Abstract
Bruton's tyrosine kinase (Btk) is crucial for B-lymphocyte activation and development. Mutations in the Btk gene cause X-linked agammaglobulinemia (XLA) in humans and X-linked immunodeficiency (Xid) in mice. Using tandem mass spectrometry, 14-3-3ζ was identified as a new binding partner and negative regulator of Btk in both B-cell lines and primary B lymphocytes. The activated serine/threonine kinase Akt/protein kinase B (PKB) phosphorylated Btk on two sites prior to 14-3-3ζ binding. The interaction sites were mapped to phosphoserine pS51 in the pleckstrin homology domain and phosphothreonine pT495 in the kinase domain. The double-alanine, S51A/T495A, replacement mutant failed to bind 14-3-3ζ, while phosphomimetic aspartate substitutions, S51D/T495D, caused enhanced interaction. The phosphatidylinositol 3-kinase (PI3-kinase) inhibitor LY294002 abrogated S51/T495 phosphorylation and binding. A newly characterized 14-3-3 inhibitor, BV02, reduced binding, as did the Btk inhibitor PCI-32765 (ibrutinib). Interestingly, in the presence of BV02, phosphorylation of Btk, phospholipase Cγ2, and NF-κB increased strongly, suggesting that 14-3-3 also regulates B-cell receptor (BCR)-mediated tonic signaling. Furthermore, downregulation of 14-3-3ζ elevated nuclear translocation of Btk. The loss-of-function mutant S51A/T495A showed reduced tyrosine phosphorylation and ubiquitination. Conversely, the gain-of-function mutant S51D/T495D exhibited intense tyrosine phosphorylation, associated with Btk ubiquitination and degradation, likely contributing to the termination of BCR signaling. Collectively, this suggests that Btk could become an important new candidate for the general study of 14-3-3-mediated regulation.
INTRODUCTION
Tec family kinases (TFKs) are nonreceptor tyrosine kinases found primarily, but not exclusively, in hematopoietic lineages, where they are differentially expressed. The family consists of five members, tyrosine kinase expressed in hepatocellular carcinoma (Tec), Bruton's tyrosine kinase (Btk), interleukin 2 (IL-2)-inducible T-cell kinase (Itk), bone marrow tyrosine kinase gene in chromosome X protein (Bmx), and resting lymphocyte kinase (Rlk/Txk) (1). Btk plays a crucial role in lymphocyte maturation and signaling. It is involved in multiple signaling pathways, such as activation of phospholipase Cγ (PLCγ), calcium mobilization, actin reorganization, adhesion, migration, survival, and apoptosis (1–3). Following the cloning of BTK as the gene that is defective in X-linked agammaglobulinemia (XLA) (4, 5), a point mutation affecting a conserved arginine residue (R28C) in the Btk pleckstrin homology (PH) domain was identified in the immunoglobulin-deficient mouse strain known as X-linked immunodeficiency (Xid) mice (1, 6, 7).
Btk contains 659 amino acids and consists of five different domains, including an N-terminal PH domain and a Tec homology (TH) domain, followed by Src homology domains 3 and 2 (SH3 and SH2) and a C-terminal kinase domain (SH1) (5). Activation of many cell surface receptors, including the B-cell receptor (BCR), as well as stimulation of the phosphatidylinositol 3-kinase (PI3-kinase) signaling pathway, triggers plasma membrane translocation of Btk (8, 9). Consequently, the tethering of Btk to the inner leaflet of the cytoplasmic membrane leads to transient phosphorylation on two tyrosine residues, pY551 and pY223 (10, 11).
The highly conserved activation loop tyrosine Y551 is transphosphorylated by a Src family tyrosine kinase and, due to a conformational change, is followed by an autophosphorylation event at Y223 (11). Protein kinase Cβ (PKCβ) negatively regulates Btk by phosphorylating it on serine 180, which results in reduced membrane recruitment, transphosphorylation, and subsequent activation (12). Interaction of Btk with caveolin 1 also leads to downregulation of Btk kinase activity (13). In contrast, PKCθ activates Btk, while Btk downregulation results in the induction of the PKCθ activity (14). Moreover, Btk phosphorylation at two serines (S21 and S115) creates a binding site for the prolylisomerase Pin1, which modulates Btk activity in a cell cycle-dependent manner (15).
14-3-3 is the name of a family of highly divergent proteins that are present in all eukaryotes, from plants to mammals. To date more than 300 proteins binding to 14-3-3 family members have been identified (16). 14-3-3 proteins modulate their targets at various levels, such as subcellular localization, stability, phosphorylation, biological activity, and/or dynamic interactions (17). Furthermore, these proteins regulate many cellular processes relevant to cancer biology, in particular, apoptosis, mitogenic signaling, and cell cycle checkpoints. The human genome contains seven 14-3-3 isoforms, β, γ, ε, ζ, η, θ, and σ (18). Their ligands share 14-3-3-binding consensus motifs and recognize serine/threonine phosphorylation sites (19). Examples of such motifs are RSXpT/SXP and RXY/FXpT/SXP , where pT/S denotes phosphothreonine/serine. Binding is mostly dependent on the phosphorylation of the serine or threonine residues on target proteins, which allows conditional association with 14-3-3 proteins (20). Moreover, another common consensus motif for 14-3-3 binding is RXRXXpS/T , which has been shown to be mainly dependent on Akt/PKB kinase activity (21, 22).
Using a proteomics approach, here, we identify 14-3-3ζ as a novel Btk-binding partner. In addition, we demonstrate two potential binding motifs (pS51 and pT495) in Btk that mediate interaction with 14-3-3ζ. We further show that these sites are phosphorylated by Akt/PKB, leading to the interaction of Btk with 14-3-3ζ. Interestingly, interaction of 14-3-3ζ with the phosphorylated S51/T495 sites seems to target Btk for ubiquitination and degradation. Finally, we demonstrate that phosphorylation of Btk at these residues (pS51/pT495) is strictly dependent on PI3-kinase signaling, as well as functional 14-3-3ζ.
MATERIALS AND METHODS
Cell lines, reagents, and transfection.
Namalwa (a Burkitt lymphoma B-cell line), K562 (human chronic myelogenous leukemia cells), RBL-2H3 (rat basophil leukemia cells with mast cell characteristics), and Cos-7 (African green monkey fibroblast-like kidney) cells were obtained from the American Type Culture Collection (ATCC). Cos-7 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Invitrogen). All other hematopoietic cells were cultured in RPMI 1640 medium with supplements. All cells were cultivated at 37°C in a humidified 5% CO2 incubator.
Preparation of primary mouse B cells.
Xid/CBA mice were obtained from Charles River Laboratories (Kungsbacka, Sweden). Btk knockout (KO)/CBA mice were created by backcrossing Btk KO SW129 mice as described previously (23). Wild-type (wt) CBA mice were used as controls. Splenic B cells from all strains were enriched essentially as described previously (23). Briefly, purified mouse splenic B cells were isolated using a magnetic-separation column (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). The spleen cells, which had been stained with anti-B220 antibody-coupled micromagnetic particles (Miltenyi Biotec), were retained on the separation column under a high-gradient magnetic field. These cells were subsequently eluted from the column. Mouse models were used with ethical approval from the Stockholm South animal ethics committee with the registration number S194-08.
Protease inhibitor Complete Mini EDTA-free tablets (Roche), phosphatase inhibitor cocktail (Sigma), protein G-Sepharose 4 Fast Flow and A–Sepharose CL-B4 (GE Healthcare), and Dynabeads (Invitrogen; LC Laboratories) were used in protein analysis. All other high-grade laboratory chemicals and reagents were obtained from Sigma. The SDS-PAGE (4 to 12% Bis-Tris-glycine) gels and nitrocellulose membranes from the iBlott Dry-Blotting system were purchased from Life Technology. PCI-32765 (ibrutinib) was a kind gift from Joseph J. Buggy (Pharmacyclics, Inc.). BV02 was a kind gift from Maria Alessandra Santucci (Italy). Transient transfections were performed in 6-well plates using polyethyleneimine (PEI) from Polysciences Inc. (Warrington, PA, USA) according to the manufacturer's protocol.
Antibodies.
The antibodies used in this work were as follows: anti-14-3-3ζ (1:2,000) and anti-pan-14-3-3 (1:2,000) were from Santa Cruz Biotechnology, antiactin (1:100,000) from Sigma, anti- RXRXXpS/T (1:1,000) and anti-NF-κB (pSer536) (1:2,000) from Cell Signaling, anti-phospho-Btk (pY551) (1:1,000) from BD Pharmingen, anti-PLCγ2 (pY759) (1:1,000) from Epitomics, and anti-phosphotyrosine 4G10 from Upstate Biotechnology. Anti-pan-Akt (1:4,000) and anti-Akt (pS473) (1:1,000) were from Invitrogen, and goat F(ab′)2 anti-human IgM were from Southern Biotechnology. Polyclonal rabbit antibody against the Btk SH3 domain has been described previously (8).
Plasmids.
The plasmids wt-Btk-GFP, GFP-Btk-NLS, wt-pSGT-Btk, and DsRed-Liar were described in our recent work (24). The Btk T495A, T495D, S51A, S51D, S51A/T495A, and S51D/T495D constructs were created by site-directed mutagenesis, and all plasmids were verified by sequencing. GFP–14-3-3ζ and 14-3-3ζ–Myc were purchased from Origene. mRFP–14-3-3ζ was a kind gift from Martin J. Humphries (University of Manchester, Manchester, United Kingdom). The wt Akt, membrane-targeted constitutive-active Akt (mCA Akt), and dominant-negative Akt (DN Akt) were kindly provided by Brian Hemmings (Friedrich Miescher Institute, Switzerland).
Immunoprecipitation and immunoblotting.
Immunoprecipitation and immunoblotting analysis was carried out as previously described (24, 25). All incubations were done at 4°C overnight. The secondary antibody was labeled with Alexa Fluor 680 (Molecular Probes) and used at 1:20,000 dilution for 1 h at room temperature. The membranes were scanned with the Odyssey infrared imaging system (Li-Cor). The densities of target bands were quantified using Image J application software.
Immunofluorescence and confocal microscopy.
