Abstract
The reported fate of Escherichia coli in the environment ranges from extended persistence to rapid decline. Incomplete understanding of factors that influence survival hinders risk assessment and modeling of the fate of fecal indicator bacteria (FIB) and pathogens. FIB persistence in subtropical aquatic environments was explored in outdoor mesocosms inoculated with five E. coli strains. The manipulated environmental factors were (i) presence or absence of indigenous microbiota (attained by natural, disinfected, and cycloheximide treatments), (ii) freshwater versus seawater, and (iii) water column versus sediment matrices. When indigenous microbes were removed (disinfected), E. coli concentrations decreased little despite exposure to sunlight. Conversely, under conditions that included the indigenous microbiota (natural), significantly greater declines in E. coli occurred regardless of the habitat. The presence of indigenous microbiota and matrix significantly influenced E. coli decline, but their relative importance differed in freshwater versus seawater. Cycloheximide, which inhibits protein synthesis in eukaryotes, significantly diminished the magnitude of E. coli decline in water but not in sediments. The inactivation of protozoa and bacterial competitors (disinfected) caused a greater decline in E. coli than cycloheximide alone in water and sediments. These results indicate that the autochthonous microbiota are an important contributor to the decline of E. coli in fresh and seawater subtropical systems, but their relative contribution is habitat dependent. This work advances our understanding of how interactions with autochthonous microbiota influence the fate of E. coli in aquatic environments and provides the framework for studies of the ecology of enteric pathogens and other allochthonous bacteria in similar environments.
INTRODUCTION
The sanitary quality of recreational waters in Florida and across the United States is currently assessed by enumeration of fecal indicator bacteria (FIB) (i.e., fecal coliforms, Escherichia coli, and enterococci), which are also intended to act as pathogen surrogates (1, 2). The validity of this paradigm is the subject of ongoing debate, and it is argued that the current regulatory standards do not adequately protect human health, due mainly to the differences in survival and transport characteristics between the FIB and pathogens (3–9). When the assumed predictive relationship is absent (e.g., FIB not detected but pathogens present), public health may be threatened by exposure of humans to pathogens. On the other hand, FIB that are detected in the absence of pathogens can lead to unnecessary beach and shellfishing area closures, which can pose economic hardships in coastal communities.
While the roles of sediments and aquatic vegetation as a refuge and a potential reservoir of FIB are the subjects of many studies (10–16), the relative influence of indigenous microbiota on the persistence and rate of decline of FIB in aquatic environments is less well characterized. Germicidal sunlight radiation is frequently implicated as a major factor in the rapid decline of E. coli and other microorganisms in aquatic habitats based on mesocosm studies conducted in shallow waters in the absence of sediments (17–20). The inactivation rates of FIB exposed to sunlight were generally smaller in freshwater (i.e., organisms survived for longer periods) than in marine mesocosms (10, 21–24). However, choice of inoculum (e.g., raw sewage or primary treated sewage) (17, 19, 24–26) and source waters (e.g., nondisinfected freshwater streams and rivers and marine waters) (17–20, 22–24, 27), both of which include a myriad of other organisms, precluded testing the effect of indigenous microbiota.
Earlier studies showed that protozoan grazing may be responsible for up to 90% of overall mortality of autochthonous organisms and allochthonous FIB from freshwater and marine environments alike (28, 29). However, studies investigating the effect of protozoan bacterivory were conducted in the laboratory setting (i.e., no ambient sunlight exposure), did not include sediments, and for the most part utilized a single FIB strain, hence creating artificial conditions not likely to be representative of the complex environmental habitats (29–32). Furthermore, bacterial mortality was determined through a physiological approach (e.g., uptake of radioactive tracers), which is useful in the strict ecological sense but offers little information about the fate of FIB from a regulatory standpoint, due mainly to differences in methodology. Even less is known about the effect of competition from indigenous microbiota, particularly in the water column, although existing accounts identify autochthonous bacteria from beach sands, soils, and storm drain sediments as important biotic stressors contributing to the decline of FIB in these habitats (33–36).
Nonetheless, many recent publications place little or no emphasis on the effect of indigenous microbiota on FIB fate in aquatic environments (37–41). Rather than ascertaining the precise mechanism of the biological control of E. coli decay in the aquatic environments, we aimed to show comparative effects of exposure to sunlight and/or indigenous microbiota on E. coli persistence in the context that is practical for the water quality managers. The objectives of this study were (i) to compare the effects of indigenous microbiota, water type, and sediment versus water column matrices on the fate of E. coli in outdoor mesocosms and (ii) to determine the relative contributions of these factors to the survival of culturable E. coli in a simulated, subtropical environment.
MATERIALS AND METHODS
Sample collection and mesocosm preparation.
Water and sediment samples used to construct mesocosms were collected at Ben T. Davis Beach (27°58′15.50″N, 82°34′43.78″W) for seawater mesocosms and a pond (28°03′42.63″N, 82°25′03.91″W) at the University of South Florida (Tampa, FL) for all freshwater mesocosms. Salinity measurements at these sites were 24.0‰ and 0.26‰ for beach and pond, respectively. Water and sediment samples from each site were collected in shallow waters (∼20- to 30-cm depth) from the swash zone of the beach and pond banks. Approximately 20 liters of water and 15 kg of sediments from each matrix were collected into sterilized containers, and large debris (e.g., leaves and branches) was manually removed. Water samples for control mesocosms lacking protozoa and indigenous bacteria (termed “disinfected”) were successively filtered through 0.45-μm and 0.22-μm-pore-size nitrocellulose membrane filters, while sediment samples were desiccated in a drying oven at 176.6°C (350°F) for 48 h (42, 43) to inactivate indigenous microorganisms. Bacterial inactivation was tested by processing 100 ml of filter-sterilized water and 50 ml of sediment suspension via a standard membrane filtration technique (described below in the “E. coli enumeration” section) on modified mTEC medium and by spread-plating on tryptic soy agar (TSA). The concentrations of culturable organisms remaining in the samples were insignificant (0 to 5 CFU per 100 ml of water or 100 g of sediment) for both types of media. In order to test the effect of predation from protozoa versus competition from indigenous bacteria, 200 mg/liter cycloheximide (final concentration) was added to the mesocosms (30, 44, 45) to inhibit activity of natural protozoa (termed “antimicrobial”). For the mesocosms containing indigenous microbiota (termed “natural”), water and sediment samples were collected 1 day prior to inoculation and held at 4°C overnight to minimize changes in indigenous microbial populations. Prior to inoculation, natural water and sediments contained negligible concentrations of culturable E. coli (0 to 10 CFU per 100 ml of water or per 100 g of sediment).
Establishment of mesocosms.
