Abstract
Mutations within the cytoplasmic tail (cytotail) of herpes simplex virus 1 (HSV-1) gH were previously observed to suppress the syncytial phenotype of gB cytoplasmic domain mutant A855V in infected cells. Here, we examined the effects of gH cytotail mutations on virus-free cell-cell fusion in transfected cells to exclude the contributions of viral proteins other than gD, gH/gL, and gB. We show that a truncation at residue 832 coupled with the point mutation V831A within the cytotail of gH reduces fusion regardless of whether the wild type (WT) or a syn gB allele is present. We hypothesize that the gH cytotail mutations either reduce activation of gB by gH/gL or suppress the fusogenicity of gB through another, as yet unknown mechanism. The gB cytodomain and the gH cytotail do not interact in vitro, suggesting that mutations in the gH cytotail may instead affect the function of the gH/gL ectodomain. Nevertheless, we cannot exclude the possibility that the gB cytodomain and the gH cytotail interact in the context of full-length membrane-anchored proteins. The observed fusion suppression in transfected cells is less prominent than what was seen in infected cells, and we propose that gH cytotail mutations may additionally suppress syncytium formation in cells infected with syn HSV-1 by acting on other viral proteins, reinforcing the idea that fusion of HSV-infected cells is a complex phenomenon. Although fusion suppression by the gH cytotail mutant in transfected cells was evident when syncytia were visualized and counted, it was not detected by the luciferase assay, highlighting the differences between the two assays.
INTRODUCTION
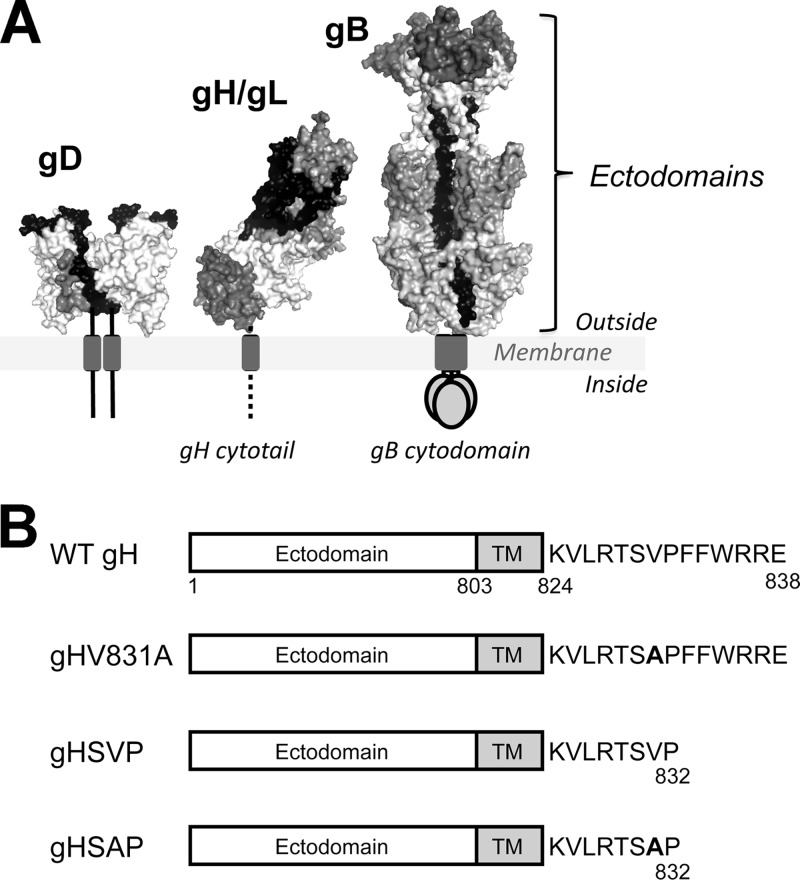
Herpes simplex virus 1 (HSV-1) and HSV-2 are enveloped, double-stranded DNA viruses that cause mucocutaneous lesions, keratitis, and encephalitis (1). HSV enters host cells by fusing its envelope with the host cell membrane, which requires four viral envelope glycoproteins: glycoprotein D (gD), gB, gH, and gL (2–4) (Fig. 1A). In conjunction with a cellular receptor, these proteins also mediate fusion of transfected cells in the absence of other viral proteins (5, 6). Membrane fusion involving these and other glycoproteins also takes place during HSV spread from infected to uninfected cells (7, 8).
Fig 1.
The HSV-1 gH cytodomain may play a role in fusion regulation. (A) The four viral proteins necessary for membrane fusion: gB, gD, gH, and gL. (B) gH mutant constructs used in this work. Point mutation V831A is denoted in boldface.
Fusion is initiated by binding of gD to one of its cellular receptors (9), which triggers a conformational change in gD (9, 10). This conformational change presumably activates the gH/gL heterodimer, which then likely activates gB (11). gB is a class III viral fusogen believed to catalyze membrane fusion by undergoing a conformational change from a prefusion to a postfusion form (12, 13). gH/gL is thought to regulate the fusogenic potential of gB, likely through direct interaction of their ectodomains (11, 14, 15). Consistent with its regulatory role, gH/gL can function in trans (11, 16) and as a soluble complex (11), albeit with reduced efficiency.
The HSV fusion machinery is subject to a multilevel regulatory mechanism. In addition to activation by gD-receptor interaction and by gH/gL, the fusion activity of gB appears to be restricted by its 109-amino-acid cytoplasmic domain (cytodomain). While cell-cell fusion is not typically observed in an HSV infection (17), some clinical isolates, such as HSV-1 strain ANG, induce extensive cell-cell fusion, generating syncytia (18, 19). The syncytial (syn) phenotype of these isolates is often due to a single point mutation in the gB cytodomain (2, 18, 20–25), e.g., the A855V mutation in HSV-1 strain ANG (26). In a virus-free cell-cell fusion system, syn gB mutants result in increased fusion (5, 23, 27), termed hyperfusion (27). The 14-amino-acid cytoplasmic tail (cytotail) of gH may also be involved in fusion, as its deletion or replacement, or an insertion mutation, abolishes fusion without altering surface expression (28, 29). Additionally, mutations within the SVP motif (residues 830 to 832) in the gH cytotail (Fig. 1B) suppress syncytium formation in cells infected with the HSV-1 strain ANG (30, 31). While truncation after residue 832 alone has a mild inhibitory effect, when coupled with a V831A mutation, it inhibits syncytium formation ∼15- to 25-fold (31).
