Abstract
Programmed death 1 (PD-1) is a potent inhibitor of T cell responses. PD-1 abrogates activation of the phosphatidylinositol 3-kinase (PI3K)/Akt pathway, but the mechanism remains unclear. We determined that during T cell receptor (TCR)/CD3- and CD28-mediated stimulation, PTEN is phosphorylated by casein kinase 2 (CK2) in the Ser380-Thr382-Thr383 cluster within the C-terminal regulatory domain, which stabilizes PTEN, resulting in increased protein abundance but suppressed PTEN phosphatase activity. PD-1 inhibited the stabilizing phosphorylation of the Ser380-Thr382-Thr383 cluster within the C-terminal domain of PTEN, thereby resulting in ubiquitin-dependent degradation and diminished abundance of PTEN protein but increased PTEN phosphatase activity. These effects on PTEN were secondary to PD-1-mediated inhibition of CK2 and were recapitulated by pharmacologic inhibition of CK2 during TCR/CD3- and CD28-mediated stimulation without PD-1. Furthermore, PD-1-mediated diminished abundance of PTEN was reversed by inhibition of ubiquitin-dependent proteasomal degradation. Our results identify CK2 as a new target of PD-1 and reveal an unexpected mechanism by which PD-1 decreases PTEN protein expression while increasing PTEN activity, thereby inhibiting the PI3K/Akt signaling axis.
INTRODUCTION
Extensive experimental evidence indicates that the pathway of the receptor programmed death 1 (PD-1) (CD279) and its ligands, PD-L1 (B7-H1; CD274) and PD-L2 (B7-DC; CD273), plays a vital role in the induction and maintenance of anergy and peripheral tolerance. This pathway also regulates the balance between stimulatory and inhibitory signals needed for effective immunity and maintenance of T cell homeostasis (1). In spite of the compelling studies on the significant functional role of PD-1 in mediating inhibitory signals on activated T cells, little is known about how PD-1 blocks T cell activation. One of the critical signaling pathways targeted by PD-1 is the phosphatidylinositol 3-kinase (PI3K)/Akt pathway, by which PD-1 mediates inhibitory effects on T cell proliferation, cytokine production, and glucose uptake (2, 3). It has been proposed that PD-1 inhibits PI3K/Akt by upregulating PTEN (phosphatase and tensin homolog deleted on chromosome 10) expression (4), but the mechanism remains unclear.
PI3K catalyzes the production of the lipid second messenger phosphatidylinositol 3,4,5-trisphosphate [PI(3,4,5)P3] (PIP3), thereby recruiting and activating several downstream kinases, including its most prominent effector, Akt (also known as protein kinase B [PKB]). The main negative regulator of the PI3K/Akt pathway is PTEN, which is thought to act predominantly as a lipid phosphatase, dephosphorylating PIP3 at the 3′ position to generate PI(4,5)P2 (5). By limiting the amount of PIP3 available within the cell, PTEN directly opposes the activation of PI3K and therefore blunts the survival and proliferative signal delivered by the PI3K/Akt pathway (6). Deletions or mutations of the PTEN gene have been observed in various cancers, ultimately resulting in decreased or absent PTEN protein expression and activity (7, 8). T cell-specific deletion of PTEN results in T cell lymphoproliferation, autoimmunity, and lymphoma-induced death (9, 10). The importance of PTEN catalytic activity in its function is underscored by the fact that the majority of PTEN missense mutations detected in tumor specimens target the phosphatase domain and cause a loss of PTEN phosphatase activity. However, a large number of PTEN nonsense or frameshift mutations found in tumors are targeted to the C-terminal domain of the protein, suggesting an important role for the domain in the regulation of the PTEN tumor suppressor activity (11, 12).
Structurally, PTEN consists of an amino-terminal phosphatase domain, a C2 domain, and a PDZ binding motif. The C2 domain directs PTEN binding to phospholipid vesicles in vitro, and mutations that abrogate vesicle binding without affecting catalysis disrupt PTEN function (13). PTEN also contains a 50-amino-acid C-terminal domain (amino acid residues 354 to 403), referred to as the “tail.” A number of studies have shown that the tail is dispensable for phosphatase activity (12, 13). Instead, the PTEN tail is necessary for maintaining protein stability and also acts to inhibit PTEN function. Removing the tail results in a loss of stability, but while unstable, the resultant protein is more active. Tail-dependent regulation of PTEN stability and activity is linked to the phosphorylation of 3 residues, S380, T382, and T383, within the tail. Phosphorylation-defective mutations of these sites lead to a loss of stability and a gain of PTEN phosphatase activity (14, 15). Therefore, the C-terminal tail regulates PTEN function through phosphorylation.
The kinase responsible for phosphorylation of the PTEN C terminus is casein kinase 2 (CK2). CK2-mediated phosphorylation stabilizes PTEN by preventing its ubiquitin-dependent degradation while decreasing PTEN lipid phosphatase activity (15, 16). This regulatory mechanism accounts for the activation of the PI3K/Akt pathway in cancers, which display unmutated and normal levels of the PTEN gene but aberrant expression or activation of CK2 (17–19). Phosphorylation and inactivation of PTEN are specific targets by which CK2 mediates activation of the PI3K/Akt pathway, because inhibition of CK2 eliminates Akt phosphorylation in PTEN+/+ but not in PTEN−/− cells (16).
We examined the effects of PD-1 signaling on the regulation of PTEN expression and lipid phosphatase activity. We determined that PD-1 regulated PTEN lipid phosphatase activity by suppressing activation of CK2, resulting in diminished phosphorylation of the Ser380-Thr382-Thr383 cluster in the C-terminal regulatory domain of PTEN. Impaired CK2-mediated phosphorylation induced by PD-1 resulted in PTEN instability, leading to ubiquitin-dependent degradation and reduced PTEN abundance but increased PTEN lipid phosphatase activity. Pharmacological inhibition of CK2 during T cell activation by T cell receptor (TCR)/CD3 and CD28 recapitulated the effects of PD-1 and resulted in hypophosphorylation of PTEN, decreased protein abundance, and increased PTEN phosphatase activity. Our results identify CK2 as a new target of PD-1 and reveal an unexpected mechanism by which PD-1 regulates PTEN lipid phosphatase activity and inhibition of the PI3K/Akt pathway.
