Abstract
Bacteriophage Φ6 contains three double-stranded RNA (dsRNA) genomic segments, L, M, and S. The RNA is located inside a core particle composed of multiple copies of a major structural protein, an RNA-dependent RNA polymerase, a hexameric NTPase, and an auxiliary protein. The virion RNA polymerase in the core particle transcribes segments M and S in vitro. Segment L is transcribed poorly because its transcript starts with GU instead of GG found on segments S and M. Transcription in vivo is modified by the binding of host protein YajQ to the outside the core particle so that segment L is transcribed well. This mechanism is the determinant of the temporal control of gene expression in Φ6. Mutants of Φ6 have been isolated that are independent of YajQ for transcription of segment L. The mutations are found in the gene of the viral polymerase or the major capsid protein or both. These mutants are capable of transcribing segment L with the GU start or GA or GC. The same is found to be true when YajQ is added to wild-type particles. Minus-strand synthesis has restrictions that are different from that of plus-strand synthesis, and YajQ or mutations to independence do not modify minus-strand synthesis behavior. Purified polymerase P2 is able to transcribe dsRNA, but transcription behavior of segment L by both wild-type and mutant polymerases is different from that seen in capsid structures. Adding YajQ to purified polymerase does not change its transcription specificity.
INTRODUCTION
Bacteriophage Φ6 is a member of the Cystoviridae, a family of bacteriophages that have genomes of three double-stranded RNA (dsRNA) molecules, L, M, and S, packaged inside a polyhedral capsid structure covered by a lipid-containing membrane. Φ6 was the first member of this family to be discovered (1). Cystoviridae infect Gram-negative bacteria, primarily Pseudomonas syringae and its relatives. The core of the virus contains an RNA-dependent RNA polymerase that synthesizes minus strands on the templates of the packaged plus-strand RNAs. The polymerase also transcribes the resulting dsRNA to form plus strands that are released from the core to program the synthesis of phage proteins and to serve as replication intermediates (2). The composition of the transcript population changes during the infection cycle (3). At very early times there are almost equal amounts of the three transcripts; while at later times transcription is limited to the S and M genomic segments. Isolated cores of the Φ6 virion have very strong transcriptase activity, but it is limited to S and M under normal conditions in magnesium-containing buffers (4). The 5′ 17-base sequences of the three plus strands are identical, with the exception that in S and M they start with GG, while in L it starts with GU. This difference is the determinant of the differences in in vitro transcription activity (5). The transcription of the L segment is activated in the presence of manganese ions (4). However, this is not involved in normal temporal control of Φ6 transcription.
We have found that a host protein, YajQ, binds to the core particle upon its entry into the host cell (6). This protein binds to protein P1, the major capsid protein, and causes a change in template specificity of the polymerase so that it now transcribes the L segment (7). The amount of protein YajQ in the cell is limited, so only a few core particles are able to bind the protein. Consequently, as more core particles are assembled they lack YajQ, and their transcriptase activity is limited primarily to segments M and S. This, then, is responsible for the temporal control of gene expression (6). In the present study, we have investigated the nature of the changes in template specificity caused by the binding of YajQ to core particles and caused by mutations in the gene for polymerase P2 that confer independence of YajQ.
MATERIALS AND METHODS
Bacterial strains, phage, and plasmids.
LM2489 is a rough derivative of P. syringae pv. phaseolicola HB10Y (1) and was used as the primary host for plating Φ6. LM4470 is a strain of P. syringae with a knockout of the gene yajQ (7). LM128, a derivative of HB10Y, was also used, and LM2691 is LM128 carrying plasmid pLM1086, which is a derivative of pRK290 (8) and pAR1219 (9) and expresses T7 RNA polymerase in pseudomonads. Plasmid transformation into Escherichia coli used strain JM109 (10) or Stratagene XL1-Blue supercompetent cells {recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac [F′ proAB lacIqZΔM15 Tn10 (Tetr)]}.
Reverse genetics of Φ6.