Three experiments, each with mesocosms containing water and sediments, were conducted in March, April, and September 2010. New samples of water and sediments were collected for each experiment, and all treatments were replicated by five separate mesocosms. Mesocosm series conducted in March (freshwater) and April (seawater) were divided into two treatments (disinfected and natural) to test the effects of indigenous microorganisms, water type, and matrix (sediment versus water column) on E. coli persistence. The mesocosm series conducted in September (freshwater) were divided into the following treatments: (i) disinfected, (ii) natural, and (iii) antimicrobial treated (cycloheximide addition) to test the effect of predation in freshwater habitats. Each mesocosm series was inoculated with the same mixture of five different E. coli strains, explained below in the “E. coli strains” section. Mesocosms were incubated for 7 to 9 days, and samples were collected at the beginning (T0), after 1 day of incubation (T1), and every other day thereafter. During the course of the experiment, cumulative averages of mean ambient air temperatures were 16.7 ± 4.20°C (March), 22.5 ± 1.21°C (April), and 28.0 ± 6.01°C (September). The average solar insolation incident on the horizontal surface (in kilowatt hours [kWh] m−2 day−1) and clearness index (i.e., cloud cover normalized 0 to 1) for the study area were obtained from NASA Langley Atmospheric Science Data Center (http://eosweb.larc.nasa.gov/cgi-bin/sse/sse.cgi) and were as follows: March, 6.08 kWh m−2 day−1, cloud cover, 0.61; April, 6.88 kWh m−2 day−1, cloud cover, 0.60; and September, 4.76 kWh m−2 day−1, cloud cover, 0.46.
For all of the experiments, mesocosms were constructed in 1.5-liter borosilicate glass beakers and filled with ∼3.0 cm sediment and 1 liter of water. All beakers were covered with translucent cover to prevent cross-contamination by rodents, insects, and rainfall. Beakers were placed in large plastic bins filled with municipal tap water (to the level of water in the beakers) to moderate temperature fluctuations. All mesocosm series were incubated outdoors exposed to ambient sunlight in the Botanical Gardens at University of South Florida Tampa campus (USF Botanical Gardens) (28°03′29.22″N, 82°25′25.77″W). Supplemental experiments (data not shown) were conducted that explored potential blockage of sunlight (and associated UV irradiation) by translucent cover. These experiments were conducted during December 2009 in freshwater and seawater (water and sediment) obtained from the same sites as the later time block series. Mesocosoms were incubated outdoors (USF Botanical Gardens) for 5 days during which there was no rainfall, and meteorological data for that period were comparable to those of the three time block series (cumulative average of mean ambient air temperatures, 20.7 ± 3.41°C; average solar insolation, 4.13 kWh m−2 day−1; and cloud cover, 0.65). Mesocosms were prepared as described earlier for disinfected controls, except that one-half of the beakers were covered, while the other half remained uncovered. Comparisons of E. coli decline over 5 days indicated no statistically significant differences in decay between covered and uncovered mesocosms.
E. coli strains.
All mesocosms contained the following E. coli strains: MG1655, ATCC 8739, SMS-35, HS, and WW6 (the latter isolated from disinfected wastewater effluent from Clearwater, FL). Strains were selected to represent diverse origins: E. coli MG1655 and ATCC 8739 are both K-12 descendants commonly used as control strains for a variety of assays (the former being a commensal inhabitant of the human gastrointestinal tract) (46). Strain HS is also a commensal inhabitant of the human gastrointestinal tract and a phage host (47), while SMS-35 was isolated from soil contaminated with heavy metals (48). All strains were simultaneously streaked for isolation on TSA and incubated overnight at 37°C. The next day, one colony of each strain was aseptically transferred to 5 ml of tryptic soy broth (TSB) and grown to stationary phase by incubation overnight at 37°C. Following incubation, 1 ml of TSB suspension was centrifuged at 14,000 rpm for 3 min, followed by two successive washing steps in 1× phosphate-buffered saline (PBS) (pH 7.4) (49) and final resuspension in 1 ml of 1× PBS. One milliliter of bacterial suspension containing ∼105 organisms was first inoculated in the sediments, stirred well, and allowed to settle by gravity. The water column inoculum was prepared in the same manner. Briefly, 1 ml of bacterial suspension in 1× PBS (containing ∼105 organisms) was inoculated in 1 liter of water and stirred well. The mixture of water and bacteria was slowly poured into the beaker; care was taken to avoid disturbing the sediments. Prepared mesocosms were allowed to settle (via gravitation) prior to collection of the initial (T0) sample.
E. coli enumeration.
In order to avoid resuspension of bacteria from sediments into the water column, the water column was always sampled first, which allowed 24 h (T1) to 48 h (all subsequent sampling events) for resuspended sediment particles to settle prior to collection of the next water sample. For all of the experiments, ∼10 ml of water and 10 g of sediment were collected into sterile 15-ml polypropylene tubes and small Ziplock bags, respectively, and transported to laboratory for immediate processing. Decimal dilution series of samples were prepared in sterile buffered water (pH 7.0) (49), and 1 ml of the suspensions was processed by standard membrane filtration methods (0.45-μm-pore-size, 47-mm-diameter nitrocellulose filters) (50). Sediment samples (5 g) were first diluted 1:10 in sterile buffered water and shaken by hand for 2 min (51) to disassociate bacteria from sediment particles, followed by serial dilution of the supernatant and membrane filtration, as described above. In the later stages of the experiment, as E. coli concentrations decreased, samples were not subjected to decimal dilutions; instead 1 ml or 10 ml of the undiluted water and sediment suspension was filtered. E. coli from water and sediment samples was enumerated on modified mTEC medium following incubation at 35°C for 2 h and 22 h at 44.5°C (50). Over the course of the experiment, constant amounts were sampled from sediment and water column in all mesocosms; hence, the ratio of water to sediment was maintained. Furthermore, less than 20% of original water and sediment volume was removed over the duration of the experiment, circumventing the need to replace the sampled materials.
Data analyses.
Prior to conducting the experiments, preliminary data were used to determine the appropriate sample size (n [e.g., number of true replicate mesocosms]) by power analysis using GraphPad StatMate version 2.00 for Windows (GraphPad Software, San Diego, CA). For all of the experiments, the E. coli concentrations were adjusted for the dilution factor and log10 transformed; the results are reported as CFU/100 ml or CFU/100 g (wet weight) for water and sediment samples, respectively. The decrease of culturable E. coli concentrations over time is presented as C/C0, calculated by dividing E. coli concentrations on the fifth day of the experiment (T5) by the initial concentrations (T0), and the resulting quotient was log10 transformed. T5 was chosen for this calculation because culturable E. coli cells were not detected at T7 and T9 in the water column of some mesocosm series. The effects of different independent variables, as well as interaction of variables, on E. coli decline were evaluated using two-way analysis of variance (ANOVA) (GraphPad Prism software version 5.00 for Windows, San Diego, CA). Analyses were organized in a 2-by-2 (March and April time blocks) or 3-by-2 (September time block) design, with treatment variables (natural, disinfected, and antimicrobial treated) presented in columns, and matrix (water/sediment) or water type (freshwater/seawater) variables in rows. The contribution of each row variable to the observed differences in column means was assessed by Bonferroni post hoc tests with 95% confidence intervals.
