Abstract
Quorum sensing (QS) regulates Phaeobacter gallaeciensis antagonism in broth systems; however, we demonstrate here that QS is not important for antagonism in algal cultures. QS mutants reduced Vibrio anguillarum to the same extent as the wild type. Consequently, a combination of probiotic Phaeobacter and QS inhibitors is a feasible strategy for aquaculture disease control.
TEXT
Bacteria predominantly exist in mixed populations, and the behavior of the complete population is often affected by highly structured and sophisticated ways of communication. Many bacteria secrete small pheromone-like substances that allow the complete population to sense its density and subsequently alter, in a coordinated manner, gene expression. This type of communication between bacteria is known as quorum sensing (QS). QS systems play an important role in the regulation of genes related to virulence and, also, in genes involved in the production of bioactive compounds, such as antibiotics and enzymes (1–4).
Roseobacter clade bacteria are some of the most common prokaryotes in oceanic and aquaculture environments (5–7). Some Roseobacter clade species, such as Phaeobacter gallaeciensis, Phaeobacter inhibens, and Ruegeria mobilis, produce a broad-spectrum antibacterial compound known as tropodithietic acid (TDA) (7–9). TDA does not induce resistance in the target organisms (10) and is the key compound for antagonism in laboratory broth cultures (11), in algae and rotifers, and in fish larval systems (12, 13). The ecological role of TDA is not known, but as TDA-producing bacteria can inhibit fish and shellfish pathogens, they have interesting prospects for application as probiotics in aquaculture (13, 14).
QS compounds of the acylated homoserine lactone (AHL) class are produced by some Roseobacter clade species (15–17), and TDA production is influenced by AHLs in Phaeobacter gallaeciensis. Genes homologous to the classical luxI (pgaI) and luxR (pgaR) genes have been identified, and a 24-h culture supernatant of a pgaI-negative mutant that is unable to produce the AHL compound did not antagonize other bacteria as the wild type did (18). Ruegeria mobilis does not produce AHLs, but in this bacterium, TDA itself can act as an autoinducer, controlling the expression of key genes for TDA production (19).
Roseobacter bacteria are frequently associated with algae, both in oceanic waters and aquaculture systems (5, 7). In aquaculture, algae are used as a direct addition to fish larva-rearing tanks and to enrich live prey organisms, such as rotifers and Artemia, with fatty acids. Both algae and live feed have been suggested as vehicles for the introduction of probiotics into rearing systems (20, 21), and a strategy of adding Phaeobacter strains to reduce pathogenic Vibrio in live feed and larval cultures has been proposed (12). As P. gallaeciensis antagonism is mainly caused by TDA and since TDA production is regulated by QS, we hypothesized that P. gallaeciensis antagonism in aquaculture settings (e.g., algal cultures) would also be affected by QS and, hence, could potentially be enhanced by the addition of synthetic QS compounds.
New approaches to control pathogenic bacteria in aquaculture systems include the use of quorum-sensing inhibition (QSI) compounds (22, 23, 24), and given the link between QS and TDA production, the use of QSI compounds could potentially limit the effectiveness of TDA-producing bacteria. Therefore, to develop effective strategies for the application of TDA-producing bacteria for disease control in aquaculture, we need to understand the effect of QS on TDA production in aquaculture systems. In the present study, we compared the antagonistic effects of P. gallaeciensis wild type and two QS mutants (pgaI and pgaR) (18) against the fish pathogen Vibrio anguillarum NB10 in cultures of the green alga Tetraselmis suecica, which is often used as live feed in aquaculture. We also determined whether the addition of the AHL compound N-3-hydroxydecanoylhomoserine lactone (3OHC10-HSL), which is naturally produced by P. gallaeciensis strain DSM17395 had any effect on the antagonism of the pgaI mutant.
Organisms and culture media.
