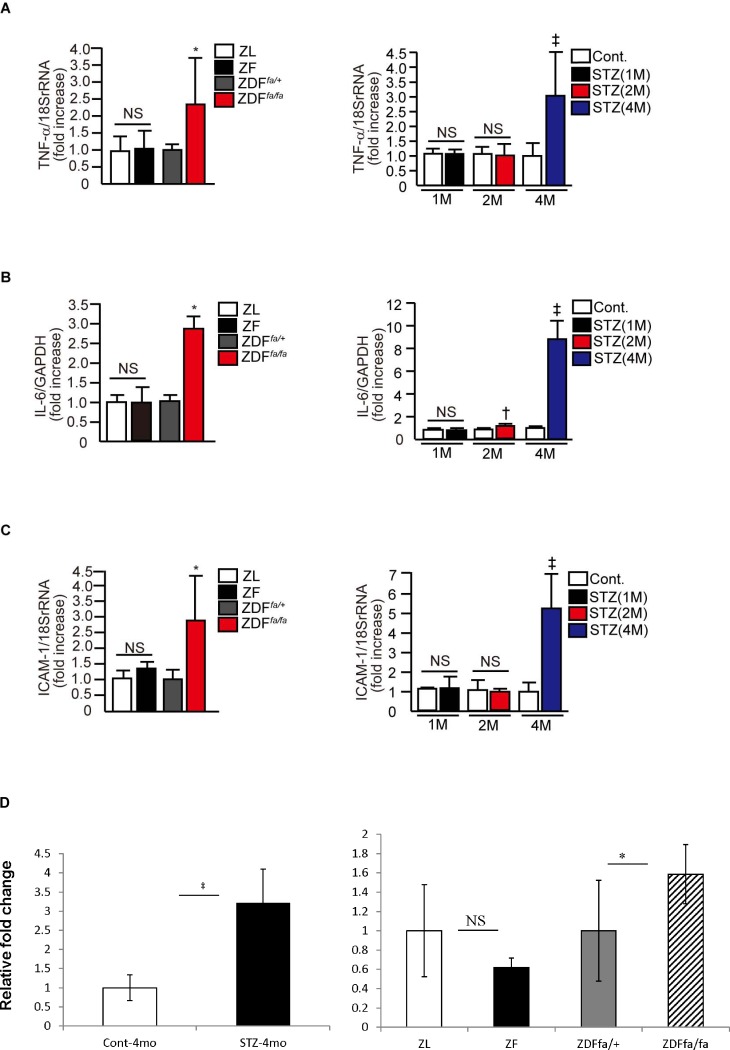
Markers of inflammation in the retina with the induction of diabetes or insulin resistant states were characterized. Expression of TNF-α mRNA levels were not increased in the retina of ZF rats when compared with ZL rats after 2 months on the fatty diet. In contrast, TNF-α mRNA levels were elevated in the retinas of ZDFfa/fa rats compared with nondiabetic ZDFfa/− rats. For Sprague-Dawley rats, the induction of diabetes by STZ for 1 and 2 months did not increase TNF-α mRNA levels in the retina, but, 4 months of diabetes did increase its levels by 3.0 ± 1.4-fold (P < 0.05, Fig. 2A). Similarly, IL-6 mRNA expression was not increased in the retina of ZF rats after 1 month of diabetes, but it did elevate significantly in the retina of ZDFfa/fa rats, after 2 and 4 months of diabetes and in Sprague-Dawley rats by 2.8 ± 0.3-fold and 9.0 ± 1.5-fold, respectively (P < 0.05, Fig. 2B). ICAM-1 mRNA expression was not increased in the retina of ZF rats, or after 1 month and 2 months of diabetes in Sprague-Dawley rats. However, ICAM-1 mRNA expression was increased in the retina of diabetic ZDFfa/fa rats and Sprague-Dawley rats after 4 months of diabetes by 2.8 ± 1.3-fold and 5.3 ± 1.8-fold, respectively (P < 0.05, Fig. 2C). Auto-inflammatory marker IL1β mRNA increased significantly after 4 months of diabetes in Sprague-Dawley rats (P < 0.05, Fig. 2D). Retinal IL1β mRNA levels did not differ between ZL and ZF rats. In contrast, levels of IL1β mRNA were increased by 1.6 ± 0.3-fold in diabetic ZDFfa/fa rats versus controls (P < 0.05, Fig. 2D).