RESULTS
The effect of indigenous microbiota in the water column of freshwater and seawater mesocosms.
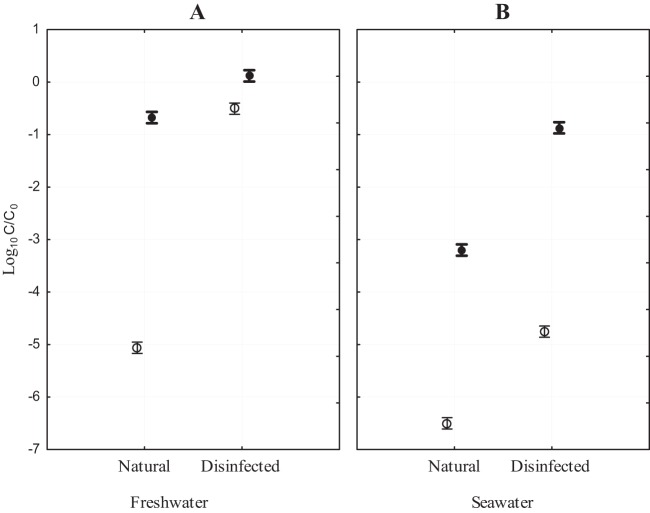
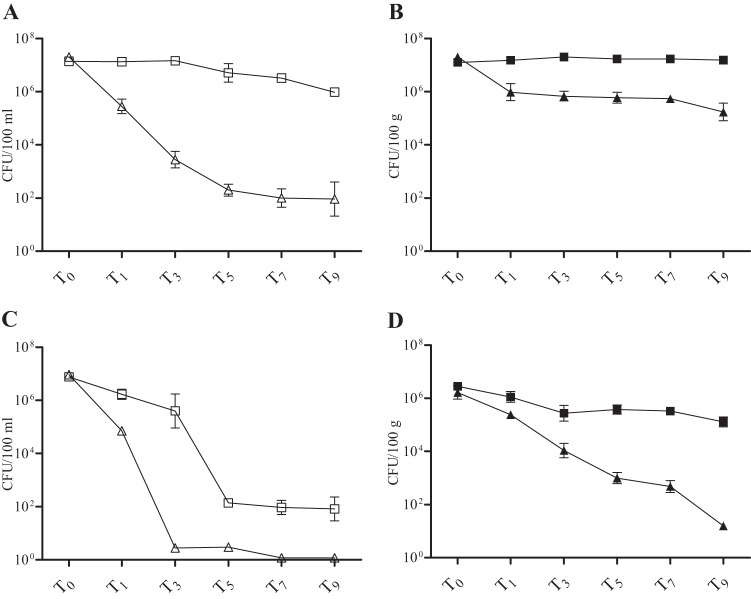
Natural and disinfected freshwater and seawater mesocosms were established (as detailed in Materials and Methods) to investigate the effect of indigenous microbiota, water type, and matrix characteristics on decay of E. coli. The magnitude of decline was significantly greater in the water column of both water types when indigenous microbiota were present compared to the disinfected controls (Table 1; also see Fig. 2 and 4). In the freshwater experiment, a large decrease (>5 orders of magnitude) of culturable E. coli occurred over 5 days in the water column of natural mesocosms and was followed by a leveling off during the last two sampling dates (i.e., T7 and T9) (Fig. 1A and 2). In contrast, little decline was observed for the matching disinfected controls. Instead, E. coli concentrations remained relatively stable over 5 days, followed by a slow decrease of less than 1 order of magnitude per day for the remainder of the experiment (Fig. 1A). The effect of the presence of indigenous microbiota on E. coli decline was an important factor (Table 2), and it contributed 41.6% to the total variation, more than any other variable tested (Table 2 and Fig. 2).
Table 1.
Comparison of the effects of indigenous microbiota and water type on E. coli decrease in the water column and sedimentsa
| Source of variation | % of total variation | P value |
|---|---|---|
| Water column (n = 5) | ||
| Freshwater vs seawater | 40.1 | <0.0001 |
| Natural vs disinfected | 49.2 | <0.0001 |
| Interaction | 9.74 | <0.0001 |
| Sediments (n = 5) | ||
| Freshwater vs seawater | 49.1 | <0.0001 |
| Natural vs disinfected | 38.9 | <0.0001 |
| Interaction | 9.38 | <0.0001 |
| Post hoc tests (natural vs disinfected) | ||
| Water column | ||
| Freshwater | <0.0001 | |
| Seawater | <0.0001 | |
| Sediment | ||
| Freshwater | <0.0001 | |
| Seawater | <0.0001 |
Shown are the effects of indigenous microbiota and water type (freshwater versus seawater) on E. coli decrease in the water column and sediments from the March and April experiments. Statistical significance was determined by two-way ANOVA.
Fig 2.
Decline of E. coli (C/C0) in the presence (natural) and absence (disinfected) of indigenous microbiota in the water column (○) and sediments (●) of freshwater (A) and saltwater (B) mesocosms. Error bars indicate standard deviations. Shown are results from the March and April experiments.
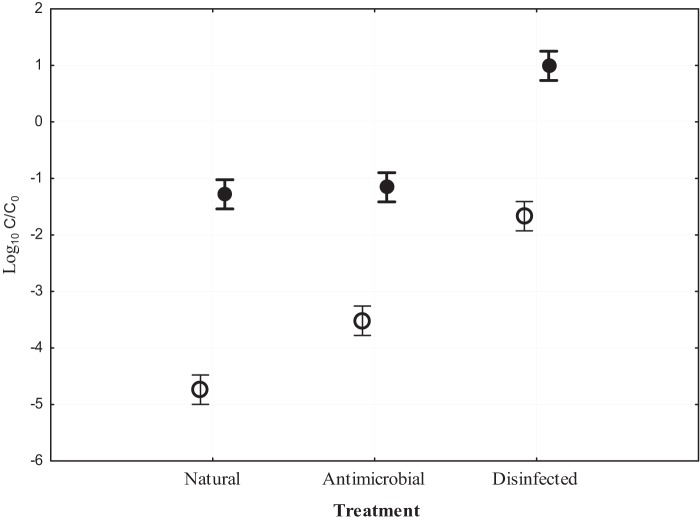
Fig 4.
Effect of removal of predation (antimicrobial treatment) or both competition and predation (disinfected treatment) on E. coli decline (C/C0) in water (○) and sediments (●) of freshwater mesocosms. Error bars represent standard deviations. Shown are results from the September experiment.
Fig 1.