Mutant forms of the phage were prepared by constructing deletions or modifications in plasmids containing cDNA copies of the genomic segments. These were then electroporated into host cells containing plasmids expressing T7 RNA polymerase (11). In general, thousands of plaques were produced and samples were purified, and their RNA was analyzed by gel behavior and by the preparation of DNA copies by reverse transcription-PCR (RT-PCR) and subsequently checked by sequence analysis at the DNA sequencing core facility of the University of Medicine and Dentistry of New Jersey (UMDNJ) or by Genewiz.
In vitro transcription with nucleocapsids, procapsids, or purified polymerase.
Nucleocapsids of Φ6 were prepared from purified wild-type and mutant virions stripped of their lipid-containing membranes by treatment with 2% Triton X-100 (12), resuspended in phosphate-buffered solution, and treated with 5 mM EGTA to remove the middle shell composed of protein P8. Transcription in 10 μl was performed in magnesium buffers with and without added YajQ. The reaction buffer contained 70 mM bicine (pH 8.8), 5 mM MgCl2, 1 mM ATP, GTP, and UTP, 0.1 mM CTP, 2 μCi of [α-32P]CTP (MP Biochemicals), 100 mM ammonium acetate, and 8 U of RNasin (Promega).
Empty procapsids were prepared by expressing plasmids with cDNA copies of segment L in E. coli JM109. Plasmid pLM687 coded for wild-type proteins, while plasmid pLM3762 contained mutation L612R in gene 2. Cells were collected and opened by French press lysis. Particles were purified by zone sedimentation through sucrose gradients and prepared as described previously (13). The reactions were composed of packaging, minus-strand synthesis, and plus-strand synthesis. Transcripts of plasmids carrying cDNA copies of the genomic segments were incubated with the particles in 10 μl of 50 mM Tris-HCl (pH 8.9), 5 mM MgCl2, 1 mM MnCl2, 80 mM ammonium acetate, 0.1 mM EDTA, 5 mM dithiothreitol (DTT), 8 U of RNasin, 6% polyethylene glycol (PEG) 4000, 2 mM ATP and GTP, 0.1 mM UTP, 0.2 mM CTP, 2.5 μCi of [α-32P]CTP, and 0.1% Triton X-100.
Wild-type polymerase protein P2 (NCBI reference sequence: NP_620346.1) was prepared from strain LM4801, which is E. coli BL21(DE3) carrying plasmid pLM3758, which is a pET28a (Novagen) derivative having a T7 promoter and an N-terminal His tag. Mutant polymerase P2 was prepared from similar cells carrying plasmid pLM3775, which contained the mutation L612R. Genes for both C-terminal and N-terminal His-tagged P2 were tested successfully for complementation of phage carrying deletions of gene 2. Neither C-terminal nor N-terminal His tags compromised the dependence of the phage on YajQ. The templates for plus-strand synthesis were dsRNA isolated from Φ6 virions with equal amounts of the three genomic segments. Templates for minus-strand synthesis were transcripts of plasmid pLM763 having various 3′ termini due to cutting the plasmid with different restriction enzymes (14). Incubation mixtures of 10 μl contained 50 mM HEPES-KOH (pH 7.5), 20 mM ammonium acetate, 0.1 mM EDTA, 5 mM MgCl2, 1 mM ATP and GTP, 0.1 mM UTP, 0.2 mM CTP, 2 μCi of [α-32P]CTP, 8 U of RNasin, 0.1% Triton X-100, and 6% PEG 4000.
Gel analysis.
Radioactive samples of RNA were applied to 0.8% SeaKem GTG agarose (FMC Bioporducts) in Tris-EDTA-borate buffer, pH 8.5, with 2 mg/ml of ethidium bromide. After electrophoresis, the gels were dried and incubated with film. Capital letters S, M, and L were used to label dsRNA bands, while lowercase letters labeled plus strands. Transcription of Φ6 RNA is semisconservative (3); therefore, dsRNA bands in procapsid packaging contain radioactivity in both plus and minus strands, while single-stranded RNA bands are radioactive plus strands. Transcription in nucleocapsids labels only plus strands in both dsRNA and single-stranded RNA (ssRNA).
RESULTS AND DISCUSSION
Transcription template specificity for particles activated by YajQ binding.
