Abstract
Leptospirillum spp. are widespread members of acidophilic microbial communities that catalyze ferrous iron oxidation, thereby increasing sulfide mineral dissolution rates. These bacteria play important roles in environmental acidification and are harnessed for bioleaching-based metal recovery. Known members of the Leptospirillum clade of the Nitrospira phylum are Leptospirillum ferrooxidans (group I), Leptospirillum ferriphilum and “Leptospirillum rubarum” (group II), and Leptospirillum ferrodiazotrophum (group III). In the Richmond Mine acid mine drainage (AMD) system, biofilm formation is initiated by L. rubarum; L. ferrodiazotrophum appears in later developmental stages. Here we used community metagenomic data from unusual, thick floating biofilms to identify distinguishing metabolic traits in a rare and uncultivated community member, the new species “Leptospirillum group IV UBA BS.” These biofilms typically also contain a variety of Archaea, Actinobacteria, and a few other Leptospirillum spp. The Leptospirillum group IV UBA BS species shares 98% 16S rRNA sequence identity and 70% average amino acid identity between orthologs with its closest relative, L. ferrodiazotrophum. The presence of nitrogen fixation and reverse tricarboxylic acid (TCA) cycle proteins suggest an autotrophic metabolism similar to that of L. ferrodiazotrophum, while hydrogenase proteins suggest anaerobic metabolism. Community transcriptomic and proteomic analyses demonstrate expression of a multicopper oxidase unique to this species, as well as hydrogenases and core metabolic genes. Results suggest that the Leptospirillum group IV UBA BS species might play important roles in carbon fixation, nitrogen fixation, hydrogen metabolism, and iron oxidation in some acidic environments.
INTRODUCTION
Some members of acidophilic microbial communities catalyze ferrous iron oxidation, accelerating ferric iron-mediated oxidative dissolution of sulfide minerals and thus formation of acid mine drainage (AMD) (1, 2). Because of this capacity, acidophilic microbial communities are harnessed to release metals from sulfide minerals in biomining (reviewed in reference 3). Microbial communities, especially those adapted to very low-pH conditions (less than pH 2), are often dominated by Leptospirillum bacteria of the phylum Nitrospira (4–6). To date, there are three recognized groups within the clade Leptospirillum based on 16S rRNA phylogeny: group I (Leptospirillum ferrooxidans), group II (Leptospirillum ferriphilum and “Leptospirillum rubarum”), and group III (Leptospirillum ferrodiazotrophum) (7–9). All three Leptospirillum groups have been observed in 16S rRNA gene surveys and metagenomic studies from acidic and bioleaching environments worldwide (5, 10–13). Based on isolate characterization studies, all are iron-oxidizing chemoautotrophs, and two groups (groups I and III) are reported to be capable of nitrogen fixation (9, 14). Near-complete genomes for Leptospirillum rubarum (UBA type), “Leptospirillum group II 5wayCG” type, and L. ferrodiazotrophum have been recovered from community genomic data sets (15–17), and the complete genomes of L. ferrooxidans and L. ferriphilum isolates are now available (18, 19). Much research has focused on floating biofilms sampled from the Richmond Mine at Iron Mountain, California; the biofilms are microns to hundred microns thick and are typically dominated by Leptospirillum group II (20, 21). Community genomics, proteomics, and transcriptomics have allowed for the cultivation-independent study of generally abundant members of these communities (16, 22–25); however, the roles of lower-abundance community members have not been well studied. Here we describe unusual, near-centimeter-thick floating biofilm communities that grow within the Richmond Mine and report the partial genome of the new species “Leptospirillum group IV UBA BS,” a representative of a new group in the Leptospirillum clade, group IV. These bacteria comprise less than 3% of the sequenced community, so this study demonstrates the power of community genomics for achieving insight into the physiology of relatively low-abundance community members.
MATERIALS AND METHODS
Biofilm samples, which we refer to as “UBA BS,” were obtained from the A-drift tunnel from within the Richmond Mine, at Iron Mountain, California (40°40′ 38.42″ N and 122″ 31′ 19.90″ W, elevation of ∼900 m). Samples were collected in November 2005 (Nov05), August 2007 (Aug07), November 2007 (Island 2 and 3), June 2008 (Jun08), and December 2011 (Dec11). Sample Nov05 was subjected to Sanger sequencing, as previously described (15, 22). Additionally, community proteomic data were obtained for Nov05 and Aug07 samples as described earlier (16, 26). Briefly, proteins were released from biofilms via sonication, fractionated based on cellular location, denatured, and reduced with 6 M guanidine–10 mM dithiothreitol (DTT), digested using sequencing-grade trypsin (Promega, Madison, WI), desalted, and analyzed via two-dimensional nanoliquid chromatography electrospray ionization coupled to tandem mass spectrometry (nano-LC-ES-MS/MS) (linear ion trap Orbitrap; Thermo Fisher Scientific). For the Nov05 samples, two protein extraction methods were used as described in reference 26: the first method used an acidic buffer referred to as M2 buffer, and the second used a 0.1 M sodium acetate (pH 5.0) buffer (S buffer). The resultant MS/MS spectra from individual runs were then used to search a database of predicted proteins from AMD genomic sequences as well as common contaminants (trypsin/keratin) with SEQUEST and filtered with DTASelect (26). For comparison of protein abundance levels for Leptospirillum ferrodiazotrophum and the Leptospirillum group IV UBA BS species, proteomics data were analyzed using clustered normalized spectral abundance factors (NSAFs), as described earlier (27, 28).
Fluorescence in situ hybridization (FISH) was done on samples Aug07, Island 2 and 3, Jun08, and Dec11 as described in reference 29 using the following probes: Eubmix (general bacteria), Arc415 (general archaea), Lf1252 (Leptospirillum group III), and Lf288CG (Leptospirillum group II 5wayCG). We have designed two probes that target the Leptospirillum group IV UBA BS species 23S rRNA gene: LIV307 (5′-CCCTCTTTGGCGGACCTTTC-3′) and LIV1191 (5′-CACTCCAGGCCGAACGCTCC-3′). FISH was performed using 40% formamide concentration.
Community genomics data obtained from sample UBA (15) and from the Nov05 biofilm (UBA BS biofilm) were used to assemble the partial genome of the Leptospirillum group IV UBA BS species. Briefly, reads belonging to L. rubarum and L. ferrodiazotrophum were removed from both data sets, and the remaining reads were coassembled using Phred/Phrap/consed as described previously (30). Contigs were binned by using ESOM (31) and by comparing coverage and sequence similarity to L. ferrodiazotrophum. Manual curation of the assembly was done using methods reported previously (16). To confirm the accuracy of the binning, reads belonging to L. rubarum and L. ferrodiazotrophum were removed from an additional community genomic data set (5wayCG [22]), and the remaining reads were mapped onto the assembled genome of the Leptospirillum group IV UBA BS species using gsMapper (Roche/454) with 90% minimum sequence identity and 40-bp minimum overlap as the parameters. Automated annotation was done using an in-house pipeline, and gene calls were manually curated using BLASTX (32) against the SwissProt database.
To assess abundance of organisms in the genomically characterized UBA BS Nov05 biofilm sample, reads were mapped to the available genomes of AMD organisms using gsMapper with 99% sequence identity and 40-bp minimum overlap as the parameters. The relative abundance of each organism was estimated based on coverage statistics. To calculate coverage, the number of reads mapping to all scaffolds binned to each organism was multiplied by the average read length (800 bp), and the result was divided by the genome size (cumulative length of scaffolds in each bin).
