Abstract
Several human cytomegalovirus (HCMV) genes encode products that modulate cellular functions in a manner likely to enhance viral pathogenesis. This includes UL111A, which encodes homologs of human interleukin-10 (hIL-10). Depending upon signals received, monocytes and macrophages become polarized to either classically activated (M1 proinflammatory) or alternatively activated (M2 anti-inflammatory) subsets. Skewing of polarization toward an M2 subset may benefit the virus by limiting the proinflammatory responses to infection, and so we determined whether HCMV-encoded viral IL-10 influenced monocyte polarization. Recombinant viral IL-10 protein polarized CD14+ monocytes toward an anti-inflammatory M2 subset with an M2c phenotype, as demonstrated by high expression of CD163 and CD14 and suppression of major histocompatibility complex (MHC) class II. Significantly, in the context of productive HCMV infection, viral IL-10 produced by infected cells polarized uninfected monocytes toward an M2c phenotype. We also assessed the impact of viral IL-10 on heme oxygenase 1 (HO-1), which is an enzyme linked with suppression of inflammatory responses. Polarization of monocytes by viral IL-10 resulted in upregulation of HO-1, and inhibition of HO-1 function resulted in a loss of capacity of viral IL-10 to suppress tumor necrosis factor alpha (TNF-α) and IL-1β, implicating HO-1 in viral IL-10-induced suppression of proinflammatory cytokines by M2c monocytes. In addition, a functional consequence of monocytes polarized with viral IL-10 was a decreased capacity to activate CD4+ T cells. This study identifies a novel role for viral IL-10 in driving M2c polarization, which may limit virus clearance by restricting proinflammatory and CD4+ T cell responses at sites of infection.
INTRODUCTION
Human cytomegalovirus (HCMV) is a highly species-specific betaherpesvirus that infects a majority of the world's population, causing significant morbidity and mortality in neonates and in immunosuppressed individuals, including solid-organ and allogeneic stem cell transplant recipients and individuals with HIV AIDS (1). HCMV encodes a number of genes involved in modulation of the host's immune system to aid virus survival and persistence. Examples include viral interference with apoptosis (2, 3) and suppression of viral antigen presentation through modulation of major histocompatibility complex (MHC) class I and MHC class II expression (4–7). In addition, HCMV encodes a number of homologs of cell receptors and other cellular proteins, including homologs of G-protein-coupled chemokine receptors and CXC chemokines (for a review, see McSharry et al. [8]).
The HCMV UL111A gene encodes homologs of the potent immunomodulatory cytokine human interleukin-10 (hIL-10) during both productive and latent phases of HCMV infection (9–12). These homologs are collectively referred to as viral IL-10, and they exhibit a range of immunomodulatory functions, including suppression of proinflammatory cytokine production and dendritic cell (DC) maturation and inhibition of MHC class I and class II expression (for a viral IL-10 review, see Slobedman et al. [12]). During latency, viral IL-10 restricts MHC class II surface expression by CD34+ myeloid progenitors, leading to the inhibition of CD4+ T cell recognition of latently infected cells (4, 13).
CD34+ myeloid progenitors undergo sequential differentiation into myeloid dendritic cells or blood monocytes. These monocytes can respond to different signals, which drives rapid migration to virtually all tissues, where they differentiate further into different types of macrophages which play multiple roles in the immune response (14). Cells of the monocyte/macrophage lineage can be functionally polarized in response to endogenous stimuli into M1 classically activated and M2 alternatively activated subsets (15). M1 monocytes/macrophages, activated mainly by stimulation with gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α), are proinflammatory effector cells with a pivotal role in the host defense against intracellular pathogens (16).
However, not all monocytes/macrophages acquire an M1 phenotype. Rather, an M2 alternatively activated monocyte/macrophage designation includes monocytes/macrophages phenotypically different from the M1 classically activated subset. These are characterized by increased phagocytic activity but suppressed production of proinflammatory cytokines and reduced killing capacity toward pathogens (15). More recently, it has been shown that there is more than one type of alternative activation of monocytes/macrophages, and this is now reflected in an expanded nomenclature for M2 alternative activation: M2a, induced by IL-4 and IL-13; M2b, induced by immune complexes and agonists of Toll-like receptors (TLRs); and M2c, stimulated by IL-10 and glucocorticoids (17). It has also been suggested that IL-4-stimulated (M2a) macrophages be named wound-healing macrophages due to their capacity to secrete components of the extracellular matrix required for wound healing and activation of TH2 CD4+ T cell responses (16). On the other hand, M2c alternatively activated cells are referred to as deactivators of the immune response (17) while M2b monocytes/macrophages are less understood and are known as a type II alternatively activated subset (18).
In this study, we defined the influence of HCMV-encoded viral IL-10 on monocyte polarization. We report that exposure of CD14+ monocytes to viral IL-10 resulted in the development of M2c alternatively activated cells. Furthermore, in the context of HCMV infection, viral IL-10 secreted from infected cells during productive infection induced the development of an M2c subset in bystander uninfected monocytes. The M2c polarization of monocytes by viral IL-10 resulted in upregulation of the anti-inflammatory enzyme heme oxygenase 1 (HO-1), and this was shown to play an important role in viral IL-10-mediated suppression of proinflammatory cytokines by M2c monocytes. Finally, we provide evidence that M2c monocyte polarization by viral IL-10 reduces the ability to stimulate CD4+ T cell activation and proliferation.
MATERIALS AND METHODS
Cells.
Peripheral blood mononuclear cells (PBMCs) were derived from healthy human whole blood by Ficoll-Hypaque Plus (GE Healthcare) gradient centrifugation. CD14+ monocytes were isolated from PBMCs by positive selection with anti-human CD14 magnetic beads (Miltenyi Biotec) and maintained in RPMI 1640 medium (Lonza) supplemented with 10% fetal calf serum (FCS; CSL). Purity of monocytes was typically >95% based on immunostaining for CD14 and flow cytometry analysis (data not shown). Human foreskin fibroblasts (HFFs) were propagated in RPMI 1640 medium supplemented with 10% FCS (CSL).
Reagents for monocyte polarization experiments.
The HCMV gene UL111A encodes two viral IL-10 homologs, cmvIL-10 and latency-associated cmvIL-10 (LAcmvIL-10) (9–11). Since deletion of UL111A in the viral IL-10 deletion viruses prevents expression of either variant of HCMV-encoded IL-10, equal amounts of cmvIL-10 and LAcmvIL-10 recombinant proteins (collectively referred to as viral IL-10) were combined to ensure that effects on monocyte polarization observed in the context of infection experiments were able to be compared with effects of purified recombinant proteins. Recombinant cmvIL-10, hIL-10, and hIL-4 proteins were purchased from R&D Systems. LAcmvIL-10 protein was generated as previously described (19). All cytokines were used at a concentration of 100 ng/ml. Lipopolysaccharide (LPS; Sigma-Aldrich) was used at a concentration of 1 μg/ml.
Viruses.