The transcript of segment L in Φ6 begins with GU, while those of segments M and S begin with GG (15). Empty core particles can be produced in strains of E. coli carrying expression plasmids coding for proteins P1, P2, P4, and P7. These particles are capable of packaging plus-strand transcripts of the genomic segments, synthesizing minus strands, and, finally, transcribing the dsRNA to form plus-strand mRNA (16). However, the in vitro transcription of the dsRNA is limited to segments S and M. In the presence of purified YajQ, transcription of segment L is activated (7). We prepared plus-strand RNA molecules of segment L that began with the wild-type sequence GU as well as GA, GC, and GG. We then incubated particles with mixtures of wild-type transcripts of segments S and M along with each member of the set of L transcripts. RNA synthesis was monitored in the absence and presence of YajQ (Fig. 1). It was found that, as expected, plus-strand transcription of L beginning with GG took place in the absence of YajQ. The 5′ GG nucleotides were both necessary and sufficient to confer YajQ-independent transcription. Neither a GU, GA, nor GC start served as a transcription template. However, in the presence of YajQ, all of the L templates were transcribed. This indicated that the change in specificity produced by the binding of YajQ was not specific for GU. We also tested the consequences of changing the 5′ sequences of the plus strands of segments S and M from GG to GU. We found that this change resulted in the loss of plus-strand synthesis in particles and that incubation with YajQ resulted in transcription activity (data not shown).
Fig 1.
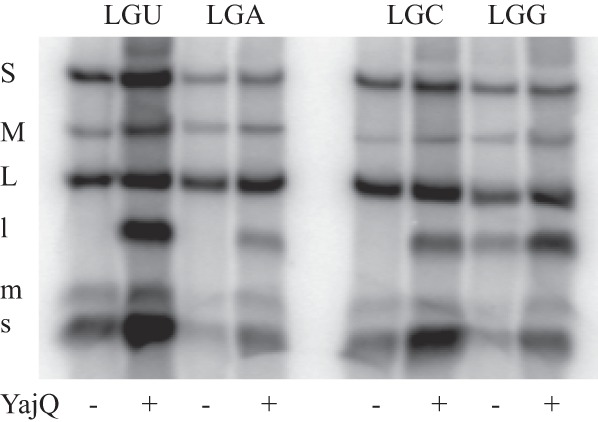
Transcriptase and minus-strand synthesis in wild-type particles packaging wild-type plus strands of segments S and M along with modified segment L plus strands that would result in transcripts starting with sequence GU, GA, GC, or GG. Incubation conditions are with or without the addition of purified protein YajQ. Bands labeled with lowercase letters are plus strands, while those labeled with uppercase letters contain dsRNA.
Isolation of additional mutants independent of YajQ.
Mutants that are independent of YajQ were isolated by plating wild-type Φ6 on strain LM4470, in which there is an insertion in gene yajQ (7). All mutations were found in segment L as single or multiple changes. Some of the mutations were in gene 1, which codes for the major capsid protein, some were in gene 2, which codes for the viral RNA polymerase, and some were in both genes. Most of the polymerase mutations were near the C terminus of the protein in the region of amino acids 603 to 612. Examples of mutations were A604E and L612R. Mutation V603A was isolated from carrier state phage in E. coli JM109 (7). Phage was produced with L segments containing YajQ-independent mutations. Nucleocapsids were then prepared and tested for transcriptase activity. The polymerase mutants were tested to determine whether their independence of YajQ resulted in transcription of the normal L segment. Figure 2 shows the results of using particles containing YajQ-independent mutations in the polymerase. It can be seen that wild-type particles transcribe segments S and M but not L unless 3 mM manganese ions are included in the reaction solution. The nucleocapsids containing mutant polymerase transcribed segment L very well.
Fig 2.