Mean E. coli concentrations over time in the natural treatments (△ and ▲) and in the disinfected controls (□ and ■) in freshwater (water column [A] and sediments [B]) and seawater (water column [C] and sediments [D]) mesocosms. Data are means of values from five individual replicate mesocosms; error bars represent standard deviations. Shown are results from the March and April experiments.
Table 2.
Comparison of the effects of the indigenous microbiota and matrix on E. coli decrease in freshwater and seawater mesocosmsa
| Source of variation | % of total variation | P value |
|---|---|---|
| Freshwater (n = 5) | ||
| Water vs sediment | 36.5 | <0.0001 |
| Natural vs disinfected | 41.6 | <0.0001 |
| Interaction | 20.5 | <0.0001 |
| Seawater (n = 5) | ||
| Water vs sediment | 74.8 | <0.0001 |
| Natural vs disinfected | 24.1 | <0.0001 |
| Interaction | 0.49 | 0.004 |
| Post hoc tests (natural vs disinfected) | ||
| Freshwater | ||
| Water | <0.0001 | |
| Sediments | <0.001 | |
| Seawater | ||
| Water | <0.0001 | |
| Sediments | <0.0001 |
Shown are the effects of the indigenous microbiota and matrix on E. coli decrease in freshwater and seawater mesocosms from the March and April experiments. Statistical significance was determined by two-way ANOVA.
Overall, the decrease in concentrations was greater in the seawater than in the freshwater water column, irrespective of the presence of indigenous microbiota (Fig. 1A and C). The general trend of greater decline in the natural mesocosms continued for seawater; a precipitous decrease (nearly 7 orders of magnitude) (Fig. 1C) was observed in the water column when indigenous microbiota were present and no culturable E. coli cells were detected during the last 2 days (T7 and T9). Significantly different dynamics were noted in the disinfected controls, where E. coli remained culturable for the duration of the experiment and decreased almost 2 orders of magnitude less than in the natural mesocosms (Fig. 1C). While the effect of the presence of natural microbiota (compared to disinfected controls) was statistically significant, it contributed less to the variation in E. coli concentrations (24.1%) than it did in the freshwater (Fig. 2 and Table 2).
The effect of indigenous microbiota in the sediments of freshwater and seawater mesocosms.
As expected, culturable E. coli persisted significantly longer in the sediments of both water types than the overlaying water column, irrespective of the presence of indigenous microbiota (Fig. 1 and 2). In the natural freshwater sediments, E. coli decrease was gradual (<1 order of magnitude), followed by a plateau during the last two sampling dates (T7 and T9) (Fig. 1B). This is in contrast to disinfected controls, where no decline in E. coli concentrations occurred; instead, a slight increase was noted over 5 days (Fig. 1B).
In natural seawater sediments, E. coli concentrations declined significantly more over a 5-day period than in the natural freshwater sediments (Fig. 1B and D). The general trend of greater decrease in seawater compared to freshwater continued in the sediments of disinfected mesocosms, where E. coli maintained relatively stable levels and declined less than 1 order of magnitude over 5 days (Fig. 1D). The difference in rates of decline of culturable E. coli between disinfected controls and natural mesocosms in both water types was statistically significant (Table 1).
The effect of water type and matrix.
In general, culturable E. coli persisted significantly longer in freshwater than in seawater and in sediments compared to the water column under all experimental conditions (Fig. 1). In the water column, the effects of water type and presence or absence of indigenous microbiota were both significant and the contributions of these two parameters to the variation in E. coli concentrations were similar (40.1% and 49.2%, respectively) (Table 1). Furthermore, these two variables showed statistically significant interactions that contributed 9.74% to the variations in E. coli concentrations, which suggests that the effect of indigenous microbiota is dependent on the water type. The effects of water type and presence of natural microbiota on variation in E. coli concentrations in the sediments were comparable to one another (49.1% and 38.9%, respectively) and to those observed for the water column (Table 1). A significant interaction of variables (presence or absence of indigenous microbiota and water type) was noted for the sediment data (9.38% contribution to variability), indicating that, similar to the water column, the effect of indigenous microbiota is dependent on the sediment type.
In the freshwater mesocosms, matrix characteristics contributed 36.5% to the variation in E. coli concentrations—less than the presence of natural microbiota (41.6%). The interaction of these two variables was significant in the freshwater mesocosms, and its contribution to the total variation was 20.5% (Table 2), implying that the effects of indigenous microbiota are dissimilar in different freshwater matrices. The interaction plot (Fig. 2) demonstrates that while the direction of the effect is the same in water or sediment (the presence of indigenous microbes results in lower E. coli concentrations), the magnitudes of the responses are different. (The response to biota is much greater in water versus sediment.) The situation was reversed in the seawater mesocosms, where matrix was a more important determinant of total variation (74.8%) than the presence of indigenous microbiota (24.1%) (Table 2). The interaction of variables (presence or absence of indigenous microbiota and matrix) was significant (0.49% contribution to total variation), implying that the effects of indigenous microbiota differ in the water column and sediments of seawater habitats.
Predation versus competition in freshwater mesocosms.
The relative influence of protozoan predation on E. coli persistence in freshwater mesocosms was assessed by adding cycloheximide (which inhibited protozoan activity) as an antimicrobial treatment (Fig. 3). The natural treatment (which included all indigenous microbiota) and the disinfected treatment were repeated according to the previous experiments. The antimicrobial treatment had a significant effect on E. coli persistence (Table 3 and Fig. 4), decreasing the decline compared to the natural treatment and contributing 35.7% to the variation in E. coli concentrations over 5 days. The matrix also had a significant effect and an even greater impact (55.4%) on the variation (Table 3 and Fig. 4). In this mesocosm series, the interaction of variables (matrix and treatment) did not contribute significantly to variability in the data set (Table 3 and Fig. 4).
Fig 3.
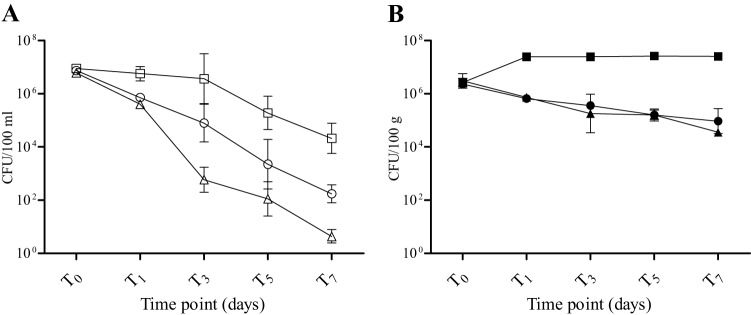
Mean E. coli concentrations over time in mesocosms with cycloheximide added (○ and ●), in the natural treatments (△ and ▲), and in disinfected controls (□ and ■) in the water column (A) and sediments (B). Data are means of log10-transformed values from five individual replicate mesocosms; error bars represent standard deviations. Shown are results from the September experiment.
