Abstract
Pseudomonas species can exhibit phenotypic variation resulting from gacS or gacA mutation. P. fluorescens Pf0-1 is a gacA mutant and exhibits pleiotropic changes following the introduction of a functional allele. GacA enhances biofilm development while reducing dissemination in soil, suggesting that alternative Gac phenotypes enable Pseudomonas sp. to exploit varied environments.
TEXT
Two-component regulators, consisting of a sensor kinase and response regulator pair, mediate bacterial adaptation to environmental perturbations. The GacS/GacA system in Pseudomonas spp., with orthologs spread across diverse members of the class Gammaproteobacteria, is a key regulator of environmentally relevant phenotypes, including motility, biofilm formation, and secondary-metabolite production. Despite the role of Gac in important and varied phenotypes, mutations in gacS or gacA arise with high frequency in a range of pseudomonads both in laboratory culture and in natural environments (1–5). The frequency with which such mutations arise suggests positive selection for loss of Gac, perhaps because of a reduced metabolic load compared to that of Gac+ species. Consistent with this hypothesis, Gac− members of the population often have a growth advantage over their Gac+ counterparts and spontaneous Gac-deficient mutants become the majority population following extended growth in nutrient-rich laboratory cultures (4, 6). Gac-deficient mutants are additionally found at high frequency in the rhizosphere; however, Gac− mutants do not displace their wild-type counterparts in nature. On the contrary, wild-type populations can benefit from the presence of Gac-deficient neighbors in mixed populations. For example, mixed-strain biofilms consisting of Gac+ and Gac− populations are more robust than those with the Gac+ wild type alone. In addition, Gac− strains are more likely to be found spatially near Gac+ neighbors, perhaps allowing exogenous “common good” metabolites produced by the wild type to be utilized by Gac-deficient members of the community (3). Such apparent mutualism may select for the maintenance of mixed-Gac populations in natural environments.
Mutations in gacS and gacA arise at high frequency in culture and soil and on plant roots (1, 2, 4). The characterization of such mutants reveals point mutations, small insertions and deletions, larger insertions of greater than 200 bp, and reversible mechanisms of phenotypic switching (5, 7). It was recently reported (8) that heterologous expression of the Pseudomonas protegens Pf-5 gacA allele in Pf0-1 induces Gac-controlled phenotypes, including swarming motility, exoprotease and chitinase activities, and hydrogen cyanide and biosurfactant production. These results suggest that Pf0-1, often used as a model of pseudomonad behavior in soil, is Gac deficient. Indeed, in silico analysis reveals an apparent N109P substitution within the signal receiver domain of Pf0-1 GacA, perhaps rendering the protein nonfunctional. DNA sequencing of the gacA region reveals that the N109P substitution is present in all of the Pf0-1 strains tested, including archived stocks spanning 2 decades and those collected from several research groups. Thus, we sought to generate Pf0-1 strains with a functional gacA allele and define Gac-related phenotypes exhibited by Pf0-1 in vitro and in the soil environment. We additionally explored the ability of Gac+ cells to compensate for Gac-deficient neighbors in mixed populations. Given the extensive use of the reference strain Pf0-1 in models of environmental persistence, biofilm formation, and biocontrol, the influence of the gacA mutation on the observed phenotypes will be crucial to consider in future research.
A point mutation in gacA renders the Gac system of P. fluorescens Pf0-1 nonfunctional.
Site-specific mutagenesis was used to generate a P109N amino acid change within the Pf0-1 gacA allele on a plasmid via overlap extension PCR (9) with primers gacAMUT1 and gacAMUT4 (Table 1). The engineered plasmid, designated pSS115 (Table 1), includes an approximately 1,800-bp DNA fragment spanning the GacA coding region and its native promoter. The introduction of pSS115 into Pf0-1 elicited the onset of multiple Gac-associated phenotypes not typical of the parent strain, including profound changes in motility, antifungal activity, and biofilm development (Fig. 1 and 2; also described below). To generate a Pf0-1 strain with a stable gacA allele and to eliminate multicopy effects, the Pf-5 gacA gene under the control of its native chromosomal promoter was cloned into the mini-Tn7 element carried by pHRB2 (Table 1) and introduced into the chromosome of Pf0-1 as described previously (10). Correct integration of the transposon into the chromosomal glmS locus was verified by PCR (11), and DNA sequencing confirmed the gacA sequence of the final strain. This strain, designated Pf0-gacA+ (Table 1), shows motility and biofilm phenotypes identical to those of the Pf0-1 strain harboring pSS115 (not shown) and was used for all additional experiments. Pf0-gacA+ exhibits a range of phenotypes consistent with proper GacS/GacA function; however, it is important to note that the strain harbors two gacA alleles—the inactive allele at the native locus and the allele from Pf-5 at a second site—and thus may show gacA expression different from that of a true wild-type strain.
Table 1.