Cells were seeded overnight at 40 to 50% confluence in 6-well plates, and 16 h later, the cells were transfected with plasmids and incubated for 48 h before processing for immunostaining and confocal microscopy, as previously described (24).
Affinity purification of Btk-Flag for mass spectrometric analysis.
To identify novel Btk complexes, we expressed epitope (Flag)-tagged Btk in two hematopoietic cell lines, Namalwa and K562. Btk-Flag was cloned into the retroviral vector pMSCV containing internal ribosome entry site (IRES)-driven green fluorescent protein (GFP) and transiently transfected into Phoenix GP cells. Viral particles were prepared from the supernatant and used to transduce Namalwa and K562 cells. GFP-positive cells were enriched to 98% purity, and the selected population was gated for cells with low expression of Btk-Flag. Purified Btk-Flag complexes, captured with anti-Flag-agarose beads, were eluted, lyophilized, and prepared for gel-free tandem mass spectrometry (MS-MS) analysis. The details of preparations for mass spectrometry analysis were previously described (24).
Transfection of siRNAs.
A small interfering RNA (siRNA) against human 14-3-3ζ was obtained from Santa Cruz (sc-29583). An siRNA directed against Bmx was used as a control, as described previously (26). Transient transfection of siRNAs (100 nM) was done using the Neon transfection system (Invitrogen) or with Lipofectamine 2000, following the manufacturer's instructions. Forty-eight hours later, cells were collected and used for further analyses. Extracts from untransfected cells, or cells transfected either with siRNA 14-3-3ζ or with the siRNA control, were harvested for immunoblot analysis.
RESULTS
Identification of 14-3-3 proteins as novel Btk-interacting partners.
To identify novel proteins that regulate Btk activity and function, proteomic analysis using mass spectrometry (MS-MS) was performed on hematopoietic cell lines stably expressing an epitope-tagged Btk (24). The peptide coverage for each 14-3-3 isoform found in the proteomic data obtained by the MS analysis is as follows: 14-3-3ζ, 37.5%; 14-3-3γ, 28.3% (Table 1; see Table S1 in the supplemental material); 14-3-3ε, 19.2%; 14-3-3θ, 18.4%; and 14-3-3β, 16.3%, while no peptide scores for the 14-3-3η and 14-3-3σ isoforms could be found. Therefore, in our study, we focused on the zeta isoform, since it represents the greatest peptide coverage (Table 1), even though interaction with other 14-3-3 isoforms might also exist. In some experiments, a panantibody was used for detecting all the 14-3-3 isoforms. It is worthwhile to mention that expression of 14-3-3ζ is pronounced in B cells, according to the human protein atlas (HPA) database (http://www.proteinatlas.org).
Table 1.
Unique peptide sequences confirmed by MS-MS analysisa
| No. of MS-MS spectra | No. of unique peptides | Mascot score | Coverage (%) | Peptide sequence |
|---|---|---|---|---|
| 5 | 4 | 182 | 19 | SVTEQGAELSNEER |
| EKIETELR | ||||
| DICNDVLSLLEK | ||||
| YLAEVAAGDDKK | ||||
| 3 | 2 | 170 | 13 | SVTEQGAELSNEER |
| TAFDEAIAELDTLSEESYK | ||||
| 3 | 2 | 133 | 11 | SVTEQGAELSNEER |
| YLAEVAAGDDKK | ||||
| 4 | 2 | 124 | 9 | LAEQAER |
| SVTEQGAELSNEER | ||||
| 3 | 2 | 123 | 11 | SVTEQGAELSNEER |
| DICNDVLSLLEK | ||||
| 3 | 2 | 117 | 11 | SVTEQGAELSNEER |
| YLAEVAAGDDKK | ||||
| 4 | 3 | 108 | 10 | SVTEQGAELSNEER |
| FLIPNASQAESK | ||||
| NLLSVAYK | ||||
| 2 | 2 | 101 | 11 | SVTEQGAELSNEER |
| YLAEVAAGDDKK |
The selections were based on high Mascot scores. The overall coverage of all amino acid sequences (92 amino acids [aa]) of unique peptides was calculated on primary sequences of the 14-3-3ζ isoform (245 aa) to be 37.5% (92 aa out of 245 aa). Proteomics analyses were based on 5 independent experiments, and each individual MS-MS data set was collected from 15 samples.
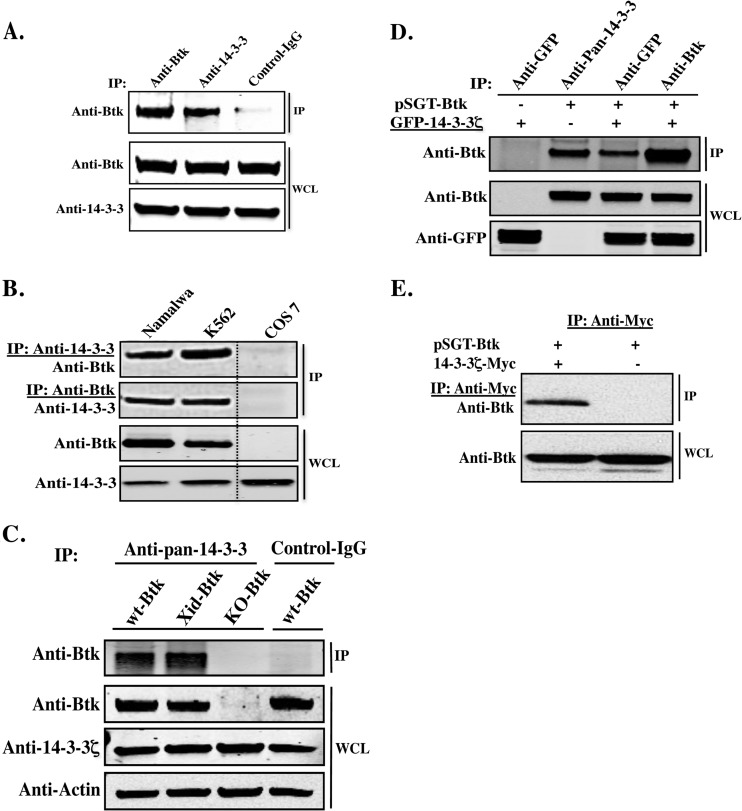
To verify the proteomics data, we investigated the interaction of endogenous Btk with 14-3-3 in both human peripheral blood mononuclear cells (PBMCs) and primary mouse B cells, as well as in hematopoietic cell lines, including K562 and Namalwa (Fig. 1). In primary human PBMCs, immunoprecipitation with a pan-anti-14-3-3 antibody resulted in the pulldown of endogenous Btk (Fig. 1A). Furthermore, reciprocal immunoprecipitation of endogenous 14-3-3 and Btk, followed by immunoblotting with Btk- and 14-3-3ζ-specific antibodies, demonstrated robust interaction between the two proteins (Fig. 1B). Second, we wondered whether the XLA/Xid Btk point mutation (Btk-R28C) would affect binding of Btk to 14-3-3. To this end, primary mouse B cells isolated from wt and Xid mice were processed for immunoprecipitation/immunoblotting analysis. There was no significant variation between wt Btk and the Btk R28C mutant with respect to their interactions with 14-3-3 proteins (Fig. 1C; see Fig. S1 in the supplemental material). These data confirm that Btk and 14-3-3 interact in vivo in both human and mouse B cells.
Fig 1.
Novel interaction of Btk with 14-3-3 proteins, predominantly the 14-3-3ζ isoform. (A) Coimmunoprecipitation (IP) of Btk and 14-3-3 from human PBMCs using rabbit polyclonal antibodies to Btk and to 14-3-3, respectively. WCL, whole-cell lysate. (B) Reciprocal coimmunoprecipitation of Btk and 14-3-3 from Namalwa and K562 cells using rabbit polyclonal antibodies to Btk and to 14-3-3, respectively. Cos-7 cells, which do not normally express Btk, were used as a negative control. (C) Endogenous Btk was coimmunoprecipitated with 14-3-3 from primary mouse cells, using pan-14-3-3 antibody. Samples were analyzed by Western blotting, using an anti-Btk antibody. Primary B cells from Btk-deficient mice (Btk−/−; KO-Btk) were used as a negative control. (D) Btk- and GFP–14-3-3ζ expression plasmids were transiently transfected into Cos-7 cells and analyzed by immunoprecipitation and Western blotting. (E) Cos-7 cells were transfected with 14-3-3ζ–Myc and –Btk plasmids. The mock pSGT plasmid served as a negative control. 14-3-3ζ–Myc proteins were immunoprecipitated with the anti-Myc antibody and probed with anti-Btk.
Btk specifically binds to the 14-3-3ζ isoform.
According to the peptide sequence coverage of the MS-MS analysis, we could predict that the 14-3-3ζ isoform predominantly interacts with Btk (Table 1). To biochemically confirm this observation, we cotransfected Btk with GFP-tagged 14-3-3ζ into Cos-7 cells, which normally do not endogenously express Btk. Immunoprecipitation and Western blot analysis with anti-Btk and anti-GFP antibodies revealed that Btk and native 14-3-3ζ strongly interact (Fig. 1D). In addition, ectopic expression of Myc-tagged 14-3-3ζ and Btk led to a robust interaction between the two proteins (Fig. 1E).
Phosphorylation of the 14-3-3ζ-binding motifs, RXRXXpT/S , in Btk.
