Abstract
The influenza virus neuraminidase H274Y substitution is a highly prevalent amino acid substitution associated with resistance to the most heavily used influenza drug, oseltamivir. Previous structural studies suggest that the group specific 252 residue (Y252 in group 1 and T252 in group 2) might be a key factor underlying H274Y resistance. However, H274Y has only been reported in N1 subtypes, which indicates that there must be additional key residues that determine H274Y resistance. Furthermore, we found that members of NA serotype N3 also possess Y252, raising the key question as to whether or not H274Y resistance may also be possible for some group 2 NAs. Here, we demonstrate that the H274Y substitution results in mild oseltamivir resistance for N3. Comparative structural analysis of N3, N1, and their 274Y variants indicates that the interaction of residue 296 (H in N1 and nonaromatic for other serotypes) with conserved W295 is another important determinant of oseltamivir resistance.
INTRODUCTION
Influenza A virus is a highly contagious pathogen, causing acute respiratory disease with high morbidity and mortality in humans, and it has been responsible for several global pandemics (1, 2). The virus is also a zoonotic pathogen infecting swine, avian, horse, and other species (3). Swine have been recognized as a primary influenza virus mixing vessel because they are susceptible to infection by both human and avian influenza viruses, facilitating re-assortment among different influenza virus strains (4, 5). The outbreak of the 2009 swine-origin pandemic influenza virus A H1N1 (2009-pH1N1) has raised more concern about swine influenza viruses and further illustrated the importance of prevention and control of potential swine influenza outbreaks. A triple reassortment swine H2N3 influenza virus strain A/Swine/Missouri/2124514/2006 (Sw/2124514) has been isolated from diseased swine and found to be experimentally infectious and highly transmissible in swine and ferrets, demonstrating its great potential to cause human disease and even pandemics (6).
Influenza virus neuraminidase (NA) is one of three membrane proteins embedded on the surface on the influenza virus. By hydrolyzing the bond of terminally linked sialic acid in the influenza virus receptor, NA facilitates the release and migration of virions (7). The nine serotypes of NA from influenza A virus (N1 to N9) have been classified into two groups based upon phylogenetic analysis (group 1 includes N1, N4, N5, and N8; group 2 includes N2, N3, N6, N7, and N9) before the divergent NA-like N10 was identified in bats (8–10). Crystal structures of seven out of nine of the influenza A virus serotypes have been solved to date (11), leaving N3 and N7 as the last two structure-unknown influenza virus NA serotypes. With the exception of the N1 from the 2009 H1N1 pandemic, all group 1 NAs have contained a 150-cavity adjacent to their active site, formed by the opening of the 150-loop (residues 147 to 150), and all group 2 NA structures have lacked this cavity (7). High-resolution NA structures have led to the successful design and worldwide approval of two NA drugs: oseltamivir and zanamivir (12, 13). Two more recently developed NA inhibitors, peramivir and laninamivir, are approved for use in Japan and under phase III clinical trials in the United States (14–16). Oseltamivir has become the most widely used NA inhibitor due to a combination of high oral bioavailability (13, 17). However, a lack of similarity to the natural NA ligand, sialic acid, renders oseltamivir vulnerable to drug resistance and the global spread of H274Y (N2 numbering is used throughout the text) oseltamivir-resistant influenza virus strains is a serious issue that should be comprehensively addressed (18–24).
Understanding the mechanisms of oseltamivir resistance is crucial for directing drug administration and next-generation influenza drug design. H274Y oseltamivir resistance has only been reported for group 1 N1 NAs. Previous structural analysis of H5N1 N1 indicates that a bulky Y252 residue, which is conserved in group 1 NAs, is a key residue that may help to explain the group 1 N1 specificity of H274Y resistance (20, 21). In N1, the combination of Y274 and Y252 leaves no room for E276 to rotate toward R224, which is necessary to create a hydrophobic pocket that can well-accommodate the oseltamivir hydrophobic pentyl ether group. A smaller T252 residue is highly conserved in group 2 NA and H274Y oseltamivir resistance has not been previously reported for any group 2 NAs (20, 21). However, we found that Sw/2124514 virus N3, which belongs to group 2 NA, also contains the previously supposed group 1 specific Y252 residue, thus raising a key question as to whether this group 2 NA member has the potential to develop H274Y oseltamivir resistance. Furthermore, the swine origin N3 contains a N344 residue, which is also present in the swine origin 2009 pandemic H1N1 N1. N344 is associated with an increase in NA activity that can help compensate for loss of activity from the H274Y mutation (20, 24).
In the present study we found that Sw/2124514 N3 can develop mild H274Y oseltamivir resistance, and we further determined multiple N3 crystal structures in order to elucidate a more comprehensive molecular mechanism of H274Y resistance. The crystal structures of N3-H274Y and its oseltamivir carboxylate complex contain novel W295 conformations, which allow for a greater range of motion of Y275 and E276. Therefore, W295 was identified as another key residue for H275Y oseltamivir resistance. More importantly, the influence of residue 296 (H for N1 and nonaromatic for most of NAs) was then identified as the key factor underlying the N1 specificity of H274Y resistance. These findings provide valuable insight into the mechanisms of oseltamivir resistance and supply valuable information to help swine origin influenza virus preparedness.
MATERIALS AND METHODS
Reagents.
Methylumbelliferyl-Neu5Ac (MUNANA) was purchased from J&K Scientific, Ltd., and used without further purification. 2,3-Difluoro-Neu5Ac, laninamivir, laninamivir octanoate, zanamivir, oseltamivir, and peramivir were readily synthesized according to the relevant literatures. All products were characterized by their nuclear magnetic resonance or mass spectrometry spectra.
Recombinant NA production.
N3, 09N1, and 57N2 were prepared using the protocol from previous reports (11, 16, 25, 26). N3-H274Y, 09N1-H274Y, and 57N2-H274Y mutants were constructed by overlap PCR (Generay, China). The cDNA encoding amino acid residues 83 to 469 were inserted into the baculovirus transfer vector pFastBac1 (Invitrogen), with a GP67 signal peptide, a 6×His tag, a tetramerizing sequence, and a thrombin cleavage site at the N terminus. Recombinant baculovirus was prepared based on the manufacturer's protocol (Invitrogen). Hi5 suspension cultures were grown in Sf-900 II SFM serum-free medium (Gibco) (28°C and 120 rpm) and transfected with high-titer recombinant baculovirus. After the growth of the transfected Hi5 suspension cultures for 2 days, centrifuged medium were applied to a 5-ml HisTrap FF column (GE Health), which was washed with 20 mM imidazole, and then NA was eluted using 300 mM imidazole. After dialysis, thrombin digestion (Sigma, 3 U of NA/mg; overnight at 4°C), and gel filtration chromatography using a Superdex-200 10/300 GL column (GE Healthcare), NA fractions were analyzed by SDS-PAGE. High-purity NA fractions were pooled and concentrated using a membrane concentrator with a molecular mass cutoff of 10 kDa (Millipore). A buffer of 20 mM Tris-HCl–50 mM NaCl (pH 8.0) was used for gel filtration and protein concentration.