Biofilms for transcriptomic exploration were grown in laboratory bioreactors, as described in reference 33, in the dark, at pH 1 and 37°C, and harvested at early and mid stages of development. Briefly, bioreactors consist of a Teflon channel (30 cm long by 5 cm wide by 3 cm deep), which allows the acidic modified 9K medium to flow at a constant rate (33). The medium was recycled through the reactor until oxidized (turning from bright green to red color), at which point the spent medium was replaced by fresh 9K medium.
Community transcriptomic data were obtained from eight environmental samples and five samples grown in a bioreactor. Biofilms were lysed using the MirVana lysis buffer (Ambion) and bead-beating. Total RNA was extracted using acid phenol-chloroform-isoamyl alcohol (Ambion), pelleted with cold isopropanol for about 1 h, and immediately purified using the RNEasy MinElute kit (Qiagen). The integrity of the RNA was confirmed using a Bioanalyzer 2100 (Agilent Technologies). An aliquot of good-quality RNA (RNA integrity number > 7) from six environmental samples and three bioreactor samples underwent rRNA depletion using the MicrobExpress kit (Ambion). Good-quality total RNA and rRNA-depleted RNA were converted to cDNA using Superscript III (Invitrogen) as described in reference 34, and the cDNA was fragmented with a Covaris S-system (Covaris, Inc.) to an average fragment size of 200 bp. Fragmented cDNA was sent to the University of California Davis sequencing facility for Illumina genomic library preparation and sequencing. Samples were indexed to sequence multiple samples in an Illumina lane.
To separate ribosomal from nonribosomal reads, transcriptomic reads were mapped to a modified Silva database containing rRNA genes from AMD bacteria and archaea (35) using Bowtie (36) with parameters –v 1 –best –y. Nonribosomal reads from the nine rRNA-depleted samples were pooled with nonribosomal reads obtained from the corresponding total RNA. Nonribosomal reads from all 13 samples were then mapped to the partial genome of the Leptospirillum group IV UBA BS species using Bowtie with parameters –v 1 –best –y. Transcript abundance per gene was normalized by dividing the read counts by the gene length, and the resulting value was divided by the total sum of the length-normalized values in each sample.
The 16S rRNA phylogenetic tree was built using ARB (37) with 1,000 bootstraps. Protein model predictions were done using the Phyre website (38) and visualized using Pymol (PyMOL molecular graphics system, version 1.2r3pre; Schrödinger, LLC). Ligand binding was predicted using the 3DLigandSite webserver (39). Clustering of NSAF values was done using the cluster v3.0 for Mac OSX, centering genes and samples by the median, using the Spearman rank correlation similarity matrix, and average linkage as the clustering method (40). The heatmaps were visualized with the TreeView software (41).
Nucleotide sequence accession number.
This Whole Genome Shotgun project has been deposited in DDBJ/EMBL/GenBank under the accession AURA00000000. The version described in this paper is version AURA01000000. The genome annotation and proteomics data sets are already publically available via the open knowledgebase ggKbase website (http://genegrabber.berkeley.edu/amd/organisms/506).
RESULTS
Diversity of UBA BS biofilms.
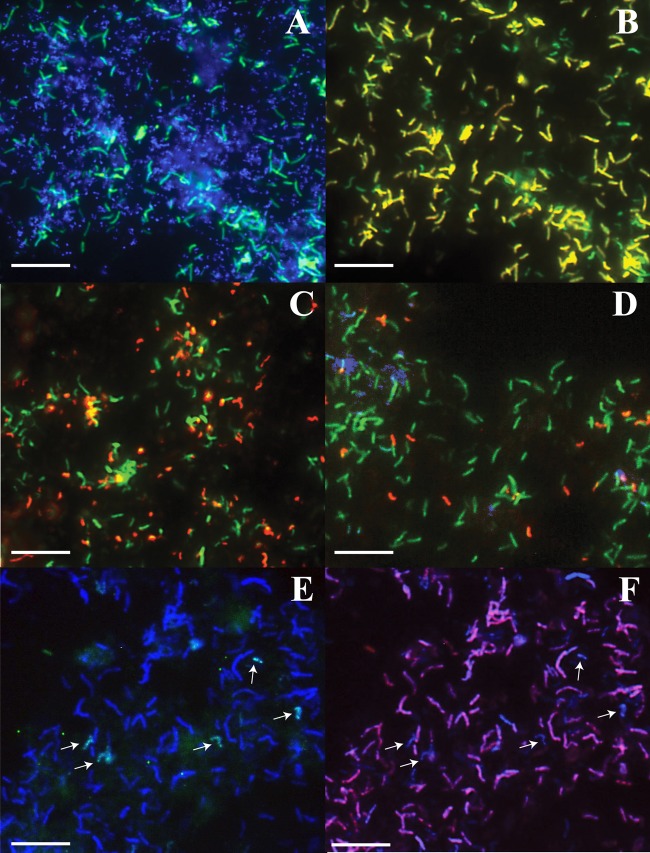
The UBA BS biofilms occur as near-centimeter-thick aggregates of fuzzy strips that float on the surfaces of slowly draining AMD ponds (see Fig. S1 in the supplemental material). The thickness of these biofilms is remarkable, given that previously described AMD biofilms are rarely more than 200 μm thick (20). Biofilm Nov05 was collected from an AMD pool in the A drift of the Richmond Mine in northern California (see Materials and Methods). The temperature of the pool was 38°C, and the pH was 0.89. DNA and proteins were recovered from this sample for metagenomic sequencing and mass spectrometry-based proteomic analysis. Community proteomic data were also obtained for biofilm Aug07, which was collected from approximately the same site (36°C and pH 1.94). In addition, five UBA BS biofilms, including Aug07, were analyzed with fluorescence in situ hybridization (FISH) for organism identification (Table 1, Fig. 1, and Fig. S2). Cell counts obtained from FISH data show that archaea appear to dominate these biofilms (average, 68% ± 9%), and Leptospirillum bacteria represent an average 34% of the organisms detected by FISH (Fig. 1A and Table 1). Only one UBA BS biofilm appeared to be dominated by Leptospirillum group III, but it also contained Leptospirillum group II 5wayCG at low abundance (sample Dec11 [Fig. S2]). Most bacteria that bound the general Leptospirillum FISH probe (data not shown) were not labeled by group II- and III-specific FISH probes, suggesting the presence of an unknown Leptospirillum type in these samples (e.g., Fig. 1C [Jun08], D [Aug07], and E [Island 2]). Leptospirillum group II 5wayCG type was detected at low abundance (Table 1, Fig. 1D, and Fig. S2), but L. rubarum (group II UBA type) was not identified in any of the biofilms. Protists were present in some biofilms; however, fungi were not observed with FISH (data not shown).
Table 1.