Strains, plasmids, and primers used in this study
| Strain, plasmid, or primer | Description or sequencea | Source or reference |
|---|---|---|
| P. fluorescens strains | ||
| Pf0-1 | Parental strain, Apr | 37 |
| Pf0-2X | Pf0-1 adnA::ΩSmSpr; nonmotile mutant | 18 |
| Pf0-2X gacA+ | Pf0-2X::mini-Tn7 gacA Kmr | This study |
| Pf0-gacA+ | Pf0-1::mini-Tn7 gacA Kmr | This study |
| Pf0-gacA+ hcn− | Pf0-1 hcnAB-ΩSmSpr mini-Tn7 gacA Kmr | This study |
| Pf0-gacA+ aprA− | Pf0-1 aprA-ΩSmSpr mini-Tn7 gacA Kmr | This study |
| Pf0-gacA+ 2211ΔS | Pf0-1 partial Δ2211-ΩSmSpr mini-Tn7 gacA Kmr | This study |
| Plasmids | ||
| pJEL5965 | gacA gene and promoter region from Pf-5 cloned into pME6000 | 8 |
| pHRP315 | pMB1 origin, Apr, source of ΩSmSpr cassette | 38 |
| pSR47S | Suicide vector, R6K origin, sacB, Kmr | 39 |
| pBBRMCS2 | Broad-host-range cloning vector, Kmr | 40 |
| pHRB2 | Kmr carried on mini-Tn7 element | 10 |
| pSS100 | gacA from Pf-5 cloned into mini-Tn7 Kmr element of pHRB2 | This study |
| pSS102 | pSR47S with Pf0-1 hcnAB fragment (3995538–3996732) interrupted by ΩSmSpr | This study |
| pSS104 | pSR47S with Pf0-1 aprA fragment (3078319–3079638) interrupted by ΩSmSpr | This study |
| pSS110 | pSR47S with Pf01 Pfl_2211ΔΩSmSpr fragment | This study |
| pSS115 | Pf0-1 gacA(P109N) allele cloned into pBBRMCS2 | This study |
| Primers | ||
| gacAMUT1 | GAT GGA CAC AGG CGC CAC CC | |
| gacAMUT2 | GAA GGG TGC CGG ATT GAA CGA AAT GGT TCA GGC C | |
| gacAMUT3 | GGC CTG AAC CAT TTC GTT CAA TCC GGC ACC CTT C | |
| gacAMUT4 | GAG CCG AGG CAC CGT TCA CC | |
| AprA_1 | TTC CAG CTG AAC GCG GCT CCC T | |
| AprA_2 | GCC GCC CGA TGA CGT CAG AAC C | |
| Hcn_1 | AGT TGC GTG CGC GGG ATG AA | |
| Hcn_2 | ACG AGC CGG CGA GGT GTA GT | |
| 2211_1 | TAC CAG CAC CGT CCC GAG CCG | |
| 2211_2 | GGG ATT CAG CCG CTA CCG CG | |
| 2211_3 | GAT CTG TGT CGA ACG CAG CCT GG | |
| 2211_4 | GGT TGG TCC GGT TCC AGT GTC |
Apr, ampicillin resistant; SmSpr, spectinomycin and streptomycin resistant; Kmr, kanamycin resistant. Nucleotides in bold are those that were engineered to introduce the desired mutation.
Fig 1.
Motility and antifungal activity. (A) The impact of gacA- and gacA-dependent CLP production on Pf0-1 swarming (A) and swimming (B) motility was assessed on 0.6 and 0.3% agar, respectively. In all cases, the size marker indicates 1 cm and the arrowhead in panel B delineates a surfactant front seen only in Pf0-gacA+. (C) An in vitro assay was used to assess the antifungal activity of Pf0-1 and its derivatives. A plug of agar containing R. solani (*) was placed adjacent to the strain of interest (between the dashed lines), and the extent of hyphal growth was used as an indirect measure of antifungal activity. Photos were taken following 4 days of fungal growth.
Fig 2.
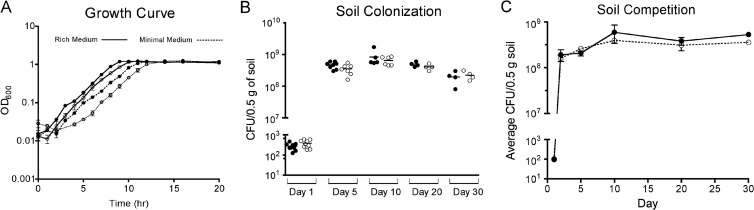
Growth and competition in laboratory medium and soil. (A) Growth curves of Pf0-1 and Pf0-gacA+ in rich medium (solid line) and a defined minimal medium (dashed line). (B) Pf0-1 and Pf0-gacA+ were inoculated into soil, and viable bacteria were monitored over a 30-day period. Data points represent individual soil columns, and horizontal lines represent median values. Differences between the parent and the Gac+ derivative are not statistically significant (P > 0.05 for all time points), as calculated with the Mann-Whitney nonparametric statistical hypothesis test. For competition assays (C), a 1:1 ratio of each strain was used to inoculate a single soil column. Cell numbers for each strain were assessed over a 30-day period, and average CFU counts are plotted. In all three panels, Pf0-1 and Pf0-gacA+ data points are represented as closed and open circles, respectively.