Figure 2. .
Evaluation of retinal inflammation. Effect of diabetes and insulin resistance on the levels of various inflammatory markers in the retina. TNF-α mRNA (A), IL-6 (B), ICAM-1 (C), and IL1β (D) expressions in the retina of ZL, ZF, ZDFfa/+ lean, ZDFfa/fa, nondiabetic Sprague-Dawley, and STZ-induced diabetic Sprague-Dawley rats. *P < 0.05 versus ZDFfa/+ lean rats. †P < 0.05 versus nondiabetic Sprague-Dawley rats (2M). ‡P < 0.05 versus nondiabetic Sprague-Dawley rats (4M). Results of three independent experiments are shown. These data are expressed as mean ± SD.
Evaluation of Oxidative Stress Markers in the Retina
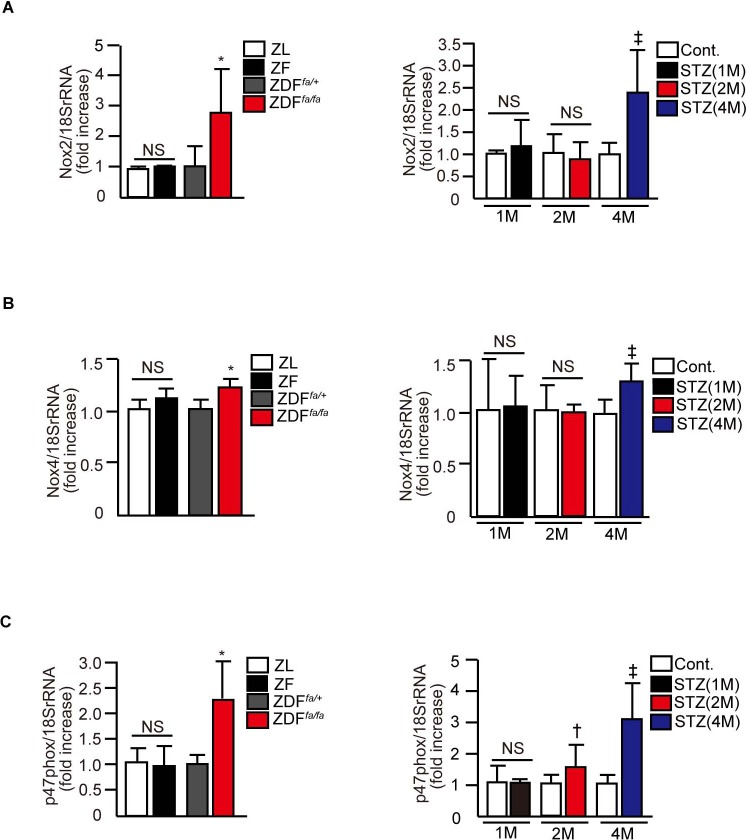
Expression of mRNA levels of NOX2, NOX4, and p47phox, assessed in the retina were not changed in insulin resistant ZF rats compared with their ZL controls. In contrast, the mRNA levels of NOX2, NOX4, and p47phox were elevated significantly by 2.5 ± 0.4-fold, 1.3 ± 0.1-fold, and 2.2 ± 0.7-fold in ZDFfa/fa compared with nondiabetic ZDFfa/wt rats. In diabetic Sprague-Dawley rats, mRNA levels of NOX2 and NOX4 were not significantly elevated until 4 months of diabetes. For p47phox mRNA levels, significant elevations of 1.5 ± 0.6-fold and 3.1 ± 1.2-fold were noted in the retina of diabetic Sprague-Dawley rats after 2 and 4 months of diabetes, respectively, but not after 1 month of disease.
Figure 3. .