NA enzymatic inhibition assay.
A neuraminidase inhibition assay using MUNANA was performed as described by Potier et al. with modifications (25). Briefly, 10 μl of purified, recombinant N3, 09N1, and 57N2 and their H274Y mutant proteins (10 nM) were mixed with 10 μl of inhibitor separately and incubated for 30 min at room temperature. NA and inhibitors were carefully diluted in fresh phosphate-buffered saline. At least five concentrations of each inhibitor at an appropriate range were used for each repeat. After incubation, 30 μl of 166 μM MUNANA in 33 mM MES (morpholineethanesulfonic acid) and 4 mM CaCl2 (pH 6.0) was added to the solution to start the reaction using a 12-tip pipette (Eppendorf). A positive and a negative control were included in each 12-well lane. After starting the reaction for each lane on the plate, the reaction mixture was immediately loaded on a SpectraMax M5 (Molecular Devices) where fluorescence was quantified over the course of 30 min at an excitation wavelength of 355 nm and an emission wavelength of 460 nm. Single time points were chosen where the positive control produced a relative fluorescence signal of approximately 1,000. At least two replicates were used for each NA-inhibitor pair, and the 50% inhibitory concentrations (IC50s) for each inhibitor were calculated with sigmoidal fitting of the log inhibitor versus inhibition percentage using GraphPad Prism.
Crystallization and inhibitor soaking.
NA crystals were grown using the hanging-drop and sitting-drop vapor diffusion methods. Initial screening was performed using a commercial kit (Hampton Research). N3 crystals were obtained by mixing 1 μl of the concentrated protein at 10 mg/ml in 20 mM Tris (pH 8.0) and 50 mM NaCl with 0.1 M Bis-Tris propane (pH 9.0)–10% (vol/vol) Jeffamine ED-2001 (pH 7.0), the same concentrations used for 57N2 crystallization. N3-H274Y crystals were obtained in the reservoir solution of 1 M bicine (pH 8.5)–15% PEG1500. All crystals were first incubated in mother liquor containing 20 mM inhibitor and then flash-cooled at 100 K. Diffraction data were collected at KEK beamline Ne3A and SSRF beamline BL17U.
Data collection, processing, and structure solution.
Diffraction data were processed and scaled using HKL2000 (26). Data collection and processing statistics are summarized in Table 2. The structures of N3 and N3-H274Y were solved by molecular replacement using Phaser from the CCP4 program suite with the structure of N2 (Protein Data Bank [PDB] identification code [ID] 2BAT) as the search model (27, 28). Initial restrained rigid-body refinement and manual model building were performed using REFMAC5 (29) and COOT (30), respectively. Further rounds of refinement were performed using the phenix.refine program implemented in the PHENIX package with coordinate refinement, isotropic ADP refinement, and bulk solvent modeling (31). The stereochemical quality of the final model was assessed with the program PROCHECK (32).
Table 2.
Crystallographic X-ray diffraction and refinement statisticsa
| Parameter | N3 | N3-laninamivir | N3-oseltamivir | N3-covalent | N3-H274Y | N3-H274Y-zanamivir | N3-H274Y-oseltamivir |
|---|---|---|---|---|---|---|---|
| Data collection statistics | |||||||
| Space group | I4 | I4 | I4 | I4 | I4 | I4 | I4 |
| Unit cell dimensions | |||||||
| a, b, c (Å) | 106.14, 106.14, 64.00 | 105.98, 105.98, 66.80 | 106.22, 106.22, 66.45 | 105.93, 105.93, 64.42 | 106.41, 106.41, 62.25 | 106.33, 106.33, 64.75 | 108.11, 108.11, 62.23 |
| α, β, γ (°) | 90, 90, 90 | 90, 90, 90 | 90, 90, 90 | 90, 90, 90 | 90, 90, 90 | 90, 90, 90 | 90, 90, 90 |
| Resolution (Å) | 50–1.80 (1.86–1.80) | 50–1.70 (1.76–1.70) | 50–2.20 (2.28–2.20) | 50–1.60 (1.66–1.60) | 50–1.60 (1.66–1.60) | 50–1.60 (1.66–1.60) | 50–1.60 (1.66–1.60) |
| Rsym or Rmerge | 0.058 (0.168) | 0.105 (0.406) | 0.155 (0.390) | 0.069 (0.491) | 0.113 (0.426) | 0.061 (0.199) | 0.057 (0.193) |
| Avg I/σI | 19.8 (11.3) | 14.5 (6.0) | 12.2 (3.2) | 25.2 (4.9) | 9.3 (5.2) | 12.2 (9.9) | 15.7 (10.7) |
| Completeness (%) | 99.2 (97.8) | 100.0 (100.0) | 97.4 (87.2) | 98.3 (97.4) | 99.5 (100.0) | 97.6 (96.2) | 99.6(100.0) |
| Redundancy | 7.2 (6.9) | 6.5 (6.6) | 6.9 (5.5) | 6.2 (6.4) | 8.1 (8.1) | 6.3 (6.4) | 7.1 (7.1) |
| Refinement statistics | |||||||
| Resolution (Å) | 33.57–1.80 | 33.51–1.70 | 38.65–2.20 | 33.50–1.60 | 33.65–1.6 | 33.63–1.60 | 34.19–1.60 |
| No. of reflections | 32203 | 39081 | 17390 | 45621 | 44906 | 46217 | 46695 |
| Rwork/Rfree | 0.1472/0.1855 | 0.1367/0.1586 | 0.1516/0.1966 | 0.1495/0.1783 | 0.1513/0.1712 | 0.1533/0.1765 | 0.1377/0.1647 |
| No. of atoms | |||||||
| Protein | 3,139 | 3,141 | 3,123 | 3,121 | 3,119 | 3,143 | 3,137 |
| Ligand/ion | 2 | 26 | 22 | 23 | 1 | 25 | 22 |
| Water | 330 | 445 | 340 | 405 | 422 | 409 | 490 |
| B-factors | |||||||
| Protein | 24.5 | 9.96 | 17.7 | 23.0 | 17.7 | 22.3 | 14.4 |
| Ligand/ion | 46.3 | 19.2 | 29.8 | 33.1 | 23.1 | 29.8 | 25.5 |
| Water | 34.9 | 24.8 | 24.4 | 36.6 | 28.0 | 36.3 | 26.9 |
| RMSD | |||||||
| Bond length (Å) | 0.005 | 0.007 | 0.004 | 0.006 | 0.007 | 0.007 | 0.007 |
| Bond angle (°) | 0.979 | 1.140 | 0.894 | 1.139 | 1.187 | 1.190 | 1.191 |
| Ramachandran plot (%) | |||||||
| Favored regions | 83.9 | 85.1 | 82.7 | 84.2 | 84.5 | 83.6 | 82.6 |
| Additional allowed regions | 15.5 | 14.3 | 16.7 | 15.2 | 15.2 | 16.1 | 15.9 |
| Generously allowed regions | 0.3 | 0.3 | 0.0 | 0.3 | 0.0 | 0.0 | 1.2 |
| Disallowed regions | 0.3 | 0.3 | 0.6 | 0.3 | 0.3 | 0.3 | 0.3 |
Values in parentheses are for the highest-resolution shell. RMSD, root mean square deviation.