GacA alters Pf0-1 motility and antifungal activity.
The GacS/GacA regulatory cascade directs motility in diverse bacterial genera (12, 13); however, specific effects vary from organism to organism. The P. protegens Pf-5 Gac system, for instance, is required for swarming but does not significantly alter swimming motility (13). Swimming by P. fluorescens F113, however, is under the negative control of the Gac system, and mutants lacking gacS or gacA produce larger swimming halos than the wild type (14, 15). We assessed the role of gacA in Pf0-1 swimming and swarming motility by using a minimal-salts medium solidified with 0.3 and 0.6% agar, respectively. Pf0-gacA+ exhibits enhanced swarming compared to that of the Pf0-1 parent (Fig. 1A). Increased swarming is abrogated by the deletion of a gene (Pfl01_2211) that encodes a nonribosomal peptide synthetase (NRPS) similar to that which specifies orfamide production in P. protegens Pf-5, confirming that its gac-dependent swarming is associated with the production of an orfamide-like cyclic lipopeptide (CLP). Swimming motility is also altered in the Pf0-gacA+ strain, with swimming halos showing an irregular shape (Fig. 1B) rather than the concentric-ring pattern exhibited by the Pf0-1 parent (16–18). While the overall sizes of the swimming halo of Pf0-1 and Pf0-gacA+ are similar, excretion of a surfactant ring (Fig. 1B) ahead of the cellular front is apparent in Pf0-gacA+ and absent from the parent strain, as well as the strain with NRPS-encoding Pfl01_2211 deleted (Fig. 1B).
P. fluorescens strains produce an array of secondary metabolites with antibiotic activity, many of which are under the control of GacS/GacA. In the case of Pf0-1, biosynthetic genes for many of the major antibiotics (phenazine, 2,4-diacetylphloroglucinol, pyrrolnitrin) are absent and the organism exhibits little in vitro antibiotic activity. Because the GacA/GacS system is closely linked to antibiosis in related pseudomonads, we tested whether gacA promotes antibiosis in Pf0-1. An in vitro assay was used to assess the activity of Pf0-1 and Pf0-gacA+ against the phytopathogenic fungus Rhizoctonia solani. The introduction of gacA increased the ability of Pf0-1 to inhibit the growth of fungal hyphae, compared to the parent (Fig. 1C). To pinpoint the gac-dependent antifungal metabolite(s) induced in Pf0-gacA, genes known for their antibiotic activity were inactivated, including hcnA (necessary for hydrogen cyanide production), aprA (encodes an extracellular protease), and Pfl01_2211 (required for CLP production, as described above). Genes of interest and flanking DNA regions were PCR amplified, interrupted via the insertion of an antibiotic resistance cassette, and cloned into the suicide vector pSR47S. Mutated alleles cloned into pSR47s were transferred to P. fluorescens Pf0-1 by conjugation as previously described (19), and subsequent allelic exchange generated Pf0-gacA+ strains that do not produce hydrogen cyanide, AprA protease, or the orfamide-like CLP (Table 1). An in vitro antifungal assay revealed that Pf0-gacA+ still inhibits fungal growth in the absence of hcnA (Fig. 1C) and aprA (not shown), indicating that these products are not essential for in vitro activity against Rhizoctonia. However, antifungal activity is reduced in the Pfl01_2211 mutant, highlighting a role for gac-dependent CLP production in antibiosis under our in vitro conditions (Fig. 1C). Although the absence of Pfl01_2211 renders Pf0-gacA+ less able to inhibit R. solani, the altered appearance of the mycelium compared to that seen in the presence of parental Pf0-1 suggests the production of at least one compound that interferes with fungal growth. It is likely that a cocktail of Gac-controlled factors, including as-yet-unidentified compounds, are produced in situ to thwart competitors. The construction of a Gac+ variant of Pf0-1 thus provides a new avenue for the discovery of novel antifungal metabolites by approaches such as the genomisotopic method used successfully with Pf-5 (20).
Effects of GacA on Pf0-1 growth and colonization of soil.
To probe the role of gacA in the environmental fitness of Pf0-1, we assessed the abilities of Pf0-1 and Pf0-gacA+ to persist and compete in soil. Soil persistence assays were done essentially as described previously (19), with an irradiated sandy loam soil of known composition (17). Five-gram soil columns were inoculated with ∼200 CFU of Pf0-1 (marked with SpSmr) or Pf0-gacA+ (marked with Kmr), and viable bacteria were monitored for 30 days. Despite the growth advantage of the gac-deficient parent strain in laboratory medium (Fig. 2A), the growth of the two strains was indistinguishable in soil (Fig. 2B). Similarly, competitive fitness was assayed by using soil columns inoculated with a 1:1 mixture of Pf0-1 and Pf0-gacA+. No significant difference in fitness during head-to-head competition under the conditions tested was observed (Fig. 2C). The role of gacA in survival in natural environments varies greatly, depending on the specific test conditions. Some reports indicate a selective advantage for Gac+ populations, especially in the presence of competing indigenous rhizosphere microbiota (2, 21). Others report a neutral (15, 22, 23) or even negative effect (15), suggesting that the Gac regulon controls traits that are beneficial under specific environmental conditions or within a particular ecological niche. Here, we assessed colonization potential in an irradiated loam soil, thus eliminating factors such as competition with indigenous bacterial populations, predation by nematodes and amoeba, and rhizosphere-dependent factors. Thus, while we see no defects in the ability of gac-deficient Pf0-1 to colonize or persist, we acknowledge that the GacA/GacS system may offer a selective advantage in more complex environments.