The viral IL-10 deletion (vIL-10 del) virus RVAdIL10C has a UL111A gene deletion in HCMV strain AD169 (20). Merlin-BAC UL111A-del virus was generated from the enhanced green fluorescent protein (eGFP)-expressing HCMV Merlin-BAC isolate pAL1160 (21) using bacterial artificial chromosome (BAC) recombineering protocols (22). The selection cassette was generated using the PCR primers: 5′-GACGCGCAGTTGGGCGGCGGATTGGGGCGGCATGCTGCGGCGGAGCTCGGTACCCGGGGATC-3′ (forward) and 5′-GTAACTGGGTGAACGACATCGGAGCGGACTGCAAATCGCAACGGAAAAGTGCCACCTGTATGC-3′ (reverse). Correct insertion of the selection cassette replacing the UL111A gene was confirmed by using the primers 5′-CCATCAAGTGGTGACGATAAC-3′ (forward) and 5′-CAACACCCACAAACAACGTC-3′ (reverse) and by restriction digest of mutant BAC DNA with HindIII (New England BioLabs) to confirm the expected restriction pattern. Productive HCMV infection of HFFs was performed at multiplicity of infection (MOI) of 3, with samples harvested 24 h postinfection (p.i.).
Immunostaining and flow cytometry.
A total of 105 cells in fluorescence-activated cell sorter (FACS) buffer (phosphate-buffered saline [PBS]–1% FCS–2 mM sodium azide) were incubated with anti-CD163-phycoerythrin (PE; BD Biosciences), anti-CD14-fluorescein isothiocyanate (FITC; BD Biosciences), and anti-HLA-DR–peridinin chlorophyll protein complex (PerCP; BD Biosciences) or their respective isotype control antibodies for 30 min. Cells were washed in FACS buffer and resuspended in 1% paraformaldehyde solution in FACS buffer. Data were acquired using a FACSCanto flow cytometer (BD Biosciences) and analyzed by FlowJo software (BD Biosciences).
qRT-PCR.
Total RNA was extracted from CD14+ monocytes using an RNaqueous kit (Ambion) and DNase treated prior to the reverse transcription step (SuperScript III kit; Invitrogen). mRNA expression was measured by quantitative reverse transcription-PCR (qRT-PCR) (Mx3000P qPCR system; Stratagene) at 50°C for 1 min and 95°C for 1 min, followed by 50 cycles consisting of 95°C for 15 s and 60°C for 45 s. mRNA levels of test genes were normalized to mRNA expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Primers used in this study were the following: GAPDH-F, 5′-TCACCAGGGCTGCTTTTAAC-3′; GAPDH-R, 5′-GACAAGCTTCCCGTTCTCAG-3′; SLAM-F, 5′-AGCAGGTCTCCACTCCAGAA-3′; SLAM-R, 5′-GCTCACGGTGCAGATGTAGA-3′; versican-F, 5′-CCTTTCTGGGGAAGAACTCC-3′; versican-R, 5′-GGTCACATAGGAAGCGTGGT-3′; SOCS3-F, 5′-ATCCTGGTGACATGCTCCTC-3′; SOCS3-R, 5′-CAAATGTTGCTTCCCCCTTA-3′; CCR7-F, 5′-GATGCGATGCTCTCTCATCA-3′; CCR7-R, 5′-TGTAGGGCAGCTGGAAGACT-3′; fibronectin 1-F, 5′-TGTTCGTGCAGCTGTTTACC-3′; fibronectin 1-R, 5′-GCCACCGTAAGTCTGGGTTA-3′; TNF-α-F, 5′-CCGTCTCCTACCAGACCAAG-3′; TNF-α-R, 5′-CTGAGTCGGTCACCCTTCTC-3′; IL-1β-F, 5′-GCTGAGGAAGATGCTGGTTC-3′; IL-1β-R, 5′-GTGATCGTACAGGTGCATCG-3′.
Western blotting.
CD14+ monocyte lysates were analyzed by Western blotting with a primary goat anti-human HO-1 antibody (R&D Systems) and secondary rabbit anti-goat horseradish peroxidase (HRP)-conjugated antibody (Dako). Primary rabbit anti-human GAPDH antibody (Santa Cruz Biotechnology) with its secondary goat anti-rabbit HRP-conjugated antibody (Cell Signaling Technology) was included as a loading control.
Treatment of CD14+ monocytes with zinc protoporphyrin.
CD14+ monocytes were pulsed for 2 h with the HO-1 inhibitor zinc protoporphyrin (ZnPP) (10 nmol/ml; Sigma-Aldrich) prior to administration of viral IL-10 (100 ng/ml) for 24 h, followed by incubation with lipopolysaccharide (LPS; 100 ng/ml) for 3 h to stimulate proinflammatory cytokine production.
Mixed leukocyte reaction.
CD4+ T cells were selected from PBMCs with anti-human CD4+ magnetic beads (Miltenyi Biotec), resulting in >95% purity (observed by flow cytometry [data not shown]). Then, 5,6-carboxyfluorescein diacetate succinimidyl ester (CFSE) staining (Invitrogen) was performed by incubating 1 × 107 CD4+ T cells/ml in serum-free RPMI 1640 medium containing 5 μM CFSE for 10 min at 37°C, followed by quenching on ice for 5 min and three washes in 10 ml of serum-free RPMI 1640 medium.
CD14+ monocytes were treated with PBS or with hIL-10 and viral IL-10 (100 ng/ml, respectively) and mixed with 2.5 × 105 allogeneic CFSE-labeled CD4+ T cells in RPMI 1640 medium with 10% human serum at monocyte-to-CD4+ T cell ratios of 1:5, 1:10, 1:20, and 1:50 in round-bottom 96-well plates. As a positive control for both activation and proliferation, CD4+ T cells were treated with 12.5 ng/ml phorbol 12-myristate 13-acetate (PMA; Sigma-Aldrich) and 250 ng/ml ionomycin (Sigma-Aldrich), while CD4+ T cells cultured in the absence of monocytes were used as a negative control. Induction of the early CD4+ T cell activation marker CD69 was determined by immunostaining with anti-CD69-allophycocyanin (APC) antibody (BD Biosciences) at 24 h of coculture, while detection of proliferating CD4+ T cells was determined 5 days later by detection of a reduction in CFSE fluorescence intensity by flow cytometry.
RESULTS
Treatment of monocytes with viral IL-10 induces expression of the M2c marker CD163.
To investigate the impact of HCMV-encoded IL-10 on monocyte polarization, we cultured CD14+ monocytes with recombinant viral IL-10 or hIL-10 protein (100 ng/ml) for 24 h. Cells were then immunostained for CD163, a scavenger receptor expressed at high levels on monocytes/macrophages exposed to hIL-10 (23, 24). Flow cytometry analysis revealed an increase in the proportion of CD163+ cells in monocyte cultures treated with either viral IL-10 or hIL-10 compared to PBS-treated monocytes (Fig. 1A). This increase was statistically significant, with approximately 90% of cells expressing CD163 following treatment with viral IL-10 or hIL-10 (Fig. 1B). Similarly, analysis of CD163 mean fluorescence intensity (MFI; presented as a fold change in CD163 MFI relative to that of untreated monocytes) demonstrated a statistically significant upregulation of CD163 MFI following treatment of monocytes with either viral IL-10 or hIL-10 (Fig. 1C). These data demonstrate that both viral IL-10 and hIL-10 strongly induce CD163 scavenger receptor expression by CD14+ monocytes.
Fig 1.
