Abstract
The majority of available monoclonal antibodies (MAbs) in the current HIV vaccine field are generated from HIV-1-infected people. In contrast, preclinical immunogenicity studies have mainly focused on polyclonal antibody responses in experimental animals. Although rabbits have been widely used for antibody studies, there has been no report of using rabbit MAbs to dissect the specificity of antibody responses for AIDS vaccine development. Here we report on the production of a panel of 12 MAbs from a New Zealand White (NZW) rabbit that was immunized with an HIV-1 JR-FL gp120 DNA prime and protein boost vaccination regimen. These rabbit MAbs recognized a diverse repertoire of envelope (Env) epitopes ranging from the highly immunogenic V3 region to several previously underappreciated epitopes in the C1, C4, and C5 regions. Nine MAbs showed cross-reactivity to gp120s of clades other than clade B. Increased somatic mutation and extended CDR3 were observed with Ig genes of several molecularly cloned rabbit MAbs. Phylogenic tree analysis showed that the heavy chains of MAbs recognizing the same region on gp120 tend to segregate into an independent subtree. At least three rabbit MAbs showed neutralizing activities with various degrees of breadth and potency. The establishment of this rabbit MAb platform will significantly enhance our ability to test optimal designs of Env immunogens to gain a better understanding of the structural specificity and evolution process of Env-specific antibody responses elicited by candidate AIDS vaccines.
INTRODUCTION
Despite 30 years of intensive research, no effective vaccine formulations are available to prevent the transmission of human immunodeficiency virus type 1 (HIV-1). The recent RV144 trial showed an estimated 31.2% efficacy of protection (1) and, most significantly, revealed a positive correlation of protection with the presence of serum IgG binding antibodies (Abs) to variable region 2 (V2) of the envelope (Env) glycoprotein of HIV-1 (2, 3). These results confirmed the role of antibodies in an effective HIV-1 vaccine but also raised serious questions about the lack of knowledge on the diversity and potential functions of Env-specific antibodies present in an immunized serum.
Antibody research in the HIV-1 vaccine field has focused for a long time on the study of neutralizing human monoclonal antibodies (HMAbs) generated from HIV-1-infected patients. While these studies have provided remarkable information on the structural requirements for HMAbs, such unusually broadly neutralizing (bnHMAbs) can be identified in only 2% to 4% of the infected population and only after 2 or 3 years of infection (4–7). In contrast, the role of nonneutralizing antibodies targeting other areas of Env was virtually unknown prior to the study of antibody responses in RV144 volunteers (2, 8). Since it is a lengthy process to advance a candidate vaccine to human trials, most preclinical vaccine studies on the diversity and quality of antibody responses are conducted first in experimental animals.
Previously, we reported the elicitation of cross-clade neutralizing antibody responses when a DNA prime-protein boost immunization approach was adopted to deliver a polyvalent Env immunogen formulation in animal and human studies (9–11). Further epitope mapping and antibody competition analyses identified quality differences between the immune sera elicited by the DNA prime-protein boost approach and the protein-alone approach (12, 13). However, these studies were conducted using polyclonal sera and results from these studies were unable to link the observed antibody activities with a particular antibody component in a polyclonal serum.
Here we report the use of a recombinant rabbit monoclonal antibody (RMAb) platform to monitor the specificity and neutralizing activities of antibodies elicited by a candidate HIV-1 Env immunogen. Historically, rabbit has been an attractive animal model for antibody studies and has been used more recently in HIV vaccine research because rabbit is highly immunogenic in responding to various immunization regimens to produce high-titer antibody responses. It was shown that only RMAbs were able to provide high-quality detection using certain difficult epitopes, such as those in tissue section samples and HIV particles (14–16). Rabbit antibodies usually carry limited background reactivity to testing antigens. Rabbits provide a large volume of immune sera for a wide range of antibody assays, while the other common experimental animal species, such as mouse or rat, can provide only a limited volume of immune sera and high background in functional antibody assays. Moreover, rabbit antibodies, but not those from mouse, are able to generate long CDR3 regions, which is important for many neutralizing antibodies against HIV-1 (17, 18).
In the current pilot study, a panel of 12 HIV-1 Env-specific rabbit hybridoma cell lines were produced that can recognize a wide range of Env epitopes. Furthermore, their heavy-chain and light-chain genes were cloned and their genetic features were analyzed. RMAbs were produced from these rabbit Ig clones, and their epitope specificity, binding affinity, and neutralization activities were determined.
MATERIALS AND METHODS
HIV-1 gp120 DNA vaccine.
The codon-optimized gene segment coding for the gp120 region of HIV-1 isolate JR-FL (19) was subcloned into the pJW4303 DNA vaccine vector for the DNA priming phase of the immunization, as previously reported (10). JR-FL gp120 DNA plasmid was produced in the HB101 strain of Escherichia coli and then purified using a Qiagen Plasmid Mega kit (catalog no. 12183).
HIV-1 gp120 protein vaccines.
Recombinant HIV-1 gp120 protein vaccine was produced from Chinese hamster ovary (CHO) cells. Secreted gp120 proteins from stably transfected CHO cell lines were harvested and purified over a lectin column.
Rabbit immunizations.
New Zealand White rabbits (6 to 8 weeks of age) were purchased from Millbrook Farm (Amherst, MA) and housed in the animal facility managed by the Department of Animal Medicine at the University of Massachusetts Medical School (UMMS). All procedures were carried out by following animal research guidelines and approved by the IACUC. Each rabbit was immunized three times at weeks 0, 2, and 4 with JR-FL gp120 DNA vaccine via a Bio-Rad Helios gene gun applied to the shaved abdominal skin (36 μg/immunization). Rabbits were then boosted twice intramuscularly at weeks 8 and 12 with 50 μg JR-FL gp120 recombinant protein formulated in incomplete Freund's adjuvant (IFA) for each boost. After a 4-month resting period, one rabbit from the study group was boosted again with the same JR-FL gp120 DNA vaccine at week 28 followed by an intravenous 400 μg JR-FL gp120 protein immunization at week 32.
Rabbit hybridoma production.
Spleens were collected from sacrificed rabbits 4 days after the final protein boost and sent to a subcontractor (Epitopmics) for the production of hybridoma cells, according to the company's established procedure. The rabbit hybridoma fusion partner cells were developed previously (20) and further optimized to facilitate the generation of rabbit hybridoma cell lines (US patents 7,575,896 B2 [21] and 7,732,168 B2 [22]).
ELISA.
Supernatant from hybridoma culture was sent back to UMMS for screening of gp120-specific antibodies. An enzyme-linked immunosorbent assay (ELISA) was conducted in 96-well microtiter plates (Corning, NY), which were first incubated with concanavalin A (ConA) (5 μg per well in 100 μl of phosphate-buffered saline [PBS], pH 7.2) for 1 h and then coated with 100 μl of gp120 antigen (1 μg/ml) from a transiently transfected 293T cell supernatant. Plates were washed five times with ELISA washing buffer (EWB; PBS containing 0.1% Triton-X) and blocked overnight at 4°C in PBS containing whey dilution buffer (4% whey by weight) and 5% powdered milk. The following morning, plates were washed five times in EWB. Hybridoma supernatants or serially diluted rabbit monoclonal antibodies were added to the wells in a volume of 100 μl. Plates were washed five times in EWB and incubated with 100 μl of biotinylated anti-rabbit secondary antibody (Vector Laboratories BA-1000) at 1.5 μg/ml for 1 h at room temperature. Plates were washed five times with EWB and incubated with 100 μl of a streptavidin-conjugated horseradish peroxidase (Vector Laboratories, catalog no. SA-5004) at 500 ng/ml. Plates were washed five times with EWB and developed for 3 min in 100 μl of a 3,3′5,5′-tetramethylbenzidine substrate solution (Sigma; catalog no. T3405). The reaction was stopped with 25 μl of 2N H2SO4.
For the peptide epitope assay, 96-well microtiter plates were coated with overlapping peptides of HIV-1 consensus subtype B Env at 4 μg/ml for 1 h and blocked overnight. Hybridoma supernatants (100 μl) were then added to the peptide wells. Bound antibody was detected using an anti-rabbit IgG secondary antibody, as described above.
Western blot analysis.
The JR-FL gp120 antigen used in the Western blot analysis was obtained from 293T cells transiently transfected with a JR-FL gp120-expressing DNA vaccine, as previously reported (12). The gp120 antigens were subjected to SDS-PAGE and blotted onto a polyvinylidene difluoride (PVDF) membrane, as previously described (11). Blocking of the PVDF membrane was done with 0.1% I-Block (Tropix, Bedford, MA). The membranes were incubated with hybridoma supernatants at a 1:100 dilution for 45 min and subsequently reacted with AP-conjugated goat anti-rabbit IgG (Tropix, Bedford, MA) at a 1:5,000 dilution for 30 min. Membranes were washed with blocking buffer after each step. Western Light substrate was then applied to the membranes for 5 min. Once the membranes were dry, X-ray films were exposed to the membrane and developed using a Kodak processor.
Cloning and expression of rabbit MAb IgG genes.
Full-length heavy-chain and light-chain gene transcripts of rabbit MAb were isolated by one-step reverse transcriptase PCR (RT-PCR) amplification of hybridoma cell RNA. Primers were designed to target the conserved regions of rabbit IgG genes using heavy-chain primers (forward, 5′-CCGTCCAAGCTTHindIIIATGGAGACTGGGCTGCGCTGGC-3′; reverse, 5′-CAACAAGGATCCBamHICTATTTACCCGGAGAGCGGGAG-3′; recognition sites for restriction enzymes are underlined) and light-chain primers (forward, 5′-CCGTCCAAGCTTHindIIIATGGACACGAGGGCCCCCACTC-3′; reverse, 5′-CAACAAGGATCCBamHICTAACAGTCACCCCTATTGAAGC-3′). Specifically, one-step RT-PCR was carried out by adding 6 μl H2O, 12.5 μl reaction mix, 2.5 μl each of the forward and backward primers, 0.1 μl RNase inhibitor, 5 μl RNA template, and 0.5 μl RT–Platinum Taq mix (Invitrogen; catalog no. 10928-042). The thermocycle program was 50°C for 30 min and 94°C for 2 min, followed by 30 cycles of 94°C for 15 s, 55°C for 30 s, 68°C for 48 s, and 68°C for 5 min, followed by 4°C for 10 min.