MATERIALS AND METHODS
In vitro T cell cultures.
Leukocytes were obtained from healthy blood donors. CD4+ T cells were isolated by negative selection using the CD4+ T cell Isolation Kit II from Miltenyi Biotec (Auburn, CA) and cultured with tosylactivated magnetic beads (1.5 × 105 beads/well) conjugated either with anti-CD3 and anti-CD28 monoclonal antibodies (MAbs) and IgG or with anti-CD3, anti-CD28, and anti-PD-1 MAbs, as previously described (20). Preparation of Dynabeads M-450 (Invitrogen) tosylactivated magnetic beads was done as previously described. Briefly, the following MAbs were used: anti-CD3 (UCHT1; R&D Systems, Inc.), anti-CD28 (CD28.2; Biolegend), agonist anti-human PD-1 (clone 17 [21], kindly provided by Pfizer Inc., Cambridge, MA), and control IgG (Jackson ImmunoResearch Laboratories, West Grove, PA). Magnetic beads (2 × 108) were coated with anti-CD3 (8%) and anti-CD28 (10%), and either anti-PD-1 (anti-PD-1/CD3/CD28) or control IgG (IgG/CD3/CD28) comprised the remaining 82% of the total protein. All incubations were performed in 0.1 M sodium phosphate buffer for 18 h at 37°C with constant rotation. The beads were then washed 3 times and stored at 4°C. In some experiments, T cells were cultured with tosylactivated magnetic beads coated with anti-CD3 and anti-CD28 MAbs, along with PD-L1–IgG2a fusion protein (kindly provided by Gordon Freeman, Dana-Farber Cancer Institute) in order to induce PD-1-mediated signaling via interaction with its natural ligand instead of anti-PD-1 agonist antibody. For inhibition of ubiquitin-dependent degradation in the proteasome pathway, the proteasome inhibitor MG132 (30 nM) or vehicle control was added for the last 6 h in cultures incubated with anti-CD3/CD28/PD-1-coated beads, and analysis for PTEN expression was performed. DNA synthesis was assessed by [3H]thymidine incorporation for the last 16 to 18 h of a 72-hour culture.
Immunoblotting.
Cell lysates were prepared as described previously (20), and equal amounts of protein from each sample were analyzed by SDS-PAGE. The antibodies for CK2α (sc-12738) and β-actin (sc-1615), as well as the immunoprecipitating PTEN (sc-7974) antibody, were from Santa Cruz Biotechnology, Santa Cruz, CA. The anti-phospho-PTEN (anti-PTEN) MAb (Ser380-Thr382-383) (number 9554) and anti-PTEN (number 9552) and anti-pAkt (number 9271) antibodies were from Cell Signaling Technologies, Danvers, MA. Where indicated, densitometric analysis was performed, and quantification of the integrated density for each band was assessed using the ImageJ 1.41o software.
PTEN lipid phosphatase activity.
For the measurement of in vitro PTEN lipid phosphatase activity, the malachite green phosphatase assay kit (Echelon Biosciences, Inc., Salt Lake City, UT) was used according to the manufacturer's instructions. Briefly, 500 μg of cell lysate was subjected to PTEN immunoprecipitation by the addition of 8 μl anti-PTEN MAb sc-7974 (Santa Cruz Biotechnology, Santa Cruz, CA), and the immunocomplex formed was captured by incubation with 20 μl protein A/G beads for 3.5 hours with gentle rotation at 4°C. The beads were then washed twice in lysis buffer and once in enzyme reaction buffer (ERB) (50 mM Tris-HCl, pH 8.0, 50 mM NaCl, 10 mM dithiothreitol [DTT], and 10 mM MgCl2) and resuspended in 80 μl prewarmed (37°C) ERB and distributed in triplicates of 20 μl in a 96-well flat-bottom plate (Echelon). The reaction was initiated by adding 30 μl of ERB containing the substratedioctanoyl phosphatidylinositol 3,4,5-trisphosphate (PIP3-DiC8) (P-3908; Echelon) to 10 μM final concentration; it was left for 1 h at 37°C and stopped by 100 μl malachite green solution (Echelon), and the absorbance was read at 620 nm after 15 min of incubation at room temperature. A PIP3-only blank was used in parallel to correct for potential nonspecific phosphate release. The remaining beads were used for SDS-PAGE, Western blotting, and densitometric quantification to confirm that equivalent amounts of PTEN were immunoprecipitated from all samples. A standard curve was made by using the phosphate solution provided with the kit. The PTEN activity was expressed as pmol phosphate released into the solution per hour per 20 μl of sample.
CK2 kinase activity.
For the measurement of in vitro CK2 activity, the Casein Kinase 2 assay kit (Millipore Corporation, Billerica. MA) was used according to the manufacturer's instructions. Briefly, 500 μg of cell lysate was subjected to CK2 immunoprecipitation by the addition of 10 μl anti-CK2 MAb sc-12738 (Santa Cruz Biotechnology, Santa Cruz, CA), and the immunocomplex formed was captured by incubation with 50 μl protein G beads for 2.5 hours with gentle rotation at 4°C. The beads were washed three times in lysis buffer without NP-40 and resuspended in 30 μl ADBI buffer (provided in the kit). Ten microliters of the sample was assayed either with or without substrate peptide in the presence of [γ-32P]ATP (PerkinElmer, Boston, MA) according to the manufacturer's instructions, and the remaining beads were used for SDS-PAGE, Western blotting, and densitometric quantification to confirm that equivalent amounts of CK2 were immunoprecipitated from all samples. The CK2 activity was expressed as pmol phosphate incorporated into the substrate peptide per min per 10 μl of sample by using the mathematical formula provided by the manufacturer of the kit. Each value was subsequently multiplied by 100 (representing pmol per min per ml of sample), and the resulting values were presented as arbitrary units (AU) in the graph. To induce pharmacologic inhibition of CK2, we used two different CK2 inhibitors, specifically, 4,5,6,7-tetrabromobenzotriazole (TBB) (EMD Millipore, Billerica, MA) or the newer inhibitor CX-4945 (Axon Medchem, Groningen, Netherlands). After testing a range of concentrations for potential nonspecific effects on cell viability, we chose the minimum concentration of TBB (50 μM) or CX-4945 (5 μM) that induced inhibition of T cell proliferation without affecting survival.