Increased CD163 expression on CD14+ monocytes in response to hIL-10 and viral IL-10 protein treatment. CD14+ monocytes were treated with hIL-10 and viral IL-10 (100 ng/ml) or with PBS (negative control) for 24 h. (A) Representative flow cytometry scatter plots of cells expressing CD163. Graphs depict the percentage of CD163+ cells (B) or fold change (relative to PBS treatment) of CD163 mean fluorescence intensity (MFI) (C). The number of independent biological replicate experiments (n) is shown. Error bars indicate the standard errors of the means. Significant differences between samples compared to PBS treatment were determined using a one-tailed, paired Student t test: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
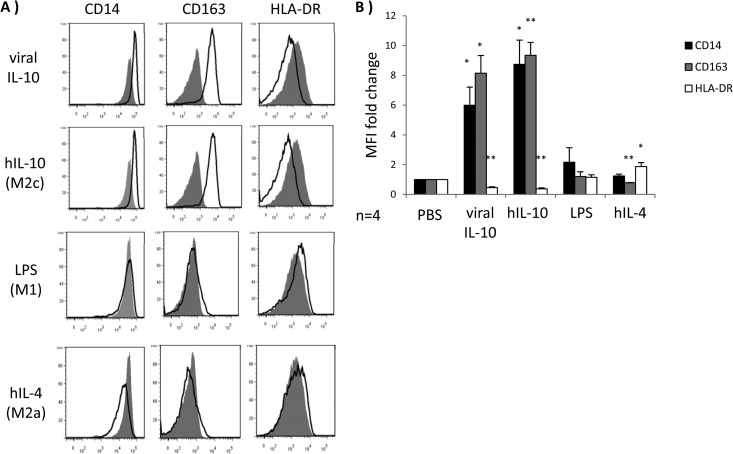
Viral IL-10 polarizes CD14+ monocytes toward an alternatively activated M2c phenotype.
To further characterize the impact of viral IL-10 on monocytes, we examined the cell surface phenotype of CD14+ monocytes treated with viral IL-10 protein in comparison to monocytes treated with stimuli which specifically induce different types of monocyte polarization: LPS (M1 classically activated monocytes), hIL-4 (M2a alternatively activated monocytes), and hIL-10 (M2c alternatively activated monocytes). The M2c alternatively activated phenotype is typified by increased cell surface levels of CD14 and CD163 and decreased cell surface expression of MHC class II (15, 17, 25). Following a 24-h incubation, CD14, CD163, and MHC class II (HLA-DR) surface expression levels were analyzed by flow cytometry, revealing that viral IL-10 protein treatment induced an expression pattern closely matched to the M2c alternatively activated monocytes induced by hIL-10 (upregulated CD14, upregulated CD163, and downregulated HLA-DR) (Fig. 2A and B). Furthermore, the profile induced by viral IL-10 was significantly different from that for monocytes treated with hIL-4 (M2a) or LPS (M1) (Fig. 2A and B). Specifically, surface expression of CD163, a key molecule associated with the induction of an anti-inflammatory environment (26, 27), was upregulated only by viral IL-10 and hIL-10, whereas hIL-4 (M2a) suppressed CD163 expression. As expected, M1 classically activated monocytes induced by LPS displayed minimal CD163 expression. The cell surface expression profiles mediated by viral IL-10, hIL-10, hIL-4, and LPS were consistent with viral IL-10 inducing an alternatively activated M2c phenotype in CD14+ monocytes. We observed a similar surface expression profile (upregulated CD14, upregulated CD163, and downregulated HLA-DR) when monocyte-derived macrophages were polarized with viral IL-10 protein (see Fig. S1 in the supplemental material).
Fig 2.
Human monocytes exhibit M2c alternatively activated phenotype in response to viral IL-10 protein treatment. CD14+ monocytes were treated with viral IL-10 (100 ng/ml), hIL-10 (100 ng/ml), lipopolysaccharide (LPS; 1 μg/ml), and hIL-4 (100 ng/ml) or with PBS (negative control) for 24 h. (A) Flow cytometry histograms of human monocytes expressing CD14, CD163, and HLA-DR. Open histograms show expression in treated samples compared to filled histograms that show expression in PBS-treated samples. (B) Graph depicting the fold change (relative to PBS treatment) of CD14, CD163, and HLA-DR mean fluorescence intensity (MFI) for hIL-10-, viral IL-10-, LPS-, and hIL-4-treated monocytes. The number of independent biological replicate experiments (n) is shown. Error bars indicate the standard errors of the means. Significant differences between samples compared to PBS treatment were determined using a one-tailed, paired Student t test: *, P < 0.05; **, P < 0.01.
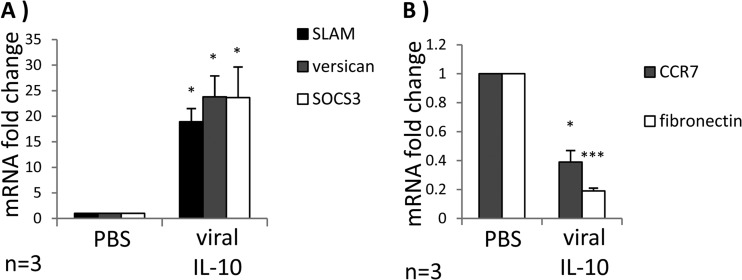
In an extension of our analyses of cell surface markers associated with an M2c phenotype, we examined mRNA expression of signaling lymphocyte activation molecule (SLAM), versican, and suppressor of cytokine signaling 3 (SOCS3) as these transcripts are upregulated by M2c alternatively activated monocytes/macrophages (17). In comparison to PBS treatment, treatment of CD14+ monocytes with viral IL-10 for 24 h resulted in significantly increased SLAM, versican, and SOCS3 mRNA expression (Fig. 3A). In contrast, expression levels of the M1-associated mRNA transcript CCR7 (28) and the M2a-specific mRNA transcript fibronectin 1 (28, 29) were downregulated in viral IL-10-treated monocytes (Fig. 3B). Together with the cell surface analyses, these data provide further evidence that viral IL-10 polarizes monocytes toward cells bearing the hallmarks of an M2c alternatively activated immunoregulatory phenotype.
Fig 3.
Viral IL-10 upregulates expression of M2c-associated mRNA transcripts in human monocytes. CD14+ monocytes were treated with viral IL-10 (100 ng/ml) or with PBS (negative control) for 24 h. (A) Fold mRNA expression change of M2c-associated transcripts SLAM, versican, and SOCS3 in monocytes treated with viral IL-10 (relative to PBS-treated samples). (B) Fold mRNA expression change of M1-associated transcript CCR7 and M2a-associated transcript fibronectin in monocytes treated with viral IL-10 (relative to PBS-treated samples). The number of independent biological replicate experiments (n) is shown. Error bars indicate the standard errors of the means. Significant differences between samples compared to PBS treatment were determined using a one-tailed, paired Student t test: *, P < 0.05; ***, P < 0.001.
Viral IL-10 expressed during productive HCMV infection induces an M2c phenotype in bystander monocytes.