Antibody heavy-chain and light-chain gene products were separately cloned into a mammalian expression vector, pJW4303. For expression, equal amounts of heavy- and light-chain plasmid DNAs were transfected with 293Fectin into Freestyle 293F cells (Invitrogen). The cell culture supernatant containing the secreted rabbit IgG was harvested 2 days after transfection and purified using protein A-Sepharose columns (GE Healthcare).
Analysis of rabbit MAb heavy-chain and light-chain sequences.
Sequencing analysis was conducted with the rabbit heavy-chain and light-chain genes of rabbit MAb. ClustalW was used, and the multiple-sequence alignments were done to construct phylogenetic trees using maximum-likelihood methods. The trees were then graphically edited (23). Antibody germ line usage, mutation frequency, and CDR3 length were determined by means of IMGT/V-QUEST (24).
Neutralization assays.
In-house neutralization assays were done as previously described (25). HIV pseudoviruses were first produced and titrated. Briefly, equimolar quantities of a plasmid expressing gp160 from the HIV-1 Env of interest and the pSG3ΔEnv HIV backbone were cotransfected into HEK 293T cells. At 48 h after transfection, supernatants were harvested and cleared of cell debris by low-speed centrifugation. Produced pseudoviruses were titrated on TZM-bl cells using a 2-fold increase above background levels of luciferase activity as a positive cutoff.
For an in-house neutralization assay, pseudovirus (200 50% tissue culture infective doses [TCID50]) was incubated with the rabbit sera or rabbit MAb for 1 h at 37°C. The virus-MAb mix was then added to 105 TZM-bl cells in a final concentration of 20 μg/ml DEAE dextran. Plates were incubated at 37°C for 48 h and developed with luciferase assay reagent according to the manufacturer's instruction (Promega). Neutralization was calculated as the percent change in luciferase activity in the presence of preimmune sera versus that of luciferase activity in the presence of immune sera as follows: [(preimmune RLUs − immune RLUs)/(preimmune RLUs)] × 100 (where RLU = relative light units).
Selected RMAbs were tested in a high-throughput neutralization assay using the PhenoSense assay at Monogram Biosciences (South San Francisco, CA) as previously reported (12).
Additional neutralization assays were conducted at Duke University and at Beth Israel Deaconess Medical Center, Harvard Medical School, according to their established protocols as described in previous reports (10).
Binding kinetics of rabbit MAbs.
The kinetics of binding of rabbit MAb to five representative gp120s from HIV-1 clades A to E were studied using a ForteBio Octet QKe instrument based on biolayer interferometry (BLI) measurements in a 96-well format following the manufacturer's instructions. Protein A-coated tips (ForteBio) were loaded with rabbit MAbs at 10 μg/ml diluted in the buffer of PBS–0.1% bovine serum albumin (BSA)–0.002% Tween 20. After capture, a 1-min wash in loading buffer removed excess unbound Ab to establish a new baseline signal. The biosensor tip was then put into wells containing the selected gp120 proteins at 3-fold serially diluted concentrations of from 100 to 11 nM in loading buffer.
On-rate rabbit MAb-gp120 association was measured using a 3-min interval followed by putting the sensor in wells containing regenerating buffer (500 mM phosphoric acid) to measure the (off-rate) dissociation rate over a 10-min interval. Equilibrium dissociation constant (KD) values were calculated as the ratio of off-rate values to on-rate values. The sensorgrams were corrected with the blank reference and fitted with ForteBio Data Analysis software package 7.0 using a 1:1 binding model with the global fitting function (grouped by color [Rmax]).
RESULTS
Generation of HIV-1 Env-specific hybridoma cell lines from a rabbit immunized with the DNA prime-protein boost JR-FL gp120 vaccine.
In this pilot study to explore the feasibility of producing recombinant rabbit MAb specific for HIV-1 Env, one NZW rabbit (9) was used for MAb isolation and characterization. During the initial immunogenicity study, this rabbit received three DNA inoculations followed by two inoculations with gp120 protein from HIV-1 JR-FL (Fig. 1). By the time we decided to produce RMAb from this rabbit, it had been 16 weeks since the last immunization. Therefore, this rabbit was boosted further with a one-time JR-FL gp120 DNA immunization and a one-time recombinant JR-FL gp120 protein immunization separated by 4 weeks. Four days after the final protein boost, the rabbit spleen was harvested and sent to a subcontractor for production of hybridoma cells (Fig. 1).
Fig 1.
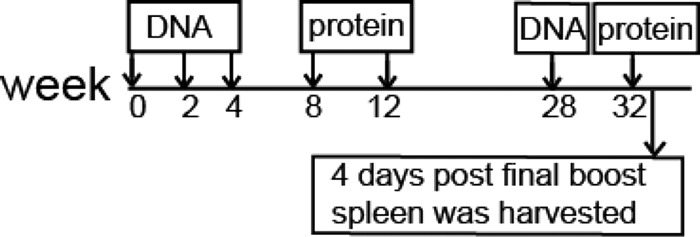
Schematic representation of the rabbit immunization schedule. The rabbit was given HIV Env-encoding DNA three times at 2-week intervals followed by two (monthly) matched protein boosts. After a 16-week resting period, this rabbit was further boosted with 1× DNA and 1× protein boost. The rabbit spleen was harvested 4 days after the final protein boost to generate hybridoma cell lines.
A total of 57 hybridoma cells (multiclonal stage) were screened for their supernatant reactivity to the JR-FL gp120 antigen (Fig. 2). Among them, 36 (63%) tested positive by ELISA, including 2 (R39 and R47) that were considered weakly positive (Fig. 2A). Western blot analysis further confirmed that a smaller fraction (17 hybridoma cells; 30%) had various levels of positivity against denatured JR-FL gp120 antigen (Fig. 2B). Based on the above screening results, a panel of 12 hybridoma cell lines (lines 13, 15, 20, 27, 28, 31, 35, 41, 43, 52, 53, and 56) was selected for further production of stable hybridoma cells (monoclonal stage), including three that recognized only native gp120 proteins (R27, R31, and R52), with the rest binding to both native and denatured gp120 proteins.
Fig 2.
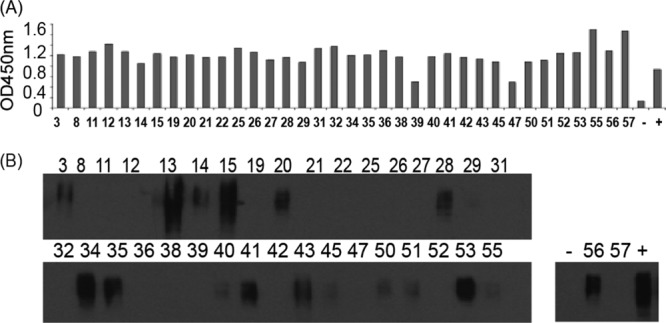
Initial screen of 36 multiclonal stages of hybridoma cell lines by their binding to HIV-1 JR-FL gp120 protein. (A) Binding of rabbit hybridoma supernatants to HIV-1 JR-FL gp120 protein was tested by ELISA. The rabbit serum samples prior to immunization and at week 14 served as the negative (−) and positive (+) controls, respectively. OD450nm, optical density at 450 nm. (B) Testing hybridoma supernatants against JR-FL gp120 protein by Western blotting. The positive and negative controls were the same as described for panel A.
Diverse epitope profiles of gp120-specific RMAbs elicited by JR-FL gp120 immunogen.
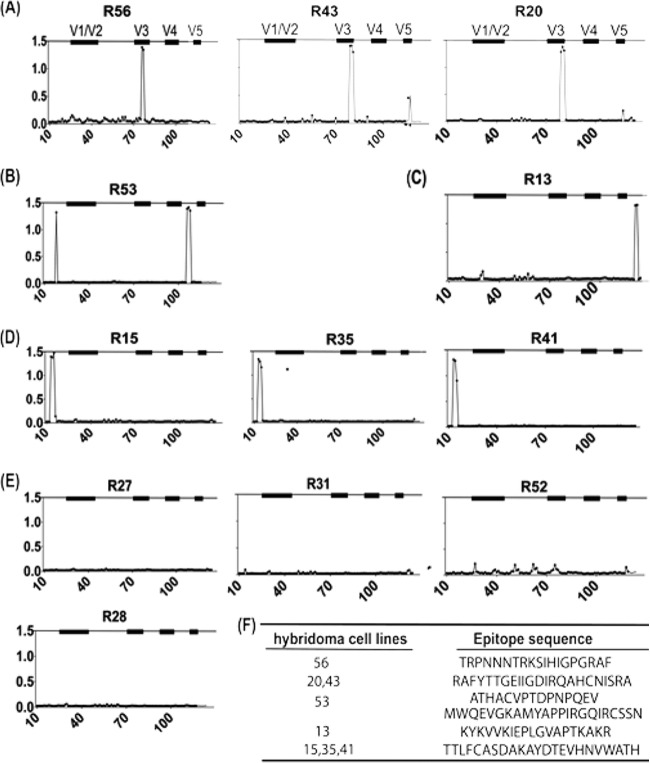
The epitope specificity of these RMAbs was determined by an ELISA against linear peptides (15 amino acid -aa) residues each with 11 aa overlapping) spanning the entire length of consensus gp120 from group B of HIV-1. These 12 rabbit hybridoma cell lines could be divided into 5 groups based on their antigen epitope specificity (Fig. 3).
Fig 3.
Diverse repertoire of gp120-specific rabbit monoclonal hybridoma supernatants revealed by ELISA with plates coated with individual consensus B gp120 overlapping peptides. RMAbs were classified into the following five groups: V3 specific (R20, R43, and R56) (A); C4 specific (R53) (B); C5 specific (R13) (C); C1 specific (R15, R35, and R41) (D); and unknown epitopes (R27, R31, R52, and R28) (E). (F) List of identified epitope sequences for selected rabbit hybridoma cell lines.