We hypothesized that gH cytotail mutations suppress the syncytial phenotype of HSV-1 strain ANG by reducing the fusogenicity of gB. The ectodomains of gB and gH/gL are thought to interact (14, 15, 32), so this suppression may be the result of direct interaction of the gH cytotail with the gB cytodomain. To evaluate the effect of gH cytotail mutations on fusion while excluding potential contributions of other viral proteins, we analyzed gH mutants in two quantitative virus-free cell-cell fusion assays in transfected cells where cell-cell fusion occurs in the presence of only gB, gD, gH, gL, and a cellular gD receptor. We generated and tested three gH mutants: the gH832 truncation mutant (gHSVP), the truncation mutant coupled with the V831A point mutation (gHSAP), and the single V831A point mutant (gHV831A) (Fig. 1B).
Of these three gH mutants, only the gHSAP mutant reduced gB-mediated fusion by 2- to 3-fold. This effect was observed regardless of whether the wild type (WT) or a syn gB allele was used, suggesting that the gHSAP mutation reduces gB fusogenicity rather than suppressing hyperfusion alone. The observed suppression was not due to altered expression of gH/gL or gB. No interaction was detected between the gB cytodomain and gH cytotail in vitro, suggesting the gHSAP mutant may suppress the fusogenicity of gB through a different mechanism, perhaps by affecting the activation of gB by the ectodomain of gH/gL. However, we cannot exclude the possibility that this interaction occurs in vivo in the context of full-length gB and gH/gL. The extent of hyperfusion suppression in transfected cells is much more modest than in infected cells, and we hypothesize that in addition to reducing the fusion activity of gB, the gHSAP mutation may suppress syncytium formation by the syn gB mutant in infected cells by acting on other viral proteins implicated in cell-cell fusion. Our results reinforce the idea that cell-cell fusion of infected cells is a complex phenomenon.
MATERIALS AND METHODS
Reagents.
1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (PO-PC) and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphate monosodium salt (PO-PA) were purchased from Avanti Polar Lipids, Inc. Streptactin Sepharose resin was purchased from IBA. A C-terminal Strep-tagged gH cytotail peptide (KVLRTSVPFFWRRESAWSHPQFEK) was synthesized by New England BioLabs, and an N-terminal Strep-tagged gH cytotail peptide (SAWSHPQFEKGSGSGKVLRTSVPFFWRRE) was synthesized at the Tufts University Core Facility. ProLong Gold Antifade Reagent, goat serum, and Geneticin were purchased from Invitrogen, and DAPI (4′,6-diamidino-2-phenylindole) stain was a gift of J. Mecsas.
Plasmids.
Plasmids carrying the HSV-1 (KOS) gB, gD, gH, and gL genes in a pCAGGS vector (pPEP98, pPEP99, pPEP100, and pPEP101), as well as plasmids carrying the T7 polymerase gene (pCAGT7) and the firefly luciferase gene (pT7EMCLuc), were gifts of P. G. Spear (33). G. H. Cohen and R. J. Eisenberg provided the pCAGGS vector and a plasmid carrying the HVEM gene (pSC386). A Clontech pAcGFP-C1 vector encoding mCherry in lieu of enhanced green fluorescent protein (GFP) (plasmid pmCherry-C1) was a gift of R. R. Isberg. Plasmid pKH52, encoding residues 801 to 904 of the gB cytodomain with an N-terminal Met and C-terminal His6 tag in a pET24b background, was generated as previously described (34).
Cells and antibodies.
CHO cells were grown in Ham's F-12 medium supplemented with 10% fetal bovine serum (FBS), and C10 cells were grown in Dulbecco's modified Eagle medium (DMEM) supplemented with 5% FBS and 250 μg/ml Geneticin. The C10 cells used in this work are B78H1 derivatives that stably express nectin 1 (35) and were a gift of G. H. Cohen and R. J. Eisenberg. The CHO cells were a gift of J. M. Coffin. Both cell lines were grown at 37°C with 5% CO2.
Goat anti-rabbit fluorescein isothiocyanate (FITC)-conjugated polyclonal antibody (PAb) was purchased from MP Biomedicals. Rabbit polyclonal antibodies R137 (anti-HSV-1 gH/gL) and R68 (anti-HSV-1 gB) were gifts of G. H. Cohen and R. J. Eisenberg.
Construction of gB mutants.
All gB sequences in this work are derived from the HSV-1 KOS strain. The full-length gB gene bearing a syncytial point mutation, A855V, in the C terminus was cloned into the pCAGGS vector, generating plasmid pJLS9, as previously described (27). The rate-of-entry point mutation, V553A (18), was introduced into the ectodomain region of full-length gB using splicing by overlap extension PCR (SOE PCR) (36). This mutation was introduced alone or in conjunction with the A855V mutation. The same forward (5′-GGAGGCGTTCCACCGGTACG-3′; the AgeI site is underlined) and reverse (5′-CCGGCACGCACGTGGAGACGG-3′; the PmlI site is underlined) primers were used to generate the 5′ and 3′ SOE PCR fragments, respectively, for both constructs. The forward primer 5′-CCATCGCCTCGGCCACCGTGGG-3′ and the reverse primer 5′-CACGGTGGCCGAGGCGATGGC-3′ were used to generate the V553A mutation. The resulting PCR products were subcloned into AgeI and PmlI restriction sites in either pPEP98 (generating plasmid pJLS17) or pJLS9 (generating plasmid pJLS18). Both plasmids were verified by sequencing.
Construction of gH mutants.
Three mutations were generated in the full-length HSV-1 KOS gH gene: the point mutation V831A (gHV831A), a C-terminal truncation at residue 832 (gHSVP), and a mutant with both the V831A point mutation and truncation at residue 832 (gHSAP) (31). The gHV831A mutant was made using SOE PCR in pPEP100. The 5′ SOE PCR fragment was generated using forward primer 5′-CAGCCCGTGGCCGCAATTGCG-3′ (the MfeI site is underlined) and reverse primer 5′-CCAAAAAAACGGGGCACTTGTCCGGAG-3′. The 3′ SOE PCR fragment was generated using forward primer 5′-CTCCGGACAAGTGCCCCGTTTTTTTG-3′ and reverse primer 5′-CTGCTAGCTCGAGGCATGCCTGCA-3′ (the SphI site is underlined). The resulting PCR product was subcloned into MfeI and SphI restriction sites of pCAGGS, producing plasmid pJLS14 (gHV831A). The gHSVP and gHSAP mutants were generated by introducing stop codons into pPEP100 and pJL14, respectively. The same forward primer, 5′-CAGCCCGTGGCCGCAATTGCG-3′ (the MfeI site is underlined), was used for both mutants, while the reverse primers were 5′-TAGCTCGAGGCATGCCTACGGGACA-3′ (gHSVP) and 5′-CTCGAGGCATGCTTACGGGGCACTT-3′ (gHSAP). The resulting PCR products were subcloned into MfeI and SphI restriction sites of pPEP100 or pJLS14, producing plasmids pJLS15 (gHSVP) and pJLS16 (gHSAP), respectively. All constructs were verified by sequencing.