P. gallaeciensis strain DSM 17395 was originally isolated from scallop-rearing systems (25). P. gallaeciensis insertion mutants with mutations of pgaI (unable to produce the AHL compound 3OHC10-HSL) and pgaR (devoid of the AHL receptor) (18) were kindly provided by Thorsten Brinkhoff, University of Oldenburg, Germany, and grown on half-strength marine agar (MA) (27.55 g MA 2216, Difco, 7.5 g agar, 1 liter deionized water) supplemented with 25 mg/liter gentamicin. The TDA-negative mutant, P. gallaeciensis MJG-G6, was created by transposon mutagenesis using the EZ-Tn5 <R6Kγori/KAN>Tnp Transposome kit (Epicentre, Madison, WI) as described by Geng et al. (38) and was cultured in 1/2 YTSS (yeast extract, tryptone, sea salts) (25) supplemented with 75 mg/liter kanamycin. Vibrio anguillarum serotype O1 strain NB10 (26, 27), which was tagged by insertion of the plasmid pNQFlaC4-gfp27 (cat gfp) into an intergenic region, was kindly provided by Debra Milton, University of Umeå, Sweden (28), and was cultured on tryptone-soy agar (TSA CM0131; Oxoid) containing 6 mg/liter chloramphenicol. Bacterial precultures were grown in 5 ml 1/2 YTSS at 25°C and 200 rpm. For serial 10-fold dilutions, autoclaved 3% Instant Ocean (IO; Aquarium Systems, Inc.) was used.
Tetraselmis suecica CCAP 66/4 (Prasinophyceae) was obtained as an axenic culture from the Culture Collection of Algae and Protozoa (Oban, United Kingdom) and cultured in B medium, prepared as follows. Solution 1 was composed of 45 g Na2EDTA, 100 g NaNO3, 33.6 g H3BO3, 20 g H2PO4, 0.36 g MnCl2·4H2O, 1.3 g FeCl3·6H2O, and 1 liter demineralized H2O; solution 2 contained 2.1 g ZnCl2, 2.0 g CoCl2·6H2O, 0.9 g (NH4)6Mo7O24·4H2O, 2.0 g CuSO4·5H2O, and 1 liter demineralized H2O, with the addition of drops of 0.1 N HCl until the solution was clear; and vitamin stock solution consisted of 200 mg thiamine, 1 mg biotin, 1 mg cyanocobalamin, and 1 liter demineralized H2O. The three solutions were autoclaved separately, and a mineral stock solution was made of 1 liter solution 1 and 1 ml solution 2. One milliliter of this stock solution and 1 ml of the vitamin stock solution were added to 1 liter 3% Sigma artificial seawater (29). Algal concentrations and axenicity were checked according to the method of D'Alvise et al. (12).
Effect of QS deficiency on P. gallaeciensis antagonism against V. anguillarum in an algal (Tetraselmis suecica) system.
T. suecica cultures (6.6 × 104 cells/ml) containing 102 CFU/ml of the appropriate P. gallaeciensis strain/mutant were prepared in B medium (150 ml). After 24 h of incubation at 25°C, the AHL compound 3OHC10-HSL (University of Nottingham, United Kingdom) was added to a concentration of 1 μM in P. gallaeciensis pgaI mutant cultures and, after 48 h, V. anguillarum was added at a level of 103 CFU/ml. T. suecica cultures inoculated with the TDA-negative mutant P. gallaeciensis MJG-G6 or without any bacteria added were used as negative controls. The growth of V. anguillarum in T. suecica cultures was checked in the absence and presence of 3OHC10-HSL. All cultures were run in duplicate at 25°C, with continuous aeration and under a light intensity of 13,000 lx. Two independent experiments were performed. Two-milliliter samples of each culture were collected in a sterile tube daily for 5 days (120 h) after V. anguillarum inoculation and used for quantification of algae and bacteria. The algal concentration was determined by optical density at 665 nm after calibration with counts of axenic reference cultures in a Neubauer counting chamber. Formaldehyde (0.5% final concentration) was used as the fixation agent. Quantification of P. gallaeciensis wild type and AHL mutants was done by plating 10-fold dilutions on MA plates and incubating them for 72 h at 25°C. The numbers of V. anguillarum organisms were determined on TSA plates supplemented with 6 mg/liter chloramphenicol and incubated at 25°C for 48 h. A one-way analysis of variance (ANOVA) and Tukey test (level of significance, 0.05) were used to analyze the differences between V. anguillarum concentrations alone or in coculture with the different P. gallaeciensis strain/mutants in algal cultures.