PDB accession codes.
All novel crystal structures from the present study have been deposited into the PDB (www.pdb.org) under the following PDB codes: N3-4HZV; N3-oseltamivir, 4HZX; N3-laninamivir, 4HZW; N3-H274Y, 4HZY; and N3-H274Y-oseltamivir, 4HZZ.
RESULTS
N3 has potential to develop mild H274Y oseltamivir resistance.
Recombinant N3 protein was successfully expressed and purified from a baculovirus expression system using a similar method described for N5 (16, 33, 34). Wild-type N3 was inhibited by oseltamivir, zanamivir, laninamivir, and peramivir at the nanomolar level, with IC50s of 0.50, 0.51, 1.31, and 0.42 nM, respectively (Table 1). The laninamivir octanoate prodrug (CS-8958) could also inhibit N3 directly with an IC50 of 350 nM (Table 1). Moreover, 2,3-difluoro-Neu5Ac was able to inhibit N3 at the same level as N2 (35) with an IC50 of 223 nM (95% confidence interval [CI] = 183 to 273 nM).These data indicate that the current NA inhibitors are indeed effective against wild-type N3.
Table 1.
IC50s and 95% CIs for the inhibition of wild-type N3 by classical NA inhibitors and for the inhibition of N1, N2, N3, and their H274Y variants by oseltamivir
| Inhibitor | Concn (nM) |
|
|---|---|---|
| IC50 | 95% CI | |
| Wild-type N3 | ||
| Oseltamivir | 0.50 | 0.42–0.61 |
| Zanamivir | 0.51 | 0.44–0.60 |
| Laninamivir | 1.31 | 1.15–1.50 |
| Peramivir | 0.42 | 0.37–0.47 |
| CS-8958 | 350 | 282–434 |
| N1, N2, and N3 and their H274Y variants | ||
| N3-H274 | 8.31 | 7.70–8.97 |
| 09N1 | 0.67 | 0.61–0.73 |
| 09N1- H274Y | 779 | 714–850 |
| 57N2 | 0.61 | 0.48–0.77 |
| 57N2-H274Y | 1.68 | 1.28–2.17 |
Analysis of the N3 sequence indicates that N3 has the potential to develop the highly prevalent H274Y oseltamivir resistance because it possesses Y252, which is usually only found in group 1 NAs. To investigate whether it is possible for a group 2 NA to develop H274Y oseltamivir resistance, N3-H274Y was also produced in our baculovirus system and purified to homogeneity. N3-H274Y displayed mild oseltamivir resistance, with an IC50 of 8.31 nM, which is 16-fold higher than that of the wild type. Inhibition of N3-H274Y by the covalent inhibitor 2,3-difluoro-Neu5Ac was slightly lower than for wild-type N3, with an IC50 of 480 nM (95% CI = 329 to 701 nM). Additionally, oseltamivir inhibition was also tested using N1 from 2009-pH1N1 (09N1), 09N1-H274Y, N2 from 1957 pandemic H2N2 (57N2), and 57N2-H274Y, with IC50s of 0.67, 779, 0.61, and 1.68 nM, respectively (Table 1). In accordance with previously reports, 09N1-H274Y shows full-blown oseltamivir resistance, and 57N2-H274Y is still highly vulnerable to oseltamivir.
N3 is a typical group 2 NA.
To investigate the structure of the second to last structure-unknown influenza A virus NA, N3 was crystallized, and its structure was solved at a resolution of 1.8 Å (Table 2). The overall structure of N3 is highly conserved relative with the other structure-solved influenza virus NAs with a typical “box-shaped” tetramer of four identical monomers, each containing six four-stranded antiparallel β-sheets in a propeller-like arrangement (Fig. 1A). Detailed analysis shows that N3 has no 150-cavity in its active site (Fig. 1B) like all other group 2 NA structures solved to date. The 150-loop (residues 147 to 152, based on N2 numbering) sequence of N3 is GTIKDR, which happens to be the same as 09N1 (PDB ID 3NSS). The 150-loop sequence of VN04N1 (PDB ID 2HTY), a typical group 1 NA, and that of N9 (PDB ID 7NN9), a typical group 2 NA, are GTVKDR and GTIHDR, respectively. Superposition of Cα atoms of the N3 150-loop with those of 09N1, VN04N1, and N9 gives average root mean square deviation (RMSD) values of 0.200, 2.259, and 1.404 Å, respectively.
Fig 1.
Crystal structure of the N3 tetramer and analysis of its active site. (A) N3 tetramer in schematic representation, viewed from above the viral surface. (B) Molecular surface of the active site of N3 (yellow); N3 has no 150-cavity. (C) Molecular surface of the active site of H5N1 N1 (red) (PDB ID 2HTY); typical group 1 N1 has a 150-cavity. (D) The covalent complex formed between 3-fluoro-Neu5Ac and N3 Tyr406. The lack of density for the C-2 fluoride leaving group indicates that it has departed, allowing for the formation of the Tyr406-inhibitor covalent bond. (E) Laninamivir bound to the N3 active site with a typical binding mode. (F) Molecular surface of the active site of N2 (blue) (PDB ID 2BAT); group 2 N2 also has no 150-cavity.
We were also able to obtain a covalent complex between N3-Tyr406 and 3-fluoro-Neu5Ac by soaking N3 crystals with 2,3-difluoro-Neu5Ac. This demonstrates that N3 also operates via a nucleophilic mechanism, just like influenza virus N2, and NAs from other microorganisms (35–37). We also soaked N3 crystals with laninamivir (4-guanidino-7-methoxy-Neu5Ac2en) and solved the complex structure at a resolution of 1.7 Å. Like all other zanamivir and laninamivir complex structures, the laninamivir C4-guanidino is buried beneath the N3 150-loop, where it hydrogen bonds with E119, D151, W178, and E227 (Fig. 1C).
N3 contains three loops with unique conformations.
Upon close comparison of the N3 structure to the other structure-solved NAs, three special loops in N3 were discovered: the 270-loop (residues 267 to 276), the 380-loop (residues 380 to 390), and the 430-loop (residues 429 to 433) (Fig. 2).