(i) V3-specific hybridomas—R20, R43, and R56 (Fig. 3A).
These hybridomas recognized two different epitopes within V3 region. One hybridoma, R56, recognized the V3 crown, the principal target for most anti-V3 MAbs isolated from patients chronically infected with HIV-1. The other hybridomas, R20 and R43, recognized the C-terminal portion of the V3 region, an epitope not commonly seen by human MAbs but recently described as part of a key neutralizing epitope for several highly potent human MAbs, such as PGT128 (26).
(ii) C4-specific hybridoma—R53 (Fig. 3B).
Based on the epitope peptide sequence, R53 binds to the bridging sheet of gp120, a rare epitope reported only in a mouse study in the early days of HIV-1 research (27, 28). One unique feature of R53 is that it can bind to two discontinuous linear epitopes in the C1 and C4 regions, although there is limited sequence homology between these two peptides.
(iii) C5-specific hybridoma—R13 (Fig. 3C).
This hybridoma binds to the far C terminus of gp120, a region known to be immunodominant (29).
(iv) C1-specific hybridomas—R15, R35, and R41 (Fig. 3D).
Supernatants from these three hybridoma cell lines were able to bind to the same three constitutive peptides in the first constant region of HIV-1 Env.
(v) Hybridomas with an unknown epitope(s)—R27, R28, R31, and R52 (Fig. 3E).
These RMAbs did not bind to any of the peptides included in the overlapping M-group Env peptide pool. R27, R31, and R52 may recognize conformational epitopes, as they showed positive ELISA binding to ConA-captured JR-FL gp120 (Fig. 2A) but no binding as observed by Western blotting to the same but denatured gp120 antigen (Fig. 2B). For R28, because it showed positive binding by both ELISA and Western blot analysis to JR-FL gp120, it is possible that R28 can recognize only a JR-FL-specific linear peptide that may be different from the sequences of the consensus clade B overlapping peptides.
Crystal structures have been produced for several RMAbs with known linear epitopes described in the current study, which confirmed the epitopes we identified in peptide-mapping ELISA. In particular, three RMAbs (R20, R56, and R53) have been cocrystalized with their respective targeted peptides, as described above for the epitope-mapping analysis, confirming the accuracy of the mapping of epitopes by ELISA (65).
Broad binding profile and high affinity of RMAbs for gp120 proteins from different clades of HIV-1.
The gene transcripts of the full-length IgG heavy chains and light chains from the above-described RMAbs were cloned from these hybridoma cells by RT-PCR and further subcloned into a mammalian protein expression vector. Individual RMAbs were produced from 293T cells transiently transfected with these RMAb-expressing molecular clones.
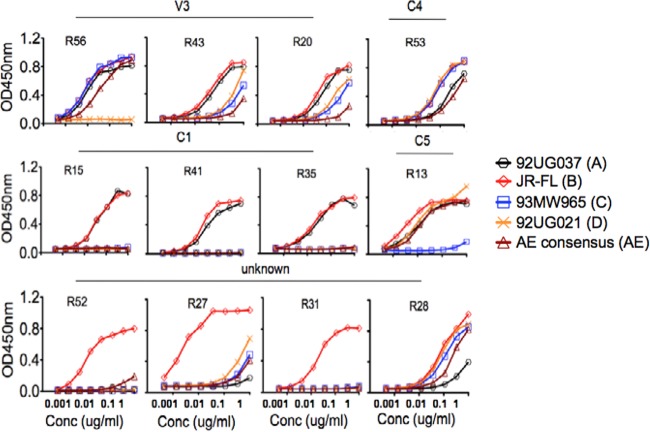
RMAbs were individually characterized for their ability to bind to a panel of five recombinant gp120 proteins (92UG037, JR-FL, 93MW965, 92UG021, and consensus AE Env, representing clades A, B, C, D, and E, respectively) (Fig. 4). All isolated rabbit MAbs were able to bind well to JR-FL gp120 by ELISA but had different levels of binding against other gp120 antigens. Among the V3-specific RMAbs, R56 binds to four gp120 proteins but not to that from the clade D isolate, 92UG021. The tip of the V3 loop for clade D gp120, 92UG021, has a sequence (GVGR) that is very different from the more common GPGR or GPGQ sequences seen with other clades, in addition to having unique sequences immediately upstream of the V3 tip (Fig. 5). The other two V3 RMAbs, R20 and R43, bind gp120 proteins from JR-FL and the clade A isolate, 92UG037, better than they bind the other three gp120 proteins from the clade C, D, and E isolates. This is also consistent with overlapping peptide ELISA (Fig. 3) and phylogenic lineage (Fig. 6) results, which showed that R20 and R43 grouped together in both analyses. Such data do not indicate that these two MAbs can be used to differentiate Env from different subtypes.
Fig 4.
The reactivity of 12 RMAbs with a panel of representative gp120 proteins revealed by ELISA. HIV-1 Env gp120 proteins were derived from the following isolates: 92UG037, JR-FL, 93MW965, 92UG021, and consensus AE. Conc, concentration.
Fig 5.

V3 region protein sequences from isolates JR-FL, 92UG037, 93MW965, 92UG021, and consensus AE (AE cons). The residue position refers to the HXB2 strain. The R56 epitope is highlighted in orange, and the R20 epitope is in blue. The amino acids within the V3 region that differed from those within the JR-FL strain are highlighted in red.
Fig 6.
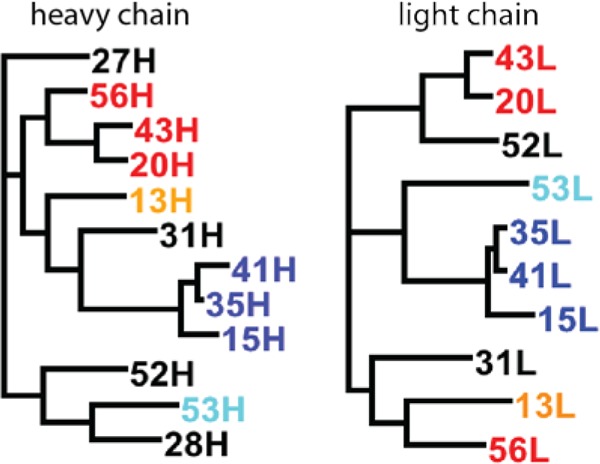
Phylogenetic tree of heavy-chain (left) and light-chain (right) gene sequences constructed using the maximum-likelihood method. V3-specific RMAbs are highlighted in red, C1-specific MAbs in orange, C4-specific MAbs in cyan, C5-specific MAbs in blue, and conformational/unknown RMAbs in black.
R53 is one RMAb that was broadly reactive with all five gp120 proteins from different clades. In contrast, C1-targeted RMAbs (R15, R41, and R35) uniformly bind to two gp120 proteins from clade B (JR-FL) and clade A (92UG037) with high efficiency.
Three RMAbs recognizing conformational epitopes, R52, R31, and R27, appeared to be highly specific to JR-FL gp120, whereas low to no detectable was observed for the other four gp120 proteins tested. R28, which targeted an unknown linear epitope, was broadly reactive to all five gp120 proteins, but the overall binding affinity was low, especially to gp120 protein from the clade A isolate, 92UG037.
A more detailed analysis of binding kinetics, including the binding affinity of these RMAbs, was further performed using a ForteBio instrument based on recently developed biolayer interferometry (BLI) technology. This system is similar to the traditional Biacore technology but is more user-friendly and can simultaneously process a large number of samples. Actual measurements for three RMAbs (R15, R53, and R56) are shown in Fig. 7. All RMAbs exhibited remarkably high binding affinity to JR-FL gp120, with KD ranging from 0.02 nM to 4.47 nM (Fig. 7).
Fig 7.
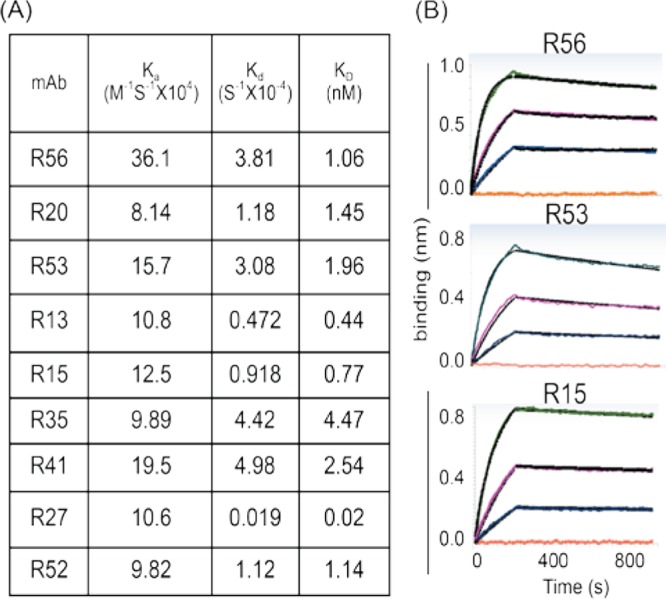
Binding affinity of rabbit MAbs to JR-FL Env gp120 protein determined by a ForteBio Octet QKe system. Rabbit MAbs were immobilized on the chip surface, and the JR-FL gp120 proteins were added in the testing solution. (A) Rate constants Ka (equilibrium association constant), Kd (dissociation constant), and KD for rabbit MAbs binding to JR-FL gp120, which were obtained by global fitting of the curves to the 1:1 binding model. (B) The binding kinetics of three selected RMAbs, R56, R53, and R15, to 3-fold serial dilution of JR-FL gp120 protein.
Gene sequence analysis of HIV-1 gp120-specific RMAbs.