After 48 h in algal coculture, P. gallaeciensis wild type and all mutants had grown to 106 CFU/ml. V. anguillarum grew to 104 CFU/ml in control cultures. After 120 h in coculture with the P. gallaeciensis wild type, the V. anguillarum concentration was significantly reduced, to 10 CFU/ml (P = 0.002), whereas in the presence of the tdaB mutant MJG-G6, which is unable to produce TDA, V. anguillarum growth was only slightly affected and reached 104 CFU/ml after 120 h (Fig. 1) (P > 0.05). Both the pgaI and pgaR mutants significantly reduced the counts of V. anguillarum (pgaI, P = 0.002; pgaR, P = 0.006), similarly to the P. gallaeciensis wild type. The addition of 3OHC10-HSL had no significant effect on the growth or antagonism of the pgaI mutant (P > 0.05), nor did the addition of the AHL compound have any effect on V. anguillarum growth (P > 0.05) (Fig. 1). None of the introduced bacteria affected the growth of the algae, which grew to a concentration of 106 to 107 cells/ml at the end of the experiment.
Fig 1.
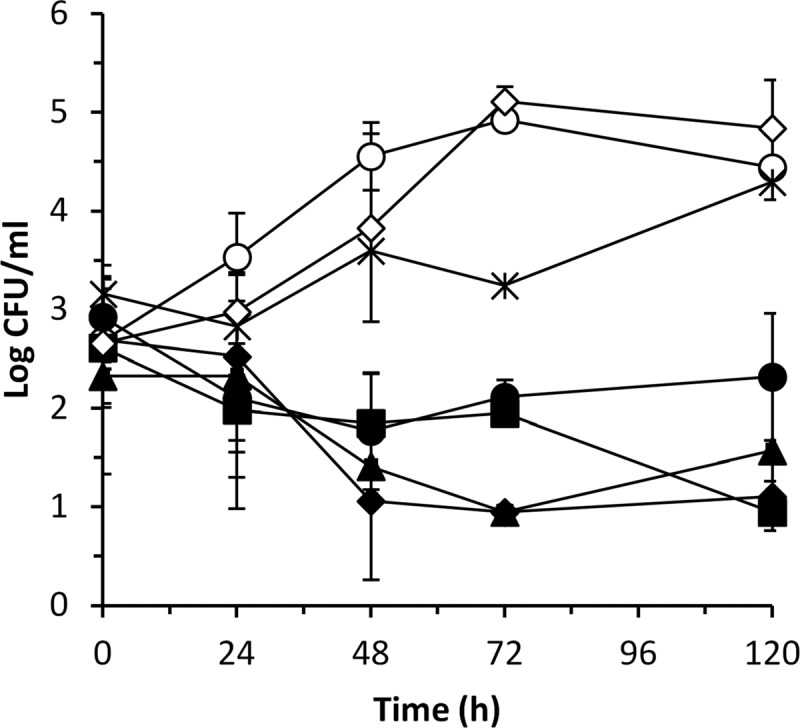
Growth of V. anguillarum in T. suecica cultures alone (○) or supplemented with 1 μM 3OHC10-HSL (♢) or in coculture with P. gallaeciensis wild type (♦), tdaB mutant ( ), pgaI mutant (■), pgaI mutant supplemented with 1 μM 3OHC10-HSL (●), or pgaR mutant (▲). Data are means and standard deviations of two independent experiments.
), pgaI mutant (■), pgaI mutant supplemented with 1 μM 3OHC10-HSL (●), or pgaR mutant (▲). Data are means and standard deviations of two independent experiments.
Antagonism of P. gallaeciensis QS mutants in broth systems.