Fig 2.
Comparison of loops in NA from different influenza viruses. (A) Superposition of NA monomers with an emphasis on the 270-loop, 380-loop, and 430-loop. NAs are differentiated by color: 18N1, green; VN04N1, cyan; 09N1, red, N4, light magenta; N5, light pink; N8, salmon; Tokyo N2, white; N6, orange; N9, light blue; and N3, yellow. (B) Stereo view of the 270-loop. All group 1 members are on the left, while group 2 members are on the right. N3 is in the middle with a longer 270-loop. (C) Ribbon representation of the 380-loop. The 380-loop of N3 is also unique compared to all other NAs. (D) Stereo image of the 430-loop. N3 also has a relative flexible 430-loop.
N3 has a longer, unique 270-loop than other structure-solved NAs due to an additional lysine residue, K271′ (Fig. 2B and Fig. 3A). The main-chain carbonyl at residue 273 of all structure known group 1 members hydrogen bonds to Gln249 and Tyr252, and the 273 main-chain nitrogen hydrogen bonds with residue 270 (Fig. 3B). In contrast, residues 272 and 273 of typical group 2 NA form hydrogen bonds with residues 314 and 316; thus, the 270-loop of group 2 NA is pulled toward the opposite direction relative to group 1 members (Fig. 3C). Unexpectedly, Gln273 in N3 forms hydrogen bonds with Tyr252 and K270, which is only observed in group 1 NA and yet also has group 2-specific interaction with Y316 (Fig. 3D). K271′ of N3 forms hydrogen bonds with the carboxyl oxygen of T314 (Fig. 3D and Table 3). Therefore, N3 possesses characteristics of both group 1 and group 2 NA, as well as unique characteristics, regarding the 270-loop.
Fig 3.
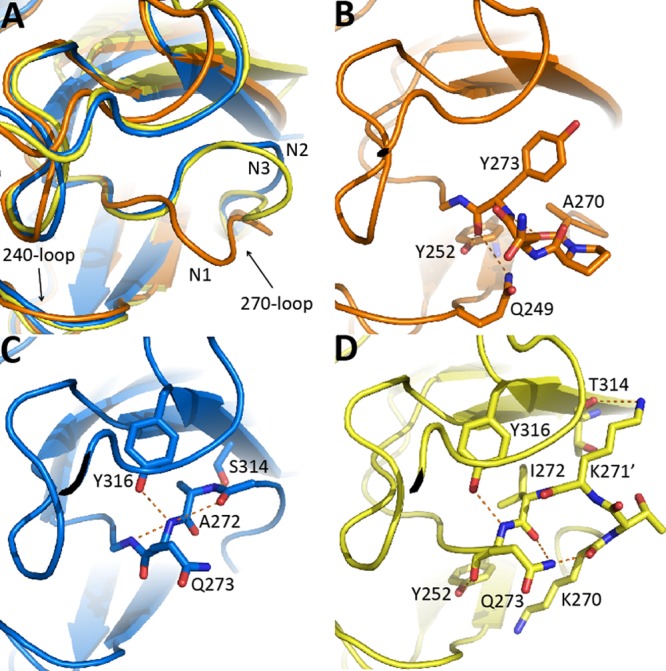
Comparison of the 270-loop in different NAs. (A) Superimposed 270-loops of VN04N1 (orange), N2 (blue), and N3 (yellow). The 270-loop of N1 is much closer to the 240-loop. (B) Y273 in VN04N1 forms hydrogen bonds with Y252, Q249, and A270, which produces a tight turn in the 270-loop. (C) Q273 and A272 in N2 hydrogen bond to Y316 and S314, respectively, thus pulling the 270-loop in N2 to turn in the opposite direction of the N1 loop. (D) Q273 in N3 hydrogen bonds with Y252, K270, Y316, and I272 and K271′ hydrogen bonds with T314.
Table 3.
Hydrogen bonds of the 270-loop
| Amino acid | Hydrogen bond(s), distance(s)a at position: |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 267 | 268 | 269 | 270 | 272 | 273 | 274 | 275 | 276 | |
| 18N1 (3BEQ) | Arg219, 3.47; Ser251, 2.67 | Tyr252, 2.83; Ala270, 3.15 | Tyr312, 2.94 | Gln249, 3.12; Tyr273, 3.08 | Gln249, 2.84; His296, 2.77 | Gln249, 3.47; Tyr252, 2.64 | Pro245, 3.04; Asn294, 3.00; Tyr316, 3.28 | Thr242, 2.69; Trp302, 2.70 | Arg224, 2.88, 3.21; Arg292, 2.91, 2.95 |
| VN04N1 (2HTY) | Ser251,2.46 | Tyr252, 2.82; Ala270, 3.12 | Tyr312, 2.85 | Gln249, 3.24; Tyr273, 2.91 | Gln249, 2.43, 3.33; His296, 3.22, 3.41 | Tyr252, 2.67; Gln249, 3.34 | Pro245, 3.15; Asn294, 3.17; Tyr316, 3.46 | Thr242, 2.63; Trp302, 2.77 | Arg224, 2.77, 2.80; Arg292, 2.80, 2.93 |
| 09N1 (3NSS) | Ser251, 2.69 | Ala270, 3.14 | Tyr312, 2.76 | Gln249, 3.27; Tyr273, 3.19 | Gln249, 2.74; His296, 2.83 | Tyr252, 2.63; Ala270, 3.19 | Pro245, 3.10; Asn294, 3.03; Tyr316, 3.38 | Thr242, 2.68; Trp302, 2.74 | Arg224, 2.77, 3.22; Arg292, 2.84, 2.84 |
| N4 (2HTV) | Tyr252, 2.86; Ala270, 3.35 | Tyr312, 3.11 | Gly272, 3.20; Phe272, 2.78 | Gln249, 2.48; Arg296, 3.15, 3.43 | Gln249, 3.44; Tyr252, 2.69; Ala270, 2.78 | Pro245, 3.07; Asn294, 3.03; Tyr316, 3.49 | Tyr252, 3.48; Thr242, 2.74 | Arg224, 3.09, 3.17; Arg292, 2.93, 3.06 | |
| N5 (3SAL) | Ser251, 2.72 | Tyr252, 2.79; Phe270, 3.44 | Tyr312, 2.77 | Gln249, 3.21; Gly273, 2.96 | Gln249, 2.81 | Gln249, 3.44; Phe270, 2.96 | Pro245, 3.18; Asn294, 3.06; Tyr316, 3.50 | Thr242, 2.70 | Arg224, 2.68, 2.98; Arg292, 2.81, 2.88 |
| N8 (2HT5) | Arg252, 2.78, 3.43; Tyr312, 3.37 | Tyr252, 2.81 | Tyr312, 2.91 | Gln249, 2.83; Gly273, 2.86 | Gln249, 2.62; Tyr252, 2.51; Phe270, 2.86 | Pro245, 3.19; Gly294, 3.02 | Thr242, 2.63 | Arg224, 2.74, 2.75; Arg292, 2.85, 2.88 | |
| N2 (1NN2) | Thr252, 3.20 | Ser314, 2.82 | Ser314, 2.79 | Lys296, 2.87; Tyr316, 3.05 | Ala246, 2.53; Gly248, 2.89 | Arg224, 2.94; Arg292, 2.83, 2.96 | |||
| N3 (4HZV) | Tyr251, 2.76 | Ser248, 2.92; Ala249, 2.61; Gln273, 2.92 | Gln273, 3.42 | Tyr252, 2.72; Lys269, 2.92; Ile272, 3.42; Lys295, 2.93; Tyr316, 3.04 | Tyr252, 3.06; Glu276, 3.39 | Thr241, 2.96 | Arg224, 2.59, 2.93; His274, 3.39; Arg292, 2.78, 2.90 | ||
| N6 (1V0Z) | Thr252, 2.88 | Ser314, 2.71 | Ser314, 2.97 | Arg249, 3.05; Tyr316, 3.04 | Glu276, 2.95 | Thr242, 2.71 | Arg224, 2.80, 2.86; His274, 2.95; Arg292, 2.74, 2.92 | ||
| N9 (7NN9) | Thr252, 2.96 | Ser314, 2.62 | Ser314, 3.08 | Tyr316, 2.86 | Ser245, 3.01; Asn294, 3.37 | Thr242, 2.67 | Arg224, 2.75, 3.21; Arg292, 2.86, 2.89 | ||
All hydrogen bond distances refer to molecule A of each structure.