Evaluation of oxidative stress in the retina. Characterization of changes in mRNA levels of Nox2 (A), Nox4 (B), and p47phox (C) expressions in the retina of ZL, ZF, ZDFfa/+ lean, ZDFfa/fa, nondiabetic Sprague-Dawley, and STZ-induced diabetic Sprague-Dawley rats. *P < 0.05 versus ZDFfa/+ lean rats. †P < 0.05 versus nondiabetic Sprague-Dawley rats (2M). ‡P < 0.05 versus nondiabetic Sprague-Dawley rats (4M). Results of three independent experiments are shown. These data are expressed as mean ± SD.
Involvement of NF-κB Activation in the Retina
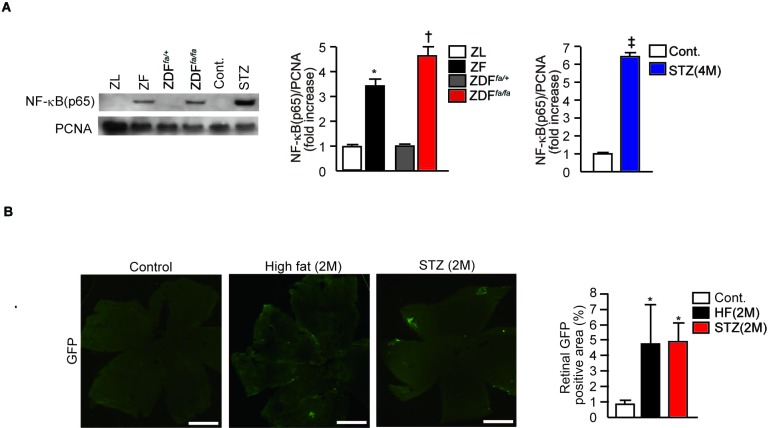
Previous reports have indicated that both increase in inflammatory cytokines and oxidants are associated with NF-κB activation, especially with diabetes or insulin resistance.12,19 In addition, high glucose levels can activate NF-κB in retinal vascular cells.20 Thus, we evaluated whether the expression of p65 subunit of NF-κB, a marker of its activation, was increased in the retina of various diabetic and insulin resistant rodent models. Immunoblot study showed increases in p65 subunit of NF-κB in the retina of ZF rats, ZDFfa/fa rats, and diabetic Sprague-Dawley rats of 4 months duration by 3.6 ± 0.3-fold, 4.6 ± 0.4-fold, and 6.4 ± 0.2-fold, respectively, when compared with ZL, ZDFfa/+ lean, and nondiabetic Sprague-Dawley rats (P < 0.05, Fig. 4A). We also evaluated the effect of insulin resistance and diabetes in cis-NF-κBEGFP mice, which will increase GFP expression in tissues that activated NF-κB.21 After 2 months of high fat feeding to induce obesity without diabetes or 2 months of STZ-induced diabetes, GFP positive areas in the retina of cis-NF-κBEGFP mice increased by 5.8 ± 2.8-fold and 5.6 ± 2.0-fold, respectively (P < 0.05, Fig. 4B). The findings of NF-κB activation were further confirmed by the measurement of NF-κB DNA binding activity, which were significantly increased in high fat feeding mice, ZF and ZDFfa/fa compared with their respective control groups (P < 0.05, see Supplementary Material and Supplementary Fig. S1, http://www.iovs.org/content/53/13/8424/suppl/DC1). These results suggest that both diabetes and insulin resistance can activate NF-κB in the retina.
Figure 4. .
Activation of NF-κB in the retina of insulin resistant and diabetic rodents. (A) Representative immunoblots of NF-κB (p65) from retinal nuclear proteins of ZL, ZF, ZDFfa/+, diabetes ZDFfa/fa, control, and STZ diabetic Sprague-Dawley rats. Data from three experiments were quantitated by densitometry. *P < 0.05 versus ZL rats. †P < 0.05 versus ZDFfa/+ lean rats. ‡P < 0.05 versus nondiabetic Sprague-Dawley rats (4M). Gel is from one of three independent experiments. These data are expressed as mean ± SD. (B) Activation of NF-κB in the retina of cis-NF-κBEGFP transgenic mice. cis-NF-κBEGFP mice were fed a high fat diet for two months or had diabetes induced by STZ for two months, EGFP fluorescence was assessed using digital fluorescence microscopy. Retina was digitally photographed, and the images were imported into Image-J software (National Institutes of Health) and analyzed morphometrically. *P < 0.05 versus control mice (2M). One of three independent experiments is shown. The results from three independent experiments were quantitated and expressed as mean ± SD. HF, high fat feeding mice.