An inverse relationship between biofilm formation and dissemination in soil.
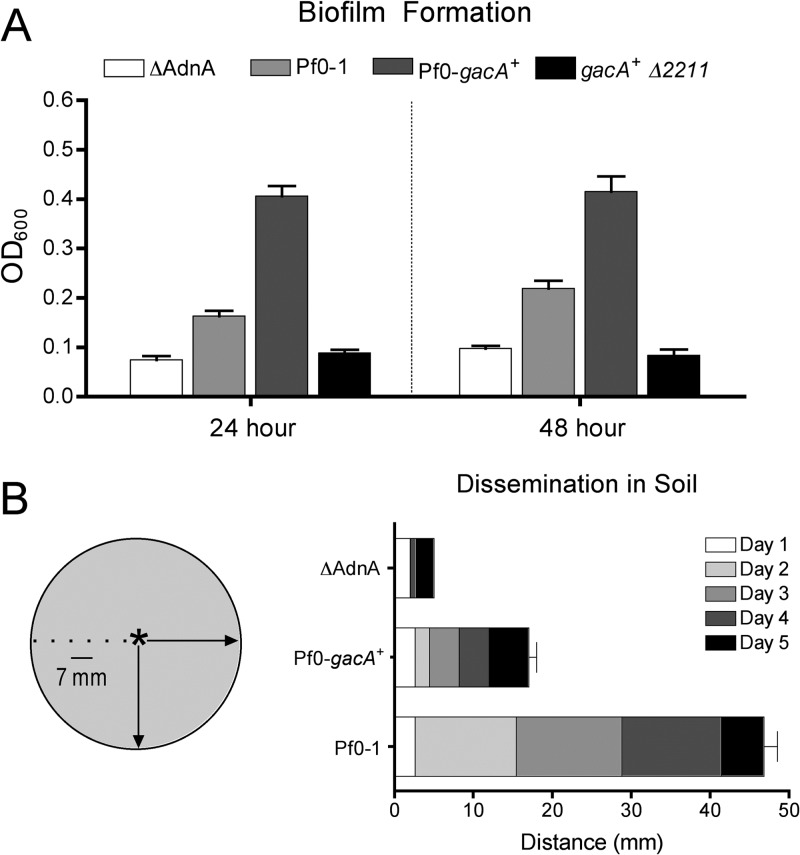
A role for the Gac system in the modulation of biofilm formation has been reported in several Pseudomonas spp. In P. aeruginosa PA14 and P. fluorescens F113, a defect in gacA leads to 10- and 5-fold reductions in biofilm density, respectively (23, 24). P. fluorescens Pf0-1 has been used as a model of biofilm development, and multiple factors have been identified as important for biofilm formation by this strain, including the lap genes, sadB, and pstC, among others (10, 17, 25). The discovery that Pf0-1 is a gacA mutant led us to investigate the potential role of the Gac system in biofilm formation by this strain. Biofilm formation was assessed on borosilicate glass by the method of O'Toole and Kolter (26). Briefly, P. fluorescens strains were grown to mid-log phase, diluted to an optical density at 600 nm (OD600) of 0.025 in Pseudomonas minimal medium (PMM) supplemented with 0.2% glucose, and incubated at 30°C without shaking. Biofilm density was measured at 24 and 48 h by crystal violet staining as described previously (17, 18). Despite the array of gac-independent factors known to play a role in Pf0-1 biofilm formation, our results indicate that the ability of Pf0-1 to form a biofilm is enhanced by the presence of functional gacA (Fig. 3A). As Gac-dependent CLP production is linked to biofilm formation by Pseudomonas strains (27, 28), we additionally assessed biofilm density in the gacA+ derivative harboring the Pfl01_2211 deletion. Indeed, the enhanced-biofilm phenotype exhibited by Pf0-gacA+ is not apparent in a strain deficient in CLP production (Fig. 3A), indicating that the gac-dependent increase in biofilm formation is due, at least in part, to the production of the orfamide-like CLP.
Fig 3.