The 380-loop of N3 is also unique relative to other structure known NAs (Fig. 2C and Fig. 4). Detailed analysis indicates that all of the known influenza virus A NA structures, including N3, have hydrogen bonds between residues in the 380-loop and residues in the 320-loop (residues 315 to 320) and 330-loop (Fig. 4 and Table 4). Relative to N3, all other structure known NAs have additional hydrogen bonds with the 320- and 330-loops (Fig. 4, Table 4, underlined text). This lack of hydrogen bonds with the N3 380-loop is likely a major factor for the unique 380-loop conformation (Fig. 4).
Fig 4.

Comparison of the N3 380-loop in with distinct NA structures. (A) Location of the 380-loop, 320-loop, and 330-loop in VN04N1 (orange), N2 (blue), and N3 (yellow). The distance between the 380-loop and 320-loop in N3 is the greatest. (B to D) Residues 319 and 320 form hydrogen bonds with the 380-loop in N1, N2, and N3. The 380-loop in N1 and N2 forms more hydrogen bonds with the 320-loop and adjacent residues relative to N3. Residues that take part in hydrogen bonds between and among the 380-loop, 320-loop, and 330-loop are labeled. Residues that are marked with an asterisk (*) indicate that they only take part in hydrogen bonds via their main chain oxygen or nitrogen.
Table 4.
Hydrogen bonds of the 380-loop with residues outside of the 380-loop
| Amino acid | Hydrogen bond(s), distance(s)a at position: |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 381 | 382 | 383 | 384 | 386 | 387 | 388 | 389 | 390 | |
| 18N1 (3BEQ) | Asp379, 3.14 | Asp379, 3.09; Ser319, 2.91 | Cys318, 3.29; Asn358, 2.95 | Asn358, 3.05 | Gly333, 2.80 | Gly320, 2.91 | |||
| N4 (2HTV) | Ser319, 2.78; Asp379, 2.70, 3.41 | Asp358, 2.98 | Gly334, 3.32 | Gly320, 2.61 | |||||
| N5 (3SAL) | Ser359, 2.90 | Ile379, 3.00; Ala319, 3.04 | Asn358, 2.90 | Gly320, 2.74 | Gly320, 2.74; Asp330, 2.84; Phe333, 2.80; Ile391, 3.28 | ||||
| N8 (2HT5) | Asp359, 2.77 | Ala319, 2.70; Ile379, 3.24 | Thr358, 2.92 | Gly335, 3.29 | Gly320, 2.59 | Gly320, 2.59 Gly320, 2.63; Phe331′, 3.56 | |||
| N2 (1NN2) | Asp359, 3.04 | Ser319, 2.88; Val379, 2.96 | Asn358, 2.81 | Asn358, 3.31 | Cys318, 3.03; Ser335, 3.00; Asn334, 2.80; Ser333, 3.49 | Gly320, 2.88 | Asp330, 2.64; Ser333, 2.68; Gln391, 3.33 | ||
| N3 (4HZV) | Asp359, 2.72 | Ser319, 2.90; Val379, 2.98 | Asp358, 2.82 | Lys320, 3.19 | Ser392, 3.05 | ||||
| N6 (1V0Z) | Gly357, 2.85; Glu358, 3.02 | Ser319, 3.03; Cys318, 3.20; Val379, 3.11 | Lys288, 2.82 | Gly334, 2.90; Lys320, 3.20 | Lys320, 3.05 | Ser319, 2.74, 3.09 | |||
| N9 (7NN9) | Gly357, 2.80 | Cys318, 3.24; Ser319, 2.89; Val379, 3.16 | Gln315, 3.22; Cys318, 3.30 | Gly335, 2.77 | Ser319, 2.95, 3.17 | Pro331, 2.82 | |||
There were no bonds at positions 380 and 385. Underlined entries indicate bonds with 320- and 330-loops. All hydrogen bond distances refer to molecule A of each structure.
In addition, N3 also possesses a unique 430-loop where the N3 residue K433 interacts with two water molecules, whereas all other NA form hydrogen bonds between E433 and G429 (Fig. 2D and Fig. 5). This results in a 430-loop in N3 that is farther away from the 150-loop; however, P431 remains in the same relative position.
Fig 5.