Assessment of PTEN expression with flow cytometry.
To assess PTEN expression by flow cytometry, we used a phycoerythrin-conjugated antibody against PTEN (number 560002; BD Biosciences, Chicago, IL) and intracellular staining according to the manufacturer's instructions. Dead cells were excluded by using the LIVE/DEAD Fixable Dead Cell Stain Kit (Life Technologies, Grand Island, NY) prior to intracellular staining.
Real-time quantitative PCR.
Total RNA extraction was performed with the RNeasy Mini Kit from Qiagen, Valencia, CA, according to the manufacturer's instructions, and 50 ng of RNA was subjected to quantitative-PCR analysis as described previously (20) for the PTEN and CK2α target genes with gene-specific primers (Applied Biosystems/Roche, Branchburg, NJ).
Statistical analysis.
In vitro assays and densitometry data were compared with the unpaired Student's t test or by analysis of variance (ANOVA) for more than two conditions. A P value of <0.05 was considered statistically significant.
RESULTS
PD-1 decreases PTEN protein expression but increases PTEN phosphatase activity.
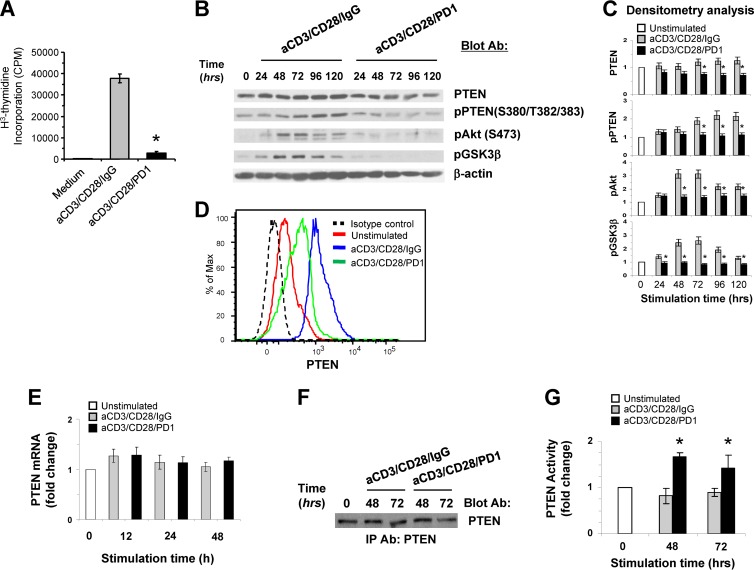
PD-1-mediated signaling inhibits activation of the PI3K/Akt pathway (2). It has been proposed that PD-1 interferes with the activation of the PI3K/Akt pathway by upregulating PTEN expression (4). However, the mechanism by which PD-1 might mediate this effect remains unclear. Previously, it was determined that PTEN protein stability and abundance inversely correlate with PTEN catalytic activity (14, 15). For this reason, we sought to dissect the molecular and biochemical effects of PD-1 signaling on PTEN expression and enzymatic function. We employed a previously established experimental system of highly purified resting human T cells and magnetic beads conjugated with anti-CD3 and anti-CD28 MAbs with or without agonist anti-PD-1 MAb (20), similar to an approach previously used in a mouse cell system (4). This approach has been designed to mimic the physiological conditions of primary T cell activation by antigen-presenting cells (APC), which leads to gradual upregulation of PD-1 in T cells during activation. As previously shown (20), culture with beads coated with anti-CD3, anti-CD28, and anti-PD-1 MAbs resulted in significant inhibition of T cell responses compared to culture with anti-CD3 and anti-CD28 in the absence of PD-1 ligation (Fig. 1A). As determined by immunoblotting, stimulation of T cells by TCR/CD3 and CD28 ligation induced an increase of PTEN protein expression that was apparent at 24 h and was further augmented during a 5-day culture period (Fig. 1B and C). In contrast, in T cells stimulated by TCR/CD3 and CD28 in the presence of PD-1 ligation, no increase of PTEN protein was observed. Instead, the abundance of PTEN protein was reduced compared to T cells stimulated by TCR/CD3 and CD28 ligation for the same time intervals (Fig. 1B and C). To determine whether such changes in PTEN protein abundance occurred at a single-cell level, we assessed PTEN expression by intracellular staining and flow cytometry. T cells stimulated by TCR/CD3 and CD28 in the presence of PD-1 displayed lower levels of PTEN expression than T cells stimulated without PD-1 signals (Fig. 1D).
Fig 1.
PD-1 stimulation results in decreased expression of PTEN protein but increased PTEN lipid phosphatase activity. (A) Purified CD4+ primary human T cells were incubated with tosylactivated magnetic beads conjugated with anti-CD3 MAb, anti-CD28 MAb, and IgG or with anti-CD3, anti-CD28, and anti-PD-1 MAbs. DNA synthesis was determined by addition of [3H]thymidine for the last 16 h of a 72-hour culture. The results are representative of seven independent experiments (*, P < 0.05, anti-CD3/CD28/PD1 versus anti-CD3/CD28/IgG). (B) Purified CD4+ primary human T cells were cultured with tosylactivated magnetic beads coated with anti-CD3/CD28/IgG or with anti-CD3/CD28/PD1 MAb. At the indicated time points, equal amounts of protein from each sample were analyzed by SDS-PAGE, and expression of PTEN, pPTEN, pAkt, and pGSK3β was assessed by immunoblotting. (C) Bar graphs showing the densitometric analysis of the abundance of each indicated protein normalized to β-actin, expressed as fold change relative to mean values before stimulation (defined as 1). The data are presented as means ± standard errors of the mean (SEM). Fold changes in the abundance of the indicated proteins for each time point of culture were compared between T cells cultured with anti-CD3/CD28/IgG and T cells cultured with anti-CD3/CD28/PD1 MAbs (*, P < 0.05; n = 5 experiments). (D) Expression of intracellular PTEN after culture under the indicated conditions for various time intervals (24 to 96 h) was examined by flow cytometry. The results at 48 h of culture are shown and are representative of three experiments. (E) PTEN mRNA was analyzed by real-time quantitative PCR, and relative expression of mRNA over the levels expressed in unstimulated cells (defined as 1) is shown. Expression levels of PTEN mRNA were comparable at each time point between cells stimulated with anti-CD3/CD28/IgG or with anti-CD3/CD28/PD1 MAb (P > 0.05; n = 3). (F and G) Equal amounts of PTEN were immunoprecipitated (IP) from the indicated samples and were subjected to SDS-PAGE and immunoblotting (F) or to malachite green-based in vitro lipid phosphatase activity (G). (G) Bar graphs showing PTEN lipid phosphatase activity expressed as fold change relative to mean values before stimulation (defined as 1). At each time point, the change in the activity of PTEN in anti-CD3/CD28/PD-1-stimulated cells was compared to that in anti-CD3/CD28/IgG-stimulated cells (*, P < 0.05; n = 3 experiments).