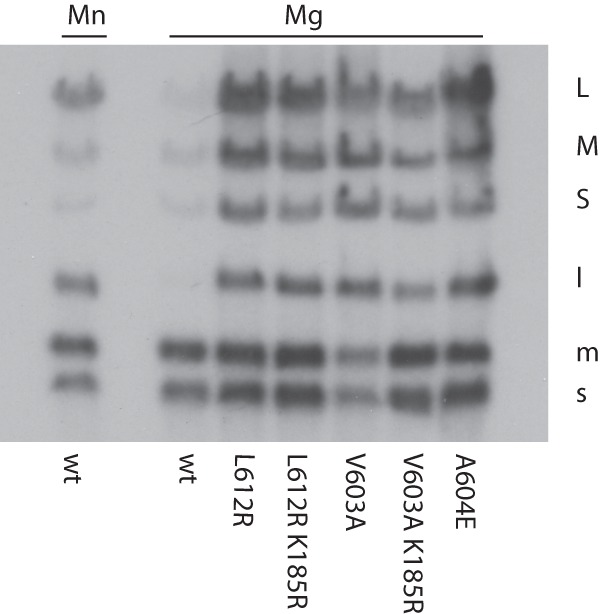
Transcriptase activity of wild-type (wt) and mutant nucleocapsids prepared from phage. Note that manganese ions activate L transcription but that YajQ-independent mutations in P2 are needed in normal magnesium buffers. The mutation K185R was found in several cases but was not essential for independence of YajQ.
Transcription template specificity for particles containing polymerase P2 carrying mutations for YajQ independence.
The protein of mutant gene 2 that changed L612 to arginine was incorporated into capsid particles and tested for transcriptase activity on segment L molecules having starting nucleotides GU, GA, GC, or GG (Fig. 3). It was found that wild-type particles packaged the plus-strand molecules and produced transcripts of segments S and M for the GU, GA, and GC samples of L. The wild-type particles did not transcribe the L segments whose transcripts began with GU, GA, or GC. The particles containing the GG L RNA produced L transcripts along with those of M and S. The particles containing the polymerase with the L612R mutation produced L transcripts for all four of the sequence varieties. This indicated again that the change in specificity is not limited to the GU sequence but is general for the second nucleotide. This is true for both the conditions wherein YajQ is added to wild-type particles and the conditions wherein particles contain mutant polymerase.
Fig 3.
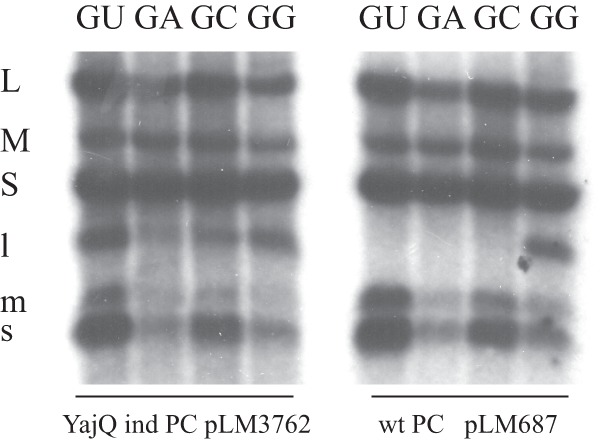
Transcriptase and minus-strand synthesis in wild-type (produced by plasmid pLM687) and YajQ-independent mutant particles (produced by plasmid pLM3762) packaging wild-type plus strands of segments S and M along with modified segment L plus strands that would result in transcripts starting with sequence GU, GA, GC, or GG. The mutant particles contain P2 with mutation L612R.
Phage could be produced by electroporating plasmids containing cDNA copies of segments S and M along with L having GA or GC as the 5′ nucleotides of L. These results suggest that viable phage can be produced that have GA or GC as the first two nucleotides of the L transcript. However, we found that the GC construct mutated to GU phage that was dependent upon YajQ, while the GA construct mutated to GU and GG, which were independent of YajQ. This indicated that although the GA- and GC-containing transcripts were functional in live phage, they were not optimal. Phages carrying YajQ-independent mutations were not found to be hypermutable.
Manganese ions change the template specificity for transcription.
Transcription of wild-type segment L was shown to be augmented by the inclusion of 3 mM manganese ions in the incubation buffer (4). We found that transcription of L segments beginning with GA and GC was enhanced by manganese ions to the same extent as transcription of wild-type segment L starting with GU. However, the level of transcription activity for mutant segment L with transcripts starting with GG was higher than that of the other modifications even in the presence of 3 mM manganese ions (Fig. 4).