Relative abundance of organisms estimated from FISH in four UBA BS biofilms
| Sample | Date collected (mo/day/yr) | pHa | Temp (°C)a | Relative total abundance (%) |
Leptospirillum abundance (%)b |
|||
|---|---|---|---|---|---|---|---|---|
| Archaea | Bacteria | LeptoIII | LeptoIV | LeptoII CG | ||||
| Aug07 | 8/22/07 | 1.94 | 36 | 72.87 | 27.13 | 3.09 | 11.10 | 0.00 |
| Island 2 | 11/7/07 | ND | ND | 57.31 | 42.69 | 5.10 | 26.34 | 1.09 |
| Island 3 | 11/7/07 | ND | ND | 77.29 | 22.71 | 5.92 | 41.01 | 1.34 |
| Jun08 | 6/26/08 | 1.08 | 37 | 64.36 | 35.64 | 27.17 | 14.74 | 0.00 |
| Avg | 67.96 | 32.04 | 10.32 | 23.30 | 0.61 | |||
ND, no data available.
LeptoIII, Leptospirillum ferrodiazotrophum; LeptoIV, Leptospirillum group IV UBA BS; LeptoII CG, Leptospirillum group II 5way CG type.
Fig 1.
Fluorescence in situ hybridization images of UBA BS biofilms. (A) Sample Aug07, showing DNA (blue; most small dots are Archaea) and general bacteria (green). (B) Sample Aug07, showing general bacteria (green) and Leptospirillum group IV UBA BS (yellow). (C) Sample Jun08, showing general bacteria (green) and L. ferrodiazotrophum (red). (D) Sample Aug07, showing general bacteria (green), L. ferrodiazotrophum (red), and Leptospirillum group II 5wayCG type (blue). (E) Sample Island 2, showing general bacteria (blue), L. ferrodiazotrophum (green/light blue). (F) Sample Island 2, showing general bacteria (blue) and Leptospirillum group IV UBA BS (purple/pink). The images in panels A and B were taken from the same field of view, as were the images in panels E and F. The white arrows indicate Leptospirillum group III cells. Bars, 1 μm.
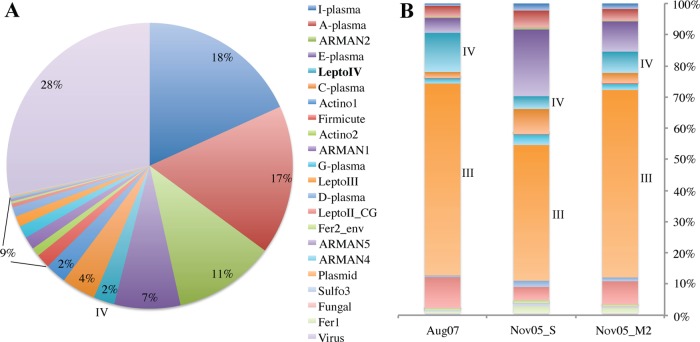
An average 84% of the reads from the Nov05 community genomics data set mapped to assembled genome fragments from bacteria or archaea, including fragments binned to known organisms (15, 16, 22, 31, 42), one bacteriophage population, three viruses of archaea (30), and one Leptospirillum megaplasmid population (Fig. 2A). The remaining reads must be associated with community members whose abundance is low enough to preclude assembly. Based on coverage, Thermoplasmatales lineage archaea E-plasma, A-plasma, and C-plasma, a related archaeon, I-plasma, and ARMAN nanoarchaea dominate the sample (61% of the reads), whereas Leptospirillum bacteria comprise ∼4%, other bacteria and fungi represent 7%, and viruses and plasmids represent 28% of the sequenced community (Fig. 2A).
Fig 2.
(A) Percentages of uniquely mapping community genomics reads from the Nov05 sample to assembled genomes from AMD organisms. LeptoIV, Leptospirillum group IV UBA BS; LeptoIII, Leptospirillum ferrodiazotrophum; LeptoII_CG, Leptospirillum group II 5way CG type; Actino1 and Actino2, actinobacterial bins 1 and 2 (from our lab), respectively; Sulfo, Sulfobacillus bin; Fer1, Ferroplasma type I; Fer2_env, Ferroplasma type II. (B) Percent NSAF values in three community proteomics data sets (III and IV refer to L. ferrodiazotrophum [group III] and Leptospirillum group IV UBA BS protein abundances, respectively).
We also evaluated community composition using proteomic data. Interestingly, community proteomic analysis of the Nov05 biofilm and of the Aug07 sample shows that L. ferrodiazotrophum proteins are the most abundant, and only 28% of the proteins are archaeal (Fig. 2B). The discrepancy between DNA and protein measures could be attributed to differences in activity levels for bacteria versus archaea (42) and/or differences in cell size (e.g., Fig. 1A). However, biases in DNA versus protein extraction could also be a factor.
Identification and “omic” sampling of the Leptospirillum group IV UBA BS species.
The partial genome of a new Leptospirillum bacterium was assembled primarily from reads from the Nov05 community genomics data set (average depth of 3.3×). Assembled genome fragments >1.6 kb were assigned to this genome in part based on some sequence similarity to the genome of Leptospirillum ferrodiazotrophum. Highly dissimilar sequences belonging to the new bacterium may have been missed in this approach. In addition, sequences were classified as belonging to this organism based on tetranucleotide sequence signatures analyzed in the context of an emergent self-organizing map (31). The partial genome contains 1,923 predicted protein-encoding genes, one rRNA operon, and 18 tRNAs on 295 scaffolds representing 1.48 Mb of sequence (Table 2). The genomes of L. ferrooxidans, L. ferriphilum, L. rubarum, Leptospirillum group II 5wayCG species, and L. ferrodiazotrophum are, on average, 2.6 ± 0.16 Mb long. Thus, we estimate that ∼56% of the newly assembled genome was recovered. The presence of 67.5% of the single-copy genes selected to measure genome completeness supports this estimation (see Fig. S3 in the supplemental material). In addition, 2.57% of the Nov05 reads mapped uniquely to the new Leptospirillum genome; hence, partial genome reconstruction was achieved for a low-abundance organism (Fig. 2A; see Table S1 in the supplemental material).
Table 2.
Statistics for the Leptospirillum group IV UBA BS genome and comparison with other Leptospirillum genomesa
| Characteristic or statistic | LeptoIV | LeptoIII | LeptoII UBA | LeptoII CG | L. ferriphilum ML-04 | L. ferrooxidans C2-3 |
|---|---|---|---|---|---|---|
| Group classification | IV | III | II | II | II | I |
| Reference | This study | 16 | 15 | 17 | 18 | 19 |
| Genome length (Mbp) | 1.48 | 2.84 | 2.65 | 2.72 | 2.41 | 2.56 |
| No. of assembled scaffolds | 295 | 25 | 10 | 77 | 1 | 1 |
| GC content (%) | 58.98 | 58.51 | 54.7 | 54.28 | 54.55 | 50.1 |
| No. of predicted proteins | 1,923 | 2,654 | 2,625 | 2,584 | 2,471 | 2,421 |
| No. of tRNAs | 18 | 46 | 48 | 47 | 48 | 51 |
| Relative values compared to Leptospirillum UBA BS | ||||||
| No. of orthologs | 1,463 | 1,382 | 1,387 | 1,310 | 1,346 | |
| % id orthologsb | 69.62 | 55.20 | 55.35 | 54.66 | 47.66 | |
| % id 16S rRNA | 98.46 | 92.43 | 92.2 | 92.3 | 92.25 | |
| % id 23S rRNA | 96.3 | 91.39 | 91.53 | 91.39 | 89.92 |
LeptoIV, Leptospirillum group IV UBA BS; LeptoIII, L. ferrodiazotrophum; LeptoII UBA, L. rubarum; LeptoII CG, Leptospirillum group II 5wayCG type.