Table 3.
Comparison of the effects of treatment and matrix on E. coli decrease in freshwater mesocosmsa
| Source of variation | % of total variation | P value |
|---|---|---|
| Water vs sediment | 55.4 | <0.0001 |
| Treatmentb | 35.7 | <0.0001 |
| Interaction | 1.48 | 0.11 |
| Post hoc tests | ||
| Natural vs disinfected | ||
| Water | <0.0001 | |
| Sediment | <0.0001 | |
| Natural vs antimicrobial treated | ||
| Water | <0.05 | |
| Sediment | >0.05 |
Shown are the effects of treatment (natural, antimicrobial treated, or disinfected) and matrix on E. coli decrease in freshwater mesocosms (n = 5) for the September experiment. Statistical significance was determined by two-way ANOVA.
Natural (all indigenous microbiota present), antimicrobial treated (cycloheximide addition), and disinfected.
DISCUSSION
Culture-dependent quantification of FIB is used throughout the world to assess the microbiological safety of drinking, recreational, and shellfishing waters. The U.S. Environmental Protection Agency currently recommends the use of E. coli and enterococci as indicator organisms for the assessment of recreational water quality (2, 50). Many questions have been raised about the validity of the FIB paradigm for predicting human health risks (7, 52, 53), and the effects of environmental factors on survival of FIB and pathogens need to be systematically investigated in order to chart a clear course for development of the next generation of tools for water quality assessment. The data presented here on E. coli survival show significant effects of the natural microbiota, water type, and matrix. The variable “water type” is not a simple one, as salinity (and associated osmotic stress), concentration of organic material, and indigenous microbial populations are all expected to vary between freshwater and seawater. The experimental design and data analysis allowed us to draw some conclusions on the relative importance of each factor under the conditions tested.
Contrary to previous studies that attributed rapid decline of FIB in environmental waters to germicidal effects of sunlight and associated UV radiation (17, 18, 20, 22–25), we observed a relatively slow change in E. coli concentrations in the absence of natural microbiota and in the presence of sunlight. A recent study conducted in the same natural pond water and sediments we used for our freshwater mesocosms found no significant differences in the decay rates of E. coli in mesocosms exposed to ambient sunlight versus darkened (aluminum-foil-covered) controls (42). Reported reductions in E. coli concentrations over 7 days in mesocosms exposed to ambient sunlight (3.6 log10 and 0.4 log10 in the water column and sediments, respectively) (42) were similar to the values we observed. This finding lends credence to the argument that indigenous microbiota (and associated predation-competition interactions) may exert more pressure on the reduction of E. coli in aquatic environments than sunlight exposure, at least under some conditions.
Overall, E. coli decayed faster in the seawater mesocosms, irrespective of the matrix and treatment type. This is not surprising, as osmotic stress posed by the elevated salinity of the marine environment is a known stressor for E. coli cells (54, 55). When indigenous microbiota were present, E. coli decay in the water column was significantly greater than that in the disinfected controls, and this trend was evident in both water types, a finding consistent with other studies (43, 56–58). Although less pronounced, the same pattern of extended E. coli persistence in disinfected mesocosms compared to natural mesocosms continued for sediments in both water types. However, a finding that is unique to our study is dissimilar magnitudes of the effects of indigenous microbiota in freshwater and seawater habitats. In freshwater mesocosms, indigenous microbiota were a more important determinant of E. coli survival than in seawater mesocosms. It is important to note that the average levels of UV insolation and cloud cover occurring during March (freshwater mesocosm) and April (seawater mesocosm) in our study location are quite similar, thus facilitating comparisons between the experiments. This observation indicates that, within the confines of our experimental design, the effect of indigenous microbiota on E. coli survival in the water column is influenced by the water type. While protozoan bacterivory is a recognized contributor to the decline of bacterial populations in aquatic environments (29, 59–63), the relative magnitudes of impact of protozoan presence on E. coli survival in sunlight-exposed freshwater and seawater systems have not been previously described.
Interestingly, in the seawater mesocosms, the matrix (water/sediment) had a greater effect on the persistence of E. coli than the presence of indigenous microbiota. Surprisingly, comparisons of the effects of freshwater versus seawater environments on bacterial survival in sediments are rare. To the best of our knowledge, only one other study (10) directly compared rates of FIB survival in natural freshwater versus seawater sediments (in the presence of all indigenous microbiota). They measured fecal coliform rather than E. coli decay rates and found that this group declined more rapidly in marine sediments than in freshwater sediments; however, the differences were not as great as those observed in this study. Our field studies have shown that fecal coliform concentrations are frequently up to 2 orders of magnitude greater than E. coli concentrations in environmental waters, suggesting that some members of the larger group are better survivors in the secondary habitat than most E. coli strains (64–67).
Inhibition of protozoan activity and therefore predation (i.e., cycloheximide treatment) in the water column of freshwater mesocosms decreased the rate of E. coli decline (increased survival) compared to natural conditions that included protozoa and other indigenous microbiota. However, E. coli declined in the cycloheximide treatments at a significantly greater rate than in the disinfected controls, suggesting that interactions with nonprotozoan microbiota, such as competition for nutrients with autochthonous bacteria, also affect E. coli survival. Addition of cycloheximide had little effect on the rate of E. coli decline in freshwater sediments, indicating that competition with autochthonous bacteria influenced survival more than predation in this matrix. These data are consistent with findings for beach sediments (34). Relatively low rates of protozoan grazing have been reported for the sediments (68–75), providing a possible mechanism for FIB persistence in such habitats. Maintenance of elevated FIB concentrations in sediments is of practical importance and has public health implications, as previous studies indicated that resuspension can lead to increased bacterial concentrations in the water column (76–82). A recent study (43), which manipulated indigenous microbiota by adding kanamycin to reduce competition from prokaryotic community, found that inclusion of the antibiotic extended E. coli survival in the river water and sediments, corroborating our results.
The data collected in this study indicate that the indigenous microbiota (and associated biotic interactions) is an important determinant of E. coli survival in subtropical waters and sediments and that the magnitudes of these interactions in different matrices and water types are dissimilar. These findings imply that wide generalizations with respect to the effect of environmental parameters on E. coli survival across habitats (i.e., water type and matrix) are unwise and that the effect of these variables on pathogen persistence should be explored. Although the utility and importance of E. coli extend beyond water quality issues, as it is a recognized prokaryotic model organism, the principles governing its survival in the environment are less than clear. Better understanding of the ecology of FIB in aquatic habitats is needed in order to improve predictions regarding their behavior in the environment, to provide a framework for similar studies on pathogen survival, and to develop better indicator systems for the assessment of recreational and environmental water quality.