Fig 4.
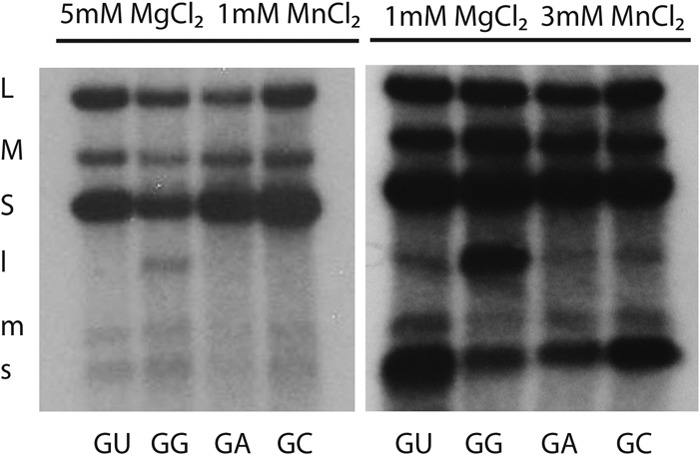
Transcriptase and minus-strand synthesis in wild-type particles packaging wild-type plus strands of segments S and M along with modified segment L plus strands that would result in transcripts starting with sequence GU, GA, GC, or GG. Reactions were performed under normal conditions of 1 mM manganese and 5 mM magnesium ions or at a high (3 mM) manganese ion concentration with 1 mM magnesium ions.
Sequence specificity for minus-strand synthesis in the wild type and mutants.
Previous reports had shown that minus-strand synthesis in Φ6 has different sequence requirements from those for transcription (14). Although the natural 5′ termini of Φ6 minus strands contain G as the second nucleotide, alterations in the sequence of plus-strand 3′ end resulted in some seemingly anomalous results. We found that minus strand beginning with the sequence GU could be synthesized in wild-type particles and that this activity was not enhanced in particles carrying a YajQ-independent mutation L612R (Fig. 5). A plus-strand copy of segment S that terminates with the sequence CU due to termination with cutting by HindIII and whose minus-strand copy would start with AG did not serve as a template for minus-strand synthesis (Fig. 6). However, cuts with XbaI and mung bean nuclease which result in a minus strand that also starts with AG constitute a good template for minus-strand synthesis as found for wild-type segments. Poor minus-strand synthesis had been seen previously with termini due to cuts with EcoRI, whose minus strand starts with AA, and PstI, which would have a minus strand starting with GG (14). A segment terminated with a SmaI cut which resulted in the sequence GGG in the minus strand was also a good template. In the cases of poor template activity, the YajQ-independent mutation did not change the template activity. The activation by YajQ or the independent mutations clearly perform differently for minus-strand synthesis than for transcription of dsRNA in the particles.
Fig 5.
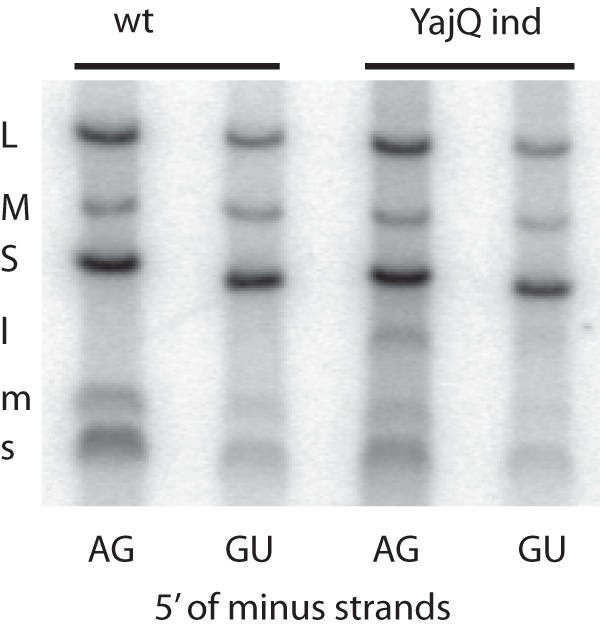
Minus-strand synthesis in wild-type (pLM687) and YajQ-independent mutant (pLM3762) particles incubated with plus strands of S, M, and L terminated so as to produce minus strands beginning with GU or the normal AG. It is seen that minus-strand synthesis is normal and is not improved in the mutant particles. Plus-strand synthesis is as expected. The level of dsRNA labeling reflects the degree of minus-strand synthesis.