% id, percent identity.
A 16S rRNA phylogenetic tree of the genus Leptospirillum, which includes the partial (>1,000-bp) sequence from the newly assembled Leptospirillum genome and two other closely related cloned sequences from other acidic environments, identifies a new group IV clade (Fig. 3). We refer to the new clade as Leptospirillum group IV. The phylogenetic distance and percent nucleotide identity of the 23S rRNA gene and the amino acid identity of the RpoB protein, ribosomal protein S3, and ribosomal protein L5 support the placement of the Leptospirillum group IV UBA BS species in a separate clade: the distance between the Leptospirillum group IV UBA BS species and Leptospirillum group III sequences is comparable to those of Leptospirillum groups I and II (see Fig. S4 in the supplemental material). We designed 23S rRNA FISH probes to specifically target ribosomes of the Leptospirillum group IV UBA BS species (see Materials and Methods). When applied to UBA BS biofilms, FISH analyses indicate that the Leptospirillum cells that did not bind the group II- and III-specific probes are the Leptospirillum group IV UBA BS species (Fig. 1B [Aug07] and F [Island 2]).
Fig 3.
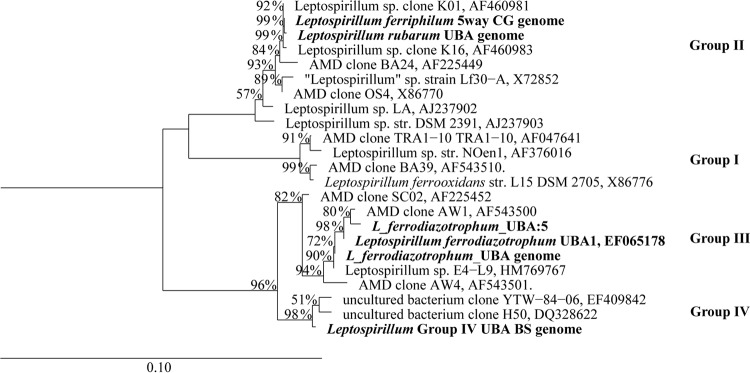
Neighbor-joining 16S rRNA phylogenetic tree of Leptospirillum spp. (1,000 bootstraps). Percent bootstrap values are shown at the nodes. The scale bar indicates 10% sequence divergence. The sequence of a Deltaproteobacterium was used as an outgroup. str., strain.
Comparison of the Leptospirillum group IV UBA BS species with other Leptospirillum spp.
Table 2 reports a comparison of the genome of the Leptospirillum group IV UBA BS species with the genomes of other Leptospirillum species. About 75% of the predicted genes in the Leptospirillum group IV UBA BS species have an ortholog in the genomes of the other Leptospirillum spp., with an average amino acid identity of 57%.
The genome of the Leptospirillum group IV UBA BS species contains several subunits for a pyruvate:ferredoxin oxidoreductase (PFOR) cluster identified by transcriptomics and proteomics (see Table S2 in the supplemental material). Cytochromes Cyt572 (cytochrome 572) and Cyt579, which were reported in L. rubarum and L. ferrodiazotrophum (16), were also identified (Table S2). Two tetraheme c-type cytochromes were predicted in the Leptospirillum group IV UBA BS genome, one of them belonging to the NapC/NirT family (UBABSL4_11800G0003 and UBABSL4_8194G0002a [Table S2]).
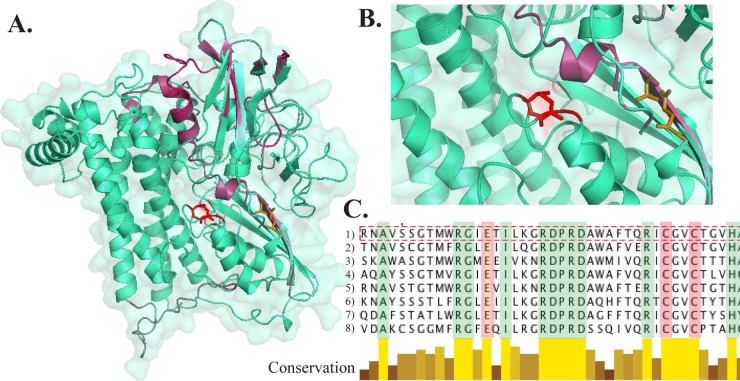
A multicopper oxidase gene with no orthologs in the other Leptospirillum bacteria is encoded in the genome of the Leptospirillum group IV UBA BS species. Multicopper oxidases (MCOs) are involved in the oxidation of ferrous iron, copper, and manganese (43). The sequence contains two predicted MCO domains, including all copper centers found in other two-domain MCOs (Fig. 4).
Fig 4.
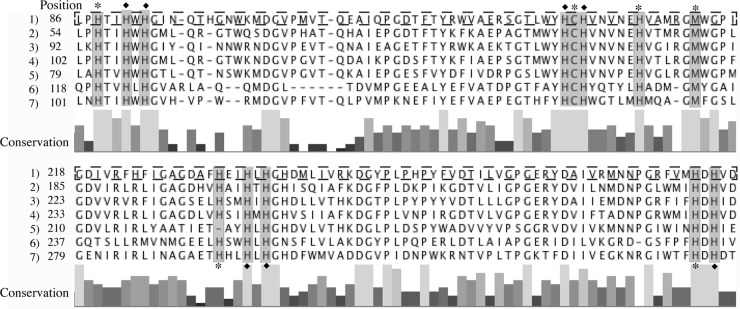
Multiple-sequence alignment of MCOs, showing the conserved copper center residues, T1 (∗) and T2/T3 (⧫). The alignment includes sequences from Leptospirillum group IV UBA BS (box outlined by a broken line) (lines 1), Nitrosomonas europaea (lines 2), Nitrosococcus halophilus (lines 3), Herminiimonas arsenicoxydans (lines 4), Shewanella woodyi ATCC 51908 (lines 5), Deinococcus maricopensis DMS 21211 (lines 6) and Flavobacterium frigoris PS1 (lines 7). Gaps introduced to maximize alignment are indicated by dashes.
As in Leptospirillum ferrodiazotrophum and L. ferrooxidans (9, 14), the Leptospirillum group IV UBA BS species contains a nitrogen (N2) fixation pathway. nif transcripts were not observed for the Leptospirillum group IV UBA BS species, and only a few transcriptomics reads were observed for L. ferrodiazotrophum in one environmental sample (see Table S2 in the supplemental material). The operon in the Leptospirillum group IV UBA BS species and L. ferrodiazotrophum contain paralogous nifZ genes (Fig. 5). The genomic assemblies were manually curated to verify the existence of paralogous nifZ genes, which share only 39% sequence identity. This duplication is not part of the L. ferrooxidans Nif operon (14, 19).
Fig 5.