Luciferase cell-cell fusion assay.
The effects of gH mutations on cell-cell fusion were assessed using a luciferase reporter gene activation cell-cell fusion assay (33, 37, 38) as previously described (27). Effector cells were transfected with gB (either pPEP98 or pJLS9), gH (pPEP100, pJLS14, pJLS15, or pJLS16), gD (pPEP99), and gL (pPEP101). In each experiment, the light output from cells transfected with only vector (pCAGGS) was subtracted from all samples (performed in triplicate), and the values were expressed as a percentage of fusion with WT gB, gD, gH, and gL. The average values from at least three experiments are reported here.
Syncytium-counting cell-cell fusion assay.
The effects of gH mutations on cell-cell fusion were also assessed by counting syncytia generated from fusion of C10 cells, as previously described (11), with some modifications. C10 cells were seeded in a 24-well plate onto Coverglass for Growth coverslips (Fisher Scientific). The next day, the cells were transfected with 125 ng each of gB (pPEP98 or pJLS9), gH (pPEP100, pJLS14, pJLS15, or pJLS16), and gL (pPEP101) in 500 μl serum-free DMEM with 4 μl Lipofectamine 2000. After 6 h, the medium was changed to DMEM plus 5% FBS containing 250 μg/ml soluble gD306, and fusion was allowed to proceed for 18 h. Immunofluorescence staining was carried out as described previously (15) using R137 diluted 1:1,000 in PBS plus 10% goat serum as the primary antibody and goat anti-rabbit FITC-conjugated PAb diluted 1:500 in PBS plus 10% goat serum as the secondary antibody. Nuclei were stained with 1 μg/ml DAPI during the secondary-antibody incubation. The coverslips were mounted onto slides using 8 μl of ProLong Gold Antifade reagent and allowed to cure overnight in the dark. The slides were stored at 4°C in the dark prior to imaging.
Two replicates for each sample were prepared and imaged with a Nikon A1R confocal microscope at the Tufts CNR Imaging Facility. Ten images per slide, representing 4% of the slide, were collected at ×20 magnification, and the syncytia in each image were quantified. Syncytia were defined as fluorescent membranes containing three or more nuclei, and only nuclei that were more than 85% enclosed by the membrane were counted. Fusion is expressed as the product of the total number of syncytia and the average number of nuclei per syncytium. The data are normalized to fusion with WT gB, gH, and gL.
FACS.
Expression of gH and gB proteins on the surfaces of CHO and C10 cells was assessed using fluorescence-activated cell sorting (FACS) as previously described (27, 39) with some modifications. To test surface expression of gH/gL alone, CHO cells were transfected with 2 μg each of gH (either pPEP100, pJLS14, pJLS15, or pJLS16) and gL (pPEP101) and 667 ng pmCherry-C1 with 2 μl Lipofectamine 2000 in 500 μl Opti-MEM for 5 h. To test expression of gH/gL or gB in the presence of all four glycoproteins, CHO cells were similarly transfected with 1 μg each of gH (either pPEP100, pJLS14, pJLS15, or pJLS16), gL (pPEP101), gB (pPEP98 or pJLS9), and gD (pPEP99) and 667 ng pmCherry-C1. C10 cells were transfected with 2 μg each of gH (either pPEP100, pJLS14, pJLS15, or pJLS16), gL (pPEP101), and gB (pPEP98 or pJLS9) with 8 μl Lipofectamine 2000 in 500 μl serum-free DMEM, and the medium was replaced with DMEM plus 5% FBS after 5 h. C10 cells were processed for FACS analysis 22 h posttransfection.
Immunofluorescence staining was carried out as previously reported (27) using a polyclonal antibody against either HSV-1 gH/gL (R137) or gB (R68) as the primary antibody and a goat anti-rabbit FITC-conjugated secondary antibody. The cells were analyzed using a Becton, Dickinson LSRII machine as previously described (27). The number of positive cells in each sample is reported as a percentage of WT gH/gL or gB expression and represents an average of at least 3 experiments. The mean fluorescence intensities (MFI) of R137-positive and R68-positive cells are also reported as averages of at least 3 experiments.
Protein expression and purification.
The gB cytodomain (residues 801 to 904 with an N-terminal Met) was expressed and purified as a soluble, C-terminally His6-tagged protein in E. coli Rosetta pLysS cells (Novagen) as previously described (27, 34). A truncated form of the gD ectodomain, gD306 (residues 1 to 306), was purified as a soluble protein from baculovirus-infected Sf9 cells as previously described (40).
Preparation of liposomes.
Large (100-nm) unilamellar vesicles (LUVs), composed of PC-PA (1:1), were prepared by extrusion as previously described (27).
Pulldown assay.
Streptactin columns were prepared by loading 1.5 ml resin into a column and washing with 4.5 ml of 20 mM Tris, pH 8.0, 150 mM NaCl, and 1 mM EDTA (gel filtration buffer). Then, 100 nmol of Strep-tagged gH peptide (either N or C terminally tagged) was loaded onto the column and incubated for 10 min, and the column was washed with 3 column volumes (CV) of gel filtration buffer. Fractions were collected in either 1.5-ml or 0.75-ml aliquots. Ten nanomoles of gB cytodomain protein preincubated for 30 min at room temperature with a 30 M excess of PC-PA LUVs was then loaded onto the column, and the column was washed with 4 CV of gel filtration buffer. Fractions were collected in either 1.5- or 0.75-ml aliquots. Protein was eluted with 3 CV of 2.5 mM desthiobiotin in gel filtration buffer, and the eluate was collected in 0.75- or 0.35-ml aliquots. Samples were then analyzed on 12.5% Tris-tricine gels by Coomassie staining.
RESULTS
Choice of gH cytotail mutants.
Mutations within residues 830 to 832 (the SVP motif [30]) in the gH cytotail affected the fusion of cells infected with a syncytial strain of HSV-1 (30, 31). A V831A mutation coupled with truncation after residue 832 had the strongest phenotype in suppressing syncytia generated by the gB syn mutant A855V (gBA855V). Suppression of syncytium formation by the single V831A point mutant has not been evaluated, but we expected it to be as substantial as that of the gHSAP mutant. We generated the original truncation at residue 832 (gHSVP), and this truncation mutant coupled with the V831A point mutation (gHSAP), as well as the single V831A point mutant (gHV831A) (Fig. 1B).
gH cytotail mutations do not suppress fusion in a virus-free cell-cell luciferase fusion assay.