GacA enhances biofilm formation and decreases soil dissemination. (A) The impact of GacA on biofilm formation was assessed on borosilicate glass by the staining method described by O'Toole and Kolter (21). Biofilm data are reported as the average of 8 to 12 replicates. (B) The motility of Pf0-1 and Pf0-gacA+ was assessed in soil microcosms. Radial dissemination from a central inoculation point was measured daily over a 5-day time course. A nonflagellated mutant lacking ΔAdnA is known to have a defect in dissemination in the soil environment (14) and was thus used as a negative control. Results are the average of four to six independent microcosms for each strain tested, with duplicate samples taken at each time point as an internal replicate.
Our in vitro assays show that in Pf0-1, GacA enhances both motility and biofilm formation. In contrast, others have suggested that phenotypic switching through Gac mutation may allow bacteria to oscillate between motile and biofilm behaviors, perhaps suggesting a transition between exploratory and plant-associated lifestyles. To test this hypothesis for Pf0-1 in a nonlaboratory environment, we assessed the abilities of Pf0-1 and Pf0-gacA+ to disseminate in natural soil. A modification of an assay described previously (29) was developed to measure the dissemination of P. fluorescens strains away from a central inoculation point in soil microcosms. Microcosms were constructed in 150-mm-diameter petri dishes and consisted of 100-g soil samples wetted to ∼50% of their water-holding capacity. Mid-log-phase cultures of Pf0-1, Pf0-gacA+, and a nonflagellated mutant (ΔAdnA) were standardized to an OD600 of 0.1, and 20 μl was dispensed into the center of the microcosm. Dissemination from the inoculation point was measured for 5 days with a sterilized sampling device with eight glass capillary tubes 7 mm apart. The results reveal that the rate of dissemination in soil is inversely correlated with functional gacA (Fig. 3B), despite the fact that Pf0-gacA+ exhibits increased swarming motility in vitro.
Motility is an important trait for rhizosphere colonization, but bacterial dissemination in natural environments is mediated by complex factors both intrinsic (motility, adherence to soil and plant matter, chemotaxis) and external (soil type, packing, moisture content, rainfall). Few studies have addressed a link between motility in vitro and dissemination in soil. Of note, Natsch and colleagues (30) report that a gac-deficient mutant of P. fluorescens CHA0 shows decreased translocation in a vertical soil column and is less abundant than the wild type in percolated water collected from the column after simulated rainfall. However, such results are complicated by the fact that the mutant also exhibits decreased survival under the conditions tested, making it difficult to determine if the difference in translocation is linked to motility per se or merely to survival. Other studies have addressed the ability of Gac+ and Gac− populations to adhere to sand, seeds, and roots (31, 32) but have done little to link this information to dissemination of the organism in natural soil. Our radial dissemination assay, while simplified, offers a link between motility phenotypes exhibited in vitro on agar and translocation in bulk soil. Results highlight the importance of swimming, but not swarming, in dissemination in soil microcosms and suggest that these two forms of motility are regulated differently by the GacS/GacA system. Our results are consistent with and extend those reported for F113, Gac-deficient mutants of which exhibit a hypermotile swimming phenotype in vitro and are more competitive than the wild-type strain in rhizosphere colonization (15). Of note, Martinez-Granero et al. (14) have suggested that the Gac-dependent repression of motility occurs during exponential growth, which is unlikely to be the physiological state of Pf0-1 in the soil dissemination assay. Our data suggest a more complex situation in situ than has been defined in vitro.
GacA-deficient strains can benefit from GacA+ neighbors in mixed populations.
Secreted public-good compounds can sometimes be used by members of a population that do not produce those compounds, for example, in the case of siderophore-negative Pseudomonas mutants that show increased survival in soil when wild-type, siderophore-producing neighbors are also present (33). Many Gac-controlled metabolites are extracellular products with the potential to be shared as public goods in heterogeneous populations. Recent attention has therefore been dedicated to the ecology of mixed Gac populations, and interaction between Gac+ and Gac− cells has, in some cases, proven mutually beneficial (3).
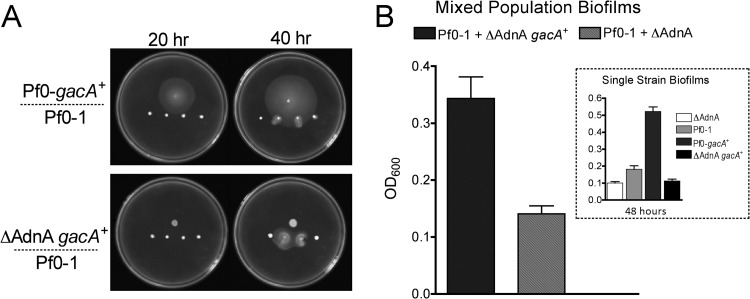
We tested whether exoproducts produced by Pf0-gacA+ may alter the physiology of the gac-deficient parent in mixed populations. As described above, swarming motility is enhanced in Pf0-gacA+ and is dependent on the production of an orfamide-like CLP. Interestingly, the presence of the Pf0-gacA+ strain induces swarming in nearby GacA-deficient Pf0-1 colonies (Fig. 4A). This effect is also seen when Pf0-gacA+ is replaced by a gacA+ strain that lacks functional flagella (Fig. 4A), indicating that the induced motility is due to the diffusion of a secreted product and not some form of physical translocation of gac-deficient cells by the gacA+ partner. We additionally assessed the impact of gac-dependent exoproducts in mixed-population biofilms. The experiment was designed as follows. A strain harboring functional gacA but lacking flagella (Pf0-gacA+ adnA−) was mixed 1:1 with the gac-deficient Pf0-1 parent strain, and biofilm formation was assessed as described above and elsewhere (18, 26). The former strain did not form robust biofilms (Fig. 4B, inset), presumably because of the absence of flagella, which aid in the physical attachment of bacteria to surfaces (18, 34). However, Pf0-gacA+ adnA− is able to enhance biofilm production when mixed with the Pf0-1 parent (Fig. 4B), supporting the hypothesis that gac-dependent exoproducts can alter the physiology of Gac− derivatives in mixed populations.