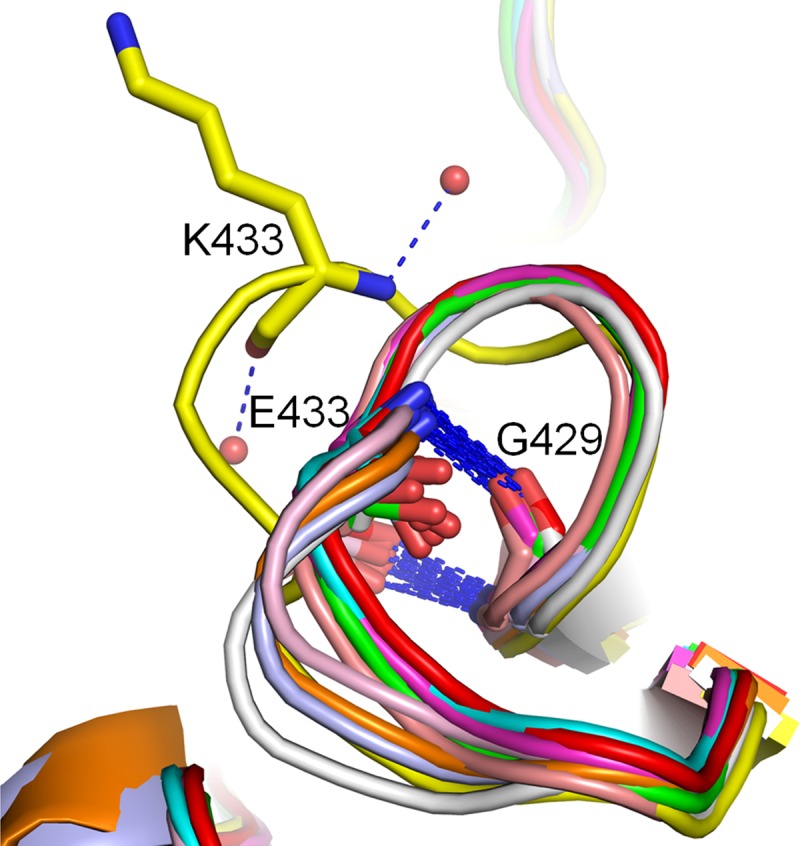
N3 contains a special 430-loop. K433 in N3 (yellow) coordinates with two water molecules, while E433 in all other structure-known NAs form a hydrogen bond with G429.
W295 and residue 296 are crucial determinants of H274Y oseltamivir resistance.
To explore the structural basis accounting for the oseltamivir resistance of N3-H274Y, structures of uncomplexed N3-H274Y, as well as N3-H274Y complexed with osletamivir and zanamivir, were solved at a resolution of 1.6 Å. Surprisingly, Y274 of uncomplexed N3-H274Y adopted two conformations: a conformation similar to other NAs where Y274 points toward to E276 and a novel conformation where Y274 points toward to the opposite direction (Fig. 6A). In the oseltamivir complex structure, Y274 is only oriented away from E276 and therefore N3-H274Y only shows mild oseltamivir resistance. W295 also adopts to a novel conformation where it is rotated by about 90° relative to other NA structures (Fig. 6B). However, in the N3-H274Y-zanamivir complex structure, Y274 and W295 are still arranged in the most common conformations, where Y274 points toward E276 (Fig. 6C).
Fig 6.

N3-H274Y contains novel Y274 and W295 conformations. (A) Y274 in N3-H274Y is flexible and adopts two conformations, one pointing toward E276, and another oriented away from E276. (B) Y274 in N3-H274Y only adopts the conformation away from E276 when binding oseltamivir, and W295 overturns to accommodate the novel Y274 conformation. (C) Y274 and W295 in the N3-H274-zanamivir complex still adopt the typical conformations found in other NAs.
Detailed structural analysis was used to investigate why N1-H274Y oseltamivir resistance is much more extreme than that of N3-H274Y. The hydrogen bond network among the 240-loop (residues 243 to 251), 270-loop (residues 267 to 276), and 290-loop (residues 290 to 301) is likely an important factor for H274Y oseltamivir resistance. In the N1-oseltamivir (PDB ID: 2HU4) and N1-H274Y-oseltamivir complex structures (PDB ID 3CL0), there is a strong hydrogen bond network consisting of H296-N272, Y273-A270, A270-Q249, A270-L268, L268-Y252, Y252-Y273, Y252-Q249, Y252-A250, and S246-N294, which limits the free rotation of residues 274 and W295 (Fig. 7A and B). Additionally, W295 and H296 in N1 form a π-π interaction which further stabilizes the conformation of W295. (Fig. 7A and B). In the N3-oseltamivir and N1-H274Y-oseltamivir complex structures, the hydrogen bond network consists of fewer hydrogen bonds than that of native N1. Furthermore, N3 K296 cannot form a π-π interaction with W295 and thus Y274 and W295 adopt novel conformations (Fig. 7C and D). This ability of W295 to adopt this novel conformation in N3 creates more space for Y274 and E276 to accommodate the oseltamivir pentyl ether group, and therefore N3-H274Y resistance is much more mild relative to N1.
Fig 7.

Group specific interactions of the 240-, 270-, and 290-loops. (A and B) VN04N1-oseltamivir (orange) and VN04N1-H274Y-oseltamivir (cyan) contain a strong hydrogen bond network among the 240-, 270-, and 290-loops, and W295 forms a π-π interaction with H296, which is only conserved in N1. (C and D) N3-oseltamivir (yellow) and N3-H274Y-oseltamivir (green) contain a weaker hydrogen bond network among these three loops, and no interaction can be formed between W295 and K296.
DISCUSSION
Comprehensive structural and functional analysis of influenza virus NA is crucial for the prevention and control of influenza virus since NA is currently the most successful anti-influenza drug target. Seven out of nine influenza A virus NA structures have been solved since 1983, leaving N3 and N7 as the last remaining unknown NA structures to be solved. Influenza virus strains containing N3 have caused several flu outbreaks in swine and birds. However, research on N3 has been very limited. Here, we not only solved the N3 structure and showed that it is a typical group 2 NA in overall structure, but we also found more insights into the molecular mechanisms of oseltamivir resistance.
Detailed structural analysis shows that three loops in N3 are special compared to other structure-known NAs: the 270-loop, 380-loop, and 430-loop. The 270-loop conformation has implications for oseltamivir resistance since the rotation of E276 toward R224 is crucial for high-avidity oseltamivir binding. The 380-loop is close to the calcium binding site and several antibody binding sites; hence, its unique conformation in N3 may have some functional implications. Our previous investigation on 09N1 illustrated that the 430-loop has some interactions with the 150-loop and may contribute to the formation or lack of the 150-cavity. The relatively more open 430-loop of N3 may have some effects on 150-loop flexibility.
Surprisingly, N3 possesses the group 1 specific Y252 residue, which is important for the N1 specificity of H274Y oseltamivir resistance. Most group 2 NAs contain a small T252 residue, which allows more freedom for E276 rotation. However, we found that N3 contains Y252 and that N3-H274Y does possess mild oseltamivir resistance. Novel Y274 and W295 conformations were observed in N3-H274Y and N3-H274Y-oseltamivir structures but not in the N3-H274Y-zanamivir complex. Y274 is still able to move away from E276 when N3-H274Y binds oseltamivir, leaving enough space for the free orientation of E276. This explains why N3-H274Y oseltamivir resistance is less pronounced than that of N1 and indicates that there are additional factors aside from residue 252 that are critical for H274Y oseltamivir resistance.