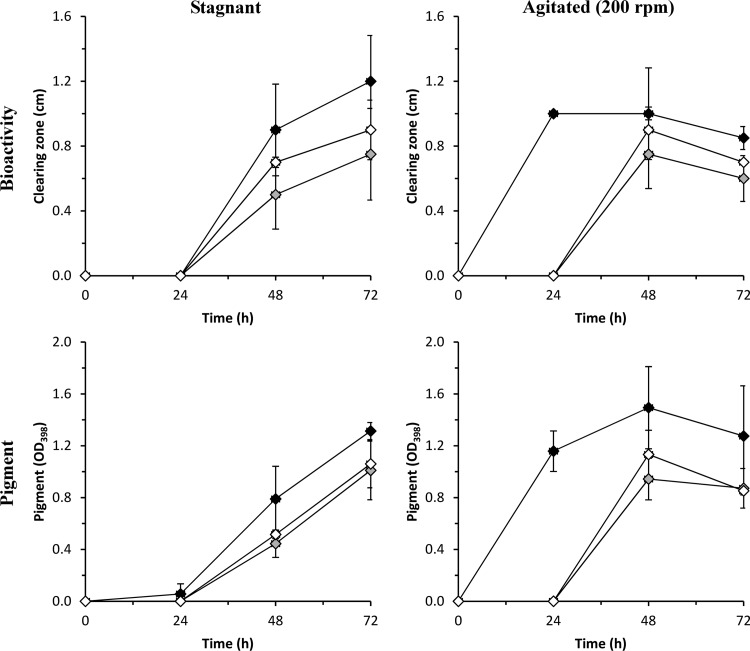
Since the QS mutants, unexpectedly, were as inhibitory as the wild type, we studied the kinetics of TDA production in P. gallaeciensis wild type and QS mutants in detail, by means of bioactivity and pigment production. The strains/mutants were grown in marine broth (MB 2216, Difco) for 3 days at 25°C. Aeration of P. gallaeciensis cultures influences TDA production in MB, and the most-pronounced TDA production occurs under stagnant conditions (15, 30). Therefore, TDA production was analyzed under both stagnant and agitated (200 rpm) conditions. Different cultures were used for each sampling point. Spent culture supernatants of P. gallaeciensis wild type and QS mutants were sampled daily for pigment measurement (optical density at 398 nm [OD398]) and analysis of antagonism against V. anguillarum (22). The bioactivities of the different strains/mutants were checked by using a previously described bioassay (6). Briefly, V. anguillarum was cultured in MB for 24 h at 25°C and infused in 3% IO agar with 0.4% glucose and 0.3% Casamino Acids at 43 to 44°C. Wells (diameter, 6 mm) were punched in the solidified agar, and sterile filtered supernatants of P. gallaeciensis wild type and QS mutants were added to the wells. The plates were incubated for 24 h at 25°C and checked for the presence of clearing zones. Pigment was measured, as its occurrence in MB cultures coincides with TDA production (15). The significance of the differences in pigment production and bioactivity between the P. gallaeciensis wild type and QS mutants grown in MB was ascertained by using a one-way ANOVA and Tukey test (level of significance, 0.05).
The supernatant from a wild-type, 24-h shaken culture was inhibitory against V. anguillarum, whereas supernatants from the pgaI and pgaR mutants were not (P < 0.05) (Fig. 2). However, when sampled after 48 and 72 h, the QS mutants were inhibitory and no significant differences in inhibitory activities were observed (P > 0.05). Under stagnant conditions, all three strains inhibited the target organism at the 48- and 72-h sampling points, and no significant differences were detected (P > 0.05). The inhibitory activity correlated with the production of pigment in all strains (Fig. 2). Pigment production in QS mutants was significantly different from that in the wild type only after 24 h of incubation under aerated conditions (P = 0.001), and the stagnant cultures of pgaI and pgaR mutants did not show pigmentation significantly different from that of the wild type (P > 0.05).
Fig 2.
Bioactivity (inhibition zone diameter) and pigment production (OD398) of P. gallaeciensis wild type (black) and pgaI (white) and pgaR (gray) knockout mutants in marine broth. Data are means and standard deviations of two independent assays.