The mechanisms that regulate PTEN expression upon TCR-mediated stimulation have not been elucidated. We examined whether the altered abundance of PTEN protein during T cell stimulation in the presence or in the absence of PD-1 might be secondary to transcriptional regulation of PTEN at the mRNA level. As determined by real-time quantitative PCR, at all time points of culture, PTEN mRNA levels were comparable in T cells stimulated either in the presence or in the absence of PD-1 ligation (Fig. 1E). This finding indicates that PD-1 alters the abundance of PTEN by posttranscriptional mechanisms. Studies in other cell types have shown that the stability and abundance of PTEN protein are regulated by phosphorylation of the serine-threonine cluster (Ser380-Thr382-Thr383) in the C-terminal regulatory region of PTEN (14, 15). Strikingly, immunoblotting with phosphospecific antibody for the C-terminal Ser380-Thr382-Thr383 cluster revealed that T cells stimulated via TCR/CD3 and CD28 displayed prominent phosphorylation of PTEN in this region. In contrast, in T cells stimulated in the presence of PD-1, phosphorylation of the C-terminal Ser380-Thr382-Thr383 cluster was significantly reduced (Fig. 1B and C). Concomitantly with the attenuated PTEN phosphorylation and the diminished abundance of PTEN protein in T cells receiving PD-1 signals, activation of the PI3K/Akt pathway was abrogated, as determined by phosphorylation of Akt and its direct downstream target, glycogen synthase kinase 3β (GSK3β) (Fig. 1B and C). This pattern of diminished PTEN expression and phosphorylation coinciding with inhibition of Akt phosphorylation and blockade of T cell proliferation was also observed when PD-L1–Ig fusion protein was used to induce PD-1-mediated signaling (data not shown).
The apparent contradiction between PTEN protein abundance/phosphorylation and the activation of the PI3K/Akt pathway in T cells receiving PD-1 signals prompted us to analyze PTEN phosphatase activity. Assessment of PTEN lipid phosphatase activity showed that the low expression of PTEN in stimulated T cells receiving PD-1 signals was not associated with diminished phosphatase activity, but rather with increased PTEN lipid phosphatase activity compared to T cells stimulated without PD-1 ligation (Fig. 1F and G).
PD-1 inhibits CK2 expression and kinase activity.
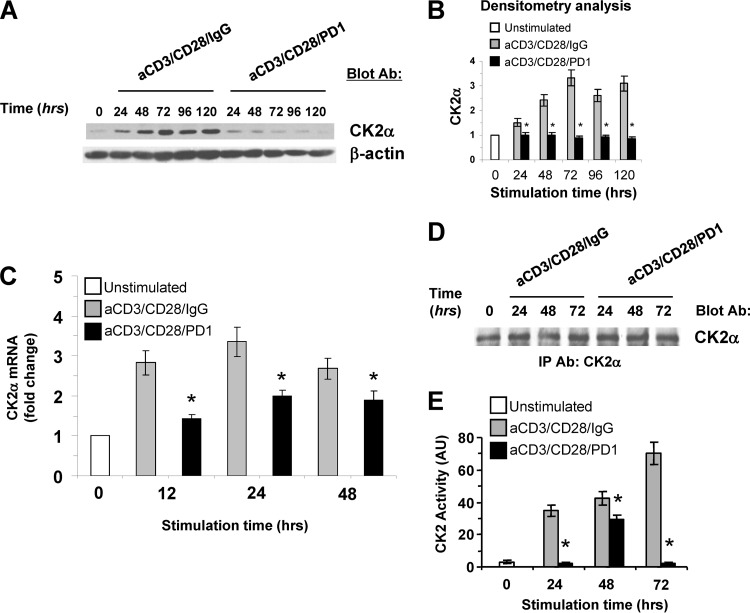
PTEN is a serine-threonine phosphoprotein and a substrate of CK2 in vitro and in vivo. CK2-mediated phosphorylation inhibits PTEN activity toward its substrate, PIP3 (15, 16). Because our studies showed that PD-1 ligation caused a decrease in PTEN phosphorylation and increased PTEN phosphatase activity, we examined the expression and activation of CK2. CK2 is generally found as a tetramer with two catalytic subunits (CK2α and/or CK2α′) and two regulatory subunits (CK2β) (22). Transgenic mice with targeted expression of the CK2α subunit in T cells develop lymphomas (23). For these reasons, we examined the expression and activation of CK2α (hereafter referred to as CK2) during T cell stimulation in the presence or in the absence of PD-1-mediated signaling. We determined that CK2 was constitutively expressed but that its abundance was increased during TCR/CD3 and CD28 stimulation. PD-1 induced a potent inhibitory effect on CK2 upregulation and resulted in significantly diminished abundance of CK2 protein compared to that in T cells stimulated without PD-1 ligation for the same time intervals (Fig. 2A and B). Because our studies showed that T cells displayed a low steady-state level of CK2 protein, which was upregulated by stimulation via TCR/CD3 and CD28, we examined whether CK2 expression was regulated at the level of mRNA. Real-time quantitative PCR showed that CK2 mRNA was increased after T cell activation. However, at all time intervals of culture, CK2 mRNA levels were significantly lower in T cells receiving PD-1 signals than in T cells stimulated by TCR/CD3 and CD28 without PD-1 (Fig. 2C).