To evaluate the effects of the gH cytotail mutations on gB fusogenicity, the contributions of other viral proteins expressed in infected cells had to be excluded. To achieve this, we tested the effects of these mutations on fusion in a quantitative virus-free cell-cell fusion assay that uses luciferase expression as a readout of cell-cell fusion (33, 37, 38). In this setting, only four viral proteins (gD, gH, gL, and gB) and a cellular gD receptor are required for fusion of transfected cells. This assay has been extensively used to study the cell-cell fusion mechanism.
We tested the effects of gH cytotail mutants on both WT gB fusion and hyperfusion due to the syn gB mutation A855V (gBA855V) found in the HSV-1 ANG strain (31). None of the mutants suppressed fusion in the presence of WT gB, although gHSVP generated a mild hyperfusion phenotype (∼150% fusion relative to WT gH) (Fig. 2A). In contrast, gHSVP suppressed hyperfusion in the presence of gBA855V, reducing it from ∼200% to ∼150%, consistent with the mild (2- to 3-fold) suppression of the HSV-1 ANG syn phenotype observed previously (31). Surprisingly, in the presence of gBA855V, neither the gHSAP mutant nor the gHV831A mutant suppressed the hyperfusogenic phenotype of gBA855V (Fig. 2B), despite the ∼15- to 25-fold suppression of HSV-1 ANG syncytium formation by gHSAP reported previously (31).
Fig 2.
Effects of gH cytotail mutations in the virus-free luciferase cell-cell fusion assay. In all experiments, the transfected glycoproteins are indicated below each bar (BDHL) and are WT unless otherwise indicated. (A) Luciferase production was used to quantify fusion of CHO cells transfected with gB (WT or A855V), gD, gL, and either WT or mutant gH, as indicated. (B) Fusion of CHO cells transfected with either WT gB, the single mutant gBA855V or gBV553A, or the double mutant gBV553A/A855V and WT gD, gL, and either WT gH or gHSAP, as indicated. In each experiment, the vector control was subtracted from each sample (performed in triplicate) and the values were expressed as percentages of the WT. The reported values represent averages of at least three experiments. The error bars indicate standard deviations.
The HSV-1 ANG strain carries an additional gB mutation, V553A, which increases the rate of viral entry (18). Its effect on cell-cell fusion has not been characterized. The presence of the single V553A point mutation in gB did not affect fusion (Fig. 2B). Likewise, the fusion phenotype of the double gB mutant V553A/A855V was similar to that of the single mutant gBA885V, regardless of whether WT gH or the gHSAP mutant was tested (Fig. 2B).
When cell-cell fusion is measured directly, gHSAP suppresses both fusion and hyperfusion.
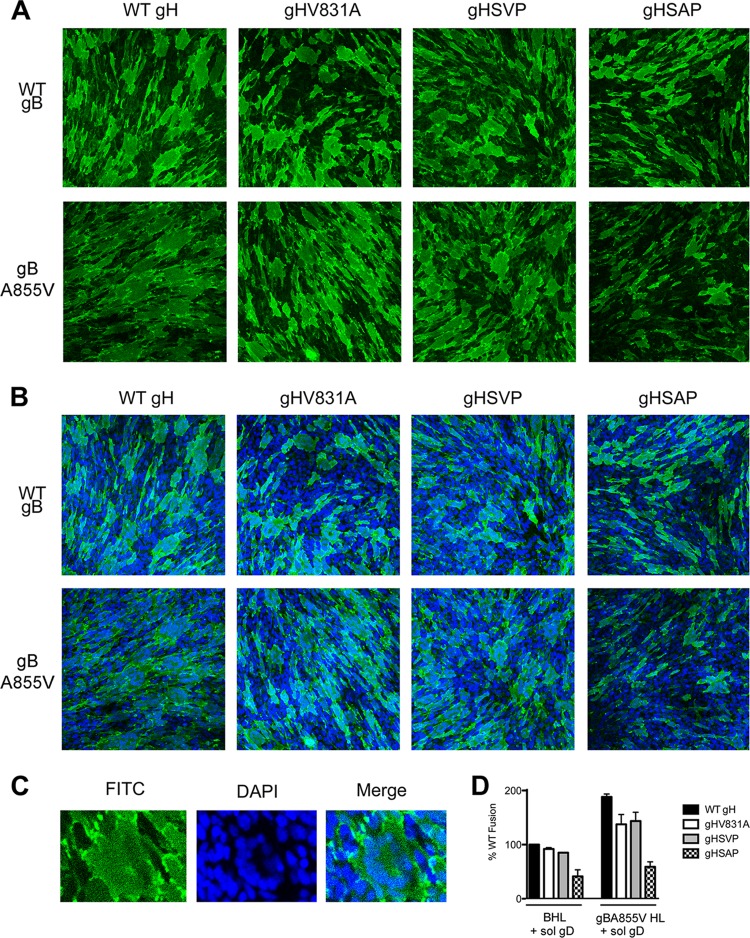
The lack of fusion suppression by the gHSAP mutant in transfected cells was surprising. While our quantitative luciferase assay recapitulates the hyperfusion phenotype of several known syn gB mutants, including gBA855V (27), it measures cell-cell fusion indirectly. In contrast, the fusion suppression phenotypes of this and other gH cytotail mutants were previously assessed by directly counting syncytia generated in infected cells (31). To ensure that the apparent lack of fusion suppression by the gHSAP mutant was not an artifact of the indirect fusion readout in the luciferase assay, we also measured the effects of the gH cytotail mutants on fusion by counting syncytia formed upon fusion of transfected cells. For this assay, C10 cells derived from B78H1 cells and expressing the gD receptor nectin 1 (35) were transfected with gB and gH/gL, and fusion between neighboring cells was triggered with soluble gD306 as described previously (11). We used soluble gD306 instead of additionally transfecting C10 cells with full-length gD because the use of the latter strategy yielded a large amount of fusion in all samples, which could not be accurately counted (data not shown). Therefore, to better quantify any potential differences in the fusion phenotypes of the gH cytotail mutants, we chose to reduce the overall amount of fusion by triggering fusion with soluble gD306. Immunofluorescence with the anti-gH PAb R137 was used to visualize the cell surface, while nuclei were visualized by DAPI staining and cells were imaged by confocal microscopy. Images were collected at ×20 magnification (Fig. 3A and B), and syncytia (Fig. 3C) were quantified in each image (Table 1 and Fig. 3D).
Fig 3.