Discussion
This study correlated, for the first time, changes in systemic and retinal indices of oxidative stress and inflammation associated with either diet induced insulin resistance or diabetes on retinal VEGF expression. Past studies have focused mainly on the activation of these pathways by diabetes alone and their correlation to increasing retinal VEGF expression, which is a pathogenic marker for diabetic macular edema and proliferative retinopathy.2,8,9,22–25 The elevated expression of VEGF in the retina or vitreous is the only retinal molecular marker of DR, which has been validated by clinical trials using anti-VEGF therapy.10,11 Multiple studies have associated changes in oxidative stress and inflammation with various retinal changes including elevated VEGF expression in DR, in animal models of diabetes, or in retinal vascular cells exposed to elevated glucose levels.13,14,24–28 However, it has not been established that these changes are essential or additive for inducing elevated retinal VEGF expression by diabetes in vivo.
The results from this comparative analysis in rodent models of diabetes and obesity associated insulin resistance showed that VEGF expression in the retina was only induced by diabetes, but not by obesity or insulin resistant states alone where hyperglycemia is not present. Surprisingly, the increases in VEGF expression were not observed until after 2 months of diabetes and were not significant in obese rats (ZF) without diabetes even though systemic markers of oxidative stress and inflammation were clearly elevated. The finding of elevated systemic inflammation markers and oxidative stress in insulin resistant states and diabetes are not surprising since obesity, hyperlipidemia, and elevated free fatty acids, in addition to hyperglycemia, have all been reported to induce the expression of inflammatory cytokines, toll-like receptors, and oxidant formation in many tissues including the blood vessels.16,29,30 We only studied ZF rats at 24 to 26 weeks, which may not be long enough to detect changes in retinal VEGF expression. However, it is likely that obesity and insulin resistance without diabetes will cause significant VEGF elevation since it is very rare that obese patients with metabolic syndrome, but without diabetes develop macular edema or proliferative diabetic retinopathy.
Elevation of retinal VEGF expression correlated only with increased levels of inflammatory cytokines and oxidases in the retina after they persisted for 2 to 4 months. This is clearly supported by the elevation of all the indicators of oxidative stress and inflammation in ZDF rats and STZ diabetic rats only after 4 months of disease duration. At 2 months of disease duration, some evidence of increased systemic oxidative stress and inflammation were present such as the elevation of p47phox in the retina, which correlated with increases in VEGF expression. In contrast to measurements of inflammatory cytokines and oxidative stress, diabetes was able to induce NF-κB activation in the retina as measured by both the binding and activation assays and NF-κB–GFP expression at 2 and 4 months of diabetes and in ZDF rats. However, our results clearly showed that insulin resistance and obesity without diabetes and hyperglycemia also activated NF-κB and possibly inflammation in the retina and shown in Figure 4 using ZF rats and high fat fed mice for 2 months. It is not surprising that insulin resistance or obesity could also induce inflammatory changes in the retina, since, it was previously reported that increases in leucocyte adhesion can be observed in both the retinal and systemic vessels in obese and insulin resistant animals similar to those observed in diabetes.14,31,32 These increases in leucocyte adhesion are correlated with the elevation of ICAM expression or inflammation markers.33,34 Thus, it is possible that increases in inflammation or oxidative stress alone are not sufficient to induce VEGF expression in vivo, without the presence of additional severe capillary endothelial dysfunction to induce hypoxia or decrease retinal perfusion induced by diabetes. These data also suggest that diabetes or hyperglycemia is activating other pathologic changes besides NF-κB, inflammation, or oxidative stress that will lead to capillary dysfunction and acellular capillaries. This conclusion is supported by clinical data, which clearly indicate that specific vascular lesions of diabetic retinopathy are rarely detected in people with obesity and severe insulin resistance without diabetes, although they clearly can manifest inflammation markers and oxidative stress.17,18 In addition, there are multiple diseases, which cause inflammation of the vasculature, yet they don't manifest retinal vascular lesions similar to DR.35 These findings strongly support the idea that hyperglycemia or diabetes is essential for the development of DR, and its progression requires hyperglycemia to activate mechanisms in addition to inflammation and oxidative stress. It is also possible that other metabolic changes induced specifically by diabetes could also play a role with hyperglycemia to induce changes in VEGF expression.