Fig 4.
Phenotypes of mixed Gac populations. (A) Gac+ strains induce swarming motility in nearby Gac− colonies, even when the Gac+ strain is itself nonmotile (ΔAdnA gacA+, bottom). Motility on PMM solidified with 0.6% agar following 20 (left) and 40 (right) h of incubation at 25°C is shown. (B) Biofilm formation in mixed Gac populations. The ΔAdnA gacA+ mutant strain does not form a biofilm with or without a functional gacA allele (inset). However, the ΔAdnA gacA+ mutant strain enhances biofilm formation when mixed with the gac-deficient Pf0-1 parent.
Much remains to be understood about the ecology of mixed Gac populations; however, our data and those from other groups suggest that there are benefits to maintaining both Gac+ and Gac− variants. Gac+ cells, because of the production of antibiotics and exoproteases, kill or suppress competing organisms (35), are less susceptible to predation by nematodes and amoeba (21, 36), and are better able to form biofilms. Gac-deficient cells enjoy a reduced metabolic load and appear more motile in the soil environment, allowing faster proliferation and increased dissemination when local conditions become unfavorable. Populations showing both phenotypes, and in particular those able to undergo high-frequency phase variation (6), capitalize on the strengths of each strategy.
Conclusions.
Phenotypic variation caused by mutation in gacS or gacA is an important driver of population heterogeneity in natural Pseudomonas populations, allowing the formation of specific subpopulations that may have increased success in the rhizosphere. Here, we confirm that the model fluorescent pseudomonad P. fluorescens Pf0-1 is a gacA-deficient variant. While this organism is well adapted to survival in natural soil, we observed the induction of multiple environmentally relevant phenotypes upon the introduction of a functional gacA allele. Thus, it is clear that the benefits of being Gac+ or Gac− depend strongly on the ecological context, and the high rates of alteration of the GacA/GacS system observed in nature may represent an adaptive mechanism enabling soil pseudomonads to exploit and explore ever-changing environments.
ACKNOWLEDGMENTS
We thank Teresa Kidarsa, Marcella Henkels, and Joyce Loper for kindly providing plasmid pJEL5965 and for making available helpful data prior to its publication.
This work was supported by Agriculture and Food Research Initiative competitive grant 2010-65110-20392 (to S.B.L. and M.W.S.) from the USDA National Institute of Food and Agriculture Microbial Functional Genomics Program and by Agriculture and Food Research Initiative competitive grant 2012-67012-19838 (to S.C.S) from the USDA National Institute of Food and Agriculture fellowship grant program.
Footnotes
Published ahead of print 28 June 2013
REFERENCES
- 1.Bull CT, Duffy B, Voisard C, Defago G, Keel C, Haas D. 2001. Characterization of spontaneous gacS and gacA regulatory mutants of Pseudomonas fluorescens biocontrol strain CHA0. Antonie Van Leeuwenhoek 79:327–336 [DOI] [PubMed] [Google Scholar]
- 2.Chancey ST, Wood DW, Pierson EA, Pierson LS., III 2002. Survival of GacS/GacA mutants of the biological control bacterium Pseudomonas aureofaciens 30-84 in the wheat rhizosphere. Appl. Environ. Microbiol. 68:3308–3314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Driscoll WW, Pepper JW, Pierson LS, III, Pierson EA. 2011. Spontaneous Gac mutants of Pseudomonas biological control strains: cheaters or mutualists? Appl. Environ. Microbiol. 77:7227–7235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duffy BK, Defago G. 2000. Controlling instability in gacS-gacA regulatory genes during inoculant production of Pseudomonas fluorescens biocontrol strains. Appl. Environ. Microbiol. 66:3142–3150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lalaouna D, Fochesato S, Sanchez L, Schmitt-Kopplin P, Haas D, Heulin T, Achouak W. 2012. Phenotypic switching in Pseudomonas brassicacearum involves GacS- and GacA-dependent Rsm small RNAs. Appl. Environ. Microbiol. 78:1658–1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van den Broek D, Bloemberg GV, Lugtenberg B. 2005. The role of phenotypic variation in rhizosphere Pseudomonas bacteria. Environ. Microbiol. 7:1686–1697 [DOI] [PubMed] [Google Scholar]
- 7.Achouak W, Conrod S, Cohen V, Heulin T. 2004. Phenotypic variation of Pseudomonas brassicacearum as a plant root-colonization strategy. Mol. Plant Microbe Interact. 17:872–879 [DOI] [PubMed] [Google Scholar]