The production of secondary metabolites, such as antifungal and antibacterial compounds, can be regulated by QS, and this regulation can be absolute (31, 32) or just a modulation (33, 34). Therefore, a mutation in the QS system can lead to the complete loss of antibiotic production (31, 32) or just cause a reduction in the production of the antagonistic compound (33, 34). Berger et al. (18) showed that TDA production in P. gallaeciensis is QS regulated. However, by extending the growth period from 24 to 48 and 72 h, we found that the production of TDA, as determined by bioactivity and pigment production, was delayed but not completely abolished. Similarly, mutations in QS genes reduced but did not totally suppress the production of biofilm-inhibiting cyclic lipopeptides in Pseudomonas putida (33) or proteases in Pseudomonas chlororaphis (34). Our results show that TDA production in P. gallaeciensis is only modulated by the PgaI-PgaR QS system, and this indicates that QS is just one of several regulatory systems involved. This is in agreement with previous observations by Berger et al. (35), who showed that pgaI and pgaR mutants are able to produce TDA in a minimal medium with phenylalanine as the sole carbon source. In some bacteria, the luxI-luxR QS system forms part of a hierarchically organized network where other regulators are integrated for the control of secondary-metabolite production (34, 36), and this type of organization has been suggested previously for TDA production in P. gallaeciensis (35). Our group has recently demonstrated that the expression of the tdaC gene, key in the synthesis of TDA in Ruegeria mobilis, coincides with high levels of the second messenger signal cyclic dimeric guanosinmonophosphate (c-di-GMP), and we have hypothesized that c-di-GMP could be involved in the regulation of TDA synthesis in Roseobacter bacteria (37). Since the genes responsible for the synthesis and degradation of c-di-GMP are present in P. gallaeciensis (37) and since TDA production is modulated by QS, one could speculate that this modulation could occur through c-di-GMP.
We can conclude that an AHL-mediated QS system modulates P. gallaeciensis antagonism in a broth system but that this regulation does not seem to be important in algal systems mimicking an aquaculture environment. Several studies have used QSI compounds to target aquaculture pathogens (22–24), and since TDA production can be regulated through QS, one could be concerned that such QSI compounds would limit the effectiveness of beneficial bacteria, such as TDA-producing Phaeobacter. However, in this study, we demonstrate that the inhibitory activity of P. gallaeciensis is independent of the QS system, and therefore, this inhibition will likely not be hampered by the possible use of QSI compounds. Hence, one can envision a dual disease control strategy, combining probiotic Phaeobacter strains with pathogen-targeted QSI compounds.
ACKNOWLEDGMENTS
This work was supported by the DTU Ørsted postdoctoral program, the DTU National Food Institute, and the Danish Research Council for Technology and Production Sciences (project 09-066524).
We thank Thorsten Brinkhoff, University of Oldenburg, for kindly providing P. gallaeciensis BS107 pgaI and pgaR mutants and Debra Milton, University of Umeå, for providing the chromosomally tagged V. anguillarum NB10. We are grateful to Jette Melchiorsen for helping with the MB experiments.
Footnotes
Published ahead of print 28 June 2013
REFERENCES
- 1.Bassler BL, Losick R. 2006. Bacterially speaking. Cell 125:237–246 [DOI] [PubMed] [Google Scholar]
- 2.von Bodman SB, Willey JM, Diggle SP. 2008. Cell-cell communication in bacteria: united we stand. J. Bacteriol. 190:4377–4391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keller L, Surette MG. 2006. Communication in bacteria: an ecological and evolutionary perspective. Nat. Rev. Microbiol. 4:249–258 [DOI] [PubMed] [Google Scholar]
- 4.Whitehead NA, Barnard AML, Slater H, Simpson NJL, Salmond GPC. 2001. Quorum-sensing in Gram-negative bacteria. FEMS Microbiol. Rev. 25:365–404 [DOI] [PubMed] [Google Scholar]