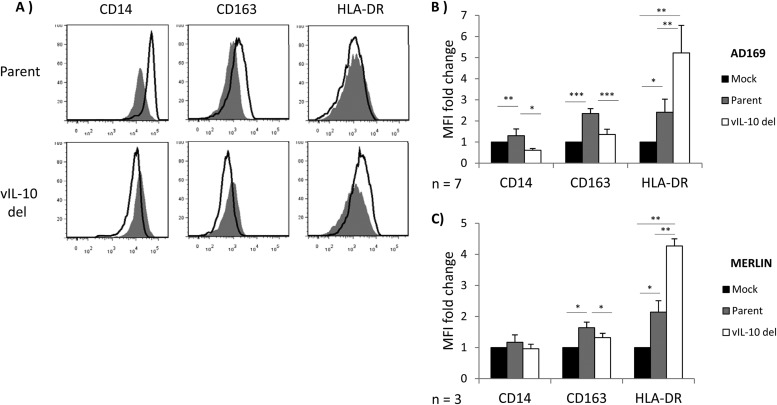
To extend the analyses of impacts on monocyte polarization by recombinant viral IL-10 proteins, we sought to determine whether viral IL-10 expressed during productive HCMV infection was able to modulate CD14+ monocyte polarization. CD14+ monocytes are refractory to the full HCMV replicative cycle (30, 31), and so to examine viral IL-10 function in the context of HCMV infection, we productively infected human foreskin fibroblasts (HFFs) and assessed the capacity of viral IL-10 secreted into the culture supernatant to modulate polarization of uninfected bystander CD14+ monocytes. To dissect the impact of viral IL-10 from other soluble factors secreted during productive HCMV infection, HFFs were infected at an MOI of 3 with either an HCMV UL111A deletion virus (RVAdIL10C), which replicates normally in HFFs but cannot express viral IL-10 (20), or its parental virus (AD169). Supernatants from mock-infected HFFs were also analyzed. At 24 h postinfection, conditioned supernatants were added to freshly isolated CD14+ monocytes for another 24 h before these cells were assessed for cell surface CD14, CD163, and HLA-DR expression (Fig. 4). In comparison to incubation of monocytes with supernatants from HFFs infected with the viral IL-10 deletion virus, monocytes incubated with supernatants from HFFs infected with parental virus displayed significant upregulation of CD14 and CD163 and downregulation of HLA-DR (Fig. 4A and B). Similar results were obtained when experiments were repeated using the clinical strain Merlin-BAC and a viral IL-10 deletion virus, Merlin-BAC UL111A-del (Fig. 4C).
Fig 4.
Viral IL-10 expressed during productive HCMV infection polarizes bystander monocytes toward an alternatively activated M2c phenotype. CD14+ monocytes were cultured for 24 h in conditioned supernatants from HFF cultures productively infected with a viral IL-10 deletion virus (vIL-10 del) or parental virus (Parent) or mock infected (Mock) for 24 h. (A) Representative flow cytometry histograms of cells expressing CD14, CD163, and HLA-DR. Open histograms show expression in monocytes cultured with conditioned supernatants from vIL-10 del-infected and parent virus-infected (both in the AD169 backbone) samples compared to filled histograms that show expression in monocytes incubated with conditioned supernatants from mock-infected samples. (B and C) Graphs depicting the mean fluorescence intensity (MFI) fold change of CD14, CD163, and HLA-DR in monocytes cultured with conditioned supernatants from vIL-10 del- and parent virus-infected samples, as indicated (relative to monocytes incubated with conditioned supernatants from mock-infected samples). The number of independent biological replicate experiments (n) is shown. Error bars indicate the standard errors of the means. Significant differences between samples compared to mock infection were determined using a one-tailed, paired Student t test: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
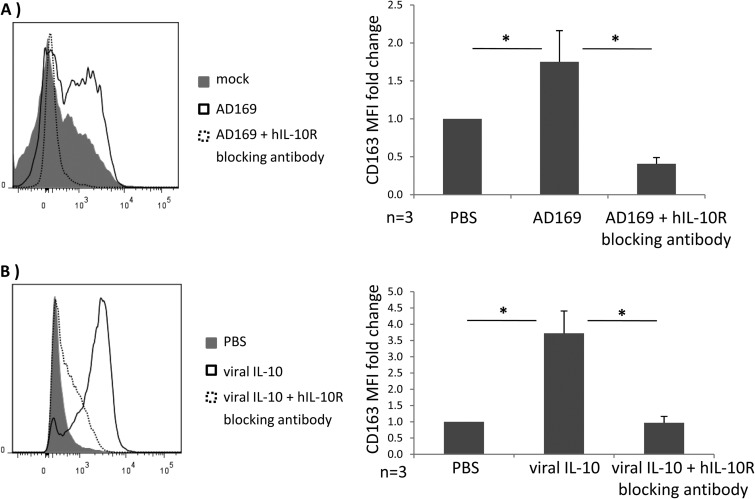
To provide further evidence that the difference in monocyte polarization induced by supernatants from parental versus viral IL-10 deletion virus was due to the expression of viral IL-10 and not other soluble mediators that may influence monocyte polarization toward an M2c phenotype, we compared CD163 surface expression by these monocytes treated with infected cell supernatants in the presence or absence of a human interleukin-10 receptor alpha (hIL-10Rα) neutralizing antibody (used at 25 μg/ml; R&D Systems). As shown in Fig. 5, CD14+ monocyte pretreatment with the hIL-10Rα neutralizing antibody completely abrogated upregulation of surface CD163 induced by supernatant from HFFs infected with parental HCMV (Fig. 5A), indicating that no other virus-encoded soluble factors released by infected HFFs could induce M2c polarization of uninfected bystander monocytes. We also performed this hIL-10R neutralizing antibody experiment using recombinant viral IL-10, rather than infected cell supernatants, to further confirm an essential role for viral IL-10 in the upregulation of CD163 by CD14+ monocytes (Fig. 5B). To exclude the possibility that HFFs respond to HCMV infection by secreting hIL-10 that could then act to mediate the observed effects, we quantified secreted hIL-10 protein levels by enzyme-linked immunosorbent assay (ELISA; R&D systems) in cultures of HFFs productively infected with either the parent or UL111A deletion virus and in mock-infected cultures. In supernatants from three independent biological replicate experiments, no hIL-10 protein was detected in any of these HFF cultures (sensitivity limit of 3.9 pg/ml).
Fig 5.
Blocking the human IL-10 receptor on CD14+ monocytes prevents surface CD163 upregulation by viral IL-10. CD14+ monocytes were pretreated with anti-hIL-10R antibody for 2 h prior to incubation for 24 h with conditioned supernatants from HFF cultures productively infected with HCMV strain AD169 (A) or recombinant viral IL-10 (100 ng/ml) (B). Surface CD163 expression with anti-hIL-10R antibody (area under the dashed line) was compared to the surface CD163 expression from the identical treatments but with anti-hIL-10R antibody omitted (A, mock) and treatment with PBS with anti-hIL-10R antibody omitted (B, PBS; negative control). Error bars indicate the standard errors of the means. Significant differences between samples compared to PBS treatment were determined using a one-tailed, paired Student t test (*, P < 0.05).
Therefore, these results provide evidence that (i) viral IL-10 expressed during the productive phase of HCMV infection polarizes bystander monocytes toward an M2c alternatively activated phenotype and (ii) that this M2c phenotype is mediated via the hIL-10R.
Heme oxygenase 1 expression is upregulated by viral IL-10-treated monocytes and is associated with viral IL-10-controlled suppression of proinflammatory cytokines.