Effects of gH cytotail mutations in the virus-free syncytium-counting cell-cell fusion assay. Fusion of C10 cells transfected with gB (WT or A855V, as indicated), gL, and gH (WT or a cytotail mutant, as indicated) was triggered with soluble gD306, and the cells were stained with the anti-gH PAb R137 and a FITC-conjugated secondary antibody. Nuclei were stained with DAPI. Ten images for each sample were collected at ×20 magnification on a Nikon A1R confocal microscope and quantified. (A) Representative images for each sample are shown with gH staining (green) alone to highlight the general syncytium morphology. (B) Merged images of both gH (A) and DAPI staining. (C) A typical syncytium, in which the nuclei display a “necklace” pattern. (D) Quantification of syncytia from C10 fusion assay samples. C10 cells were transfected with gB (WT or A855V), gL, and either WT or mutant gH, as indicated, and incubated with soluble gD306. Syncytia were defined as fluorescent membranes containing three or more nuclei, and only nuclei that were more than 85% enclosed by the membrane were counted. Fusion is expressed as the product of the total number of syncytia and the average number of nuclei per syncytium. The data are normalized to fusion with WT gB, gH, gL, and gD. The reported values represent the means of two experiments. The error bars indicate standard deviations.
Table 1.
Quantification of cell-cell fusion by transfected C10 cells triggered with soluble gD306
| Transfected glycoproteina | Total no. of syncytia | Avg. no. of nuclei/ syncytium |
Total no. of fusion events | % of WT fusionb |
|---|---|---|---|---|
| BLH | 265 | 6.4 | 1,696 | 100 |
| 169 | 5.0 | 845 | 100 | |
| 234 | 5.7 | 1,334 | 100 | |
| BL gHV831A | 300 | 5.3 | 1,590 | 93.8 |
| 241 | 5.0 | 1,205 | 90.3 | |
| BL gHSVP | 286 | 5.0 | 1,441 | 85.0 |
| 215 | 5.3 | 1,140 | 85.4 | |
| BL gHSAP | 142 | 3.9 | 554 | 32.7 |
| 111 | 3.8 | 422 | 49.9 | |
| gBA855V LH | 407 | 8.0 | 3,256 | 192.0 |
| 264 | 5.9 | 1,558 | 184.3 | |
| gBA855V L gHV831A | 385 | 5.5 | 2,118 | 124.9 |
| 329 | 6.1 | 2,007 | 150.5 | |
| gBA855V L gHSVP | 446 | 5.9 | 2,631 | 155.2 |
| 304 | 5.8 | 1,763 | 132.2 | |
| gBA855V L gHSAP | 179 | 5.0 | 895 | 52.8 |
| 123 | 4.5 | 554 | 65.5 |
All proteins are WT unless otherwise indicated.
Different amounts of fusion were observed for some sample replicates due to the confluence of cells at the time of transfection. To account for these differences in overall fusion, samples were normalized to the corresponding WT sample prepared on the same date.
As with the luciferase assay, no significant suppression of either WT fusion or gBA855V hyperfusion was observed with either the gHSVP or gHV831A mutant (Table 1 and Fig. 3D). However, when the gHSAP mutant was present, we observed more than 2-fold suppression of fusion with WT gB and more than 3-fold suppression of hyperfusion with gBA855V (Table 1 and Fig. 3D). Reduced fusion was evident in both the reduced number of syncytia and the reduced average number of nuclei per cell (Table 1). Thus, when cell-cell fusion was measured directly, the gHSAP mutant recapitulated the fusion suppression phenotype observed in infected cells, even if to a lesser extent.
C-terminal truncation slightly decreases surface expression of gH in CHO cells, but not in C10 cells.
To determine the effect of surface expression of the gH cytotail mutants on fusion, we measured the expression of WT and mutant gH on the surfaces of transiently transfected CHO or C10 cells using an established FACS protocol (27). Surface levels of gHSAP and gHV831A were indistinguishable from WT gH levels in both cell types, as judged both by the percentage of positive cells and by their mean fluorescence intensity (Fig. 4A to C), but the truncation mutant gHSVP was expressed at only 60% of WT levels in CHO cells (Fig. 4A and C). Lower expression of gHSVP was observed in CHO cells regardless of whether it was coexpressed with gL alone or with gL, gB (WT or A855V), and gD (Fig. 4A and C).
Fig 4.
gH cytotail mutations have a minimal effect on surface expression of gB and gH. (A) Surface expression of gH/gL on CHO cells, as assessed by FACS with the PAb R137. CHO cells were transfected with either 2 μg each of gH (WT or mutant) and WT gL DNA or 1 μg each of gB (WT or A855V), WT gD, WT gL, and either WT or mutant gH DNA, as indicated. For each sample, the number of transfected cells that bound R137 (R137+) was quantified and normalized to the WT, and the average of at least three experiments is shown. (B) Surface expression of gH/gL on C10 cells as assessed by FACS. C10 cells were transfected with gB (WT or gBA855V), WT gL, and either WT or mutant gH, as indicated. (C) Surface expression of gH/gL on CHO or C10 cells was also assessed by measuring the MFI of R137+ cells. Cells were transfected as described for panels A and B with WT gL and either WT gB or gBA855V and either WT gH or a gH cytotail mutant, as indicated. CHO cells were also transfected with WT gD. The MFI of each R137+ population was calculated, and the results of at least three experiments were averaged. (D) Surface expression of gB on CHO cells, as assessed by FACS with the PAb R68. CHO cells were transfected with gB (WT or gBA855V), WT gD, WT gL, and either WT or mutant gH. (E) Surface expression of gB on C10 cells as assessed by FACS. C10 cells were transfected with gB (WT or gBA855V), WT gL, and either WT or mutant gH. (F) Surface expression of gB on CHO or C10 cells as assessed by FACS. The bars are labeled as in panel C. The error bars indicate standard deviations.
gB surface expression is not altered by gH cytotail mutations.
We tested whether the reduced-fusion phenotype of gHSAP was due to altered surface expression of gB by measuring binding of the polyclonal anti-gB antibody R68 to gB (WT or A855V) expressed in transiently transfected CHO or C10 cells in a FACS assay. To mimic the fusion assay conditions, CHO cells were additionally transfected with gD, gL, and gH (either the WT or one of the three mutants), while C10 cells were transfected with gL and gH (WT or mutant). The surface levels of gB, as judged both by the percentage of positive cells and by their mean fluorescence intensity, were similar whether WT or mutant gH was expressed together with gB (WT or A855V), WT gL, or, in CHO cells, gD (Fig. 4D to F), indicating gH cytotail mutations do not exert their effect on fusion by altering surface levels of gB.
The gB cytodomain and the gH cytotail do not interact in vitro.