Fig 6.
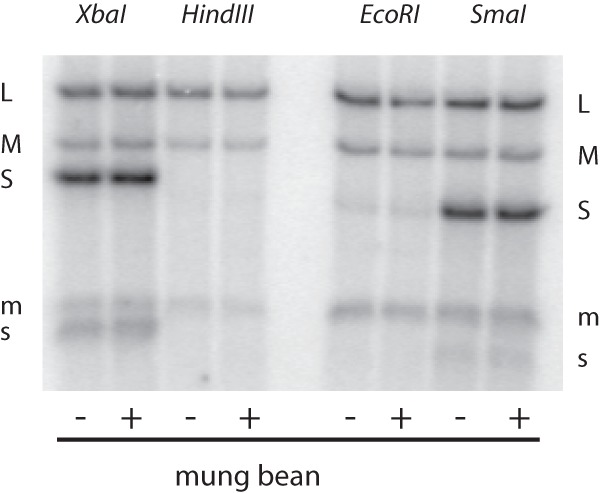
Minus- and plus-strand synthesis by core particles with mutant polymerase using S templates with altered 3′ termini resulting in varied 5′ sequences of the new minus strands. Plasmid pLM763 was cut with XbaI, HindIII, EcoRI, or SmaI with or without subsequent treatment with mung bean nuclease. All of the cuts treated with mung bean nuclease would have minus-strand transcripts with G as the second nucleotide. XbaI and EcoRI cuts without mung bean nuclease treatment would not have G as the second nucleotide. Segments M and L were wild type.
Activities of isolated polymerase P2.
The above-described results were obtained with intact capsid particles. The behavior of isolated polymerase might be expected to differ in some ways from the activity inside procapsids. Makayev and Bamford (17) showed that isolated P2 was able to transcribe dsRNA but that transcriptase activity on L templates was poor, even for a heat-denatured template. They also showed that at low protein concentrations, template activity of single-stranded RNA whose complement began with GU was a much poorer template than one whose complement began with GG. But at higher protein concentrations, the differences diminished. We find that purified P2 transcription of dsRNA is primarily that of M and S. At higher concentrations of P2, L transcription becomes more active. YajQ has no effect on the activity of purified P2. Mutant polymerase also shows a concentration dependence on L transcription, but the activation of L transcription takes place at lower P2 concentrations (Fig. 7). At P2 concentrations below 1 μM, the ratio of L transcription to that of M and S with the L612R mutant polymerase is about three times that found with the wild-type P2. The ratio of L transcription to that of M and S is about 0.3, while the ratio of molecular weights is 0.9. Although the mutation to YajQ independence seems to enhance the transcriptase activity of purified P2 on segment L, it is clear that the enhancement is not as great as found in the viral particles. The synthesis of minus strands by purified P2 is similar to that seen in particles, and mutant polymerase does not behave differently than wild-type molecules on single-stranded templates (Fig. 8).
Fig 7.
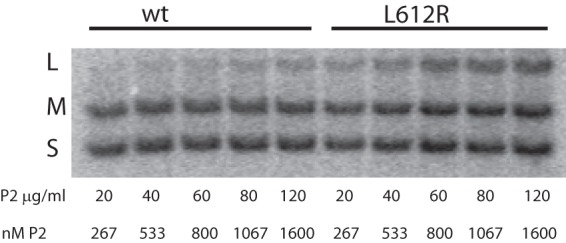
Transcription of normal dsRNA by purified wild-type (LM4801) and mutant (LM4820) polymerase at increasing protein concentrations.
Fig 8.