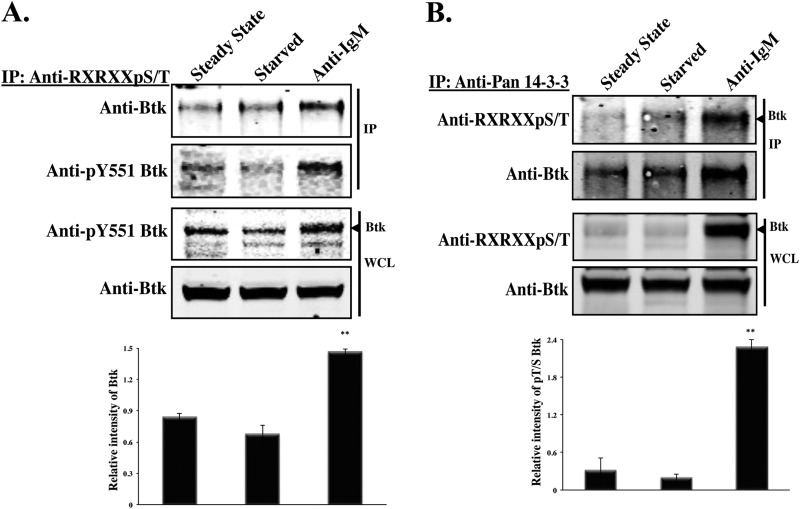
Next, we sought to identify the residues that may be responsible for the binding between Btk and 14-3-3ζ. Visual inspection of the primary sequence of Btk identified two putative 14-3-3-binding motifs with the consensus sequence RXRXXpT/S . One motif is located in the PH domain (46-RGRRGS-51), while the other is in the kinase domain (490-RHRFQT-495). To determine whether these potential 14-3-3ζ-binding sites are subject to phosphorylation, a phospho-specific antibody directed against the RXRXXpT/S motif was used. Namalwa cells were either starved or activated with F(ab′)2 anti-human IgM (20 μg/ml) for 15 min, and whole-cell lysates were immunoprecipitated using an antibody against the RXRXXpT/S motif. Interestingly, the phosphospecific antibody was strongly reactive with Btk in activated cells but not in cells that had been serum starved or kept under steady-state conditions, indicating that following B-cell receptor stimulation, phosphorylation of Btk on serine or threonine is augmented (P < 0.004) (Fig. 2A). Similarly, immunoblot analysis with anti- RXRXXpT/S antibody showed enhanced Btk phosphorylation (P < 0.004) on this motif in anti-14-3-3 immunoprecipitation complexes obtained from stimulated cells (Fig. 2B). Hence, 14-3-3ζ binds to Btk in a phosphorylation-dependent manner. In addition, we showed that the interaction of Btk and 14-3-3ζ increases in proportion to the level of Btk phosphorylation. After stimulation of RBL cells with pervanadate, the interaction between Btk and 14-3-3ζ was induced and reached its peak at 10 min, with a slight decay thereafter over a period of 30 min (see Fig. S4 in the supplemental material).
Fig 2.
14-3-3ζ binds more efficiently to serine/threonine-phosphorylated Btk. (A) Namalwa cells at steady state, starved or activated using anti-IgM (20 μg/ml) for 15 min. Btk phosphorylated on S51/T495 was immunoprecipitated from Namalwa cell extracts with an anti- RXRXXpT/S antibody, and the precipitated proteins were detected with an antibody against Btk. (B) The 14-3-3 proteins in Namalwa cells were immunoprecipitated with the anti-pan-14-3-3 antibody and probed for the presence of the phosphorylated S51/T495 by immunoblotting with anti- RXRXXpT/S . Btk was detected by immunoblotting with anti-Btk antibody. Quantitative analysis of the bands indicated by the arrowheads was performed by densitometry analysis using Image J software. The bars represent the means and standard errors (SE) of two independent experiments. **, P < 0.004.
The Akt/PKB kinase phosphorylates the RXRXXpT/S motif on Btk.
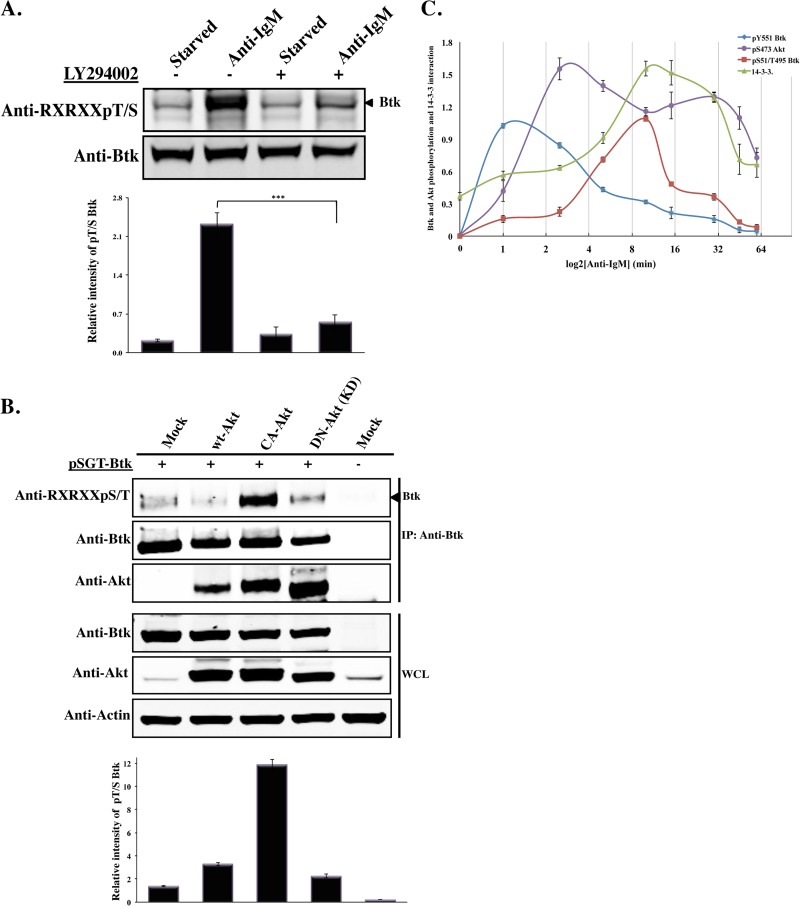
These results show that the RXRXXpT/S motif in Btk is phosphorylated following B-cell activation and that such posttranslationally modified Btk interacts with 14-3-3ζ. These sequences are also known consensus phosphorylation sites for Akt/PKB (21, 22). Previously, it has been reported that in B cells, a physical interaction between Btk and Akt might occur (27). In light of these facts, we carried out biochemical analysis to address the origin of the serine/threonine phosphorylation in Namalwa cells, following serum starvation or anti-IgM activation, in the presence or absence of the PI3-kinase inhibitor LY294002. Treatment of cells with the anti-IgM antibody led to robust phosphorylation of the RXRXXpT/S motif on Btk (Fig. 3A). However, phosphorylation was reduced when cells were activated in the presence of the PI3-kinase inhibitor LY294002 (P < 0.001) (Fig. 3A). Second, we coexpressed three different Akt constructs with Btk, and the phosphorylation status of Btk and its interaction with Akt were monitored. As expected, phosphorylation of Btk was considerably enhanced upon heterologous expression of the constitutively active Akt (mCA Akt; P < 0.001). In contrast, there was no detectable phosphorylation when a dominant-negative form of Akt (DN Akt) was used (Fig. 3B). In addition, Btk was found to interact with the ectopically expressed Akt constructs (Fig. 3B). Together, these data indicate that Akt and Btk are downstream of PI3-kinase (8). Thus, treatment of cells with a PI3-kinase inhibitor, as well as expression of a construct encoding DN Akt, prevents serine/threonine phosphorylation of Btk.
Fig 3.
Akt/PKB kinase phosphorylates the RXRXXpT/S motif on Btk. (A) Namalwa cells were serum starved or activated with anti-IgM (20 μg/ml) for 15 min in the presence or absence of the PI3-kinase inhibitor LY294002. Western blot analysis of the induced phosphorylation of Btk S51/T495 was detected using phospho-specific antibody. The arrowhead shows the position of the Btk band. (B) Akt constructs (wt Akt, mCA Akt, and DN Akt) were transiently coexpressed with Btk in Cos-7 cells. WCL was immunoprecipitated with anti-Btk antibody, and the precipitated proteins were identified using anti- RXRXXpT/S antibody. Pan-Akt antibody was used to demonstrate Akt's interaction with Btk. Quantitative analysis of the bands indicated by arrowheads was performed by densitometry analysis using Image J software. The data shown are representative of three independent experiments (the error bars indicate SE). ***, P < 0.001. (C) Time course of Btk (pY551 and pS51/T495) and Akt (pS473) phosphorylation and of 14-3-3ζ interaction with phosphorylated Btk during anti-IgM (20 μg/ml) stimulation. The cells were starved for 3 h and then subjected to anti-IgM activation. The error bars represent the means ± SE of two independent experiments.
Next, we carried out experiments to assess the time course correlation between phosphorylation events on Btk and Akt/PKB and Btk's capacity to interact with 14-3-3. Btk phosphorylation on pY551, pS51, and pT495 was monitored for 1 h after activation of cells with anti-IgM. Cells from the Namalwa B-cell line were starved for 3 h prior to anti-IgM stimulation and followed for 1 h (1 min to 60 min). Tyrosine phosphorylation of pY551 robustly peaked after 1 min, while the pS51/pT495 phosphorylation optimum appeared later, after 10 min. Interestingly, phosphorylation on pS51/pT495 appeared after the activation of the Akt/PKB kinase, as measured by the level of pS473 phosphorylation. Remarkably, the level of 14-3-3 interaction with Btk coincided with the S51/T495 phosphorylation status of Btk, both yielding a very potent increase (Fig. 3C).
Collectively, this analysis confirms that the activating Y551 phosphorylation of the Btk kinase domain is a very early response and demonstrates that this event is followed by Akt/PKB phosphorylation. Subsequently the bona fide Akt/PKB target sites on Btk are phosphorylated, with binding to 14-3-3 being simultaneously increased.
PCI-32765 (ibrutinib) and BV02 inhibitors block the interaction of Btk and 14-3-3.