The presence of hyperglycemia in inducing retinal VEGF expression is clearly very important, which is likely due to its adverse effects on retinal pericytes and endothelial cells to cause pericyte apoptosis, acellular capillaries, and reduction in retinal perfusion.5,20,35–38 However, unlike pericyte apoptosis, the formation of acellular capillaries may not be specific to DR since other inhibitors such as ischemic reperfusion may also induce acellular capillary formation. Previously, we have reported ZF rats, derived from a different rodent colony, which had mean plasma glucose levels that were significantly higher, by 50%, than its control ZL rats, had significant increases in VEGF expression.39 In contrast, the mean plasma glucose levels in the present study were not different between ZL and ZF rats, resulting in the lack of VEGF levels being elevated. These data again supported the necessity of hyperglycemia to cause significant retinal vascular pathologies in order to induce VEGF expression in the retina. We have reported that one potential mechanism to induce pericyte dysfunction and apoptosis, independent of oxidative and inflammatory stress, is the activation of SHP-1 by hyperglycemia.40 The activation of SHP-1, a tyrosine phosphatase, which can be induced by hyperglycemia and PKC-δ, inhibited the actions of platelet derived growth factor, a pericyte survival factor, causing pericyte apoptosis by mechanisms independent of inflammation, oxidative stress and NF-κB.40
In summary, both hyperglycemia and obesity related insulin resistance can elevate systemic and retinal oxidative stress and inflammation. However, hyperglycemia or diabetes are necessary to increase retinal VEGF expression, possibly by activating additional mechanisms that can induce significant capillary pathology, hypoperfusion, and hypoxia. Thus, future effective therapeutic interventions for diabetic retinopathy will require neutralizing hyperglycemia's multiple adverse effects in addition to anti-oxidative and anti-inflammatory agents.
Supplementary Material
Footnotes
Supported by Grants DK053105 and R01EY016150 from the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (GLK) and EY018677 from the National Institutes of Health (CRM), a Research Fellowship (Manpei Suzuki Diabetes Foundation, Kanzawa Medical Research Foundation, NOVARTIS Foundation, Japan [AM]), Diabetes Endocrinology Research Center (P30DK036836), and a Juvenile Diabetes Research Foundation Postdoctural Fellowship (WQ).