Mutations in the gH cytotail can potentially influence the fusion activity of gB through a direct interaction between the gB cytodomain and the gH cytotail, which we evaluated by testing binding of the purified gB cytodomain to Strep-tagged gH cytotail peptides (Fig. 5A) in an in vitro pulldown assay.
Fig 5.
The gH cytotail and the gB cytodomain do not interact in vitro. (A) Sequences of the N- and C-terminally Strep-tagged gH cytotail peptides used in the pulldown assays. (B) Coomassie-stained Tris-tricine gel of collected fractions from a Streptactin column loaded with C-terminally Strep-tagged gH peptide. MM, molecular mass standards (kDa); *, purified gB cytodomain, LUVs, and gH-s peptide; gBcyto, gB cytodomain; gH-s, C-terminally tagged gHcyto-Strep peptide. (C) Coomassie-stained Tris-tricine gels of collected fractions from a Streptactin column loaded with N-terminally tagged gH cytotail peptide. gB+LUVs, gB cytodomain-LUV mixture loaded onto the column; s-gH, N-terminally tagged Strep-gHcyto peptide.
In an attempt to induce folding of its native conformation (27), gB cytodomain protein was incubated with LUVs before being applied to a Streptactin column preloaded with gH cytotail peptide. No interaction was observed between the gB cytodomain and the gH cytotail, regardless of whether the Strep tag was attached to the N (Fig. 5B) or the C (Fig. 5C) terminus of the gH cytotail. While no interaction was observed in vitro, we cannot rule out the possibility that the gB cytodomain may interact with the gH cytotail in vivo in the context of full-length gB and gH/gL.
DISCUSSION
In this study, we show that the gH cytotail mutation V831A, when combined with truncation at residue 832 (gHSAP), reduced cell-cell fusion of transfected cells by 2- to 3-fold. This effect is independent of whether the WT or a syn gB allele is used, suggesting that the gHSAP mutation reduces gB fusogenicity rather than suppressing hyperfusion alone.
Reduced fusion in the presence of the gHSAP mutation is not due to diminished surface expression of either gH/gL or gB. Additionally, no interaction was detected between the gB cytodomain and the gH cytotail in vitro, suggesting that the gHSAP mutant may suppress the fusogenicity of gB through a different mechanism. The gH cytotail is 14 amino acids long and is predicted to be unstructured at physiological temperatures (41), so gH cytotail mutations are unlikely to function by disrupting its secondary structure. However, being short, the gH cytotail is positioned close to the membrane and may control the function of the gH/gL ectodomain across the membrane by affecting its conformation through “inside-out” signaling, which occurs in the fusion proteins of some retroviruses (42–44). In this way, the mutations in the gH cytotail may prevent gH from efficiently activating gB, leading to reduced activation of fusion. Alternatively, the gH cytotail could influence the clustering of gH/gL or gB in the membrane, which has been found to restrict syncytium formation by HIV Env (45). Nevertheless, we cannot rule out the possibility that the gB cytodomain and the gH cytotail may interact in vivo in the context of the full-length, membrane-anchored gB and gH/gL. Further research is needed to discriminate between this and other possible mechanisms leading to reduced fusion in the presence of the gHSAP mutation.
Although the gHSAP mutation suppresses fusion of transfected cells, the extent of this suppression, ∼2- to 3-fold, is much more modest than the ∼15- to 25-fold suppression of syncytium formation previously observed in both a transient-transfection/virus complementation system (31) and a significant, albeit not quantified, suppression in cells infected with recombinant viruses (30). Our assays were carried out in the presence of only four viral proteins, gB, gH/gL, and gD, while previous studies (30, 31) were conducted with infected cells in which all viral proteins were present. This suggests that although the gHSAP mutation can reduce the fusion activity of gB under the “minimal” conditions of virus-free cell-cell fusion, this effect is heightened in infected cells, possibly due to its effect on other viral proteins absent from our assays. We propose that the gH cytotail mutation gHSAP may additionally suppress cell-cell fusion in infected cells, i.e., syncytium formation by syn gB, by acting on other viral proteins involved in cell-cell fusion. For example, glycoproteins gE, gI, and gM appear to be necessary for the syncytial phenotype (46–48) due to syn gB, so perhaps the gHSAP mutation reduces syncytia by influencing the function of one or several of these or other proteins. Nonetheless, we cannot exclude the possibility that the differences in fusion suppression observed in our assay versus infected cells may be influenced by higher surface levels of gD receptor in transfected cells (39).
Importantly, we show that the gH cytotail affects the fusion activity of gB during cell-cell fusion even in the absence of such potentially regulatory proteins. An insertion between the TM and the cytotail of gH has been reported to abolish cell-cell fusion (29), suggesting that the gH cytotail is critical for cell-cell fusion. Although the TM anchor and the cytotail of gH are not absolutely required for this process, in their absence, the extent of fusion is greatly reduced (11, 28). Our results further confirm the important function of the gH cytotail in cell-cell fusion.
This study provides a side-by-side comparison with HSV glycoproteins of the two commonly used quantitative virus-free cell-cell fusion assays in transfected cells, the luciferase assay and the syncytium-counting assay, and demonstrates a difference in their dynamic ranges. In the syncytium-counting assay, fusion is measured directly, while in the luciferase assay, fusion is measured indirectly by the luciferase signal. We show here that both assays measure a 2-fold increase in fusion with the known syn mutant gBA855V, suggesting that the two assays are capable of detecting comparable differences in fusion in the upper range of their signal. However, our inability to detect the fusion suppression phenotype of the gHSAP mutant by using the luciferase assay suggests that the assay may be insensitive to differences in fusion below a certain threshold level. These results indicate the importance of caution when interpreting the results of this indirect fusion assay.
Our findings reinforce the idea that cell-cell fusion of infected cells is a complex phenomenon in which viral glycoproteins other than gD, gB, and gH/gL play a significant role. In cells transfected with the four WT glycoproteins, we detect cell-cell fusion that is increased 2- to 3-fold with syn gB (27). In contrast, no cell-cell fusion is observed during a typical infection with HSV-1 encoding WT gB, and extensive syncytia form only in the presence of syn gB. This suggests that cell-cell fusion is negatively regulated during a WT HSV infection and may be positively regulated or derepressed when syn gB is present. We surmise that in transfected cells, there are only a few levels of regulation: the positive effect of the gD-receptor interaction and of gH/gL plus the negative effect of the gB cytodomain. Additional regulatory mechanisms must be present in infected cells to prevent cell-cell fusion under WT conditions and to generate the high levels of fusion observed with syn gB. Our results suggest additional, but as yet not well understood, levels of regulation that govern the fusion of infected cells.