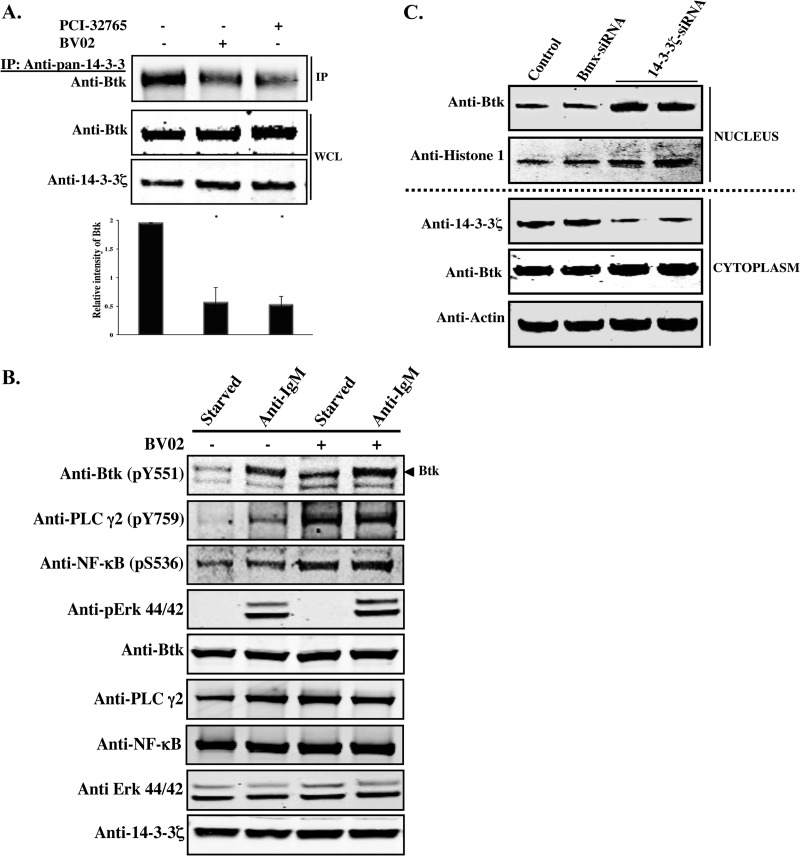
Since the PI3-kinase–Akt pathway induces serine/threonine phosphorylation and subsequent binding of Btk to 14-3-3, we wondered whether specific inhibition of Btk and/or 14-3-3 would affect the physical interaction between the two proteins. Therefore, we employed two newly developed inhibitors—PCI-32765 (ibrutinib), an irreversible Btk inhibitor (28), and BV02, a 14-3-3 inhibitor (29), and performed a coimmunoprecipitation analysis in primary mouse B cells (Fig. 4A). Purified primary B lymphocytes were treated with the drugs, and 24 h later, whole-cell lysates were prepared and subsequently processed for immunoprecipitation/immunoblotting analysis as described in Materials and Methods. Interestingly, interaction of Btk with 14-3-3 was reduced (P < 0.015) in both the PCI-32765- and BV02-treated cells (Fig. 4A). In addition, the BV02 inhibitor was used in two hematopoietic cell lines (Namalwa and K562), and a similar effect was observed (see Fig. S2 in the supplemental material). Quantitative analysis showed a reduction of the interaction by two-thirds in the presence of PCI-32765 and/or BV02 inhibitor compared to untreated cells (Fig. 4A).
Fig 4.
Inhibition of Btk and 14-3-3ζ. (A) Primary mouse B cells were treated with the Btk inhibitor PCI-32765 and the nonpeptide 14-3-3 inhibitor BV02 and incubated for 24 h. Control samples were incubated with DMSO. WCLs were processed for Western blot analysis. Quantitative analysis of Btk bands was performed by densitometry analysis using Image J software. The bars represent the means and SE of two independent experiments. *, P < 0.015. (B) Namalwa cells were starved or activated using anti-IgM (20 μg/ml) for 15 min in the presence or absence of BV02. WCLs were processed for Western blotting using phospho-specific antibodies against Btk (pY551), PLCγ2 (pY759), NF-κB (pS536), or pErk p44/42 (Thr202/Tyr204). (C) siRNA-mediated depletion of endogenous 14-3-3ζ in Namalwa cells. Cells were transfected with a 14-3-3ζ siRNA (two independent experiments) or with control siRNA targeting Bmx, another Tec family member. The siRNA-treated cells were collected 48 h after transfection. Western blot analysis was performed using lysates prepared from nuclear and cytoplasmic fractions. The purity of the nuclear and cytoplasmic fractions was verified by probing with antibodies against H1 histone and β-actin, respectively.
Btk phosphorylation correlates with activation of PLCγ2 and NF-κB, and this pathway participates in 14-3-3-regulated tonic signaling.
The above data demonstrate that the BV02 inhibitor interferes with the interaction between 14-3-3 and Btk. Therefore, we sought to assess the phosphorylation status of the unbound fraction of Btk as a consequence of BV02 treatment and compared it to that of Btk complexed with 14-3-3. BV02 potently inhibited the interaction between the two proteins in the Namalwa cell line under both serum-starved and -activated conditions using F(ab′)2 anti-human IgM (Fig. 4B). Interestingly, phosphorylation of Btk at the conserved activation loop Y551 was augmented following treatment of cells with the 14-3-3 inhibitor BV02 under both conditions (Fig. 4B). Moreover, Btk activation following treatment with BV02 leads to activation and phosphorylation of both PLCγ2 and NF-κB, both well-known downstream targets of Btk, while the phosphorylation status of Erk44/42 was essentially unaffected (Fig. 4B). Importantly, both PLCγ2 and NF-κB phosphorylation levels are potently enhanced following BV02 treatment. This suggests that tonic signaling, which is crucial for B-cell survival, is strongly regulated by 14-3-3.
Downregulation of 14-3-3ζ by siRNA causes nuclear accumulation of Btk.
To further investigate the implications of the 14-3-3ζ and Btk interaction, we employed an siRNA targeting the endogenous 14-3-3ζ isoform. To deplete 14-3-3ζ in B cells, siRNA was introduced into Namalwa cells, and 48 h later, the cells were fractionated into nuclear and cytoplasmic compartments (Fig. 4C). Immunoblot analysis showed that 14-3-3ζ knockdown led to a marked increase of Btk protein in the nucleus. In contrast, a control siRNA directed against the Tec family kinase (Bmx) did not exhibit any effect on the nuclear distribution of Btk (Fig. 4C).
Colocalization of 14-3-3ζ and Btk.
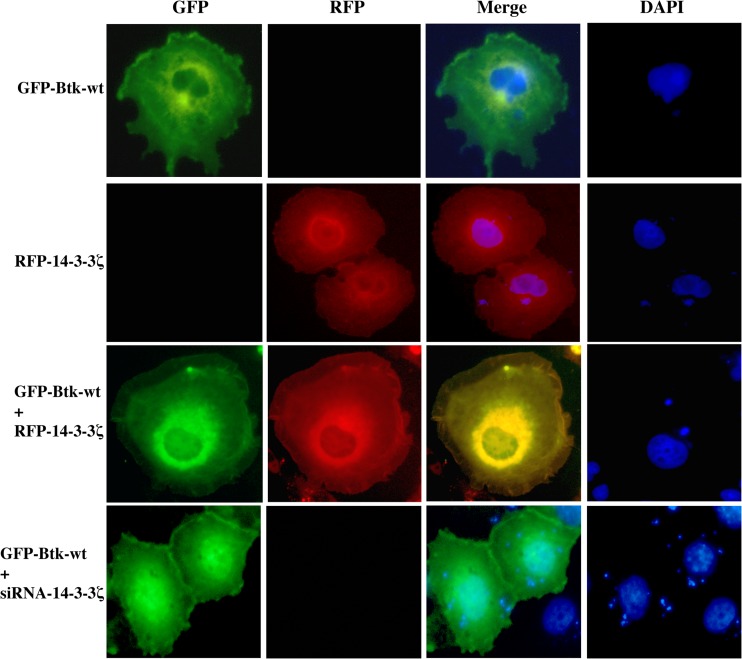
Our previous studies have shown that Btk is predominantly cytoplasmic and translocates to the inner leaflet of the cytoplasmic membrane upon PI3-kinase activation (8). Moreover, nucleocytoplasmic shuttling of Btk was also observed and investigated in detail, but the shuttling mechanism and the functional consequences still remain elusive (25). We therefore sought to analyze the effect of coexpressing a fusion construct consisting of 14-3-3ζ and red fluorescent protein (RFP–14-3-3ζ) with wt Btk fused to green fluorescent protein (GFP-Btk) or with Btk equipped with a nuclear localization signal (GFP-Btk-NLS). We also wanted to study the influence of ankyrin repeat domain 54 (ANKRD54)/Liar fused to pDsRed-monomer-C1 (DsRed-Liar), since ANKRD54 has been recently demonstrated to modulate Btk shuttling (24). RFP–14-3-3ζ and wt GFP-Btk colocalized in both the cytoplasmic and perinuclear regions (Fig. 5).
Fig 5.
Colocalization of 14-3-3ζ with Btk. Cos-7 cells were cotransfected with GFP-Btk-wt and RFP–14-3-3ζ plasmids in the presence or absence of siRNA–14-3-3ζ. Forty-eight hours posttransfection, the cells were fixed and stained with DAPI (4′,6-diamidino-2-phenylindole). GFP-Btk is shown in green, while RFP–14-3-3ζ is shown in red. The merged images indicate colocalization of Btk and 14-3-3ζ in membrane ruffles and in the perinuclear region (yellow). The data are representative of at least 3 different experiments.
In contrast, depletion of 14-3-3ζ by siRNA resulted in a significant increase of Btk in the nucleus (Fig. 5), whereas conversely, overexpression of ANKRD54, as expected, reduced Btk levels in the compartment (Table 2). Nevertheless, while nuclear-targeted GFP-Btk-NLS is entirely nuclear resident (24), coexpression of 14-3-3ζ with GFP-Btk-NLS yielded cytoplasmic colocalization and, surprisingly, significant nuclear exclusion of Btk (P < 0.0001), as shown in Table 2. Similar influence on Btk localization has been shown with the ANKRD54 protein (24). Consequently, these observations strongly suggest the existence of a functional link between 14-3-3ζ and Btk regulating nucleocytoplasmic shuttling and subcellular localization.
Table 2.