Structural analysis of N3-H274Y and its oseltamivir complex revealed W295 and the N1 unique, aromatic H296 residue as two additional amino acid residues that are critical for H274Y oseltamivir resistance. These residues are both located near N294, which is responsible for the prevalent N294S form of oseltamivir resistance. This further emphasizes the importance of interactions between the 270-loop and 290-loop on E276 mobility. The hydrogen bond network between not only the 270-loop and 290-loop, but also the 240-loop, was further identified as another important factor critical for H274Y resistance.
In conclusion, the structure of the N3 NA of influenza A virus was solved, bringing us closer to a comprehensive understanding of the most successful influenza drug target. Our results contribute to the comprehensive understanding of influenza virus NA structural diversity and provide insight into the molecular mechanisms of oseltamivir resistance.
ACKNOWLEDGMENTS
This study was supported by the National 973 Project (grant 2011CB504703) and the National Natural Science Foundation of China (NSFC; grant 81021003), and G.F.G. is a leading principal investigator of the NSFC Innovative Research Group. C.J.V. is a recipient of the Chinese Academy of Sciences Young International Scientist Research Fellowship (2011Y2SA01) and the NSFC Research Fund for Young International Scientists (31150110147).
The assistance of the staff at Shanghai Synchrotron Radiation Facility (SSRF-Beamline 17U) is acknowledged. We thank Mingyang Wang, Yanfang Zhang, and Yue Liu for help with protein preparation and discussion. We also thank Liu Di and Li Wei at the Network Information Center, Institute of Microbiology, Chinese Academy of Sciences, for help with sequence analysis.
G.F.G. conceived and supervised the project. G.F.G. and Q.L. designed the project. Q.L. carried out the protein expression, purification, and crystallization. J.Q. solved the structures. Q.L. and C.J.V. carried out the functional analysis. H.K., K.T., H.O., Y.S., and Y.S. provided the covalent inhibitor. All of the authors analyzed the data. Q.L., C.J.V., and G.F.G. wrote the paper.
Footnotes
Published ahead of print 3 July 2013
REFERENCES
- 1. Kobasa D, Takada A, Shinya K, Hatta M, Halfmann P, Theriault S, Suzuki H, Nishimura H, Mitamura K, Sugaya N, Usui T, Murata T, Maeda Y, Watanabe S, Suresh M, Suzuki T, Suzuki Y, Feldmann H, Kawaoka Y. 2004. Enhanced virulence of influenza A viruses with the haemagglutinin of the 1918 pandemic virus. Nature 431:703–707 [DOI] [PubMed] [Google Scholar]
- 2. Tumpey TM, Garcia-Sastre A, Taubenberger JK, Palese P, Swayne DE, Basler CF. 2004. Pathogenicity and immunogenicity of influenza viruses with genes from the 1918 pandemic virus. Proc. Natl. Acad. Sci. U. S. A. 101:3166–3171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Suzuki Y, Ito T, Suzuki T, Holland RE, Chambers TM, Kiso M, Ishida H, Kawaoka Y. 2000. Sialic acid species as a determinant of the host range of influenza A viruses. J. Virol. 74:11825–11831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ma W, Lager KM, Vincent AL, Janke BH, Gramer MR, Richt JA. 2009. The role of swine in the generation of novel influenza viruses. Zoonoses Public Health 56:326–337 [DOI] [PubMed] [Google Scholar]
- 5. Scholtissek C, Burger H, Kistner O, Shortridge KF. 1985. The nucleoprotein as a possible major factor in determining host specificity of influenza H3N2 viruses. Virology 147:287–294 [DOI] [PubMed] [Google Scholar]
- 6. Ma W, Vincent AL, Gramer MR, Brockwell CB, Lager KM, Janke BH, Gauger PC, Patnayak DP, Webby RJ, Richt JA. 2007. Identification of H2N3 influenza A viruses from swine in the United States. Proc. Natl. Acad. Sci. U. S. A. 104:20949–20954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vavricka CJ, Liu Y, Li Q, Shi Y, Wu Y, Sun Y, Qi J, Gao GF. 2011. Special features of the 2009 pandemic swine-origin influenza A H1N1 hemagglutinin and neuraminidase. Chin. Sci. Bull. 56:1747–1752 [Google Scholar]
- 8. Tong S, Li Y, Rivailler P, Conrardy C, Castillo DA, Chen L, Recuenco S, Ellison JA, Davis CT, York IA, Turmelle AS, Moran D, Rogers S, Shi M, Tao Y, Weil MR, Tang K, Rowe LA, Sammons S, Xu X, Frace M, Lindblade KA, Cox NJ, Anderson LJ, Rupprecht CE, Donis RO. 2012. A distinct lineage of influenza A virus from bats. Proc. Natl. Acad. Sci. U. S. A. 109:4269–4274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li Q, Sun X, Li Z, Liu Y, Vavricka CJ, Shi Y, Wei J, Feng E, Shen J, Chen J, Liu D, He J, Yan J, Liu H, Jiang H, Teng M, Li X, Gao GF. 2012. Structural and functional characterization of neuraminidase-like molecule N10 derived from bat influenza A virus. Proc. Natl. Acad. Sci. U. S. A. 109:18897–18902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhu X, Yang H, Guo Z, Yu W, Carney PJ, Li Y, Chen LM, Paulson JC, Donis RO, Tong S, Stevens J, Wilson IA. 2012. Crystal structures of two subtype N10 neuraminidase-like proteins from bat influenza A viruses reveal a diverged putative active site. Proc. Natl. Acad. Sci. U. S. A. 109:18903–18908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang M, Qi J, Liu Y, Vavricka CJ, Wu Y, Li Q, Gao GF. 2011. Influenza A virus neuraminidase N5 has an extended 150-cavity. J. Virol. 85:8431–8435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. von Itzstein M, Wu WY, Kok GB, Pegg MS, Dyason JC, Jin B, Van Phan T, Smythe ML, White HF, Oliver SW, Colman PM, Varghese JN, Ryan DM, Woods JM, Bethell RC, Hotham VJ, Cameron JM, Penn CR. 1993. Rational design of potent sialidase-based inhibitors of influenza virus replication. Nature 363:418–423 [DOI] [PubMed] [Google Scholar]
- 13. Kim CU, Lew W, Williams MA, Liu H, Zhang L, Swaminathan S, Bischofberger N, Chen MS, Mendel DB, Tai CY, Laver WG, Stevens RC. 1997. Influenza neuraminidase inhibitors possessing a novel hydrophobic interaction in the enzyme active site: design, synthesis, and structural analysis of carbocyclic sialic acid analogues with potent anti-influenza activity. J. Am. Chem. Soc. 119:681–690 [DOI] [PubMed] [Google Scholar]