ACKNOWLEDGMENTS
We thank Stephen Kwok and Allen Parmelee at the Tufts Laser Cytometry Core facility for their assistance with FACS experiments and Alenka Lovy at the Tufts CNR Imaging Facility for assistance with confocal microscopy. We also thank Arti Tewari for her assistance with cloning and in vitro pulldown assays, Laurice Jackson for her assistance with FACS and luciferase fusion assay experiments, and Doina Atanasiu for her guidance on syncytium-counting experiments. Additionally, we thank Henry Rogalin for supplying gD306 for cell-cell fusion assays and Julia Murphy for her expertise in microscopy sample preparation and data analysis.
This work was funded by NIH grant 1DP20D001996, by the Burroughs Wellcome Fund Investigators in the Pathogenesis of Infectious Disease Award, and by the Pew Scholar Program in Biomedical Sciences (E.E.H.).
Footnotes
Published ahead of print 10 July 2013
REFERENCES
- 1. Heldwein EE, Krummenacher C. 2008. Entry of herpesviruses into mammalian cells. Cell. Mol. Life Sci. 65:1653–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cai WH, Gu B, Person S. 1988. Role of glycoprotein B of herpes simplex virus type 1 in viral entry and cell fusion. J. Virol. 62:2596–2604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Forrester A, Farrell H, Wilkinson G, Kaye J, Davis-Poynter N, Minson T. 1992. Construction and properties of a mutant of herpes simplex virus type 1 with glycoprotein H coding sequences deleted. J. Virol. 66:341–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ligas MW, Johnson DC. 1988. A herpes simplex virus mutant in which glycoprotein D sequences are replaced by b-galactosidase sequences binds to but is unable to penetrate into cells. J. Virol. 62:1486–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Muggeridge MI. 2000. Characterization of cell-cell fusion mediated by herpes simplex virus 2 glycoproteins gB, gD, gH and gL in transfected cells. J. Gen. Virol. 81:2017–2027 [DOI] [PubMed] [Google Scholar]
- 6. Turner A, Bruun B, Minson T, Browne H. 1998. Glycoproteins gB, gD, and gHgL of herpes simplex virus type 1 are necessary and sufficient to mediate membrane fusion in a Cos cell transfection system. J. Virol. 72:873–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dingwell KS, Brunetti CR, Hendricks RL, Tang Q, Tang M, Rainbow AJ, Johnson DC. 1994. Herpes simplex virus glycoproteins E and I facilitate cell-to-cell spread in vivo and across junctions of cultured cells. J. Virol. 68:834–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Uchida H, Chan J, Shrivastava I, Reinhart B, Grandi P, Glorioso JC, Cohen JB. 2013. Novel mutations in gB and gH circumvent the requirement for known gD receptors in herpes simplex virus 1 entry and cell-to-cell spread. J. Virol. 87:1430–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eisenberg RJ, Atanasiu D, Cairns TM, Gallagher JR, Krummenacher C, Cohen GH. 2012. Herpes virus fusion and entry: a story with many characters. Viruses 4:800–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Krummenacher C, Supekar VM, Whitbeck JC, Lazear E, Connolly SA, Eisenberg RJ, Cohen GH, Wiley DC, Carfi A. 2005. Structure of unliganded HSV gD reveals a mechanism for receptor-mediated activation of virus entry. EMBO J. 24:4144–4153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Atanasiu D, Saw WT, Cohen GH, Eisenberg RJ. 2010. Cascade of events governing cell-cell fusion induced by herpes simplex virus glycoproteins gD, gH/gL, and gB. J. Virol. 84:12292–12299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hannah BP, Heldwein EE, Bender FC, Cohen GH, Eisenberg RJ. 2007. Mutational evidence of internal fusion loops in herpes simplex virus glycoprotein B. J. Virol. 81:4858–4865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Heldwein EE, Lou H, Bender FC, Cohen GH, Eisenberg RJ, Harrison SC. 2006. Crystal structure of glycoprotein B from herpes simplex virus 1. Science 313:217–220 [DOI] [PubMed] [Google Scholar]
- 14. Atanasiu D, Whitbeck JC, Cairns TM, Reilly B, Cohen GH, Eisenberg RJ. 2007. Bimolecular complementation reveals that glycoproteins gB and gH/gL of herpes simplex virus interact with each other during cell fusion. Proc. Natl. Acad. Sci. U. S. A. 104:18718–18723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Atanasiu D, Whitbeck JC, de Leon MP, Lou H, Hannah BP, Cohen GH, Eisenberg RJ. 2010. Bimolecular complementation defines functional regions of Herpes simplex virus gB that are involved with gH/gL as a necessary step leading to cell fusion. J. Virol. 84:3825–3834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vanarsdall AL, Ryckman BJ, Chase MC, Johnson DC. 2008. Human cytomegalovirus glycoproteins gB and gH/gL mediate epithelial cell-cell fusion when expressed either in cis or in trans. J. Virol. 82:11837–11850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ejercito PM, Kieff ED, Roizman B. 1968. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J. Gen. Virol. 2:357–364 [DOI] [PubMed] [Google Scholar]
- 18. Bzik DJ, Fox BA, DeLuca NA, Person S. 1984. Nucleotide sequence of a region of the herpes simplex virus type 1 gB glycoprotein gene: mutations affecting rate of virus entry and cell fusion. Virology 137:185–190 [DOI] [PubMed] [Google Scholar]
- 19. Weise K, Kaerner HC, Glorioso J, Schroder CH. 1987. Replacement of glycoprotein B gene sequences in herpes simplex virus type 1 strain ANG by corresponding sequences of the strain KOS causes changes of plaque morphology and neuropathogenicity. J. Gen. Virol. 68:1909–1919 [DOI] [PubMed] [Google Scholar]
- 20. Baghian A, Huang L, Newman S, Jayachandra S, Kousoulas KG. 1993. Truncation of the carboxy-terminal 28 amino acids of glycoprotein B specified by herpes simplex virus type 1 mutant amb1511-7 causes extensive cell fusion. J. Virol. 67:2396–2401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Diakidi-Kosta A, Michailidou G, Kontogounis G, Sivropoulou A, Arsenakis M. 2003. A single amino acid substitution in the cytoplasmic tail of the glycoprotein B of herpes simplex virus 1 affects both syncytium formation and binding to intracellular heparan sulfate. Virus Res. 93:99–108 [DOI] [PubMed] [Google Scholar]
- 22. Engel JP, Boyer EP, Goodman JL. 1993. Two novel single amino acid syncytial mutations in the carboxy terminus of glycoprotein B of herpes simplex virus type 1 confer a unique pathogenic phenotype. Virology 192:112–120 [DOI] [PubMed] [Google Scholar]