ACKNOWLEDGMENTS
We thank Laurie Walker (USF Botanical Gardens) and her staff for generously allowing us unrestricted access to the Botanical Gardens for the duration of mesocosm studies. Additionally, we acknowledge Jacques Ravel of the Institute for Genome Sciences for kindly providing two of the E. coli strains (HS and SMS-35) utilized in these experiments.
The funding for this study was provided by the Gulf of Mexico Alliance Regional Partnership Projects MX-96478707-0.
Footnotes
Published ahead of print 28 June 2013
REFERENCES
- 1.Florida Administrative Code 1998. Surface water quality standards. FAC 62-603. Florida Department of Environmental Protection, Tallahassee, FL [Google Scholar]
- 2.US Environmental Protection Agency 1986. Ambient water quality criteria for bacteria. EPA 440/5-84-002. US Environmental Protection Agency, Washington, DC [Google Scholar]
- 3.Ashbolt NJ, Grabow OK, Snozzi M. 2001. Indicators of microbial water quality, p 289–315 In Fewtrell L, Bartram J. (ed), Water quality: guidelines, standards and health. IWA Publishing, London, United Kingdom [Google Scholar]
- 4.Craig DL, Fallowfield HJ, Cromar NJ. 2003. Effectiveness of guideline faecal indicator organism values in estimation of exposure risk at recreational coastal sites. Water Sci. Technol. 47:191–198 [PubMed] [Google Scholar]
- 5.Ferguson C, Husman AMD, Altavilla N, Deere D, Ashbolt N. 2003. Fate and transport of surface water pathogens in watersheds. Crit. Rev. Environ. Sci. Technol. 33:299–361 [Google Scholar]
- 6.Field KG, Samadpour M. 2007. Fecal source tracking, the indicator paradigm, and managing water quality. Water Res. 41:3517–3538 [DOI] [PubMed] [Google Scholar]
- 7.Harwood VJ, Levine AD, Scott TM, Chivukula V, Lukasik J, Farrah SR, Rose JB. 2005. Validity of the indicator organism paradigm for pathogen reduction in reclaimed water and public health protection. Appl. Environ. Microbiol. 71:3163–3170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leclerc H, Mossel DAA, Edberg SC, Struijk CB. 2001. Advances in the bacteriology of the coliform group: their suitability as markers of microbial water safety. Annu. Rev. Microbiol. 55:201–234 [DOI] [PubMed] [Google Scholar]
- 9.Savichtcheva O, Okabe S. 2006. Alternative indicators of fecal pollution: relations with pathogens and conventional indicators, current methodologies for direct pathogen monitoring and future application perspectives. Water Res. 40:2463–2476 [DOI] [PubMed] [Google Scholar]
- 10.Anderson KL, Whitlock JE, Harwood VJ. 2005. Persistence and differential survival of fecal indicator bacteria in subtropical waters and sediments. Appl. Environ. Microbiol. 71:3041–3048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Badgley BD, Nayak BS, Harwood VJ. 2010. The importance of sediment and submerged aquatic vegetation as potential habitats for persistent strains of enterococci in a subtropical watershed. Water Res. 44:5857–5866 [DOI] [PubMed] [Google Scholar]
- 12.Badgley BD, Thomas FI, Harwood VJ. 2011. Quantifying environmental reservoirs of fecal indicator bacteria associated with sediment and submerged aquatic vegetation. Environ. Microbiol. 13:932–942 [DOI] [PubMed] [Google Scholar]
- 13.Badgley BD, Thomas FIM, Harwood VJ. 2010. The effects of submerged aquatic vegetation on the persistence of environmental populations of Enterococcus spp. Environ. Microbiol. 12:1271–1281 [DOI] [PubMed] [Google Scholar]
- 14.Byappanahalli MN, Shively DA, Nevers MB, Sadowsky MJ, Whitman RL. 2003. Growth and survival of Escherichia coli and enterococci populations in the macro-alga Cladophora (Chlorophyta). FEMS Microbiol. Ecol. 46:203–211 [DOI] [PubMed] [Google Scholar]
- 15.Desmarais TR, Solo-Gabriele HM, Palmer CJ. 2002. Influence of soil on fecal indicator organisms in a tidally influenced subtropical environment. Appl. Environ. Microbiol. 68:1165–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishii S, Hansen DL, Hicks RE, Sadowsky MJ. 2007. Beach sand and sediments are temporal sinks and sources of Escherichia coli in Lake Superior. Environ. Sci. Technol. 41:2203–2209 [DOI] [PubMed] [Google Scholar]
- 17.Davies-Colley RJ, Bell RG, Donnison AM. 1994. Sunlight inactivation of enterococci and fecal coliforms in sewage effluent diluted in seawater. Appl. Environ. Microbiol. 60:2049–2058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujioka RS, Hashimoto HH, Siwak EB, Young RH. 1981. Effect of sunlight on survival of indicator bacteria in seawater. Appl. Environ. Microbiol. 41:690–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sinton LW, Davies-Colley RJ, Bell RG. 1994. Inactivation of enterococci and fecal coliforms from sewage and meatworks effluents in seawater chambers. Appl. Environ. Microbiol. 60:2040–2048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sinton LW, Finlay RK, Lynch PA. 1999. Sunlight inactivation of fecal bacteriophages and bacteria in sewage-polluted seawater. Appl. Environ. Microbiol. 65:3605–3613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davies CM, Evison LM. 1991. Sunlight and the survival of enteric bacteria in natural waters. J. Appl. Bacteriol. 70:265–274 [DOI] [PubMed] [Google Scholar]
- 22.Fujioka RS, Narikawa OT. 1982. Effect of sunlight on enumeration of indicator bacteria under field conditions. Appl. Environ. Microbiol. 44:395–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sinton L, Hall C, Braithwaite R. 2007. Sunlight inactivation of Campylobacter jejuni and Salmonella enterica, compared with Escherichia coli, in seawater and river water. J. Water Health 5:357–365 [DOI] [PubMed] [Google Scholar]
- 24.Sinton LW, Hall CH, Lynch PA, Davies-Colley RJ. 2002. Sunlight inactivation of fecal indicator bacteria and bacteriophages from waste stabilization pond effluent in fresh and saline waters. Appl. Environ. Microbiol. 68:1122–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davies-Colley RJ, Donnison AM, Speed DJ. 1997. Sunlight wavelengths inactivating faecal indicator microorganisms in waste stabilisation ponds. Water Sci. Technol. 35:219–225 [Google Scholar]
- 26.Davies-Colley RJ, Donnison AM, Speed DJ, Ross CM, Nagels JW. 1999. Inactivation of faecal indicator microorganisms in waste stabilisation ponds: interactions of environmental factors with sunlight. Water Res. 33:1220–1230 [Google Scholar]