Disclosure: A. Mima, None; W. Qi, None; J. Hiraoka-Yamomoto, None; K. Park, None; M. Matsumoto, None; M. Kitada, None; Q. Li, None; K. Mizutani, None; E. Yu, None; T. Shimada, None; J. Lee, None; S.E. Shoelson, None; C. Jobin, None; C. Rask-Madsen, None; G.L. King, None
References
- 1.Klein R, Klein BE, Moss SE. Epidemiology of proliferative diabetic retinopathy. Diabetes Care. 1992;15:1875–1891 [DOI] [PubMed] [Google Scholar]
- 2.Miller JW, Adamis AP, Aiello LP. Vascular endothelial growth factor in ocular neovascularization and proliferative diabetic retinopathy. Diabetes Metab Rev. 1997;13:37–50 [DOI] [PubMed] [Google Scholar]
- 3.Kohner EM, Stratton IM, Aldington SJ, Holman RR, Matthews DR. Relationship between the severity of retinopathy and progression to photocoagulation in patients with type 2 diabetes mellitus in the UKPDS (UKPDS 52). Diabet Med. 2001;18:178–184 [DOI] [PubMed] [Google Scholar]
- 4.Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. N Engl J Med. 2000;342:1376. [DOI] [PubMed] [Google Scholar]
- 5.Antonetti DA, Klein R, Gardner TW. Mechanisms of disease: diabetic retinopathy. N Engl J Med. 2012;366:1227–1239 [DOI] [PubMed] [Google Scholar]
- 6.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611 [DOI] [PubMed] [Google Scholar]
- 7.Pierce EA, Avery RL, Foley ED, Aiello LP, Smith LE. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci U S A. 1995;92:905–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480–1487 [DOI] [PubMed] [Google Scholar]
- 9.Adamis AP, Miller JW, Bernal MT, et al. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994;118:445–450 [DOI] [PubMed] [Google Scholar]
- 10.Diabetic Retinopathy Clinical Research Network, Elman MJ, Aiello LP, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117:1064–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avery RL, Pearlman J, Pieramici DJ, et al. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology. 2006;113:1695.e1–e15 [DOI] [PubMed] [Google Scholar]
- 12.Kowluru RA, Odenbach S. Role of interleukin-1 beta in the development of retinopathy in rats: effect of antioxidants. Invest Ophthalmol Vis Sci. 2004;45:4161–4166 [DOI] [PubMed] [Google Scholar]
- 13.Hammes HP, Feng Y, Pfister F, Brownlee M. Diabetic retinopathy: targeting vasoregression. Diabetes. 2011;60:9–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joussen AM, Poulaki V, Le ML, et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEBJ. 2004;18:1450–1452 [DOI] [PubMed] [Google Scholar]
- 15.Zhang B, Hu Y, Ma JX. Anti-inflammatory and antioxidant effects of SERPINA3K in the retina. Invest Ophthalmol Vis Sci. 2009;50:3943–3952 [DOI] [PubMed] [Google Scholar]
- 16.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klein R, Barrett-Connor EL, Blunt BA, Wingard DL. Visual impairment and retinopathy in people with normal glucose tolerance, impaired glucose tolerance, and newly diagnosed NIDDM. Diabetes Care. 1991;14:914–918 [DOI] [PubMed] [Google Scholar]
- 18.Singleton JR, Smith AG, Russell JW, Feldman EL. Microvascular complications of impaired glucose tolerance. Diabetes. 2003;52:2867–2873 [DOI] [PubMed] [Google Scholar]
- 19.Siebenlist U, Franzoso G, Brown K. Structure, regulation and function of NF-kappa B. Annu Rev Cell Biol. 1994;10:405–455 [DOI] [PubMed] [Google Scholar]
- 20.Romeo G, Liu WH, Asnaghi V, Kern TS, Lorenzi M. Activation of nuclear factor-kappaB induced by diabetes and high glucose regulates a proapoptotic program in retinal pericytes. Diabetes. 2002;51:2241–2248 [DOI] [PubMed] [Google Scholar]
- 21.Magness ST, Jijon H, Van Houten Fisher N, Sharpless NE, Brenner DA, Jobin C. In vivo pattern of lipopolysaccharide and anti-CD3-induced NF-kappa B activation using a novel gene-targeted enhanced GFP reporter gene mouse. J Immunol. 2004;173:1561–1570 [DOI] [PubMed] [Google Scholar]
- 22.Nguyen QD, Tatlipinar S, Shah SM, et al. Vascular endothelial growth factor is a critical stimulus for diabetic macular edema. Am J Ophthalmol. 2006;142:961–969 [DOI] [PubMed] [Google Scholar]