Fig 2.
PD-1 negatively regulates protein kinase CK2. (A) Purified CD4+ primary human T cells were cultured with tosylactivated magnetic beads coated with anti-CD3/CD28/IgG or with anti-CD3/CD28/PD1 MAb. Cell lysates were prepared at the indicated time points, equal amounts of protein were analyzed by SDS-PAGE, and expression of CK2α was assessed by immunoblotting. (B) Bar graphs showing densitometric analysis of the abundance of CK2α normalized to β-actin, expressed as the fold change relative to mean values before stimulation (defined as 1). The data are presented as means ± SEM. Fold changes in the abundance of CK2a for each time point of culture were compared between T cells cultured with anti-CD3/CD28/IgG and T cells cultured with anti-CD3/CD28/PD1 MAbs (*, P < 0.05; n = 3 experiments). (C) mRNA for CK2α was analyzed by real-time quantitative PCR, and relative expression of mRNA over the levels expressed in unstimulated cells (defined as 1) is shown. At each time point, comparisons were made between T cells cultured with anti-CD3/CD28/IgG and T cells cultured with anti-CD3/CD28/PD1 MAbs (*, P < 0.05; n = 3). (D and E) Equal amounts of CK2α were immunoprecipitated from the indicated samples and subjected to SDS-PAGE and immunoblotting (D) or to in vitro kinase assay (E) as described in Materials and Methods. At each time point, comparisons were made between T cells cultured with anti-CD3/CD28/IgG and T cells cultured with anti-CD3/CD28/PD1 MAbs (*, P < 0.05; n = 3).
To determine whether, in addition to expression, PD-1 regulated CK2 activation, we examined kinase activity in equal amounts of CK2 immunoprecipitated from these samples, using an antibody specific for the CK2α catalytic subunit (Fig. 2D). As determined by in vitro kinase reaction, at all time points tested, PD-1 induced potent suppression of CK2 activity, which displayed a limited and transient upregulation at 48 h but was comparable to that in unstimulated cells at 24 and 72 h of culture (Fig. 2E). These results identify CK2 as a new target of PD-1.
Pharmacological inhibition of CK2 during T cell stimulation via TCR/CD3 and CD28 results in diminished PTEN expression and increased PTEN phosphatase activity.
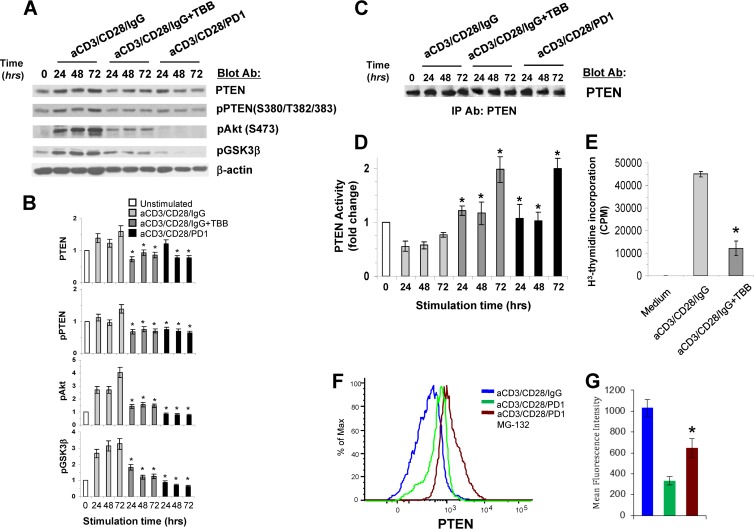
To further investigate the role of PD-1-mediated inhibition of CK2 in regulation of PTEN, we used TBB, a specific inhibitor of CK2 (24). We examined whether addition of TBB during T cell stimulation via TCR/CD3 and CD28 would recapitulate the effects of PD-1 in reducing PTEN abundance and C-terminal phosphorylation and in increasing PTEN phosphatase activity. Inhibition of CK2 during stimulation via TCR/CD3 and CD28 recapitulated the effects of PD-1 and resulted in diminished PTEN expression and phosphorylation in the C-terminal regulatory region. These events were associated with diminished activation of the PI3K/Akt pathway, as determined by impaired phosphorylation of Akt and its downstream target, GSK3β (Fig. 3A and B). Assessment of PTEN activity showed that T cell stimulation via TCR/CD3 and CD28 in the presence of TBB resulted in significantly increased lipid phosphatase activity of PTEN compared to T cells stimulated without TBB (Fig. 3C and D). Furthermore, CK2 inhibition by TBB during stimulation via TCR/CD3 and CD28 inhibited T cell proliferation (Fig. 3E). CX-4945, a newer and more potent CK2 inhibitor, also induced a similar pattern of diminished PTEN expression and PTEN phosphorylation in the C-terminal S380-T382-T383 cluster while increasing PTEN lipid phosphatase activity and inhibiting T cell proliferation induced via TCR/CD3- and CD28-mediated stimulation (data not shown). Thus, inhibition of CK2 during stimulation via TCR/CD3 and CD28 recapitulated the effects of PD-1 on PTEN expression and activity and the biological effects of PD-1 on T cell proliferation and expansion. These findings suggest that at least one mechanism by which PD-1 inhibits PI3K/Akt depends on PD-1-mediated inhibition of CK2.
Fig 3.