- 14. Sidwell RW, Smee DF. 2002. Peramivir (BCX-1812, RWJ-270201): potential new therapy for influenza. Expert Opin. Invest. Drugs 11:859–869 [DOI] [PubMed] [Google Scholar]
- 15. Yamashita M, Tomozawa T, Kakuta M, Tokumitsu A, Nasu H, Kubu S. 2009. CS-8958, a prodrug of the new neuraminidase inhibitor R-125489, shows long-acting anti-influenza virus activity. Antimicrob. Agents Chemother. 53:186–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vavricka CJ, Qi J, Wu Y, Qi J, Wang M, Liu Y, Gao F, Liu J, Feng E, He J, Wang J, Liu H, Jiang H, Gao GF. 2011. Structural and functional analysis of laninamivir and its octanoateprodrug reveals group specific mechanisms for influenza NA inhibition. PLoS Pathog. 7:e1002249. 10.1371/journal.ppat.1002249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li Q, Feng E, Liu H, Gao GF. 2010. The design and development of influenza virus neuraminidase inhibitors on the basis of crystal structures. Biotech. Bus. 6:42–48 (In Chinese) [Google Scholar]
- 18. Hurt AC, Chotpitayasunondh T, Cox NJ, Daniels R, Fry AM, Gubareva LV, Hayden FG, Hui DS, Hungnes O, Lackenby A, Lim W, Meijer A, Penn C, Tashiro M, Uyeki TM, Zambon M, WHO Consultation on Pandemic Influenza A (H1N1) 2009 Virus Resistance to Antivirals 2012. Antiviral resistance during the 2009 influenza A H1N1 pandemic: public health, laboratory, and clinical perspectives. Lancet Infect. Dis. 12:240–248 [DOI] [PubMed] [Google Scholar]
- 19. Varghese JN, Smith PW, Sollis SL, Blick TJ, Sahasrabudhe A, McKimm-Breschkin JL, Colman PM. 1998. Drug design against a shifting target: a structural basis for resistance to inhibitors in a variant of influenza virus neuraminidase. Structure 6:735–746 [DOI] [PubMed] [Google Scholar]
- 20. Collins PJ, Haire LF, Lin YP, Liu J, Russell RJ, Walker PA, Martin SR, Daniels RS, Gregory V, Skehel JJ, Gamblin SJ, Hay AJ. 2009. Structural basis for oseltamivir resistance of influenza viruses. Vaccine 27:6317–6323 [DOI] [PubMed] [Google Scholar]
- 21. Collins PJ, Haire LF, Lin YP, Liu J, Russell RJ, Walker PA, Skehel JJ, Martin SR, Hay AJ, Gambling SJ. 2008. Crystal structures of oseltamivir-resistant influenza virus neuraminidase mutants. Nature 453:1258–1261 [DOI] [PubMed] [Google Scholar]
- 22. Gubareva LV, Kaiser L, Matrosovich MN, Soo-Hoo Y, Hayden FG. 2001. Selection of influenza virus mutants in experimentally infected volunteers treated with oseltamivir. J. Infect. Dis. 183:523–531 [DOI] [PubMed] [Google Scholar]
- 23. Lackenby A, Hungnes O, Dudman SG, Meijer A, Paget WJ, Hay AJ, Zambon MC. 2008. Emergence of resistance to oseltamivir among influenza A(H1N1) viruses in Europe. Euro Surveill. 13(5):Article 2. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=8026 [DOI] [PubMed] [Google Scholar]
- 24. Rameix-Welti MA, Enouf V, Cuvelier F, Jeannin P, van der Werf S. 2008. Enzymatic properties of the neuraminidase of seasonal H1N1 influenza viruses provide insights for the emergence of natural resistance to oseltamivir. PLoS Pathog. 4:e1000103. 10.1371/journal.ppat.1000103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Potier M, Mameli L, Belisle M, Dallaire L, Melancon SB. 1979. Fluorometric assay of neuraminidase with a sodium (4-methylumbelliferyl-α-d-N-acetylneuraminate) substrate. Anal. Biochem. 94:287–296 [DOI] [PubMed] [Google Scholar]
- 26. Otwinowski Z, Minor W. 1997. Processing of X-ray diffraction data collected in oscillation mode. Method. Enzymol. 276:307–326 [DOI] [PubMed] [Google Scholar]
- 27. Read RJ. 2001. Pushing the boundaries of molecular replacement with maximum likelihood. Acta Crystallogr. D Biol. Crystallogr. 57:1373–1382 [DOI] [PubMed] [Google Scholar]
- 28.Collaborative Computational Project Number 4 1994. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D Biol. Crystallogr. 50:760–763 [DOI] [PubMed] [Google Scholar]
- 29. Murshudov GN, Vagin AA, Dodson EJ. 1997. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D Biol. Crystallogr. 53:240–255 [DOI] [PubMed] [Google Scholar]
- 30. Emsley P, Cowtan K. 2004. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60:2126–2132 [DOI] [PubMed] [Google Scholar]
- 31. Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH. 2010. PHENIX: a comprehensive python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66:213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Laskowski RA, Macarthur MW, Moss DS, Thornton JM. 1993. Procheck: a program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 26:283–291 [Google Scholar]
- 33. Xu X, Zhu X, Dwek RA, Stevens J, Wilson IA. 2008. Structural characterization of the 1918 influenza virus H1N1 neuraminidase. J. Virol. 82:10493–10501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li Q, Qi J, Zhang W, Vavricka CJ, Shi Y, Wei J, Feng E, Shen J, Chen J, Liu D, He J, Yan J, Liu H, Jiang H, Teng M, Li X, Gao GF. 2010. The 2009 pandemic H1N1 neuraminidase N1 lacks the 150-cavity in its active site. Nat. Struct. Mol. Biol. 17:1266–1268 [DOI] [PubMed] [Google Scholar]
- 35. Vavricka CJ, Liu Y, Kiyota H, Sriwilaijaroen N, Qi J, Tanaka K, Wu Y, Li Q., Li Y, Yan J, Suzuki Y, Gao GF. 2013. Influenza neuraminidase operates via a nucleophilic mechanism and can be targeted by covalent inhibitors. Nat. Commun. 2013:e2487. 10.1038/ncomms2487 [DOI] [PubMed] [Google Scholar]
- 36. Watts AG, Oppezzo P, Withers SG, Alzari PM, Buschiazzo A. 2006. Structural and kinetic analysis of two covalent sialosyl-enzyme intermediates on trypanosomarangelisialidase. J. Biol. Chem. 281:4149–4155 [DOI] [PubMed] [Google Scholar]
- 37. Newstead SL, Potter JA, Wilson JC, Xu G, Chien CH, Watts AG, Withers SG, Taylor GL. 2008. The structure of Clostridium perfringens NanI sialidase and its catalytic intermediates. J. Biol. Chem. 283:9080–9088 [DOI] [PMC free article] [PubMed] [Google Scholar]