Distribution of expressed proteins in nuclear, cytoplasmic, membrane, and perinuclear regionsa
| Protein(s) expressed | % of cells expressing protein(s)b |
% of cells with cytoplasmic protein(s) showing membrane or perinuclear localization |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| GFP-Btk or GFP-Btk-NLS |
DsRed–14-3-3ζ or DsRed-Liar |
Membrane |
Perinuclear |
|||||||
| N Only | C Only | N + C | N Only | C Only | N + C | Btk | 14-3-3ζ | Btk | 14-3-3ζ | |
| GFP-Btk | 0 | 91 | 9 | 82 | 13 | |||||
| DsRed–14-3-3ζ | 0 | 92 | 8 | 4 | 86 | |||||
| GFP-Btk-NLS | 100 | 0 | 0 | |||||||
| GFP-Btk + DsRed–14-3-3ζ | 0 | 94 | 6 | 0 | 95 | 5 | 58 | 26 | 41 | 71 |
| GFP-Btk-NLS + DsRed–14-3-3ζ | 12 | 0 | 88 | 0 | 78 | 22 | ||||
| GFP-Btk + DsRed-Liar | 0 | 100 | 0 | 19 | 0 | 81 | ||||
| GFP-Btk + siRNA–14-3-3ζ | 1 | 70 | 29 | |||||||
| GFP-Btk + DsRed-Liar + siRNA–14-3-3ζ | 0 | 100 | 0 | 11 | 0 | 89 | ||||
Mean values were calculated for 100 cells from 3 independent experiments.
N, nuclear; C, cytoplasmic.
Effect of mutating the 14-3-3ζ docking sites in Btk.
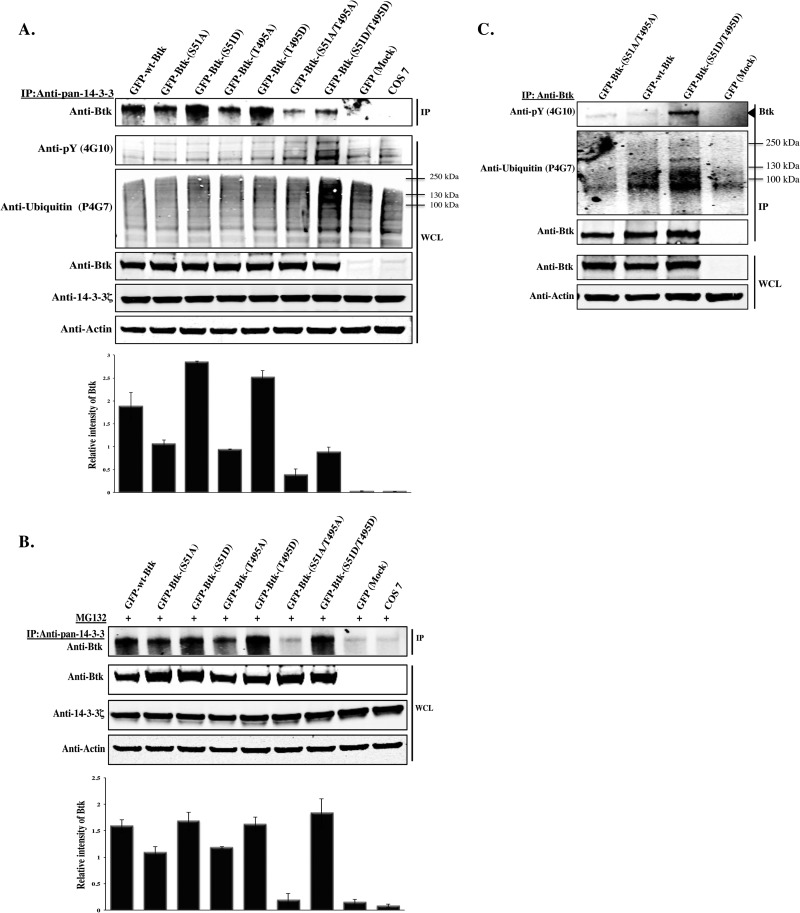
Next, we wanted to delineate the effect of mutating the putative 14-3-3ζ docking sites in Btk. Following identification of the two potential Akt phosphorylation and 14-3-3-binding motifs, we performed a sequence alignment of the 14-3-3-binding region in Btk and found that both sites are phylogenetically conserved (see Fig. S3 in the supplemental material). To determine the roles of these residues in the activation and signaling of Btk, we mutated them to alanine or aspartic acid, singly or doubly. Accordingly, DN and CA mutants were generated as follows: S51A, T495A, and S51A/T495A as DN forms and S51D, T495D, and S51D/T495D as potentially phosphomimetic CA forms. To biochemically assess their functions, constructs encoding these mutants were ectopically expressed together with control plasmids in Cos-7 cells. Forty-eight hours later, whole-cell lysates were processed for an immunoprecipitation/immunoblotting analysis using antibodies against 14-3-3 and Btk. Compared to 14-3-3 binding to wt Btk, binding to the S51A/T495A double mutant was strongly diminished in the precipitates. Moreover, the single-site mutants showed an intermediate interaction. Surprisingly, in comparison with the single mutants, steady-state levels of the S51D/T495D mutant were reduced (Fig. 6A, top). We conclude that maximal binding of 14-3-3 to Btk requires both the S51 and the T495 sites and that the interaction is strictly dependent on their phosphorylation status.
Fig 6.
S51- and T495-to-alanine substitution disrupts binding of Btk to 14-3-3ζ, while S51- and T495-to-aspartic-acid substitution promotes degradation. (A) Plasmids encoding GFP-Btk-wt and 14-3-3-binding-deficient mutants S51A, T495A, and S51A/T495A, as well as constructs encoding phosphomimetic mutants S51D, T495D, and S51D/T495D, were transfected into Cos-7 cells. Endogenous 14-3-3 was immunoprecipitated and probed with anti-Btk. WCL was immunoblotted with anti-pY (4G10), antiubiquitin (P4G7), anti-Btk, anti-14-3-3ζ, or antiactin antibody. (B) Ectopically expressed Btk was immunoprecipitated from whole-cell extracts of Cos-7 with anti-Btk antibody, and the precipitated proteins were detected with phospho-specific antibodies against global phosphoproteins (4G10) or with an antiubiquitin antibody (P4G7). Total Btk protein was detected by immunoblotting with anti-Btk. (C) Endogenous 14-3-3 proteins were immunoprecipitated with the anti-pan-14-3-3 antibody from Cos-7 cells transfected with Btk constructs and treated overnight with a proteasome inhibitor (MG132; 10 μm) and probed for the presence of Btk by immunoblotting with anti-Btk. Quantitative analysis of the top band in panels A and C was performed by densitometry analysis using Image J software. The data shown are representative of two independent experiments (the error bars indicate SE).
Constitutive 14-3-3ζ binding to Btk causes ubiquitination and degradation.
The surprising finding that steady-state levels of the double phosphomimetic S51D/T495D mutant was reduced (Fig. 6A, top) prompted us to do further experiments that might shed light on the underlying mechanism. Therefore, we wanted to study the fate of phosphorylated Btk following constitutive 14-3-3ζ binding. It is possible that the strong binding of the S51D/T495D mutant to 14-3-3ζ renders it unstable, leading to its degradation. To also determine whether the reduced levels of the mutant could be due to ubiquitination, we immunoprecipitated ectopically expressed Btk in Cos-7 cells transfected with the S51A/T495A or S51D/T495D construct and probed the immunoblotted filters with an antiphosphotyrosine antibody (4G10) or an anti-ubiquitin (P4G7) antibody, respectively. We demonstrate here that the S51D/T495D mutant is highly tyrosine phosphorylated and also ubiquitinated (Fig. 6A, second and third gels from top). In stark contrast, the S51A/T495A double mutant was neither phosphorylated nor ubiquitinated, suggesting that posttranslational modification at these two residues is critical for the degradation of Btk (Fig. 6B). These findings are compatible with the idea that the decrease in the steady-state levels of S51D/T495D is due to ubiquitination, targeting Btk for proteasomal degradation. In support of this, when the S51D/T495D mutant was overexpressed in Cos-7 cells, followed by overnight incubation with the proteasome inhibitor MG132, accumulation of the phosphomimetic mutant form of Btk was observed (Fig. 6C, top).
DISCUSSION
Using an MS-MS-based technique, we identified a novel partner for Btk, namely, 14-3-3ζ, and subsequently verified their interaction in B cells. Two Akt phosphorylation sites (pS51 and pT495) were found to be critical for this interaction. The inducible phosphorylation resulting in 14-3-3ζ binding was shown to regulate Btk ubiquitination and degradation. Moreover, the nuclear translocation of Btk is compromised following interaction with 14-3-3ζ.
14-3-3 proteins bind to different consensus motifs, which have been classified as mode I ( RSXpSXP ) and mode II ( RXXpS/TXP ) (30, 31). The presence of phosphorylated serine or threonine, common consensus motifs, characterizes 14-3-3 interaction sites (20, 32, 33). However, there is also another consensus motif for 14-3-3 binding, RXRXXpT/S , which is a known target site for Akt phosphorylation (21, 34). Close inspection of the Btk sequence reveals two potential 14-3-3-binding sites located in the PH and kinase domains, respectively, both of which are well conserved across mammals. The S51 motif is also conserved in birds and amphibians (see Fig. S3 in the supplemental material). However, with the possible exception of Bmx, these sites are not conserved in other Tec family kinases (data not shown), suggesting that this mode of regulation is unique to Btk. Akt kinase activation is mainly induced by PI3-kinase and is known to activate a strong survival signaling pathway that is defective in many human disorders (35–37). Inhibition of PI3-kinase by wortmannin or LY294002 induces apoptosis, mainly due to inhibition of Akt signaling (38–40). A previous report from this laboratory has also suggested that Akt interacts with Btk (27). In addition, we observed that the Btk inhibitor PCI-32765 abolishes Btk's interaction with 14-3-3. One possible mechanism for how PCI-32765 could block Btk's binding to 14-3-3ζ would be by inhibiting Btk phosphorylation and activation, which seem to be a prerequisite for 14-3-3ζ's interaction.
BCR ablation leads to death in most of the peripheral B cells, which indicates a need for continuous “tonic” signals through the BCR (41, 42). To this end, we observed that there are clearly increased levels of activated PLCγ2 and NF-κB in the presence of the 14-3-3 inhibitor BV02 (Fig. 4B). This is especially noticeable in the unstimulated, serum-starved cultures, clearly suggesting that there is ongoing activation followed by 14-3-3-mediated degradation of Btk under these tonic signaling conditions. Collectively, our data suggest that 14-3-3 is a key regulator of both induced and tonic signaling in B cells.