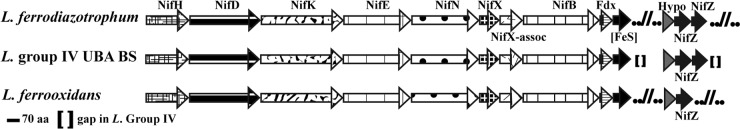
Diagram of the nitrogen fixation operon in Leptospirillum group IV UBA BS, L. ferrodiazotrophum, and L. ferrooxidans. NifX-assoc, NifX-associated protein; Fdx, ferredoxin; [FeS], FeS cluster assembly protein. Gaps (brackets) in the Leptospirillum group IV UBA BS operon correspond to genes: (i) genes encoding two hypothetical (Hypo) proteins, nifS, aminotransferase gene; and (ii) nifU, nifT, genes encoding two hypothetical proteins, ferredoxin gene, nifW, and genes encoding two final nitrogen regulatory proteins P-II. aa, amino acids.
The genome of the Leptospirillum group IV UBA BS species contains genes for osmoprotection (ectoine operon and trehalose synthase), biosynthesis of vitamins and cofactors, amino acid biosynthesis, polar flagella, chemotaxis, nitrite/sulfite reductase and sulfur assimilation, ATP synthase, 20S proteasome cluster, an arsenic resistance operon, phosphate transport, and formate hydrogenlyase cluster (see Table S2 in the supplemental material), all of which are present in L. rubarum and L. ferrodiazotrophum (16). Similar to L. rubarum, the Leptospirillum group IV UBA BS species contains a cellulose synthase gene, suggesting that it too may be involved in biofilm formation. A clustered regularly interspaced short palindromic repeat (CRISPR) system type I (44) implicated in phage/plasmid resistance (45) has also been identified in the Leptospirillum group IV UBA BS species, and all CRISPR-associated Cas genes were generally detected at low levels by transcriptomics (Table S2).
The genomes of Leptospirillum ferriphilum, L. ferrooxidans, L. rubarum, and L. ferrodiazotrophum contain two mercury (Hg) resistance genes in a cluster, merA and merR, and the first three species also contain the Hg transporter merT gene. The Leptospirillum group IV UBA BS species carries in a cluster the merR and merA genes and the mercury transporter merC gene, which contains four transmembrane domains and a conserved metal-binding motif MXCXXC at the C terminus. The operon was identified by transcriptomics in four biofilm samples (see Table S2 in the supplemental material). At low concentrations, Hg can enter the cell where it is volatilized by the mercuric reductase MerA, but MerC may be necessary when high concentrations of Hg are encountered and efficient reduction is required (reviewed in reference 46).
The Leptospirillum group IV UBA BS species contains genes for a respiratory [NiFe]-hydrogenase. HydB is the large subunit of the complex, which catalyzes the reversible oxidation of H2 (reviewed in reference 47). The predicted structure model for HydB based on the structure of Escherichia coli HydB contains perfectly conserved active site residues (cysteine 75 and cysteine 78, which bind nickel, and glutamate 56 and alanine 528, which bind ferrous iron and magnesium) (Fig. 6), and transmembrane helix prediction suggests that it is membrane bound. The small subunit, HydA, is encoded in the cluster with HydB. It contains the conserved motif (S/T)RRXFXK within the signal peptide and shares high sequence identity over the full length of the protein with a membrane-bound hydrogenase small subunit from Ralstonia eutropha (data not shown). The genes encoding hydrogenase maturation proteins HypBFCDE are located in a cluster with a nickel transporter. Hydrogenases have not yet been reported for the Leptospirillum spp., but the recently deposited genome of a Leptospirillum ferrooxidans isolate contains a single cluster of hydrogenase and maturation genes (19), which share an average 66% amino acid identity with the Leptospirillum group IV UBA BS orthologs.
Fig 6.
(A and B) Three-dimensional (3D) structure representations of the hydrogenase HydB in Leptospirillum group IV modeled on the Escherichia coli protein. (A) HydB full model; (B) higher-magnification image of the active site highlighting catalytic residues (NiFe-binding residues [red] and Fe2-binding residues [brown]). Conserved structural regions are shown in blue-green, while less-conserved regions are shown in magenta. (C) Multiple-alignment screenshot showing catalytic residues (Cys75 and Cys78 [rose] and Glu56 [peach]) and other perfectly conserved residues around the active site (green). Alignment includes hydrogenase sequences from Leptospirillum group IV UBA BS (line 1), E. coli (lines 2 and 3), Allocromatium vinosum (line 4), Bradyrhizobium japonicum (line 5), Desulfovibrio vulgaris (line 6), Wolinella succinogenes (line 7), and Desulfovibrio baculatus (line 8). The shades of yellow and brown indicate degrees of conservation.
A second [NiFe]-hydrogenase large subunit (likely HoxH) and a hydrogenase maturation protein gene in the genome of Leptospirillum group IV UBA BS have homologs in Acidithiobacillus ferrooxidans, sharing an average 55% identity. Ligand binding prediction shows that HoxH is also able to bind nickel through four conserved cysteines (Cys64, Cys67, Cys415, and Cys418) as well as magnesium and ferrous iron (Glu45, Ile370, and His421). However, no transmembrane helices were predicted for this hydrogenase, suggesting that it is a cytoplasmic hydrogenase involved in H2 uptake during N2 fixation (reviewed in reference 47).
HydB was identified by proteomics in one UBA BS biofilm, and the hydA, hydB, and hoxH genes and genes encoding maturation proteins and the nickel transporter were identified by transcriptomics only in the sample in which the nitrogen fixation operon in L. ferrodiazotrophum was transcribed (see Table S2 in the supplemental material).
Proteomics and transcriptomics of Leptospirillum group IV UBA BS.
A total of 22.9% of Leptospirillum group IV UBA BS predicted proteins were identified by mass spectrometry-based proteomics (see Materials and Methods) in three UBA BS biofilms (see Table S2 in the supplemental material). In order to compare the functional profiles of L. ferrodiazotrophum and the Leptospirillum group IV UBA BS species, we clustered NSAF values for orthologous proteins identified in these three biofilms (Fig. 7). All ribosomal proteins, the ATPase and PFOR clusters, chemotaxis and flagellar proteins, and phosphate uptake proteins were largely overrepresented in Leptospirillum group IV UBA BS (Fig. 7, cluster 2), whereas lipid and fatty acid biosynthesis and peptide-processing proteins were overrepresented in L. ferrodiazotrophum. Proteins involved in carbohydrate metabolism, amino acid, vitamin and cofactor, and nucleotide biosyntheses were overrepresented in both complexes.
Fig 7.
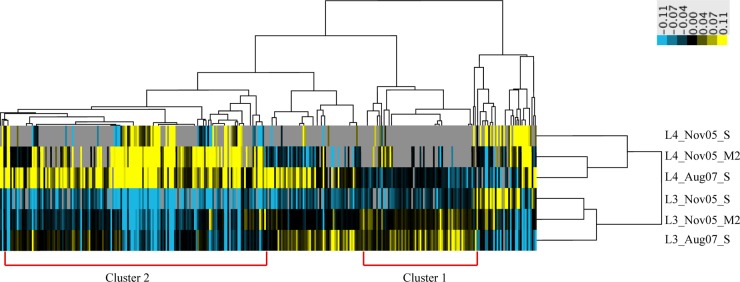
NSAF clustering in Leptospirillum group IV UBA BS compared to L. ferrodiazotrophum in three UBA BS biofilms. Cluster 1 was overrepresented by L. ferrodiazotrophum, and cluster 2 was overrepresented by Leptospirillum group IV UBA BS. Clusters 1 and 2 contained genes involved in the following functional categories (number of genes in cluster 1; number in cluster 2): energy-related genes (8; 17), carbohydrate metabolism (10; 20), nucleotide metabolism (2; 5), amino acid metabolism (10; 16), lipid and fatty acid metabolism (4; 0), vitamin and cofactor biosynthesis (5; 9), ribosomal proteins (0; 20), peptide processing (7; 4), chemotaxis and flagella (1; 9) and phosphate uptake (0; 3).