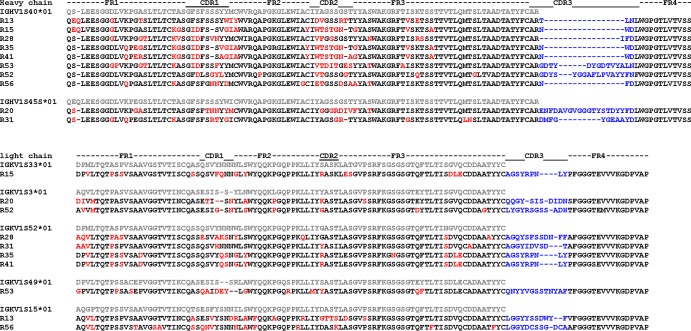
The full-length IgG heavy-chain and light-chain genes of the RMAbs were sequenced. Germ line family usage, mutation frequency, and CDR3 length in heavy-chain and light-chain genes were determined using IMGT and V-QUEST (Fig. 8 and Table 1). Consistent with a recent report (30), most of the isolated RMAb heavy-chain genes preferentially utilized IGHV1S40*01 (IMGT numbering system) or a3-VH1 (Kabat numbering system) as the germ line family gene whereas only R13 and R31 used IGHV1S45*01 (IMGT numbering system) or a3-VH4 (Kabat numbering system). For the light-chain genes, diverse germ line families were utilized to generate gp120-specific MAbs.
Fig 8.
Protein sequences of heavy- and light-chain variable regions of gp120-specific rabbit MAbs. The sequences are aligned to their corresponding putative germ line genes. Framework regions (FR) and CDRs are indicated based on IMGT (http://www.imgt.org/) nomenclature. Somatic mutated amino acids compared to the putative germ line are in red and CDR3 regions in blue.
Table 1.
Characteristics of the variable region of heavy chains and light chains of the vaccine-elicited rabbit MAbs
| Epitope | RMAb | Heavy chain |
Light chain |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IGHV |
IGHJ |
CDR3 length (aa) | IGKV |
IGKJ |
CDR3 length (aa) | ||||||
| Germ line | Mutation (%) | Germ line | Mutation (%) | Germ line | Mutation (%) | Germ line | Mutation (%) | ||||
| V3 | R56 | S40*01 | 6 | 4*01 | 9 | 6 | S15*01 | 9 | 2*01 | 10 | 13 |
| R20 | S40*01 | 6 | 4*01 | 5 | 23 | S3*01 | 8 | 2*01 | 3 | 12 | |
| R43 | S40*01 | 6 | 4*01 | 6 | 6 | S37*01 | 8 | 2*01 | 3 | 11 | |
| C4 | R53 | S40*01 | 10 | 4*01 | 9 | 17 | S49*01 | 9 | 2*01 | 13 | 15 |
| C1 | R15 | S40*01 | 27 | 4*01 | 11 | 6 | S33*01 | 12 | 2*01 | 10 | 10 |
| R35 | S40*01 | 26 | 4*01 | 11 | 6 | S52*01 | 10 | 2*01 | 3 | 9 | |
| R41 | S40*01 | 26 | 4*01 | 11 | 6 | S52*01 | 9 | 2*01 | 9 | 10 | |
| C5 | R13 | S45*01 | 5 | 4*01 | 9 | 6 | S15*01 | 11 | 2*01 | 8 | 12 |
| Conformational/unknown | R27 | S40*01 | 23 | 4*01 | 15 | 15 | S10*01 | 11 | 2*01 | 10 | 10 |
| R28 | S40*01 | 10 | 4*01 or 2*01 | 11 | 6 | S52*01 | 9 | 2*01 | 10 | 13 | |
| R31 | S45*01 | 22 | 2*01 | 12 | 14 | S52*01 or S64*01 | 7 | 2*01 | 5 | 11 | |
| R52 | S40*01 | 8 | 4*01 or 4*02 | 3 | 20 | S3*01 | 8 | 2*01 | 3 | 12 | |
Somatic hypermutation (SHM) is a key parameter in evaluating antibody affinity maturation. In the most studied model of influenza immunization, the mean heavy-chain variable (VH) gene mutation frequency of human antibodies induced by repeated vaccination is ∼5% (31, 32). Among 12 gp120-specific RMAbs identified in the current study, heavy-chain V genes exhibited an average SHM rate of 14.6% ± 9.2% (ranging from 5% to 27%) at the amino acid level, while light-chain V genes exhibited an average SHM rate of 9.25% ± 1.5% (ranging from 7% to 12%), indicating that the immunization regimen used in this study was effective in stimulating a high SHM rate for gp120 antigen-specific Ab genes (Table 1). The mutated amino acids for heavy chains were mainly distributed in the CDR1 and CDR2 regions, while SHM occurred across the light-chain kappa gene (Fig. 8). Examination of the heavy-chain CDR3 region revealed an average length of 10.9 ± 6.5 aa (ranging from 6 to 23 aa), and the light-chain CDR3 region contained an average of 11.5 ± 1.7 aa (ranging from 8 to14 aa) (Table 1), compatible with rabbit MAbs elicited from immunization, as previously reported (33).
To define the genetic relationship of these RMAbs, phylogenetic trees were constructed based on heavy-chain and light-chain gene sequences using the maximum-likelihood method (Fig. 6). Interestingly, RMAbs targeting the same region of gp120 tend to cluster to form an independent subtree. For example, heavy chains and light chains from R15, R35, and R41 (all targeting the C1 region) formed independent subtrees. Similarly, the heavy chains from three V3-specific RMAbs, R56, R20, and R43, grouped into another subtree, whereas the light-chain genes of R43 and R20 clustered in a subtree but not with R56. This demonstrates a process of clonally related B cell lineage development and affinity maturation. At the same time, building a phylogenetic tree may help determine the biological relevance of Env-specific RMAbs.
The breadth and potency of neutralizing activities with individual gp120-specific RMAbs.
A screening neutralization assay was conducted using an in-house assay at the University of Massachusetts Medical School to first identify those RMAbs with possible neutralizing activities (Table 2). Because this particular rabbit was immunized with a clade B gp120 antigen, a standardized in-house TZM-bl assay was used with a small panel of pseudotyped viruses expressing HIV-1 Env antigens from clade B viruses representing both neutralization-sensitive (tier 1) and neutralization-resistant (tier 2) primary isolates. Among 12 isolated RMAbs, 3 showed positive neutralizing activities in this initial screening assay. V3-specific R56 displayed potent neutralizing activity against SF162 (50% inhibitory concentration [IC50] = 0.02 μg/ml) and to a lesser degree against another sensitive tier 1 virus, SS1196 (IC50 = 4.0 μg/ml), and a tier 2 virus, QH0692 (IC50 = 8.6 μg/ml). C4-specific R53 had potent neutralizing activity against SF162 (IC50 = 0.5 μg/ml) but much lower activity against SS1196 (IC50 = 31.9 μg/ml). It is interesting to see that R15, a C1-specific RMAb, was also able to neutralize SF162 and SS1196, suggesting a novel neutralizing epitope within the C1 region, at least with respect to certain sensitive viruses.
Table 2.
Neutralization titers of rabbit MAbs by in-house TZM-bl assaya
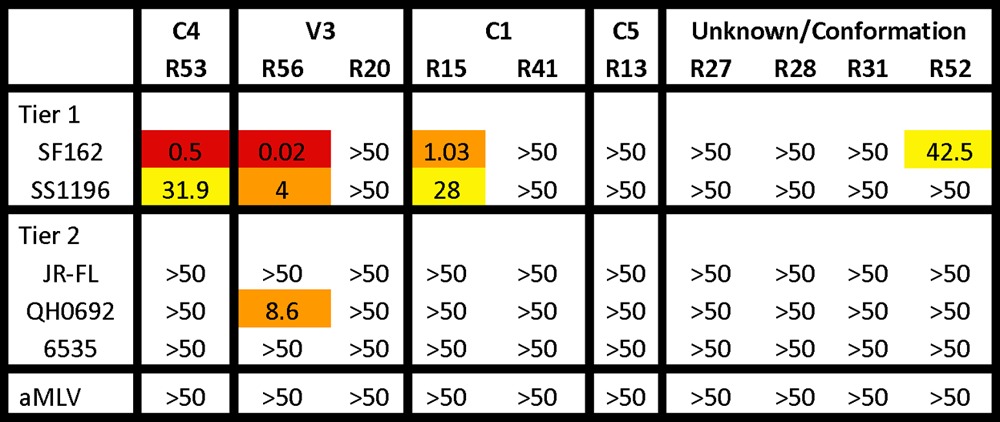
Data represent IC50 values in μg/ml. Red, <1 μg/ml; orange, >1 to <10; yellow, >10 to <50.
To further map the breadth of neutralizing activities of RMAbs, a high-throughput neutralizing assay was conducted at Monogram, Inc., using its PhenoSense assay system. R53 and R56 were tested against three highly sensitive viruses in addition to 13 primary isolates covering clades A to AE (Table 3). V3-specific R56 showed neutralizing activities against SF162 and QH0692, confirming the screening assay results, but was unable to neutralize other pseudotyped viruses. Interestingly, C4-specific R53 neutralized all three sensitive viruses with high potency (IC50 < 1.9 μg/ml) and a wide range of primary isolates from various clades, although at low potency.
Table 3.