- 5.Buchan A, González JM, Moran MA. 2005. Overview of the marine Roseobacter lineage. Appl. Environ. Microbiol. 71:5665–5677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hjelm M, Bergh Ø Riaza A, Nielsen J, Melchiorsen J, Jensen S, Duncan H, Ahrens P, Birkbeck H, Gram L. 2004. Selection and identification of autochthonous potential probiotic bacteria from turbot larvae (Scolphthalmus maximus) rearing units. Syst. Appl. Microbiol. 27:360–371 [DOI] [PubMed] [Google Scholar]
- 7.Porsby CH, Nielsen KF, Gram L. 2008. Phaeobacter and Ruegeria species of the Roseobacter clade colonize different niches in a Danish turbot (Scophthalmus maximus) rearing farm and antagonize turbot larval pathogens under different growth conditions. Appl. Environ. Microbiol. 74:2356–2364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brinkhoff T, Bach G, Heidorn T, Liang L, Schlingloff A, Simon M. 2004. Antibiotic production by a Roseobacter clade-affiliated species from the German Wadden Sea and its antagonistic effects on indigenous isolates. Appl. Environ. Microbiol. 70:2560–2565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruhn JB, Nielsen KF, Hjelm M, Hansen M, Bresciani J, Schultz S, Gram L. 2005. Ecology, inhibitory activity and morphogenesis of a potential marine fish larvae probiotic bacteria, Roseobacter strain 27-4. Appl. Environ. Microbiol. 71:7263–7270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Porsby CH, Webber MA, Nielsen KF, Piddock LJV, Gram L. 2011. Resistance and tolerance to tropodithietic acid, an antimicrobial in aquaculture, is hard to select. Antimicrob. Agents Chemother. 55:1332–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Alvise P, Melchiorsen J, Porsby CH, Nielsen KF, Gram L. 2010. Inactivation of Vibrio anguillarum by attached and planktonic Roseobacter cells. Appl. Environ. Microbiol. 76:2366–2370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D'Alvise PW, Lillebø S, Prol-Garcia MJ, Wergeland HI, Nielsen KF, Bergh Ø, Gram L. 2012. Phaeobacter gallaeciensis reduces Vibrio anguillarum in cultures of microalgae and rotifers, and prevents vibriosis in cod larvae. PLoS One 7:e43996. 10.1371/journal.pone.0043996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D'Alvise PW, Lillebø S, Wergeland HI, Gram L, Bergh Ø. 2013. Protection of cod larvae from vibriosis by Phaeobacter spp.: a comparison of strains and introduction times. Aquaculture 384–387:82–86 [Google Scholar]
- 14.Planas M, Pérez-Lorenzo M, Hjelm M, Gram L, Fiksdal IU, Bergh Ø, Pintado J. 2006. Probiotic effect in vivo of Roseobacter strain 27-4 against Vibrio anguillarum infections in turbot (Scophthalmus maximus L.) larvae. Aquaculture 255:323–333 [Google Scholar]
- 15.Bruhn JB, Gram L, Belas R. 2007. Production of antibacterial compound and biofilm formation in dinoflagellate-associated Roseobacter species are influenced by culture conditions. Appl. Environ. Microbiol. 73:442–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gram L, Grossart HP, Schlingloff A, Kiørboe T. 2002. Quorum sensing in marine snow bacteria: production of acylated homoserine lactones by Roseobacter strains isolated from marine snow. Appl. Environ. Microbiol. 68:4111–4116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martens T, Gram L, Grossart HP, Kessler D, Müller R, Simon M, Wenzel SC, Brinkhoff T. 2007. Bacteria of the Roseobacter clade show potential for secondary metabolite production. Microb. Ecol. 54:31–42 [DOI] [PubMed] [Google Scholar]
- 18.Berger M, Neumann A, Schulz S, Simon M, Brinkhoff T. 2011. Tropodithietic acid production in Phaeobacter gallaeciensis is regulated by N-acyl homoserine lactone-mediated quorum sensing. J. Bacteriol. 193:6576–6585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geng H, Belas R. 2010. Expression of tropodithietic acid biosynthesis is controlled by a novel autoinducer. Appl. Environ. Microbiol. 192:4377–4387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tinh NTN, Dierckens K, Sorgeloos P, Bossier P. 2007. A review of the functionality of probiotics in the larviculture food chain. Mar. Biotechnol. 10:1–12 [DOI] [PubMed] [Google Scholar]
- 21.Pintado J, Pérez-Lorenzo M, Luna-González A, Sotelo CG, Prol MJ, Planas M. 2010. Monitoring of the bioencapsulation of a probiotic Phaeobacter strain in the rotifers Brachionus plicatilis using denaturing gradient gel electrophoresis. Aquaculture 302:182–194 [Google Scholar]