The specific CK2 inhibitor TBB decreases PTEN expression and phosphorylation during TCR/CD3- and CD28-mediated stimulation of T cells. (A) Purified CD4+ primary human T cells were cultured with tosylactivated magnetic beads coated with anti-CD3/CD28/IgG with or without the CK2-specific inhibitor TBB (50 μM) or with anti-CD3/CD28/PD1 MAbs. At the indicated time points, equal amounts of protein were analyzed by SDS-PAGE, and expression of PTEN, pPTEN, pAkt, and pGSK3β was assessed by immunoblotting. (B) Bar graphs showing the densitometric analysis of the abundance of each indicated protein normalized to that of β-actin, expressed as the fold change relative to mean values before stimulation (defined as 1). The data are presented as means ± SEM. Fold changes in the abundance of the indicated proteins for each time point of culture were compared between T cells cultured with anti-CD3/CD28/IgG and T cells cultured with anti-CD3/CD28/PD1 MAbs (*, P < 0.05; n = 3 experiments). (C and D) Equal amounts of PTEN were immunoprecipitated from the indicated samples and subjected to SDS-PAGE and immunoblotting (C) or to malachite green-based in vitro lipid phosphatase activity (D). Fold changes in PTEN phosphatase activity in T cells cultured with anti-CD3/CD28/IgG plus TBB or with anti-CD3/CD28/PD-1 were compared to those of T cells cultured with anti-CD3/CD28/IgG beads (*, P < 0.05; n = 3 experiments). (E) CD4+ primary T cells were cultured with tosylactivated magnetic beads coated with anti-CD3/CD28/IgG with or without the CK2-specific inhibitor TBB (50 μM), and DNA synthesis was determined by addition of [3H]thymidine for the last 16 h of a 72-hour culture. The results are representative of three independent experiments (*, P < 0.05). (F and G) Proteasome inhibition reverses PD-1-mediated decrease of PTEN abundance. (F) T cells were cultured under the indicated conditions for various time intervals (24 to 96 h), and the amounts of intracellular PTEN were examined by flow cytometry. The results at 48 h of culture are shown and are representative of three independent experiments. (G) Bar graphs depicting means ± SEM of the mean fluorescence intensity (MFI) of PTEN expression. Comparisons of MFIs were made between T cells cultured with anti-CD3/CD28/PD1 and T cells cultured with anti-CD3/CD28/PD1 and MG132 (*, P < 0.05; n = 3).
PD-1 induces PTEN instability and ubiquitin-dependent proteasomal degradation.
Phosphorylation of PTEN regulates PTEN protein stability. Specifically, phosphorylation of the C-terminal region renders PTEN resistant to ubiquitin-dependent degradation in the proteasome and results in PTEN protein stability. In contrast, impaired phosphorylation of the PTEN tail results in loss of PTEN protein stability and increased ubiquitin-dependent degradation (15). Because our studies showed that PD-1-mediated signaling suppressed PTEN phosphorylation in the C-terminal Ser380-Thr382-Thr383 cluster, we examined whether decreased abundance of PTEN protein in T cells receiving PD-1 signals might be mediated via ubiquitin-dependent proteasomal degradation. Incubation with the proteasome inhibitor MG132 significantly diminished the downregulation of PTEN expression induced in stimulated T cells receiving PD-1 signals (Fig. 3F and G). Thus, PD-1 decreases PTEN protein expression by promoting PTEN instability and ubiquitin-dependent degradation.
DISCUSSION
Our studies revealed an unexpected mechanism by which PD-1 inhibits activation of the PI3K/Akt pathway, which involves regulation of PTEN stability and phosphatase activity via CK2-mediated phosphorylation. Because PD-1 inhibits the PI3K/Akt pathway (2), initially, we were surprised to observe a decrease rather than an increase of PTEN protein abundance in T cells receiving PD-1 signals. Our experiments revealed that this paradox is due to PD-1-induced inhibition of CK2-mediated phosphorylation of the PTEN C-terminal serine-threonine cluster S380-T382-T383, which promotes PTEN protein stability while reducing PTEN lipid phosphatase activity. Consequently, in T cells receiving PD-1 signals, PTEN is susceptible to ubiquitin-dependent degradation, but the remaining PTEN protein has increased phosphatase activity, leading to inhibition of the PI3K/Akt pathway. Our studies also reveal a physiological mechanism by which TCR-mediated activation increases PTEN stability while decreasing PTEN phosphatase activity. This mechanism is supported by our finding that, after T cell activation, high levels of Akt phosphorylation coincide with increased PTEN protein expression and phosphorylation of the PTEN C-terminal serine-threonine cluster S380-T382-T383. These findings are also consistent with the regulation of PTEN expression and activity in other cell systems (14, 15). A previous study reported that, compared to stimulation without PD-1, culture in the presence of PD-1 ligation resulted in increased PTEN expression while decreasing Akt activation (4). Although in that system PTEN phosphorylation and PTEN lipid phosphatase activity were not examined, we hypothesize that the discrepancy between those observations and our present results is rather due to methodological reasons, because in all our experiments, we observed that during stimulation of primary T cells, Akt activation correlated and temporally coincided with increased PTEN protein levels, assessed by either Western blotting or flow cytometry, and with phosphorylation of the C-terminal serine-threonine cluster S380-T382-T383.
PTEN contains two PEST sequences that serve as targets of ubiquitination. In cancer cells, it has been proposed that polyubiquitination of PTEN by the E3 ubiquitin ligase NEDD4-1 leads to its degradation (25). In contrast monoubiquitination does not promote its degradation, but rather, results in increased nuclear import of PTEN with a subsequent role in the maintenance of chromosomal stability (26). We investigated whether such differential subcellular localization of PTEN might also be operative in our experimental system, but we were unable to detect any differences in the cytoplasmic versus nuclear distributions of PTEN in T cells under any culture conditions.
Our studies revealed that in T cells receiving TCR-mediated activation signals, PTEN is not regulated predominantly at the mRNA level but rather by posttranslational modification and inactivation mediated by CK2. Remarkably, the CK2 phosphorylation sites in PTEN are conserved in species from mammals to Xenopus laevis, and clusters of putative CK2 phosphorylation sites are also present at the C terminus of PTEN in Drosophila melanogaster, Caenorhabditis elegans, and Saccharomyces cerevisiae (22). Thus, CK2-mediated phosphorylation of PTEN is an evolutionarily conserved dominant mechanism regulating PTEN phosphatase activity, and this mechanism is directly targeted by PD-1 to mediate an inhibitory effect on the PI3K/Akt pathway in T cells.