Neutralization titers of rabbit MAbs by PhenoSense assay at Monogram Biosciencesa
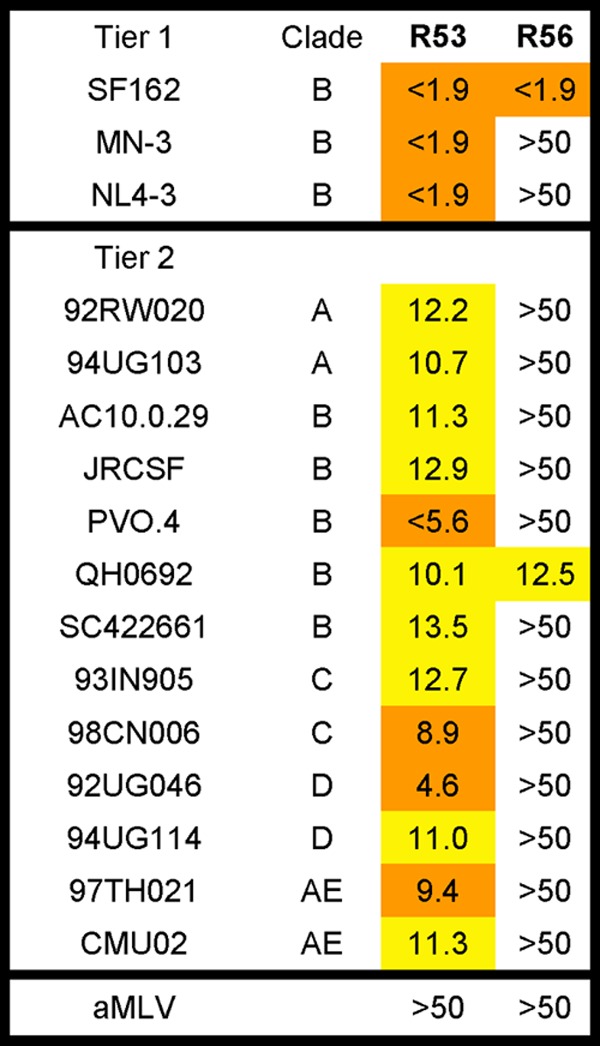
Data represent IC50 values in μg/ml. Orange, >1 to <10 μg/ml; yellow, >10 to <50.
Final neutralization assays were conducted by the Comprehensive Antibody Vaccine Immune Monitoring Consortium (CAVIMC) of the Collaboration for AIDS Vaccine Discovery (CAVD) program supported by the Bill & Melinda Gates Foundation. RMAb R56, R53, and R15 were tested against a panel of well-characterized pseudotyped viruses expressing Env from a wide range of primary HIV-1 isolates, including 19 tier 1 viruses (2 clade A, 7 clade B, 6 clade C, 1 clade AE, and 3 clade AG) and 25 tier 2 viruses (3 clade A, 6 clade B, 3 clade C, 6 clade AE, 3 clade AG, 2 clade BC, and 2 clade G) (Table 4). V3-specific R56 showed potent neutralizing activities (IC50 titer < 1 μg/ml) against tier 1 clade B virus SF162.LS, clade C virus MW965.26, and clade AG virus DJ263.8, while it exhibited moderate to low neutralizing activities against three tier 1 clade B viruses (SS1196.1, Bal.26, and Bx.08.16), one tier 1 clade C virus (TV1.21), one clade AE virus (Th023.6), and one clade AG virus (T271-11). C4-specific R53 was also able to neutralize 3 tier 1 viruses (SF162.LS, MW965.26, and Bx08.16) and 1 tier 2 virus (Thro4156.18) at low potency. No other viruses on this panel were neutralized by R53. C1-specific R15 was able to neutralize seven tier 1 viruses, including SF162.LS, SS1196.1, and Bx.08.16 (clade B), MW965.26 and TV1.21 (both are clade C), TH023 (clade AE), and 242-14 (clade AG), and 1 clade C tier 2 virus, ZM135M.PL10a.
Table 4.
Neutralization titers of rabbit MAbs by TZM-bl assay at CAVIMCa
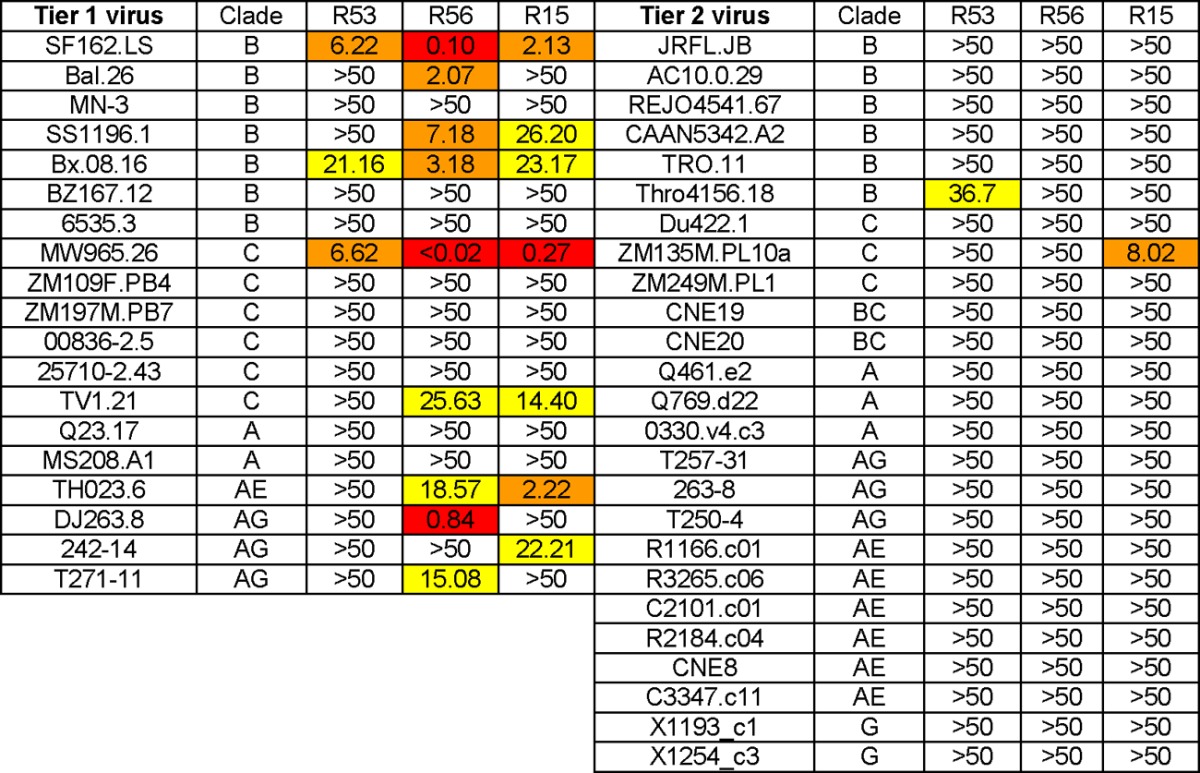
Data represent IC50 values in μg/ml. Red, <1 μg/ml; orange, >1 to <10; yellow, >10 to <50.
Comparing the neutralization results determined by different laboratories, the Monogram PhenoSense assay revealed that R53 had broader neutralization activities against some tier 2 viruses but that none of them were neutralized by R53 in the in-house test and CAVIMC assay. This discrepancy may be due to differences in target cells and assay systems: the Monogram PhenoSense assay used U87 cells, while both the CAVIMC test and the in-house test used TZM-bl cells. Of note, although PVO.4 was used as a tier 2 virus, it was usually considered a tier 3 virus and was neutralized by R53 at relatively high potency. Several tier 1 isolates, such as SS1196 and 6535, are much less sensitive to neutralization than the highly sensitive SF162 strain. R56 and R15 were able to neutralize SS1196 in both the in-house test and the CAVIMC test, while the R53 result was positive in the in-house test.
DISCUSSION
Human monoclonal antibodies against HIV-1 Env have played an important role in helping scientists understand the structural features of potential immunogen designs (34). During the first 20 years of HIV research, only four broadly cross-reactive neutralizing HMAbs (2F5, 4E10, 2G12, and b12) have been identified from HIV-1-infected individuals via the traditional Epstein-Barr virus (EBV) transformation or phage display library technologies (35–38). There are also V3-specific human MAbs that can neutralize multiple HIV-1 isolates (39, 40), but, in general, the breadth of neutralizing activities is more limited for V3 MAbs than for the four MAbs mentioned above.
New progress in antigen-specific single B cell sorting (32, 41), high-throughput microneutralization screens (42), and deep sequencing of Ig genes (43) has produced more broadly neutralizing Abs (bnAbs) in recent years. These included those targeting CD4 binding sites such as VRC01 to VRC03 (41), PGV04 (44), and HAAD motif (43) antibodies. The discovery of PG9 and PG16 revealed new bnAb targets at V1 and V2 or the quaternary conformational epitopes (42, 45), which were confirmed by other similar MAbs, such as CH01 to CH04 (46) and PGT141 to PGT145 (47). Another new group of bnAbs, exemplified by PGT125 to PGT128 and PGT130 and PGT131, recognized the C-terminal part of the V3 epitope along with two conserved glycans (47).
One caveat concerning these bnAbs is that they were isolated from chronically infected individuals. Therefore, it is not clear whether findings from infected humans can be directly translated into vaccines designed to be tested in a healthy uninfected human population. In vaccine settings, much lower immunogen dosing and a shorter exposure time are used for the healthy population than for those with chronic HIV-1 infection. The unique features of Env-specific B cells observed in HIV-1-infected patients, such as hyperactivation of B cells and continuously elevated immunoglobin levels (48), which are responsible for the high degree of somatic mutation, long CDR3, and high autoreactivity of Env-specific antibodies, are lacking in healthy humans. Recently, a small panel of antibodies isolated from the RV144 clinical trial vaccines was reported (8). They targeted the V2 region and have only modest neutralization, and yet they may be correlated to protection (2).
Such progress indicates that (i) there is a need to model future HIV vaccine designs according to these novel human bnAbs and (ii) further investigation of the full spectrum of antibodies elicited by HIV vaccines is warranted to understand whether there are additional targets on Env that have not been discovered. More importantly, these new studies have to be first conducted in animal models before the next generation of HIV vaccines can be tested in the more expensive and time-consuming human studies. Therefore, production of MAb should be included in such preclinical studies to investigate the functional and genetic differences between infection-elicited MAbs and vaccine-induced MAbs.