- 23. Foster TP, Melancon JM, Kousoulas KG. 2001. An alpha-helical domain within the carboxyl terminus of herpes simplex virus type 1 (HSV-1) glycoprotein B (gB) is associated with cell fusion and resistance to heparin inhibition of cell fusion. Virology 287:18–29 [DOI] [PubMed] [Google Scholar]
- 24. Gage PJ, Levine M, Glorioso JC. 1993. Syncytium-inducing mutations localize to two discrete regions within the cytoplasmic domain of herpes simplex virus type 1 glycoprotein B. J. Virol. 67:2191–2201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lin E, Spear PG. 2007. Random linker-insertion mutagenesis to identify functional domains of herpes simplex virus type 1 glycoprotein B. Proc. Natl. Acad. Sci. U. S. A. 104:13140–13145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Haanes EJ, Nelson CM, Soule CL, Goodman JL. 1994. The UL45 gene product is required for herpes simplex virus type 1 glycoprotein B-induced fusion. J. Virol. 68:5825–5834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Silverman JL, Greene NG, King DS, Heldwein EE. 2012. Membrane requirement for folding of the herpes simplex virus 1 gB cytodomain suggests a unique mechanism of fusion regulation. J. Virol. 86:8171–8184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Harman A, Browne H, Minson T. 2002. The transmembrane domain and cytoplasmic tail of herpes simplex virus type 1 glycoprotein H play a role in membrane fusion. J. Virol. 76:10708–10716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jackson JO, Lin E, Spear PG, Longnecker R. 2010. Insertion mutations in herpes simplex virus 1 glycoprotein H reduce cell surface expression, slow the rate of cell fusion, or abrogate functions in cell fusion and viral entry. J. Virol. 84:2038–2046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Browne HM, Bruun BC, Minson AC. 1996. Characterization of herpes simplex virus type 1 recombinants with mutations in the cytoplasmic tail of glycoprotein H. J. Gen. Virol. 77:2569–2573 [DOI] [PubMed] [Google Scholar]
- 31. Wilson DW, Davis-Poynter N, Minson AC. 1994. Mutations in the cytoplasmic tail of herpes simplex virus glycoprotein H suppress cell fusion by a syncytial strain. J. Virol. 68:6985–6993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chowdary TK, Cairns TM, Atanasiu D, Cohen GH, Eisenberg RJ, Heldwein EE. 2010. Crystal structure of the conserved herpesvirus fusion regulator complex gH-gL. Nat. Struct. Mol. Biol. 17:882–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pertel PE, Fridberg A, Parish ML, Spear PG. 2001. Cell fusion induced by herpes simplex virus glycoproteins gB, gD, and gH-gL requires a gD receptor but not necessarily heparan sulfate. Virology 279:313–324 [DOI] [PubMed] [Google Scholar]
- 34. Chowdary TK, Heldwein EE. 2010. Syncytial phenotype of C-terminally truncated herpes simplex virus type 1 gB is associated with diminished membrane interactions. J. Virol. 84:4923–4935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Krummenacher C, Baribaud I, Sanzo JF, Cohen GH, Eisenberg RJ. 2002. Effects of herpes simplex virus on structure and function of nectin-1/HveC. J. Virol. 76:2424–2433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Heckman KL, Pease LR. 2007. Gene splicing and mutagenesis by PCR-driven overlap extension. Nat. Protoc. 2:924–932 [DOI] [PubMed] [Google Scholar]
- 37. Connolly SA, Landsburg DJ, Carfi A, Wiley DC, Cohen GH, Eisenberg RJ. 2003. Structure-based mutagenesis of herpes simplex virus glycoprotein D defines three critical regions at the gD-HveA/HVEM binding interface. J. Virol. 77:8127–8140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Okuma K, Nakamura M, Nakano S, Niho Y, Matsuura Y. 1999. Host range of human T-cell leukemia virus type I analyzed by a cell fusion-dependent reporter gene activation assay. Virology 254:235–244 [DOI] [PubMed] [Google Scholar]
- 39. Krummenacher C, Baribaud F, Ponce De Leon M, Baribaud I, Whitbeck JC, Xu R, Cohen GH, Eisenberg RJ. 2004. Comparative usage of herpesvirus entry mediator A and nectin-1 by laboratory strains and clinical isolates of herpes simplex virus. Virology 322:286–299 [DOI] [PubMed] [Google Scholar]
- 40. Bender FC, Whitbeck JC, Lou H, Cohen GH, Eisenberg RJ. 2005. Herpes simplex virus glycoprotein B binds to cell surfaces independently of heparan sulfate and blocks virus entry. J. Virol. 79:11588–11597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kamen DE, Gross ST, Girvin ME, Wilson DW. 2005. Structural basis for the physiological temperature dependence of the association of VP16 with the cytoplasmic tail of herpes simplex virus glycoprotein H. J. Virol. 79:6134–6141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Aguilar HC, Anderson WF, Cannon PM. 2003. Cytoplasmic tail of Moloney murine leukemia virus envelope protein influences the conformation of the extracellular domain: implications for mechanism of action of the R peptide. J. Virol. 77:1281–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Loving R, Li K, Wallin M, Sjoberg M, Garoff H. 2008. R-Peptide cleavage potentiates fusion-controlling isomerization of the intersubunit disulfide in Moloney murine leukemia virus Env. J. Virol. 82:2594–2597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Waning DL, Russell CJ, Jardetzky TS, Lamb RA. 2004. Activation of a paramyxovirus fusion protein is modulated by inside-out signaling from the cytoplasmic tail. Proc. Natl. Acad. Sci. U. S. A. 101:9217–9222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Roy NH, Chan J, Lambele M, Thali M. 2013. Clustering and mobility of HIV-1 Env at viral assembly sites predict its propensity to induce cell-cell fusion. J. Virol. 87:7516–7525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Balan P, Davis-Poynter N, Bell S, Atkinson H, Browne H, Minson T. 1994. An analysis of the in vitro and in vivo phenotypes of mutants of herpes simplex virus type 1 lacking glycoproteins gG, gE, gI or the putative gJ. J. Gen. Virol. 75:1245–1258 [DOI] [PubMed] [Google Scholar]
- 47. Davis-Poynter N, Bell S, Minson T, Browne H. 1994. Analysis of the contribution of herpes simplex virus type 1 membrane proteins to the induction of cell-cell fusion. J. Virol. 68:7586–7590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Johnson DC, Huber MT. 2002. Directed egress of animal viruses promotes cell-to-cell spread. J. Virol. 76:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]