- 27.Craggs RJ, Sukias JP, Tanner CT, Davies-Colley RJ. 2004. Advanced pond system for dairy-farm effluent treatment. N. Z. J. Agric. Res. 47:449–460 [Google Scholar]
- 28.Anderson A, Larsson U, Hagstrom A. 1986. Size selective grazing by a microflagellate on pelagic bacteria. Mar. Ecol. Prog. Ser. 33:51–57 [Google Scholar]
- 29.Menon P, Billen G, Servais P. 2003. Mortality rates of autochthonous and fecal bacteria in natural aquatic ecosystems. Water Res. 37:4151–4158 [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Lara J, Menon P, Servais P, Billen G. 1991. Mortality of fecal bacteria in seawater. Appl. Environ. Microbiol. 57:885–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pirlot S, Unrein F, Descy JP, Servais P. 2007. Fate of heterotrophic bacteria in Lake Tanganyika (East Africa). FEMS Microbiol. Ecol. 62:354–364 [DOI] [PubMed] [Google Scholar]
- 32.Servais P, Garcia-Armisen T, George I, Billen G. 2007. Fecal bacteria in the rivers of the Seine drainage network (France): sources, fate and modelling. Sci. Total Environ. 375:152–167 [DOI] [PubMed] [Google Scholar]
- 33.Byappanahalli M, Fujioka R. 2004. Indigenous soil bacteria and low moisture may limit but allow faecal bacteria to multiply and become a minor population in tropical soils. Water Sci. Technol. 50:27–32 [PubMed] [Google Scholar]
- 34.Feng F, Goto D, Yan T. 2010. Effects of autochthonous microbial community on the die-off of fecal indicators in tropical beach sand. FEMS Microbiol. Ecol. 74:214–225 [DOI] [PubMed] [Google Scholar]
- 35.Marino RP, Gannon JJ. 1991. Survival of fecal coliforms and fecal streptococci in storm drain sediment. Water Res. 25:1089–1098 [Google Scholar]
- 36.Unc A, Gardner J, Springthorpe S. 2006. Recovery of Escherichia coli from soil after addition of sterile organic wastes. Appl. Environ. Microbiol. 72:2287–2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boehm AB, Yamahara KM, Love DC, Peterson BM, McNeill K, Nelson KL. 2009. Covariation and photoinactivation of traditional and novel indicator organisms and human viruses at a sewage-impacted marine beach. Environ. Sci. Technol. 43:8046–8052 [DOI] [PubMed] [Google Scholar]
- 38.Kashefipour SM, Lin B, Falconer RA. 2006. Modelling the fate of faecal indicators in a coastal basin. Water Res. 40:1413–1425 [DOI] [PubMed] [Google Scholar]
- 39.Sassoubre LM, Walters SP, Russell TL, Boehm AB. 2011. Sources and fate of Salmonella and fecal indicator bacteria in an urban creek. J. Environ. Monit. 13:2206–2212 [DOI] [PubMed] [Google Scholar]
- 40.Schultz-Fademrecht C, Wichern M, Horn H. 2008. The impact of sunlight on inactivation of indicator microorganisms both in river water and benthic biofilms. Water Res. 42:4771–4779 [DOI] [PubMed] [Google Scholar]
- 41.van Elsas JD, Semenov AV, Costa R, Trevors JT. 2011. Survival of Escherichia coli in the environment: fundamental and public health aspects. ISME J. 5:173–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Staley ZR, Rohr JR, Harwood VJ. 2011. Test of direct and indirect effects of agrochemicals on the survival of fecal indicator bacteria. Appl. Environ. Microbiol. 77:8765–8774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wanjugi P, Harwood VJ. 2012. The influence of predation and competition on the survival of commensal and pathogenic fecal bacteria in aquatic habitats. Environ. Microbiol. 15:517–526 [DOI] [PubMed] [Google Scholar]
- 44.Chabaud S, Andres Y, Lakel A, Le Cloirec P. 2006. Bacteria removal in septic effluent: influence of biofilm and protozoa. Water Res. 40:3109–3114 [DOI] [PubMed] [Google Scholar]
- 45.Hurst CJ. (ed). 1997. Manual of environmental microbiology, 2nd ed ASM Press, Washington, DC [Google Scholar]
- 46.Blattner FR, Plunkett G, III, Bloch CA, Perna NT, Burland V, Riley M, Collado-Vides J, Glasner JD, Rode CK, Mayhew GF, Gregor J, Davis NW, Kirkpatrick HA, Goeden MA, Rose DJ, Mau B, Shao Y. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453–1469 [DOI] [PubMed] [Google Scholar]
- 47.Levine MM, Nalin DR, Hornick RB, Bergquist EJ, Waterman DH, Young CR, Sotman S, Rowe B. 1978. Escherichia coli strains that cause diarrhea but do not produce heat-labile or heat-stable enterotoxins and are non-invasive. Lancet i:1119–1122 [DOI] [PubMed] [Google Scholar]
- 48.Fricke WF, Wright MS, Lindell AH, Harkins DM, Baker-Austin C, Ravel J, Stepanauskas R. 2008. Insights into the environmental resistance gene pool from the genome sequence of the multidrug-resistant environmental isolate Escherichia coli SMS-3-5. J. Bacteriol. 190:6779–6794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.American Public Health Association 1999. Standard methods for the examination of water and wastewater. Standard method 9222D. American Public Health Association, Washington, DC [Google Scholar]
- 50.US Environmental Protection Agency 2002. Method 1603: Escherichia coli (E. coli) in water by membrane filtration using modified membrane-thermotolerant Escherichia coli agar (modified mTEC). EPA 821-R-02-023. US Environmental Protection Agency, Washington, DC [Google Scholar]
- 51.Boehm AB, Griffith J, McGee C, Edge TA, Solo-Gabriele HM, Whitman R, Cao Y, Getrich M, Jay JA, Ferguson D, Goodwin KD, Lee CM, Madison M, Weisberg SB. 2009. Faecal indicator bacteria enumeration in beach sand: a comparison study of extraction methods in medium to coarse sands. J. Appl. Microbiol. 107:1740–1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ashbolt NJ. 2004. Microbial contamination of drinking water and disease outcomes in developing regions. Toxicology 198:229–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boehm AB, Ashbolt NJ, Colford JM, Jr, Dunbar LE, Fleming LE, Gold MA, Hansel JA, Hunter PR, Ichida AM, McGee CD, Soller JA, Weisberg SB. 2009. A sea change ahead for recreational water quality criteria. J. Water Health 7:9–20 [DOI] [PubMed] [Google Scholar]
- 54.Gunasekera TS, Csonka LN, Paliy O. 2008. Genome-wide transcriptional responses of Escherichia coli K-12 to continuous osmotic and heat stresses. J. Bacteriol. 190:3712–3720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shabala L, Bowman J, Brown J, Ross T, McMeekin T, Shabala S. 2009. Ion transport and osmotic adjustment in Escherichia coli in response to ionic and non-ionic osmotica. Environ. Microbiol. 11:137–148 [DOI] [PubMed] [Google Scholar]