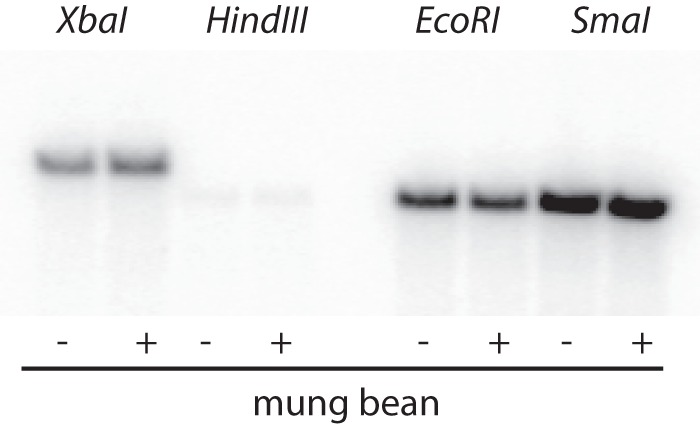
Minus-strand synthesis by purified wild-type polymerase P2 on templates of S prepared from plasmid pLM763 as in Fig. 5.
Roles of capsid and polymerase structure in transcription specificity.
The activity of the Φ6 RNA polymerase is highly dependent upon its associations with the viral core structure. Minus-strand synthesis does not take place during plus-strand packaging until the three segments have been packaged (18). We believe that this reflects the expansion of the core to a point where the association with P2 has been altered. Plus-strand synthesis does not begin until minus-strand synthesis is completed (18). We believe that this involves further expansion of the core particle. The binding of the limited amount of YajQ molecules to the capsids of the incoming virus particles turns on the transcription of segment L. The lack of sufficient YajQ to bind to newly assembled particles results in a temporal change in the viral transcription activity. The change in transcription activity of segment L upon the binding of YajQ might not involve additional expansion but could involve rotation or alterations of conformation of molecules of P1, the major structural protein of the viral core. The observation that the change in transcription template specificity is similar in the cases obtained with YajQ binding, YajQ-independent mutation, and manganese ion supplementation suggests that these three conditions result in similar alterations in the conformation of polymerase P2. A high-affinity manganese binding site has been identified in the Φ6 polymerase, and structural equivalents of this site have been shown in other RNA-dependent RNA polymerases (19). Because of the low concentration of manganese in bacterial cells, it is felt that this ion species does not normally play a role in the template specificity of the Φ6 polymerase.
Structural studies of the Φ6 polymerase P2 (20) have suggested that the first base pairing in the synthesis of transcript is that of GTP with the penultimate base of the template, while the first base of the template is bound in a pocket of the C-terminal domain of the polymerase. Following this, the terminal base pairs with GTP, which then forms a bond with the other GTP. The interaction of these two nucleotides with the template involves interaction with a platform composed of the C-terminal domain of the polymerase. The C-terminal domain then must move out of the way since it is blocking the exit pore of the polymerase (20). Most of the mutations to YajQ independence in the polymerase P2 have been found in amino acids 603 to 612. This sequence has been characterized as a hinge region in the C-terminal domain that affects the placement of the stacking platform involved in the presentation of the first two nucleotides to the active site prior to elongation (21). The stacking platform, which is part of the C-terminal domain, moves out of the way for elongation to occur. Therefore, the specificity of nucleotide placement apart from complementarity functions only for the first two nucleotides of the nascent chain.
These results suggest that the difference in the template specificity in plus- and minus-strand synthesis probably depends more on structural differences than sequence. It is frustrating to consider that the most striking changes in template specificity are manifested in complete core particles and not in the isolated polymerase. This results in a much greater challenge to determine the conformational consequences in P2 of YajQ binding to P1.