To more broadly evaluate the activity of Leptospirillum group IV UBA BS in AMD biofilms, we obtained community transcriptomics data from eight relatively thin floating biofilms from a range of locations in the Richmond Mine, and five laboratory-grown thin floating biofilms. Leptospirillum group IV UBA BS accounts for less than 1% of the total community RNA on average in all of these transcriptomic samples (data not shown). Despite the low abundance, up to 46% of its predicted genes were identified by at least one Illumina read (see Table S2 in the supplemental material). Clustering of normalized transcriptomic values shows that Leptospirillum group IV UBA BS transcript abundance is generally higher in bioreactor-grown samples than in environmental samples (see Fig. S5 in the supplemental material). Bioreactor solutions tend to have a higher pH and higher concentration of ferric iron compared to natural AMD solutions (Shufen Ma, personal communication). Interestingly, Leptospirillum group IV UBA BS genes encoded in mobile elements (transposases, hypothetical proteins, secretion proteins, and transporters) are overrepresented in bioreactor transcriptomic data sets (Fig. S5). Core metabolism genes are generally similarly transcribed in natural and bioreactor-grown biofilms (Table S2).
DISCUSSION
Bacteria generally dominate Richmond Mine AMD biofilms, and only recently were Archaea reported to dominate sunken biofilms degrading under microaerophilic and anaerobic conditions (48). Here we described an unusual Archaea-dominated acidophilic biofilm community that contains a novel bacterial species, Leptospirillum group IV UBA BS. The identity of its 16S rRNA gene sequence compared to sequences of the other Leptospirillum spp. is sufficiently low to warrant its designation as a distinct species (<98.7% identity, as recommended by Stackebrandt and Ebers [49]). Enrichment of this species has not been observed in cultures from the Richmond Mine, possibly due to its habitat within an extraordinarily thick polymer environment and its association with uncultivated Archaea. Notably, Leptospirillum group IV UBA BS is most abundant in biofilms that contain only very low abundances of other cultivated Leptospirillum spp. Consistent with its designation as a distinct species, among the leptospirilli, the Leptospirillum group IV UBA BS species is unique in its capacity for mercury resistance, the presence of a multicopper oxidase, and many unique hypothetical proteins. As in Leptospirillum ferrooxidans, it has the potential for hydrogen metabolism.
Its genomic content suggests that cells are likely motile, capable of both carbon and nitrogen fixation. It has been suggested that motility allows L. ferrodiazotrophum to redistribute into microcolonies (16). Motility may be particularly important for Leptospirillum group IV UBA BS growing in the gel-like environment of the thick floating biofilms, allowing it to find a suitable habitat as conditions change during biofilm development. Leptospirillum group IV UBA BS might be capable of anaerobic growth using H2 as electron donor, as shown for Acidithiobacillus ferrooxidans (50), although experimental validation is required to confirm this function. Hydrogen metabolism could be associated with growth under anaerobic conditions predicted within AMD biofilms thicker than a few microns (51).
Leptospirillum bacteria are known iron oxidizers, likely using cytochrome 572 (Cyt572) to take electrons from Fe(II) (52, 53). Cytochrome 579 (Cyt579) is involved in electron transport (54, 55). The presence of a multicopper oxidase, as well as Cyt572 and Cyt579, whose biochemical function was verified in Leptospirillum group II from the Richmond Mine (52, 54), provides strong evidence supporting the role of Leptospirillum group IV UBA BS in iron oxidation. The Leptospirillum group IV UBA BS species likely fixes CO2 via the reverse tricarboxylic acid (TCA) cycle using pyruvate:ferredoxin oxidoreductase (PFOR), as shown in other Leptospirillum spp. (reviewed in reference 56).
Community transcriptomics shows that the fraction of Leptospirillum group IV UBA BS genes for which a transcript was detected was highest in the environmental sample A-drift GS0 (see Table S2 in the supplemental material). This early developmental stage biofilm, dominated by L. ferrodiazotrophum, was collected from the A-drift tunnel in September 2010. The temperature at that location was 40°C, the pH was 1.27, and the solution contained unusually high concentrations of ferric iron (Shufen Ma, personal communication). The sample in which the lowest fraction of genes for which a transcript was detected was the environmental sample C75 GS1 (Table S2). This mid-developmental stage biofilm, collected from a pool in the C-drift tunnel with pH 0.86 and temperature 46°C, was dominated by Leptospirillum rubarum (group II, UBA type), and L. ferrodiazotrophum was detected at low abundance (21). Therefore, both physiologically and in terms of certain environmental preferences, the Leptospirillum group IV UBA BS species is most similar to L. ferrodiazotrophum (group III), to which it is most closely related based on phylogenetic analysis. It is notable that expression of hydrogenases in the Leptospirillum group IV UBA BS species was detected only in the sample in which the nitrogen fixation operon is expressed in L. ferrodiazotrophum. H2 is a by-product of nitrogen fixation, but there is no evidence that it can be used by L. ferrodiazotrophum. Thus, consumption of H2 may be the basis for cooperative interactions between the Leptospirillum group IV UBA BS species and L. ferrodiazotrophum.
Supplementary Material
ACKNOWLEDGMENTS
We thank T. W. Arman, President, Iron Mountain Mines Inc., R. Sugarek (U.S. Environmental Protection Agency) for site access, and R. Carver for on-site assistance. We thank Christine Sun for help with ruby scripts, and Edward Ralston for access to, and help with, the Covaris system. DNA sequencing was carried out at The Joint Genome Institute. Transcriptomic sequencing was done at the University of California Davis.
This research was supported by the U.S. Department of Energy through the Genomic Sciences (DE-FG02-05ER64134) and Carbon-Cycling (DE-FG02-10ER64996) programs. D.S.A.G. was supported by an NSF GRFP fellowship.
We declare that we have no conflicts of interest.
Footnotes
Published ahead of print 3 May 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.00202-13.