Moreover, not only PI3-kinase, Akt, and Btk, but also the transcription factor FOXO1, are functionally connected to 14-3-3ζ. Thus, PI3-kinase induces phosphorylation of both Btk (43) and the transcription factor FOXO1 (44). It has also been reported that activated Btk contributes to the downregulation of FOXO1 (45), and an interaction between FOXO1 and 14-3-3ζ has been established (46). Similar to that of Btk (this study), Akt-induced phosphorylation of FOXO1 results in ubiquitination and proteasomal degradation (47). Thus, the downregulation of FOXO1 by Btk may be related to a common 14-3-3ζ-mediated mechanism for the termination of signaling as a result of Akt activation.
Btk has been shown to shuttle between the cytoplasm and the nucleus. However, the molecular mechanisms controlling this phenomenon remain elusive (25, 48). Our most recent study on this topic has shown that Btk interacts with a novel ankyrin repeat domain protein, ANKRD54/Liar. This association was found to exclude both proteins from the nucleus (24). It has been shown that 14-3-3 affects localization of target proteins, such as Ataxin-1 (49), c-Abl (50), and FOXO3a (51). Our results are consistent with several functions for Akt and 14-3-3ζ in regulating the activity and subcellular localization of target proteins. Thus, 14-3-3ζ was found to actively retain Btk in the cytoplasm, while siRNA-mediated depletion of 14-3-3ζ resulted in nuclear translocation of Btk. In contrast, overexpression of ANKRD54 caused export of Btk from the nucleus. Thus, ANKRD54 overexpression seems to have a dominant effect over 14-3-3ζ on Btk shuttling and redistribution (Table 2). In this scenario, ANKRD54 and 14-3-3ζ affect Btk shuttling in an independent manner. At present, we have no evidence suggesting that there is direct interaction between ANKRD54 and 14-3-3. To further explore nucleocytoplasmic shuttling of Btk, we employed a nucleus-targeted GFP-Btk fusion protein containing a synthetic NLS located at the C terminus of Btk (24). Indeed, overexpression of 14-3-3ζ led to exclusion of the nucleus-resident Btk from the nucleus (Table 2). Moreover, subcellular localization analysis in Cos-7 cells did not reveal any detectable differences between wt Btk and the S51A/T495A or S51D/T495D double mutant (data not shown). This might be due to the fact that the mutated sites confer functional alterations only when expressed in B cells. Hence, this phenomenon needs to be explored further in order to determine the mechanism of shuttling.
Btk is not the only protein where 14-3-3-dependent shuttling between the nucleus and the cytoplasm has been observed. Thus, several histone deacetylases (HDACs) are regulated by serine/threonine phosphorylation, and these residues bind to 14-3-3. The best-characterized acetylase in this regard is HDAC5, for which several phosphorylation sites have been identified to date. PKD- and calmodulin-dependent protein kinase (CaMK)-induced phosphorylation of Ser259 and Ser498, flanking the NLS, provides docking sites for 14-3-3 chaperone binding, which triggers nuclear export, thereby reducing HDAC5's repressor activity (52, 53). In contrast, it has recently been demonstrated that cyclic AMP (cAMP)-activated PKA specifically phosphorylates HDAC5 at serine 280, which prevents its export from the nucleus, leading to suppression of gene transcription (54).
In this work, we mapped the 14-3-3ζ interaction sites to pS51/T495 in Btk. Mutation of S51 and T495 to alanine resulted in complete loss of interaction, while a phosphomimetic mutant (S51D/T495D) enhanced interaction with 14-3-3ζ. Phosphorylation of the sites by Akt and subsequent Btk phosphorylation are sufficient for the interaction with 14-3-3ζ, suggesting that either 14-3-3ζ binds to only a single site in Btk or there is cooperative binding. The single phosphomimetic mutants, S51D and T495D, both display stronger interaction with 14-3-3ζ than wt Btk but weaker interaction than the double mutant, suggesting that the two sites cooperate in the binding of 14-3-3ζ.
The ubiquitin proteasome system is a pathway regulating many cellular processes (55). Recently, we have shown that Btk expression is reduced in primary B cells and B-cell lines following treatment with proteasome inhibitors. This downregulation is due to the inactivation of NF-κB, which binds to the Btk promoter and induces transcription of Btk (56). Moreover, it has been reported that 14-3-3 binding can stimulate ubiquitination, resulting in degradation of its partner proteins, such as SLP-76 and Mdmx (57, 58). Interestingly, BLNK, also known as SLP-65, which in B cells has a function related to that of SLP-76 in T cells, is also regulated by 14-3-3. Thus, it was recently reported that hematopoietic progenitor kinase 1 (HPK1), by phosphorylating threonine 152 in BLNK, attenuates BCR-mediated activation, since 14-3-3 binding to T152 causes ubiquitination and degradation of BLNK (59). This also suggests that other components than Btk can contribute to the enhanced activation that we observed upon BV02 treatment. However, given that increased levels of Btk Y551 phosphorylation were also readily detectable, this implies that Btk plays an important role in tonic signaling. To our knowledge, a role for the proteasomal degradation of Btk has not been previously established. However, as mentioned above, proteasomal degradation of NF-κB reduces the activity of the Btk promoter (56). Thus, proteasomal degradation downregulates the activity of Btk at profoundly different levels. The fact that both BLNK and Btk are inactivated by 14-3-3-mediated ubiquitination and degradation suggests that this is a common mechanism for attenuating signaling from the BCR, as well as fine-tuning tonic signaling. By simultaneously degrading more than one of the key components, signaling may be regulated more robustly.
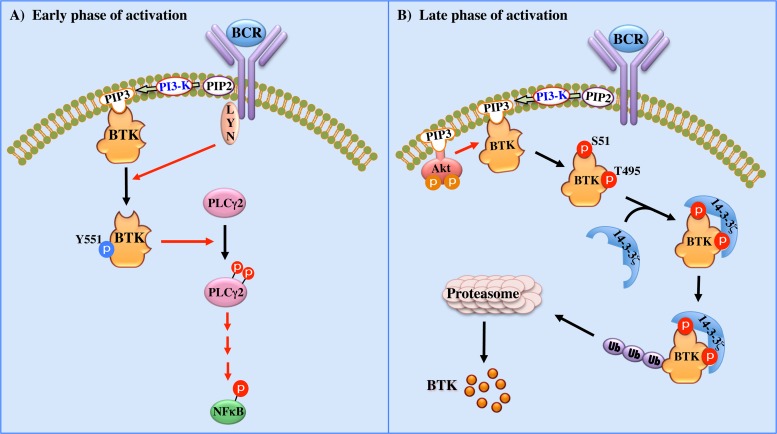
Figure 7 shows a schematic model of our current understanding regarding the role of Btk in regulating and fine-tuning the BCR signaling pathway. During the early phase of BCR activation, Btk-Y551 phosphorylation reaches a peak, which correlates with the activation of PLCγ2 and NF-κB (Fig. 7A). Moreover, this activation is significantly increased following treatment with the BV02 inhibitor, particularly under unstimulated conditions (Fig. 4B). During an intermediate phase of BCR activation, activated Akt catalyzes Btk phosphorylation on S51 and T495, leading to the association with 14-3-3ζ proteins (Fig. 7B). Since PI3-kinase activation, which is downstream of a number of receptors, such as the BCR, also results in membrane translocation of Btk, PI3-kinase regulates several important aspects of Btk activity (8). Another essential aspect of this model is that there is a feedback loop whereby phosphorylated Btk is targeted for degradation and, in parallel, its translocation to the nucleus is severely affected (Fig. 7B). We have previously postulated that dephosphorylation of Btk may be related to its translocation to the nucleus (25). Accordingly, interaction of 14-3-3ζ with phospho-Btk-S51/T495 seems to interfere with translocation of Btk into the nucleus, which in turn promotes ubiquitination and degradation of activated Btk. These events were verified using a 14-3-3ζ inhibitor (BV02), an siRNA directed against 14-3-3ζ, and a PI3-kinase inhibitor (LY294002), as well as a dominant-negative form of Akt. Although many 14-3-3-interacting proteins have been described (60–62), to our knowledge, this is the first report demonstrating their interaction with Btk.
Fig 7.
Schematic representation of the 14-3-3–Btk interaction. (A) Stimulation of the BCR first induces Btk-Y551 phosphorylation, which leads to activation of downstream signaling (PLCγ2 and NF-κB). (B) PI3-kinase-mediated activation of Akt/PKB leads to phosphorylation of Btk at S51 and T495. Subsequently, 14-3-3ζ interacts with phospho-Btk-S51/T495 and prevents translocation of Btk to the nucleus. Moreover, binding of 14-3-3ζ to activated Btk stimulates ubiquitination and degradation of active Btk, which is followed by termination of BCR signaling.
A single protein kinase can sometimes phosphorylate more than a single tandem phosphorylation site in its substrates, as exemplified by BAD, FOXO1, FOXO2, FOXO3, Mdm2, and TBC1D4 (63). Our study adds Btk to this growing list of substrates. To date, more than 60 proteins are known to harbor two phosphorylated 14-3-3 target sites. It is therefore of considerable interest that Btk contains two putative consensus motifs for 14-3-3 binding. We believe that this finding strengthens the idea that this interaction is biologically relevant, since 14-3-3 proteins often bind their targets as dimers, and the serendipitous finding of two conserved consensus sites in the same molecule would be rather unlikely. Furthermore, some phosphomimetic mutant proteins, where serines/threonines were substituted for a negatively charged amino acid, enhance their interaction with 14-3-3. Conversely, other phosphomimetic mutations abolish the interaction with 14-3-3. Here, we report for the first time that phosphomimetic mutants of Btk strongly bind to 14-3-3 and stimulate degradation of the active protein.
Even though Btk is a new member of the family of 14-3-3-regulated proteins, it could potentially serve as an important new candidate for the general analysis of 14-3-3-mediated events. Thus, Btk fulfills essentially all the known criteria that characterize a core group of 14-3-3 targets, while most known targets fulfill only some; Btk is phosphorylated on two sites by the same kinase (Akt/PKB), both sites are functionally relevant, and there are defined B-cell phenotypes when either or both sites are mutated. Btk is also an attractive candidate, since not only are the alanine replacement mutants inactive, but phosphomimetic mutants behave like their phosphorylated counterparts.