Footnotes
Published ahead of print 17 July 2013
REFERENCES
- 1. Vidaver AK, Koski RK, Van Etten JL. 1973. Bacteriophage Φ6: a lipid-containing virus of Pseudomonas phaseolicola. J. Virol. 11:799–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Van Etten JL, Vidaver AK, Koski RK, Semancik JS. 1973. RNA polymerase activity associated with bacteriophage Φ6. J. Virol. 12:464–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Coplin DL, Van Etten JL, Koski RK, Vidaver AK. 1975. Intermediates in the biosynthesis of double-stranded ribonucleic acids of bacteriophage Φ6. Proc. Natl. Acad. Sci. U. S. A. 72:849–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Emori Y, Iba H, Okada Y. 1983. Transcriptional regulation of three double-stranded RNA segments of bacteriophage Φ6 in vitro. J. Virol. 46:196–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Frilander M, Poranen M, Bamford DH. 1995. The large genome segment of dsRNA bacteriophage Φ6 is the key regulator in the in vitro minus and plus strand synthesis. RNA 1:510–518 [PMC free article] [PubMed] [Google Scholar]
- 6. Qiao X, Sun Y, Qiao J, Mindich L. 2010. Interaction of a host protein with core complexes of bacteriophage Phi6 to control transcription. J. Virol. 84:4821–4825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Qiao X, Sun Y, Qiao J, Mindich L. 2008. The role of host protein YajQ in the temporal control of transcription in bacteriophage Phi6. Proc. Natl. Acad. Sci. U. S. A. 105:15956–15960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ditta G, Stanfield S, Corbin D, Helinski DR. 1980. Broad host range DNA cloning system for gram-negative bacteria; construction of a gene bank of Rhizobium meliloti. Proc. Natl. Acad. Sci. U. S. A. 77:7347–7351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Davanloo P, Rosenberg AH, Dunn JJ, Studier FW. 1984. Cloning and expression of the gene for bacteriophage T7 RNA polymerase. Proc. Natl. Acad. Sci. U. S. A. 81:2035–2039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yanisch-Perron C, Vieira J, Messing J. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103–119 [DOI] [PubMed] [Google Scholar]
- 11. Sun Y, Qiao X, Mindich L. 2004. Construction of carrier state viruses with partial genomes of the segmented dsRNA bacteriophages. Virology 319:274–279 [DOI] [PubMed] [Google Scholar]
- 12. Van Etten JL, Lane L, Gonzalez C, Partridge J, Vidaver A. 1976. Comparative properties of bacteriophage Φ6 and Φ6 nucleocapsid. J. Virol. 18:652–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gottlieb P, Strassman J, Bamford DH, Mindich L. 1988. Production of a polyhedral particle in E. coli from a cDNA copy of the large genomic segment of bacteriophage Φ6. J. Virol. 62:181–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mindich L, Qiao X, Onodera S, Gottlieb P, Frilander M. 1994. RNA structural requirements for stability and minus-strand synthesis in the dsRNA bacteriophage phi 6. Virology 202:258–263 [DOI] [PubMed] [Google Scholar]
- 15. Iba H, Watanabe T, Emori Y, Okada Y. 1982. Three double-stranded RNA genome segments of bacteriophage Φ6 have homologous terminal sequences. FEBS Lett. 141:111–115 [DOI] [PubMed] [Google Scholar]
- 16. Mindich L. 2004. Packaging, replication and recombination of the segmented genome of bacteriophage Phi6 and its relatives. Virus Res. 101:83–92 [DOI] [PubMed] [Google Scholar]
- 17. Makeyev EV, Bamford DH. 2000. The polymerase subunit of a dsRNA virus plays a central role in the regulation of viral RNA metabolism. EMBO J. 19:6275–6284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Frilander M, Gottlieb P, Strassman J, Bamford DH, Mindich L. 1992. Dependence of minus strand synthesis upon complete genomic packaging in the dsRNA bacteriophage Φ6. J. Virol. 66:5013–5017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Poranen MM, Salgado PS, Koivunen MR, Wright S, Bamford DH, Stuart DI, Grimes JM. 2008. Structural explanation for the role of Mn2+ in the activity of phi6 RNA-dependent RNA polymerase. Nucleic Acids Res. 36:6633–6644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Butcher SJ, Grimes JM, Makeyev EV, Bamford DH, Stuart DI. 2001. A mechanism for initiating RNA-dependent RNA polymerization. Nature 410:235–240 [DOI] [PubMed] [Google Scholar]
- 21. Sarin LP, Wright S, Chen Q, Degerth LH, Stuart DI, Grimes JM, Bamford DH, Poranen MM. The C-terminal priming domain is strongly associated with the main body of bacteriophage varphi6 RNA-dependent RNA polymerase. Virology 432:184–193 [DOI] [PubMed] [Google Scholar]