- 23.Aiello LP, Bursell SE, Clermont A, et al. Vascular endothelial growth factor-induced retinal permeability is mediated by protein kinase C in vivo and suppressed by an orally effective beta-isoform-selective inhibitor. Diabetes. 1997;46:1473–1480 [DOI] [PubMed] [Google Scholar]
- 24.Aiello LP. Clinical implications of vascular growth factors in proliferative retinopathies. Curr Opin Ophthalmol. 1997;8:19–31 [DOI] [PubMed] [Google Scholar]
- 25.Caldwell RB, Bartoli M, Behzadian MA, et al. Vascular endothelial growth factor and diabetic retinopathy: role of oxidative stress. Curr Drug Targets. 2005;6:511–524 [DOI] [PubMed] [Google Scholar]
- 26.Al-Shabrawey M, Rojas M, Sanders T, et al. Role of NADPH oxidase in retinal vascular inflammation. Invest Ophthalmol Vis Sci. 2008;49:3239–3244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang B, Abreu JG, Zhou K, et al. Blocking the Wnt pathway, a unifying mechanism for an angiogenic inhibitor in the serine proteinase inhibitor family. Proc Natl Acad Sci U S A. 2010;107:6900–6905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang J, Xu X, Elliott MH, Zhu M, Le YZ. Müller cell-derived VEGF is essential for diabetes-induced retinal inflammation and vascular leakage. Diabetes. 2010;59:2297–2305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mas S, Martinez-Pinna R, Martin-Ventura JL, et al. Local non-esterified fatty acids correlate with inflammation in atheroma plaques of patients with type 2 diabetes. Diabetes. 2010;59:1292–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saberi M, Woods NB, de Luca C, et al. Hematopoietic cell-specific deletion of toll-like receptor 4 ameliorates hepatic and adipose tissue insulin resistance in high-fat-fed mice. Cell Metab. 2009;10:419–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kowluru RA, Zhong Q, Kanwar M. Metabolic memory and diabetic retinopathy: role of inflammatory mediators in retinal pericytes. Exp Eye Res. 2010;90:617–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abiko T, Abiko A, Clermont AC, et al. Characterization of retinal leukostasis and hemodynamics in insulin resistance and diabetes: role of oxidants and protein kinase-C activation. Diabetes. 2003;52:829–837 [DOI] [PubMed] [Google Scholar]
- 33.Roberts CK, Won D, Pruthi S, Lin SS, Barnard RJ. Effect of a diet and exercise intervention on oxidative stress, inflammation and monocyte adhesion in diabetic men. Diabetes Res Clin Pract. 2006;73:249–259 [DOI] [PubMed] [Google Scholar]
- 34.Leal EC, Martins J, Voabil P, et al. Calcium dobesilate inhibits the alterations in tight junction proteins and leukocyte adhesion to retinal endothelial cells induced by diabetes. Diabetes. 2010;59:2637–2645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuusniemi AM, Lapatto R, Holmberg C, Karikoski R, Rapola J, Jalanko H. Kidneys with heavy proteinuria show fibrosis, inflammation, and oxidative stress, but no tubular phenotypic change. Kidney Int. 2005;68:121–132 [DOI] [PubMed] [Google Scholar]
- 36.Darland DC, Massingham LJ, Smith SR, Piek E, Sant-Geniez M, D'Amore PA. Pericyte production of cell-associated VEGF is differentiation-dependent and is associated with endothelial survival. Dev Biol. 2003;264:275–288 [DOI] [PubMed] [Google Scholar]
- 37.Vidro EK, Gee S, Unda R, Ma JX, Tsin A. Glucose and TGFbeta2 modulate the viability of cultured human retinal pericytes and their VEGF release. Curr Eye Res. 2008;33:984–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cukiernik M, Hileeto D, Evans T, Mukherjee S, Downey D, Chakrabarti S. Vascular endothelial growth factor in diabetes induced early retinal abnormalities. Diabetes Res Clin Pract. 2004;65:197–208 [DOI] [PubMed] [Google Scholar]
- 39.Zheng L, Gong B, Hatala DA, Kern TS. Retinal ischemia and reperfusion causes capillary degeneration: similarities to diabetes. Invest Ophthalmol Vis Sci. 2007;48:361–367 [DOI] [PubMed] [Google Scholar]
- 40.Chou E, Suzuma I, Way KJ, et al. Decreased cardiac expression of vascular endothelial growth factor and its receptors in insulin-resistant and diabetic states: a possible explanation for impaired collateral formation in cardiac tissue. Circulation. 2002;105:373–379 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.