Notably, the Akt-induced phosphorylation of Btk is not the only serine/threonine kinase-regulated inhibitory mechanism for Btk. Since Btk does not have a negative autoregulatory mechanism, similar to Src family kinases, for modulating its own activity, it requires interacting partners for this regulation. Interestingly, a number of Btk-interacting proteins have been demonstrated to inhibit Btk activation, possibly fine-tuning the BCR signaling pathway (12, 15). Thus, Btk is downregulated and sequestered in the cytoplasm upon PKCβ-mediated phosphorylation at S180 (12). Similarly, the prolylisomerase Pin1 was shown to inhibit Btk following phosphorylation at two key residues, S21 and S115 in the PH domain. Although the enzyme responsible for phosphorylating these serines is currently unknown, the subsequent isomerization induced by Pin1 results in negative regulation of Btk. Phosphorylation of Btk at S21 leads to Pin1 binding during mitosis, whereas phosphorylation at S115 is critical for the interaction of the proteins in interphase cells (15). We now report two other serine/threonine motifs involved in the negative regulation of Btk, namely, S51 and T495. Further studies are required to fully understand the functional implications of the interaction between Akt, Btk, and 14-3-3ζ in B cells and their potential interplay with other negative regulators.
Supplementary Material
ACKNOWLEDGMENTS
We are indebted to Vivian Nguyen, Pavel Metalnikov, Karen Colwill, and Tony Pawson, Samuel Lunenfeld Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada, and Department of Molecular and Medical Genetics, University of Toronto, Toronto, Ontario, Canada, for assistance in the proteomics analysis. We thank Martin J. Humphries for the mRFP–14-3-3ζ plasmid and Brian Hemmings for the Akt (PKB) plasmids.
Dara Khorshed Mohammad holds a Ph.D. fellowship from the Ministry of Higher Education and Scientific Research in the Kurdistan Regional Government (ERBIL-Iraq). Alamdar Hussein has received a Ph.D. fellowship from COMSATS, Institute of Information Technology, Islamabad, Pakistan, and Manuela O. Gustafsson receives a Ph.D. scholarship from Södertörn University College. This work was further supported by the Swedish Cancer Society, the Swedish Research Council, the Stockholm County Council (research grant ALF), and the European Council FP7 grant EURO-PADnet.
We declare no competing financial interests.
Dara K. Mohammad performed and conceived the majority of the experiments, analyzed data, and wrote the paper; Beston F. Nore conceived the research, designed the experiments and revised the manuscript; Alamdar Hussain and Manuela O. Gustafsson conducted some laboratory work; Abdalla J. Mohamed analyzed the data and revised the manuscript; C. I. Edvard Smith conceived the project, provided supervision throughout, interpreted data, assisted with manuscript editing, and obtained research funding.
Footnotes
Published ahead of print 10 June 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/MCB.00247-13.
REFERENCES
- 1. Smith CI, Islam TC, Mattsson PT, Mohamed AJ, Nore BF, Vihinen M. 2001. The Tec family of cytoplasmic tyrosine kinases: mammalian Btk, Bmx, Itk, Tec, Txk and homologs in other species. Bioessays 23: 436– 446 [DOI] [PubMed] [Google Scholar]
- 2. Kurosaki T, Hikida M. 2009. Tyrosine kinases and their substrates in B lymphocytes. Immunol. Rev. 228: 132– 148 [DOI] [PubMed] [Google Scholar]
- 3. Yang WC, Collette Y, Nunes J, Olive D. 2000. Tec kinases: a family with multiple roles in immunity. Immunity 12: 373– 382 [DOI] [PubMed] [Google Scholar]
- 4. Tsukada S, Saffran DC, Rawlings DJ, Parolini O, Allen RC, Klisak I, Sparkes RS, Kubagawa H, Mohandas T, Quan S, Belmont JW, Cooper MD, Conley ME, Witte ON. 1993. Deficient expression of a B cell cytoplasmic tyrosine kinase in human X-linked agammaglobulinemia. Cell 72: 279– 290 [DOI] [PubMed] [Google Scholar]
- 5. Vetrie D, Vorechovsky I, Sideras P, Holland J, Davies A, Flinter F, Hammarstrom L, Kinnon C, Levinsky R, Bobrow M, Edvard Smith CI, Bentley DR. 1993. The gene involved in X-linked agammaglobulinaemia is a member of the src family of protein-tyrosine kinases. Nature 361: 226– 233 [DOI] [PubMed] [Google Scholar]
- 6. Rawlings DJ, Saffran DC, Tsukada S, Largaespada DA, Grimaldi JC, Cohen L, Mohr RN, Bazan JF, Howard M, Copeland NG, Jenkins NA, Witte ON. 1993. Mutation of unique region of Bruton's tyrosine kinase in immunodeficient XID mice. Science 261: 358– 361 [DOI] [PubMed] [Google Scholar]
- 7. Thomas JD, Sideras P, Smith CI, Vorechovsky I, Chapman V, Paul WE. 1993. Colocalization of X-linked agammaglobulinemia and X-linked immunodeficiency genes. Science 261: 355– 358 [DOI] [PubMed] [Google Scholar]
- 8. Nore BF, Vargas L, Mohamed AJ, Branden LJ, Backesjo CM, Islam TC, Mattsson PT, Hultenby K, Christensson B, Smith CI. 2000. Redistribution of Bruton's tyrosine kinase by activation of phosphatidylinositol 3-kinase and Rho-family GTPases. Eur. J. Immunol. 30: 145– 154 [DOI] [PubMed] [Google Scholar]
- 9. Reth M, Brummer T. 2004. Feedback regulation of lymphocyte signalling. Nat. Rev. Immunol. 4: 269– 277 [DOI] [PubMed] [Google Scholar]
- 10. Mohamed AJ, Yu L, Backesjo CM, Vargas L, Faryal R, Aints A, Christensson B, Berglof A, Vihinen M, Nore BF, Smith CI. 2009. Bruton's tyrosine kinase (Btk): function, regulation, and transformation with special emphasis on the PH domain. Immunol. Rev. 228: 58– 73 [DOI] [PubMed] [Google Scholar]
- 11. Park H, Wahl MI, Afar DE, Turck CW, Rawlings DJ, Tam C, Scharenberg AM, Kinet JP, Witte ON. 1996. Regulation of Btk function by a major autophosphorylation site within the SH3 domain. Immunity 4: 515– 525 [DOI] [PubMed] [Google Scholar]
- 12. Kang SW, Wahl MI, Chu J, Kitaura J, Kawakami Y, Kato RM, Tabuchi R, Tarakhovsky A, Kawakami T, Turck CW, Witte ON, Rawlings DJ. 2001. PKCbeta modulates antigen receptor signaling via regulation of Btk membrane localization. EMBO J. 20: 5692– 5702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vargas L, Nore BF, Berglof A, Heinonen JE, Mattsson PT, Smith CI, Mohamed AJ. 2002. Functional interaction of caveolin-1 with Bruton's tyrosine kinase and Bmx. J. Biol. Chem. 277: 9351– 9357 [DOI] [PubMed] [Google Scholar]
- 14. Crosby D, Poole AW. 2002. Interaction of Bruton's tyrosine kinase and protein kinase Ctheta in platelets. Cross-talk between tyrosine and serine/threonine kinases. J. Biol. Chem. 277: 9958– 9965 [DOI] [PubMed] [Google Scholar]
- 15. Yu L, Mohamed AJ, Vargas L, Berglof A, Finn G, Lu KP, Smith CI. 2006. Regulation of Bruton tyrosine kinase by the peptidylprolyl isomerase Pin1. J. Biol. Chem. 281: 18201– 18207 [DOI] [PubMed] [Google Scholar]
- 16. Moore BW, Perez VJ. 1967. Specific acidic proteins of the nervous system, p 343–359 In Carlson FD. (ed), Physiological and biochemical aspects of nervous integration. Prentice-Hall, Englewood Cliffs, NJ [Google Scholar]
- 17. Muslin AJ, Tanner JW, Allen PM, Shaw AS. 1996. Interaction of 14-3-3 with signaling proteins is mediated by the recognition of phosphoserine. Cell 84: 889– 897 [DOI] [PubMed] [Google Scholar]
- 18. Morrison DK. 2009. The 14-3-3 proteins: integrators of diverse signaling cues that impact cell fate and cancer development. Trends Cell Biol. 19: 16– 23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fu H, Subramanian RR, Masters SC. 2000. 14-3-3 proteins: structure, function, and regulation. Annu. Rev. Pharmacol. Toxicol. 40: 617– 647 [DOI] [PubMed] [Google Scholar]
- 20. Yaffe MB, Rittinger K, Volinia S, Caron PR, Aitken A, Leffers H, Gamblin SJ, Smerdon SJ, Cantley LC. 1997. The structural basis for 14-3-3:phosphopeptide binding specificity. Cell 91: 961– 971 [DOI] [PubMed] [Google Scholar]
- 21. Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. 1999. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96: 857– 868 [DOI] [PubMed] [Google Scholar]
- 22. Kovacina KS, Park GY, Bae SS, Guzzetta AW, Schaefer E, Birnbaum MJ, Roth RA. 2003. Identification of a proline-rich Akt substrate as a 14-3-3 binding partner. J. Biol. Chem. 278: 10189– 10194 [DOI] [PubMed] [Google Scholar]
- 23. Lindvall JM, Blomberg KE, Berglof A, Yang Q, Smith CI, Islam TC. 2004. Gene expression profile of B cells from Xid mice and Btk knockout mice. Eur. J. Immunol. 34: 1981– 1991 [DOI] [PubMed] [Google Scholar]
- 24. Gustafsson MO, Hussain A, Mohammad DK, Mohamed AJ, Nguyen V, Metalnikov P, Colwill K, Pawson T, Smith CI, Nore BF. 2012. Regulation of nucleocytoplasmic shuttling of Bruton's tyrosine kinase (Btk) through a novel SH3-dependent interaction with ankyrin repeat domain 54 (ANKRD54). Mol. Cell. Biol. 32: 2440– 2453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mohamed AJ, Vargas L, Nore BF, Backesjo CM, Christensson B, Smith CI. 2000. Nucleocytoplasmic shuttling of Bruton's tyrosine kinase. J. Biol. Chem. 275: 40614– 40619 [DOI] [PubMed] [Google Scholar]