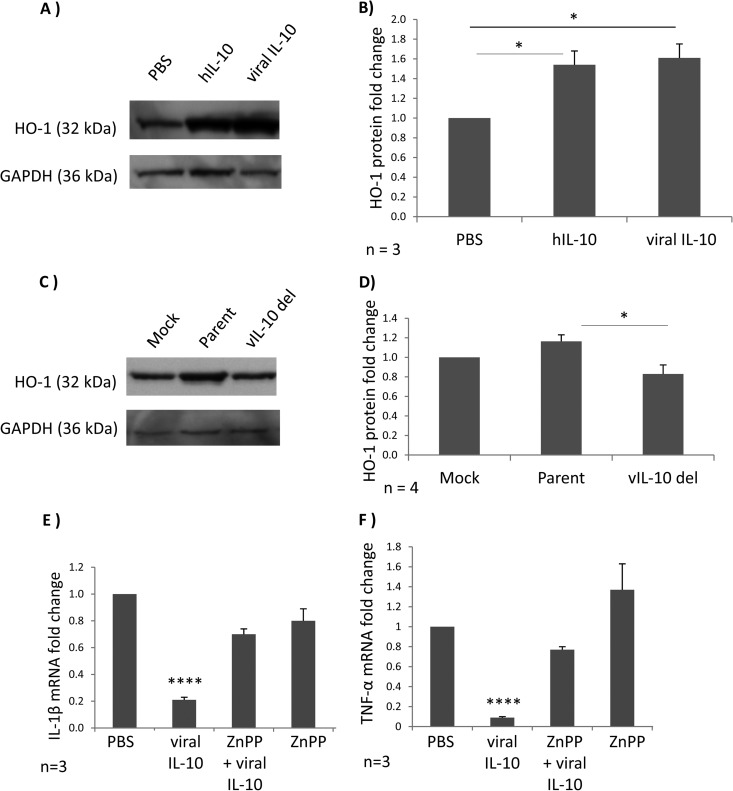
Heme oxygenase 1 (HO-1) is an enzyme linked with suppression of inflammatory responses (32). Cellular IL-10 is a potent inducer of HO-1 (33, 34). To further explore the mechanistic basis for viral IL-10-mediated polarization of monocytes to an M2c phenotype, we examined the impact of viral IL-10 on HO-1 expression by measuring HO-1 protein by Western blotting in cell lysates derived from CD14+ monocytes treated with viral IL-10 protein, as well as in uninfected CD14+ monocytes cultured with conditioned supernatants from HFFs productively infected with either parent (AD169) or viral IL-10 deletion (RVAdIL10C) virus.
Recombinant viral IL-10 protein upregulated HO-1 protein in CD14+ monocytes in a manner comparable to that of hIL-10 (Fig. 6A and B). In the context of HCMV infection, CD14+ monocytes cultured in conditioned supernatants from HFFs productively infected with parental virus displayed a modest but statistically significant increase of HO-1 protein in comparison to CD14+ monocytes cultured in conditioned supernatants from HFFs infected with viral IL-10 deletion virus (Fig. 6C and D). Thus, recombinant viral IL-10 protein and viral IL-10 expressed in the context of productive infection functioned to upregulate HO-1 protein expression by uninfected monocytes.
Fig 6.
Heme oxygenase 1 (HO-1) is upregulated in viral IL-10-polarized M2c monocytes and plays a role in viral IL-10-driven suppression of proinflammatory cytokines. (A) Western blot showing HO-1 protein levels in CD14+ monocytes treated with viral IL-10 (100 ng/ml), hIL-10 (100 ng/ml), or PBS (negative control) for 24 h. Expression of GAPDH was used as a protein loading control. (B) The fold change of HO-1 protein expression in monocytes treated with recombinant proteins was determined by densitometry normalized to the expression of GAPDH. (C) Western blot showing HO-1 protein levels in uninfected CD14+ monocytes cultured for 24 h with conditioned supernatants from HFF cultures productively infected with viral IL-10 deletion virus (vIL-10 del), parental virus (Parent), or with supernatants from mock-infected HFFs (Mock). Expression of GAPDH was used as a protein loading control. (D) The fold change of HO-1 protein expression in uninfected monocytes treated with supernatants was determined by densitometry normalized to the expression of GAPDH. Quantitative RT-PCR-based analysis of IL-1β mRNA (E) and TNF-α mRNA (F) in LPS-stimulated human CD14+ monocytes treated with viral IL-10 (100 ng/ml), with or without the HO-1 competitive inhibitor ZnPP (10 nmol/ml) is shown. Graphs depict fold change of mRNA expression relative to cells treated with PBS. Error bars indicate the standard errors of the means. Significant differences were determined using a one-tailed, paired Student t test: *, P < 0.05; ****, P < 0.0001.
HCMV-encoded viral IL-10 can inhibit production of proinflammatory cytokines by PBMCs, monocytes, and DCs (19, 35, 36). We investigated whether viral IL-10 requires HO-1 for this inhibition of proinflammatory cytokine production. Monocytes were cultured with or without the HO-1 competitive inhibitor ZnPP prior to polarization with viral IL-10. Polarized monocytes were then stimulated to express proinflammatory cytokines by LPS treatment, and the ability of these cells to induce mRNA transcription of the proinflammatory cytokines TNF-α and IL-1β was examined. These analyses demonstrated that HO-1 induced by viral IL-10 is required for suppression of mRNA transcription of TNF-α and IL-1β in viral IL-10-polarized monocytes, as indicated by restoration of TNF-α and IL-1β mRNA transcription by treatment with the HO-1 inhibitor ZnPP prior to addition of viral IL-10 (Fig. 6E and F). Monocytes treated with ZnPP in the absence of viral IL-10 treatment did not upregulate TNF-α and IL-1β mRNA compared to control (PBS-treated) monocytes, demonstrating that ZnPP itself does not significantly alter the expression of TNF-α and IL-1β. These results implicate viral IL-10-mediated upregulation of HO-1 as being an important step in the inhibition of proinflammatory cytokines by viral IL-10-polarized M2c monocytes.
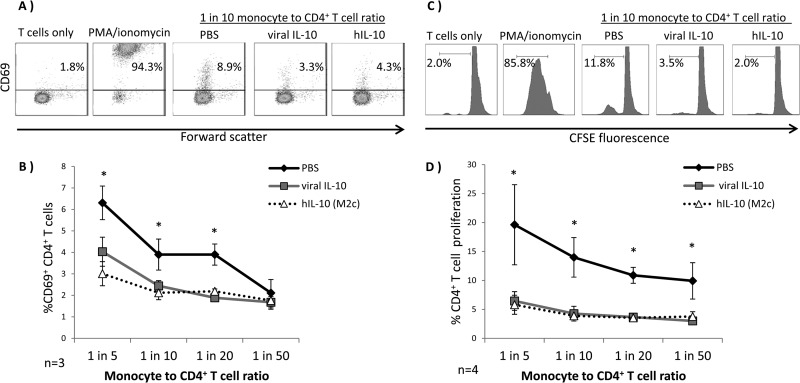
Viral IL-10-polarized M2c monocytes have a decreased capacity to stimulate CD4+ T cell activation and proliferation.
To assess downstream functional consequences of M2c monocyte polarization by viral IL-10, we examined the ability of viral IL-10-polarized monocytes to induce allogeneic CD4+ T cell activation and proliferation in a mixed leukocyte reaction (MLR). CD4+ T cell activation was assessed by measuring the surface expression of the early T cell activation marker CD69, whereas CD4+ T cell proliferation was measured by a CFSE T cell proliferation assay. CD14+ monocytes were either treated with PBS or polarized with viral IL-10 or hIL-10 (100 ng/ml) for 24 h prior to coculture with allogeneic CD4+ T cells. CD4+ T cells cultured alone or stimulated with PMA-ionomycin in the absence of monocytes were included as negative and positive controls, respectively, for T cell activation and proliferation. These analyses revealed that CD4+ T cells cocultured with monocytes treated with viral IL-10 or hIL-10 displayed significantly lower levels of CD69 expression than CD4+ T cells cocultured with control monocytes treated with PBS (Fig. 7A and B). Similarly, in comparison to CD4+ T cells cocultured with monocytes treated with PBS, proliferation of CD4+ T cells was almost completely abolished when they were cocultured with monocytes polarized with either viral IL-10 or hIL-10 (Fig. 7C and D). Thus, human monocytes polarized with viral IL-10 were poor stimulators of CD4+ T cell activation and proliferation, with the level of inhibition of T cell function by viral IL-10-polarized monocytes comparable to that of hIL-10-polarized M2c monocytes. Thus, viral IL-10 polarizes monocytes in a manner which inhibits the CD4+ T cell response.