Protein kinase CK2 is a highly conserved, ubiquitously expressed serine-threonine kinase that phosphorylates a wide variety of substrates involved in essential cell processes, including the cell cycle and cell growth (22). Alterations in CK2 expression have been found in tumors (17–19, 27, 28), and overexpression of CK2 affects cell growth and transformation (23, 29). Conversely, CK2 depletion inhibits cell cycle progression and growth (30, 31). The role of CK2 in T cell proliferation and growth by regulating PTEN is underlined by the observation that CK2 overexpression and hyperactivation are responsible for posttranslational inactivation of PTEN and hyperactivation of the PI3K/Akt pathway in the absence of PTEN gene mutations or deletions in the majority of T cell acute lymphoblastic leukemia (T-ALL) cases (17).
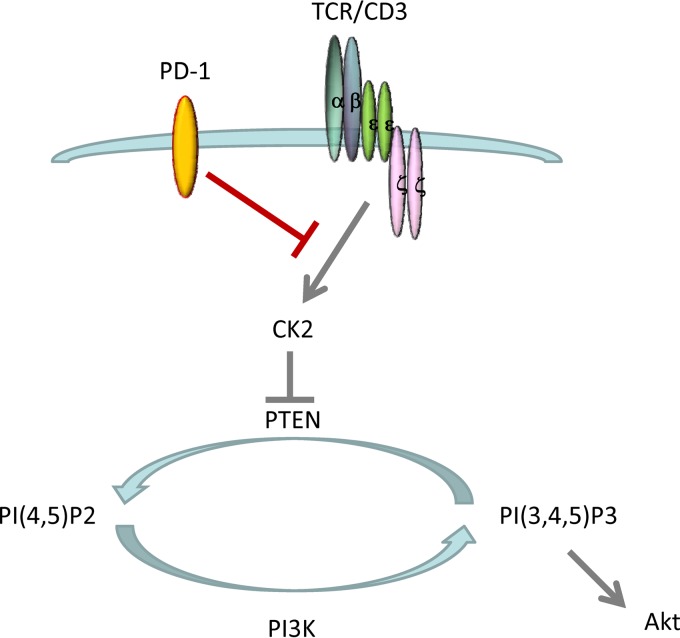
We determined that, in contrast to cancer cells, in which CK2 is constitutively highly activated, in T lymphocytes, CK2 activation is regulated by TCR-mediated signals. Upregulation and activation of CK2 lead to phosphorylation of the PTEN C-terminal region and inhibit PTEN activity. Thus, during TCR-mediated stimulation, PTEN phosphatase activity is inhibited in a CK2-dependent manner, allowing sustained activation of the PI3K/Akt pathway to support glucose uptake, survival, expansion, and generation of T effector cells. In contrast, PD-1 suppresses CK2 and results in impaired phosphorylation of the PTEN C-terminal serine-threonine cluster S380-T382-T383, increased PTEN phosphatase activity, and inhibition of PI3K/Akt (Fig. 4). The identification of CK2 as a new target of PD-1 will provide new insights into the elusive mechanisms by which PD-1 inhibits TCR proximal signaling.
Fig 4.
Model for TCR- and PD-1-mediated regulation of PTEN activity via CK2. TCR-mediated signals induce CK2 activation. CK2 leads to phosphorylation of the PTEN C-terminal serine-threonine cluster S380-T382-T383 and inhibits PTEN phosphatase activity. During TCR-mediated activation, PTEN phosphatase activity is inhibited in a CK2-dependent manner, allowing activation of the PI3K/Akt pathway. PD-1 suppresses CK2 and results in impaired phosphorylation of the PTEN C-terminal serine-threonine cluster S380-T382-T383, increased PTEN phosphatase activity, and inhibition of PI3K/Akt.
ACKNOWLEDGMENTS
This work was supported by NIH grants HL107997-01 and R56AI43552 and by the Leukemia and Lymphoma Society Translational Research Program (TRP 6222-11).
Footnotes
Published ahead of print 3 June 2013
REFERENCES
- 1. Francisco LM, Sage PT, Sharpe AH. 2010. The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 236:219–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Riley JL. 2009. PD-1 signaling in primary T cells. Immunol. Rev. 229:114–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, Linsley PS, Thompson CB, Riley JL. 2005. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol. Cell. Biol. 25:9543–9553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. 2009. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 206:3015–3029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maehama T, Dixon JE. 1998. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem. 273:13375–13378 [DOI] [PubMed] [Google Scholar]
- 6. Ramaswamy S, Nakamura N, Vazquez F, Batt DB, Perera S, Roberts TM, Sellers WR. 1999. Regulation of G1 progression by the PTEN tumor suppressor protein is linked to inhibition of the phosphatidylinositol 3-kinase/Akt pathway. Proc. Natl. Acad. Sci. U. S. A. 96:2110–2115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Podsypanina K, Ellenson LH, Nemes A, Gu J, Tamura M, Yamada KM, Cordon-Cardo C, Catoretti G, Fisher PE, Parsons R. 1999. Mutation of Pten/Mmac1 in mice causes neoplasia in multiple organ systems. Proc. Natl. Acad. Sci. U. S. A. 96:1563–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sansal I, Sellers WR. 2004. The biology and clinical relevance of the PTEN tumor suppressor pathway. J. Clin. Oncol. 22:2954–2963 [DOI] [PubMed] [Google Scholar]
- 9. Liu X, Karnell JL, Yin B, Zhang R, Zhang J, Li P, Choi Y, Maltzman JS, Pear WS, Bassing CH, Turka LA. 2010. Distinct roles for PTEN in prevention of T cell lymphoma and autoimmunity in mice. J. Clin. Invest. 120:2497–2507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Suzuki A, Yamaguchi MT, Ohteki T, Sasaki T, Kaisho T, Kimura Y, Yoshida R, Wakeham A, Higuchi T, Fukumoto M, Tsubata T, Ohashi PS, Koyasu S, Penninger JM, Nakano T, Mak TW. 2001. T cell-specific loss of Pten leads to defects in central and peripheral tolerance. Immunity 14:523–534 [DOI] [PubMed] [Google Scholar]
- 11. Tolkacheva T, Chan AM. 2000. Inhibition of H-Ras transformation by the PTEN/MMAC1/TEP1 tumor suppressor gene. Oncogene 19:680–689 [DOI] [PubMed] [Google Scholar]