- 26. Heinonen JE, Smith CI, Nore BF. 2002. Silencing of Bruton's tyrosine kinase (Btk) using short interfering RNA duplexes (siRNA). FEBS Lett. 527: 274– 278 [DOI] [PubMed] [Google Scholar]
- 27. Lindvall J, Islam TC. 2002. Interaction of Btk and Akt in B cell signaling. Biochem. Biophys. Res. Commun. 293: 1319– 1326 [DOI] [PubMed] [Google Scholar]
- 28. Honigberg LA, Smith AM, Sirisawad M, Verner E, Loury D, Chang B, Li S, Pan Z, Thamm DH, Miller RA, Buggy JJ. 2010. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc. Natl. Acad. Sci. U. S. A. 107: 13075– 13080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mancini M, Corradi V, Petta S, Barbieri E, Manetti F, Botta M, Santucci MA. 2011. A new nonpeptidic inhibitor of 14-3-3 induces apoptotic cell death in chronic myeloid leukemia sensitive or resistant to imatinib. J. Pharmacol. Exp. Ther. 336: 596– 604 [DOI] [PubMed] [Google Scholar]
- 30. Gu M, Du X. 1998. A novel ligand-binding site in the zeta-form 14-3-3 protein recognizing the platelet glycoprotein Ibalpha and distinct from the c-Raf-binding site. J. Biol. Chem. 273: 33465– 33471 [DOI] [PubMed] [Google Scholar]
- 31. Tzivion G, Shen YH, Zhu J. 2001. 14-3-3 proteins; bringing new definitions to scaffolding. Oncogene 20: 6331– 6338 [DOI] [PubMed] [Google Scholar]
- 32. Dubois T, Howell S, Amess B, Kerai P, Learmonth M, Madrazo J, Chaudhri M, Rittinger K, Scarabel M, Soneji Y, Aitken A. 1997. Structure and sites of phosphorylation of 14-3-3 protein: role in coordinating signal transduction pathways. J. Protein Chem. 16: 513– 522 [DOI] [PubMed] [Google Scholar]
- 33. Liu YC, Liu Y, Elly C, Yoshida H, Lipkowitz S, Altman A. 1997. Serine phosphorylation of Cbl induced by phorbol ester enhances its association with 14-3-3 proteins in T cells via a novel serine-rich 14-3-3-binding motif. J. Biol. Chem. 272: 9979– 9985 [DOI] [PubMed] [Google Scholar]
- 34. Obata T, Yaffe MB, Leparc GG, Piro ET, Maegawa H, Kashiwagi A, Kikkawa R, Cantley LC. 2000. Peptide and protein library screening defines optimal substrate motifs for AKT/PKB. J. Biol. Chem. 275: 36108– 36115 [DOI] [PubMed] [Google Scholar]
- 35. Fayard E, Xue G, Parcellier A, Bozulic L, Hemmings BA. 2010. Protein kinase B (PKB/Akt), a key mediator of the PI3K signaling pathway. Curr. Top. Microbiol. Immunol. 346: 31– 56 [DOI] [PubMed] [Google Scholar]
- 36. Manning BD, Cantley LC. 2007. AKT/PKB signaling: navigating downstream. Cell 129: 1261– 1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yoeli-Lerner M, Toker A. 2006. Akt/PKB signaling in cancer: a function in cell motility and invasion. Cell Cycle 5: 603– 605 [DOI] [PubMed] [Google Scholar]
- 38. Barnett SF, Bilodeau MT, Lindsley CW. 2005. The Akt/PKB family of protein kinases: a review of small molecule inhibitors and progress towards target validation. Curr. Top. Med. Chem. 5: 109– 125 [DOI] [PubMed] [Google Scholar]
- 39. Song G, Ouyang G, Bao S. 2005. The activation of Akt/PKB signaling pathway and cell survival. J. Cell. Mol. Med. 9: 59– 71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. St-Germain ME, Gagnon V, Parent S, Asselin E. 2004. Regulation of COX-2 protein expression by Akt in endometrial cancer cells is mediated through NF-kappaB/IkappaB pathway. Mol. Cancer 3: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lam KP, Kuhn R, Rajewsky K. 1997. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell 90:1073– 1083 [DOI] [PubMed] [Google Scholar]
- 42. Monroe JG. 2006. ITAM-mediated tonic signalling through pre-BCR and BCR complexes. Nat. Rev. Immunol. 6: 283– 294 [DOI] [PubMed] [Google Scholar]
- 43. Deane JA, Fruman DA. 2004. Phosphoinositide 3-kinase: diverse roles in immune cell activation. Annu. Rev. Immunol. 22: 563– 598 [DOI] [PubMed] [Google Scholar]
- 44. Yusuf I, Zhu X, Kharas MG, Chen J, Fruman DA. 2004. Optimal B-cell proliferation requires phosphoinositide 3-kinase-dependent inactivation of FOXO transcription factors. Blood 104: 784– 787 [DOI] [PubMed] [Google Scholar]
- 45. Hinman RM, Bushanam JN, Nichols WA, Satterthwaite AB. 2007. B cell receptor signaling down-regulates forkhead box transcription factor class O 1 mRNA expression via phosphatidylinositol 3-kinase and Bruton's tyrosine kinase. J. Immunol. 178: 740– 747 [DOI] [PubMed] [Google Scholar]
- 46. Huang H, Regan KM, Lou Z, Chen J, Tindall DJ. 2006. CDK2-dependent phosphorylation of FOXO1 as an apoptotic response to DNA damage. Science 314: 294– 297 [DOI] [PubMed] [Google Scholar]
- 47. Kops GJ, de Ruiter ND, De Vries-Smits AM, Powell DR, Bos JL, Burgering BM. 1999. Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature 398: 630– 634 [DOI] [PubMed] [Google Scholar]
- 48. Webb CF, Yamashita Y, Ayers N, Evetts S, Paulin Y, Conley ME, Smith EA. 2000. The transcription factor Bright associates with Bruton's tyrosine kinase, the defective protein in immunodeficiency disease. J. Immunol. 165: 6956– 6965 [DOI] [PubMed] [Google Scholar]
- 49. Lai S, O'Callaghan B, Zoghbi HY, Orr HT. 2011. 14-3-3 binding to ataxin-1(ATXN1) regulates its dephosphorylation at Ser-776 and transport to the nucleus. J. Biol. Chem. 286: 34606– 34616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mancini M, Veljkovic N, Corradi V, Zuffa E, Corrado P, Pagnotta E, Martinelli G, Barbieri E, Santucci MA. 2009. 14-3-3 ligand prevents nuclear import of c-ABL protein in chronic myeloid leukemia. Traffic 10: 637– 647 [DOI] [PubMed] [Google Scholar]
- 51. Singh A, Ye M, Bucur O, Zhu S, Tanya Santos M, Rabinovitz I, Wei W, Gao D, Hahn WC, Khosravi-Far R. 2010. Protein phosphatase 2A reactivates FOXO3a through a dynamic interplay with 14-3-3 and AKT. Mol. Biol. Cell 21: 1140– 1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. McKinsey TA, Zhang CL, Lu J, Olson EN. 2000. Signal-dependent nuclear export of a histone deacetylase regulates muscle differentiation. Nature 408: 106– 111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang AH, Kruhlak MJ, Wu J, Bertos NR, Vezmar M, Posner BI, Bazett-Jones DP, Yang XJ. 2000. Regulation of histone deacetylase 4 by binding of 14-3-3 proteins. Mol. Cell. Biol. 20: 6904– 6912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ha CH, Kim JY, Zhao J, Wang W, Jhun BS, Wong C, Jin ZG. 2010. PKA phosphorylates histone deacetylase 5 and prevents its nuclear export, leading to the inhibition of gene transcription and cardiomyocyte hypertrophy. Proc. Natl. Acad. Sci. U. S. A. 107: 15467– 15472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ciechanover A, Orian A, Schwartz AL. 2000. Ubiquitin-mediated proteolysis: biological regulation via destruction. Bioessays 22: 442– 451 [DOI] [PubMed] [Google Scholar]
- 56. Yu L, Mohamed AJ, Simonson OE, Vargas L, Blomberg KE, Bjorkstrand B, Arteaga HJ, Nore BF, Smith CI. 2008. Proteasome-dependent autoregulation of Bruton tyrosine kinase (Btk) promoter via NF-kappaB. Blood 111: 4617– 4626 [DOI] [PubMed] [Google Scholar]
- 57. LeBron C, Chen L, Gilkes DM, Chen J. 2006. Regulation of MDMX nuclear import and degradation by Chk2 and 14-3-3. EMBO J. 25: 1196– 1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wang X, Li JP, Chiu LL, Lan JL, Chen DY, Boomer J, Tan TH. 2012. Attenuation of T cell receptor signaling by serine phosphorylation-mediated lysine-30 ubiquitination of SLP-76. J. Biol. Chem. 287: 34091– 34100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wang X, Li JP, Kuo HK, Chiu LL, Dement GA, Lan JL, Chen DY, Yang CY, Hu H, Tan TH. 2012. Down-regulation of B cell receptor signaling by hematopoietic progenitor kinase 1 (HPK1)-mediated phosphorylation and ubiquitination of activated B cell linker protein (BLNK). J. Biol. Chem. 287: 11037– 11048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Porter GW, Khuri FR, Fu H. 2006. Dynamic 14-3-3/client protein interactions integrate survival and apoptotic pathways. Semin. Cancer Biol. 16: 193– 202 [DOI] [PubMed] [Google Scholar]
- 61. Pozuelo Rubio M, Geraghty KM, Wong BH, Wood NT, Campbell DG, Morrice N, Mackintosh C. 2004. 14-3-3-affinity purification of over 200 human phosphoproteins reveals new links to regulation of cellular metabolism, proliferation and trafficking. Biochem. J. 379: 395– 408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. van Heusden GP. 2005. 14-3-3 proteins: regulators of numerous eukaryotic proteins. IUBMB Life 57: 623– 629 [DOI] [PubMed] [Google Scholar]
- 63. Johnson C, Crowther S, Stafford MJ, Campbell DG, Toth R, MacKintosh C. 2010. Bioinformatic and experimental survey of 14-3-3-binding sites. Biochem. J. 427: 69– 78 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.