Fig 7.
CD4+ T cell activation and proliferation are inhibited by viral IL-10-polarized M2c monocytes in a mixed leukocyte reaction. Human CD14+ monocytes polarized with viral IL-10 (100 ng/ml) and hIL-10 (100 ng/ml) or with PBS (no polarization control) were cultured with CD4+ T cells in an allogeneic setting for 24 h prior to examination of an early T cell activation marker, CD69, or for 5 days with CFSE-labeled CD4+ T cells prior to assessment of CD4+ T cell proliferation by flow cytometry. CD4+ T cells with no monocytes added were used as a negative control while CD4+ T cells cultured with PMA-ionomycin were used as a positive control. (A) Representative flow cytometry scatter plots of CD4+ T cells expressing CD69. (B) Graph depicting the percentages of CD69+ CD4+ T cells. (C) Representative flow cytometry histograms showing percentage of proliferating CD4+ T cells. (D) Graph depicting the percentages of proliferating CD4+ T cells. The number of independent biological replicate experiments (n) is shown. Error bars indicate the standard errors of the means. Significant differences between viral IL-10- and hIL-10-treated samples compared to PBS-treated samples were determined using a one-tailed, paired Student t test (*, P < 0.05).
DISCUSSION
Cells of the myeloid lineage represent a key component of the immune system required for the control and clearance of pathogens (16). However, despite a strong host immune response, infectious HCMV often continues to be shed for many months following infection, arguing in favor of virus-mediated inhibition of the host immune response (1). Indeed, HCMV encodes genes proposed to play important roles in limiting host defenses, including a number of homologs of cytokines, chemokines, and their receptors (8). Monocyte-derived macrophages are capable of presenting antigens to CD4+ T cells and clearing intracellular pathogens through high expression of IL-12, IL-23, nitric oxide, and reactive oxygen intermediates, but this effect is dependent on their functional polarization into M1 classically activated cells (16, 17). Here, we present evidence that HCMV-encoded viral IL-10 modulates monocyte polarization and promotes an alternatively activated M2c phenotype, characterized by downregulated MHC class II and upregulated expression of molecules associated with anti-inflammatory functions, namely, CD163 and HO-1. Functionally, viral IL-10-polarized monocytes were capable of inhibiting proinflammatory cytokine synthesis via upregulation of HO-1. In addition, these M2c monocytes were very poor stimulators of CD4+ T cell activation. Thus, viral IL-10 appears to fundamentally influence monocyte/macrophage polarization in a manner likely to enhance the capacity of HCMV to limit virus clearance by the host's immune system.
CD163 is a member of the scavenger receptor cysteine-rich domain-containing superfamily (SRCR), expressed predominantly on monocytes and macrophages (37, 38). CD163 is a scavenger receptor for heme-containing hemoglobin-haptoglobin (Hb:Hp) complexes (39). Cell surface CD163 expression is significantly upregulated by the known inducers of M2c monocyte/macrophage polarization, hIL-10 (23, 24) and glucocorticoids (40, 41). Our finding that CD14+ monocytes stimulated with viral IL-10 upregulated surface CD163 is consistent with viral IL-10-mediated promotion of an M2c phenotype. The downregulation of MHC class II (HLA-DR) by monocytes exposed to viral IL-10 provides additional evidence that viral IL-10 promotes formation of cells that are anti- rather than proinflammatory in nature.
Significantly, the capacity of viral IL-10 to mediate polarization toward an M2c phenotype was demonstrated in the infection setting when monocytes were cultured with supernatants from HFFs productively infected with parental viruses which could express viral IL-10 but not when monocytes were cultured with supernatants from HFFs productively infected with viral IL-10 deletion viruses (constructed using both laboratory and clinical viral strains). In addition, neither supernatants containing viral IL-10 (from HFFs productively infected with parental viruses) nor purified recombinant viral IL-10 proteins were able to induce surface CD163 on monocytes when the human IL-10 receptor was blocked. While an ELISA to quantify secreted viral IL-10 protein levels from infected HFFs was not available, as little as 200 pg/ml of recombinant viral IL-10 was sufficient to strongly induce cell surface CD163 expression (data not shown). Our data indicate that, apart from viral IL-10 expressed during the productive phase of HCMV infection in HFFs, no other virus-derived soluble factor could directly induce an M2c phenotype in bystander monocytes. However, it remains to be determined whether monocyte M2c polarization is due exclusively to the direct effect of viral IL-10 or whether viral IL-10 may also induce the expression of cellular IL-10 by monocytes, which may add to the overall impact on M2c polarization.
The scavenging of the Hb:Hp complexes by CD163 plays a major role in dampening of the inflammatory response, predominantly mediated by end products of HO-1-mediated metabolism of heme from Hb:Hp complexes (42, 43). Here, we show that viral IL-10 upregulates HO-1 synthesis both in CD14+ monocytes stimulated with recombinant viral IL-10 and in bystander monocytes exposed to supernatant from cells productively infected with parental HCMV but not in monocytes exposed to supernatant from cells productively infected with viral IL-10 deletion virus. While control of proinflammatory cytokines by viral IL-10 appears to involve multiple pathways (44), our findings that expression of the proinflammatory cytokines TNF-α and IL-1β was suppressed by viral IL-10-polarized monocytes and that inhibition of HO-1 function abrogated this suppression provide evidence that HO-1 plays an important role in the anti-inflammatory function of monocytes polarized by viral IL-10.
A functional consequence of M2c monocyte polarization by viral IL-10 identified in this study was the altered ability to stimulate CD4+ T cell responses, with both early events of CD4+ T cell activation as well as CD4+ T cell proliferation almost completely abolished when CD4+ T cells were cocultured with viral IL-10-polarized monocytes. Thus, viral IL-10 exerts a striking impact on monocytes, which is likely to enhance virus-mediated inhibition of CD4+ T cell immunity. Whether the response of CD8+ T cells is similarly inhibited will be an important focus of future studies to further define the extent of the pleiotropic immunomodulatory functions of viral IL-10.
In addition to surface CD163 expression, continuous shedding of the extracellular domain of CD163 may result in release of soluble CD163 (sCD163), which has been linked with inhibition of T cell proliferation (45, 46). However, we did not detect any significant increase in shedding of sCD163 by M2c monocytes polarized with viral IL-10 or by bystander monocytes treated with conditioned supernatants from HFFs productively infected with HCMV (data not shown), so the inhibition of CD4+ T cell proliferation by viral IL-10-polarized M2c monocytes does not appear to be dependent upon release of sCD163. It seems more likely that the suppression of surface MHC class II expression by viral IL-10-polarized M2c monocytes results in limited antigen presentation by these monocytes, thus leading to reduced CD4+ T cell activation and proliferation.
While we examined monocytes exposed to recombinant viral IL-10 protein or to supernatants from infected HFFs, it has been reported that monocytes directly exposed to HCMV display a differentiation pattern with features of M1 polarization, although some transcripts associated with M2 polarization were also upregulated (30, 47, 48). Monocytes do not support productive HCMV replication (31), and Chan and colleagues (30) confirmed in their study that at 4 h p.i., no HCMV immediate-early gene expression was detectable. Thus, while monocytes exposed directly to HCMV acquire a unique M1 biased polarization phenotype triggered by virus binding and entry, bystander monocytes exposed to recombinant viral IL-10 proteins or viral IL-10-containing supernatants from HCMV-infected HFFs acquire a more definitive, functional M2c phenotype, with no evidence of M1 polarization. These findings highlight a fundamental difference in monocyte polarization which is dependent on whether polarization signals are provided by direct virus infection or by interaction with extracellular viral IL-10.