REFERENCES
- 1.Edwards KJ, Bond PL, Druschel GK, McGuire MM, Hamers RJ, Banfield JF. 2000. Geochemical and biological aspects of sulfide mineral dissolution: lessons from Iron Mountain, California. Chem. Geol. 169:383–397 [Google Scholar]
- 2.Rawlings DE, Dew D, du Plessis C. 2003. Biomineralization of metal-containing ores and concentrates. Trends Biotechnol. 21:38–44 [DOI] [PubMed] [Google Scholar]
- 3.Rawlings DE, Johnson DB. 2007. The microbiology of biomining: development and optimization of mineral-oxidizing microbial consortia. Microbiology 153:315–324 [DOI] [PubMed] [Google Scholar]
- 4.Bond PL, Druschel GK, Banfield JF. 2000. Comparison of acid mine drainage microbial communities in physically and geochemically distinct ecosystems. Appl. Environ. Microbiol. 66:4962–4971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker BJ, Banfield JF. 2003. Microbial communities in acid mine drainage. FEMS Microbiol. Ecol. 44:139–152 [DOI] [PubMed] [Google Scholar]
- 6.Druschel GK, Baker BJ, Gihring TM, Banfield JF. 2004. Acid mine drainage biogeochemistry at Iron Mountain, California. Geochem. Trans. 5:13. 10.1186/1467-4866-5-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coram NJ, Rawlings DE. 2002. Molecular relationship between two groups of the genus Leptospirillum and the finding that Leptospirillum ferriphilum sp. nov. dominates South African commercial biooxidation tanks that operate at 40°C. Appl. Environ. Microbiol. 68:838–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hippe H. 2000. Leptospirillum gen. nov. (ex Markosyan 1972), nom. rev., including Leptospirillum ferrooxidans sp. nov. (ex Markosyan 1972), nom. rev. and Leptospirillum thermoferrooxidans sp. nov. (Golovacheva et al 1992). Int. J. Syst. Evol. Microbiol. 50:501–503 [DOI] [PubMed] [Google Scholar]
- 9.Tyson GW, Lo I, Baker BJ, Allen EE, Hugenholtz P, Banfield JF. 2005. Genome-directed isolation of the key nitrogen fixer Leptospirillum ferrodiazotrophum sp. nov. from an acidophilic microbial community. Appl. Environ. Microbiol. 71:6319–6324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diaby N, Dold B, Pfeifer HR, Holliger C, Johnson DB, Hallberg KB. 2007. Microbial communities in a porphyry copper tailings impoundment and their impact on the geochemical dynamics of the mine waste. Environ. Microbiol. 9:298–307 [DOI] [PubMed] [Google Scholar]
- 11.Xie X, Xiao S, He Z, Liu J, Qiu G. 2007. Microbial populations in acid mineral bioleaching systems of Tong Shankou copper mine, China. J. Appl. Microbiol. 103:1227–1238 [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Moyano A, Gonzalez-Toril E, Aguilera A, Amils R. 2007. Prokaryotic community composition and ecology of floating macroscopic filaments from an extreme acidic environment, Rio Tinto (SW, Spain). Syst. Appl. Microbiol. 30:601–614 [DOI] [PubMed] [Google Scholar]
- 13.Galleguillos PA, Hallberg KB, Johnson DB. 2009. Microbial diversity and genetic response to stress conditions of extremophilic bacteria isolated from the Escondida copper mine. Adv. Materials Res. 71-73:55–58 [Google Scholar]
- 14.Parro V, Moreno-Paz M. 2004. Nitrogen fixation in acidophile iron-oxidizing bacteria: the nif regulon of Leptospirillum ferrooxidans. Res. Microbiol. 155:703–709 [DOI] [PubMed] [Google Scholar]
- 15.Lo I, Denef VJ, Verberkmoes NC, Shah MB, Goltsman D, DiBartolo G, Tyson GW, Allen EE, Ram RJ, Detter JC, Richardson P, Thelen MP, Hettich RL, Banfield JF. 2007. Strain-resolved community proteomics reveals recombining genomes of acidophilic bacteria. Nature 446:537–541 [DOI] [PubMed] [Google Scholar]
- 16.Goltsman DSA, Denef VJ, Singer SW, VerBerkmoes NC, Lefsrud M, Mueller RS, Dick GJ, Sun CL, Wheeler KE, Zemla A, Baker BJ, Hauser L, Land M, Shah MB, Thelen MP, Hettich RL, Banfield JF. 2009. Community genomic and proteomic analyses of chemoautotrophic iron-oxidizing “Leptospirillum rubarum” (group II) and “Leptospirillum ferrodiazotrophum” (group III) bacteria in acid mine drainage biofilms. Appl. Environ. Microbiol. 75:4599–4615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simmons SL, Dibartolo G, Denef VJ, Goltsman DS, Thelen MP, Banfield JF. 2008. Population genomic analysis of strain variation in Leptospirillum group II bacteria involved in acid mine drainage formation. PLoS Biol. 6:e177. 10.1371/journal.pbio.0060177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mi S, Song J, Lin J, Che Y, Zheng H, Lin J. 2011. Complete genome of Leptospirillum ferriphilum ML-04 provides insight into its physiology and environmental adaptation. J. Microbiol. 49:890–901 [DOI] [PubMed] [Google Scholar]
- 19.Fujimura R, Sato Y, Nishizawa T, Oshima K, Kim SW, Hattori M, Kamijo T, Ohta H. 2012. Complete genome sequence of Leptospirillum ferrooxidans strain C2-3, isolated from a fresh volcanic ash deposit on the island of Miyake, Japan. J. Bacteriol. 194:4122–4123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilmes P, Remis JP, Hwang M, Auer M, Thelen MP, Banfield JF. 2009. Natural acidophilic biofilm communities reflect distinct organismal and functional organization. ISME J. 3:266–270 [DOI] [PubMed] [Google Scholar]
- 21.Denef VJ, Banfield JF. 2012. In situ evolutionary rate measurements show ecological success of recently emerged bacterial hybrids. Science 336:462–466 [DOI] [PubMed] [Google Scholar]
- 22.Tyson GW, Chapman J, Hugenholtz P, Allen EE, Ram RJ, Richardson PM, Solovyev VV, Rubin EM, Rokhsar DS, Banfield JF. 2004. Community structure and metabolism through reconstruction of microbial genomes from the environment. Nature 428:37–43 [DOI] [PubMed] [Google Scholar]
- 23.Denef VJ, Kalnejais LH, Mueller RS, Wilmes P, Baker BJ, Thomas BC, VerBerkmoes NC, Hettich RL, Banfield JF. 2010. Proteogenomic basis for ecological divergence of closely related bacteria in natural acidophilic microbial communities. Proc. Natl. Acad. Sci. U. S. A. 107:2383–2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mueller RS, Dill BD, Pan C, Belnap CP, Thomas BC, VerBerkmoes NC, Hettich RL, Banfield JF. 2011. Proteome changes in the initial bacterial colonist during ecological succession in an acid mine drainage biofilm community. Environ. Microbiol. 13:2279–2292 [DOI] [PubMed] [Google Scholar]
- 25.Moreno-Paz M, Gomez MJ, Arcas A, Parro V. 2010. Environmental transcriptome analysis reveals physiological differences between biofilm and planktonic modes of life of the iron oxidizing bacteria Leptospirillum spp. in their natural microbial community. BMC Genomics 11:404. 10.1186/1471-2164-11-404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ram RJ, Verberkmoes NC, Thelen MP, Tyson GW, Baker BJ, Blake RC, II, Shah M, Hettich RL, Banfield JF. 2005. Community proteomics of a natural microbial biofilm. Science 308:1915–1920 [PubMed] [Google Scholar]