Unfortunately, experience in producing high-quality HIV-1 Env-specific MAb from animal models is limited. Recently, a panel of CD4bs-directed macaque MAbs elicited by an Env vaccine was identified (49) but the high cost of NHP studies prevents the wide adoption of this system. Historically, murine MAbs were unable to achieve good neutralizing activities, partially due to the poor efficacy in generating a long CDR3 in mouse MAbs (17, 18). Transgenic mouse systems expressing human antibodies are well established; however, many of these systems are under the control of major pharmaceutical companies, preventing scientists not affiliated with these companies from having easy access to these models due to intellectual property barriers. Newly developed humanized mice models are making progress in HIV infection studies, including the analysis of T cell responses, but their use for B cell and antibody development has not been fully developed (63, 64).
Results presented in this report established the feasibility of producing high-quality Env-specific and potentially functional rabbit MAbs. Rabbit is an attractive model to study antibody responses to a wide range of emerging infectious diseases vaccines, including HIV-1 vaccines (9, 10, 12, 13, 50, 51). Rabbits share features in B cell development similar to those in mice and human and those of gut-associated lymphoid tissue (GALT) species, such as chickens, sheep, and cows (52). B lymphopoiesis in rabbit occurs in a manner similar to that in the human and mice classical pathway in the bone marrow; however, since rabbit is a member of the GALT species, rabbit B lymphopoiesis terminates during early development (53). The exogenous antigen further expands and diversifies B cells in GALT species through somatic hypermutation and gene conversion. Furthermore, phylogenetic analysis showed that human variable heavy-chain (VH) genes can be divided into three major groups, A, B, and C, and all rabbit VH genes are closely related to one another and form one monophyletic group, which is included in VH group C (54). Among those VH genes, most rabbit B lineage cells rearranged the same VH gene, VH1, which encodes the VHa allotypic sequence, and rabbits preferentially use it to produce functional antibodies (55). The rabbit humoral system can serve as a simplified testing platform to study the evolution pathway of antibody genes, since rabbit adopted a much less complicated germ line usage than mouse and human. On the other hand, it was speculated that no VRC01-like antibody would be raised in rabbits due to the lack of an optimal germ line VH segment (56).
The phylogenetic analysis suggests that the human and rabbit Ig genes evolved at nearly the same rate. In contrast, most mouse genes evolved considerably faster than human and rabbit genes (54). Gene conversion and somatic hypermutation not only occur in the germinal center (GC) of the young rabbit appendix but are also used for diversification during the immune response in secondary lymphoid tissues, such as the spleen (57). Rabbit B cells see antigen similarly to cells of the human immune system (58). A rabbit hybridoma fusion partner was developed previously (20), and optimization of the traditional rabbit fusion partner cells and fusion methods greatly facilitated the generation of monoclonal antigen-specific rabbit hybridoma cell lines (US patents 7,575,896 B2 [21] and 7,732,168 B2 [22]), allowing relatively efficient production of RMAbs and use in our current study as a contracted service.
In this first report of HIV-1 Env-specific rabbit MAb, a wide diversity of epitopes was identified from a single immunized rabbit, which showed high levels of serum polyclonal antibodies against the autologous JR-FL gp120 antigen. Besides the common V3 loop-specific rabbit MAbs, other rabbit MAbs were produced against both linear epitopes at either the N-terminal or C-terminal region of gp120 and conformational epitopes. Several epitopes were not previously known. At the time of this study, the role of V2-specific antibodies was not known; therefore, efforts were not devoted to cloning V2-specific RMAbs in this study. However, serum from this rabbit did show positive antibody responses, recognizing gp70-V1V2 antigen and indicating the presence of V2-specific antibodies.
Three of 12 RMAbs cloned in this pilot study showed positive neutralizing activities. R15 indicated a previously unknown neutralizing epitope in the C1 region of Env, as it was able to neutralize several viral isolates across subtypes B, C, AE, and AG, although these viruses are from tier 1 groups and the potency of R15 was low. V3-specific R56 demonstrated high potency against those viral isolates that were sensitive to V3 neutralization, but the breadth of the activity of R56 was limited, as would be expected from MAbs recognizing the tip of the V3 loop.
The third neutralizing MAb, R53, was the only one in this pilot study that was able to bind all five gp120 antigens from subtype A to subtype E and also showed broad neutralizing activities in Monogram assays despite low potency. Interestingly, previous studies reported that several murine MAbs (28), as well as rat MAbs (27), isolated from gp120 protein immunization were able to prevent gp120 binding to CD4. They mainly bound to a region on aa 430 to 447 of the C4 region, which is now known as part of the Env bridging sheet. Crystal structure analysis showed that R53 actually binds to the beta strand 21 region within the bridging sheet (data not shown). The bridging sheet, along with the interface of the outer domain and interdomain of Env, composes the CD4 binding site. Upon CD4 binding, the bridging sheet undergoes a conformational change and enables the formation and exposure of the coreceptor binding site. Given the broad reactivity of R53 to a wide range of primary gp120 proteins across different subtypes and its ability to neutralize multiple tier 1 pseudoviruses from different clades, future Env vaccine studies should further investigate the protection potential of this class of bridging sheet-targeting antibodies.
In this pilot study, RMAbs were first screened by conventional ELISA against recombinant monomer gp120 antigens. Screening based on recognition of viral particles or positive neutralizing antibody activities will improve the chance of identifying more functionally relevant MAbs. Furthermore, since the study was limited by the cost restraints associated with the contracted service used, not all gp120-positive hybridomas from the initial screening were further cloned, which reduced the understanding of the full spectrum of gp120-specific RMAbs elicited by immunization. Alternative technologies that can isolate individual gp120-positive B cells, similar to those developed for human MAbs, should be applied to RMAb production in future studies.
Studies of HIV-1-specific human MAbs have suggested that somatic hypermutation and unusually long heavy-chain CDR3, as two key indicators for affinity maturation, are usually required for broad and potent neutralizing activities of human MAbs (59–62). Rabbit MAbs identified in the current study showed various degrees of hypermutation and lengths of CDR3, suggesting that HIV-1 vaccines delivered in the current prime-boost format were able to initiate the antibody maturation process. It is not known whether the low potency observed in several neutralizing RMAbs could be improved if the affinity maturation process were further enhanced, such as by a different adjuvant. Also, the current study examined the status of affinity maturation only at the end of a full vaccination schedule. Observing the induction of gp120-specific antibody responses throughout the process starting from time points before immunization or early in immunization, by using the new RMAb platform, will provide more insights into this process. It is also possible that the observed high degree of somatic mutations in the derived MAbs is due to the multiple immunizations at the end, which deserves future study.
As learned from studies following the RV144 trial, antibody activities detected in vitro can link to correlates of immune protection, and identifying the effector MAbs would be helpful to evaluate vaccine-elicited humoral immune response. Isolation of MAbs from preclinical vaccine studies using small-animal or nonhuman primate models will help us understand the antibody response and B cell development driven by immunization. The RMAb platform described in the current report will provide a useful tool to achieve such goals.
ACKNOWLEDGMENTS
The study reported here was funded in part by NIH grants AI082274, AI082676, AI065250, and AI087191 and the Bill & Melinda Gates Foundation Collaboration for AIDS Vaccine Discovery/Comprehensive Antibody-Vaccine Immune Monitoring Consortium, grant OPP1033112.
We thank Jill M. Serrano for critical reading and editing of the manuscript.