- 8.Loper JE, Hassan KA, Mavrodi DV, Davis EW, II, Lim CK, Shaffer BT, Elbourne LD, Stockwell VO, Hartney SL, Breakwell K, Henkels MD, Tetu SG, Rangel LI, Kidarsa TA, Wilson NL, van de Mortel JE, Song C, Blumhagen R, Radune D, Hostetler JB, Brinkac LM, Durkin AS, Kluepfel DA, Wechter WP, Anderson AJ, Kim YC, Pierson LS, III, Pierson EA, Lindow SE, Kobayashi DY, Raaijmakers JM, Weller DM, Thomashow LS, Allen AE, Paulsen IT. 2012. Comparative genomics of plant-associated Pseudomonas spp.: insights into diversity and inheritance of traits involved in multitrophic interactions. PLoS Genet. 8:e1002784. 10.1371/journal.pgen.1002784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Urban A, Neukirchen S, Jaeger KE. 1997. A rapid and efficient method for site-directed mutagenesis using one-step overlap extension PCR. Nucleic Acids Res. 25:2227–2228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monds RD, Newell PD, Gross RH, O'Toole GA. 2007. Phosphate-dependent modulation of c-di-GMP levels regulates Pseudomonas fluorescens Pf0-1 biofilm formation by controlling secretion of the adhesin LapA. Mol. Microbiol. 63:656–679 [DOI] [PubMed] [Google Scholar]
- 11.Lambertsen L, Sternberg C, Molin S. 2004. Mini-Tn7 transposons for site-specific tagging of bacteria with fluorescent proteins. Environ. Microbiol. 6:726–732 [DOI] [PubMed] [Google Scholar]
- 12.Goodier RI, Ahmer BM. 2001. SirA orthologs affect both motility and virulence. J. Bacteriol. 183:2249–2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hassan KA, Johnson A, Shaffer BT, Ren Q, Kidarsa TA, Elbourne LD, Hartney S, Duboy R, Goebel NC, Zabriskie TM, Paulsen IT, Loper JE. 2010. Inactivation of the GacA response regulator in Pseudomonas fluorescens Pf-5 has far-reaching transcriptomic consequences. Environ. Microbiol. 12:899–915 [DOI] [PubMed] [Google Scholar]
- 14.Martinez-Granero F, Navazo A, Barahona E, Redondo-Nieto M, Rivilla R, Martin M. 2012. The Gac-Rsm and SadB signal transduction pathways converge on AlgU to downregulate motility in Pseudomonas fluorescens. PLoS One 7:e31765. 10.1371/journal.pone.0031765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez-Granero F, Rivilla R, Martin M. 2006. Rhizosphere selection of highly motile phenotypic variants of Pseudomonas fluorescens with enhanced competitive colonization ability. Appl. Environ. Microbiol. 72:3429–3434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casaz P, Happel A, Keithan J, Read DL, Strain SR, Levy SB. 2001. The Pseudomonas fluorescens transcription activator AdnA is required for adhesion and motility. Microbiology 147:355–361 [DOI] [PubMed] [Google Scholar]
- 17.Mastropaolo MD, Silby MW, Nicoll JS, Levy SB. 2012. Novel genes involved in Pseudomonas fluorescens Pf0-1 motility and biofilm formation. Appl. Environ. Microbiol. 78:4318–4329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robleto EA, Lopez-Hernandez I, Silby MW, Levy SB. 2003. Genetic analysis of the AdnA regulon in Pseudomonas fluorescens: nonessential role of flagella in adhesion to sand and biofilm formation. J. Bacteriol. 185:453–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silby MW, Levy SB. 2004. Use of in vivo expression technology to identify genes important in growth and survival of Pseudomonas fluorescens Pf0-1 in soil: discovery of expressed sequences with novel genetic organization. J. Bacteriol. 186:7411–7419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gross H, Stockwell VO, Henkels MD, Nowak-Thompson B, Loper JE, Gerwick WH. 2007. The genomisotopic approach: a systematic method to isolate products of orphan biosynthetic gene clusters. Chem. Biol. 14:53–63 [DOI] [PubMed] [Google Scholar]
- 21.Jousset A, Lara E, Wall LG, Valverde C. 2006. Secondary metabolites help biocontrol strain Pseudomonas fluorescens CHA0 to escape protozoan grazing. Appl. Environ. Microbiol. 72:7083–7090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidt-Eisenlohr H, Gast A, Baron C. 2003. Inactivation of gacS does not affect the competitiveness of Pseudomonas chlororaphis in the Arabidopsis thaliana rhizosphere. Appl. Environ. Microbiol. 69:1817–1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barahona E, Navazo A, Yousef-Coronado F, Aguirre de Carcer D, Martinez-Granero F, Espinosa-Urgel M, Martin M, Rivilla R. 2010. Efficient rhizosphere colonization by Pseudomonas fluorescens F113 mutants unable to form biofilms on abiotic surfaces. Environ. Microbiol. 12:3185–3195 [DOI] [PubMed] [Google Scholar]