- 27.Florens L, Carozza MJ, Swanson SK, Fournier M, Coleman MK, Workman JL, Washburn MP. 2006. Analyzing chromatin remodeling complexes using shotgun proteomics and normalized spectral abundance factors. Methods 40:303–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mueller RS, Denef VJ, Kalnejais LH, Suttle KB, Thomas BC, Wilmes P, Smith RL, Nordstrom DK, McCleskey RB, Shah MB, Verberkmoes NC, Hettich RL, Banfield JF. 2010. Ecological distribution and population physiology defined by proteomics in a natural microbial community. Mol. Syst. Biol. 6:374. 10.1038/msb.2010.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hugenholtz P, Tyson GW, Blackall LL. 2002. Design and evaluation of 16S rRNA-targeted oligonucleotide probes for fluorescence in situ hybridization. Methods Mol. Biol. 179:29–42 [DOI] [PubMed] [Google Scholar]
- 30.Andersson AF, Banfield JF. 2008. Virus population dynamics and acquired virus resistance in natural microbial communities. Science 320:1047–1050 [DOI] [PubMed] [Google Scholar]
- 31.Dick GJ, Andersson AF, Baker BJ, Simmons SL, Thomas BC, Yelton AP, Banfield JF. 2009. Community-wide analysis of microbial genome sequence signatures. Genome Biol. 10:R85. 10.1186/gb-2009-10-8-r85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403–410 [DOI] [PubMed] [Google Scholar]
- 33.Belnap CP, Pan C, VerBerkmoes NC, Power ME, Samatova NF, Carver RL, Hettich RL, Banfield JF. 2010. Cultivation and quantitative proteomic analyses of acidophilic microbial communities. ISME J. 4:520–530 [DOI] [PubMed] [Google Scholar]
- 34.Parkhomchuk D, Borodina T, Amstislavskiy V, Banaru M, Hallen L, Krobitsch S, Lehrach H, Soldatov A. 2009. Transcriptome analysis by strand-specific sequencing of complementary DNA. Nucleic Acids Res. 37:e123. 10.1093/nar/gkp596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller CS, Baker BJ, Thomas BC, Singer SW, Banfield JF. 2011. EMIRGE: reconstruction of full-length ribosomal genes from microbial community short read sequencing data. Genome Biol. 12:R44. 10.1186/gb-2011-12-5-r44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Langmead B. 2010. Aligning short sequencing reads with Bowtie. Curr. Protoc. Bioinformatics Chapter 11:Unit 11.7. 10.1002/0471250953.bi1107s32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ludwig W, Strunk O, Westram R, Richter L, Meier H, Yadhukumar Buchner A, Lai T, Steppi S, Jobb G, Forster W, Brettske I, Gerber S, Ginhart AW, Gross O, Grumann S, Hermann S, Jost R, Konig A, Liss T, Lussmann R, May M, Nonhoff B, Reichel B, Strehlow R, Stamatakis A, Stuckmann N, Vilbig A, Lenke M, Ludwig T, Bode A, Schleifer KH. 2004. ARB: a software environment for sequence data. Nucleic Acids Res. 32:1363–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kelley LA, Sternberg MJ. 2009. Protein structure prediction on the Web: a case study using the Phyre server. Nat. Protoc. 4:363–371 [DOI] [PubMed] [Google Scholar]
- 39.Wass MN, Kelley LA, Sternberg MJ. 2010. 3DLigandSite: predicting ligand-binding sites using similar structures. Nucleic Acids Res. 38:W469–W473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eisen MB, Spellman PT, Brown PO, Botstein D. 1998. Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. U. S. A. 95:14863–14868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saldanha AJ. 2004. Java Treeview–extensible visualization of microarray data. Bioinformatics 20:3246–3248 [DOI] [PubMed] [Google Scholar]
- 42.Baker BJ, Comolli LR, Dick GJ, Hauser LJ, Hyatt D, Dill BD, Land ML, Verberkmoes NC, Hettich RL, Banfield JF. 2010. Enigmatic, ultrasmall, uncultivated Archaea. Proc. Natl. Acad. Sci. U. S. A. 107:8806–8811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kosman DJ. 2010. Multicopper oxidases: a workshop on copper coordination chemistry, electron transfer, and metallophysiology. J. Biol. Inorg. Chem. 15:15–28 [DOI] [PubMed] [Google Scholar]
- 44.Makarova KS, Haft DH, Barrangou R, Brouns SJ, Charpentier E, Horvath P, Moineau S, Mojica FJ, Wolf YI, Yakunin AF, van der Oost J, Koonin EV. 2011. Evolution and classification of the CRISPR-Cas systems. Nat. Rev. Microbiol. 9:467–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barrangou R, Fremaux C, Deveau H, Richards M, Boyaval P, Moineau S, Romero DA, Horvath P. 2007. CRISPR provides acquired resistance against viruses in prokaryotes. Science 315:1709–1712 [DOI] [PubMed] [Google Scholar]
- 46.Barkay T, Miller SM, Summers AO. 2003. Bacterial mercury resistance from atoms to ecosystems. FEMS Microbiol. Rev. 27:355–384 [DOI] [PubMed] [Google Scholar]
- 47.Vignais PM, Colbeau A. 2004. Molecular biology of microbial hydrogenases. Curr. Issues Mol. Biol. 6:159–188 [PubMed] [Google Scholar]
- 48.Justice NB, Pan C, Mueller R, Spaulding SE, Shah V, Sun CL, Yelton AP, Miller CS, Thomas BC, Shah M, VerBerkmoes N, Hettich R, Banfield JF. 2012. Heterotrophic archaea contribute to carbon cycling in low-pH, suboxic biofilm communities. Appl. Environ. Microbiol. 78:8321–8330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stackebrandt E, Ebers J. 2006. Taxonomic parameters revisited: tarnished gold standards. Microbiology Today 33:152–155 [Google Scholar]
- 50.Ohmura N, Sasaki K, Matsumoto N, Saiki H. 2002. Anaerobic respiration using Fe(3+), S(0), and H(2) in the chemolithoautotrophic bacterium Acidithiobacillus ferrooxidans. J. Bacteriol. 184:2081–2087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ma S, Banfield JF. 2011. Micron-scale Fe2+/Fe3+, intermediate sulfurspecies and O2 gradients across the biofilm–solution–sediment interface control biofilm organization. Geochim. Cosmochim. Acta 75:3568–3580 [Google Scholar]
- 52.Jeans C, Singer SW, Chan CS, Verberkmoes NC, Shah M, Hettich RL, Banfield JF, Thelen MP. 2008. Cytochrome 572 is a conspicuous membrane protein with iron oxidation activity purified directly from a natural acidophilic microbial community. ISME J. 2:542–550 [DOI] [PubMed] [Google Scholar]
- 53.Bonnefoy V, Holmes DS. 2012. Genomic insights into microbial iron oxidation and iron uptake strategies in extremely acidic environments. Environ. Microbiol. 14:1597–1611 [DOI] [PubMed] [Google Scholar]
- 54.Singer SW, Chan CS, Zemla A, VerBerkmoes NC, Hwang M, Hettich RL, Banfield JF, Thelen MP. 2008. Characterization of cytochrome 579, an unusual cytochrome isolated from an iron-oxidizing microbial community. Appl. Environ. Microbiol. 74:4454–4462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Blake RC, II, Griff MN. 2012. In situ spectroscopy on intact Leptospirillum ferrooxidans reveals that reduced cytochrome 579 is an obligatory intermediate in the aerobic iron respiratory chain. Front. Microbiol. 3:136. 10.3389/fmicb.2012.00136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cardenas JP, Valdes J, Quatrini R, Duarte F, Holmes DS. 2010. Lessons from the genomes of extremely acidophilic bacteria and archaea with special emphasis on bioleaching microorganisms. Appl. Microbiol. Biotechnol. 88:605–620 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.