Footnotes
Published ahead of print 17 July 2013
REFERENCES
- 1. Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH, MOPH-TAVEG Investigators 2009. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 361:2209–2220 [DOI] [PubMed] [Google Scholar]
- 2. Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. 2012. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N. Engl. J. Med. 366:1275–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rolland M, Edlefsen PT, Larsen BB, Tovanabutra S, Sanders-Buell E, Hertz T, deCamp AC, Carrico C, Menis S, Magaret CA, Ahmed H, Juraska M, Chen L, Konopa P, Nariya S, Stoddard JN, Wong K, Zhao H, Deng W, Maust BS, Bose M, Howell S, Bates A, Lazzaro M, O'Sullivan A, Lei E, Bradfield A, Ibitamuno G, Assawadarachai V, O'Connell RJ, de Souza MS, Nitayaphan S, Rerks-Ngarm S, Robb ML, McLellan JS, Georgiev I, Kwong PD, Carlson JM, Michael NL, Schief WR, Gilbert PB, Mullins JI, Kim JH. 2012. Increased HIV-1 vaccine efficacy against viruses with genetic signatures in Env V2. Nature 490:417–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Doria-Rose NA, Klein RM, Manion MM, O'Dell S, Phogat A, Chakrabarti B, Hallahan CW, Migueles SA, Wrammert J, Ahmed R, Nason M, Wyatt RT, Mascola JR, Connors M. 2009. Frequency and phenotype of human immunodeficiency virus envelope-specific B cells from patients with broadly cross-neutralizing antibodies. J. Virol. 83:188–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gray ES, Madiga MC, Hermanus T, Moore PL, Wibmer CK, Tumba NL, Werner L, Mlisana K, Sibeko S, Williamson C, Abdool Karim SS, Morris L, CAPRISA002 Study Team 2011. The neutralization breadth of HIV-1 develops incrementally over four years and is associated with CD4+ T cell decline and high viral load during acute infection. J. Virol. 85:4828–4840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sather DN, Armann J, Ching LK, Mavrantoni A, Sellhorn G, Caldwell Z, Yu X, Wood B, Self S, Kalams S, Stamatatos L. 2009. Factors associated with the development of cross-reactive neutralizing antibodies during human immunodeficiency virus type 1 infection. J. Virol. 83:757–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Simek MD, Rida W, Priddy FH, Pung P, Carrow E, Laufer DS, Lehrman JK, Boaz M, Tarragona-Fiol T, Miiro G, Birungi J, Pozniak A, McPhee DA, Manigart O, Karita E, Inwoley A, Jaoko W, Dehovitz J, Bekker LG, Pitisuttithum P, Paris R, Walker LM, Poignard P, Wrin T, Fast PE, Burton DR, Koff WC. 2009. Human immunodeficiency virus type 1 elite neutralizers: individuals with broad and potent neutralizing activity identified by using a high-throughput neutralization assay together with an analytical selection algorithm. J. Virol. 83:7337–7348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liao HX, Bonsignori M, Alam SM, McLellan JS, Tomaras GD, Moody MA, Kozink DM, Hwang KK, Chen X, Tsao CY, Liu P, Lu X, Parks RJ, Montefiori DC, Ferrari G, Pollara J, Rao M, Peachman KK, Santra S, Letvin NL, Karasavvas N, Yang ZY, Dai K, Pancera M, Gorman J, Wiehe K, Nicely NI, Rerks-Ngarm S, Nitayaphan S, Kaewkungwal J, Pitisuttithum P, Tartaglia J, Sinangil F, Kim JH, Michael NL, Kepler TB, Kwong PD, Mascola JR, Nabel GJ, Pinter A, Zolla-Pazner S, Haynes BF. 2013. Vaccine induction of antibodies against a structurally heterogeneous site of immune pressure within HIV-1 envelope protein variable regions 1 and 2. Immunity 38:176–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vaine M, Wang S, Hackett A, Arthos J, Lu S. 2010. Antibody responses elicited through homologous or heterologous prime-boost DNA and protein vaccinations differ in functional activity and avidity. Vaccine 28:2999–3007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang S, Arthos J, Lawrence JM, Van Ryk D, Mboudjeka I, Shen S, Chou TH, Montefiori DC, Lu S. 2005. Enhanced immunogenicity of gp120 protein when combined with recombinant DNA priming to generate antibodies that neutralize the JR-FL primary isolate of human immunodeficiency virus type 1. J. Virol. 79:7933–7937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang S, Kennedy JS, West K, Montefiori DC, Coley S, Lawrence J, Shen S, Green S, Rothman AL, Ennis FA, Arthos J, Pal R, Markham P, Lu S. 2008. Cross-subtype antibody and cellular immune responses induced by a polyvalent DNA prime-protein boost HIV-1 vaccine in healthy human volunteers. Vaccine 26:3947–3957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vaine M, Wang S, Crooks ET, Jiang P, Montefiori DC, Binley J, Lu S. 2008. Improved induction of antibodies against key neutralizing epitopes by human immunodeficiency virus type 1 gp120 DNA prime-protein boost vaccination compared to gp120 protein-only vaccination. J. Virol. 82:7369–7378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vaine M, Wang S, Liu Q, Arthos J, Montefiori D, Goepfert P, McElrath MJ, Lu S. 2010. Profiles of human serum antibody responses elicited by three leading HIV vaccines focusing on the induction of Env-specific antibodies. PLoS One 5:e13916. 10.1371/journal.pone.0013916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hellerstein M, Xu Y, Marino T, Lu S, Yi H, Wright ER, Robinson HL. 2012. Co-expression of HIV-1 virus-like particles and granulocyte-macrophage colony stimulating factor by GEO-D03 DNA vaccine. Hum. Vaccin Immunother. 8:1654–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Puri R, Suzuki T, Yamakawa K, Ganesh S. 2009. Hyperphosphorylation and aggregation of Tau in laforin-deficient mice, an animal model for Lafora disease. J. Biol. Chem. 284:22657–22663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shaftel SS, Kyrkanides S, Olschowka JA, Miller JN, Johnson RE, O'Banion MK. 2007. Sustained hippocampal IL-1 beta overexpression mediates chronic neuroinflammation and ameliorates Alzheimer plaque pathology. J. Clin. Invest. 117:1595–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Burton DR, Desrosiers RC, Doms RW, Koff WC, Kwong PD, Moore JP, Nabel GJ, Sodroski J, Wilson IA, Wyatt RT. 2004. HIV vaccine design and the neutralizing antibody problem. Nat. Immunol. 5:233–236 [DOI] [PubMed] [Google Scholar]
- 18. Gao F, Scearce RM, Alam SM, Hora B, Xia S, Hohm JE, Parks RJ, Ogburn DF, Tomaras GD, Park E, Lomas WE, Maino VC, Fiscus SA, Cohen MS, Moody MA, Hahn BH, Korber BT, Liao HX, Haynes BF. 2009. Cross-reactive monoclonal antibodies to multiple HIV-1 subtype and SIVcpz envelope glycoproteins. Virology 394:91–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Peters PJ, Bhattacharya J, Hibbitts S, Dittmar MT, Simmons G, Bell J, Simmonds P, Clapham PR. 2004. Biological analysis of human immunodeficiency virus type 1 R5 envelopes amplified from brain and lymph node tissues of AIDS patients with neuropathology reveals two distinct tropism phenotypes and identifies envelopes in the brain that confer an enhanced tropism and fusigenicity for macrophages. J. Virol. 78:6915–6926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Spieker-Polet H, Sethupathi P, Yam PC, Knight KL. 1995. Rabbit monoclonal antibodies: generating a fusion partner to produce rabbit-rabbit hybridomas. Proc. Natl. Acad. Sci. U. S. A. 92:9348–9352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yu GL. August 2009. Cell fusion method. US patent 7,575,896 B2
- 22. Pytela R, Zhu W, Ke Y, Qian R, Au HC. June 2010. Fusion partner for production of monoclonal rabbit antibodies. US patent 7,732,168 B2
- 23. Huson DH, Richter DC, Rausch C, Dezulian T, Franz M, Rupp R. 2007. Dendroscope: an interactive viewer for large phylogenetic trees. BMC Bioinformatics 8:460. 10.1186/1471-2105-8-460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lefranc MP, Giudicelli V, Ginestoux C, Jabado-Michaloud J, Folch G, Bellahcene F, Wu Y, Gemrot E, Brochet X, Lane J, Regnier L, Ehrenmann F, Lefranc G, Duroux P. 2009. IMGT, the international ImMunoGeneTics information system. Nucleic Acids Res. 37:D1006–D1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Montefiori DC. 2004. Evaluating neutralizing antibodies against HIV, SIV and SHIV in luciferase reporter gene assays, p 1–15 In Coligan JE, Kruisbeek AM, Margullies DH, Shevach EM, Strober W. (ed), Current protocols in immunology, vol 12 John Wiley, Hoboken, NJ: [DOI] [PubMed] [Google Scholar]
- 26. Pejchal R, Doores KJ, Walker LM, Khayat R, Huang PS, Wang SK, Stanfield RL, Julien JP, Ramos A, Crispin M, Depetris R, Katpally U, Marozsan A, Cupo A, Maloveste S, Liu Y, McBride R, Ito Y, Sanders RW, Ogohara C, Paulson JC, Feizi T, Scanlan CN, Wong CH, Moore JP, Olson WC, Ward AB, Poignard P, Schief WR, Burton DR, Wilson IA. 2011. A potent and broad neutralizing antibody recognizes and penetrates the HIV glycan shield. Science 334:1097–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cordell J, Moore JP, Dean CJ, Klasse PJ, Weiss RA, McKeating JA. 1991. Rat monoclonal antibodies to nonoverlapping epitopes of human immunodeficiency virus type 1 gp120 block CD4 binding in vitro. Virology 185:72–79 [DOI] [PubMed] [Google Scholar]
- 28. Sun NC, Ho DD, Sun CR, Liou RS, Gordon W, Fung MS, Li XL, Ting RC, Lee TH, Chang NT, Chang TW. 1989. Generation and characterization of monoclonal antibodies to the putative CD4-binding domain of human immunodeficiency virus type 1 gp120. J. Virol. 63:3579–3585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Palker TJ, Matthews TJ, Clark ME, Cianciolo GJ, Randall RR, Langlois AJ, White GC, Safai B, Snyderman R, Bolognesi DP, Haynes BF. 1987. A conserved region at the COOH terminus of human immunodeficiency virus gp120 envelope protein contains an immunodominant epitope. Proc. Natl. Acad. Sci. U. S. A. 84:2479–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cheung WC, Beausoleil SA, Zhang X, Sato S, Schieferl SM, Wieler JS, Beaudet JG, Ramenani RK, Popova L, Comb MJ, Rush J, Polakiewicz RD. 2012. A proteomics approach for the identification and cloning of monoclonal antibodies from serum. Nat. Biotechnol. 30:447–452 [DOI] [PubMed] [Google Scholar]