- 22.Defoirdt T, Boon N, Bossier P. 2010. Can bacteria evolve resistance to quorum sensing disruption? PLoS Pathog. 6:e1000989. 10.1371/journal.ppat.1000989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Natrah FMI, Defoirdt T, Sorgeloos P, Bossier P. 2011. Disruption of bacterial cell-to-cell communication by marine organisms and its relevance to aquaculture. Mar. Biotechnol. 13:109–126 [DOI] [PubMed] [Google Scholar]
- 24.Ruíz-Ponte C, Samain JF, Sánchez JL, Nicolas JL. 1999. The benefit of Roseobacter species on the survival of scallop larvae. Mar. Biol. 1:52–59 [DOI] [PubMed] [Google Scholar]
- 25.Sobecky PA, Mincer TJ, Chang MC, Helinski DR. 1997. Plasmids isolated from marine sediment microbial communities contain replication and incompatibility regions unrelated to those of known plasmid groups. Appl. Environ. Microbiol. 63:888–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Norqvist A, Hagstrom A, Wolfwatz H. 1989. Protection of rainbow trout against vibriosis and furunculosis by the use of attenuated strains of Vibrio anguillarum. Appl. Environ. Microbiol. 55:1400–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Norqvist A, Norrman B, Wolfwatz H. 1990. Identification and characterization of a zinc metalloprotease associated with invasion by the fish pathogen Vibrio anguillarum. Infect. Immun. 58:3731–3736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Croxatto A, Lauritz J, Chen C, Milton DL. 2007. Vibrio anguillarum colonization of rainbow trout integument requires a DNA locus involved in exopolysaccharide transport and biosynthesis. Environ. Microbiol. 9:370–382 [DOI] [PubMed] [Google Scholar]
- 29.Hansen PJ. 1989. The red tide dinoflagellate Alexandrium tamarense. Effects on behaviour and growth of a tintinnid ciliate. Mar. Ecol. Prog. Ser. 53:105–116 [Google Scholar]
- 30.Porsby CH, Nielsen KF, Gram L. 2008. Phaeobacter and Ruegeria species of the Roseobacter clade colonize separate niches in a Danish turbot (Scophthalmus maximus)-rearing farm and antagonize Vibrio anguillarum under different growth conditions. Appl. Environ. Microbiol. 74:7356–7364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu X, Bimerew M, Ma Y, Müller H, Ovadis M, Eberl L, Berg G, Chernin L. 2007. Quorum-sensing signalling is required for production of the antibiotic pyrrolnitrin in a rhizospheric biocontrol strain Serratia plymuthica. FEMS Microbiol. Lett. 270:299–305 [DOI] [PubMed] [Google Scholar]
- 32.Schmidt S, Blom JF, Pernthaler J, Berg G, Baldwin A, Mahenthiralingam E, Eberl L. 2009. Production of the antifungal compound pyrrolnitrin is quorum sensing-regulated in members of the Burkholderia cepacia complex. Environ. Microbiol. 11:1422–1437 [DOI] [PubMed] [Google Scholar]
- 33.Dubern J, Lugtenberg JJ, Bloemberg GV. 2006. The ppuI-rsaL-ppuR quorum sensing system regulates biofilm formation of Pseudomonas putida PCL1445 by controlling biosynthesis of the cyclic lipopeptides putisolvins I and II. J. Bacteriol. 188:2898–2906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Selin C, Dilantha Fernando WG, de Kievit T. 2012. The PhzI/PhzR quorum-sensing system is required for pyrrolnitrin and phenazine production, and exhibits cross-regulation with RpoS in Pseudomonas chlororaphis PA23. Microbiology 158:896–907 [DOI] [PubMed] [Google Scholar]
- 35.Berger M, Brock NL, Liesegang H, Dogs M, Preuth I, Simon M, Dickschat JS, Brinkhoff T. 2012. Genetic analysis of the upper phenylacetate catabolic pathway in the production of tropodithietic acid by Phaeobacter gallaeciensis. Appl. Environ. Microbiol. 78:3539–3551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu J, Huang X, Zhang M, Li S, Jiang H, Xu Y. 2009. The distinct quorum sensing hierarchy of las and rhl in Pseudomonas sp. M18. Curr. Microbiol. 59:621–627 [DOI] [PubMed] [Google Scholar]
- 37.D'Alvise PW. 2013. Aquaculture application and ecophysiology of marine bacteria from the Roseobacter clade. Ph.D. thesis. Technical University of Denmark, Kongens Lyngby, Denmark [Google Scholar]
- 38.Geng H, Bruhn JB, Nielsen KF, Gram L, Belas R. 2008. Genetic dissection of tropodithietic acid biosynthesis by marine roseobacters. Appl. Environ. Microbiol. 74:1535–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]