- 12. Georgescu MM, Kirsch KH, Akagi T, Shishido T, Hanafusa H. 1999. The tumor-suppressor activity of PTEN is regulated by its carboxyl-terminal region. Proc. Natl. Acad. Sci. U. S. A. 96:10182–10187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee JO, Yang H, Georgescu MM, Di Cristofano A, Maehama T, Shi Y, Dixon JE, Pandolfi P, Pavletich NP. 1999. Crystal structure of the PTEN tumor suppressor: implications for its phosphoinositide phosphatase activity and membrane association. Cell 99:323–334 [DOI] [PubMed] [Google Scholar]
- 14. Vazquez F, Ramaswamy S, Nakamura N, Sellers WR. 2000. Phosphorylation of the PTEN tail regulates protein stability and function. Mol. Cell. Biol. 20:5010–5018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Torres J, Pulido R. 2001. The tumor suppressor PTEN is phosphorylated by the protein kinase CK2 at its C terminus. Implications for PTEN stability to proteasome-mediated degradation. J. Biol. Chem. 276:993–998 [DOI] [PubMed] [Google Scholar]
- 16. Miller SJ, Lou DY, Seldin DC, Lane WS, Neel BG. 2002. Direct identification of PTEN phosphorylation sites. FEBS Lett. 528:145–153 [DOI] [PubMed] [Google Scholar]
- 17. Silva A, Yunes JA, Cardoso BA, Martins LR, Jotta PY, Abecasis M, Nowill AE, Leslie NR, Cardoso AA, Barata JT. 2008. PTEN posttranslational inactivation and hyperactivation of the PI3K/Akt pathway sustain primary T cell leukemia viability. J. Clin. Invest. 118:3762–3774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. He L, Fan C, Gillis A, Feng X, Sanatani M, Hotte S, Kapoor A, Tang D. 2007. Co-existence of high levels of the PTEN protein with enhanced Akt activation in renal cell carcinoma. Biochim. Biophys. Acta 1772:1134–1142 [DOI] [PubMed] [Google Scholar]
- 19. Cheong JW, Eom JI, Maeng HY, Lee ST, Hahn JS, Ko YW, Min YH. 2003. Phosphatase and tensin homologue phosphorylation in the C-terminal regulatory domain is frequently observed in acute myeloid leukaemia and associated with poor clinical outcome. Br. J. Haematol. 122:454–456 [DOI] [PubMed] [Google Scholar]
- 20. Patsoukis N, Brown J, Petkova V, Liu F, Li L, Boussiotis VA. 2012. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci. Signal. 5:ra46. 10.1126/scisignal.2002796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bennett F, Luxenberg D, Ling V, Wang IM, Marquette K, Lowe D, Khan N, Veldman G, Jacobs KA, Valge-Archer VE, Collins M, Carreno BM. 2003. Program death-1 engagement upon TCR activation has distinct effects on costimulation and cytokine-driven proliferation: attenuation of ICOS, IL-4, and IL-21, but not CD28, IL-7, and IL-15 responses. J. Immunol. 170:711–718 [DOI] [PubMed] [Google Scholar]
- 22. Litchfield DW. 2003. Protein kinase CK2: structure, regulation and role in cellular decisions of life and death. Biochem. J. 369:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Seldin DC, Leder P. 1995. Casein kinase II alpha transgene-induced murine lymphoma: relation to theileriosis in cattle. Science 267:894–897 [DOI] [PubMed] [Google Scholar]
- 24. Sarno S, Reddy H, Meggio F, Ruzzene M, Davies SP, Donella-Deana A, Shugar D, Pinna LA. 2001. Selectivity of 4,5,6,7-tetrabromobenzotriazole, an ATP site-directed inhibitor of protein kinase CK2 (‘casein kinase-2′). FEBS Lett. 496:44–48 [DOI] [PubMed] [Google Scholar]
- 25. Wang X, Trotman LC, Koppie T, Alimonti A, Chen Z, Gao Z, Wang J, Erdjument-Bromage H, Tempst P, Cordon-Cardo C, Pandolfi PP, Jiang X. 2007. NEDD4-1 is a proto-oncogenic ubiquitin ligase for PTEN. Cell 128:129–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shen WH, Balajee AS, Wang J, Wu H, Eng C, Pandolfi PP, Yin Y. 2007. Essential role for nuclear PTEN in maintaining chromosomal integrity. Cell 128:157–170 [DOI] [PubMed] [Google Scholar]
- 27. ole-MoiYoi OK, Brown WC, Iams KP, Nayar A, Tsukamoto T, Macklin MD. 1993. Evidence for the induction of casein kinase II in bovine lymphocytes transformed by the intracellular protozoan parasite Theileria parva. EMBO J. 12:1621–1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Munstermann U, Fritz G, Seitz G, Lu YP, Schneider HR, Issinger OG. 1990. Casein kinase II is elevated in solid human tumours and rapidly proliferating non-neoplastic tissue. Eur. J. Biochem. 189:251–257 [DOI] [PubMed] [Google Scholar]
- 29. Li D, Dobrowolska G, Aicher LD, Chen M, Wright JH, Drueckes P, Dunphy EL, Munar ES, Krebs EG. 1999. Expression of the casein kinase 2 subunits in Chinese hamster ovary and 3T3 L1 cells provides information on the role of the enzyme in cell proliferation and the cell cycle. J. Biol. Chem. 274:32988–32996 [DOI] [PubMed] [Google Scholar]
- 30. Lorenz P, Pepperkok R, Ansorge W, Pyerin W. 1993. Cell biological studies with monoclonal and polyclonal antibodies against human casein kinase II subunit beta demonstrate participation of the kinase in mitogenic signaling. J. Biol. Chem. 268:2733–2739 [PubMed] [Google Scholar]
- 31. Ulloa L, Diaz-Nido J, Avila J. 1993. Depletion of casein kinase II by antisense oligonucleotide prevents neuritogenesis in neuroblastoma cells. EMBO J. 12:1633–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]