- 31. Moody MA, Zhang R, Walter EB, Woods CW, Ginsburg GS, McClain MT, Denny TN, Chen X, Munshaw S, Marshall DJ, Whitesides JF, Drinker MS, Amos JD, Gurley TC, Eudailey JA, Foulger A, DeRosa KR, Parks R, Meyerhoff RR, Yu JS, Kozink DM, Barefoot BE, Ramsburg EA, Khurana S, Golding H, Vandergrift NA, Alam SM, Tomaras GD, Kepler TB, Kelsoe G, Liao HX, Haynes BF. 2011. H3N2 influenza infection elicits more cross-reactive and less clonally expanded anti-hemagglutinin antibodies than influenza vaccination. PLoS One 6:e25797. 10.1371/journal.pone.0025797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wrammert J, Smith K, Miller J, Langley WA, Kokko K, Larsen C, Zheng NY, Mays I, Garman L, Helms C, James J, Air GM, Capra JD, Ahmed R, Wilson PC. 2008. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature 453:667–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Popkov M, Mage RG, Alexander CB, Thundivalappil S, Barbas CF, III, Rader C. 2003. Rabbit immune repertoires as sources for therapeutic monoclonal antibodies: the impact of kappa allotype-correlated variation in cysteine content on antibody libraries selected by phage display. J. Mol. Biol. 325:325–335 [DOI] [PubMed] [Google Scholar]
- 34. Clapham PR, Lu S. 2011. Vaccinology: precisely tuned antibodies nab HIV. Nature 477:416–417 [DOI] [PubMed] [Google Scholar]
- 35. Burton DR, Pyati J, Koduri R, Sharp SJ, Thornton GB, Parren PW, Sawyer LS, Hendry RM, Dunlop N, Nara PL, et al. 1994. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science 266:1024–1027 [DOI] [PubMed] [Google Scholar]
- 36. Conley AJ, Kessler JA, II, Boots LJ, Tung JS, Arnold BA, Keller PM, Shaw AR, Emini EA. 1994. Neutralization of divergent human immunodeficiency virus type 1 variants and primary isolates by IAM-41 to 2F5, an anti-gp41 human monoclonal antibody. Proc. Natl. Acad. Sci. U. S. A. 91:3348–3352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Trkola A, Purtscher M, Muster T, Ballaun C, Buchacher A, Sullivan N, Srinivasan K, Sodroski J, Moore JP, Katinger H. 1996. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J. Virol. 70:1100–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zwick MB, Labrijn AF, Wang M, Spenlehauer C, Saphire EO, Binley JM, Moore JP, Stiegler G, Katinger H, Burton DR, Parren PW. 2001. Broadly neutralizing antibodies targeted to the membrane-proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. J. Virol. 75:10892–10905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hioe CE, Wrin T, Seaman MS, Yu X, Wood B, Self S, Williams C, Gorny MK, Zolla-Pazner S. 2010. Anti-V3 monoclonal antibodies display broad neutralizing activities against multiple HIV-1 subtypes. PLoS One 5:e10254. 10.1371/journal.pone.0010254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zolla-Pazner S, Zhong P, Revesz K, Volsky B, Williams C, Nyambi P, Gorny MK. 2004. The cross-clade neutralizing activity of a human monoclonal antibody is determined by the GPGR V3 motif of HIV type 1. AIDS Res. Hum. Retroviruses 20:1254–1258 [DOI] [PubMed] [Google Scholar]
- 41. Wu X, Yang ZY, Li Y, Hogerkorp CM, Schief WR, Seaman MS, Zhou T, Schmidt SD, Wu L, Xu L, Longo NS, McKee K, O'Dell S, Louder MK, Wycuff DL, Feng Y, Nason M, Doria-Rose N, Connors M, Kwong PD, Roederer M, Wyatt RT, Nabel GJ, Mascola JR. 2010. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science 329:856–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Walker LM, Phogat SK, Chan-Hui PY, Wagner D, Phung P, Goss JL, Wrin T, Simek MD, Fling S, Mitcham JL, Lehrman JK, Priddy FH, Olsen OA, Frey SM, Hammond PW, Protocol G Principal Investigators. Kaminsky S, Zamb T, Moyle M, Koff WC, Poignard P, Burton DR. 2009. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science 326:285–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Scheid JF, Mouquet H, Ueberheide B, Diskin R, Klein F, Oliveira TY, Pietzsch J, Fenyo D, Abadir A, Velinzon K, Hurley A, Myung S, Boulad F, Poignard P, Burton DR, Pereyra F, Ho DD, Walker BD, Seaman MS, Bjorkman PJ, Chait BT, Nussenzweig MC. 2011. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science 333:1633–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Falkowska E, Ramos A, Feng Y, Zhou T, Moquin S, Walker LM, Wu X, Seaman MS, Wrin T, Kwong PD, Wyatt RT, Mascola JR, Poignard P, Burton DR. 2012. PGV04, an HIV-1 gp120 CD4 binding site antibody, is broad and potent in neutralization but does not induce conformational changes characteristic of CD4. J. Virol. 86:4394–4403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kimura T, Wang XH, Williams C, Zolla-Pazner S, Gorny MK. 2009. Human monoclonal antibody 2909 binds to pseudovirions expressing trimers but not monomeric HIV-1 envelope proteins. Hum. Antibodies 18:35–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bonsignori M, Hwang KK, Chen X, Tsao CY, Morris L, Gray E, Marshall DJ, Crump JA, Kapiga SH, Sam NE, Sinangil F, Pancera M, Yongping Y, Zhang B, Zhu J, Kwong PD, O'Dell S, Mascola JR, Wu L, Nabel GJ, Phogat S, Seaman MS, Whitesides JF, Moody MA, Kelsoe G, Yang X, Sodroski J, Shaw GM, Montefiori DC, Kepler TB, Tomaras GD, Alam SM, Liao HX, Haynes BF. 2011. Analysis of a clonal lineage of HIV-1 envelope V2/V3 conformational epitope-specific broadly neutralizing antibodies and their inferred unmutated common ancestors. J. Virol. 85:9998–10009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julien JP, Wang SK, Ramos A, Chan-Hui PY, Moyle M, Mitcham JL, Hammond PW, Olsen OA, Phung P, Fling S, Wong CH, Phogat S, Wrin T, Simek MD, Protocol G Principal Investigators. Koff WC, Wilson IA, Burton DR, Poignard P. 2011. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature 477:466–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. De Milito A, Nilsson A, Titanji K, Thorstensson R, Reizenstein E, Narita M, Grutzmeier S, Sonnerborg A, Chiodi F. 2004. Mechanisms of hypergammaglobulinemia and impaired antigen-specific humoral immunity in HIV-1 infection. Blood 103:2180–2186 [DOI] [PubMed] [Google Scholar]
- 49. Sundling C, Li Y, Huynh N, Poulsen C, Wilson R, O'Dell S, Feng Y, Mascola JR, Wyatt RT, Karlsson Hedestam GB. 2012. High-resolution definition of vaccine-elicited B cell responses against the HIV primary receptor binding site. Sci. Transl. Med. 4:142ra96. 10.1126/scitranslmed.3003752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang S, Chou TH, Sakhatskyy PV, Huang S, Lawrence JM, Cao H, Huang X, Lu S. 2005. Identification of two neutralizing regions on the severe acute respiratory syndrome coronavirus spike glycoprotein produced from the mammalian expression system. J. Virol. 79:1906–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang S, Taaffe J, Parker C, Solorzano A, Cao H, Garcia-Sastre A, Lu S. 2006. Hemagglutinin (HA) proteins from H1 and H3 serotypes of influenza A viruses require different antigen designs for the induction of optimal protective antibody responses as studied by codon-optimized HA DNA vaccines. J. Virol. 80:11628–11637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mage RG, Lanning D, Knight KL. 2006. B cell and antibody repertoire development in rabbits: the requirement of gut-associated lymphoid tissues. Dev. Comp. Immunol. 30:137–153 [DOI] [PubMed] [Google Scholar]
- 53. Jasper PJ, Zhai SK, Kalis SL, Kingzette M, Knight KL. 2003. B lymphocyte development in rabbit: progenitor B cells and waning of B lymphopoiesis. J. Immunol. 171:6372–6380 [DOI] [PubMed] [Google Scholar]
- 54. Su C, Nei M. 1999. Fifty-million-year-old polymorphism at an immunoglobulin variable region gene locus in the rabbit evolutionary lineage. Proc. Natl. Acad. Sci. U. S. A. 96:9710–9715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Knight KL, Becker RS. 1990. Molecular basis of the allelic inheritance of rabbit immunoglobulin VH allotypes: implications for the generation of antibody diversity. Cell 60:963–970 [DOI] [PubMed] [Google Scholar]
- 56. West AP, Jr, Diskin R, Nussenzweig MC, Bjorkman PJ. 2012. Structural basis for germ-line gene usage of a potent class of antibodies targeting the CD4-binding site of HIV-1 gp120. Proc. Natl. Acad. Sci. U. S. A. 109:E2083–E2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mage RG, Sehgal D, Schiaffella E, Anderson AO. 1999. Gene-conversion in rabbit B-cell ontogeny and during immune responses in splenic germinal centers. Vet. Immunol. Immunopathol. 72:7–15 [DOI] [PubMed] [Google Scholar]
- 58. McClain MT, Lutz CS, Kaufman KM, Faig OZ, Gross TF, James JA. 2004. Structural availability influences the capacity of autoantigenic epitopes to induce a widespread lupus-like autoimmune response. Proc. Natl. Acad. Sci. U. S. A. 101:3551–3556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bonsignori M, Alam SM, Liao HX, Verkoczy L, Tomaras GD, Haynes BF, Moody MA. 2012. HIV-1 antibodies from infection and vaccination: insights for guiding vaccine design. Trends Microbiol. 20:532–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Haynes BF, Fleming J, St Clair EW, Katinger H, Stiegler G, Kunert R, Robinson J, Scearce RM, Plonk K, Staats HF, Ortel TL, Liao HX, Alam SM. 2005. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science 308:1906–1908 [DOI] [PubMed] [Google Scholar]
- 61. Huang J, Ofek G, Laub L, Louder MK, Doria-Rose NA, Longo NS, Imamichi H, Bailer RT, Chakrabarti B, Sharma SK, Alam SM, Wang T, Yang Y, Zhang B, Migueles SA, Wyatt R, Haynes BF, Kwong PD, Mascola JR, Connors M. 2012. Broad and potent neutralization of HIV-1 by a gp41-specific human antibody. Nature 491:406–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wu X, Zhou T, Zhu J, Zhang B, Georgiev I, Wang C, Chen X, Longo NS, Louder M, McKee K, O'Dell S, Perfetto S, Schmidt SD, Shi W, Wu L, Yang Y, Yang ZY, Yang Z, Zhang Z, Bonsignori M, Crump JA, Kapiga SH, Sam NE, Haynes BF, Simek M, Burton DR, Koff WC, Doria-Rose N, Connors M, NISC Comparative Sequencing Program. Mullikin JC, Nabel GJ, Roederer M, Shapiro L, Kwong PD, Mascola JR. 2011. Focused evolution of HIV-1 neutralizing antibodies revealed by structures and deep sequencing. Science 333:1593–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Shultz LD, Ishikawa F, Greiner DL. 2007. Humanized mice in translational biomedical research. Nat. Rev. Immunol. 7:118–130 [DOI] [PubMed] [Google Scholar]
- 64. Shultz LD, Brehm MA, Garcia-Martinez JV, Greiner DL. 2012. Humanized mice for immune system investigation: progress, promise and challenges. Nat. Rev. Immunol. 12:786–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pan R, Sampson JM, Chen Y, Vaine M, Wang S, Lu S, Kong X-P. 2013. Rabbit anti-HIV-1 monoclonal antibodies raised by immunization can mimic the antigen-binding modes of antibodies derived from HIV-1-infected humans. J. Virol. 87:10221–10231 [DOI] [PMC free article] [PubMed] [Google Scholar]