- 56.Anderson IC, Rhodes MW, Kator HI. 1983. Seasonal variation in survival of Escherichia coli exposed in situ in membrane-diffusion chambers containing filtered and nonfiltered estuarine water. Appl. Environ. Microbiol. 45:1877–1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Korajkic A, McMinn BR, Shanks OC, Harwood VJ, Fout GS, Ashbolt NJ. 2013. Differential decay of enterococci and Escherichia coli originating from two fecal pollution sources. Appl. Environ. Microbiol. 79:2488–2492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rhodes MW, Kator H. 1988. Survival of Escherichia coli and Salmonella spp. in estuarine environments. Appl. Environ. Microbiol. 54:2902–2907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beardsley C, Pernthaler J, Wosniok W, Amann R. 2003. Are readily culturable bacteria in coastal North Sea waters suppressed by selective grazing mortality? Appl. Environ. Microbiol. 69:2624–2630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gonzalez JM, Iriberri J, Egea L, Barcina I. 1992. Characterization of culturability, protistan grazing, and death of enteric bacteria in aquatic ecosystems. Appl. Environ. Microbiol. 58:998–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iriberri J, Azua I, Labiruaiturburu A, Artolozaga I, Barcina I. 1994. Differential elimination of enteric bacteria by protists in a fresh-water system. J. Appl. Bacteriol. 77:476–483 [DOI] [PubMed] [Google Scholar]
- 62.Rhodes MW, Kator HI. 1990. Effects of sunlight and autochthonous microbiota on Escherichia coli survival in an estuarine environment. Curr. Microbiol. 21:65–73 [Google Scholar]
- 63.Surbeck CQ, Jiang SC, Grant SB. 2010. Ecological control of fecal indicator bacteria in an urban stream. Environ. Sci. Technol. 44:631–637 [DOI] [PubMed] [Google Scholar]
- 64.Chase E, Hunting J, Staley C, Harwood VJ. 2011. Microbial source tracking to identify human and ruminant sources of faecal pollution in an ephemeral Florida river. J. Appl. Microbiol. 113:1396–1406 [DOI] [PubMed] [Google Scholar]
- 65.Korajkic A, Badgley BD, Brownell MJ, Harwood VJ. 2009. Application of microbial source tracking methods in a Gulf of Mexico field setting. J. Appl. Microbiol. 107:1518–1527 [DOI] [PubMed] [Google Scholar]
- 66.Korajkic A, Brownell MJ, Harwood VJ. 2011. Investigation of human sewage pollution and pathogen analysis at Florida Gulf coast beaches. J. Appl. Microbiol. 110:174–183 [DOI] [PubMed] [Google Scholar]
- 67.Staley C, Reckhow KH, Lukasik J, Harwood VJ. 2012. Assessment of sources of human pathogens and fecal contamination in a Florida freshwater lake. Water Res. 46:5799–5812 [DOI] [PubMed] [Google Scholar]
- 68.Epstein SS, Shiaris MP. 1992. Rates of microbenthic and meiobenthic bacterivory in a temperate muddy tidal flat community. Appl. Environ. Microbiol. 58:2426–2431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.First MR, Hollibaugh JT. 2008. Protistan bacterivory and benthic microbial biomass in an intertidal creek mudflat. Mar. Ecol. Prog. Ser. 361:59–68 [Google Scholar]
- 70.Gucker B, Fischer H. 2003. Flagellate and ciliate distribution in sediments of a lowland river: relationships with environmental gradients and bacteria. Aquat. Microb. Ecol. 31:67–76 [Google Scholar]
- 71.Hamels I, Muylaert K, Casteleyn G, Vyverman W. 2001. Uncoupling of bacterial production and flagellate grazing in aquatic sediments: a case study from an intertidal flat. Aquat. Microb. Ecol. 25:31–42 [Google Scholar]
- 72.Konigs S, Cleven EJ. 2007. The bacterivory of interstitial ciliates in association with bacterial biomass and production in the hyporheic zone of a lowland stream. FEMS Microbiol. Ecol. 61:54–64 [DOI] [PubMed] [Google Scholar]
- 73.Starink M, Bar Gilissen MJ, Bak RPM, Cappenberg TE. 1996. Seasonal and spatial variations in heterotrophic nanoflagellate and bacteria abundances in sediments of a freshwater littoral zone. Limnol. Oceanogr. 41:234–242 [Google Scholar]
- 74.Wieltschnig C, Fischer UR, Kirschner AKT, Velimirov B. 2003. Benthic bacterial production and protozoan predation in a silty freshwater environment. Microb. Ecol. 46:62–72 [DOI] [PubMed] [Google Scholar]
- 75.Wieltschnig C, Fischer UR, Velimirov B, Kirschner AKT. 2008. Effects of deposit-feeding macrofauna on benthic bacteria, viruses, and protozoa in a silty freshwater sediment. Microb. Ecol. 56:1–12 [DOI] [PubMed] [Google Scholar]
- 76.An YJ, Kampbell DH, Breidenbach GP. 2002. Escherichia coli and total coliforms in water and sediments at lake marinas. Environ. Pollut. 120:771–778 [DOI] [PubMed] [Google Scholar]
- 77.Boehm AB, Weisberg SB. 2005. Tidal forcing of enterococci at marine recreational beaches at fortnightly and semidiurnal frequencies. Environ. Sci. Technol. 39:5575–5583 [DOI] [PubMed] [Google Scholar]
- 78.Craig DL, Fallowfield HJ, Cromar NJ. 2004. Use of microcosms to determine persistence of Escherichia coli in recreational coastal water and sediment and validation with in situ measurements. J. Appl. Microbiol. 96:922–930 [DOI] [PubMed] [Google Scholar]
- 79.Ferguson DM, Moore DF, Getrich MA, Zhowandai MH. 2005. Enumeration and speciation of enterococci found in marine and intertidal sediments and coastal water in southern California. J. Appl. Microbiol. 99:598–608 [DOI] [PubMed] [Google Scholar]
- 80.Fries JS, Characklis GW, Noble RT. 2008. Sediment-water exchange of Vibrio sp. and fecal indicator bacteria: implications for persistence and transport in the Neuse River estuary, North Carolina, USA. Water Res. 42:941–950 [DOI] [PubMed] [Google Scholar]
- 81.Kinzelman J, McLellan SL, Daniels AD, Cashin S, Singh A, Gradus S, Bagley R. 2004. Non-point source pollution: determination of replication versus persistence of Escherichia coli in surface water and sediments with correlation of levels to readily measurable environmental parameters. J. Water Health 2:103–114 [PubMed] [Google Scholar]
- 82.LaLiberte P, Grimes DJ. 1982. Survival of Escherichia coli in lake bottom sediment. Appl. Environ. Microbiol. 43:623–628 [DOI] [PMC free article] [PubMed] [Google Scholar]