Unlike monocytes, macrophages do support productive HCMV infection, and recent work points to fundamental differences in the nature of HCMV infection of M1 and M2 macrophages. For example, M2 alternatively activated macrophages productively infected with HCMV are ineffective in stimulation of natural killer (NK) cells, while M1 macrophages are able to efficiently promote NK cell-mediated IFN-γ secretion (49). Furthermore, this and other studies reported more efficient HCMV replication in M2 alternatively activated macrophages than in M1 classically activated macrophages (49–51). Taken together with the findings we present here, the increased permissiveness of M2 macrophages to HCMV productive infection raises the intriguing possibility that viral IL-10-mediated polarization of monocytes to an M2c phenotype not only results in deactivation of the CD4+ T cell function but also may enhance the efficiency of subsequent HCMV infection when viral IL-10-polarized monocytes differentiate into M2c macrophages at sites of infection.
In conclusion, we have identified a novel role for viral IL-10 in the modulation of monocyte polarization toward an immunoregulatory, deactivated M2c phenotype. We propose that viral IL-10-mediated induction of an M2c phenotype is likely to enhance the capacity of HCMV to limit immune clearance by interfering with activation of other immune cells.
Supplementary Material
ACKNOWLEDGMENTS
We thank Bodo Plachter and Sandra Pepperl-Klindworth (Institute for Virology, University Medical Center of the University of Mainz) for providing the viral IL-10 deletion virus RVAdIL10C.
Flow cytometry was performed in the Flow Cytometry Core Facility supported by the Westmead Millennium Institute, National Health and Medical Research Council (NHMRC), and Cancer Institute New South Wales. This work was supported by Australian NHMRC project grant funding awarded to B.S. and A.A.
Footnotes
Published ahead of print 17 July 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.00912-13.
REFERENCES
- 1. Mocarski ES, Shenk T, Pass RF. 2007. Cytomegaloviruses, p 2701–2772 In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE. (ed), Fields virology, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 2. Goldmacher VS, Bartle LM, Skaletskaya A, Dionne CA, Kedersha NL, Vater CA, Han JW, Lutz RJ, Watanabe S, Cahir McFarland ED, Kieff ED, Mocarski ES, Chittenden T. 1999. A cytomegalovirus-encoded mitochondria-localized inhibitor of apoptosis structurally unrelated to Bcl-2. Proc. Natl. Acad. Sci. U. S. A. 96:12536–12541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Skaletskaya A, Bartle LM, Chittenden T, McCormick AL, Mocarski ES, Goldmacher VS. 2001. A cytomegalovirus-encoded inhibitor of apoptosis that suppresses caspase-8 activation. Proc. Natl. Acad. Sci. U. S. A. 98:7829–7834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cheung AK, Gottlieb DJ, Plachter B, Pepperl-Klindworth S, Avdic S, Cunningham AL, Abendroth A, Slobedman B. 2009. The role of the human cytomegalovirus UL111A gene in downregulating CD4+ T cell recognition of latently infected cells: implications for virus elimination during latency. Blood 114:4128–4137 [DOI] [PubMed] [Google Scholar]
- 5. Hegde NR, Johnson DC. 2003. Human cytomegalovirus US2 causes similar effects on both major histocompatibility complex class I and II proteins in epithelial and glial cells. J. Virol. 77:9287–9294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jones TR, Hanson LK, Sun L, Slater JS, Stenberg RM, Campbell AE. 1995. Multiple independent loci within the human cytomegalovirus unique short region down-regulate expression of major histocompatibility complex class I heavy chains. J. Virol. 69:4830–4841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Slobedman B, Mocarski ES, Arvin AM, Mellins ED, Abendroth A. 2002. Latent cytomegalovirus down-regulates major histocompatibility complex class II expression on myeloid progenitors. Blood 100:2867–2873 [DOI] [PubMed] [Google Scholar]
- 8. McSharry BP, Avdic S, Slobedman B. 2012. Human cytomegalovirus encoded homologs of cytokines, chemokines and their receptors: roles in immunomodulation. Viruses 4:2448–2470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jenkins C, Abendroth A, Slobedman B. 2004. A novel viral transcript with homology to human interleukin-10 is expressed during latent human cytomegalovirus infection. J. Virol. 78:1440–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kotenko SV, Saccani S, Izotova LS, Mirochnitchenko OV, Pestka S. 2000. Human cytomegalovirus harbors its own unique IL-10 homolog (cmvIL-10). Proc. Natl. Acad. Sci. U. S. A. 97:1695–1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lockridge KM, Zhou SS, Kravitz RH, Johnson JL, Sawai ET, Blewett EL, Barry PA. 2000. Primate cytomegaloviruses encode and express an IL-10-like protein. Virology 268:272–280 [DOI] [PubMed] [Google Scholar]
- 12. Slobedman B, Barry PA, Spencer JV, Avdic S, Abendroth A. 2009. Virus-encoded homologs of cellular interleukin-10 and their control of host immune function. J. Virol. 83:9618–9629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Avdic S, Cao JZ, Cheung AK, Abendroth A, Slobedman B. 2011. Viral interleukin-10 expressed by human cytomegalovirus during the latent phase of infection modulates latently infected myeloid cell differentiation. J. Virol. 85:7465–7471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gordon S, Taylor PR. 2005. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 5:953–964 [DOI] [PubMed] [Google Scholar]
- 15. Gordon S. 2003. Alternative activation of macrophages. Nat. Rev. Immunol. 3:23–35 [DOI] [PubMed] [Google Scholar]
- 16. Mosser DM, Edwards JP. 2008. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8:958–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. 2004. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 25:677–686 [DOI] [PubMed] [Google Scholar]
- 18. Mosser DM. 2003. The many faces of macrophage activation. J. Leukoc. Biol. 73:209–212 [DOI] [PubMed] [Google Scholar]
- 19. Jenkins C, Garcia W, Godwin MJ, Spencer JV, Stern JL, Abendroth A, Slobedman B. 2008. Immunomodulatory properties of a viral homolog of human interleukin-10 expressed by human cytomegalovirus during the latent phase of infection. J. Virol. 82:3736–3750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pepperl-Klindworth S, Besold K, Frankenberg N, Farkas M, Kuball J, Theobald M, Plachter B. 2006. Cytomegalovirus interleukin-10 expression in infected cells does not impair MHC class I restricted peptide presentation on bystanding antigen-presenting cells. Viral Immunol. 19:92–101 [DOI] [PubMed] [Google Scholar]
- 21. Stanton RJ, Baluchova K, Dargan DJ, Cunningham C, Sheehy O, Seirafian S, McSharry BP, Neale ML, Davies JA, Tomasec P, Davison AJ, Wilkinson GW. 2010. Reconstruction of the complete human cytomegalovirus genome in a BAC reveals RL13 to be a potent inhibitor of replication. J. Clin. Invest. 120:3191–3208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thomason L, Court DL, Bubunenko M, Costantino N, Wilson H, Datta S, Oppenheim A. 2007. Recombineering: genetic engineering in bacteria using homologous recombination. Curr. Protoc. Mol. Biol. Chapter 1:Unit 1.16. 10.1002/0471142727.mb0116s78 [DOI] [PubMed] [Google Scholar]