- 24.Parkins MD, Ceri H, Storey DG. 2001. Pseudomonas aeruginosa GacA, a factor in multihost virulence, is also essential for biofilm formation. Mol. Microbiol. 40:1215–1226 [DOI] [PubMed] [Google Scholar]
- 25.Newell PD, Monds RD, O'Toole GA. 2009. LapD is a bis-(3′,5′)-cyclic dimeric GMP-binding protein that regulates surface attachment by Pseudomonas fluorescens Pf0-1. Proc. Natl. Acad. Sci. U. S. A. 106:3461–3466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Toole GA, Kolter R. 1998. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol. Microbiol. 28:449–461 [DOI] [PubMed] [Google Scholar]
- 27.de Bruijn I, de Kock MJ, Yang M, de Waard P, van Beek TA, Raaijmakers JM. 2007. Genome-based discovery, structure prediction and functional analysis of cyclic lipopeptide antibiotics in Pseudomonas species. Mol. Microbiol. 63:417–428 [DOI] [PubMed] [Google Scholar]
- 28.Raaijmakers JM, de Bruijn I, de Kock MJ. 2006. Cyclic lipopeptide production by plant-associated Pseudomonas spp.: diversity, activity, biosynthesis, and regulation. Mol. Plant Microbe Interact. 19:699–710 [DOI] [PubMed] [Google Scholar]
- 29.Marshall B, Robleto EA, Wetzler R, Kulle P, Casaz P, Levy SB. 2001. The adnA transcriptional factor affects persistence and spread of Pseudomonas fluorescens under natural field conditions. Appl. Environ. Microbiol. 67:852–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Natsch A, Keel C, Pfirter HA, Haas D, Defago G. 1994. Contribution of the global regulator gene gacA to persistence and dissemination of Pseudomonas fluorescens biocontrol strain CHA0 introduced into soil microcosms. Appl. Environ. Microbiol. 60:2553–2560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deflaun MF, Tanzer AS, McAteer AL, Marshall B, Levy SB. 1990. Development of an adhesion assay and characterization of an adhesion-deficient mutant of Pseudomonas fluorescens. Appl. Environ. Microbiol. 56:112–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Espinosa-Urgel M, Salido A, Ramos JL. 2000. Genetic analysis of functions involved in adhesion of Pseudomonas putida to seeds. J. Bacteriol. 182:2363–2369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Weger LA, van Arendonk JJ, Recourt K, van der Hofstad GA, Weisbeek PJ, Lugtenberg B. 1988. Siderophore-mediated uptake of Fe3+ by the plant growth-stimulating Pseudomonas putida strain WCS358 and by other rhizosphere microorganisms. J. Bacteriol. 170:4693–4698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klausen M, Heydorn A, Ragas P, Lambertsen L, Aaes-Jorgensen A, Molin S, Tolker-Nielsen T. 2003. Biofilm formation by Pseudomonas aeruginosa wild type, flagella and type IV pili mutants. Mol. Microbiol. 48:1511–1524 [DOI] [PubMed] [Google Scholar]
- 35.Johansen JE, Binnerup SJ, Lejbolle KB, Mascher F, Sorensen J, Keel C. 2002. Impact of biocontrol strain Pseudomonas fluorescens CHA0 on rhizosphere bacteria isolated from barley with special reference to Cytophaga-like bacteria. J. Appl. Microbiol. 93:1065–1074 [DOI] [PubMed] [Google Scholar]
- 36.Neidig N, Paul RJ, Scheu S, Jousset A. 2011. Secondary metabolites of Pseudomonas fluorescens CHA0 drive complex non-trophic interactions with bacterivorous nematodes. Microb. Ecol. 61:853–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Compeau G, Al-Achi BJ, Platsouka E, Levy SB. 1988. Survival of rifampin-resistant mutants of Pseudomonas fluorescens and Pseudomonas putida in soil systems. Appl. Environ. Microbiol. 54:2432–2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parales RE, Harwood CS. 1993. Construction and use of a new broad-host-range lacZ transcriptional fusion vector, pHRP309, for gram− bacteria. Gene 133:23–30 [DOI] [PubMed] [Google Scholar]
- 39.Matthews M, Roy CR. 2000. Identification and subcellular localization of the Legionella pneumophila IcmX protein: a factor essential for establishment of a replicative organelle in eukaryotic host cells. Infect. Immun. 68:3971–3982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kovach ME, Elzer PH, Hill DS, Robertson GT, Farris MA, Roop RM, II, Peterson KM. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175–176 [DOI] [PubMed] [Google Scholar]