- 23. Sulahian TH, Hogger P, Wahner AE, Wardwell K, Goulding NJ, Sorg C, Droste A, Stehling M, Wallace PK, Morganelli PM, Guyre PM. 2000. Human monocytes express CD163, which is upregulated by IL-10 and identical to p155. Cytokine 12:1312–1321 [DOI] [PubMed] [Google Scholar]
- 24. Williams L, Jarai G, Smith A, Finan P. 2002. IL-10 expression profiling in human monocytes. J. Leukoc. Biol. 72:800–809 [PubMed] [Google Scholar]
- 25. Zizzo G, Hilliard BA, Monestier M, Cohen PL. 2012. Efficient clearance of early apoptotic cells by human macrophages requires M2c polarization and MerTK induction. J. Immunol. 189:3508–3520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moestrup SK, Moller HJ. 2004. CD163: a regulated hemoglobin scavenger receptor with a role in the anti-inflammatory response. Ann. Med. 36:347–354 [DOI] [PubMed] [Google Scholar]
- 27. Philippidis P, Mason JC, Evans BJ, Nadra I, Taylor KM, Haskard DO, Landis RC. 2004. Hemoglobin scavenger receptor CD163 mediates interleukin-10 release and heme oxygenase-1 synthesis: antiinflammatory monocyte-macrophage responses in vitro, in resolving skin blisters in vivo, and after cardiopulmonary bypass surgery. Circ. Res. 94:119–126 [DOI] [PubMed] [Google Scholar]
- 28. Martinez FO, Gordon S, Locati M, Mantovani A. 2006. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J. Immunol. 177:7303–7311 [DOI] [PubMed] [Google Scholar]
- 29. Gratchev A, Guillot P, Hakiy N, Politz O, Orfanos CE, Schledzewski K, Goerdt S. 2001. Alternatively activated macrophages differentially express fibronectin and its splice variants and the extracellular matrix protein βIG-H3. Scand. J. Immunol. 53:386–392 [DOI] [PubMed] [Google Scholar]
- 30. Chan G, Bivins-Smith ER, Smith MS, Smith PM, Yurochko AD. 2008. Transcriptome analysis reveals human cytomegalovirus reprograms monocyte differentiation toward an M1 macrophage. J. Immunol. 181:698–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Taylor-Wiedeman J, Sissons P, Sinclair J. 1994. Induction of endogenous human cytomegalovirus gene expression after differentiation of monocytes from healthy carriers. J. Virol. 68:1597–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Otterbein LE, Soares MP, Yamashita K, Bach FH. 2003. Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol. 24:449–455 [DOI] [PubMed] [Google Scholar]
- 33. Lee TS, Chau LY. 2002. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat. Med. 8:240–246 [DOI] [PubMed] [Google Scholar]
- 34. Ricchetti GA, Williams LM, Foxwell BM. 2004. Heme oxygenase 1 expression induced by IL-10 requires STAT-3 and phosphoinositol-3 kinase and is inhibited by lipopolysaccharide. J. Leukoc. Biol. 76:719–726 [DOI] [PubMed] [Google Scholar]
- 35. Chang WL, Baumgarth N, Yu D, Barry PA. 2004. Human cytomegalovirus-encoded interleukin-10 homolog inhibits maturation of dendritic cells and alters their functionality. J. Virol. 78:8720–8731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Spencer JV, Lockridge KM, Barry PA, Lin G, Tsang M, Penfold ME, Schall TJ. 2002. Potent immunosuppressive activities of cytomegalovirus-encoded interleukin-10. J. Virol. 76:1285–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Backe E, Schwarting R, Gerdes J, Ernst M, Stein H. 1991. Ber-MAC3: new monoclonal antibody that defines human monocyte/macrophage differentiation antigen. J. Clin. Pathol. 44:936–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pulford K, Micklem K, McCarthy S, Cordell J, Jones M, Mason DY. 1992. A monocyte/macrophage antigen recognized by the four antibodies GHI/61, Ber-MAC3, Ki-M8 and SM4. Immunology 75:588–595 [PMC free article] [PubMed] [Google Scholar]
- 39. Kristiansen M, Graversen JH, Jacobsen C, Sonne O, Hoffman HJ, Law SK, Moestrup SK. 2001. Identification of the haemoglobin scavenger receptor. Nature 409:198–201 [DOI] [PubMed] [Google Scholar]
- 40. Hogger P, Dreier J, Droste A, Buck F, Sorg C. 1998. Identification of the integral membrane protein RM3/1 on human monocytes as a glucocorticoid-inducible member of the scavenger receptor cysteine-rich family (CD163). J. Immunol. 161:1883–1890 [PubMed] [Google Scholar]
- 41. Morganelli PM, Guyre PM. 1988. IFN-gamma plus glucocorticoids stimulate the expression of a newly identified human mononuclear phagocyte-specific antigen. J. Immunol. 140:2296–2304 [PubMed] [Google Scholar]
- 42. Abraham NG, Drummond G. 2006. CD163-mediated hemoglobin-heme uptake activates macrophage HO-1, providing an antiinflammatory function. Circ. Res. 99:911–914 [DOI] [PubMed] [Google Scholar]
- 43. Schaer CA, Schoedon G, Imhof A, Kurrer MO, Schaer DJ. 2006. Constitutive endocytosis of CD163 mediates hemoglobin-heme uptake and determines the noninflammatory and protective transcriptional response of macrophages to hemoglobin. Circ. Res. 99:943–950 [DOI] [PubMed] [Google Scholar]
- 44. Spencer JV. 2007. The cytomegalovirus homolog of interleukin-10 requires phosphatidylinositol 3-kinase activity for inhibition of cytokine synthesis in monocytes. J. Virol. 81:2083–2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Frings W, Dreier J, Sorg C. 2002. Only the soluble form of the scavenger receptor CD163 acts inhibitory on phorbol ester-activated T-lymphocytes, whereas membrane-bound protein has no effect. FEBS Lett. 526:93–96 [DOI] [PubMed] [Google Scholar]
- 46. Hogger P, Sorg C. 2001. Soluble CD163 inhibits phorbol ester-induced lymphocyte proliferation. Biochem. Biophys. Res. Commun. 288:841–843 [DOI] [PubMed] [Google Scholar]
- 47. Chan G, Bivins-Smith ER, Smith MS, Yurochko AD. 2009. NF-κB and phosphatidylinositol 3-kinase activity mediates the HCMV-induced atypical M1/M2 polarization of monocytes. Virus Res. 144:329–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chan G, Nogalski MT, Yurochko AD. 2012. Human cytomegalovirus stimulates monocyte-to-macrophage differentiation via the temporal regulation of caspase 3. J. Virol. 86:10714–10723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Romo N, Magri G, Muntasell A, Heredia G, Baia D, Angulo A, Guma M, Lopez-Botet M. 2011. Natural killer cell-mediated response to human cytomegalovirus-infected macrophages is modulated by their functional polarization. J. Leukoc. Biol. 90:717–726 [DOI] [PubMed] [Google Scholar]
- 50. Bayer C, Varani S, Wang L, Walther P, Zhou S, Straschewski S, Bachem M, Soderberg-Naucler C, Mertens T, Frascaroli G. 2013. Human cytomegalovirus infection of M1 and M2 macrophages triggers inflammation and autologous T-cell proliferation. J. Virol. 87:67–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Poglitsch M, Weichhart T, Hecking M, Werzowa J, Katholnig K, Antlanger M, Krmpotic A, Jonjic S, Horl WH, Zlabinger GJ, Puchhammer E, Saemann MD. 2012. CMV late phase-induced mTOR activation is essential for efficient virus replication in polarized human macrophages. Am. J. Transplant. 12:1458–1468 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.